Dual Glyoxalase-1 and β-Klotho Gene-Activated Scaffold Reduces Methylglyoxal and Reprograms Diabetic Adipose-Derived Stem Cells: Prospects in Improved Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Gene-Activated Scaffold (GAS)
2.2. Cell Seeding
2.3. Immunofluorescence Imaging
2.4. Image Quantification
2.5. Statistical Analysis
3. Results
3.1. Dual GAS Reduces Methylglyoxal Production of dADSCs
3.2. Dual GAS Reduces the Expression of Pro-Fibrotic Markers
3.3. GAS Shows Decrease in Collagen
3.4. GAS Shows Contradictory Results in Basement Membrane Regeneration
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Innovations in gene and growth factor delivery systems for diabetic wound healing. J. Tissue Eng. Regen. Med. 2018, 12, e296–e312. [Google Scholar] [CrossRef]
- Wang, X.; Yuan, C.X.; Xu, B.; Yu, Z. Diabetic foot ulcers: Classification, risk factors and management. World J. Diabetes 2022, 13, 1049–1065. [Google Scholar] [CrossRef]
- Liao, X.; Li, S.H.; El Akkawi, M.M.; Fu, X.B.; Liu, H.W.; Huang, Y.S. Surgical amputation for patients with diabetic foot ulcers: A Chinese expert panel consensus treatment guide. Front. Surg. 2022, 9, 1003339. [Google Scholar] [CrossRef]
- Lin, H.; BoLatai, A.; Wu, N. Application Progress of Nano Silver Dressing in the Treatment of Diabetic Foot. Diabetes Metab. Syndr. Obes. 2021, 14, 4145–4154. [Google Scholar] [CrossRef]
- Bouly, M.; Laborne, F.X.; Tourte, C.; Henry, E.; Penfornis, A.; Dardari, D. Post-healing follow-up study of patients in remission for diabetic foot ulcers Pied-REM study. PLoS ONE 2022, 17, e0268242. [Google Scholar] [CrossRef]
- Zhang, J.; Qu, X.; Li, J.; Harada, A.; Hua, Y.; Yoshida, N.; Ishida, M.; Sawa, Y.; Liu, L.; Miyagawa, S. Tissue Sheet Engineered Using Human Umbilical Cord-Derived Mesenchymal Stem Cells Improves Diabetic Wound Healing. Int. J. Mol. Sci. 2022, 23, 12697. [Google Scholar] [CrossRef]
- Stolzing, A.; Colley, H.; Scutt, A. Effect of age and diabetes on the response of mesenchymal progenitor cells to fibrin matrices. Int. J. Biomater. 2011, 2011, 378034. [Google Scholar] [CrossRef]
- Matsiko, A.; Levingstone, T.J.; O’Brien, F.J.; Gleeson, J.P. Addition of hyaluronic acid improves cellular infiltration and promotes early-stage chondrogenesis in a collagen-based scaffold for cartilage tissue engineering. J. Mech. Behav. Biomed. Mater. 2012, 11, 41–52. [Google Scholar] [CrossRef] [PubMed]
