Mesenchymal Stem Cell (MSC)-Based Drug Delivery into the Brain across the Blood–Brain Barrier
Abstract
1. Introduction
2. Discussion
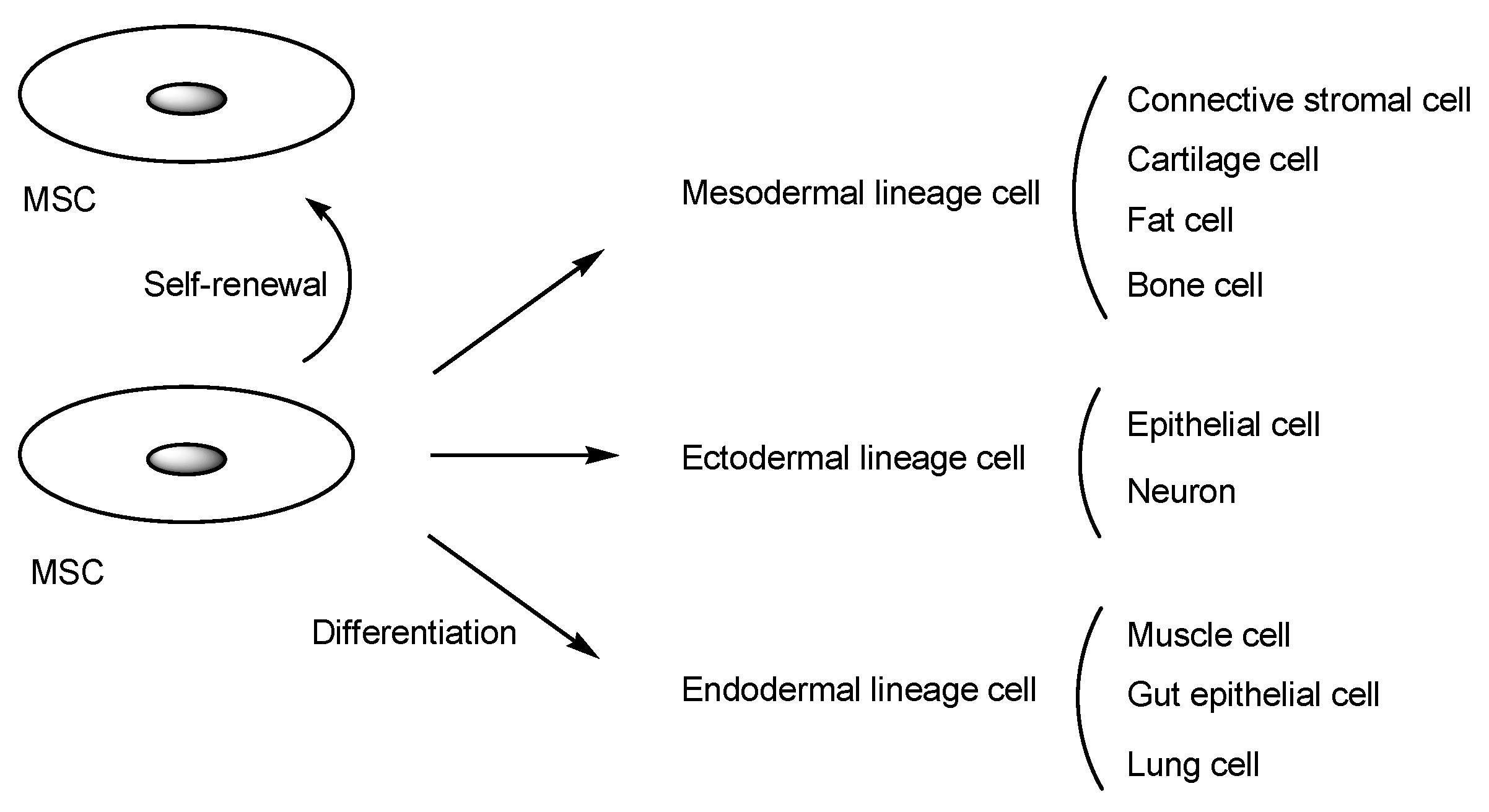
2.1. Human MSCs for Regenerative Medicine
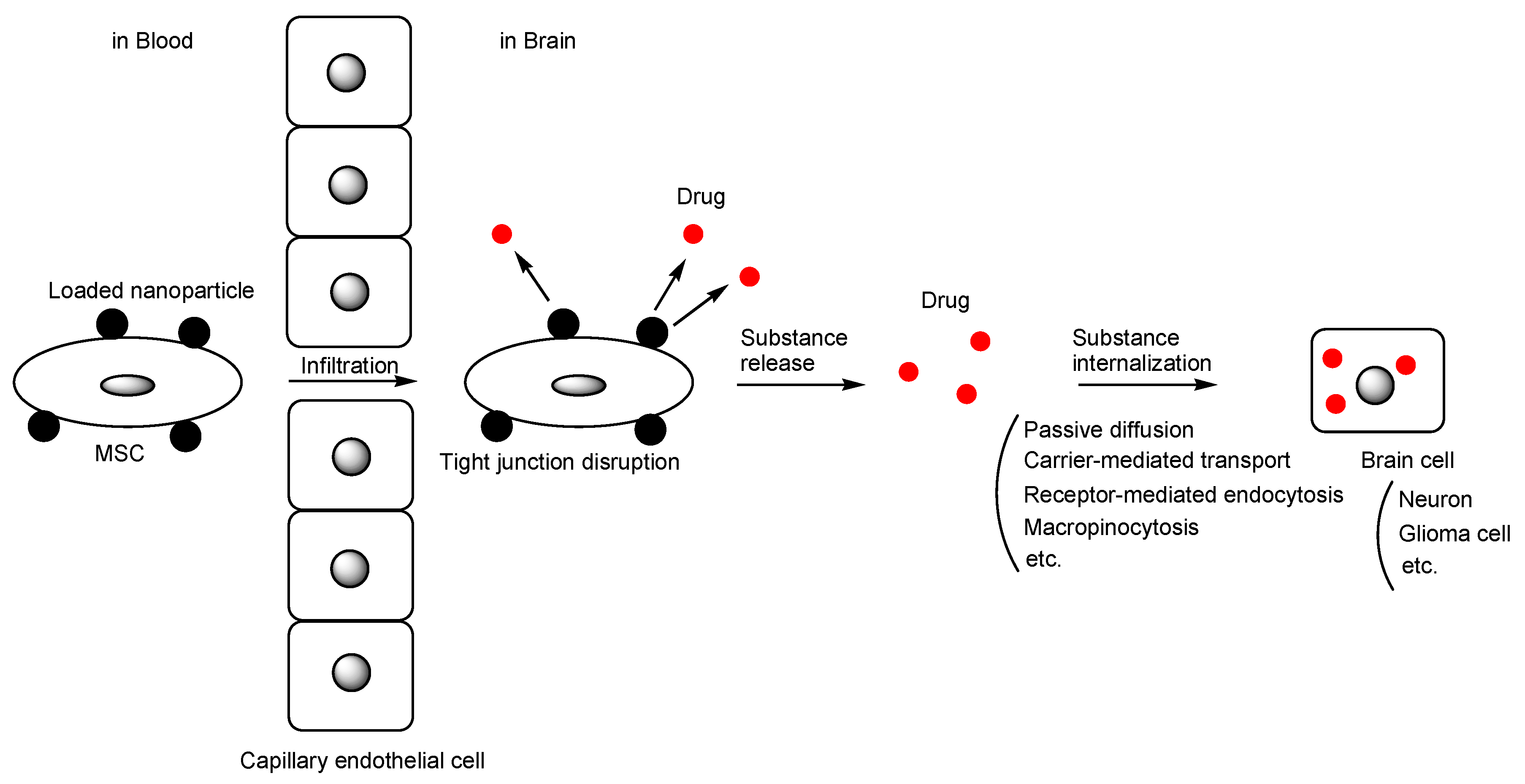
2.2. The Potential of MSC-Mediated Drug Delivery
2.3. The Implementation of MSC Delivery into the Brain across the BBB
2.3.1. Glioma
2.3.2. Parkinson’s Disease (PD)
2.3.3. Alzheimer’s Disease (AD)
2.3.4. Stroke
2.3.5. Traumatic Brain Injury
2.3.6. Amyotrophic Lateral Sclerosis (ALS)
2.3.7. Multiple Sclerosis
2.4. MSCs as a Carrier of Nanoparticles
3. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alexander, P.; Thomson, H.A.; Luff, A.J.; Lotery, A.J. Retinal pigment epithelium transplantation: Concepts, challenges, and future prospects. Eye 2015, 29, 992–1002. [Google Scholar] [CrossRef]
- Tashima, T. Smart Strategies for Therapeutic Agent Delivery into Brain across the Blood–Brain Barrier Using Receptor-Mediated Transcytosis. Chem. Pharm. Bull. 2020, 68, 316–325. [Google Scholar] [CrossRef]
- Stimulus package. Nat. Med. 2018, 24, 247. [CrossRef]
- Tashima, T. Delivery of Intravenously Administered Antibodies Targeting Alzheimer’s Disease-Relevant Tau Species into the Brain Based on Receptor-Mediated Transcytosis. Pharmaceutics 2022, 14, 411. [Google Scholar] [CrossRef] [PubMed]
- Ghasempour, E.; Hesami, S.; Movahed, E.; Keshel, S.H.; Doroudian, M. Mesenchymal stem cell-derived exosomes as a new therapeutic strategy in the brain tumors. Stem Cell Res. Ther. 2022, 13, 527. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Wang, Z. Recent Advances in the Application of Mesenchymal Stem Cell-Derived Exosomes for Cardiovascular and Neurodegenerative Disease Therapies. Pharmaceutics 2022, 14, 618. [Google Scholar] [CrossRef] [PubMed]
- Rao, D.; Huang, D.; Sang, C.; Zhong, T.; Zhang, Z.; Tang, Z. Advances in Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles. Front. Bioeng. Biotechnol. 2022, 9, 797359. [Google Scholar] [CrossRef]
