Biomaterial-Based Responsive Nanomedicines for Targeting Solid Tumor Microenvironments
Abstract
:1. Introduction
2. Solid Tumor Nanomedicine: Distribution in Tumor Microenvironment
| Carrier Type | Product Name | Therapeutic Agent | Cancer Type | Stage | Ref. |
|---|---|---|---|---|---|
| Liposomes | Zolsketil® | Doxorubicin | Metastatic breast cancer, advanced ovarian cancer, multiple myeloma, AIDS-related Kaposi’s sarcoma (https://www.ema.europa.eu/en/medicines/human/EPAR/zolsketil-pegylated-liposomal (accessed on 15 January 2024) | Approved (EMA, 2022) | [56,57] |
| Vyxeos® | Cytarabine: daunorubicin | Newly diagnosed therapy-related acute myeloid leukemia, acute myeloid leukemia with myelodysplasia related changes (https://www.ema.europa.eu/en/medicines/human/EPAR/vyxeos-liposomal-previously-known-vyxeos, accessed on 15 January 2024) | Approved (EMA, 2018) (FDA, 2017) | [56,57] | |
| Onivyde®/CPX-351 | Irinotecan | Pancreatic cancer (https://www.ema.europa.eu/en/medicines/human/EPAR/onivyde-pegylated-liposomal-previously-known-onivyde, accessed on 15 January 2024) | Approved (EMA, 2016) (FDA, 2015) | [56,57] | |
| Mepact® | Mifamurtide | Osteosarcoma (https://www.ema.europa.eu/en/medicines/human/EPAR/mepact, accessed on 15 January 2024) | Approved (EMA, 2009) | [56,57] | |
| Ameluz® | 5-aminolevulinic acid | Superficial and/or nodular basal cell carcinoma (https://www.ema.europa.eu/en/medicines/human/EPAR/ameluz, accessed on 15 January 2024) | Approved (EMA, 2011) | [56,57] | |
| DaunoXome® | Daunorubicin | Kaposi’s sarcoma | Approved (FDA 1996) Discontinued (FDA, 2021) | [56,57] | |
| Iron Oxide nanoparticles | NanoTherm® | Fe2O3 | Glioblastoma, prostate, and pancreatic cancer (https://www.eib.org/en/stories/new-cancer-treatments, accessed on 15 January 2024) | Approved (EMA, 2013) | [57] |
| Albumin nanoparticles | Abraxane® | Paclitaxel | Metastatic breast cancer, locally advanced or metastatic non-small cell lung cancer, metastatic adenocarcinoma of the pancreas (https://www.ema.europa.eu/en/medicines/human/EPAR/abraxane, accessed on 15 January 2024) | Approved (EMA 2008) (FDA 2005) | [57,58] |
| Pazenir® | Paclitaxel | Metastatic breast cancer, metastatic adenocarcinoma of the pancreas, non-small cell lung cancer (https://www.ema.europa.eu/en/medicines/human/EPAR/pazenir, accessed on 15 January 2024) | Approved (EMA 2019) | [58] | |
| Vaccines | Adstiladrin® | Adenoviral vector-based gene therapy | Bacillus Calmette–Guérin unresponsive non-muscle invasive bladder cancer with carcinoma in situ with or without papillary tumors (https://www.fda.gov/drugs/resources-information-approved-drugs/fda-disco-burst-edition-fda-approval-adstiladrin-nadofaragene-firadenovec-vncg-patients-high-risk, accessed on 15 January 2024) | Approved (FDA 2022) | [59] |
| Provenge® | Autologous peripheral-blood mononuclear cells | Metastatic castration-resistant prostate cancer (mCRPC) (https://www.drugs.com/history/provenge.html, accessed on 15 January 2024) | Approved (EMA 2013) (FDA 2010) Discontinued (EMA, 2015) | [59] |
3. Nanomedicines for Targeting TME: Application of Natural and Synthetic Biomaterials
3.1. The Heterogenic Vasculature
3.1.1. VEGF Therapeutic Targeting
3.1.2. Targeting Molecular Markers for Vasculature Regulation
3.1.3. Targeting Formulation Based on FDA-Approved Drugs
3.1.4. Responsive Targeting and Combinational Therapies
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| VEGF | Hydroxyapatite (HA) | Sulfated s-HA | Non-selective binding of VEGF165a | [75] |
| Chitosan (CS) | Sulfated s-CS | Inhibition of VEGF/VEGFR2 signaling pathway | [76] | |
| CS/siRNA nanoplexes | siRNA | Silencing effect of siVEGF-A, siVEGFR-1, siVEGFR-2, and NRP-1 inhibiting proliferation with improved immune functions | [77] | |
| Carboxymethyl chitosan (CMCS) | CMCS | Regulate dexpression of VEGF levels, MMP-1, and CD34, and promoted inhibition of angiogenesis | [78] | |
| Endothelial Cell Regulation | HA-P123/F127 Polymeric nanoparticles | Thymoquinone | Modulating expression of miR-362/Rac1/RhoA and miR-361/VEGF-A pathways for inhibiting angiogenesis | [85] |
| CS nanoplexes | siRNA | Targeting PDGF-D and PDGFR-β expressions | [86] | |
| Hydroxyapatite nanoparticles (HANP) | HANP | Regulating ECs function by the PI3K/Akt/eNOS signaling pathway | [87] | |
| Hydroxyapatite nanoparticles | p53 plasmid and candesartan | Downregulation of VEGF protein secretion and functional microvessel density | [88] | |
| PLGA nanoparticles | P28 peptide and gefitinib | Inhibit tumor angiogenesis, primary tumor growth, and metastasis | [89] | |
| FDA-Approved Drugs | Mesoporous silica nanoparticles (PEG-MSNs) | Sunitinib (anti-VEGFR) | Increased VEGFR targeting specificity, efficient inhibition of angiogenesis | [93] |
| Lipid-chitosan nanoparticles | Bevacizumab (VEGF-A antibody) | Suppressing proliferation and endothelial cells angiogenesis | [94] | |
| PLGA-PEG nanoparticles | Bevacizumab | Higher internalization and bevacizumab delivery into CD44v6+ ECs | [95] | |
| Human serum albumin nanoparticles | Bevacizumab | Decreased glycolysis and metabolic tumor volume, inhibition of tumor growth | [96] | |
| Chitosan nanoparticles | Sorafenib (Tie2 inhibitor) | Superior antitumor activity | [97] | |
| Combinational Therapies | PEG-PCL-PAEA-SA nanoparticles | Gambogenic acid/charge-reversible effect | Suppressed tumor angiogenesis, very little to no vascular tubes inside tumor models | [99] |
| PLGA nanoparticles | Sorafenib/Sunitinib/siRNA | Synergistic effect inhibiting cell proliferation | [101] | |
| PLGA-PEG nanoparticles | Anlotinib/pH-sensitivity | Inhibited tumor growth and metastasis suppressing lymphangiogenesis | [102] | |
| Polycation liposomes | siRNA/calcium phosphate particles | Suppressed tumor growth and angiogenesis | [103] | |
| PEG-liposomes | Doxorubicin/curcumin | Suppressed tumor growth, invasion, and metastasis | [104] | |
| Au nanorods | NRP-1 peptide/PDT | Inhibition of angiogenesis | [108] |
3.2. The Tumor Stroma Extracellular Matrix
3.2.1. Hyaluronidase for ECM Targeting
3.2.2. Extracellular Matrix Degradation
3.2.3. Targeting Biomolecules for Extracellular Matrix
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| Hyaluronidase | PEGPH20 | PEGPH20/gemcitabine/nab-paclitaxel | Phase III trial (HALO-109-301) | [117] |
| PLGA-PEG nanoparticles | rHUPH20/doxorubicin | Effective tumor accumulation enhanced antitumor effect | [118] | |
| ECM Degradation | Doxorubicin liposome (Doxosome) | Bromelain/Hyaluronic acid linked collagen type IV-binding peptide | Decayed the density of collagen fibers and advanced the tumor distribution | [119] |
| PLGA-polydopamine-PEG nanoparticles | Collagenase I/Doxorubicin | Degradation, enhanced the intratumoral distribution, and enhanced antitumor immunity | [120] | |
| LOXL2 antibody | LOXL2 antibody | Control of collagen assembly in ECM, potentially control tumor progression | [123] | |
| PLGA-PEG-PLGA thermosensitive hydrogel | Trastuzumab (Herceptin)/collagenase | Degradation of intratumoral collagen promoting the antibody effect | [124] | |
| mPEG-PLGA nanoparticles | LOL2 and DDR1 inhibitors/Nab-paclitaxel | Enhanced penetration and accumulation in tumor | [125] | |
| Ultrasmall superparamagnetic iron oxide (USPIO) nanoparticles | MMP9-sensitive peptide/Doxorubicin | Effective bioimaging and synergistic chemo-photothermal antitumor effect | [126] | |
| ECM Biomolecules | Peptide nanoparticles | Laminin (LN) mimic peptide | Increased distribution in the tumor site and simultaneous transformation into nanofibers surrounding the tumor site | [128] |
| Hyaluronic acid mesoporous silica nanoparticles | siRNA suppressing CTGF expression/Doxorubicin | Inhibition of multidrug resistance and increased susceptibility of tumor cells to drug-induced apoptosis | [131] |
3.3. The Tumor Stroma Cancer Associated Fibroblasts
3.3.1. Targeting Nanomedicine for CAFs Depletion
3.3.2. Synergistic CAFs Inactivation with Antitumor Targeting
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| CAFs depletion | Anti-FAP-IL liposomes | Single-chain Fv fragments against FAP (scFv’FAP) | Specifically and efficiently respond to FAP-α cell surface biomarker | [139] |
| Cleavable amphiphilic peptide (CAP) nanoparticles | Doxorubicin/CAP | Disturbed the stromal barrier and increased drug intratumoral accumulation | [140] | |
| Thermosensitive liposomes (CAP-TSL) | IR-780 photothermal agent/paclitaxel/human serum albumin | Increased cells apoptosis, expanded tumor interstitial space, promoted deep tumor penetration | [141] | |
| Poly(amidoamine) (PAMAM) hyaluronic acid nanoparticles | Doxorubicin/CAP peptide | Deep intratumoral penetration, suppression of TGF-β, α-SMA, and FAP-α, degradation of tumor fibrotic stroma | [143] | |
| Vaccines | FAP targeting | Synergistic antitumor immunity effect | [136,144,145] | |
| Synergistic inactivation | Glycol chitosan–DEAP nanodrug | Methotrexate/quercetin | Inhibition of pre-metastatic initiation, downregulation of metastasis promoting factors inactivation of CAFs | [146] |
| Hydroxyethyl starch PLA nanoparticles | Doxorubicin/TGF-β receptor inhibitor | Suppression of tumor growth and metastasis | [147] | |
| Au nanoparticles | Photodynamic therapy | Inhibit the expression of pro-fibrotic signaling via Akt pathway | [154] |
3.4. The Tumor Hypoxia
3.4.1. GLUT Targeting Nanomedicines
3.4.2. Multidrug Resistance Targeting Therapeutics
3.4.3. Increasing Chemo-Sensitivity
3.4.4. Targeting M1/M2 Macrophage Polarization
3.4.5. Combinational Targeting for Increasing Tumor Oxygenation
3.4.6. Synergistic Targeting with Antioxidants
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| GLUT | PLGA-chitosan particles | GLUT-1 | Glucose deprivation, increased apoptotic enzymes expression | [161] |
| Glucose-Methacrylate-OEGMA nanoparticles | Interferon-α | Tumor targeting and antitumor immunity | [162] | |
| Cu particles/tumor cell membrane coating | HIF-1α inhibitor/disulfiram | Enhanced tumor sensitivity | [167] | |
| Zn-imidazole–hyaluronic acid particles | DNAzymes | Antitumor effects inhibiting glucose energy | [168] | |
| Nanopipette sensors | Glucose Oxidase | Identification of intracellular glucose level | [169] | |
| Multidrug Resistance | Se/chitosan nanoparticles | Cisplatin | Suppressed ROS formation, inhibited HIF-1α, MDR-2, P-gp | [175] |