- Holm, J.S.; Toyserkani, N.M.; Sorensen, J.A. Adipose-derived stem cells for treatment of chronic ulcers: Current status. Stem Cell Res. Ther. 2018, 9, 142. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Bai, B.; Zhang, H.; Zhang, W.; Tang, S. Adipose stem cell secretion combined with biomaterials facilitates large-area wound healing. Regen. Med. 2020, 15, 2311–2323. [Google Scholar] [CrossRef] [PubMed]
- Mizuno, H.; Tobita, M.; Uysal, A.C. Concise review: Adipose-derived stem cells as a novel tool for future regenerative medicine. Stem Cells 2012, 30, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Alicka, M.; Major, P.; Wysocki, M.; Marycz, K. Adipose-Derived Mesenchymal Stem Cells Isolated from Patients with Type 2 Diabetes Show Reduced “Stemness” through an Altered Secretome Profile, Impaired Anti-Oxidative Protection, and Mitochondrial Dynamics Deterioration. J. Clin. Med. 2019, 8, 765. [Google Scholar] [CrossRef] [PubMed]
- Li, C.W.; Young, T.H.; Wang, M.H.; Pei, M.Y.; Hsieh, T.Y.; Hsu, C.L.; Cheng, N.C. Low-glucose culture environment can enhance the wound healing capability of diabetic adipose-derived stem cells. Stem Cell Res. Ther. 2023, 14, 236. [Google Scholar] [CrossRef] [PubMed]
- Spiekman, M.; Przybyt, E.; Plantinga, J.A.; Gibbs, S.; van der Lei, B.; Harmsen, M.C. Adipose tissue-derived stromal cells inhibit TGF-beta1-induced differentiation of human dermal fibroblasts and keloid scar-derived fibroblasts in a paracrine fashion. Plast. Reconstr. Surg. 2014, 134, 699–712. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Srivastava, S.; Singh, M.R.; Singh, D. Mechanistic insight into diabetic wounds: Pathogenesis, molecular targets and treatment strategies to pace wound healing. Biomed. Pharmacother. 2019, 112, 108615. [Google Scholar] [CrossRef] [PubMed]
- Saki, N.; Jalalifar, M.A.; Soleimani, M.; Hajizamani, S.; Rahim, F. Adverse Effect of High Glucose Concentration on Stem Cell Therapy. Int. J. Hematol. Oncol. Stem Cell Res. 2013, 7, 34–40. [Google Scholar] [PubMed]
- Syed, N.A.; Bhatti, A.; John, P. Molecular Link between Glo-1 Expression and Markers of Hyperglycemia and Oxidative Stress in Vascular Complications of Type 2 Diabetes Mellitus. Antioxidants 2023, 12, 1663. [Google Scholar] [CrossRef]
- Rabbani, N.; Thornalley, P.J. Emerging Glycation-Based Therapeutics-Glyoxalase 1 Inducers and Glyoxalase 1 Inhibitors. Int. J. Mol. Sci. 2022, 23, 2453. [Google Scholar] [CrossRef]