- Uccelli, A.; Moretta, L.; Pistoia, V. Mesenchymal stem cells in health and disease. Nat. Rev. Immunol. 2008, 8, 726–736. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Gao, J.; Liang, Z.; Gao, C.; Niu, Q.; Wu, F.; Zhang, L. Mesenchymal stem cells and their microenvironment. Stem Cell Res. Ther. 2022, 13, 429. [Google Scholar] [CrossRef] [PubMed]
- Wei, W.; Ao, Q.; Wang, X.; Cao, Y.; Liu, Y.; Zheng, S.G.; Tian, X. Mesenchymal Stem Cell–Derived Exosomes: A Promising Biological Tool in Nanomedicine. Front. Pharmacol. 2021, 11, 590470. [Google Scholar] [CrossRef] [PubMed]
- Honda, T.; Yasui, M.; Shikamura, M.; Kubo, T.; Kawamata, S. What kind of impact does the Cell and Gene Therapy Product have on the medical and manufacturing industry? Part 3. Pharm. Tech. Jpn. 2023, 39, 2367–2374. [Google Scholar]
- Fan, Y.; Goh, E.L.K.; Chan, J.K.Y. Neural Cells for Neurodegenerative Diseases in Clinical Trials. Stem Cells Transl. Med. 2023, 12, 510–526. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Robbins, S.; Wang, X.; Virk, S.; Schuck, K.; Deveza, L.A.; Oo, W.M.; Carmichael, K.; Antony, B.; Eckstein, F.; et al. Efficacy and cost-effectiveness of Stem Cell injections for symptomatic relief and strUctural improvement in people with Tibiofemoral knee OsteoaRthritis: Protocol for a randomised placebo-controlled trial (the SCUlpTOR trial). BMJ Open 2021, 11, e056382. [Google Scholar] [CrossRef] [PubMed]
- Oka, S.; Yamaki, T.; Sasaki, M.; Ukai, R.; Takemura, M.; Yokoyama, T.; Kataoka-Sasaki, Y.; Onodera, R.; Ito, Y.M.; Kobayashi, S.; et al. Intravenous Infusion of Autoserum-Expanded Autologous Mesenchymal Stem Cells in Patients with Chronic Brain Injury: Protocol for a Phase 2 Trial. JMIR Res. Protoc. 2022, 11, e37898. [Google Scholar] [CrossRef] [PubMed]
- Cyranoski, D. Japan’s approval of stem-cell treatment for spinal-cord injury concerns scientists. Nature 2019, 565, 544–545. [Google Scholar] [CrossRef] [PubMed]
- Japan should put the brakes on stem-cell sales. Nature 2019, 565, 535–536. [CrossRef]
