Piperacillin/Tazobactam Co-Delivery by Micellar Ionic Conjugate Systems Carrying Pharmaceutical Anions and Encapsulated Drug
Abstract
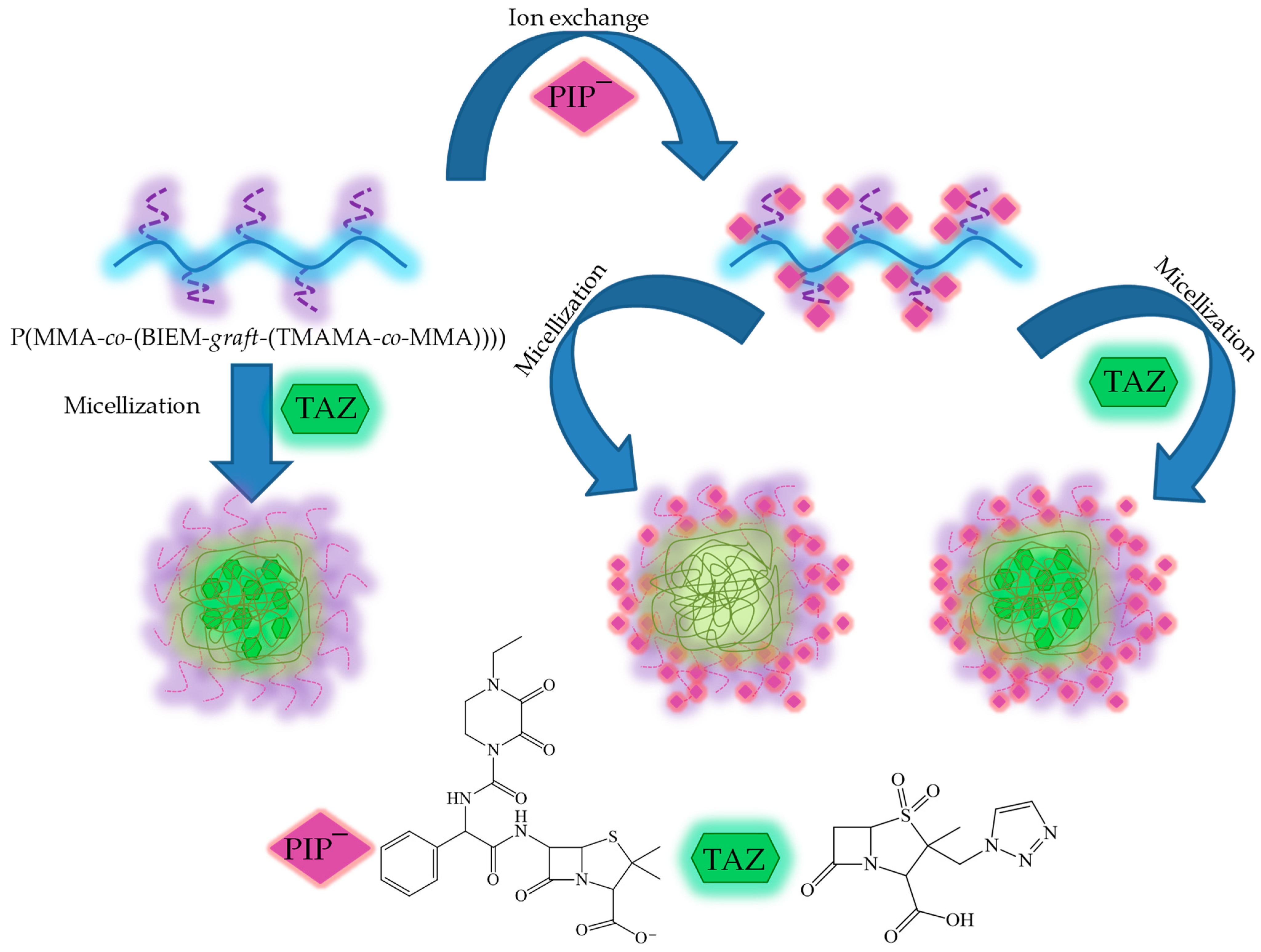
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Ionic Conjugates Bearing PIP Anions (Example of GP1_PIP−)
2.2. Encapsulation of TAZ
2.3. In Vitro Drug Release Studies
2.4. Cell Growth and MTT Cytotoxicity Assay
2.5. Characterization
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Coelho, J.F.; Ferreira, P.C.; Alves, P.; Cordeiro, R.; Fonseca, A.C.; Góis, J.R.; Gil, M.H. Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J. 2010, 1, 164–209. [Google Scholar] [CrossRef]
- Zhang, Y.; Chan, H.F.; Leong, K.W. Advanced materials and processing for drug delivery: The past and the future. Adv. Drug Deliv. Rev. 2013, 65, 104–120. [Google Scholar] [CrossRef]
- Tiwari, G.; Tiwari, R.; Bannerjee, S.; Bhati, L.; Pandey, S.; Pandey, P.; Sriwastawa, B. Drug delivery systems: An updated review. Int. J. Pharm. Investig. 2012, 2, 2. [Google Scholar] [CrossRef]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, 17488. [Google Scholar] [CrossRef]
- Pushpamalar, J.; Meganathan, P.; Tan, H.L.; Dahlan, N.A.; Ooi, L.T.; Neerooa, B.N.H.M.; Essa, R.Z.; Shameli, K.; Teow, S.Y. Development of a Polysaccharide-Based Hydrogel Drug Delivery System (DDS): An Update. Gels 2021, 7, 153. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Wang, J.; Wang, Y.; Gao, H.; Wei, G.; Huang, Y.; Haijun Yu, H.; Gan, Y.; Wang, Y.; Mei, L.; et al. Recent progress in drug delivery. Acta Pharm. Sin. B 2019, 9, 1145–1162. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Ramos Campos, E.V.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Lei, Z.; Chen, B.; Koo, M.-K.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 10, 6633–6635. [Google Scholar] [CrossRef]
- Clare, B.; Sirwardana, A.; MacFarlane, D.R. Synthesis, Purification and Characterization of Ionic Liquids. In Ionic Liquids; Topics in Current Chemistry; Sprinder: Berlin/Heidelberg, Germany, 2010; Volume 290, pp. 1–40. [Google Scholar]
- Forsyth, S.A.; Pringle, J.M.; MacFarlane, D.R. Ionic Liquids—An Overview. Aust. J. Chem. 2004, 57, 113. [Google Scholar] [CrossRef]
- Kausar, A. Research Progress in Frontiers of Poly(Ionic Liquid)s: A Review. Polym. Plast. Technol. Eng. 2017, 56, 1823–1838. [Google Scholar] [CrossRef]
- Yuan, J.; Markus Antonietti, M. Poly(ionic liquid)s: Polymers expanding classical property profiles. Polymer 2011, 52, 1469–1482. [Google Scholar] [CrossRef]