- Stratmann, B.; Goldstein, B.; Thornalley, P.J.; Rabbani, N.; Tschoepe, D. Intracellular Accumulation of Methylglyoxal by Glyoxalase 1 Knock down Alters Collagen Homoeostasis in L6 Myoblasts. Int. J. Mol. Sci. 2017, 18, 480. [Google Scholar] [CrossRef]
- Van Putte, L.; De Schrijver, S.; Moortgat, P. The effects of advanced glycation end products (AGEs) on dermal wound healing and scar formation: A systematic review. Scars Burn. Heal. 2016, 2, 2059513116676828. [Google Scholar] [CrossRef]
- Asbun, J.; Manso, A.M.; Villarreal, F.J. Profibrotic influence of high glucose concentration on cardiac fibroblast functions: Effects of losartan and vitamin E. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H227–H234. [Google Scholar] [CrossRef]
- Duran-Jimenez, B.; Dobler, D.; Moffatt, S.; Rabbani, N.; Streuli, C.H.; Thornalley, P.J.; Tomlinson, D.R.; Gardiner, N.J. Advanced glycation end products in extracellular matrix proteins contribute to the failure of sensory nerve regeneration in diabetes. Diabetes 2009, 58, 2893–2903. [Google Scholar] [CrossRef] [PubMed]
- Nigro, C.; Leone, A.; Raciti, G.A.; Longo, M.; Mirra, P.; Formisano, P.; Beguinot, F.; Miele, C. Methylglyoxal-Glyoxalase 1 Balance: The Root of Vascular Damage. Int. J. Mol. Sci. 2017, 18, 188. [Google Scholar] [CrossRef] [PubMed]
- Bierhaus, A.; Nawroth, P.P. Multiple levels of regulation determine the role of the receptor for AGE (RAGE) as common soil in inflammation, immune responses and diabetes mellitus and its complications. Diabetologia 2009, 52, 2251–2263. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Glyoxalase I—Structure, function and a critical role in the enzymatic defence against glycation. Biochem. Soc. Trans. 2003, 31 Pt 6, 1343–1348. [Google Scholar] [CrossRef] [PubMed]
- Schalkwijk, C.G.; Stehouwer, C.D.A. Methylglyoxal, a Highly Reactive Dicarbonyl Compound, in Diabetes, Its Vascular Complications, and Other Age-Related Diseases. Physiol. Rev. 2020, 100, 407–461. [Google Scholar] [CrossRef] [PubMed]
- Moraru, A.; Wiederstein, J.; Pfaff, D.; Fleming, T.; Miller, A.K.; Nawroth, P.; Teleman, A.A. Elevated Levels of the Reactive Metabolite Methylglyoxal Recapitulate Progression of Type 2 Diabetes. Cell Metab. 2018, 27, 926–934.e8. [Google Scholar] [CrossRef] [PubMed]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. SDF-1alpha Gene-Activated Collagen Scaffold Restores Pro-Angiogenic Wound Healing Features in Human Diabetic Adipose-Derived Stem Cells. Biomedicines 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, F.J.; Harley, B.A.; Yannas, I.V.; Gibson, L.J. The effect of pore size on cell adhesion in collagen-GAG scaffolds. Biomaterials 2005, 26, 433–441. [Google Scholar] [CrossRef]