- Omae, K.; Yamamoto, K.; Teramukai, S.; Fukushima, M. The Principles of Regulatory Science in Regenerative Medicine Products. Pharma. Med. Device Regul. Sci. 2019, 50, 770–778. Available online: https://www.lhsi.jp/docs/05RS50-12_N-1.pdf (accessed on 1 January 2024).
- Murata, M.; Teshima, T. Treatment of Steroid-Refractory Acute Graft-Versus-Host Disease Using Commercial Mesenchymal Stem Cell Products. Front. Immunol. 2021, 12, 724380. [Google Scholar] [CrossRef]
- Scott, L.J. Darvadstrocel: A Review in Treatment-Refractory Complex Perianal Fistulas in Crohn’s Disease. BioDrugs 2018, 32, 627–634. [Google Scholar] [CrossRef]
- Tate, C.C.; Fonck, C.; McGrogan, M.; Case, C.C. Human mesenchymal stromal cells and their derivative, SB623 cells, rescue neural cells via trophic support following in vitro ischemia. Cell Transplant. 2010, 19, 973–984. [Google Scholar] [CrossRef]
- Sanz-Nogués, C.; O’Brien, T. Current good manufacturing practice considerations for mesenchymal stromal cells as therapeutic agents. Biomater. Biosyst. 2021, 2, 100018. [Google Scholar] [CrossRef] [PubMed]
- Schu, S.; Nosov, M.; O’Flynn, L.; Shaw, G.; Treacy, O.; Barry, F.; Murphy, M.; O’Brien, T.; Ritter, T. Immunogenicity of allogeneic mesenchymal stem cells. J. Cell. Mol. Med. 2012, 16, 2094–2103. [Google Scholar] [CrossRef]
- Fischer, U.M.; Harting, M.T.; Jimenez, F.; Monzon-Posadas, W.O.; Xue, H.; Savitz, S.I.; Laine, G.A.; Cox, C.S., Jr. Pulmonary Passage is a Major Obstacle for Intravenous Stem Cell Delivery: The Pulmonary First-Pass Effect. Stem Cells Dev. 2009, 18, 683–692. [Google Scholar] [CrossRef]
- Toma, C.; Wagner, W.R.; Bowry, S.; Schwartz, A.; Villanueva, F. Fate of Culture-Expanded Mesenchymal Stem Cells in the Microvasculature. Circ. Res. 2009, 104, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Kvernebo, K. Chapter 33—Microcirculation and Tissue Perfusion Assessment for Complex Cardiovascular Disease Care. In Advances in Cardiovascular Technology. New Devices and Concepts; Academic Press: Cambridge, MA, USA, 2022; pp. 501–513. [Google Scholar] [CrossRef]
- Turgeon, M.L. Clinical Hematology: Theory and Procedures; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2004; p. 100. ISBN 9780781750073. [Google Scholar]