| Organosilica particles | Cisplatin/Acriflavine | Inhibition of tumor growth and metastasis | [176] | |
| Silk fibroin particles | Doxorubicin/PX478 HIF inhibitor | Downregulation of MDR1 and P-gp | [177] | |
| PLA-diazobenzene-PEG polymersomes | iRGD peptide/Doxorubicin | Increased accumulation, inhibition of tumor growth | [179] | |
| Chemo-Sensitivity | Hyaluronic acid nanogels/DSPE-PEG nano-micelles | Doxorubicin/TRPA-1 inhibitor | Enhanced tumors sensitivity, antitumor and antimetastatic effects | [183] |
| Chitosan-FA particles | Nitroreductase/Doxorubicin | Hypoxia triggered effective antitumor action | [184] | |
| CM-chitosan-maleimide particles | Dihydroartemicin/PDT | Suppression of HIF-1α and VEGF, inhibition of tumor metastasis | [185] | |
| M1/M2 polarization | Iron oxide-hyaluronic acid-chitosan nanoparticle | HIF-1α siRNA/PGE2 receptor antagonist | Suppression of proliferation, migration, angiogenesis, decreased protein levels | [189] |
| Combinational | MnO2–hyaluronic acid nanoparticles | Doxorubicin | Inhibiting tumor growth and cell proliferation | [193] |
| Human serum albumin MnO2 nanoparticles | Chlorin e6/PDT | Tumor targeting ability, increased accumulation, elevated oxygen levels, tumor necrosis and apoptosis | [194] | |
| MnO2–albumin nanoparticles | Indocyanine green/PDT | Enhanced oxygen production, antitumor effect | [195] | |
| DSPE-PEG liposomes/MnO2-BSA nanoparticles | Atovaquone/hypericin/PDT | Suppressing hypoxia, increased antitumor effect | [196] | |
| Lipid-PLGA-MnO2 particles | Sorafenib | Hypoxia suppression, inhibited tumor cells proliferation, suppressed angiogenesis and metastasis | [198] | |
| Solid lipid calcium peroxide (CaO2) nanocarriers | Doxorubicin/iron-oleate/Chemodynamic theapy | Oxidative damage to tumor tissues | [199] | |
| pH-sensitive methacrylate–CaO2 particles | CaO2 particles/PDT | Increased tumor oxygenation | [200] | |
| Liposome nanoparticles | Cu-oleate/Acriflavine | Immunogenic cell death, combined antitumor immune responses | [201] | |
| Antioxidants | PEGylated liposomes | Palmitoyl ascorbate | Suppressed tumor growth | [206] |
| Liposomes | Doxorubicin/Palmitoyl ascorbate | Suppressed tumor growth | [207] |
3.5. The Tumor Acidosis
3.5.1. pH-Sensitive Peptides in Acidic Tumor Targeting
3.5.2. Metals and Metal Oxides in Acidic Tumor Targeting
3.5.3. Biomaterial Based Polymeric Nanomedicines in Acidic Tumor Targeting
| Targeting Effects | Carrier Type | Therapeutic Agent | Characteristics | Ref. |
|---|---|---|---|---|
| pH-sensitive peptides | Chitosan nanoparticles/cRGD peptide | Raloxifene | Increased accumulation, enhanced antitumor effect inhibiting angiogenesis and migration | [226] |
| Glycogen nanoparticles/hydrazine-based bond | Doxorubicin/β-galactose | Enhanced accumulation, inhibiting tumor growth | [227] | |
| PLGA–BSA particles ATRAM peptide | Doxorubicin/TPP | Enhanced mitochondria targeting, inhibited tumor volume and mass | [228] | |
| Hyaluronic acid nanogels E3/K3 peptides | Cytochrome C (CC)/saporin proteins | Inhibition of protein synthesis in the cytosol, efficient antitumor effect | [230] | |
| Metals/Metal Oxides Chemo-Sensitivity | Cerium oxide–glycol chitosan nanoparticles | CXCR4 antagonist/Doxorubicin | Elevated internalization, increased ROS production at acidic pH, tumor size suppression, and reduced blood vessel leakage | [233] |
| PEG–MnO2 nanoparticles | Doxorubicin/Ce6 PDT | Tumor oxygenation, inhibition of tumor growth, elevated antitumor immune responses | [234] | |
| MnO2-coated mesoporous silicon nanoparticles | Metformin/fluvastatin sodium | Induced intracellular acidosis promoting tumor cell death, suppressed tumor growth and metastasis | [235] | |
| Au nanorods/P(Glu-co-Lys) polypetides | Au nanorods | Enhanced accumulation in tumors periphery and hypoxic core | [236] | |
| Iron oxide SPIONs/cystamine-dextran | Doxorubicin | Increased pH-triggered internalization, inhibition of tumor volume | [237] | |
| Iron oxide SPIONs/PMAA-g-PEGMA | Canagliflozin/Radiotherapy | Accumulation in tumor tissue, inhibition of tumor growth | [238] | |
| pH-sensitive Polymeric particles | Carboxyethyl chitosan–PEGDA hydrogels | Doxorubicin | Self-healing properties, antitumor effect | [243] |
| Chitosan-PEG niosomes | Tamoxifen | Increased drug accumulation and antitumor efficacy | [244] | |
| Chitosan microparticles | - | Screening of tumor progression | [245] | |
| FA-PMgDP-PDPA-PDEMA particles | Doxorubicin/Galactose | Efficient internalization, increased toxicity, and apoptosis | [247] | |
| PCL-b-PAEP-TMA-Cya/DMA micelles | Doxorubicin | Enhanced internalization, inhibition of tumor growth | [248] | |
| Iron oxide-PDPA particles | PEG-polycamptothecin prodrug | Effective antitumor activities, effective antitumor activities | [249] | |
| Graphene quantum dots-PLGA-BSA particles | Doxorubicin | Sufficient internalization and in vitro toxicity | [250] | |
| PLGA particles | Doxorubicin/sodium carbonate/liquid perfluorocarbon | Tumor accumulating ability, and inhibited tumor growth | [251] | |
| PEG-b-PHMA particles | Doxorubicin-P85 prodrug/iRGD peptide/Ce6 PDT | Elevated antitumor effect and complete suppression of tumor growth | [253] | |
| PCL-PEG particles | Paclitaxel/Acetazolamide | Inhibitory effect on tumor growth, increasing the survival rate | [254] | |
| DSPE-PEOz liposomes in platelet membrane particles | Doxorubicin | Enhanced antitumor effect | [255] | |
| Zeolitic imidazolate framework-8 nanoparticles | Doxorubicin/hemoglobin/LOX | Tumor targeting effect, suppressed tumor hypoxia, remodeled tumor acidity and inhibited tumor growth | [256] |
3.6. Tumor Immunotherapies
4. Discussion
5. Conclusions
6. Future Directions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics 2023. CA Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef]
- World Health Organization. World Health Statistics 2022: Monitoring Health for the SDGs, Sustainable Development Goals; World Health Organization: Geneva, Switzerland, 2022; p. 23. [Google Scholar]
- Peng, S.; Xiao, F.; Chen, M.; Gao, H. Tumor-Microenvironment-Responsive Nanomedicine for Enhanced Cancer Immunotherapy. Adv. Sci. 2022, 9, 2103836. [Google Scholar] [CrossRef]
- Miao, L.; Lin, C.M.; Huang, L. Stromal barriers and strategies for the delivery of nanomedicine to desmoplastic tumors. J. Control. Release 2015, 219, 192–204. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Q.; Feng, L.; Liu, Z. Nanomedicine for tumor microenvironment modulation and cancer treatment enhancement. Nano Today 2018, 21, 55–73. [Google Scholar] [CrossRef]
- Yang, S.; Gao, H. Nanoparticles for modulating tumor microenvironment to improve drug delivery and tumor therapy. Pharmacol. Res. 2017, 126, 97–108. [Google Scholar] [CrossRef]
- Das, M.; Law, S. Role of tumor microenvironment in cancer stem cell chemoresistance and recurrence. Int. J. Biochem. Cell Biol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Ribeiro Franco, P.I.; Perillo Rodrigues, A.; Borges de Menezes, L.; Pacheco Miguel, M. Tumor microenvironment components: Allies of cancer progression. Pathol. Res. Pract. 2020, 216, 152729. [Google Scholar] [CrossRef]
- Wei, R.; Liu, S.; Zhang, S.; Min, L.; Zhu, S. Cellular and Extracellular Components in Tumor Microenvironment and Their Application in Early Diagnosis of Cancers. Anal. Cell. Pathol. 2020, 2020, 6283796. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable Polymeric Nanoparticles for Drug Delivery to Solid Tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Martin, J.D.; Miyazaki, T.; Cabral, H. Remodeling tumor microenvironment with nanomedicines. WIREs Nanomed. Nanobiotechnol. 2021, 13, 1730. [Google Scholar] [CrossRef]
- Saini, H.; Eliato, K.R.; Veldhuizen, J.; Zare, A.; Allam, M.; Silva, C.; Kratz, A.; Truong, D.; Mouneimne, G.; LaBaer, J.; et al. The role of tumor-stroma interactions on desmoplasia and tumorigenicity within a microengineered 3D platform. Biomaterials 2020, 247, 119975. [Google Scholar] [CrossRef]
- Klemm, F.; Joyce, J.A. Microenvironmental regulation of therapeutic response in cancer. Trends Cell Biol. 2015, 25, 198–213. [Google Scholar] [CrossRef]
- Deasy, S.K.; Erez, N. A glitch in the matrix: Organ-specific matrisomes in metastatic niches. Trends Cell Biol. 2022, 32, 110–123. [Google Scholar] [CrossRef]
- Lu, P.; Weaver, V.M.; Werb, Z. The extracellular matrix: A dynamic niche in cancer progression. J. Cell Biol. 2012, 196, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Anand, U.; Dey, A.; Singh Chandel, A.K.; Sanyal, R.; Mishra, A.; Kumar Pandey, D.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A.; et al. Cancer chemotherapy and beyond: Current status, drug candidates, associated risks and progress in targeted therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Kifle, Z.D.; Tadele, M.; Alemu, E.; Gedamu, T.; Ayele, A.G. A recent development of new therapeutic agents and novel drug targets for cancer treatment. SAGE Open Med. 2021, 23, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Boohaker, R.J.; Lee, M.W.; Vishnubhotla, P.; Perez, J.M.; Khaled, A.R. The Use of Therapeutic Peptides to Target and to Kill Cancer Cells. Curr. Med. Chem. 2012, 19, 3794. [Google Scholar] [CrossRef]
- Khongorzul, P.; Ling, C.J.; Khan, F.U.; Ihsan, A.U.; Zhang, J. Antibody-drug conjugates: A comprehensive review. Mol. Cancer Res. 2020, 18, 3–19. [Google Scholar] [CrossRef]
- Labrijn, A.F.; Janmaat, M.L.; Reichert, J.M.; Parren, P.W.H.I. Bispecific antibodies: A mechanistic review of the pipeline. Nat. Rev. Drug Discov. 2019, 18, 585–608. [Google Scholar] [CrossRef]
- Muthiah, G.; Jaiswal, A. Can the Union of Prodrug Therapy and Nanomedicine Lead to Better Cancer Management? Adv. NanoBiomed Res. 2022, 2, 2100074. [Google Scholar] [CrossRef]
- Kashkooli, F.M.; Soltani, M.; Hamedi, M.-H. Drug delivery to solid tumors with heterogeneous microvascular networks: Novel insights from image-based numerical modeling. Eur. J. Pharm. Sci. 2020, 151, 105399. [Google Scholar] [CrossRef]
- Wu, M.; Frieboes, H.B.; Chaplain, M.A.J.; McDougall, S.R.; Cristini, V.; Lowengrub, J.S. The effect of interstitial pressure on therapeutic agent transport: Coupling with the tumor blood and lymphatic vascular systems. J. Theor. Biol. 2014, 355, 194–207. [Google Scholar] [CrossRef] [PubMed]