- Qader, I.B.; Prasad, K. Recent developments on Ionic Liquids and Deep Eutectic Solvents for Drug Delivery Applications. Pharm. Res. 2022, 39, 2367–2377. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xie, F.; Chen, L.; Li, X. Ionic liquids for preparation of biopolymer materials for drug/gene delivery: A review. Green Chem. 2018, 20, 4169–4200. [Google Scholar] [CrossRef]
- Pedro, S.N.; Freire, C.S.R.; Silvestre, A.J.D.; Freire, M.G. Ionic Liquids in Drug Delivery. Encyclopedia 2021, 1, 324–339. [Google Scholar] [CrossRef]
- Md Moshikur, R.; Chowdhury, M.R.; Moniruzzaman, M.; Goto, M. Biocompatible ionic liquids and their application in pharmaceutics. Green Chem. 2020, 22, 8116–8139. [Google Scholar] [CrossRef]
- Lu, B.; Bo, Y.; Yi, M.; Wang, Z.; Zhang, J.; Zhu, Z.; Zhao, Y.; Zhang, J. Enhancing the Solubility and Transdermal Delivery of Drugs Using Ionic Liquid-In-Oil Microemulsions. Adv. Funct. Mater. 2021, 31, 2102794. [Google Scholar] [CrossRef]
- Ait-Touchente, Z.; Zine, N.; Jaffrezic-Renault, N.; Errachid, A.; Lebaz, N.; Fessi, H.; Elaissari, A. Exploring the Versatility of Microemulsions in Cutaneous Drug Delivery: Opportunities and Challenges. Nanomaterials 2023, 13, 1688. [Google Scholar] [CrossRef]
- Liu, C.; Chen, B.; Shi, W.; Huang, W.; Qian, H. Ionic Liquids for Enhanced Drug Delivery: Recent Progress and Prevailing Challenges. Mol. Pharm. 2022, 19, 1033–1046. [Google Scholar] [CrossRef]
- Shukla, M.K.; Tiwari, H.; Verma, R.; Dong, W.-L.; Azizov, S.; Kumar, B.; Pandey, S.; Kumar, D. Role and Recent Advancements of Ionic Liquids in Drug Delivery Systems. Pharmaceutics 2023, 15, 702. [Google Scholar] [CrossRef]
- Cojocaru, O.A.; Bica, K.; Gurau, G.; Narita, A.; McCrary, P.D.; Shamshina, J.L.; Barber, P.S.; Rogers, R.D. Prodrug ionic liquids: Functionalizing neutral active pharmaceutical ingredients to take advantage of the ionic liquid form. Med. Chem. Comm. 2013, 4, 559. [Google Scholar] [CrossRef]
- Zhang, W.; Guo, Y.; Yang, J.; Tang, G.; Zhang, J.; Cao, Y. Prodrug Based on Ionic Liquids for Dual-Triggered Release of Thiabendazole. ACS Omega 2023, 8, 3484–3492. [Google Scholar] [CrossRef] [PubMed]
- Moshikur, R.M.; Chowdhury, M.R.; Wakabayashi, R.; Tahara, Y.; Moniruzzaman, M.; Goto, M. Ionic liquids with methotrexate moieties as a potential anticancer prodrug: Synthesis, characterization and solubility evaluation. J. Mol. Liq. 2019, 278, 226–233. [Google Scholar] [CrossRef]
