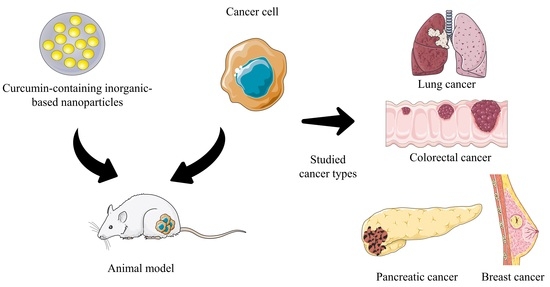
Recent Trends in Curcumin-Containing Inorganic-Based Nanoparticles Intended for In Vivo Cancer Therapy
Abstract
1. Introduction
2. Curcumin and Its Anticancer Effects
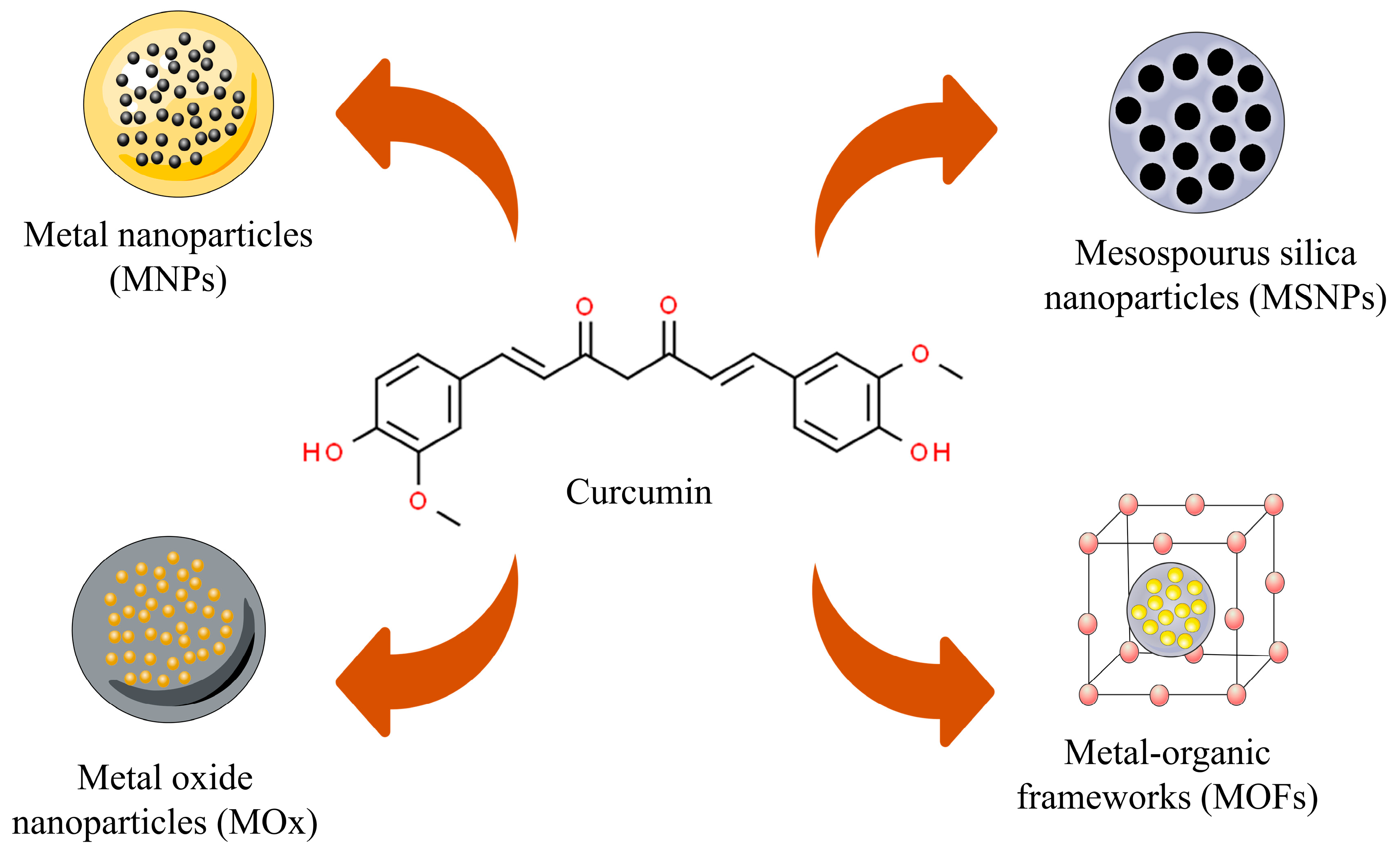
3. Curcumin-Based Inorganic Nanoparticles—Physicochemical Characteristics
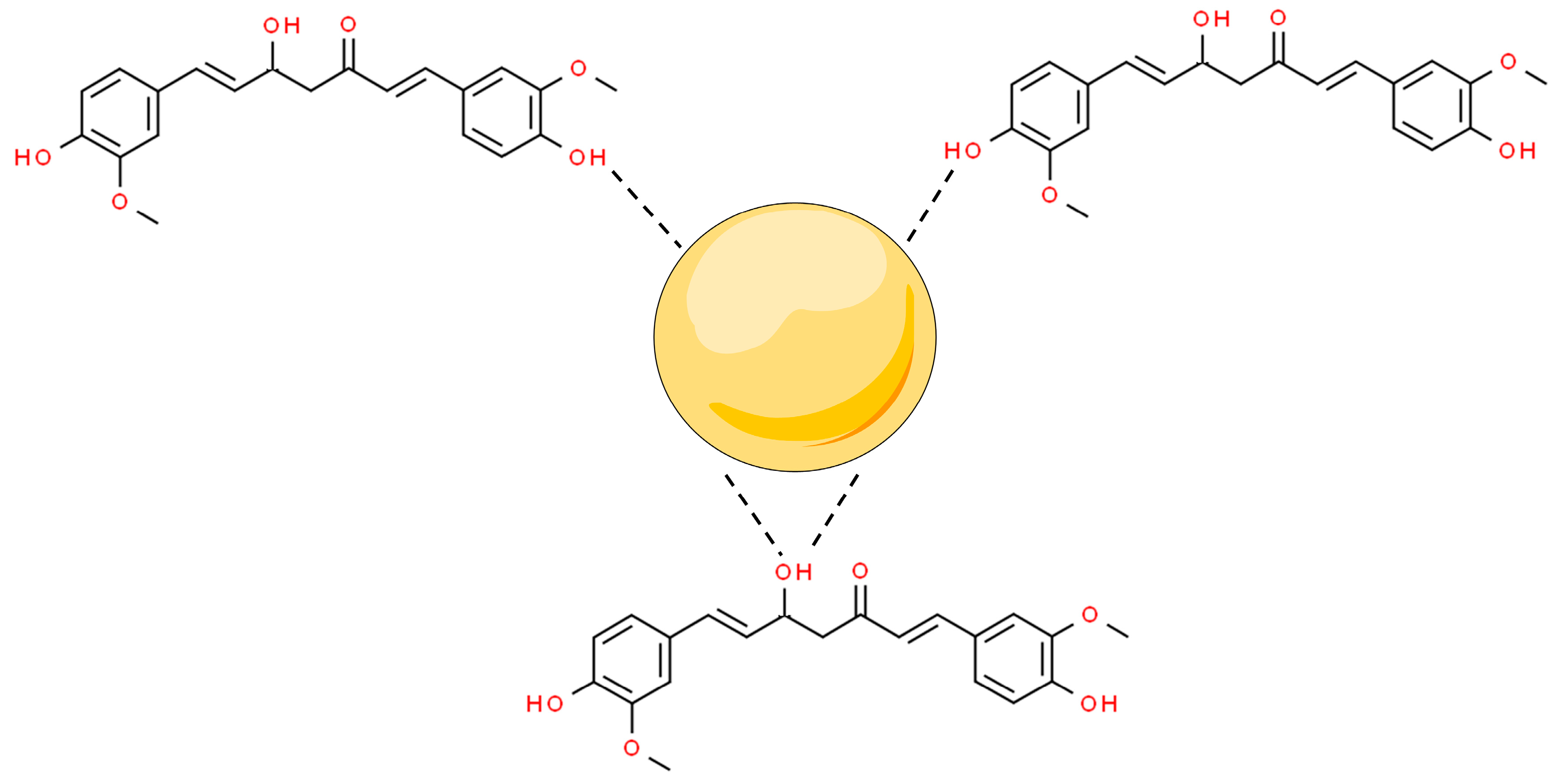
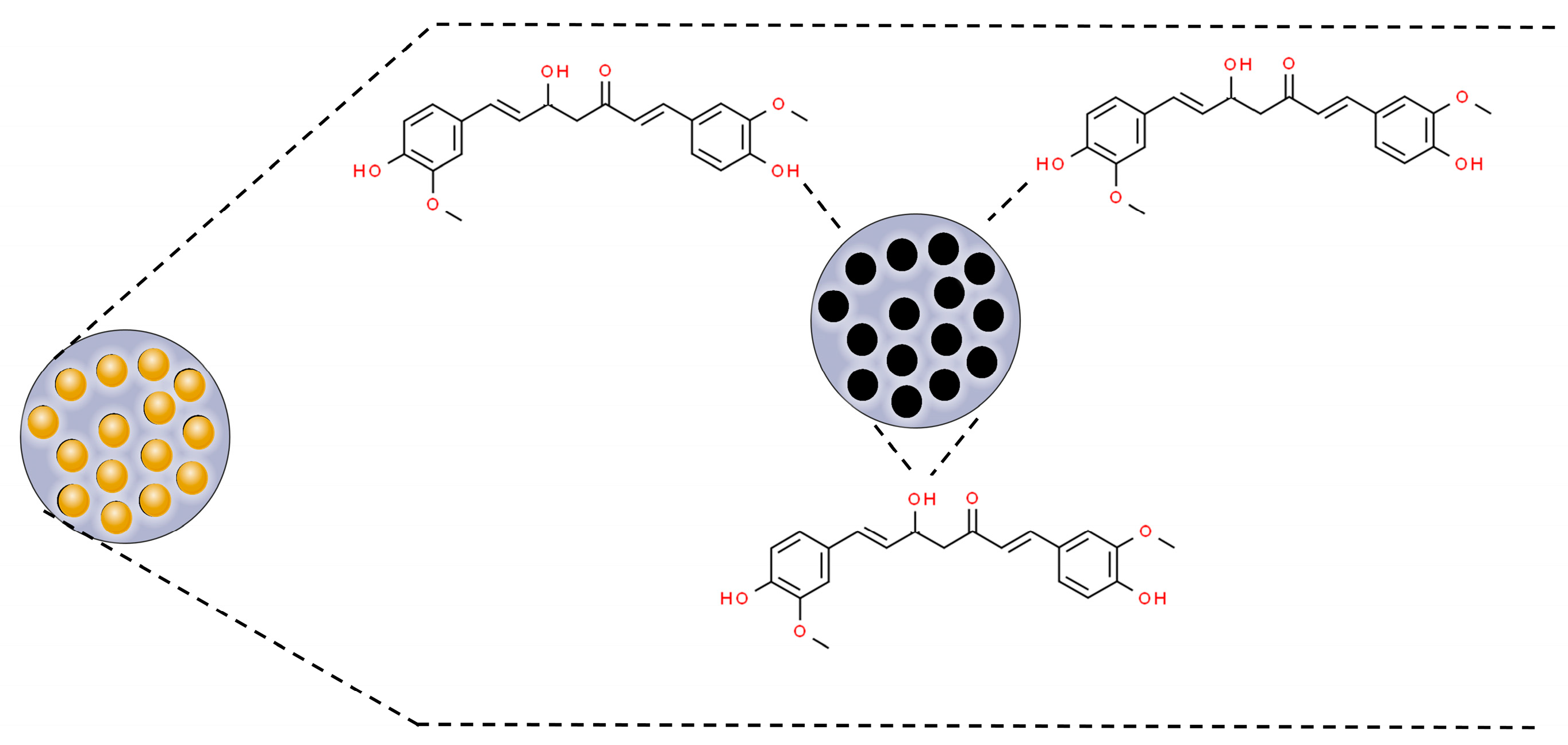
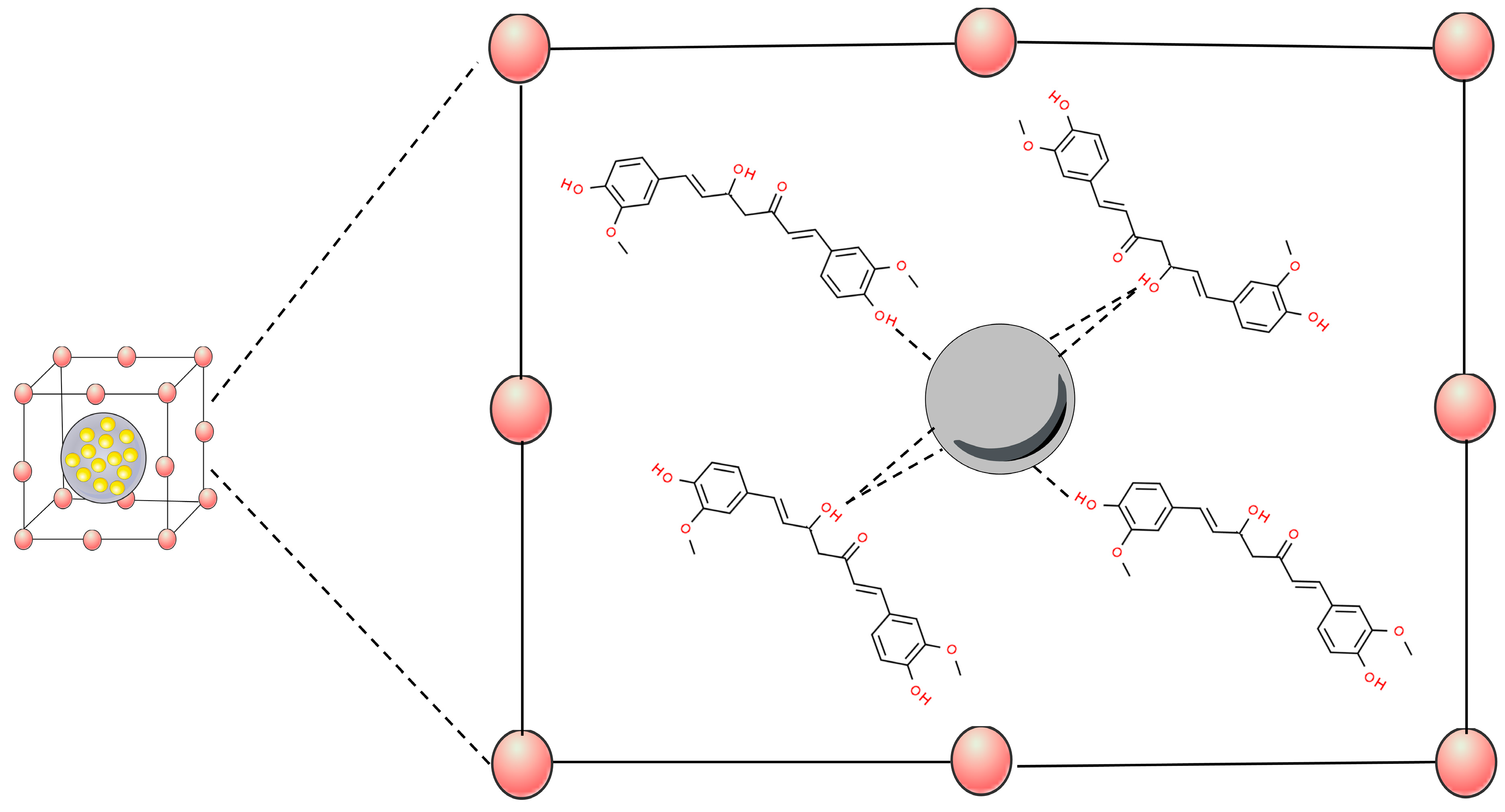
3.1. Metal Nanoparticles
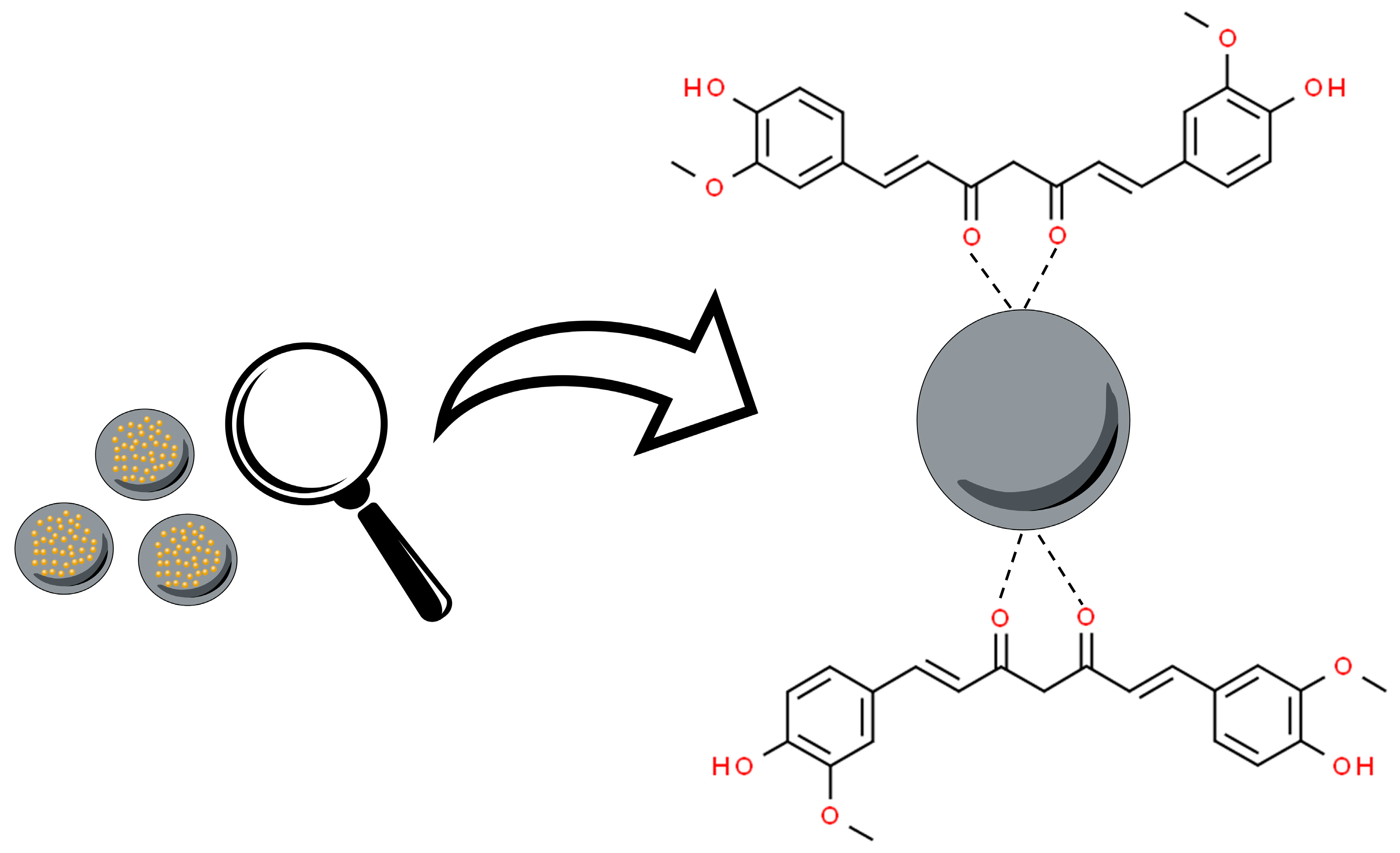
3.2. Metal Oxide Nanoparticles
3.3. Mesoporous Silica Nanoparticles (MSNPs)
3.4. Metal–Organic Frameworks (MOFs)
4. Curcumin In Vivo Cancer Studies
4.1. Pancreatic Cancer
4.2. Lung Cancer
4.3. Breast Cancer
4.4. Colorectal Cancer
5. Limitations and Future Directions
6. Final Considerations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Alzahrani, S.M.; Al Doghaither, H.A.; Al-Ghafari, A.B. General insight into cancer: An overview of colorectal cancer (Review). Mol. Clin. Oncol. 2021, 15, 271. [Google Scholar] [CrossRef]
- Diori Karidio, I.; Sanlier, S.H. Reviewing cancer’s biology: An eclectic approach. J. Egypt. Natl. Canc Inst. 2021, 33, 32. [Google Scholar] [CrossRef]
- Li, T.; Kang, G.; Wang, T.; Huang, H. Tumor angiogenesis and anti-angiogenic gene therapy for cancer. Oncol. Lett. 2018, 16, 687–702. [Google Scholar] [CrossRef]
- Kerr, A.J.; Dodwell, D.; McGale, P.; Holt, F.; Duane, F.; Mannu, G.; Darby, S.C.; Taylor, C.W. Adjuvant and neoadjuvant breast cancer treatments: A systematic review of their effects on mortality. Cancer Treat. Rev. 2022, 105, 102375. [Google Scholar] [CrossRef]
- Xuan, W.; Haiyun, Z.; Xiaozhuo, C. Drug resistance and combating drug resistance in cancer. Cancer Drug Resist. 2019, 2, 141–160. [Google Scholar] [CrossRef]
- Huang, M.; Lu, J.-J.; Ding, J. Natural Products in Cancer Therapy: Past, Present and Future. Nat. Prod. Bioprospect 2021, 11, 5–13. [Google Scholar] [CrossRef]
- Yang, Z.-J.; Huang, S.-Y.; Zhou, D.-D.; Xiong, R.-G.; Zhao, C.-N.; Fang, A.-P.; Zhang, Y.-J.; Li, H.-B.; Zhu, H.-L. Effects and Mechanisms of Curcumin for the Prevention and Management of Cancers: An Updated Review. Antioxidants 2022, 11, 1481. [Google Scholar] [CrossRef]
- Celani, L.A.-O.; Egito, E.A.-O.; Azevedo, Í.M.A.-O.; Oliveira, C.A.-O.X.; Dourado, D.A.-O.; Medeiros, A.A.-O.X. Treatment of colitis by oral negatively charged nanostructured curcumin in rats. Acta Cir. Bras. 2022, 37, 1678–2674. [Google Scholar] [CrossRef]
- Dytrych, P.; Kejík, Z.; Hajduch, J.; Kaplánek, R.; Veselá, K.; Kučnirová, K.; Skaličková, M.; Venhauerová, A.; Hoskovec, D.; Martásek, P.; et al. Therapeutic potential and limitations of curcumin as antimetastatic agent. Biomed. Pharmacother. 2023, 163, 114758. [Google Scholar] [CrossRef]
- Dourado, D.; Batista, F.P.; Philadelpho, B.O.; de Souza, M.L.; de Cerqueira e Silva, M.B.; de Grandis, R.A.; Miranda, P.A.; Colauto, N.B.; Pereira, D.T.; Formiga, F.R.; et al. Resveratrol-Loaded Attalea funifera Oil Organogel Nanoparticles: A Potential Nanocarrier against A375 Human Melanoma Cells. Int. J. Mol. Sci. 2023, 24, 2112. [Google Scholar] [CrossRef]
- Gong, G.; Fu, B.; Ying, C.; Zhu, Z.; He, X.; Li, Y.; Shen, Z.; Xuan, Q.; Huang, Y.; Lin, Y.; et al. Targeted delivery of paclitaxel by functionalized selenium nanoparticles for anticancer therapy through ROS-mediated signaling pathways. RSC Adv. 2018, 8, 39957–39966. [Google Scholar] [CrossRef]