- Lim, Z.-F.; Ma, P.C. Emerging insights of tumor heterogeneity and drug resistance mechanisms in lung cancer targeted therapy. J. Hematol. Oncol. 2019, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.; Levi-Polyachenko, N. Conjugated polymer nano-systems for hyperthermia, imaging and drug delivery. Adv. Drug Deliv. Rev. 2020, 163–164, 40–64. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Jia, Y.; Wu, Y.; Shi, K.; Yang, D.; Li, P.; Qian, Z. Physical-, chemical-, and biological-responsive nanomedicine for cancer therapy. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1581. [Google Scholar] [CrossRef] [PubMed]
- Granja, A.; Pinheiro, M.; Sousa, C.T.; Reis, S. Gold nanostructures as mediators of hyperthermia therapies in breast cancer. Biochem. Pharmacol. 2021, 190, 114639. [Google Scholar] [CrossRef] [PubMed]
- Angelopoulou, A.; Voulgari, E.; Kolokithas-Ntoukas, A.; Bakandritsos, A.; Avgoustakis, K. Magnetic Nanoparticles for the Delivery of Dapagliflozin to Hypoxic Tumors: Physicochemical Characterization and Cell Studies. AAPS PharmSciTech 2018, 19, 2. [Google Scholar] [CrossRef] [PubMed]
- Seynhaeve, A.L.B.; Amin, M.; Haemmerich, D.; van Rhoon, G.C.; ten Hagen, T.L.M. Hyperthermia and smart drug delivery systems for solid tumor therapy. Adv. Drug Deliv. Rev. 2020, 163–164, 125–144. [Google Scholar] [CrossRef]
- Majumder, N.; Das, N.G.; Das, S.K. Polymeric micelles for anticancer drug delivery. Ther. Deliv. 2020, 11, 613–635. [Google Scholar] [CrossRef]
- Perez-Herrero, E.; Fernandez-Medarde, A. Advanced targeted therapies in cancer: Drug nanocarriers, the future of chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Klein, O.; Kee, D.; Markman, B.; Carlino, M.S.; Underhill, C.; Palmer, J.; Power, D.; Cebon, J.; Behren, A. Evaluation of TMB as a predictive biomarker in patients with solid cancers treated with anti-PD-1/CTLA-4 combination immunotherapy. Cancer Cell 2021, 39, 592–593. [Google Scholar] [CrossRef] [PubMed]
- Marofi, F.; Motavalli, R.; Safonov, V.A.; Thangavelu, L.; Yumashev, A.V.; Alexander, M.; Shomali, N.; Chartrand, M.S.; Pathak, Y.; Jarahian, M.; et al. CAR T cells in solid tumors: Challenges and opportunities. Stem Cell Res. Ther. 2021, 12, 81. [Google Scholar] [CrossRef] [PubMed]
- Kirtane, K.; Elmariah, H.; Chung, C.H.; Abate-Daga, D. Adoptive cellular therapy in solid tumor malignancies: Review of the literature and challenges ahead. J. ImmunoTher. Cancer. 2021, 9, e002723. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Y.; Zheng, H.; He, Y.; Jia, H.; Zhang, L.; Lin, C.; Chen, S.; Zheng, J.; Yang, Q.; et al. In Situ biomimetic Nanoformulation for metastatic cancer immunotherapy. Acta Biomater. 2021, 134, 633–648. [Google Scholar] [CrossRef] [PubMed]
- Catalano, V.; Turdo, A.; Di Franco, S.; Dieli, F.; Todaro, M.; Stassi, G. Tumor and its microenvironment: A synergistic interplay. Semin. Cancer Biol. 2013, 23P, 522–532. [Google Scholar] [CrossRef] [PubMed]
- Ribatti, D.; Pezzella, F. Overview on the Different Patterns of Tumor Vascularization. Cells 2021, 10, 639. [Google Scholar] [CrossRef] [PubMed]
- Egeblad, M.; Nakasone, E.S.; Werb, Z. Tumors as organs: Complex tissues that interface with the entire organism. Dev. Cell 2010, 18, 884–901. [Google Scholar] [CrossRef] [PubMed]
- Siemann, D.W. The Unique Characteristics of Tumor Vasculature and Preclinical Evidence for its Selective Disruption by Tumor-Vascular Disrupting Agents. Cancer Treat. Rev. 2011, 37, 63–74. [Google Scholar] [CrossRef]
- Nagl, L.; Horvath, L.; Pircher, A.; Wolf, D. Tumor Endothelial Cells (TECs) as Potential Immune Directors of the Tumor Microenvironment—New Findings and Future Perspectives. Front. Cell Dev. Biol. 2020, 8, 766. [Google Scholar] [CrossRef]
- Salavati, H.; Debbaut, C.; Pullens, P.; Ceelen, W. Interstitial fluid pressure as an emerging biomarker in solid tumors. BBA–Rev. Cancer 2022, 1877, 188792. [Google Scholar] [CrossRef]
- Jiao, D.; Cai, Z.; Choksi, S.; Ma, D.; Choe, M.; Kwon, H.-J.; Baik, J.Y.; Rowan, B.G.; Liu, C.; Liu, Z.-G. Necroptosis of tumor cells leads to tumor necrosis and promotes tumor metastasis. Cell Res. 2018, 28, 868–870. [Google Scholar] [CrossRef] [PubMed]
- Seok Youn, Y.; Han Bae, Y. Perspectives on the past, present, and future of cancer nanomedicine. Adv. Drug Deliv. Rev. 2018, 130, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Al-Abd, A.M.; Aljehani, Z.K.; Gazzaz, R.W.; Fakhri, S.H.; Jabbad, A.H.; Alahdal, A.M.; Torchilin, V.P. Pharmacokinetic strategies to improve drug penetration and entrapment within solid tumors. J. Control. Release 2015, 219, 269–277. [Google Scholar] [CrossRef] [PubMed]
- Lammers, T.; Kiessling, F.; Ashford, M.; Hennink, W.; Crommelin, D.; Storm, G. Cancer nanomedicine: Is targeting our target? Nat. Rev. Mater. 2016, 1, 16069. [Google Scholar] [CrossRef] [PubMed]
- Wu, J. The Enhanced Permeability and Retention (EPR) Effect: The Significance of the Concept and Methods to Enhance Its Application. J. Pers. Med. 2021, 11, 771. [Google Scholar] [CrossRef] [PubMed]
- Nicolas-Boluda, A.; Silva, A.K.A.; Fournei, S.; Gazeau, F. Physical oncology: New targets for nanomedicine. Biomaterials 2018, 150, 87–99. [Google Scholar] [CrossRef] [PubMed]
- Dhaliwal, A.; Zheng, G. Improving accessibility of EPR-insensitive tumor phenotypes using EPR-adaptive strategies: Designing a new perspective in nanomedicine delivery. Theranostics 2019, 9, 8091–8108. [Google Scholar] [CrossRef]
- Dai, W.; Wang, X.; Song, G.; Liu, T.; He, B.; Zhang, H.; Wang, X.; Zhang, Q. Combination antitumor therapy with targeted dual-nanomedicines. Adv. Drug Deliv. Rev. 2017, 115, 23–45. [Google Scholar] [CrossRef]
- Danhier, F. To exploit the tumor microenvironment: Since the EPR effect fails in the clinic, what is the future of nanomedicine? J. Control. Release 2016, 244, 108–121. [Google Scholar] [CrossRef]
- Khot, V.M.; Salunkhe, A.B.; Pricl, S.; Bauer, J.; Thorat, N.D.; Townley, H. Nanomedicine-driven molecular targeting, drug delivery, and therapeutic approaches to cancer chemoresistance. Drug Discov. Today 2021, 26, 724–739. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.J.Y.; Chan, S.Y.; Goh, Y.-Y.; Luo, Z.; Lau, J.W.; Liu, X. Emerging strategies in developing multifunctional nanomaterials for cancer nanotheranostics. Adv. Drug Deliv. Rev. 2021, 178, 113907. [Google Scholar] [CrossRef] [PubMed]
- Anjali Das, C.G.; Ganesh Kumar, V.; Stalin Dhas, T.; Karthick, V.; Vineeth Kumar, C.M. Nanomaterials in anticancer applications and their mechanism of action—A review. Nanomed. Nanotechnol. Biol. Med. 2023, 47, 102613. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Kashkooli, F.M.; Shahvandi, M.K. Engineered strategies to enhance tumor penetration of drug-loaded nanoparticles. J. Control. Release 2022, 341, 227–246. [Google Scholar] [CrossRef] [PubMed]
- Fulton, M.D.; Najahi-Missaoui, W. Liposomes in Cancer Therapy: How Did We Start and Where Are We Now. Int. J. Mol. Sci. 2023, 24, 6615. [Google Scholar] [CrossRef]
- Rodriguez, F.; Caruana, P.; De la Fuente, N.; Espanol, P.; Gamez, M.; Balart, J.; Llurba, E.; Rovira, R.; Ruiz, R.; Martín-Lorente, C.; et al. Nano-Based Approved Pharmaceuticals for Cancer Treatment: Present and Future Challenges. Biomolecules 2022, 12, 784. [Google Scholar] [CrossRef] [PubMed]
- Choo, H.; Jeon, S.I.; Ahn, C.-H.; Shim, M.K.; Kim, K. Emerging Albumin-Binding Anticancer Drugs for Tumor-Targeted Drug Delivery: Current Understandings and Clinical Translation. Pharmaceutics 2022, 14, 728. [Google Scholar] [CrossRef]
- Pallerla, S.; Abdul, A.R.M.; Comeau, J.; Jois, S. Cancer Vaccines, Treatment of the Future: With Emphasis on HER2-Positive Breast Cancer. Int. J. Mol. Sci. 2021, 22, 779. [Google Scholar] [CrossRef]
- Micale, N.; Molonia, M.S.; Citarella, A.; Cimino, F.; Saija, A.; Cristami, M.; Speciale, A. Natural Product-Based Hybrids as Potential Candidates for the Treatment of Cancer: Focus on Curcumin and Resveratrol. Molecules 2021, 26, 4665. [Google Scholar] [CrossRef]
- George, A.; Shah, P.A.; Shrivastav, P.S. Natural biodegradable polymers based nano-formulations for drug delivery: A review. Int. J. Pharm. 2019, 561, 244–264. [Google Scholar] [CrossRef]
- Tong, X.; Pan, W.; Su, T.; Zhang, M.; Dong, W.; Qi, X. Recent advances in natural polymer-based drug delivery systems. React. Funct. Polym. 2020, 148, 104501. [Google Scholar] [CrossRef]
- Van Vierken, L.E.; Amiji, M.M. Multi-functional polymeric nanoparticles for tumour-targeted drug delivery. Expert Opin. Drug Deliv. 2006, 3, 205. [Google Scholar] [CrossRef] [PubMed]
- Kuang, Y.; Zhai, J.; Xiao, Q.; Zhao, S.; Li, C. Polysaccharide/mesoporous silica nanoparticle-based drug delivery systems: A review. Int. J. Biol. Macromol. 2021, 193, 457. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Han, J.; Gong, Y.; Liu, C.; Yu, H.; Xie, N. Nanoparticle-Based Drug Delivery Systems Targeting Tumor Microenvironment for Cancer Immunotherapy Resistance: Current Advances and Applications. Pharmaceutics 2022, 14, 1990. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, S.K.; Jain, A.; Jain, A.; Jain, S. Biodegradable polymers and constructs: A novel approach in drug delivery. Eur. Polym. J. 2019, 120, 109191. [Google Scholar] [CrossRef]
- Fathi, M.; Abdolahinia, E.D.; Bara, J.; Omidi, Y. Smart stimuli-responsive biopolymeric nanomedicines for targeted therapy of solid tumors. Nanomedicine 2020, 15, 2171–2200. [Google Scholar] [CrossRef] [PubMed]
- Salavati, H.; Soltani, M.; Amanpour, S. The pivotal role of angiogenesis in a multi-scale modeling of tumor growth exhibiting the avascular and vascular phases. Microvasc. Res. 2018, 119, 105–116. [Google Scholar] [CrossRef] [PubMed]
- Aguilar-Cazares, D.; Chavez-Dominguez, R.; Carlos-Reyes, A.; Lopez-Camarillo, C.; Hernadez de la Cruz, O.N.; Lopez-Gonzalez, J.S. Contribution of Angiogenesis to Inflammation and Cancer. Front. Oncol. 2019, 9, 1399. [Google Scholar] [CrossRef]
- Al-Ostoot, F.H.; Salah, S.; Khamees, H.A.; Khanum, S.A. Tumor angiogenesis: Current challenges and therapeutic opportunities. Cancer Treat. Res. Commun. 2021, 28, 100422. [Google Scholar] [CrossRef]
- Teleanu, R.I.; Chircov, C.; Grumezescu, A.M.; Teleanu, D.M. Tumor Angiogenesis and Anti-Angiogenic Strategies for Cancer Treatment. J. Clin. Med. 2020, 9, 84. [Google Scholar] [CrossRef]