- Peng, Z.; Yang, X.; Qin, J.; Ye, K.; Wang, X.; Shi, H.; Jiang, M.; Liu, X.; Lu, X. Glyoxalase-1 Overexpression Reverses Defective Proangiogenic Function of Diabetic Adipose-Derived Stem Cells in Streptozotocin-Induced Diabetic Mice Model of Critical Limb Ischemia. Stem Cells Transl. Med. 2017, 6, 261–271. [Google Scholar] [CrossRef]
- Donate-Correa, J.; Martin-Nunez, E.; Mora-Fernandez, C.; Muros-de-Fuentes, M.; Perez-Delgado, N.; Navarro-Gonzalez, J.F. Klotho in cardiovascular disease: Current and future perspectives. World J. Biol. Chem. 2015, 6, 351–357. [Google Scholar] [CrossRef]
- Typiak, M.; Kulesza, T.; Rachubik, P.; Rogacka, D.; Audzeyenka, I.; Angielski, S.; Saleem, M.A.; Piwkowska, A. Role of Klotho in Hyperglycemia: Its Levels and Effects on Fibroblast Growth Factor Receptors, Glycolysis, and Glomerular Filtration. Int. J. Mol. Sci. 2021, 22, 7867. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Q.; Ren, Q.; Li, L.; Tan, H.; Lu, M.; Tian, Y.; Huang, L.; Zhao, B.; Fu, H.; Hou, F.F.; et al. Author Correction: A Klotho-derived peptide protects against kidney fibrosis by targeting TGF-beta signaling. Nat. Commun. 2022, 13, 6640. [Google Scholar] [CrossRef] [PubMed]
- Sopjani, M.; Rinnerthaler, M.; Kruja, J.; Dermaku-Sopjani, M. Intracellular signaling of the aging suppressor protein Klotho. Curr. Mol. Med. 2015, 15, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Anti-Aging beta-Klotho Gene-Activated Scaffold Promotes Rejuvenative Wound Healing Response in Human Adipose-Derived Stem Cells. Pharmaceuticals 2021, 14, 1168. [Google Scholar] [CrossRef] [PubMed]
- Suku, M.; Laiva, A.L.; O’Brien, F.J.; Keogh, M.B. Anti-Ageing Protein beta-Klotho Rejuvenates Diabetic Stem Cells for Improved Gene-Activated Scaffold Based Wound Healing. J. Pers. Med. 2020, 11, 4. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; O’Brien, F.J. Novel freeze-drying methods to produce a range of collagen-glycosaminoglycan scaffolds with tailored mean pore sizes. Tissue Eng. Part C Methods 2010, 16, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Haugh, M.G.; Murphy, C.M.; McKiernan, R.C.; Altenbuchner, C.; O’Brien, F.J. Crosslinking and mechanical properties significantly influence cell attachment, proliferation, and migration within collagen glycosaminoglycan scaffolds. Tissue Eng. Part A 2011, 17, 1201–1208. [Google Scholar] [CrossRef] [PubMed]