- Cojocaru, O.; Shamshina, J.; Rogers, R. Review/Preview: Prodrug Ionic Liquids Combining the Prodrug and Ionic Liquid Strategies to Active Pharmaceutical Ingredients. Chim. Oggi-Chem. Today 2013, 31, 24–29. [Google Scholar]
- Pedro, S.; Freire, C.; Silvestre, A.; Freire, M. The Role of Ionic Liquids in the Pharmaceutical Field: An Overview of Relevant Applications. Int. J. Mol. Sci. 2020, 21, 8298. [Google Scholar] [CrossRef]
- Liu, C.; Raza, F.; Qian, H.; Tian, X. Recent advances in poly(ionic liquid)s for biomedical application. Biomater. Sci. 2022, 10, 2524–2539. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Zhou, Y.; Qiu, L.; Yuan, C.; Yan, F. Self-assembly of amphiphilic random co-poly(ionic liquid)s: The effect of anions, molecular weight, and molecular weight distribution. Polym. Chem. 2013, 4, 4004–4009. [Google Scholar] [CrossRef]
- Lu, B.; Zhou, G.; Xiao, F.; He, Q.; Zhang, J. Stimuli-Responsive Poly(ionic liquid) Nanoparticle for Controlled Drug Delivery. J. Mater. Chem. B. 2020, 8, 7994–8001. [Google Scholar] [CrossRef]
- Fan, S.-Y.; Hao, Y.-N.; Zhang, W.-X.; Kapasi, A.; Shu, Y.; Wang, J.-H.; Chen, W. Poly(ionic liquid)-Gated CuCo2S4 for pH-/Thermo-Triggered Drug Release and Photoacoustic Imaging. ACS Appl. Mater. Interfaces 2020, 12, 9000–9007. [Google Scholar] [CrossRef]
- Niesyto, K.; Neugebauer, D. Synthesis and Characterization of Ionic Graft Copolymers: Introduction and In Vitro Release of Antibacterial Drug by Anion Exchange. Polymers 2020, 12, 2159. [Google Scholar] [CrossRef]
- Mazur, A.; Niesyto, K.; Neugebauer, D. Pharmaceutical Functionalization of Monomeric Ionic Liquid for the Preparation of Ionic Graft Polymer Conjugates. Int. J. Mol. Sci. 2022, 23, 14731. [Google Scholar] [CrossRef]
- Niesyto, K.; Mazur, A.; Neugebauer, D. Dual-Drug Delivery via the Self-Assembled Conjugates of Choline-Functionalized Graft Copolymers. Materials 2022, 15, 4457. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Zheng, R.; Yu, F.; Xiao, X.; Jiang, M.; Yuan, Y. Dual drug delivery system with flexible and controllable drug ratios for synergistic chemotherapy. Sci. China Chem. 2021, 64, 1020–1030. [Google Scholar] [CrossRef]
- Xiao, Y.; Gao, Y.; Li, F.; Deng, Z. Combinational dual drug delivery system to enhance the care and treatment of gastric cancer patients. Drug Deliv. 2020, 27, 1491–1500. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Cai, C.; Lin, J.; Chen, T. Dual-drug delivery system based on hydrogel/micelle composites. Biomaterials 2009, 30, 2606–2613. [Google Scholar] [CrossRef] [PubMed]