- Bhat, S.; Viswanathan, P.; Chandanala, S.; Prasanna, S.J.; Seetharam, R.N. Expansion and characterization of bone marrow derived human mesenchymal stromal cells in serum-free conditions. Sci. Rep. 2021, 11, 3403. [Google Scholar] [CrossRef]
- Tashima, T. Delivery of Drugs into Cancer Cells Using Antibody–Drug Conjugates Based on Receptor-Mediated Endocytosis and the Enhanced Permeability and Retention Effect. Antibodies 2022, 11, 78. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Pan, X.; Wang, L.; Yu, G. Alveolar cells under mechanical stressed niche: Critical contributors to pulmonary fibrosis. Mol. Med. 2020, 26, 95. [Google Scholar] [CrossRef]
- Hartz, A.M.S.; Schulz, J.A.; Sokola, B.S.; Edelmann, S.E.; Shen, A.N.; Rempe, R.G.; Zhong, Y.; Seblani, N.E.; Bauer, B. Isolation of Cerebral Capillaries from Fresh Human Brain Tissue. J. Vis. Exp. 2018, 139, 57346. [Google Scholar] [CrossRef]
- Laughlin, C.D.; D’Aquili, E.G. Biogenetic Structuralism; Columbia University Press: New York, NY, USA, 1974. [Google Scholar]
- Leavy, S.A. Biogenetic Structuralism. Yale J. Biol. Med. 1976, 49, 420–421. [Google Scholar]
- Zhang, T.; Lin, R.; Wu, H.; Jiang, X.; Gao, J. Mesenchymal stem cells: A living carrier for active tumor-targeted delivery. Adv. Drug Deliv. Rev. 2022, 185, 114300. [Google Scholar] [CrossRef]
- Litvinova, L.S.; Shupletsova, V.V.; Khaziakhmatova, O.G.; Daminova, A.G.; Kudryavtseva, V.L.; Yurova, K.A.; Malashchenko, V.V.; Todosenko, N.M.; Popova, V.; Litvinov, R.I.; et al. Human Mesenchymal Stem Cells as a Carrier for a Cell-Mediated Drug Delivery. Front. Bioeng. Biotechnol. 2022, 10, 796111. [Google Scholar] [CrossRef]
- Weller, M.; Wick, W.; Aldape, K.; Brada, M.; Berger, M.; Pfister, S.M.; Nishikawa, R.; Rosentha, M.; Wen, P.Y.; Stupp, R.; et al. Glioma. Nat. Rev. Dis. Prim. 2015, 1, 15017. [Google Scholar] [CrossRef]
- Paget, S. The Distribution of Secondary Growths in Cancer of the Breast. Lancet 1889, 133, 571–573. [Google Scholar] [CrossRef]