- Fu, L.-Q.; Du, W.-L.; Cai, M.-H.; Yao, J.-Y.; Zhao, Y.-Y.; Mou, X.-Z. The roles of tumor-associated macrophages in tumor angiogenesis and metastasis. Cell. Immun. 2020, 353, 104119. [Google Scholar] [CrossRef] [PubMed]
- Vimalraj, S. A concise review of VEGF, PDGF, FGF, Notch, angiopoietin, and HGF signalling in tumor angiogenesis with a focus on alternative approaches and future directions. Int. J. Biol. Macromol. 2022, 221, 1428–1438. [Google Scholar] [CrossRef] [PubMed]
- Banik, N.; Yang, S.-B.; Kang, T.-B.; Lim, J.-H.; Park, J. Heparin and Its Derivatives: Challenges and Advances in Therapeutic Biomolecules. Int. J. Mol. Sci. 2021, 22, 10524. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.-K.; Wylie, R.G.; Lange, R.; Kohane, D.S. Selective binding of C-6 OH sulfated hyaluronic acid to the angiogenic isoform of VEGF165. Biomaterials 2016, 77, 130. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Wang, W.; Zhang, Y.; Wang, X.; Gao, X.; Yuan, Z.; Li, Y. Chitosan sulfate inhibits angiogenesis via blocking the VEGF/VEGFR2 pathway and suppresses tumor growth in vivo. Biomater. Sci. 2019, 7, 1584–1597. [Google Scholar] [CrossRef] [PubMed]
- Salva, E.; Kabasakai, L.; Eren, F.; Ozkan, N.; Cakalagaoglu, F.; Akbuga, J. Local Delivery of Chitosan/VEGF siRNA Nanoplexes Reduces Angiogenesis and Growth of Breast Cancer In Vivo. Nucleic Acid Ther. 2012, 22, 40. [Google Scholar] [CrossRef]
- Jiang, Z.; Han, B.; Li, H.; Yang, Y.; Liu, W. Carboxymethyl chitosan represses tumor angiogenesis in vitro and in vivo. Carbohydr. Polym. 2015, 129, 1–8. [Google Scholar] [CrossRef]
- Picoli, C.C.; Goncalves, B.O.P.; Santos, G.S.P.; Rocha, B.G.S.; Costa, A.C.; Resende, R.R.; Birbrair, A. Pericytes cross-talks within the tumor microenvironment. BBA–Rev. Cancer 2021, 1876, 188608. [Google Scholar] [CrossRef]
- Ma, X.; Geng, Z.; Wang, S.; Yu, Z.; Liu, T.; Guan, S.; Du, S.; Zhu, C. The driving mechanism and targeting value of mimicry between vascular endothelial cells and tumor cells in tumor progression. Biomed. Pharmacother. 2023, 165, 115029. [Google Scholar] [CrossRef]
- Zalpoor, H.; Aziziyan, F.; Liaghat, M.; Bakhtiyari, M.; Akbari, A.; Nabi-Afjadi, M.; Forghaniesfidvajani, R.; Rezaei, N. The roles of metabolic profiles and intracellular signaling pathways of tumor microenvironment cells in angiogenesis of solid tumors. Cell Commun. Signal. 2022, 20, 186. [Google Scholar] [CrossRef]
- Larionova, I.; Kazakova, E.; Gerashchenko, T.; Kzhyshkowska, J. New Angiogenic Regulators Produced by TAMs: Perspective for Targeting Tumor Angiogenesis. Cancers 2021, 13, 3253. [Google Scholar] [CrossRef]
- Seyyednia, E.; Oroojalian, F.; Baradaran, B.; Mojarrad, J.S.; Mokhtarzadeh, A.; Valizadeh, H. Nanoparticles modified with vasculature-homing peptides for targeted cancer therapy and angiogenesis imaging. J. Control. Release 2021, 338, 367. [Google Scholar] [CrossRef] [PubMed]
- Kargozar, S.; Baino, F.; Hamzehiou, S.; Hamblin, M.R.; Mozafari, M. Nanotechnology for angiogenesis: Opportunities and challenges. Chem. Soc. Rev. 2020, 49, 5008. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Ghosh, A.; Maiti, S.; Ahir, M.; Debnath, G.H.; Gupta, P.; Bhattacharjee, M.; Chosh, S.; Chattopadhyay, S.; Mukherjee, P.; et al. Delivery of thymoquinone through hyaluronic acid-decorated mixed Pluronic® nanoparticles to attenuate angiogenesis and metastasis of triple-negative breast cancer. J. Control. Release 2020, 322, 357. [Google Scholar] [CrossRef] [PubMed]
- Salva, E.; Ozbas, S.; Alan, S.; Ozkan, N.; Ekentok-Atici, C.; Kabasakal, L.; Akbuga, J. Combination therapy with chitosan/siRNA nanoplexes targeting PDGF-D and PDGFR-β reveals anticancer effect in breast cancer. J. Gene Med. 2023, 25, 3465. [Google Scholar] [CrossRef] [PubMed]
- Shi, X.; Zhou, K.; Huang, F.; Wang, C. Interaction of hydroxyapatite nanoparticles with endothelial cells: Internalization and inhibition of angiogenesis in vitro through the PI3K/Akt pathway. Int. J. Nanomed. 2017, 12, 5781. [Google Scholar] [CrossRef]
- Zhao, L.; Zhao, W.; Liu, Y.; Chen, X.; Wang, Y. Nano-Hydroxyapatite-Derived Drug and Gene Co-Delivery System for Anti-Angiogenesis Therapy of Breast Cancer. Med. Sci. Monit. 2017, 23, 4723. [Google Scholar] [CrossRef] [PubMed]
- Garizo, A.R.; Castro, F.; Martins, C.; Almeida, A.; Dias, T.P.; Fernardes, F.; Barrias, C.C.; Bernardes, N.; Fialho, A.M.; Sarmento, B. p28-functionalized PLGA nanoparticles loaded with gefitinib reduce tumor burden and metastases formation on lung cancer. J. Control. Release 2021, 337, 329. [Google Scholar] [CrossRef]
- Micaily, I.; Johnson, J.; Argiris, A. An update on angiogenesis targeting in head and neck squamous cell carcinoma. Cancers Head Neck 2020, 5, 5. [Google Scholar] [CrossRef]
- Hu, H.; Chen, Y.; Tan, S.; Wu, S.; Huang, Y.; Fu, S.; Luo, F.; He, J. The Research Progress of Antiangiogenic Therapy, Immune Therapy and Tumor Microenvironment. Front. Immunol. 2022, 13, 802846. [Google Scholar] [CrossRef]
- Taylor, M.H.; Schmidt, E.V.; Dutcus, C.; Pinheiro, E.M.; Funahashi, Y.; Lubiniecki, G.; Rasco, D. The LEAP program: Lenvatinib plus pembrolizumab for the treatment of advanced solid tumors. Future Oncol. 2021, 17, 637–647. [Google Scholar] [CrossRef] [PubMed]
- Goel, S.; Chen, F.; Hong, H.; Valdovinos, H.F.; Hernandez, R.; Shi, S.; Barnhart, T.E.; Cai, W. VEGF121-Conjugated Mesoporous Silica Nanoparticle: A Tumor Targeted Drug Delivery System. Appl. Mater. Interfaces 2014, 6, 21677. [Google Scholar] [CrossRef] [PubMed]
- Abdi, F.; Arkan, E.; Eidizadeh, M.; Valipour, E.; Naseriyeh, T.; Gamizgy, Y.H.; Mansouri, K. The possibility of angiogenesis inhibition in cutaneous melanoma by bevacizumab-loaded lipid-chitosan nanoparticles. Drug Deliv. Transl. 2023, 13, 568. [Google Scholar] [CrossRef] [PubMed]
- Balao, A.; Sousa, F.; Oliveira, V.; Oliveira, C.; Sarmento, B. Effective intracellular delivery of bevacizumab via PEGylated polymeric nanoparticles targeting the CD44v6 receptor in colon cancer cells. Biomater. Sci. 2020, 8, 3720. [Google Scholar] [CrossRef]
- Luis de Redin, I.; Exposito, F.; Agueros, M.; Collantes, M.; Penuelas, I.; Allemandi, D.; Llabot, J.M.; Calvo, A.; Irache, J.M. In vivo efficacy of bevacizumab-loaded albumin nanoparticles in the treatment of colorectal cancer. Drug Deliv. Transl. 2020, 10, 635. [Google Scholar] [CrossRef] [PubMed]
- Ruman, U.; Buskaran, K.; Pastorin, G.; Masarudin, M.J.; Fakurazi, S.; Hussein, M.Z. Synthesis and Characterization of Chitosan-Based Nanodelivery Systems to Enhance the Anticancer Effect of Sorafenib Drug in Hepatocellular Carcinoma and Colorectal Adenocarcinoma Cells. Nanomaterials 2021, 11, 497. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chen, M.; Ran, X.; Tang, H.; Cao, D. Sorafenib-Based Drug Delivery Systems: Applications and Perspectives. Polymers 2023, 15, 2638. [Google Scholar] [CrossRef]
- Du, M.; Geng, T.; Yu, R.; Song, G.; Cheng, H.; Cao, Y.; He, W.; Haleem, A.; Li, Q.; Hu, R.; et al. Smart anti-vascular nanoagent induces positive feedback loop for self-augmented tumor accumulation. J. Control. Release 2023, 356, 595. [Google Scholar] [CrossRef]
- Zhang, J.; Hu, H.; Chen, E. The combination of MnO2@Lipo-coated gefitinib and bevacizumab inhibits the development of non-small cell lung cancer. Drug Deliv. 2022, 29, 466. [Google Scholar] [CrossRef]
- Punuch, K.; Wongwan, C.; Jantana, S.; Somboonyosdech, C.; Rodponthukwaji, K.; Kunwong, N.; Nguyen, K.T.; Sirivatanauksorn, V.; Sirivatanauksorn, Y.; Srisawat, C.; et al. Study of siRNA Delivery via Polymeric Nanoparticles in Combination with Angiogenesis Inhibitor for The Treatment of AFP-Related Liver Cancer. Int. J. Mol. Sci. 2022, 23, 12666. [Google Scholar] [CrossRef]
- Cong, X.; Chen, J.; Xu, R. Tumor-Acidity Responsive Polymeric Nanoparticles for Targeting Delivery of Angiogenesis Inhibitor for Enhanced Antitumor Efficacy with Decreased Toxicity. Front. Bioeng. Biotechnol. 2021, 9, 664051. [Google Scholar] [CrossRef]
- Chen, J.; Sun, X.; Shao, R.; Xu, Y.; Gao, J.; Liang, W. VEG F siRNA delivered by polycation liposome-encapsulated calcium phosphate nanoparticles for tumor angiogenesis inhibition in breast cancer. Int. J. Nanomed. 2017, 12, 6075. [Google Scholar] [CrossRef] [PubMed]
- Barui, S.; Saha, S.; Mondal, G.; Haseena, S.; Chaudhuri, A. Simultaneous delivery of doxorubicin and curcumin encapsulated in liposomes of pegylated RGDK-lipopeptide to tumor vasculature. Biomaterials 2014, 35, 1643. [Google Scholar] [CrossRef] [PubMed]
- Barui, A.K.; Nethi, S.K.; Haque, S.; Basuthakur, P.; Patra, C.R. Recent Development of Metal Nanoparticles for Angiogenesis Study and Their Therapeutic Applications. ACS Appl. Bio Mater. 2019, 2, 5492. [Google Scholar] [CrossRef] [PubMed]
- Darweesh, R.S.; Ayoub, N.M.; Nazzal, S. Gold nanoparticles and angiogenesis: Molecular mechanisms and biomedical applications. Int. J. Nanomed. 2019, 14, 7643. [Google Scholar] [CrossRef] [PubMed]
- Roma-Rodrigues, C.; Heuer-Jungemann, A.; Fernandes, A.R.; Kanaras, A.G.; Baptista, P.V. Peptide-coated gold nanoparticles for modulation of angiogenesis in vivo. Int. J. Nanomed. 2016, 11, 2633. [Google Scholar] [CrossRef]
- Bartczak, D.; Muskens, O.L.; Nitti, S.; Millar, T.M.; Kanaras, A.G. Nanoparticles for inhibition of in vitro tumour angiogenesis: Synergistic actions of ligand function and laser irradiation. Biomater. Sci. 2015, 3, 733. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Zhou, X.; Yang, J.; Shi, H.; Li, H.; Zhao, X.; Ma, X. The Role of Tumor-Stroma Interactions in Drug Resistance Within Tumor Microenvironment. Front. Cell Dev. Biol. 2021, 9, 637675. [Google Scholar] [CrossRef] [PubMed]
- Xu, M.; Zhang, T.; Xia, R.; Wei, Y.; Wei, X. Targeting the tumor stroma for cancer therapy. Mol. Cancer 2022, 21, 208. [Google Scholar] [CrossRef]
- Zhao, X.; He, Y.; Chen, H. Autophagic tumor stroma: Mechanisms and roles in tumor growth and progression. Int. J. Cancer. 2013, 132, 1–8. [Google Scholar] [CrossRef]
- Simsek, H.; Klotzsch, E. The solid tumor microenvironment Breaking the barrier for T cells How the solid tumor microenvironment influences T cells. BioEssays 2022, 44, 2100285. [Google Scholar] [CrossRef] [PubMed]
- Gargalionis, A.N.; Papavassiliou, K.A.; Papavassiliou, A.G. Mechanobiology of solid tumors. BBA–Mol. Basis Dis. 2022, 1868, 166555. [Google Scholar] [CrossRef]