- Hsiao, C.-H.; Huang, H.-L.; Chen, Y.-H.; Chen, M.-L.; Lin, Y.-H. Enhanced antitumor effect of doxorubicin through active-targeted nanoparticles in doxorubicin-resistant triple-negative breast cancer. J. Drug Deliv. Sci. Technol. 2022, 77, 103845. [Google Scholar] [CrossRef]
- Raheem, M.A.; Rahim, M.A.; Gul, I.; Zhong, X.; Xiao, C.; Zhang, H.; Wei, J.; He, Q.; Hassan, M.; Zhang, C.Y.; et al. Advances in nanoparticles-based approaches in cancer theranostics. OpenNano 2023, 12, 100152. [Google Scholar] [CrossRef]
- Gavas, S.; Quazi, S.; Karpiński, T.A.-O. Nanoparticles for Cancer Therapy: Current Progress and Challenges. Nanoscale Res. Lett. 2021, 16, 173. [Google Scholar] [CrossRef]
- Yeo, S.; Kim, M.J.; Shim, Y.K.; Yoon, I.; Lee, W.K. Solid Lipid Nanoparticles of Curcumin Designed for Enhanced Bioavailability and Anticancer Efficiency. ACS Omega 2022, 7, 35875–35884. [Google Scholar] [CrossRef]
- Hu, H.; Liao, Z.; Xu, M.; Wan, S.; Wu, Y.; Zou, W.; Wu, J.; Fan, Q. Fabrication, Optimization, and Evaluation of Paclitaxel and Curcumin Coloaded PLGA Nanoparticles for Improved Antitumor Activity. ACS Omega 2023, 8, 976–986. [Google Scholar] [CrossRef]
- Valencia, M.S.; da Silva Júnior, M.F.; Xavier-Júnior, F.H.; de Oliveira Veras, B.; de Albuquerque, P.B.S.; de Oliveira Borba, E.F.; da Silva, T.G.; Xavier, V.L.; de Souza, M.P.; das Graças Carneiro-da-Cunha, M. Characterization of curcumin-loaded lecithin-chitosan bioactive nanoparticles. Carbohydr. Polym. Technol. Appl. 2021, 2, 100119. [Google Scholar] [CrossRef]
- López-Lorente, Á.I.; Valcárcel, M. Analytical Nanoscience and Nanotechnology. In Comprehensive Analytical Chemistry; Valcárcel, M., López-Lorente, Á.I., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 66, pp. 3–35. [Google Scholar]
- Pugazhendhi, A.; Edison, T.; Karuppusamy, I.; Kathirvel, B. Inorganic nanoparticles: A potential cancer therapy for human welfare. Int. J. Pharm. 2018, 539, 104–111. [Google Scholar] [CrossRef]
- Tuli, H.S.; Joshi, R.; Kaur, G.; Garg, V.K.; Sak, K.; Varol, M.; Kaur, J.; Alharbi, S.A.; Alahmadi, T.A.; Aggarwal, D.; et al. Metal nanoparticles in cancer: From synthesis and metabolism to cellular interactions. J. Nanosctructure Chem. 2023, 13, 321–348. [Google Scholar] [CrossRef]
- Amaldoss, M.J.N.; Yang, J.-L.; Koshy, P.; Unnikrishnan, A.; Sorrell, C.C. Inorganic nanoparticle-based advanced cancer therapies: Promising combination strategies. Drug Discov. Today 2022, 27, 103386. [Google Scholar] [CrossRef]
- Sahne, F.; Mohammadi, M.; Najafpour, G.D. Single-Layer Assembly of Multifunctional Carboxymethylcellulose on Graphene Oxide Nanoparticles for Improving in Vivo Curcumin Delivery into Tumor Cells. ACS Biomater. Sci. Eng. 2019, 5, 2595–2609. [Google Scholar] [CrossRef]
- Farghadani, R.; Naidu, R. The anticancer mechanism of action of selected polyphenols in triple-negative breast cancer (TNBC). Biomed. Pharmacother. 2023, 165, 115170. [Google Scholar] [CrossRef]
- Briguglio, G.; Costa, C.; Pollicino, M.; Giambò, F.; Catania, S.; Fenga, C. Polyphenols in cancer prevention: New insights (Review). Int. J. Funct. Nutr. 2020, 1, 9. [Google Scholar] [CrossRef]
- Bhosale, P.B.; Ha, S.E.; Vetrivel, P.; Kim, H.H.; Kim, S.M.; Kim, G.S. Functions of polyphenols and its anticancer properties in biomedical research: A narrative review. Transl. Cancer Res. 2020, 9, 7619–7631. [Google Scholar] [CrossRef] [PubMed]
- Abd Wahab, N.A.; Lajis, N.H.; Abas, F.; Othman, I.; Naidu, R. Mechanism of Anti-Cancer Activity of Curcumin on Androgen-Dependent and Androgen-Independent Prostate Cancer. Nutrients 2020, 12, 679. [Google Scholar] [CrossRef] [PubMed]