- Farrell, E.; Both, S.K.; Odorfer, K.I.; Koevoet, W.; Kops, N.; O’Brien, F.J.; Baatenburg de Jong, R.J.; Verhaar, J.A.; Cuijpers, V.; Jansen, J.; et al. In-Vivo generation of bone via endochondral ossification by in-vitro chondrogenic priming of adult human and rat mesenchymal stem cells. BMC Musculoskelet. Disord. 2011, 12, 31. [Google Scholar] [CrossRef]
- Tierney, E.G.; Duffy, G.P.; Hibbitts, A.J.; Cryan, S.A.; O’Brien, F.J. The development of non-viral gene-activated matrices for bone regeneration using polyethyleneimine (PEI) and collagen-based scaffolds. J. Control. Release 2012, 158, 304–311. [Google Scholar] [CrossRef]
- Barker, T.H.; Engler, A.J. The provisional matrix: Setting the stage for tissue repair outcomes. Matrix Biol. 2017, 60, 1–4. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Tamaki, Z.; Wang, W.; Hinchcliff, M.; Hoover, P.; Getsios, S.; White, E.S.; Varga, J. FibronectinEDA promotes chronic cutaneous fibrosis through Toll-like receptor signaling. Sci. Transl. Med. 2014, 6, 232ra50. [Google Scholar] [CrossRef]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef]
- Rodrigues, M.; Kosaric, N.; Bonham, C.A.; Gurtner, G.C. Wound Healing: A Cellular Perspective. Physiol. Rev. 2019, 99, 665–706. [Google Scholar] [CrossRef]
- Huang, Y.; Kyriakides, T.R. The role of extracellular matrix in the pathophysiology of diabetic wounds. Matrix Biol. Plus. 2020, 6–7, 100037. [Google Scholar] [CrossRef]
- Vulesevic, B.; McNeill, B.; Geoffrion, M.; Kuraitis, D.; McBane, J.E.; Lochhead, M.; Vanderhyden, B.C.; Korbutt, G.S.; Milne, R.W.; Suuronen, E.J. Glyoxalase-1 overexpression in bone marrow cells reverses defective neovascularization in STZ-induced diabetic mice. Cardiovasc. Res. 2014, 101, 306–316. [Google Scholar] [CrossRef]
- Molgat, A.S.; Tilokee, E.L.; Rafatian, G.; Vulesevic, B.; Ruel, M.; Milne, R.; Suuronen, E.J.; Davis, D.R. Hyperglycemia inhibits cardiac stem cell-mediated cardiac repair and angiogenic capacity. Circulation 2014, 130 (Suppl. S1), S70–S76. [Google Scholar] [CrossRef]