- Oliveira, F.D.; Castanho, M.A.R.B.; Neves, V. Exosomes and Brain Metastases: A Review on Their Role and Potential Applications. Int. J. Mol. Sci. 2021, 22, 10899. [Google Scholar] [CrossRef] [PubMed]
- Mo, F.; Pellerino, A.; Soffietti, R.; Rudà, R. Blood–Brain Barrier in Brain Tumors: Biology and Clinical Relevance. Int. J. Mol. Sci. 2021, 22, 12654. [Google Scholar] [CrossRef] [PubMed]
- Arvanitis, C.D.; Ferraro, G.B.; Jain, R.K. The blood–brain barrier and blood–tumour barrier in brain tumours and metastases. Nat. Rev. Cancer 2020, 20, 26–41. [Google Scholar] [CrossRef]
- Li, L.; Guan, Y.; Liu, H.; Hao, N.; Liu, T.; Meng, X.; Fu, C.; Li, Y.; Qu, Q.; Zhang, Y.; et al. Silica nanorattle-doxorubicin-anchored mesenchymal stem cells for tumor-tropic therapy. ACS Nano 2011, 5, 7462–7470. [Google Scholar] [CrossRef]
- Wang, X.-L.; Zhao, W.-Z.; Fan, J.-Z.; Jia, L.-C.; Lu, Y.-N.; Zeng, L.-H.; Lv, Y.-Y.; Sun, X.-Y. Tumor Tropic Delivery of Hyaluronic Acid-Poly (D,L-lactide-co-glycolide) Polymeric Micelles Using Mesenchymal Stem Cells for Glioma Therapy. Molecules 2022, 27, 2419. [Google Scholar] [CrossRef]
- Muthukutty, P.; Yoo, S.Y. Oncolytic Virus Engineering and Utilizations: Cancer Immunotherapy Perspective. Viruses 2023, 15, 1645. [Google Scholar] [CrossRef]
- Asija, S.; Chatterjee, A.; Goda, J.S.; Yadav, S.; Chekuri, G.; Purwar, R. Oncolytic immunovirotherapy for high-grade gliomas: A novel and an evolving therapeutic option. Front. Immunol. 2023, 14, 1118246. [Google Scholar] [CrossRef] [PubMed]
- Frampton, J.E. Teserpaturev/G47Δ: First Approval. BioDrugs 2022, 36, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Chen, H.; Chen, C.; Liu, H.; He, Y.; Zhao, J.; Yang, P.; Mao, Q.; Xia, H. Systemic administration of mesenchymal stem cells loaded with a novel oncolytic adenovirus carrying IL-24/endostatin enhances glioma therapy. Cancer Lett. 2021, 509, 26–38. [Google Scholar] [CrossRef]
- Zhu, W.; Wei, L.; Zhang, H.; Chen, J.; Qin, X. Oncolytic adenovirus armed with IL-24 Inhibits the growth of breast cancer in vitro and in vivo. J. Exp. Clin. Cancer Res. 2012, 31, 51. [Google Scholar] [CrossRef] [PubMed]