- Hafez Ghoran, S.A.-O.; Calcaterra, A.A.-O.; Abbasi, M.; Taktaz, F.A.-O.; Nieselt, K.; Babaei, E.A.-O. Curcumin-Based Nanoformulations: A Promising Adjuvant towards Cancer Treatment. Molecules 2022, 27, 5236. [Google Scholar] [CrossRef] [PubMed]
- Basnet, P.; Skalko-Basnet, N. Curcumin: An anti-inflammatory molecule from a curry spice on the path to cancer treatment. Molecules 2011, 16, 4567–4598. [Google Scholar] [CrossRef] [PubMed]
- Cheng, A.-L.; HSU, C.-H.; Lin, J.-K.; Hsu, M.-M.; Ho, Y.-F. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer. Res. 2001, 21, e2900. [Google Scholar]
- Dcodhar, S.; Sethi, R.; Srimal, R. Preliminary study on antirheumatic activity of curcumin (diferuloyl methane). Indian. J. Med. Res. 1980, 138, 632. [Google Scholar]
- Sahebkar, A.; Mohammadi, A.; Atabati, A.; Rahiman, S.; Tavallaie, S.; Iranshahi, M.; Akhlaghi, S.; Ferns, G.A.; Ghayour-Mobarhan, M. Curcuminoids modulate pro-oxidant-antioxidant balance but not the immune response to heat shock protein 27 and oxidized LDL in obese individuals. Phytother. Res. 2013, 27, 1883–1888. [Google Scholar] [CrossRef] [PubMed]
- Adeluola, A.; Zulfiker, A.H.M.; Brazeau, D.; Amin, A. Perspectives for synthetic curcumins in chemoprevention and treatment of cancer: An update with promising analogues. Eur. J. Pharmacol. 2021, 906, 174266. [Google Scholar] [CrossRef]
- Phillips, J.M.; Clark, C.; Herman-Ferdinandez, L.; Moore-Medlin, T.; Rong, X.; Gill, J.R.; Clifford, J.L.; Abreo, F.; Nathan, C.O. Curcumin inhibits skin squamous cell carcinoma tumor growth in vivo. Otolaryngol. Head. Neck Surg. 2011, 145, 58–63. [Google Scholar] [CrossRef]
- Sun, S.; Fang, H. Curcumin inhibits ovarian cancer progression by regulating circ-PLEKHM3/miR-320a/SMG1 axis. J. Ovarian Res. 2021, 14, 158. [Google Scholar] [CrossRef]
- Tian, S.; Liao, L.; Zhou, Q.; Huang, X.; Zheng, P.; Guo, Y.; Deng, T.; Tian, X. Curcumin inhibits the growth of liver cancer by impairing myeloid-derived suppressor cells in murine tumor tissues. Oncol. Lett. 2021, 21, 286. [Google Scholar] [CrossRef]
- Yang, Q.-Q.; Farha, A.K.; Kim, G.; Gul, K.; Gan, R.-Y.; Corke, H. Antimicrobial and anticancer applications and related mechanisms of curcumin-mediated photodynamic treatments. Trends Food Sci. Technol. 2020, 97, 341–354. [Google Scholar] [CrossRef]
- Fu, H.; Wang, C.; Yang, D.; Wei, Z.; Xu, J.; Hu, Z.; Zhang, Y.; Wang, W.; Yan, R.; Cai, Q. Curcumin regulates proliferation, autophagy, and apoptosis in gastric cancer cells by affecting PI3K and P53 signaling. J. Cell Physiol. 2018, 233, 4634–4642. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Li, M.; Guo, T.; Wang, S.; Huang, W.; Yang, K.; Liao, Z.; Wang, J.; Zhang, F.; Wang, H. Anti-metastasis activity of curcumin against breast cancer via the inhibition of stem cell-like properties and EMT. Phytomedicine 2019, 58, 152740. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhai, Y.; Heng, X.; Che, F.Y.; Chen, W.; Sun, D.; Zhai, G. Oral bioavailability of curcumin: Problems and advancements. J. Drug Target. 2016, 24, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Wahlang, B.; Pawar, Y.B.; Bansal, A.K. Identification of permeability-related hurdles in oral delivery of curcumin using the Caco-2 cell model. Eur. J. Pharm. Biopharm. 2011, 77, 275–282. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Ma, W.; Tu, P. The mechanism of self-assembled mixed micelles in improving curcumin oral absorption: In vitro and in vivo. Colloids Surf. B Biointerfaces 2015, 133, 108–119. [Google Scholar] [CrossRef] [PubMed]