- Jiang, M.; Yakupu, A.; Guan, H.; Dong, J.; Liu, Y.; Song, F.; Tang, J.; Tian, M.; Niu, Y.; Lu, S. Pyridoxamine ameliorates methylglyoxal-induced macrophage dysfunction to facilitate tissue repair in diabetic wounds. Int. Wound J. 2022, 19, 52–63. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, U.; Dobler, D.; Larkin, S.J.; Rabbani, N.; Thornalley, P.J. Reversal of hyperglycemia-induced angiogenesis deficit of human endothelial cells by overexpression of glyoxalase 1 in vitro. Ann. N. Y. Acad. Sci. 2008, 1126, 262–264. [Google Scholar] [CrossRef] [PubMed]
- Stratmann, B.; Engelbrecht, B.; Espelage, B.C.; Klusmeier, N.; Tiemann, J.; Gawlowski, T.; Mattern, Y.; Eisenacher, M.; Meyer, H.E.; Rabbani, N.; et al. Glyoxalase 1-knockdown in human aortic endothelial cells—Effect on the proteome and endothelial function estimates. Sci. Rep. 2016, 6, 37737. [Google Scholar] [CrossRef] [PubMed]
- Antognelli, C.; Cecchetti, R.; Riuzzi, F.; Peirce, M.J.; Talesa, V.N. Glyoxalase 1 sustains the metastatic phenotype of prostate cancer cells via EMT control. J. Cell. Mol. Med. 2018, 22, 2865–2883. [Google Scholar] [CrossRef] [PubMed]
- Yuen, A.; Laschinger, C.; Talior, I.; Lee, W.; Chan, M.; Birek, J.; Young, E.W.K.; Sivagurunathan, K.; Won, E.; Simmons, C.A.; et al. Methylglyoxal-modified collagen promotes myofibroblast differentiation. Matrix Biol. 2010, 29, 537–548. [Google Scholar] [CrossRef] [PubMed]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of alpha-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Torr, E.E.; Ngam, C.R.; Bernau, K.; Tomasini-Johansson, B.; Acton, B.; Sandbo, N. Myofibroblasts exhibit enhanced fibronectin assembly that is intrinsic to their contractile phenotype. J. Biol. Chem. 2015, 290, 6951–6961. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Wang, X.; Sun, K.H.; Zhou, L. alpha-smooth muscle actin is not a marker of fibrogenic cell activity in skeletal muscle fibrosis. PLoS ONE 2018, 13, e0191031. [Google Scholar]
- McKleroy, W.; Lee, T.H.; Atabai, K. Always cleave up your mess: Targeting collagen degradation to treat tissue fibrosis. Am. J. Physiol. Lung Cell. Mol. Physiol. 2013, 304, L709–L721. [Google Scholar] [CrossRef]
- Marshall, C.D.; Hu, M.S.; Leavitt, T.; Barnes, L.A.; Lorenz, H.P.; Longaker, M.T. Cutaneous Scarring: Basic Science, Current Treatments, and Future Directions. Adv. Wound Care 2018, 7, 29–45. [Google Scholar] [CrossRef]
- Nystrom, A.; Velati, D.; Mittapalli, V.R.; Fritsch, A.; Kern, J.S.; Bruckner-Tuderman, L. Collagen VII plays a dual role in wound healing. J. Clin. Investig. 2013, 123, 3498–3509. [Google Scholar] [CrossRef] [PubMed]