- Rianna, C.; Kumar, P.; Radmacher, M. The role of the microenvironment in the biophysics of cancer. Semin. Cell Dev. Biol. 2018, 73, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Abyaneh, H.S.; Regenold, M.; McKee, T.D.; Allen, C.; Gauthier, M.A. Towards extracellular matrix normalization for improved treatment of solid tumors. Theranostics 2020, 10, 1960–1980. [Google Scholar] [CrossRef] [PubMed]
- Henke, E.; Nandigama, R.; Ergun, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef]
- Doherty, G.J.; Tempero, M.; Corrie, P.G. HALO-109–301: A Phase III trial of PEGPH20 (with gemcitabine and nab-paclitaxel) in hyaluronic acid-high stage IV pancreatic cancer. Future Oncol. 2018, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Fan, Z.; Deng, J.; Lemons, P.K.; Arhontoulis, D.C.; Bowne, W.B.; Cheng, H. Hyaluronidase Embedded in Nanocarrier PEG Shell for Enhanced Tumor Penetration and Highly Efficient Antitumor Efficacy. Nano Lett. 2016, 16, 3268. [Google Scholar] [CrossRef]
- Ikeda-Imafuku, M.; Gao, Y.; Shaha, S.; Wang, L.L.-W.; Soo Park, K.; Nakajima, M.; Adebowale, O.; Mitragotri, S. Extracellular matrix degrading enzyme with stroma-targeting peptides enhance the penetration of liposomes into tumors. J. Control. Release 2022, 352, 1093–1103. [Google Scholar] [CrossRef]
- Amoozgar, Z.; Goldberg, M.S. Surface modulation of polymeric nanocarriers enhances the stability and delivery of proteins and small molecules. Nanomedicine 2017, 12, 729. [Google Scholar] [CrossRef]
- Ding, M.; Zhang, Y.; Li, J.; Pu, K. Bioenzyme-based nanomedicines for enhanced cancer therapy. Nano Converg. 2022, 9, 7. [Google Scholar] [CrossRef]
- Setargew, Y.F.I.; Wyllie, K.; Grant, R.D.; Chitty, J.L.; Cox, T.R. Targeting Lysyl Oxidase Family Meditated Matrix Cross-Linking as an Anti-Stromal Therapy in Solid Tumours. Cancers 2021, 13, 491. [Google Scholar] [CrossRef] [PubMed]
- Grossman, M.; Ben-Chetrit, N.; Zhuravlev, A.; Afik, R.; Bassat, E.; Solomonov, I.; Yarden, Y.; Sagi, I. Tumor Cell Invasion Can Be Blocked by Modulators of Collagen Fibril Alignment That Control Assembly of the Extracellular Matrix. Cancer Res. 2016, 76, 4249. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Wang, Z.; Chen, B.; Dai, W.; Zhang, H.; He, B.; Wang, X.; Wang, Y.; Zhang, Q. Localized co-delivery of collagenase and trastuzumab by thermosensitive hydrogels for enhanced antitumor efficacy in human breast xenograft. Drug Deliv. 2018, 25, 1495. [Google Scholar] [CrossRef] [PubMed]
- Wei, D.; Cheng, X.; Du, C.; Wang, Y.; Sun, J.; Li, C.; Wu, J.; Tian, X.; Zhao, Y.; Nie, G.; et al. Stroma-targeted nanoparticles that remodel stromal alignment to enhance drug delivery and improve the antitumor efficacy of Nab-paclitaxel in pancreatic ductal adenocarcinoma models. Nano Today 2022, 45, 101533. [Google Scholar] [CrossRef]
- Chen, A.; Lu, H.; Cao, R.; Zhu, Y.; Li, Y.; Ge, R.; Zhang, S.; Li, Y.; Xiao, L.; Su, L.; et al. A novel MMP-responsive nanoplatform with transformable magnetic resonance property for quantitative tumor bioimaging and synergetic chemo-photothermal therapy. Nano Today 2022, 45, 101524. [Google Scholar] [CrossRef]
- Guo, Z.; Hu, K.; Sun, J.; Zhang, T.; Zhang, Q.; Song, L.; Zhang, X.; Gu, N. Fabrication of Hydrogel with Cell Adhesive Micropatterns for Mimicking the Oriented Tumor-Associated Extracellular Matrix. ACS Appl. Mater. Interfaces 2014, 6, 10963. [Google Scholar] [CrossRef]
- Hu, X.-X.; He, P.-P.; Qi, G.-B.; Gao, Y.-J.; Lin, Y.-X.; Yang, C.; Yang, P.-P.; Hao, H.; Wang, L.; Wang, H. Transformable Nanomaterials as an Artificial Extracellular Matrix for Inhibiting Tumor Invasion and Metastasis. ACS Nano 2017, 11, 4086. [Google Scholar] [CrossRef]
- Hellmud, K.S.; Koksch, B. Self-Assembling Peptides as Extracellular Matrix Mimics to Influence Stem Cell’s Fate. Front. Chem. 2019, 7, 172. [Google Scholar] [CrossRef]
- Richeldi, L.; Perez, E.R.; Costabel, U.; Albera, C.; Lederer, D.J.; Flaherty, K.R.; Ettinger, N.; Perez, R.; Scholand, M.B.; Goldin, J.; et al. Pamrevlumab, an anti-connective tissue growth factor therapy, for idiopathic pulmonary fibrosis (PRAISE): A phase 2, randomised, double-blind, placebo-controlled trial. Lancet Respir. Med. 2020, 8, 25–33. [Google Scholar] [CrossRef]
- Ding, J.; Liang, T.; Zhou, Y.; He, Z.; Min, Q.; Jiang, L.; Zhu, J. Hyaluronidase-triggered anticancer drug and siRNA delivery from cascaded targeting nanoparticles for drug resistant breast cancer therapy. Nano Res. 2017, 10, 690. [Google Scholar] [CrossRef]
- Cheng, B.; Yu, Q.; Wang, W. Intimate communications within the tumor microenvironment: Stromal factors function as an orchestra. J. Biomed. Sci. 2023, 30, 1. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zheng, Z.; Li, C.; Li, Z.; Wu, J.; Zhang, B. Therapeutic drugs and drug delivery systems targeting stromal cells for cancer therapy: A review. J. Drug Deliv. 2020, 28, 714–726. [Google Scholar] [CrossRef] [PubMed]
- Hurtado, P.; Martinez-Pena, I.; Pineiro, R. Dangerous Liaisons: Circulating Tumor Cells (CTCs) and Cancer-Associated Fibroblasts (CAFs). Cancers 2020, 12, 2861. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer- associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Shahvali, S.; Rahiman, N.; Jaafari, M.R.; Arabi, L. Targeting fibroblast activation protein (FAP): Advances in CAR-T cell, antibody, and vaccine in cancer immunotherapy. Drug Deliv. Transl. Res. 2023, 13, 2041–2056. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Keller, E.T.; Garfield, D.H.; Shen, K.; Wang, J. Stromal cells in tumor microenvironment and breast cancer. Cancer Metastasis Rev. 2013, 32, 303–315. [Google Scholar] [CrossRef]
- Li, F.; Zhao, S.; Wei, C.; Hu, Y.; Xu, T.; Xin, X.; Zhu, T.; Shang, L.; Ke, S.; Zhou, J.; et al. Development of Nectin4/FAP-targeted CAR-T cells secreting IL-7, CCL19, and IL-12 for malignant solid tumors. Front. Immunol. 2022, 13, 958082. [Google Scholar] [CrossRef]
- Ruger, R.; Tansi, F.L.; Rabenhold, M.; Steiniger, F.; Kontermann, R.E.; Fahr, A.; Hilger, I. In vivo near-infrared fluorescence imaging of FAP-expressing tumors with activatable FAP-targeted, single-chain Fv-immunoliposomes. J. Control. Release 2014, 186, 1–10. [Google Scholar] [CrossRef]
- Ji, T.; Zhao, Y.; Ding, Y.; Wang, J.; Zhao, R.; Lang, J.; Qin, H.; Liu, X.; Shi, J.; Tao, N.; et al. Transformable Peptide Nanocarriers for Expeditious Drug Release and Effective Cancer Therapy via Cancer-Associated Fibroblast Activation. Angew. Chem. Int. Ed. 2016, 55, 1050. [Google Scholar] [CrossRef]
- Yu, Q.; Qiu, Y.; Li, J.; Tang, X.; Wang, X.; Cun, X.; Xu, S.; Liu, Y.; Li, M.; Zhang, Z.; et al. Targeting cancer-associated fibroblasts by dual-responsive lipid-albumin nanoparticles to enhance drug perfusion for pancreatic tumor therapy. J. Control. Release 2020, 321, 564. [Google Scholar] [CrossRef]
- Liu, H.; Shi, Y.; Qian, F. Opportunities and delusions regarding drug delivery targeting pancreatic cancer-associated fibroblasts. Adv. Drug Deliv. Rev. 2021, 172, 37. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Chen, D.; Hao, L.; Tian, C.; Yan, Y.; Zhu, L.; Zhang, H.; Zhang, Y.; Zhang, Z. Transformable nanoparticles triggered by cancer associated fibroblasts for improving drug permeability and efficacy in desmoplastic tumors. Nanoscale 2019, 11, 20030. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Xiang, R.; Wen, Y.; Xu, G.; Wang, C.; Luo, S.; Yin, T.; Wei, X.; Shao, B.; Liu, N.; et al. A whole-cell tumor vaccine modified to express fibroblast activation protein induces antitumor immunity against both tumor cells and cancer-associated fibroblasts. Sci. Rep. 2015, 5, 14421. [Google Scholar] [CrossRef] [PubMed]
- Chintala, N.K.; Restle, D.; Quach, H.; Saini, J.; Bellis, R.; Offin, M.; Beattie, J.; Adusumilli, P.S. CAR T-cell therapy for pleural mesothelioma: Rationale, preclinical development, and clinical trials. Lung Cancer 2021, 157, 48–59. [Google Scholar] [CrossRef]
- Huo, M.; Zhou, J.; Wang, H.; Zheng, Y.; Tong, Y.; Zhou, J.; Liu, J.; Yin, T. A pHe sensitive nanodrug for collaborative penetration and inhibition of metastatic tumors. J. Control. Release 2022, 352, 893. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Li, Y.; Zhu, Y.; Yu, C.; Jia, H.; Bao, B.; Hu, H.; Xiao, C.; Zhang, J.; Zeng, X.; et al. Co-delivery nanoparticle to overcome metastasis promoted by insufficient chemotherapy. J. Control. Release 2018, 275, 67. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Luco, A.-L.; Camirand, A.; St-Arnaud, R.; Kremer, R. Vitamin D regulates CXCL12/CXCR4 and epithelial-to-mesenchymal transition in a model of breast cancer metastasis to lung. Endocrinology 2021, 162, bqab049. [Google Scholar] [CrossRef] [PubMed]
- Gorchs, L.; Ahmed, S.; Mayer, C.; Knauf, A.; Fernández Moro, C.; Svensson, M.; Heuchel, R.; Rangelova, E.; Bergman, P.; Kaipe, H. The vitamin D analogue calcipotriol promotes an anti-tumorigenic phenotype of human pancreatic CAFs but reduces T cell mediated immunity. Sci. Rep. 2020, 10, 17444. [Google Scholar] [CrossRef]
- Han, X.; Xu, Y.; Geranpayehvaghei, M.; Anderson, G.J.; Li, Y.; Nie, G. Emerging nanomedicines for anti-stromal therapy against desmoplastic tumors. Biomaterials 2020, 232, 119745. [Google Scholar] [CrossRef]
- Mortezaee, K.; Majidpoor, J. Key promoters of tumor hallmarks. Int. J. Clin. Oncol. 2022, 27, 45–58. [Google Scholar] [CrossRef]
- Meng, H.; Nel, A.E. Use of nano engineered approaches to overcome the stromal barrier in pancreatic cancer. Adv. Drug Deliv. Rev. 2018, 130, 50. [Google Scholar] [CrossRef]
- Guo, J.; Zeng, H.; Chen, Y. Emerging Nano Drug Delivery Systems Targeting Cancer-Associated Fibroblasts for Improved Antitumor Effect and Tumor Drug Penetration. Mol. Pharm. 2020, 17, 1028. [Google Scholar] [CrossRef]
- Zhao, X.; Pan, J.; Li, W.; Yang, W.; Qin, L.; Pan, Y. Gold nanoparticles enhance cisplatin delivery and potentiate chemotherapy by decompressing colorectal cancer vessels. Int. J. Nanomed. 2018, 13, 6207. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Bermudez, J.; Williams, R.T.; Guarecuco, R.; Birsoy, K. Targeting extracellular nutrient dependencies of cancer cells. Mol. Metab. 2020, 33, 67–82. [Google Scholar] [CrossRef] [PubMed]
- Horsman, M.R.; Sorensen, B.S.; Busk, M.; Siemann, D.W. Therapeutic Modification of Hypoxia. Clin. Oncol. 2021, 33, e492–e509. [Google Scholar] [CrossRef] [PubMed]