- Dourado, D.; Oliveira, M.C.d.; Araujo, G.R.S.d.; Amaral-Machado, L.; Porto, D.L.; Aragão, C.F.S.; Alencar, E.d.N.; Egito, E.S.T.d. Low-surfactant microemulsion, a smart strategy intended for curcumin oral delivery. Colloids Surf. A Physicochem. Eng. Asp. 2022, 652, 129720. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Campos, M.L.; Arcaro, C.A.; Assis, R.P.; Baldan-Cimatti, H.M.; Peccinini, R.G.; Paula-Gomes, S.; Kettelhut, I.C.; Baviera, A.M.; Brunetti, I.L. Curcumin Pharmacokinetic and Pharmacodynamic Evidences in Streptozotocin-Diabetic Rats Support the Antidiabetic Activity to Be via Metabolite(s). J. Evid. Based Complement. Altern. Med. 2015, 2015, 678218. [Google Scholar] [CrossRef] [PubMed]
- Wanninger, S.; Lorenz, V.; Subhan, A.; Edelmann, F.T. Metal complexes of curcumin--synthetic strategies, structures and medicinal applications. Chem. Soc. Rev. 2015, 44, 4986–5002. [Google Scholar] [CrossRef]
- Prasad, S.; DuBourdieu, D.; Srivastava, A.; Kumar, P.; Lall, R. Metal-Curcumin Complexes in Therapeutics: An Approach to Enhance Pharmacological Effects of Curcumin. Int. J. Mol. Sci. 2021, 22, 7094. [Google Scholar] [CrossRef]
- Meena, J.; Gupta, A.; Ahuja, R.; Singh, M.; Bhaskar, S.; Panda, A.K. Inorganic nanoparticles for natural product delivery: A review. Environ. Chem. Lett. 2020, 18, 2107–2118. [Google Scholar] [CrossRef]
- Tiwari, A.P.; Rohiwal, S.S. Chapter 2—Synthesis and Bioconjugation of Hybrid Nanostructures for Biomedical Applications. In Hybrid Nanostructures for Cancer Theranostics; Ashok Bohara, R., Thorat, N., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 17–41. [Google Scholar]
- Saleh, T.A. Chapter 8—Properties of nanoadsorbents and adsorption mechanisms. In Interface Science and Technology; Saleh, T.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2022; Volume 34, pp. 233–263. [Google Scholar]
- Cobley, C.M.; Chen, J.; Cho, E.C.; Wang, L.V.; Xia, Y. Gold nanostructures: A class of multifunctional materials for biomedical applications. Chem. Soc. Rev. 2011, 40, 44–56. [Google Scholar] [CrossRef]
- Mahalunkar, S.; Yadav, A.S.; Gorain, M.; Pawar, V.; Braathen, R.; Weiss, S.; Bogen, B.; Gosavi, S.W.; Kundu, G.C. Functional design of pH-responsive folate-targeted polymer-coated gold nanoparticles for drug delivery and in vivo therapy in breast cancer. Int. J. Nanomed. 2019, 14, 8285–8302. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, S.V.; Nikam, D.S.; Khot, V.M.; Thorat, N.D.; Phadatare, M.R.; Ningthoujam, R.S.; Salunkhe, A.B.; Pawar, S.H. Studies on colloidal stability of PVP-coated LSMO nanoparticles for magnetic fluid hyperthermia. New J. Chem. 2013, 37, 3121–3130. [Google Scholar] [CrossRef]
- Koczkur, K.M.; Mourdikoudis, S.; Polavarapu, L.; Skrabalak, S.E. Polyvinylpyrrolidone (PVP) in nanoparticle synthesis. Dalton Trans. 2015, 44, 17883–17905. [Google Scholar] [CrossRef]
- Panda, J.J.; Kaul, A.; Kumar, S.; Alam, S.; Mishra, A.K.; Kundu, G.C.; Chauhan, V.S. Modified dipeptide-based nanoparticles: Vehicles for targeted tumor drug delivery. Nanomedicine 2013, 8, 1927–1942. [Google Scholar] [CrossRef]
- Lowry, G.V.; Hill, R.J.; Harper, S.; Rawle, A.F.; Hendren, C.O.; Klaessig, F.; Nobbmann, U.; Sayre, P.; Rumble, J. Guidance to improve the scientific value of zeta-potential measurements in nanoEHS. Environ. Sci. Nano 2016, 3, 953–965. [Google Scholar] [CrossRef]