- Mathew-Steiner, S.S.; Roy, S.; Sen, C.K. Collagen in Wound Healing. Bioengineering 2021, 8, 63. [Google Scholar] [CrossRef] [PubMed]
- Siregar, S.; Herlambang, M.S.; Reza, M.; Mustafa, A.; Stefanus, D. Role of human adipose-derived stem cells (hADSC) on TGF-beta1, type I collagen, and fibrosis degree in bladder obstruction model of Wistar rats. BMC Urol. 2022, 22, 69. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Zhang, W.; Gao, J.; Liu, J.; Wang, H.; Li, J.; Yang, X.; He, T.; Guan, H.; Zheng, Z.; et al. Adipose tissue-derived stem cells suppress hypertrophic scar fibrosis via the p38/MAPK signaling pathway. Stem Cell Res. Ther. 2016, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Ma, Y.; Gao, Z.; Yang, J. Human adipose-derived stem cells inhibit bioactivity of keloid fibroblasts. Stem Cell Res. Ther. 2018, 9, 40. [Google Scholar] [CrossRef]
- Hamill, K.J.; Kligys, K.; Hopkinson, S.B.; Jones, J.C. Laminin deposition in the extracellular matrix: A complex picture emerges. J. Cell Sci. 2009, 122 Pt 24, 4409–4417. [Google Scholar] [CrossRef] [PubMed]
- Iorio, V.; Troughton, L.D.; Hamill, K.J. Laminins: Roles and Utility in Wound Repair. Adv. Wound Care 2015, 4, 250–263. [Google Scholar] [CrossRef]
- Fournet, M.; Bonte, F.; Desmouliere, A. Glycation Damage: A Possible Hub for Major Pathophysiological Disorders and Aging. Aging Dis. 2018, 9, 880–900. [Google Scholar] [CrossRef] [PubMed]
- Takeichi, M. Dynamic contacts: Rearranging adherens junctions to drive epithelial remodelling. Nat. Rev. Mol. Cell Biol. 2014, 15, 397–410. [Google Scholar] [CrossRef] [PubMed]
- Ibi, T.; Usuda, J.; Inoue, T.; Sato, A.; Takegahara, K. Klotho expression is correlated to molecules associated with epithelial-mesenchymal transition in lung squamous cell carcinoma. Oncol. Lett. 2017, 14, 5526–5532. [Google Scholar] [CrossRef]
- Chang, D.K.; Louis, M.R.; Gimenez, A.; Reece, E.M. The Basics of Integra Dermal Regeneration Template and its Expanding Clinical Applications. Semin. Plast. Surg. 2019, 33, 185–189. [Google Scholar] [CrossRef]
- Tu, Z.; Zhong, Y.; Hu, H.; Shao, D.; Haag, R.; Schirner, M.; Lee, J.; Sullenger, B.; Leong, K.W. Design of therapeutic biomaterials to control inflammation. Nat. Rev. Mater. 2022, 7, 557–574. [Google Scholar] [CrossRef]

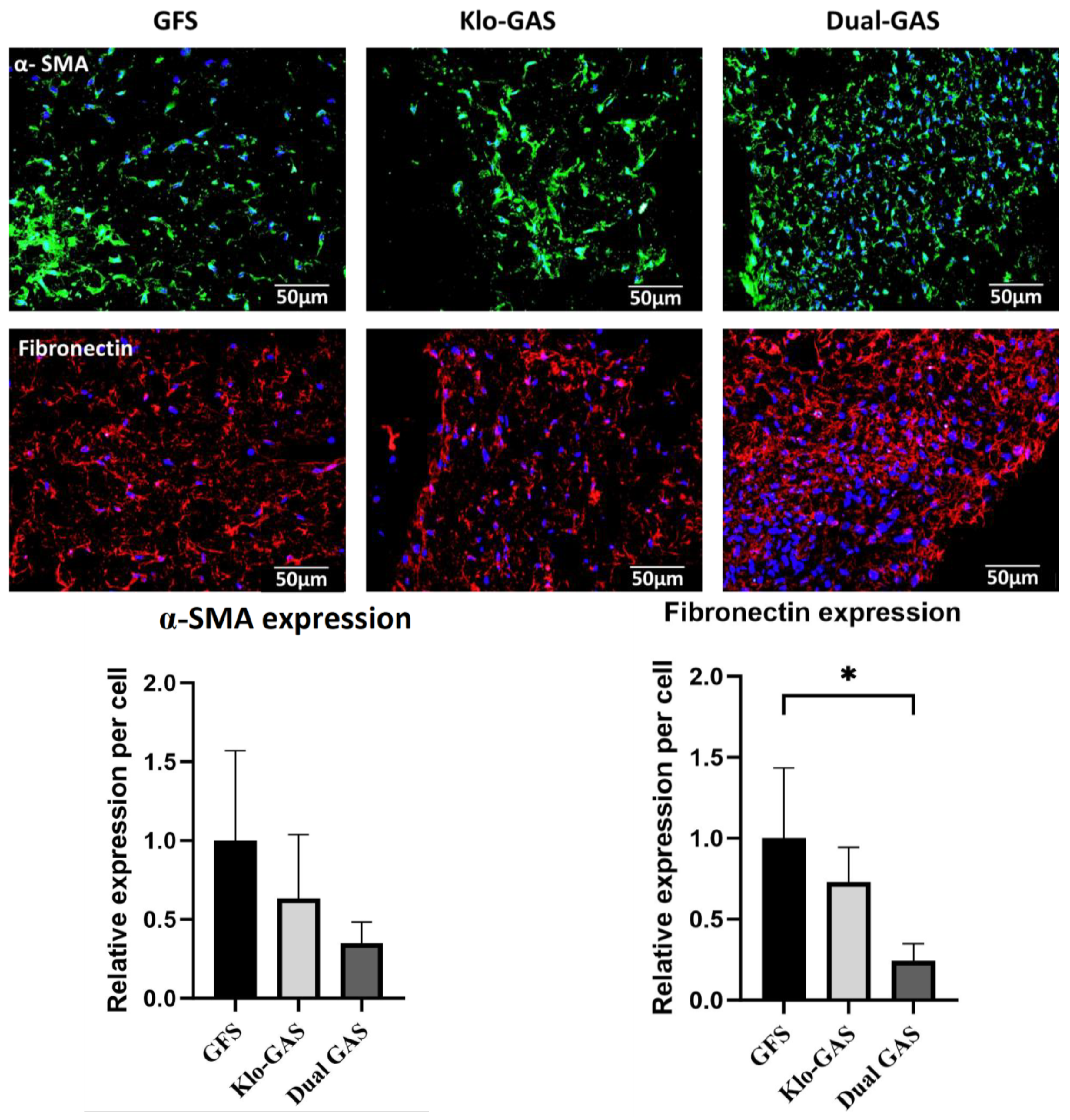
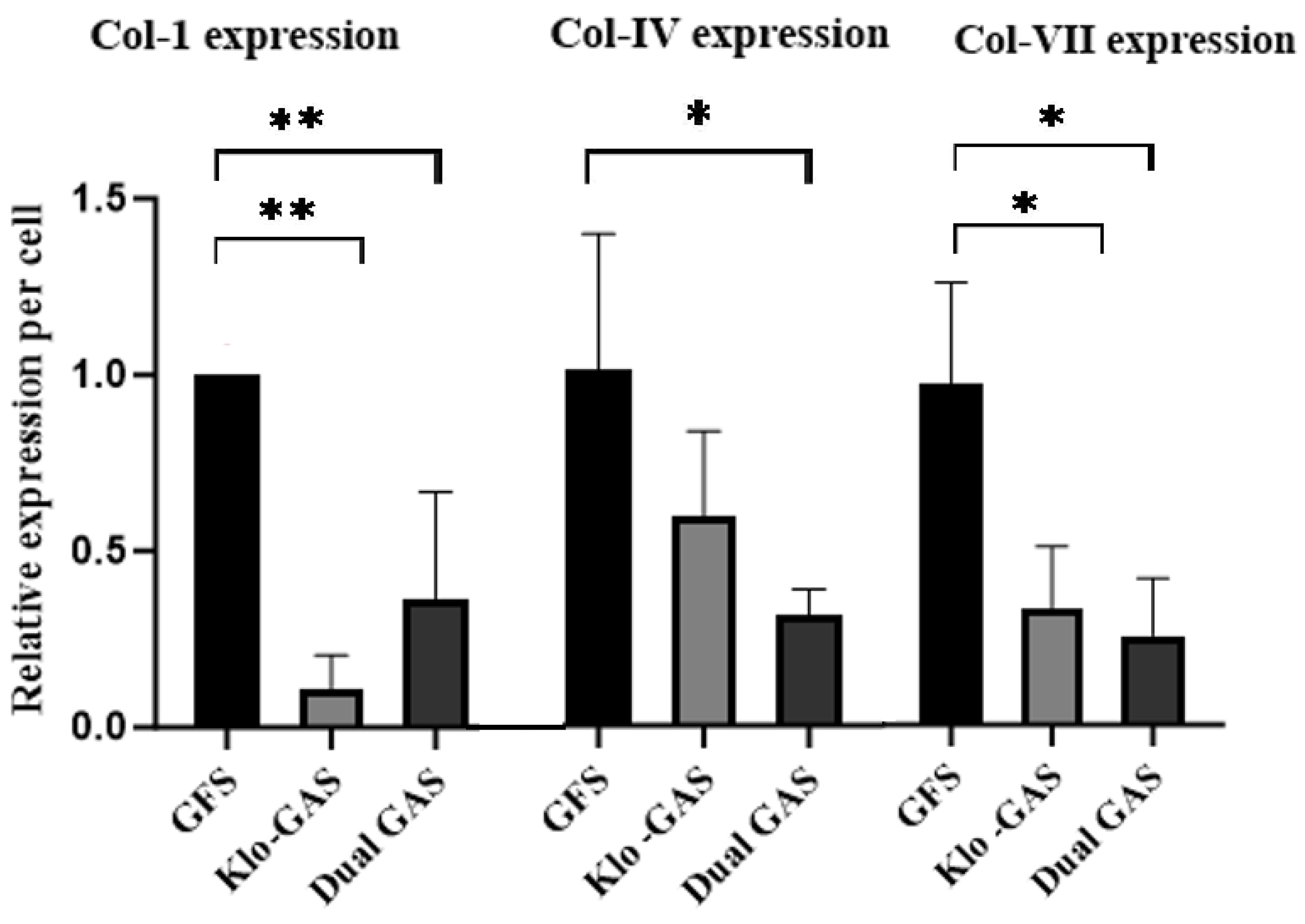

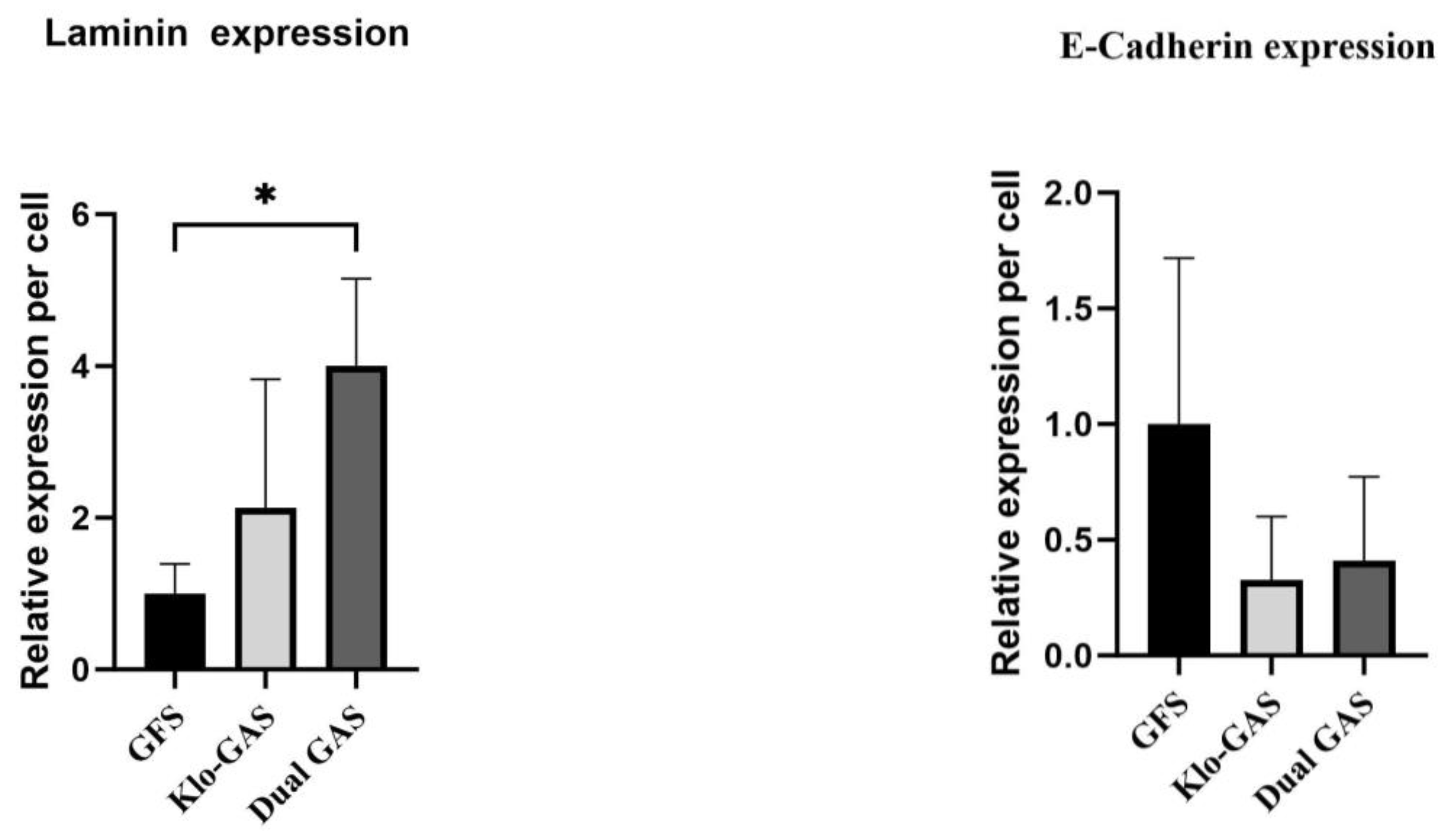