- McAleese, C.E.; Choudhury, C.; Butcher, N.J.; Minchin, R.F. Hypoxia-mediated drug resistance in breast cancers. Cancer Lett. 2021, 502, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Khoshinani, H.M.; Afshar, S.; Najafi, R. Hypoxia: A Double-Edged Sword in Cancer Therapy. Cancer Investig. 2016, 34, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Wiechec, E.; Matic, N.; Ali, A.; Roberg, K. Hypoxia induces radioresistance, epithelial-mesenchymal transition, cancer stem cell-like phenotype and changes in genes possessing multiple biological functions in head and neck squamous cell carcinoma. Oncol. Rep. 2022, 47, 58. [Google Scholar] [CrossRef]
- Zeng, W.; Liu, P.; Pan, W.; Singh, S.R.; Wei, Y. Hypoxia and hypoxia inducible factors in tumor metabolism. Cancer Lett. 2015, 356, 263–267. [Google Scholar] [CrossRef]
- Abolhasani, A.; Biria, D.; Abolhasani, H.; Zarrabi, A.; Komeili, T. Investigation of the Role of Glucose Decorated Chitosan and PLGA Nanoparticles as Blocking Agents to Glucose Transporters of Tumor Cells. Int. J. Nanomed. 2019, 14, 9535. [Google Scholar] [CrossRef]
- Sun, J.; Guo, J.; Zhang, L.; Gong, L.; Sun, Y.; Deng, X.; Gao, W. Active-targeting long-acting protein-glycopolymer conjugates for selective cancer therapy. J. Control. Release 2023, 356, 175. [Google Scholar] [CrossRef]
- Torres-Perez, S.A.; Torres-Perez, C.E.; Pedraza-Escalona, M.; Perez-Tapia, S.M.; Ramon-Gallegos, E. Glycosylated Nanoparticles for Cancer-Targeted Drug Delivery. Front. Oncol. 2020, 10, 605037. [Google Scholar] [CrossRef]
- Geng, C.; Pang, S.; Ye, R.; Shi, J.; Yang, Q.; Chen, C.; Wang, W. Glycolysis-based drug delivery nanosystems for therapeutic use in tumors and applications. Biomed. Pharmacother. 2023, 165, 115009. [Google Scholar] [CrossRef] [PubMed]
- Heddleston, J.M.; Li, Z.; Lathia, J.D.; Bao, S.; Hjelmeland, A.B.; Rich, J.N. Hypoxia inducible factors in cancer stem cells. Br. J. Cancer 2010, 102, 789. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, K.; Okada, M.; Suzuki, S.; Seino, M.; Seino, S.; Takeda, H.; Kitanaka, C. Targeting the facilitative glucose transporter GLUT1 inhibits the self-renewal and tumor-initiating capacity of cancer stem cells. Oncotarget 2015, 6, 651. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, Y.; Bao, B.; Hu, C.; Li, Z. Boosting the anti-tumor performance of disulfiram against glioblastoma by using ultrasmall nanoparticles and HIF-1α inhibitor. Compos. B Eng. 2022, 243, 110117. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, K.; Liang, Y.; Wei, Y.; An, J.; Wang, Y.; Yang, J.; Zhang, H.; Zhang, Z.; Liu, J.; et al. Nano-enabled Tumor Systematic Energy Exhaustion via Zinc (II) Interference Mediated Glycolysis Inhibition and Specific GLUT1 Depletion. Adv. Sci. 2022, 9, 2103534. [Google Scholar] [CrossRef]
- Nascimento, R.A.S.; Ozel, R.E.; Han Mak, W.; Mulato, M.; Singaram, B.; Pourmand, N. Single Cell “Glucose Nanosensor” Verifies Elevated Glucose Levels in Individual Cancer Cells. Nano Lett. 2016, 16, 1194. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Coleman, M.; Brekken, R.A. Perspectives on Hypoxia Signaling in Tumor Stroma. Cancers 2021, 13, 3070. [Google Scholar] [CrossRef]
- Shirai, Y.; Chow, C.C.T.; Kambe, G.; Suwa, T.; Kobayashi, M.; Takahashi, I.; Harada, H.; Nam, J.-M. An Overview of the Recent Development of Anticancer Agents Targeting the HIF-1 Transcription Factor. Cancers 2021, 13, 2813. [Google Scholar] [CrossRef]
- Xu, X.-X.; Chen, S.-Y.; Yi, N.-B.; Li, X.; Chen, S.-L.; Lei, Z.; Cheng, D.-B.; Sun, T. Research progress on tumor hypoxia-associative nanomedicine. J. Control. Release 2022, 350, 829–840. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Xiang, G. Engineering nanomedicines to inhibit hypoxia-inducible Factor-1 for cancer therapy. Cancer Lett. 2022, 530, 110. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of Multidrug Resistance in Cancer Chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; He, C.; Yan, R.; Chen, Y.; Zhao, P.; Li, M.; Fan, T.; Yang, T.; Lu, Y.; Luo, J.; et al. HIF-1 dependent reversal of cisplatin resistance via anti-oxidative nano selenium for effective cancer therapy. Chem. Eng. J. 2020, 380, 122540. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; Liu, X.; Zhao, P.; Chen, C.; Yan, R.; Li, M.; Fan, T.; Altine, B.; Yang, T.; et al. One-pot synthesis of a microporous organosilica-coated cisplatin nanoplatform for HIF-1–targeted combination cancer therapy. Theranostics 2020, 10, 2918. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, G.; Zhang, Q.; Wu, W.; Zhang, Y.; Wu, B.; Liu, Z.; Tong, X.; Xiao, B.; Cheng, L.; et al. PX478-loaded silk fibroin nanoparticles reverse multidrug resistance by inhibiting the hypoxia-inducible factor. Int. J. Biol. Macromol. 2022, 222, 2309. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Palanca, P.; Payo-Serafin, T.; San-Miguel, B.; Mendez-Blanco, C.; Tunon, M.J.; Gonzalez-Gallego, J.; Mauriz, J.L. Hepatocellular carcinoma cells loss lenvatinib efficacy in vitro through autophagy and hypoxia response-derived neuropilin-1 degradation. Acta Pharmacol. Sin. 2022, 44, 1066. [Google Scholar] [CrossRef] [PubMed]
- Mamnoon, B.; Loganathan, J.; Confeld, M.I.; De Fonseka, N.; Feng, L.; Froberg, J.; Choi, Y.; Tuvin, D.M.; Sathish, V.; Mallik, S. Targeted Polymeric Nanoparticles for Drug Delivery to Hypoxic, Triple-Negative Breast Tumors. ACS Appl. Bio Mater. 2021, 4, 1450. [Google Scholar] [CrossRef] [PubMed]
- Telarovic, I.; Wenger, R.H.; Pruschy, M. Interfering with Tumor Hypoxia for Radiotherapy Optimization. J. Exp. Clin. Cancer Res. 2021, 40, 197. [Google Scholar] [CrossRef]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Faris, P.; Rumolo, A.; Pellavio, G.; Tanzi, M.; Vismara, M.; Berra-Romani, R.; Gerbino, A.; Corallo, S.; Pedrazzoli, P.; Laforenza, U.; et al. Transient receptor potential ankyrin 1 (TRPA1) mediates reactive oxygen species-induced Ca2+ entry, mitochondrial dysfunction, and caspase-3/7 activation in primary cultures of metastatic colorectal carcinoma cells. Cell Death Discov. 2023, 9, 213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yin, S.; Mei, L.; Yang, Y.; Xu, S.; He, X.; Wang, M.; Li, M.; Zhang, Z.; He, Q. A dual receptors-targeting and size-switchable “cluster bomb” co-loading chemotherapeutic and transient receptor potential ankyrin 1 (TRPA-1) inhibitor for treatment of triple negative breast cancer. J. Control. Release 2020, 321, 71. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.H.; Shim, M.K.; Kim, G.L.; Kim, S.-H.; Kang, H.; Kim, J.-H. Hypoxia-responsive folic acid conjugated glycol chitosan nanoparticle for enhanced tumor targeting treatment. Int. J. Pharm. 2020, 580, 119237. [Google Scholar] [CrossRef] [PubMed]
- Luo, R.; Zhang, Z.; Han, L.; Xue, Z.; Zhang, K.; Liu, F.; Feng, F.; Xue, J.; Liu, W.; Qu, W. An albumin-binding dimeric prodrug nanoparticle with long blood circulation and light-triggered drug release for chemo-photodynamic combination therapy against hypoxia-induced metastasis of lung cancer. Biomater. Sci. 2021, 9, 3718. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-associated macrophages: An accomplice in solid tumor progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar] [CrossRef] [PubMed]
- Cebola, I.; Custodio, J.; Munoz, M.; Diez-Villanueva, A.; Pare, L.; Prieto, P.; Ausso, S.; Coll-Mulet, L.; Bosca, L.; Moreno, V.; et al. Epigenetics override pro-inflammatory PTGS transcriptomic signature towards selective hyperactivation of PGE2 in colorectal cancer. Clin. Epigenetics 2015, 7, 74. [Google Scholar] [CrossRef] [PubMed]
- Jun, J.C.; Rathore, A.; Younas, H.; Gilkes, D.; Polotsky, V.Y. Hypoxia-Inducible Factors and Cancer. Curr. Sleep Med. Rep. 2017, 3, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Karpisheh, V.; Afjadi, J.F.; Afjadi, M.N.; Haeri, M.S.; Sough, T.S.A.; Asl, S.H.; Edalati, M.; Atyabi, F.; Masjedi, A.; Hajizadeh, F.; et al. Inhibition of HIF-1α/EP4 axis by hyaluronate-trimethyl chitosan-SPION nanoparticles markedly suppresses the growth and development of cancer cells. Int. J. Biol. Macromol. 2021, 167, 1006. [Google Scholar] [CrossRef]
- Hughes, V.S.; Wiggins, J.M.; Siemann, D.W. Tumor oxygenation and cancer therapy—Then and now. Br. J. Radiol. 2019, 92, 20170955. [Google Scholar] [CrossRef]
- Moen, I.; Stuhr, L.E.B. Hyperbaric oxygen therapy and cancer—A review. Target. Oncol. 2012, 7, 233. [Google Scholar] [CrossRef]
- Wang, X.; Ye, N.; Xu, C.; Xiao, C.; Zhang, Z.; Deng, Q.; Li, S.; Li, J.; Li, Z.; Yang, X. Hyperbaric oxygen regulates tumor mechanics and augments Abraxane and gemcitabine antitumor effects against pancreatic ductal adenocarcinoma by inhibiting cancer-associated fibroblasts. Nano Today 2022, 44, 101458. [Google Scholar] [CrossRef]
- Song, M.; Liu, T.; Shi, C.; Zhang, X.; Chen, X. Bioconjugated Manganese Dioxide Nanoparticles Enhance Chemotherapy Response by Priming Tumor-Associated Macrophages toward M1-like Phenotype and Attenuating Tumor Hypoxia. ACS Nano 2016, 10, 633. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Zhao, X.; Zhao, S.; Yu, H.; Cao, W.; Chen, W.; Wei, H.; Guo, H. O2-generating MnO2 nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia. Theranostics 2018, 8, 990. [Google Scholar] [CrossRef] [PubMed]
- Jiang, N.; Zhou, Z.; Xiong, W.; Chen, J.; Shen, J.; Li, R.; Ye, R. Tumor microenvironment triggered local oxygen generation and photosensitizer release from manganese dioxide mineralized albumin-ICG nanocomplex to amplify photodynamic immunotherapy efficacy. Chin. Chem. Lett. 2021, 32, 3948. [Google Scholar] [CrossRef]
- Ren, C.; Xu, X.; Yan, D.; Gu, M.; Zhang, J.; Zhang, H.; Han, C.; Kong, L. Dual-action nanoplatform with a synergetic strategy to promote oxygen accumulation for enhanced photodynamic therapy against hypoxic tumors. Acta Biomater. 2022, 146, 465. [Google Scholar] [CrossRef] [PubMed]
- Zhou, R.; Zeng, X.; Zhao, H.; Chen, Q.; Wu, P. Combating the hypoxia limit of photodynamic therapy through reversing the survival-related pathways of cancer cells. Coord. Chem. Rev. 2022, 452, 214306. [Google Scholar] [CrossRef]
- Chang, C.-C.; Dinh, T.K.; Lee, Y.-A.; Wang, F.-N.; Sung, Y.-C.; Yu, P.-L.; Chiu, S.-C.; Shih, Y.-C.; Wu, C.-Y.; Huang, Y.-D.; et al. Nanoparticle Delivery of MnO2 and Anti-angiogenic Therapy to Overcome Hypoxia-Driven Tumor Escape and Suppress Hepatocellular Carcinoma. ACS Appl. Mater. Interfaces 2020, 12, 44407. [Google Scholar] [CrossRef]