- Alibolandi, M.; Hoseini, F.; Mohammadi, M.; Ramezani, P.; Einafshar, E.; Taghdisi, S.M.; Ramezani, M.; Abnous, K. Curcumin-entrapped MUC-1 aptamer targeted dendrimer-gold hybrid nanostructure as a theranostic system for colon adenocarcinoma. Int. J. Pharm. 2018, 549, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Mohd Amin, M.C.I.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Kesharwani, P.; Choudhury, H.; Meher, J.G.; Pandey, M.; Gorain, B. Dendrimer-entrapped gold nanoparticles as promising nanocarriers for anticancer therapeutics and imaging. Prog. Mater. Sci. 2019, 103, 484–508. [Google Scholar] [CrossRef]
- Kesharwani, P.; Banerjee, S.; Gupta, U.; Mohd Amin, M.C.I.; Padhye, S.; Sarkar, F.H.; Iyer, A.K. PAMAM dendrimers as promising nanocarriers for RNAi therapeutics. Mater. Today 2015, 18, 565–572. [Google Scholar] [CrossRef]
- Thakur, S.; Kesharwani, P.; Tekade, R.K.; Jain, N.K. Impact of pegylation on biopharmaceutical properties of dendrimers. Polymer 2015, 59, 67–92. [Google Scholar] [CrossRef]
- Xu, Z.; Joshi, N.; Agarwal, A.; Dahiya, S.; Bittner, P.; Smith, E.; Taylor, S.; Piwnica-Worms, D.; Weber, J.; Leonard, J.R. Knocking down nucleolin expression in gliomas inhibits tumor growth and induces cell cycle arrest. J. Neurooncol. 2012, 108, 59–67. [Google Scholar] [CrossRef]
- Alibolandi, M.; Taghdisi, S.M.; Ramezani, P.; Hosseini Shamili, F.; Farzad, S.A.; Abnous, K.; Ramezani, M. Smart AS1411-aptamer conjugated pegylated PAMAM dendrimer for the superior delivery of camptothecin to colon adenocarcinoma in vitro and in vivo. Int. J. Pharm. 2017, 519, 352–364. [Google Scholar] [CrossRef]
- Khandelwal, P.; Alam, A.; Choksi, A.; Chattopadhyay, S.; Poddar, P. Retention of Anticancer Activity of Curcumin after Conjugation with Fluorescent Gold Quantum Clusters: An in Vitro and in Vivo Xenograft Study. ACS Omega 2018, 3, 4776–4785. [Google Scholar] [CrossRef]
- Bhandari, R.; Gupta, P.; Dziubla, T.; Hilt, J.Z. Single step synthesis, characterization and applications of curcumin functionalized iron oxide magnetic nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 67, 59–64. [Google Scholar] [CrossRef]
- Yallapu, M.M.; Ebeling, M.C.; Khan, S.; Sundram, V.; Chauhan, N.; Gupta, B.K.; Puumala, S.E.; Jaggi, M.; Chauhan, S.C. Novel curcumin-loaded magnetic nanoparticles for pancreatic cancer treatment. Mol. Cancer Ther. 2013, 12, 1471–1480. [Google Scholar] [CrossRef]
- Bilal, M.; Mehmood, S.; Rasheed, T.; Iqbal, H.M.N. Bio-catalysis and biomedical perspectives of magnetic nanoparticles as versatile carriers. Magnetochemistry 2019, 5, 42. [Google Scholar] [CrossRef]
- Colombo, M.; Carregal-Romero, S.; Casula, M.F.; Gutierrez, L.; Morales, M.P.; Bohm, I.B.; Heverhagen, J.T.; Prosperi, D.; Parak, W.J. Biological applications of magnetic nanoparticles. Chem. Soc. Rev. 2012, 41, 4306–4334. [Google Scholar] [CrossRef]
- Issa, B.; Obaidat, I.M.; Albiss, B.A.; Haik, Y. Magnetic nanoparticles: Surface effects and properties related to biomedicine applications. Int. J. Mol. Sci. 2013, 14, 21266–21305. [Google Scholar] [CrossRef]
- Ahmed, S.; Ikram, S.; Kanchi, S.; Bisetty, K. Biocomposites Biomedical and Environmental Applications; Pan Stanford: Stanford, CA, USA, 2018. [Google Scholar]
- Kundu, M.; Sadhukhan, P.; Ghosh, N.; Chatterjee, S.; Manna, P.; Das, J.; Sil, P.C. pH-responsive and targeted delivery of curcumin via phenylboronic acid-functionalized ZnO nanoparticles for breast cancer therapy. J. Adv. Res. 2019, 18, 161–172. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Priyadarsini, K. The chemistry of curcumin: From extraction to therapeutic agent. Molecules 2014, 19, 20091–20112. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, X.; Jin, S.; Xue, X.; Zhang, C.; Wei, T.; Guo, W.; Liang, X.J. Zinc oxide nanoparticles as adjuvant to facilitate doxorubicin intracellular accumulation and visualize pH-responsive release for overcoming drug resistance. Mol. Pharm. 2016, 13, 1723–1730. [Google Scholar] [CrossRef] [PubMed]