- Pufe, T.; Petersen, W.J.; Miosge, N.; Goldring, M.B.; Mentlein, R.; Varoga, D.J.; Tillmann, B.N. Endostatin/collagen XVIII—An inhibitor of angiogenesis—is expressed in cartilage and fibrocartilage. Matrix Biol. 2004, 23, 267–276. [Google Scholar] [CrossRef] [PubMed]
- Chastkofsky, M.I.; Pituch, K.C.; Katagi, H.; Zannikou, M.; Ilut, L.; Xiao, T.; Han, Y.; Sonabend, A.M.; Curiel, D.T.; Bonner, E.R.; et al. Mesenchymal Stem Cells Successfully Deliver Oncolytic Virotherapy to Diffuse Intrinsic Pontine Glioma. Clin. Cancer Res. 2021, 27, 1766–1777. [Google Scholar] [CrossRef] [PubMed]
- Kalimuthu, S.; Oh, J.M.; Gangadaran, P.; Zhu, L.; Lee, H.W.; Rajendran, R.L.; Baek, S.H.; Jeon, Y.H.; Jeong, S.Y.; Lee, S.W.; et al. In Vivo Tracking of Chemokine Receptor CXCR4-Engineered Mesenchymal Stem Cell Migration by Optical Molecular Imaging. Stem Cells Int. 2017, 2017, 8085637. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharboosh, R.; ReFaey, K.; Lara-Velazquez, M.; Grewal, S.S.; Imitola, J.; Quiñones-Hinojosa, A. Inflammatory Mediators in Glioma Microenvironment Play a Dual Role in Gliomagenesis and Mesenchymal Stem Cell Homing: Implication for Cellular Therapy. Mayo Clin. Proc. Innov. Mayo Clin. Proc. Innov. Qual. Outcomes 2020, 4, 443–459. [Google Scholar] [CrossRef]
- Bloem, B.R.; Okun, M.S.; Klein, C. Parkinson’s disease. Lancet 2021, 397, 2284–2303. [Google Scholar] [CrossRef]
- Ma, J.; Shi, X.; Li, M.; Chen, S.; Gu, Q.; Zheng, J.; Li, D.; Wu, S.; Yang, H.; Li, X. MicroRNA-181a–2–3p shuttled by mesenchymal stem cell-secreted extracellular vesicles inhibits oxidative stress in Parkinson’s disease by inhibiting EGR1 and NOX4. Cell Death Discov. 2022, 8, 33. [Google Scholar] [CrossRef]
- Heris, R.M.; Shirvaliloo, M.; Abbaspour-Aghdam, S.; Hazrati, A.; Shariati, A.; Youshanlouei, H.R.; Niaragh, F.J.; Valizadeh, H.; Ahmadi, M. The potential use of mesenchymal stem cells and their exosomes in Parkinson’s disease treatment. Stem Cell Res. Ther. 2022, 13, 371. [Google Scholar] [CrossRef]