- Rahimi, M.; Shafiei-Irannejad, V.; Safa, K.D.; Salehi, R. Multi-branched ionic liquid-chitosan as a smart and biocompatible nano-vehicle for combination chemotherapy with stealth and targeted properties. Carbohydr. Polym. 2018, 196, 299–312. [Google Scholar] [CrossRef]
- Bielas, R.; Siewniak, A.; Skonieczna, M.; Adamiec, M.; Mielańczyk, Ł.; Neugebauer, D. Choline based polymethacrylate matrix with pharmaceutical cations as co-delivery system for antibacterial and anti-inflammatory combined therapy. J. Mol. Liq. 2019, 285, 114–122. [Google Scholar] [CrossRef]
- Niesyto, K.; Neugebauer, D. Linear Copolymers Based on Choline Ionic Liquid Carrying Anti-Tuberculosis Drugs: Influence of Anion Type on Physicochemical Properties and Drug Release. Int. J. Mol. Sci. 2021, 22, 284. [Google Scholar] [CrossRef]
- Milani, M.; Salehi, R.; Hamishehkar, H.; Zarebkohan, A.; Akbarzadeh, A. Synthesis and evaluation of polymeric micelle containing piperacillin/tazobactam for enhanced antibacterial activity. Drug Delivery 2019, 26, 1292–1299. [Google Scholar]
- Pulat, M.; Tan, N.; Onurdağ, F.K. Swelling dynamics of IPN hydrogels including acrylamide-acrylic acid-chitosan and evaluation of their potential for controlled release of piperacillin-tazobactam. J. Appl. Polym. Sci. 2011, 120, 441–450. [Google Scholar] [CrossRef]
- Mahata, D.; Jana, M.; Jana, A.; Mukherjee, A.; Mondal, N.; Saha, T.; Sen, S.; Nando, G.B.; Mukhopadhyay, C.K.; Chakraborty, R.; et al. Lignin-graft-Polyoxazoline Conjugated Triazole a Novel Anti-Infective Ointment to Control Persistent Inflammation. Sci. Rep. 2017, 7, 46412. [Google Scholar] [CrossRef] [PubMed]
- Niesyto, K.; Łyżniak, W.; Skonieczna, M.; Neugebauer, D. Biological in vitro evaluation of PIL graft conjugates: Cytotoxicity characteristics. Int. J. Mol. Sci. 2021, 22, 7741. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Villodres, Á.; Gutiérrez Linares, A.; Gálvez-Benitez, L.; Pachón, J.; Lepe, J.A.; Smani, Y. Semirapid Detection of Piperacillin/Tazobactam Resistance and Extended-Spectrum Resistance to β-Lactams/β-Lactamase Inhibitors in Clinical Isolates of Escherichia coli. Microbiol. Spectr. 2021, 9, e0080121. [Google Scholar] [CrossRef] [PubMed]






| No. | nsc | DG (mol. %) | DPsc | FTMAMA (mol. %) | Mn × 10−3 (g/mol) | Mw/Mn |
|---|---|---|---|---|---|---|
| GP1 | 48 | 26 | 35 | 39 | 273.1 | 1.15 |
| GP2 | 133 | 46 | 28 | 36 | 583.5 | 1.03 |
| GP3 | 65 | 18 | 1090.5 | 1.11 |
| No. | CMC a (mg/mL) | Contact Angle b (°) | ||
|---|---|---|---|---|
| Cl (23) | PIP | Cl (23) | PIP | |