- Paul, S.; Hmar, E.B.L.; Pathak, H.; Sharma, H.K. An overview on nanocarriers. In Nanocarriers for Drug-Targeting Brain Tumors; Kumar, L., Pathak, Y.Y., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 145–204. [Google Scholar]
- Lind, A.; Spliethoff, B.; Lindén, M. Unusual, vesicle-like patterned, mesoscopically ordered silica. Chem. Mater. 2003, 15, 813–818. [Google Scholar] [CrossRef]
- Xu, C.; Lei, C.; Yu, C. Mesoporous silica nanoparticles for protein protection and delivery. Front. Chem. 2019, 7, 290. [Google Scholar] [CrossRef] [PubMed]
- Koohi Moftakhari Esfahani, M.; Alavi, S.E.; Cabot, P.J.; Islam, N.; Izake, E.L. Application of Mesoporous Silica Nanoparticles in Cancer Therapy and Delivery of Repurposed Anthelmintics for Cancer Therapy. Pharmaceutics 2022, 14, 1579. [Google Scholar] [CrossRef]
- Manzano, M.; Vallet-Regí, M. Mesoporous Silica Nanoparticles for Drug Delivery. Adv. Funct. Mater. 2019, 30, 1902634. [Google Scholar] [CrossRef]
- Mekaru, H.; Lu, J.; Tamanoi, F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv. Drug Deliv. Rev. 2015, 95, 40–49. [Google Scholar] [CrossRef]
- Jambhrunkar, S.; Karmakar, S.; Popat, A.; Yu, M.; Yu, C. Mesoporous silica nanoparticles enhance the cytotoxicity of curcumin. Rsc Adv. 2014, 4, 709–712. [Google Scholar] [CrossRef]
- Lungare, S.; Hallam, K.; Badhan, R.K. Phytochemical-loaded mesoporous silica nanoparticles for nose-to-brain olfactory drug delivery. Int. J. Pharm. 2016, 513, 280–293. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, T.d.C.; Sábio, R.M.; Luiz, M.T.; de Souza, L.C.; Fonseca-Santos, B.; Cides da Silva, L.C.; Fantini, M.C.d.A.; Planeta, C.d.S.; Chorilli, M. Curcumin-Loaded Mesoporous Silica Nanoparticles Dispersed in Thermo-Responsive Hydrogel as Potential Alzheimer Disease Therapy. Pharmaceutics 2022, 14, 1976. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Wang, Z.; Zhang, Y.; Zhang, K.; Xie, J.; Liu, Y.; Li, W.; Feng, N. Curcumin-loaded redox-responsive mesoporous silica nanoparticles for targeted breast cancer therapy. Artif. Cells Nanomed. Biotechnol. 2018, 46, 921–935. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, X.; Nie, W.; Zhang, Q.; Wang, W.; Zhang, Y.; He, C. Multifunctional redox-responsive mesoporous silica nanoparticles for efficient targeting drug delivery and magnetic resonance imaging. ACS Appl. Mater. Interfaces 2016, 8, 33829–33841. [Google Scholar] [CrossRef]
- Yaghi, O.M.; O’Keeffe, M.; Ockwig, N.W.; Chae, H.K.; Eddaoudi, M.; Kim, J. Reticular synthesis and the design of new materials. Nature 2003, 423, 705–714. [Google Scholar] [CrossRef]
- Zhang, F.; Zhang, J.; Zhang, B.; Zheng, L.; Cheng, X.; Wan, Q.; Han, B.; Zhang, J. CO(2) controls the oriented growth of metal-organic framework with highly accessible active sites. Nat. Commun. 2020, 11, 1431. [Google Scholar] [CrossRef]
- Phang, W.J.; Jo, H.; Lee, W.R.; Song, J.H.; Yoo, K.; Kim, B.; Hong, C.S. Superprotonic conductivity of a UiO-66 framework functionalized with sulfonic acid groups by facile postsynthetic oxidation. Angew. Chem. Int. Ed. Engl. 2015, 54, 5142–5146. [Google Scholar] [CrossRef]
- Cai, M.; Chen, G.; Qin, L.; Qu, C.; Dong, X.; Ni, J.; Yin, X. Metal Organic Frameworks as Drug Targeting Delivery Vehicles in the Treatment of Cancer. Pharmaceutics 2020, 12, 232. [Google Scholar] [CrossRef]