- He, C.; Zhang, X.; Chen, C.; Liu, X.; Chen, Y.; Yan, R.; Fan, T.; Gai, Y.; Lee, R.J.; Ma, X.; et al. A solid lipid coated calcium peroxide nanocarrier enables combined cancer chemo/chemodynamic therapy with O2/H2O2 self-sufficiency. Acta Biomater. 2021, 122, 354. [Google Scholar] [CrossRef]
- Ou, M.; Lin, C.; Wang, Y.; Lu, Y.; Wang, W.; Li, Z.; Zeng, W.; Zeng, X.; Ji, X.; Mei, L. Heterojunction engineered bioactive chlorella for cascade promoted cancer therapy. J Control. Release 2022, 345, 755. [Google Scholar] [CrossRef]
- Dong, Z.Z.; Yang, C.; Wang, Z.; Zhong, Z.; Wong, M.-S.; Li, H.-W. Tumor microenvironment-responsive Zn/Cu nanoparticles for enhanced chemodynamic therapy. Smart Mater. Med. 2023, 4, 286. [Google Scholar] [CrossRef]
- Sheng, Y.; Nesbitt, H.; Callan, B.; Taylor, M.A.; Love, M.; McHale, A.P.; Callan, J.F. Oxygen generating nanoparticles for improved photodynamic therapy of hypoxic tumours. J. Control. Release 2017, 264, 333. [Google Scholar] [CrossRef]
- Zhang, X.; He, C.; He, X.; Fan, S.; Ding, B.; Lu, Y.; Xiang, G. HIF-1 inhibitor-based one-stone-two-birds strategy for enhanced cancer chemodynamic-immunotherapy. J. Control. Release 2023, 356, 649. [Google Scholar] [CrossRef]
- Li, Y.; Jeon, J.; Park, J.H. Hypoxia-responsive nanoparticles for tumor-targeted drug delivery. Cancer Lett. 2020, 490, 31. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, J.; Wang, Y.; Gong, X.; Xu, X.; Wang, J.; Li, Y.; Sha, X.; Zhang, Z. Nanoparticles-mediated reoxygenation strategy relieves tumor hypoxia for enhanced cancer therapy. J. Control. Release 2020, 319, 25. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Sant, S. Hypoxic tumor microenvironment: Opportunities to develop targeted therapies. Biotechnol. Adv. 2016, 34, 803. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Ren, Z.; Zhang, J.; Chuang, C.-C.; Kandaswamy, E.; Zhou, T.; Zuo, L. Role of ROS and Nutritional Antioxidants in Human Diseases. Front. Rhysiol. 2018, 9, 477. [Google Scholar] [CrossRef] [PubMed]
- Sawant, R.R.; Vaze, O.S.; Wang, T.; D’Souza, G.G.M.; Rockwell, K.; Gada, K.; Khaw, B.-A.; Torchilin, V.P. Palmitoyl Ascorbate Liposomes and Free Ascorbic Acid: Comparison of Anticancer Therapeutic Effects Upon Parenteral Administration. Pharm. Res. 2012, 29, 375. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Lu, X.; Liu, Q.; Dai, Y.; Zhu, X.; Wen, Y.; Xu, J.; Lu, J.; Lu, Y.; Zhao, D.; et al. Palmitoyl ascorbate and doxorubicin co-encapsulated liposome for synergistic anticancer therapy. Eur. J. Pharm. Sci. 2017, 105, 219. [Google Scholar] [CrossRef]
- Razi, S.; Haghparast, A.; Khameneh, S.C.; Sadrabadi, A.E.; Aziziyan, F.; Bakhtiyari, M.; Nabi-Afjadi, M.; Tarhriz, V.; Jalili, A.; Zalpoor, H. The role of tumor microenvironment on cancer stem cell fate in solid tumors. Cell Commun. Signal. 2023, 21, 143. [Google Scholar] [CrossRef] [PubMed]
- Vaupel, P.; Multhoff, G. Fatal Alliance of Hypoxia-/HIF-1α-Driven Microenvironmental Traits Promoting Cancer Progression. In Oxygen Transport to Tissue XLI; Advances in Experimental Medicine and, Biology; Ryu, P.D., LaManna, J., Harrison, D., Lee, S.S., Eds.; Springer: Cham, Switzerland, 2020; Volume 1232. [Google Scholar] [CrossRef]
- Kes, M.M.G.; Van den Bossche, J.; Griffioen, A.W.; Huijbers, E.J.M. Oncometabolites lactate and succinate drive pro-angiogenic macrophage response in tumors. BBA–Rev. Cancer 2020, 1874, 188427. [Google Scholar] [CrossRef]
- Singh, L.; Nair, L.; Kumar, D.; Arora, M.K.; Bajaj, S.; Gadewar, M.; Mishra, S.S.; Rath, S.K.; Dubey, A.K.; Kaithwas, G.; et al. Hypoxia induced lactate acidosis modulates tumor microenvironment and lipid reprogramming to sustain the cancer cell survival. Front. Oncol. 2023, 13, 1034205. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Matisevic, S. Functional and metabolic targeting of natural killer cells to solid tumors. Cell. Oncol. 2020, 43, 577–600. [Google Scholar] [CrossRef]
- Chen, C.; Wang, Z.; Ding, Y.; Qin, Y. Manipulating T-cell metabolism to enhance immunotherapy in solid tumor. Front. Immunol. 2022, 13, 1090429. [Google Scholar] [CrossRef] [PubMed]
- Goswami, K.K.; Banerjee, S.; Bose, A.; Baral, R. Lactic acid in alternative polarization and function of macrophages in tumor microenvironment. Hum. Immunol. 2022, 83, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Koltai, T. The complex relationship between multiple drug resistance and the tumor pH gradient: A review. Cancer Drug Resist. 2022, 5, 277. [Google Scholar] [CrossRef] [PubMed]
- Vaidya, F.U.; Chhipa, A.S.; Mishra, V.; Gupta, V.K.; Rawat, S.G.; Kumar, A.; Pathak, C. Molecular and cellular paradigms of multidrug resistance in cancer. Cancer Rep. 2022, 5, e1291. [Google Scholar] [CrossRef] [PubMed]
- AlSawaftah, N.M.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. pH-Responsive Nanocarriers in Cancer Therapy. Polymers 2022, 14, 936. [Google Scholar] [CrossRef] [PubMed]
- Zhou, W.; Jia, Y.; Liu, Y.; Chen, Y.; Zhao, P. Tumor Microenvironment-Based Stimuli-Responsive Nanoparticles for Controlled Release of Drugs in Cancer Therapy. Pharmaceutics 2022, 14, 2346. [Google Scholar] [CrossRef]
- Yan, Y.; Ding, H. pH-Responsive Nanoparticles for Cancer Immunotherapy: A Brief Review. Nanomaterials 2020, 10, 1613. [Google Scholar] [CrossRef]
- Lim, E.-K.; Chung, B.H.; Chung, S. Recent Advances in pH-Sensitive Polymeric Nanoparticles for Smart Drug Delivery in Cancer Therapy. Curr. Drug Targets 2018, 19, 300. [Google Scholar] [CrossRef]
- Sethuraman, V.; Janakiraman, K.; Krishnaswami, V.; Kandasamy, R. Recent Progress in Stimuli-Responsive Intelligent Nano Scale Drug Delivery Systems: A Special Focus Towards pH-Sensitive Systems. Curr. Drug Targets 2021, 22, 947. [Google Scholar] [CrossRef]
- Corbet, C.; Feron, O. Tumour acidosis: From the passenger to the driver’s seat. Nat. Rev. Cancer 2017, 17, 577. [Google Scholar] [CrossRef]
- Wang, C.; Ding, S.; Wang, S.; Shi, Z.; Pandey, N.K.; Chudal, L.; Wang, L.; Zhang, Z.; Wen, Y.; Yao, H.; et al. Endogenous tumor microenvironment-responsive multifunctional nanoplatforms for precision cancer theranostics. Coord. Chem. Rev. 2021, 426, 213529. [Google Scholar] [CrossRef]
- Yadav, A.S.; Venkata Radharani, N.N.; Gorain, M.; Bulbule, A.; Shetti, D.; Roy, G.; Baby, T.; Kundu, G.C. RGD functionalized chitosan nanoparticle mediated targeted delivery of raloxifene selectively suppresses angiogenesis and tumor growth in breast cancer. Nanoscale 2020, 12, 10664. [Google Scholar] [CrossRef]
- Han, Y.; Hu, B.; Wang, M.; Yang, Y.; Zhang, L.; Zhou, J.; Chen, J. pH-Sensitive tumor-targeted hyperbranched system based on glycogen nanoparticles for liver cancer therapy. Appl. Mater. Today 2020, 18, 100521. [Google Scholar] [CrossRef]
- Palanikumar, L.; Al-Hosani, S.; Kalmouni, M.; Nguyen, V.P.; Ali, L.; Pasricha, R.; Barrera, F.N.; Magzoub, M. pH-responsive high stability polymeric nanoparticles for targeted delivery of anticancer therapeutics. Commun. Biol. 2020, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Huang, J.; Wu, J. pH-Sensitive nanogels for drug delivery in cancer therapy. Biomater. Sci. 2021, 9, 574. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Jiang, Y.; Zhang, J.; Klok, H.-A.; Zhong, Z. pH-Sensitive Coiled-Coil Peptide-Cross-Linked Hyaluronic Acid Nanogels: Synthesis and Targeted Intracellular Protein Delivery to CD44 Positive Cancer Cells. Biomacromolecules 2018, 19, 555. [Google Scholar] [CrossRef] [PubMed]
- Vinardell, M.P.; Mitjans, M. Metal/Metal Oxide Nanoparticles for Cancer Therapy. In Nanooncology; Nanomedicine and Nanotoxicology; Gonçalves, G., Tobias, G., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Subhan, M.A. Advances with metal oxide-based nanoparticles as MDR metastatic breast cancer therapeutics and diagnostics. RSC Adv. 2022, 12, 32956. [Google Scholar] [CrossRef]
- Gao, R.; Mitra, R.N.; Zheng, M.; Wang, K.; Dahringer, J.C.; Han, Z. Developing Nanoceria-Based pH-Dependent Cancer-Directed Drug Delivery System for Retinoblastoma. Adv. Funct. Mater. 2018, 28, 1806248. [Google Scholar] [CrossRef]
- Yang, G.; Xu, L.; Chao, Y.; Xu, J.; Sun, X.; Wu, Y.; Peng, R.; Liu, Z. Hollow MnO2 as a tumor-microenvironment-responsive biodegradable nano-platform for combination therapy favoring antitumor immune responses. Nat. Commun. 2017, 8, 902. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.-X.; Liu, M.-D.; Guo, D.-K.; Zou, M.-Z.; Wang, S.-B.; Cheng, H.; Zhong, Z.; Zhang, X.-Z. A MSN-Based Tumor-Targeted Nanoplatform to Interfere with Lactate Metabolism to Induce Tumor Cell Acidosis for Tumor Suppression and Anti-Metastasis. Nanoscale 2020, 12, 2966. [Google Scholar] [CrossRef] [PubMed]
- Rauta, P.R.; Mackeyev, Y.; Sanders, K.; Kim, J.B.K.; Gonzalez, V.V.; Zahra, Y.; Shohayeb, M.A.; Abousaida, B.; Vijay, G.V.; Tezcan, O.; et al. Pancreatic tumor microenvironmental acidosis and hypoxia transform gold nanorods into cell-penetrant particles for potent radiosensitization. Sci. Adv. 2022, 8, eabm9729. [Google Scholar] [CrossRef] [PubMed]
- Xue, Y.; Tao, L.; Zhou, Y.; Liu, J.; Yu, B.; Long, S.; Huang, S.; Yu, F. Enhanced Targeted Delivery of Doxorubicin Based on Acid Induced Charge Reversal and Combinational Stimuli-Responsive Nanocarrier. Adv. Eng. Mater. 2018, 20, 1701151. [Google Scholar] [CrossRef]
- Angelopoulou, A.; Kolokithas-Ntoukas, A.; Papaioannou, L.; Kakazanis, Z.; Khoury, N.; Zoumpourlis, V.; Papatheodorou, S.; Kardamakis, D.; Bakandritsos, A.; Hatziantoniou, S.; et al. Canagliflozin-loaded magnetic nanoparticles as potential treatment of hypoxic tumors in combination with radiotherapy. Nanomedicine 2018, 13, 2435. [Google Scholar] [CrossRef]
- Ma, Q.; Li, Q.; Cai, X.; Zhou, P.; Wu, Z.; Wang, B.; Ma, W.; Fu, S. Injectable hydrogels as drug delivery platform for in-situ treatment of malignant tumor. J. Drug Deliv. Sci. Technol. 2022, 76, 103817. [Google Scholar] [CrossRef]
- Deirram, N.; Zhang, C.; Kermaniyan, S.S.; Johnston, A.P.R.; Such, G.K. pH-Responsive Polymer Nanoparticles for Drug Delivery. Macromol. Rapid Commun. 2019, 40, 1800917. [Google Scholar] [CrossRef]
- Sim, T.M. Nanoparticle-assisted targeting of the tumour microenvironment. OpenNano 2022, 8, 100097. [Google Scholar] [CrossRef]