| Indicators | Primary Antibodies (Catalog No.) | Functional Roles | Dilutions in 1% BSA Solution |
|---|---|---|---|
| Glyoxylate | Methylglyoxal (NBP2-59368, Novus Biologicals, Abingdon, UK) | Non-enzymatic glycation of proteins; promotes diabetic vascular dysfunctions | 1:100 |
| Pro-fibrotic | Alpha-smooth muscle actin (ab7817, Abcam, Cambridge, UK) | Structural filament protein; promotes contraction and scarring | 1:200 |
| Fibronectin (ab2413, Abcam, UK) | Provisional matrix protein; promotes fibrosis [41,42] | 1:200 | |
| Basement membrane | E-cadherin (ab1416, Abcam, UK) | Mediate cell–cell adhesion; regulating contact formation and stability | 1:200 |
| Laminin (ab11575, Abcam, UK) | Nascent basement membrane (BM) protein; BM assembly | 1:200 | |
| Collagen IV (ab6586, Abcam, UK) | Mature BM protein; BM stability [43] | 1:200 | |
| Dermal matrix | Collagen I (NB600-408, Novusbio, Centennial, CO, USA) | Major body collagen; provide structure to tissue and skin | 1:200 |
| Collagen VII (ab6312, Abcam, UK) | Epidermal basement membrane; dermal–epidermal adhesion | 1:200 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pang, N.; Laiva, A.L.; Sulaiman, N.Z.; Das, P.; O’Brien, F.J.; Keogh, M.B. Dual Glyoxalase-1 and β-Klotho Gene-Activated Scaffold Reduces Methylglyoxal and Reprograms Diabetic Adipose-Derived Stem Cells: Prospects in Improved Wound Healing. Pharmaceutics 2024, 16, 265. https://doi.org/10.3390/pharmaceutics16020265
Pang N, Laiva AL, Sulaiman NZ, Das P, O’Brien FJ, Keogh MB. Dual Glyoxalase-1 and β-Klotho Gene-Activated Scaffold Reduces Methylglyoxal and Reprograms Diabetic Adipose-Derived Stem Cells: Prospects in Improved Wound Healing. Pharmaceutics. 2024; 16(2):265. https://doi.org/10.3390/pharmaceutics16020265
Chicago/Turabian StylePang, Nadia, Ashang L. Laiva, Noof Z. Sulaiman, Priya Das, Fergal J. O’Brien, and Michael B. Keogh. 2024. "Dual Glyoxalase-1 and β-Klotho Gene-Activated Scaffold Reduces Methylglyoxal and Reprograms Diabetic Adipose-Derived Stem Cells: Prospects in Improved Wound Healing" Pharmaceutics 16, no. 2: 265. https://doi.org/10.3390/pharmaceutics16020265
APA StylePang, N., Laiva, A. L., Sulaiman, N. Z., Das, P., O’Brien, F. J., & Keogh, M. B. (2024). Dual Glyoxalase-1 and β-Klotho Gene-Activated Scaffold Reduces Methylglyoxal and Reprograms Diabetic Adipose-Derived Stem Cells: Prospects in Improved Wound Healing. Pharmaceutics, 16(2), 265. https://doi.org/10.3390/pharmaceutics16020265