- Munasinghe, V.K.; Manawadu, D.; de Silva, R.M.; de Silva, K.N. Impact of active sites on encapsulation of curcumin in Metal Organic Frameworks. Mater. Res. Express 2023, 10, 035102. [Google Scholar] [CrossRef]
- Dehghani, S.; Hosseini, M.; Haghgoo, S.; Changizi, V.; Akbari Javar, H.; Khoobi, M.; Riahi Alam, N. Multifunctional MIL-Cur@FC as a theranostic agent for magnetic resonance imaging and targeting drug delivery: In vitro and in vivo study. J. Drug Target. 2020, 28, 668–680. [Google Scholar] [CrossRef] [PubMed]
- Laha, D.; Pal, K.; Chowdhuri, A.R.; Parida, P.K.; Sahu, S.K.; Jana, K.; Karmakar, P. Fabrication of curcumin-loaded folic acid-tagged metal organic framework for triple negative breast cancer therapy in and systems. New J. Chem. 2019, 43, 217–229. [Google Scholar] [CrossRef]
- Yu, S.; Wang, S.; Xie, Z.; Yu, S.; Li, L.; Xiao, H.; Song, Y. Hyaluronic acid coating on the surface of curcumin-loaded ZIF-8 nanoparticles for improved breast cancer therapy: An in vitro and in vivo study. Colloids Surf. B Biointerfaces 2021, 203, 111759. [Google Scholar] [CrossRef] [PubMed]
- Kleeff, J.; Korc, M.; Apte, M.; La Vecchia, C.; Johnson, C.D.; Biankin, A.V.; Neale, R.E.; Tempero, M.; Tuveson, D.A.; Hruban, R.H.; et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2016, 2, 16022. [Google Scholar] [CrossRef] [PubMed]
- Halbrook, C.J.; Lyssiotis, C.A.; Pasca di Magliano, M.; Maitra, A. Pancreatic cancer: Advances and challenges. Cell 2023, 186, 1729–1754. [Google Scholar] [CrossRef]
- Zhu, Y.; Bu, S. Curcumin Induces Autophagy, Apoptosis, and Cell Cycle Arrest in Human Pancreatic Cancer Cells. Evid. Based Complement. Altern. Med. 2017, 2017, 5787218. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef]
- WHO. Lung Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/lung-cancer (accessed on 3 December 2023).
- Kumar, G.; Mittal, S.; Sak, K.; Tuli, H.S. Molecular mechanisms underlying chemopreventive potential of curcumin: Current challenges and future perspectives. Life Sci. 2016, 148, 313–328. [Google Scholar] [CrossRef]
- Smolarz, B.; Nowak, A.Z.; Romanowicz, H. Breast Cancer-Epidemiology, Classification, Pathogenesis and Treatment (Review of Literature). Cancers 2022, 14, 2569. [Google Scholar] [CrossRef]
- Hong, R.; Xu, B. Breast cancer: An up-to-date review and future perspectives. Cancer Commun. 2022, 42, 913–936. [Google Scholar] [CrossRef] [PubMed]
- Farghadani, R.; Naidu, R. Curcumin as an Enhancer of Therapeutic Efficiency of Chemotherapy Drugs in Breast Cancer. Int. J. Mol. Sci. 2022, 23, 2144. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Ho, Y.S. Anticancer effect of curcumin on breast cancer and stem cells. Food Sci. Hum. Well 2018, 7, 134–137. [Google Scholar] [CrossRef]
- Talib, W.H.; Al-Hadid, S.A.; Ali, M.B.W.; Al-Yasari, I.H.; Ali, M.R.A. Role of curcumin in regulating p53 in breast cancer: An overview of the mechanism of action. Breast Cancer (Dove Med. Press) 2018, 10, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Julien, O.; Wells, J.A. Caspases and their substrates. Cell Death Differ. 2017, 24, 1380–1389. [Google Scholar] [CrossRef]
- Thornberry, N.A.; Lazebnik, Y. Caspases: Enemies within. Science 1998, 281, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- WHO. Colorectal Cancer. Available online: https://www.who.int/news-room/fact-sheets/detail/colorectal-cancer#:~:text=Key%20facts,people%20aged%2050%20and%20above (accessed on 3 December 2023).