- Wang, Q.; Atluri, K.; Tiwari, A.K.; Babu, R.J. Exploring the Application of Micellar Drug Delivery Systems in Cancer Nanomedicine. Pharmaceuticals 2023, 16, 433. [Google Scholar] [CrossRef]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. pH-responsive self-healing injectable hydrogel based on N-carboxyethyl chitosan for hepatocellular carcinoma therapy. Acta Biomater. 2017, 58, 168. [Google Scholar] [CrossRef]
- Megahed, M.A.; El-Sawy, H.S.; Reda, A.M.; Abd-Allah, F.I.; Abu Elyazid, S.K.; Lila, A.E.; Ismael, H.R.; El-Say, H.M. Effect of nanovesicular surface-functionalization via chitosan and/or PEGylation on cytotoxicity of tamoxifen in induced-breast cancer model. Life Sci. 2022, 307, 120908. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, D.; Tacheva, T.; Semkova, S.; Panovska, R.; Yaneva, Z. In Vitro Model for Evaluation of Cancer Cell Proliferative Activity under Simulated Acidosis and Using Chitosan Microparticles. Appl. Sci. 2022, 12, 12029. [Google Scholar] [CrossRef]
- Zhou, T.; Lu, W.; Mezhuev, Y.; Lan, M.; Li, L.; Liu, F.; Cai, T.; Wu, X.; Cai, Y. A review of nanoparticle drug delivery systems responsive to endogenous breast cancer microenvironment. Eur. J. Pharm. Biopharm. 2021, 166, 30. [Google Scholar] [CrossRef]
- Zhao, J.; Yan, C.; Chen, Z.; Liu, J.; Song, H.; Wang, W.; Liu, J.; Yang, N.; Zhao, Y.; Chen, L. Dual-targeting nanoparticles with core-crosslinked and pH/redox bioresponsive properties for enhanced intracellular drug delivery. J. Colloid Interface Sci. 2019, 540, 66. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.-Y.; Mao, C.-Q.; Du, X.-J.; Du, J.-Z.; Wang, F.; Wang, J. Surface Charge Switchable Nanoparticles Based on Zwitterionic Polymer for Enhanced Drug Delivery to Tumor. Adv. Mater. 2012, 24, 5476. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Z.; Yu, G.; Zhou, Z.; Jacobson, O.; Liu, Y.; Ma, Y.; Zhang, F.; Chen, Z.-Y.; Chen, X. Tumor-Specific Drug Release and Reactive Oxygen Species Generation for Cancer Chemo/Chemodynamic Combination Therapy. Adv. Sci. 2019, 6, 1801986. [Google Scholar] [CrossRef]
- Liang, J.; Huang, Q.; Hua, C.; Hu, J.; Chen, B.; Wan, J.; Hu, Z.; Wang, B. pH-Responsive Nanoparticles Loaded with Graphene Quantum Dots and Doxorubicin for Intracellular Imaging, Drug Delivery and Efficient Cancer Therapy. ChemistrySelect 2019, 4, 6004. [Google Scholar] [CrossRef]
- Meng, X.; Xu, Y.; Lu, Q.; Sun, L.; An, X.; Zhang, J.; Chen, J.; Dao, Y.; Zhang, Y.; Ning, X. Ultrasound-responsive alkaline nanorobots for the treatment of lactic acidosis-mediated doxorubicin resistance. Nanoscale 2020, 12, 13801. [Google Scholar] [CrossRef]
- Shi, Z.; Li, Q.; Mei, L. pH-Sensitive nanoscale materials as robust drug delivery systems for cancer therapy. Chin. Chem. Lett. 2020, 31, 1345. [Google Scholar] [CrossRef]
- Wang, T.; Wang, D.; Liu, J.; Feng, B.; Zhou, F.; Zhang, H.; Zhou, L.; Yin, Q.; Zhang, Z.; Cao, Z.; et al. Acidity-Triggered Ligand-Presenting Nanoparticles To Overcome Sequential Drug Delivery Barriers to Tumors. Nano Lett. 2017, 17, 5429. [Google Scholar] [CrossRef]
- Liu, S.; Luo, X.; Liu, S.; Xu, P.; Wang, J.; Hu, Y. Acetazolamide-Loaded pH-Responsive Nanoparticles Alleviating Tumor Acidosis to Enhance Chemotherapy Effects. Macromol. Biosci. 2019, 19, 1800366. [Google Scholar] [CrossRef]
- Liu, G.; Zhao, X.; Zhang, Y.; Xu, J.; Xu, J.; Li, Y.; Min, H.; Shi, J.; Zhao, Y.; Wei, J.; et al. Engineering Biomimetic Platesomes for pH-Responsive Drug Delivery and Enhanced Antitumor Activity. Adv. Mater. 2019, 31, 1900795. [Google Scholar] [CrossRef] [PubMed]
- Luo, X.; Cao, J.; Yu, J.; Dai, D.; Jiang, W.; Feng, Y.; Hu, Y. Regulating Acidosis and Relieving Hypoxia by Platelet Membrane-Coated Nanoparticle for Enhancing Tumor Chemotherapy. Front. Bioeng. Biotechnol. 2022, 10, 885105. [Google Scholar] [CrossRef] [PubMed]
- Ruan, S.; Huang, Y.; He, M.; Gao, H. Advanced Biomaterials for Cell-Specific Modulation and Restore of Cancer Immunotherapy. Adv. Sci. 2022, 9, 2200027. [Google Scholar] [CrossRef] [PubMed]
- Cai, L.; Xu, J.; Yang, Z.; Tong, R.; Dong, Z.; Wang, C.; Leong, K.W. Engineered biomaterials for cancer immunotherapy. Med. Comm. 2020, 1, 35. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.-Q.; Wei, L.; Tang, L. Immunoengineering with biomaterials for enhanced cancer immunotherapy. WIREs Nanomed. Nanobiotechnol. 2018, 10, e1506. [Google Scholar] [CrossRef] [PubMed]
- Bo, Y.; Wang, H. Biomaterial-Based In Situ Cancer Vaccines. Adv. Mater. 2023, 2210452. [Google Scholar] [CrossRef] [PubMed]
- Abdou, P.; Wang, Z.; Chen, Q.; Chan, A.; Zhou, D.R.; Gunadhi, V.; Gu, Z. Advances in engineering local drug delivery systems for cancer immunotherapy. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1632. [Google Scholar] [CrossRef]
- Hu, L.; Cao, Z.; Ma, L.; Liu, Z.; Liao, G.; Wang, J.; Shen, S.; Li, D.; Yang, X. The potentiated checkpoint blockade immunotherapy by ROS-responsive nanocarrier-mediated cascade chemo-photodynamic therapy. Biomaterials 2019, 223, 119469. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hu, Q.; Bellotti, A.; Gu, Z. Tailoring Biomaterials for Cancer Immunotherapy: Emerging Trends and Future Outlook. Adv. Mater. 2017, 29, 1606036. [Google Scholar] [CrossRef]
- Rosalia, R.A.; Cruz, L.J.; van Duikeren, S.; Tromp, A.T.; Silva, A.L.; Jiskoot, W.; de Gruijl, T.; Lowik, C.; Oostendorp, J.; van der Burg, A.; et al. CD40-targeted dendritic cell delivery of PLGA-nanoparticle vaccines induce potent anti-tumor responses. Biomaterials 2015, 40, 88. [Google Scholar] [CrossRef]
- Wang, C.; Li, P.; Liu, L.I.; Pan, H.; Li, H.; Cai, L.; Ma, Y. Self-adjuvanted nanovaccine for cancer immunotherapy: Role of lysosomal rupture-induced ROS in MHC class I antigen presentation. Biomaterials 2016, 79, 88. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Luo, Z.; Li, Z.; Wang, Y.; Tao, J.; Gong, C.; Liu, X. Improving Cancer Immunotherapy Outcomes Using Biomaterials. Angew. Chem. 2020, 132, 17484. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, C. Regulation of cancer-immunity cycle and tumor microenvironment by nanobiomaterials to enhance tumor immunotherapy. WIREs Nanomed. Nanobiotechnol. 2020, 12, e1612. [Google Scholar] [CrossRef] [PubMed]
- Overchuk, M.; Zheng, G. Overcoming obstacles in the tumor microenvironment: Recent advancements in nanoparticle delivery for cancer theranostics. Biomaterials 2018, 156, 217. [Google Scholar] [CrossRef]
- Li, Y.; Tang, K.; Zhang, X.; Pan, W.; Li, N.; Tang, B. Tumor microenvironment responsive nanocarriers for gene therapy. Chem. Commun. 2022, 58, 8754. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Park, O.K.; Kim, J.; Shin, K.; Pack, C.G.; Kim, K.; Ko, G.; Lee, N.; Kwon, S.-H.; Hyeon, T. Deep Tumor Penetration of Drug-Loaded Nanoparticles by Click Reaction-Assisted Immune Cell Targeting Strategy. J. Am. Chem. Soc. 2019, 141, 13829. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Ma, Z.; Xiang, Z.; Xia, Y.; Shi, Q.; Wong, S.-C.; Yin, J. Hydrogen Peroxide and Glutathione Dual Redox-Responsive Nanoparticles for Controlled DOX Release. Macromol. Biosci. 2020, 20, 1900331. [Google Scholar] [CrossRef]
- Chen, X.; Jia, F.; Li, Y.; Deng, Y.; Huang, Y.; Liu, W.; Jin, Q.; Ji, J. Nitric oxide-induced stromal depletion for improved nanoparticle penetration in pancreatic cancer treatment. Biomaterials 2020, 246, 119999. [Google Scholar] [CrossRef]
- Dabaghi, M.; Rasa, S.M.M.; Cirri, E.; Ori, A.; Neri, F.; Quaas, R.; Hilger, I. Iron Oxide Nanoparticles Carrying 5-Fluorouracil in Combination with Magnetic Hyperthermia Induce Thrombogenic Collagen Fibers, Cellular Stress, and Immune Responses in Heterotopic Human Colon Cancer in Mice. Pharmaceutics 2021, 13, 1625. [Google Scholar] [CrossRef]
- Paholak, H.J.; Stevers, N.O.; Chen, H.; Burnett, J.P.; He, M.; Korkaya, H.; McDermott, S.P.; Deol, Y.; Clouthier, S.G.; Luther, T.; et al. Elimination of epithelial-like and mesenchymal-like breast cancer stem cells to inhibit metastasis following nanoparticle-mediated photothermal therapy. Biomaterials 2016, 104, 145. [Google Scholar] [CrossRef]
- Tan, T.; Wang, Y.; Wang, J.; Wang, Z.; Wang, H.; Cao, H.; Li, J.; Zhang, Z.; Wang, S. Targeting peptide-decorated biomimetic lipoproteins improve deep penetration and cancer cells accessibility in solid tumor. Acta Pharm. Sin. B 2020, 10, 529. [Google Scholar] [CrossRef]
- Kola, P.; Nagesh, P.K.B.; Roy, P.K.; Deepak, K.; Reis, R.L.; Kundu, S.C.; Mandal, M. Innovative nanotheranostics: Smart nanoparticles based approach to overcome breast cancer stem cells mediated chemo- and radioresistances. Wiley Interdiscip. Rev. Nanomed. 2023, 15, e1876. [Google Scholar] [CrossRef]
- Souri, M.; Soltani, M.; Moradi Kashkooli, F.; Kiani Shahvandi, M.; Chiani, M.; Sadat Shariatic, F.; Reza Mehrabi, M.; Munn, L.L. Towards principled design of cancer nanomedicine to accelerate clinical translation. Mater. Today Bio 2022, 13, 100208. [Google Scholar] [CrossRef]
- Zhang, P.; Xiao, Y.; Sun, X.; Lin, X.; Koo, S.; Yaremenko, A.V.; Qin, D.; Kong, N.; Farokhzad, O.C.; Tao, W. Cancer nanomedicine toward clinical translation: Obstacles, opportunities, and future prospects. Med 2023, 4, 147. [Google Scholar] [CrossRef] [PubMed]
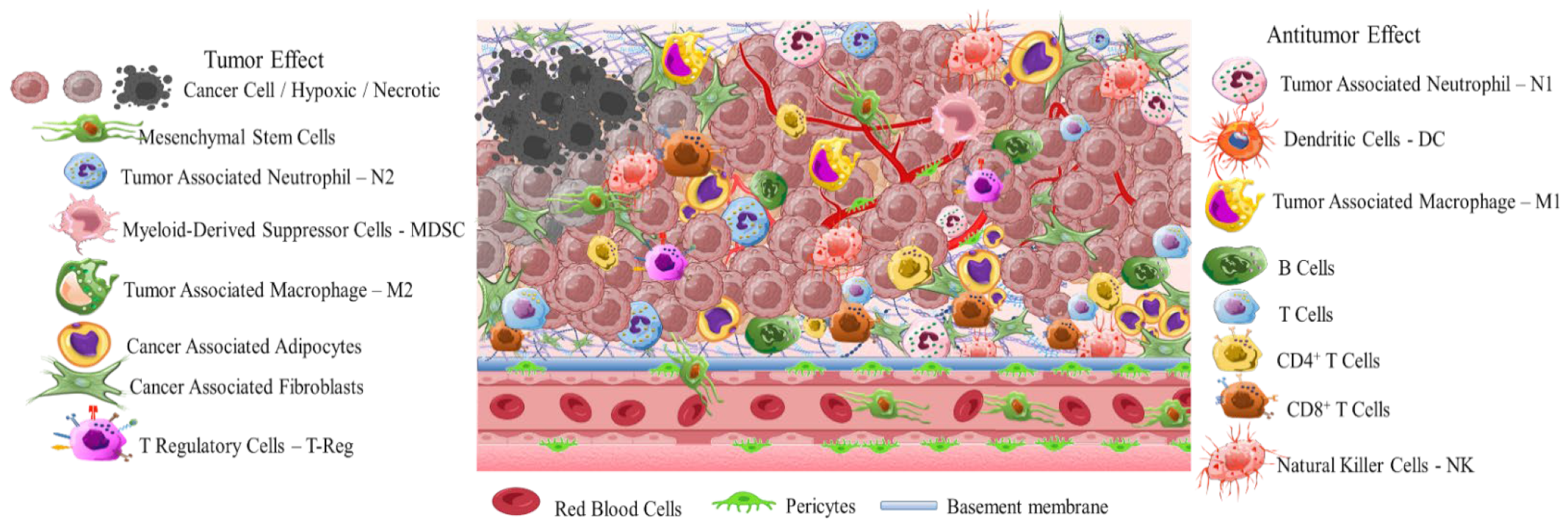
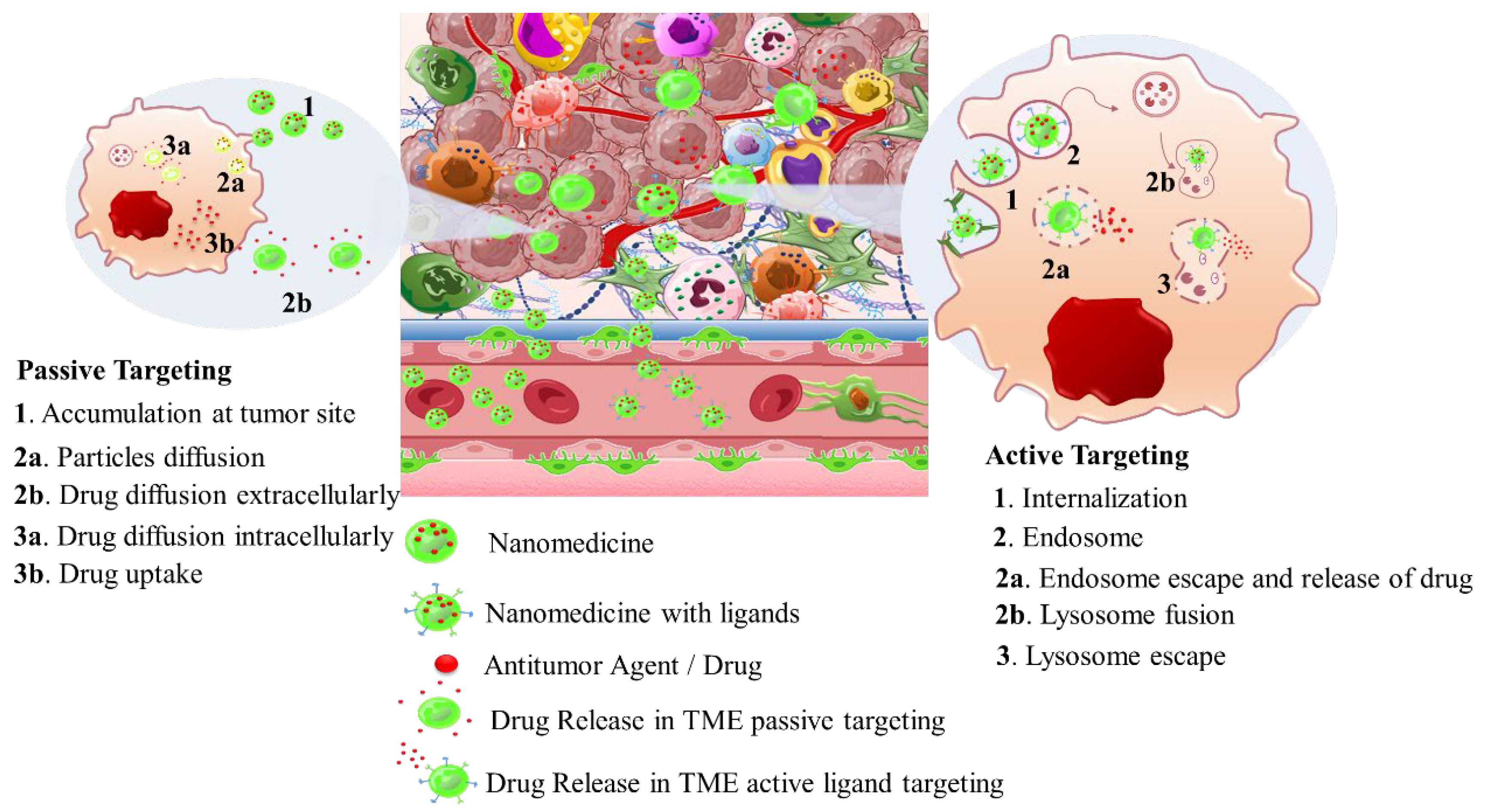
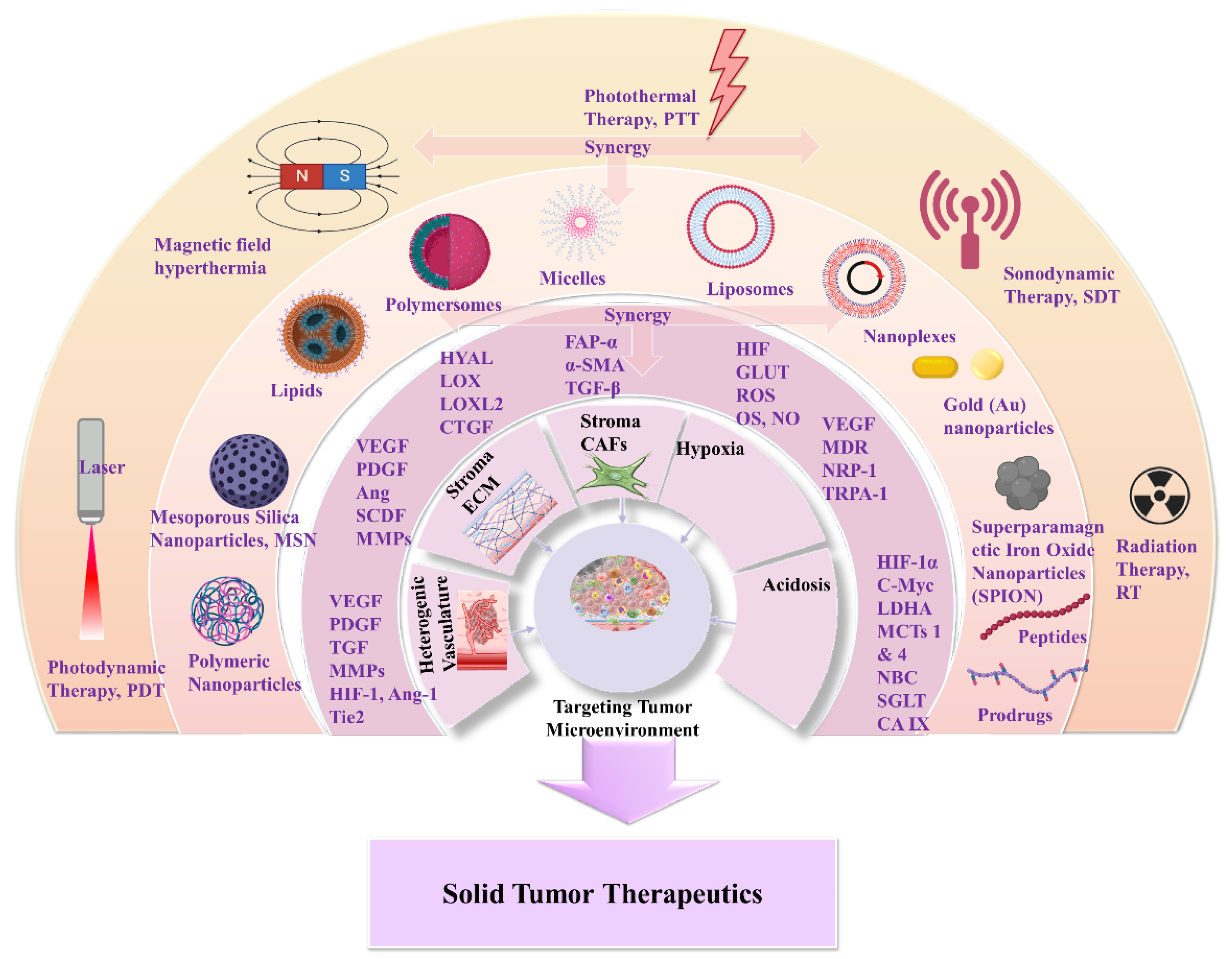
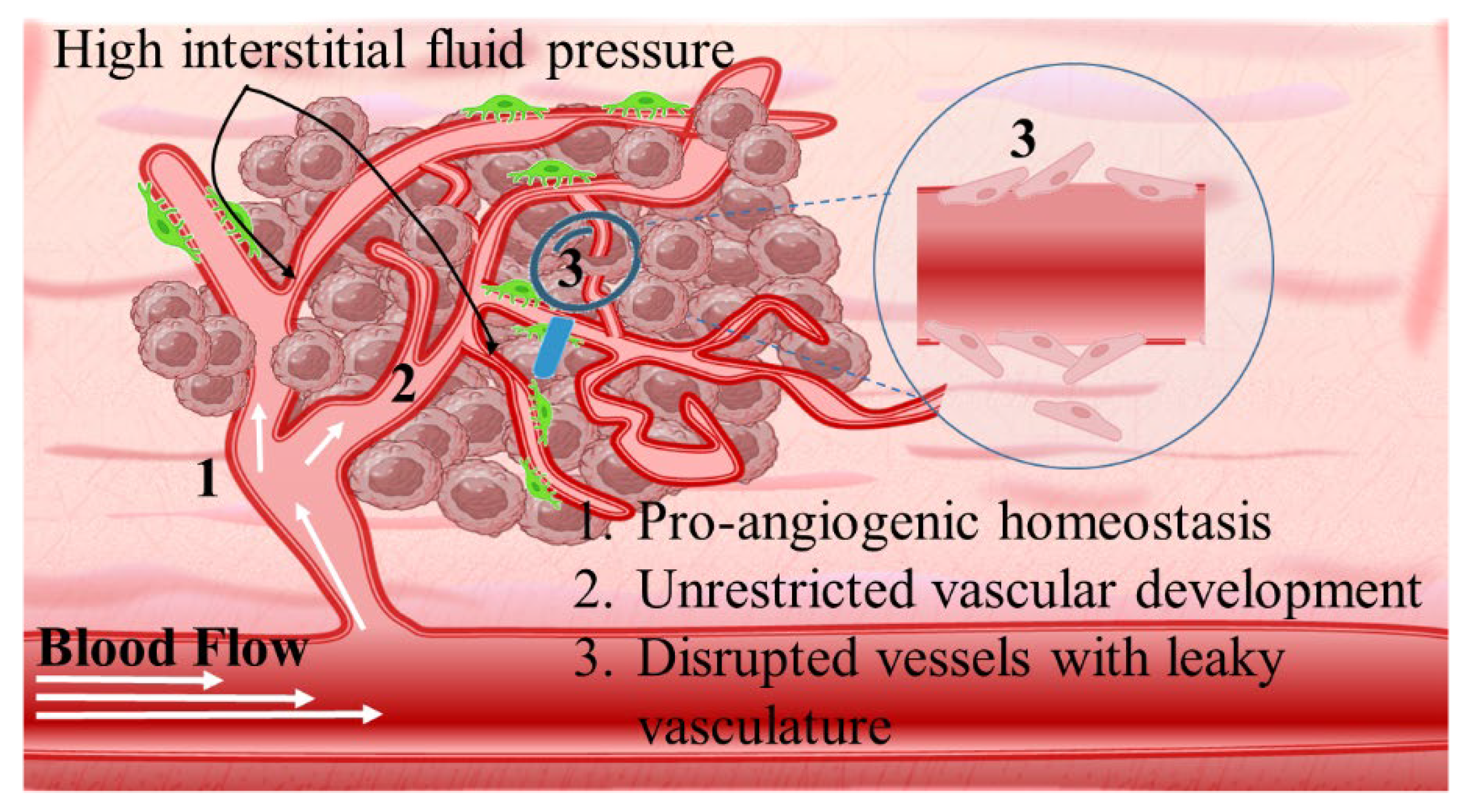
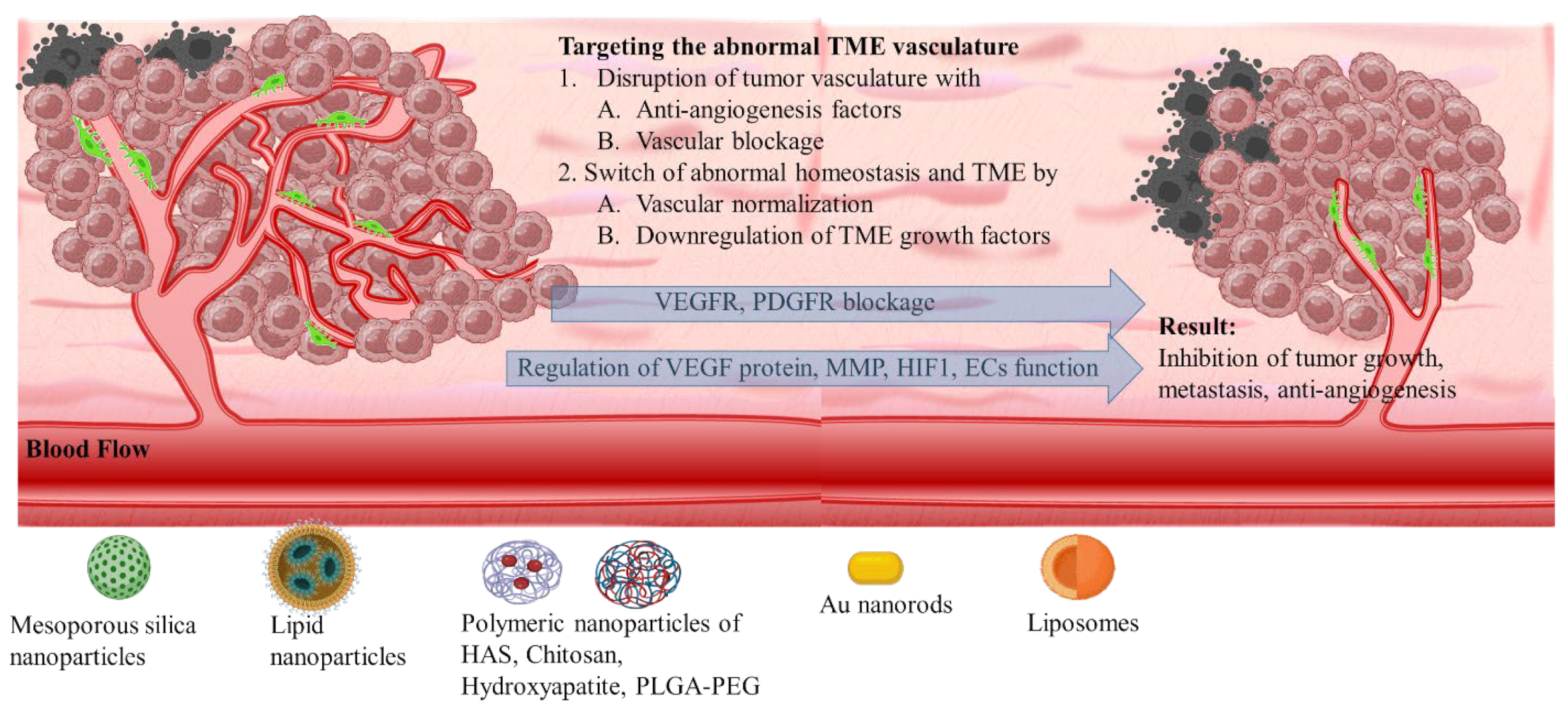
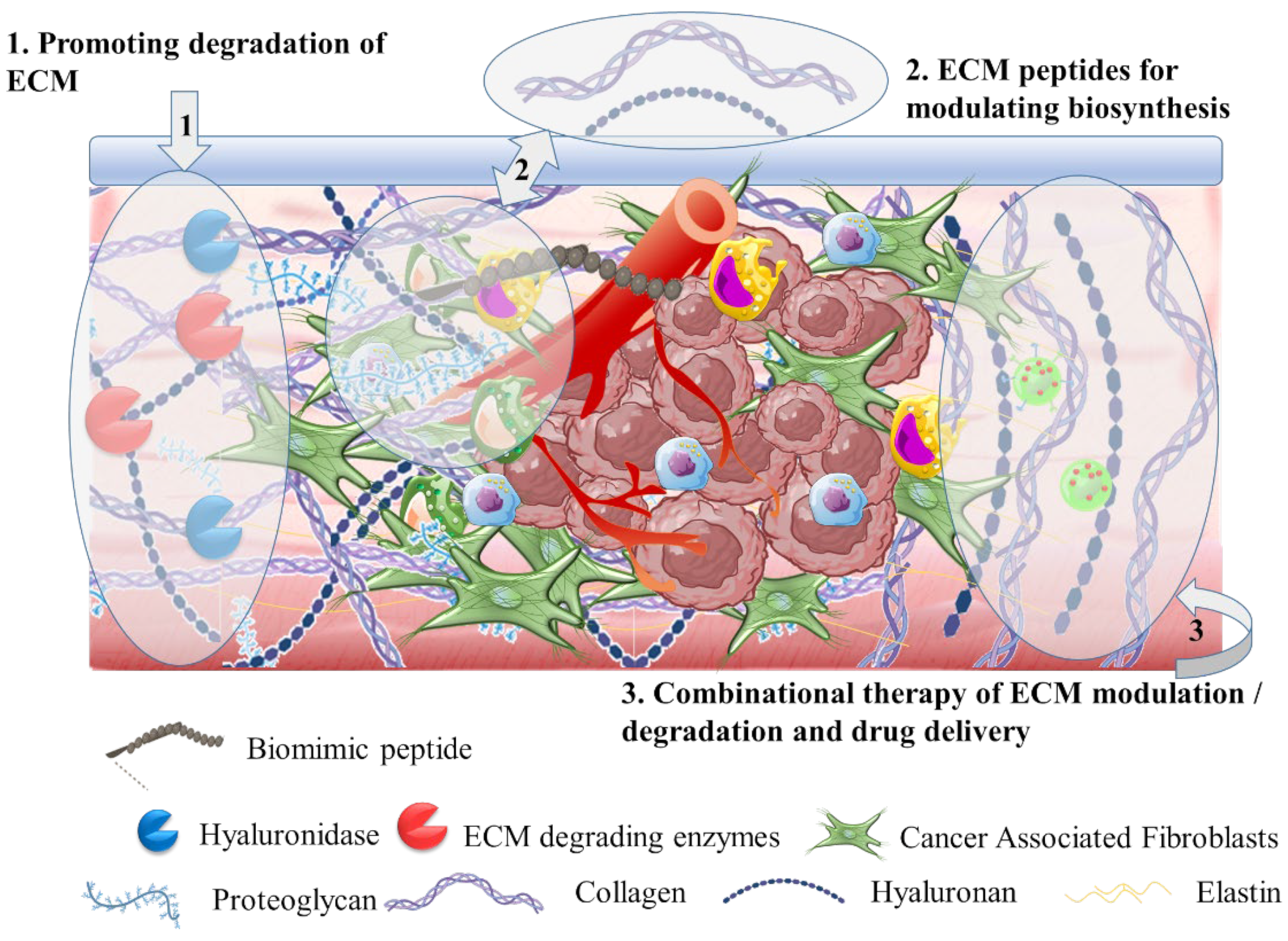

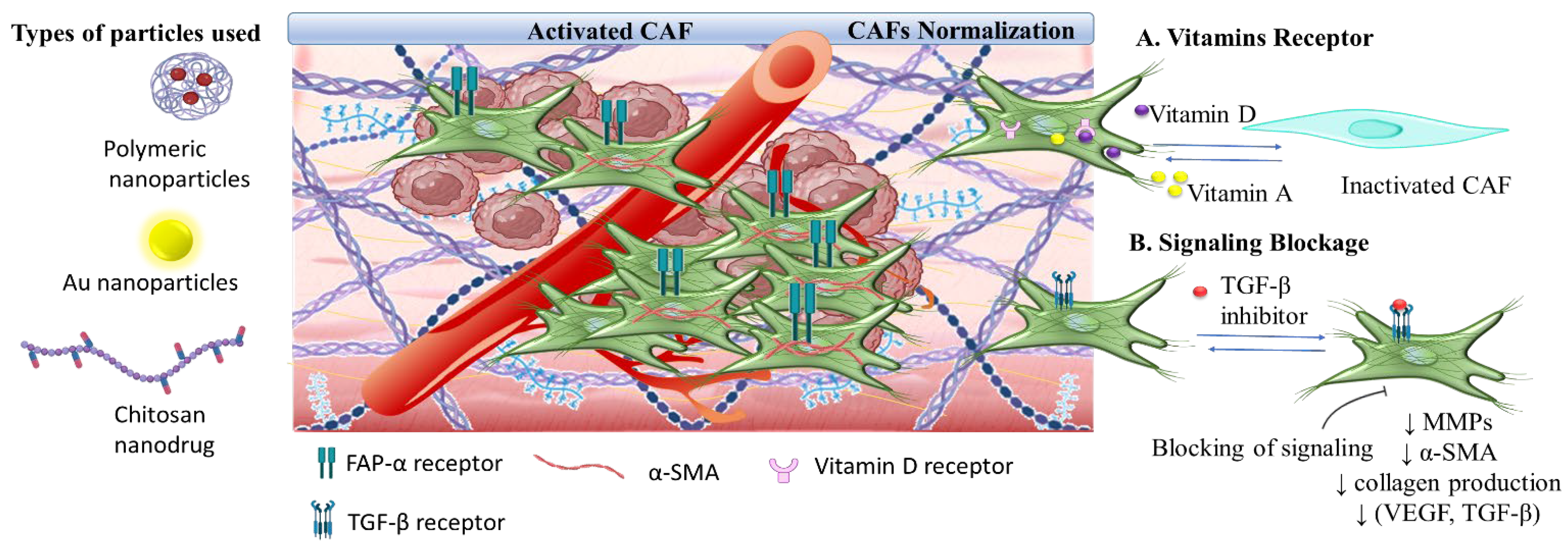
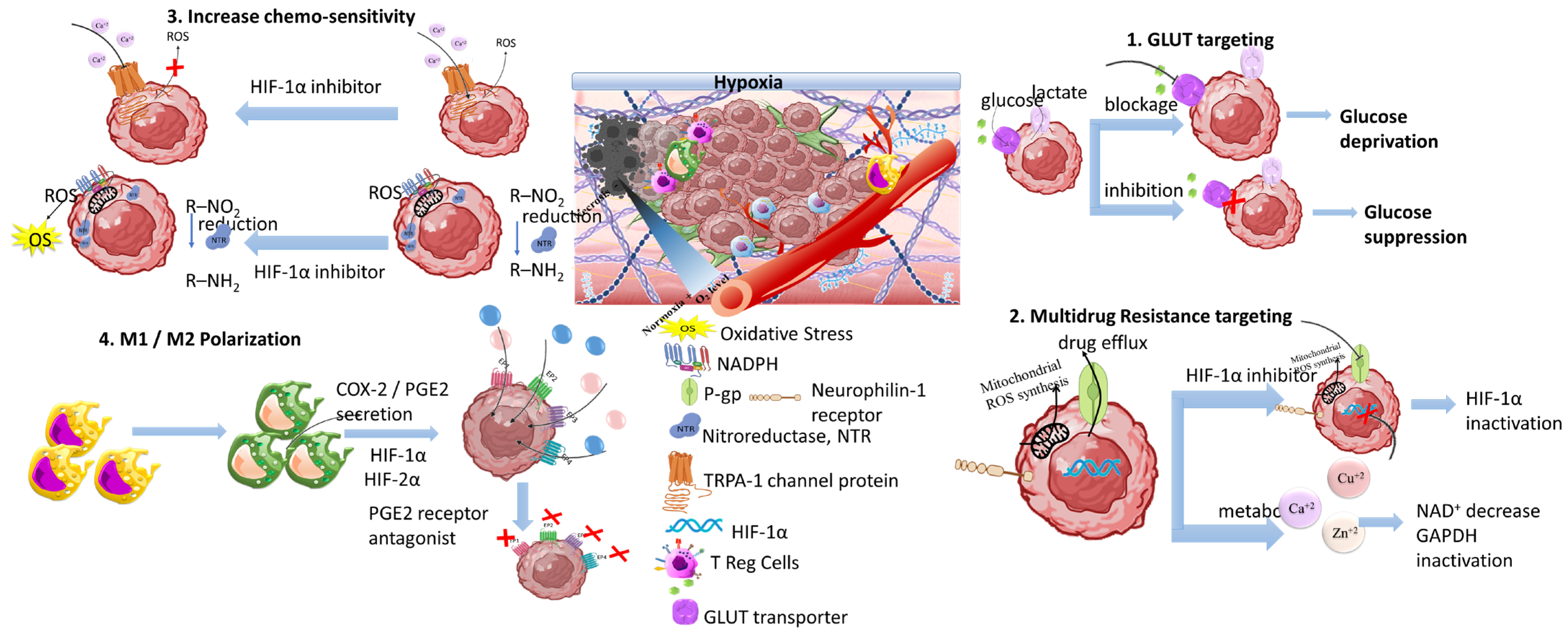
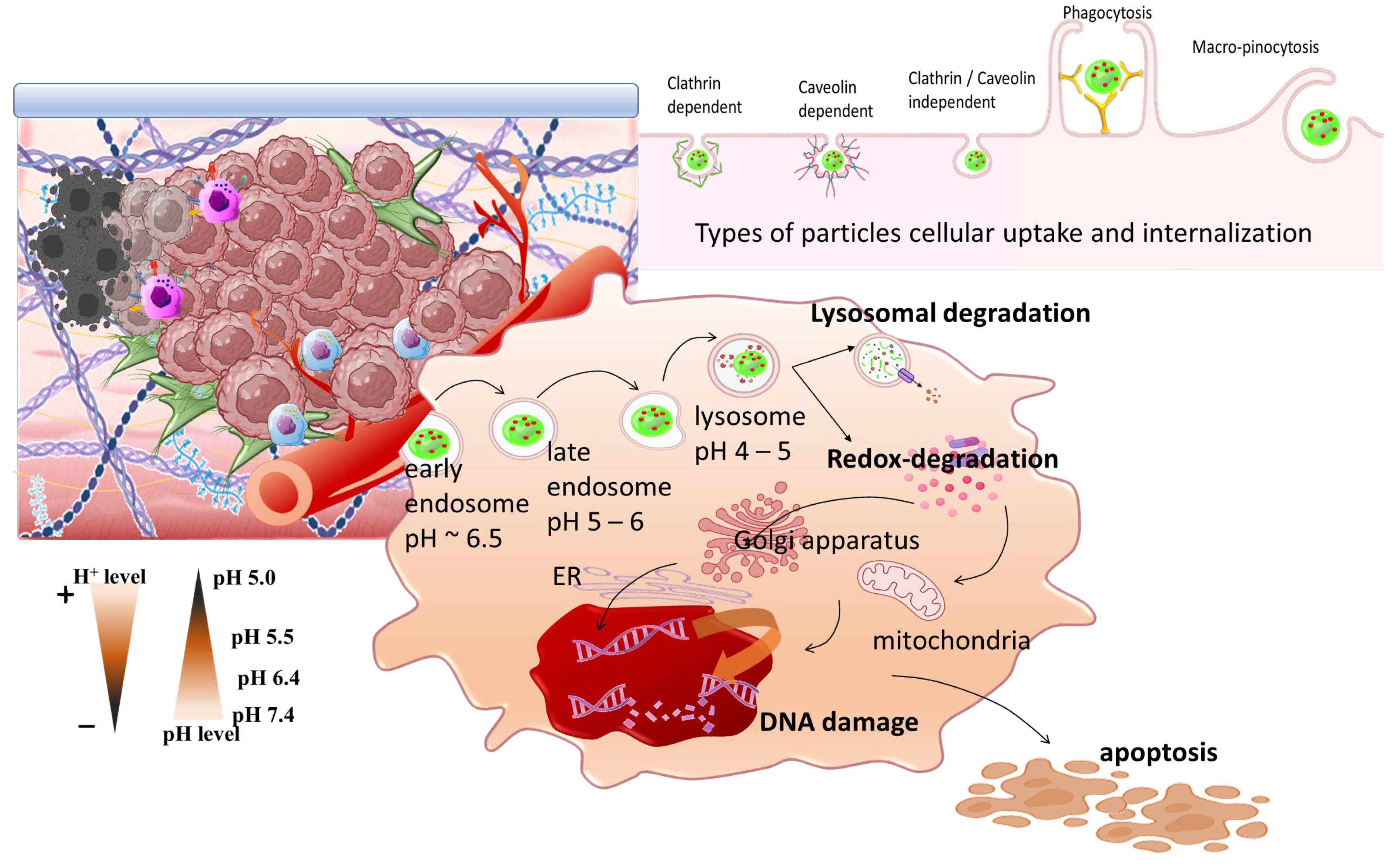
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avgoustakis, K.; Angelopoulou, A. Biomaterial-Based Responsive Nanomedicines for Targeting Solid Tumor Microenvironments. Pharmaceutics 2024, 16, 179. https://doi.org/10.3390/pharmaceutics16020179
Avgoustakis K, Angelopoulou A. Biomaterial-Based Responsive Nanomedicines for Targeting Solid Tumor Microenvironments. Pharmaceutics. 2024; 16(2):179. https://doi.org/10.3390/pharmaceutics16020179
Chicago/Turabian StyleAvgoustakis, Konstantinos, and Athina Angelopoulou. 2024. "Biomaterial-Based Responsive Nanomedicines for Targeting Solid Tumor Microenvironments" Pharmaceutics 16, no. 2: 179. https://doi.org/10.3390/pharmaceutics16020179
APA StyleAvgoustakis, K., & Angelopoulou, A. (2024). Biomaterial-Based Responsive Nanomedicines for Targeting Solid Tumor Microenvironments. Pharmaceutics, 16(2), 179. https://doi.org/10.3390/pharmaceutics16020179