| GP1 | 0.013 | 0.073 | 56.3 | 36.1 |
| GP2 | 0.020 | 0.041 | 48.9 | 35.5 |
| GP3 | 0.011 | 0.044 | 44.3 | 44.3 |
| TAZ | PIP− | PIP−/TAZ | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Dh (nm) | Fraction (%) | PDI | Dh (nm) | Fraction (%) | PDI | Dh (nm) | Fraction (%) | PDI | |
| GP1 | 0.004 | 20 | 77.8 | 0.26 | 24 | 42.4 | 0.36 | ||
| 319 | 100 | 244 | 11.7 | 192 | 55.2 | ||||
| 1714 | 10.5 | 1875 | 2.4 | ||||||
| GP2 | 16 | 24.4 | 0.17 | 26 | 67.1 | 0.42 | |||
| 97 | 41.3 | ||||||||
| 504 | 24.9 | 83 | 32.9 | ||||||
| 5430 | 9.4 | ||||||||
| GP3 | 247 | 97.4 | 0.09 | 31 | 68.3 | 1.15 | 48 | 54.3 | 1.06 |
| 6000 | 2.6 | 451 | 31.7 | 770 | 45.7 | ||||
| Single System | Dual System | Single System | Dual System | |||||
|---|---|---|---|---|---|---|---|---|
| TAZ | TAZ | PIP | PIP | |||||
| % | C (μg/mL) | % | C (μg/mL) | % | C (μg/mL) | % | C (μg/mL) | |
| GP1 | 47 | 8.30 | 47 | 9.60 | 79 | 10.81 | 25 | 3.41 |
| GP2 | 68 | 7.52 | 69 | 10.41 | 66 | 7.76 | 23 | 2.64 |
| GP3 | 98 | 9.04 | 64 | 8.27 | 81 | 15.04 | 21 | 3.94 |
| Drug | Type | Matrix | First-Order Model | Higuchi Model | Korsmeyer–Peppas Model | |
|---|---|---|---|---|---|---|
| R2 | R2 | R2 | n | |||
| TAZ | Single | GP1 | 0.88 | 0.89 | 0.87 | 0.35 |
| GP2 | 0.96 | 0.98 | 0.99 | 0.60 | ||
| GP3 | 0.77 | 0.96 | 0.96 | 0.48 | ||
| Dual | GP1 | 0.83 | 0.84 | 0.77 | 0.46 | |
| GP2 | 0.88 | 0.91 | 0.92 | 0.38 | ||
| GP3 | 0.93 | 0.94 | 0.79 | 0.80 | ||
| PIP− | Single | GP1 | 0.99 | 0.98 | 0.94 | 0.29 |
| GP2 | 0.94 | 0.98 | 0.98 | 0.36 | ||
| GP3 | 0.95 | 0.97 | 0.99 | 0.38 | ||
| Dual | GP1 | 0.91 | 0.94 | 0.90 | 0.48 | |
| GP2 | 0.90 | 0.97 | 0.97 | 0.64 | ||
| GP3 | 0.91 | 0.99 | 0.95 | 0.56 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niesyto, K.; Mazur, A.; Neugebauer, D. Piperacillin/Tazobactam Co-Delivery by Micellar Ionic Conjugate Systems Carrying Pharmaceutical Anions and Encapsulated Drug. Pharmaceutics 2024, 16, 198. https://doi.org/10.3390/pharmaceutics16020198
Niesyto K, Mazur A, Neugebauer D. Piperacillin/Tazobactam Co-Delivery by Micellar Ionic Conjugate Systems Carrying Pharmaceutical Anions and Encapsulated Drug. Pharmaceutics. 2024; 16(2):198. https://doi.org/10.3390/pharmaceutics16020198
Chicago/Turabian StyleNiesyto, Katarzyna, Aleksy Mazur, and Dorota Neugebauer. 2024. "Piperacillin/Tazobactam Co-Delivery by Micellar Ionic Conjugate Systems Carrying Pharmaceutical Anions and Encapsulated Drug" Pharmaceutics 16, no. 2: 198. https://doi.org/10.3390/pharmaceutics16020198