- Ismail, N.I.; Othman, I.; Abas, F.; Lajis, N.H.; Naidu, R. Mechanism of Apoptosis Induced by Curcumin in Colorectal Cancer. Int. J. Mol. Sci. 2019, 20, 2454. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Adeyemo, T.R.; Rotimi, D.; Batiha, G.E.; Mostafa-Hedeab, G.; Iyobhebhe, M.E.; Elebiyo, T.C.; Atunwa, B.; Ojo, A.B.; Lima, C.M.G.; et al. Anticancer Properties of Curcumin Against Colorectal Cancer: A Review. Front. Oncol. 2022, 12, 881641. [Google Scholar] [CrossRef]
- Lammers, T.; Aime, S.; Hennink, W.E.; Storm, G.; Kiessling, F. Theranostic nanomedicine. Acc. Chem. Res. 2011, 44, 1029–1038. [Google Scholar] [CrossRef] [PubMed]
| Nanoparticles | Composition | Method | Physicochemical Characteristics | References |
|---|---|---|---|---|
| MNPs | FA-Au-PVP | LBL assembly | Size: 72–350 nm and zeta potential: −9 mV | [50] |
| MNPs | PEG-AuPAMAM | Synthesis and dialysis | Size: 5 nm, zeta potential: ~4 mV, and EE: 88% | [55] |
| MNPs | Au QCs | Synthesis | Size: ~2.3–~20.2 nm | [62] |
| MOx | Iron | Co-precipitation | Size: ~100 nm and zeta potential: −0.99 mV | [64] |
| MOx | CMC, PVP, graphene oxide | Synthesis, acetylation, and polymerization | Size: 60 nm, zeta potential: −48 mV, EE: 94%, and DLC: 47% | [22] |
| MOx | PBA–ZnO | Precipitation | Size (TEM): 30–40 nm and zeta potential: −16 mV | [69] |
| MSNPs | Silica, polyethyleneimine, folic acid or hyaluronic acid | Synthesis | Size: 90 nm | [82] |
| MOFs | Iron–MOF | Hydrothermal synthesis reaction | Size: ~100 nm, zeta potential: −30 mV, and EE: 98% | [89] |
| MOFs | IRMOF-3, folic acid | Synthesis and precipitation | Size: 255.3–371.7 nm, zeta potential: −0.907 to −10.09 mV, EE: 98%, and DLC: 52% | [90] |
| MOFs | ZIF-8 and hyaluronic acid | Synthesis and precipitation | Size: 184.1 nm and DLC: ~10% | [91] |
| Cancer | Nanoparticles | Administration Regimen | In Vivo Model | In Vivo Performance | References |
|---|---|---|---|---|---|
| Pancreatic | MNP | 20 µg/100 mL for 40 days | HPAF-II human pancreatic cancer cells inoculated into male athymic nude (nu/nu) mice | Increase in tumor inhibition | [64] |
| Lung | FC-coated MIL | Intravenous (5 mg/kg) | Subcutaneously M109 cells (2 × 106) in Balb/C mice | Decrease in tumor size; high uptake | [89] |
| Breast | PBA-ZnO | Intravenous for 14 days every other day (10 mg/kg) | Ehrlich ascites carcinoma was injected into mice (107 cells per 50 mL/mouse) | Decrease in tumor; increase in caspases-9 and caspases-3 cleaved apoptosis induction mechanism | [69] |
| Breast | FA-Au-PVP | Intratumoral per 2 weeks (10 mg/kg) | 4T1 mammary carcinoma injection in female BALB/C mice | Tumor growth was inhibited | [50] |
| Breast | Au QCs | Breast cancer cells injected subcutaneously into the flank areas (20 mg/kg) | Mouse xenograft model MDA-MB-231 cells female BALB/C nude mice | Tumor growth was inhibited | [62] |
| Breast | Cur-FA-CMC/PVP GO NPs | Subcutaneous injection via tail (200 µL, 4 mg of curcumin/kg) | 4T1 breast cancer model in BALB/C mice | Tumor volume supression; tumor weight decrease | [22] |
| Breast | IRMOF-3 FA | 100 µL injected subcutaneously into mammary | Triple negative tumor-bearing BALB/C model | Tumor volume supression; tumor weight decrease | [90] |
| Breast | ZIF-8-HA | Subcutaneous injection | 4T1 xenograft model in BALB/C mice | Tumor growth inhibited; tumor weight decrease | [91] |
| Breast | PEI-FA MSN | Intravenous by the tail every three days (8 mg/kg) | Mouse xenograft model MDA-MB-231 cells female BALB/C nude mice | Tumor growth was inhibited | [82] |
| Colorectal | Apt-PEG-AuPAMAM | Intravenous per week for three weeks (2 mg/kg) | C26 tumor-bearing mice model was used for anticancer activity after 14 days of subcutaneous inoculation | Higher cellular uptake; higher cytotoxicity | [55] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dourado, D.; Miranda, J.A.; de Oliveira, M.C.; Freire, D.T.; Xavier-Júnior, F.H.; Paredes-Gamero, E.J.; Alencar, É.d.N. Recent Trends in Curcumin-Containing Inorganic-Based Nanoparticles Intended for In Vivo Cancer Therapy. Pharmaceutics 2024, 16, 177. https://doi.org/10.3390/pharmaceutics16020177
Dourado D, Miranda JA, de Oliveira MC, Freire DT, Xavier-Júnior FH, Paredes-Gamero EJ, Alencar ÉdN. Recent Trends in Curcumin-Containing Inorganic-Based Nanoparticles Intended for In Vivo Cancer Therapy. Pharmaceutics. 2024; 16(2):177. https://doi.org/10.3390/pharmaceutics16020177
Chicago/Turabian StyleDourado, Douglas, Júlio Abreu Miranda, Matheus Cardoso de Oliveira, Danielle Teixeira Freire, Francisco Humberto Xavier-Júnior, Edgar Julian Paredes-Gamero, and Éverton do Nascimento Alencar. 2024. "Recent Trends in Curcumin-Containing Inorganic-Based Nanoparticles Intended for In Vivo Cancer Therapy" Pharmaceutics 16, no. 2: 177. https://doi.org/10.3390/pharmaceutics16020177
APA StyleDourado, D., Miranda, J. A., de Oliveira, M. C., Freire, D. T., Xavier-Júnior, F. H., Paredes-Gamero, E. J., & Alencar, É. d. N. (2024). Recent Trends in Curcumin-Containing Inorganic-Based Nanoparticles Intended for In Vivo Cancer Therapy. Pharmaceutics, 16(2), 177. https://doi.org/10.3390/pharmaceutics16020177