- Li, J.; Li, N.; Wei, J.; Feng, C.; Chen, Y.; Chen, T.; Ai, Z.; Zhu, X.; Ji, W.; Li, T. Genetically engineered mesenchymal stem cells with dopamine synthesis for Parkinson’s disease in animal models. NPJ Park. Dis. 2022, 8, 175. [Google Scholar] [CrossRef]
- Shin, J.Y.; Kim, D.Y.; Lee, J.; Shin, Y.J.; Kim, Y.S.; Lee, P.H. Priming mesenchymal stem cells with α-synuclein enhances neuroprotective properties through induction of autophagy in Parkinsonian models. Stem Cell Res. Ther. 2022, 13, 483. [Google Scholar] [CrossRef]
- Oh, S.H.; Kim, H.N.; Park, H.J.; Shin, J.Y.; Kim, D.Y.; Lee, P.H. The Cleavage Effect of Mesenchymal Stem Cell and Its Derived Matrix Metalloproteinase-2 on Extracellular α-Synuclein Aggregates in Parkinsonian Models. Stem Cells Transl. Med. 2017, 6, 949–961. [Google Scholar] [CrossRef]
- Uehara, T.; Choong, C.J.; Nakamori, M.; Hayakawa, H.; Nishiyama, K.; Kasahara, Y.; Baba, K.; Nagata, T.; Yokota, T.; Tsuda, H.; et al. Amido-bridged nucleic acid (AmNA)-modified antisense oligonucleotides targeting α-synuclein as a novel therapy for Parkinson’s disease. Sci. Rep. 2019, 21, 7567. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood-Brain Barrier and Delivery of Protein and Gene Therapeutics to Brain. Front. Aging Neurosci. 2020, 11, 373. [Google Scholar] [CrossRef] [PubMed]
- van Dyck, C.H.; Swanson, C.J.; Aisen, P.; Bateman, R.J.; Chen, C.; Gee, M.; Kanekiyo, M.; Li, D.; Reyderman, L.; Cohen, S.; et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023, 388, 9–21. [Google Scholar] [CrossRef]
- Rashad, A.; Rasool, A.; Shaheryar, M.; Sarfraz, A.; Sarfraz, Z.; Robles-Velasco, K.; Cherrez-Ojeda, I. Donanemab for Alzheimer’s Disease: A Systematic Review of Clinical Trials. Healthcare 2023, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Hernández, A.E.; García, E. Mesenchymal Stem Cell Therapy for Alzheimer’s Disease. Stem Cells Int. 2021, 2021, 7834421. [Google Scholar] [CrossRef] [PubMed]
- Sobue, A.; Komine, O.; Yamanaka, K. Neuroinflammation in Alzheimer’s disease: Microglial signature and their relevance to disease. Inflamm. Regen. 2023, 43, 26. [Google Scholar] [CrossRef]
- Weston, L.L.; Jiang, S.; Chisholm, D.; Jantzie, L.L.; Bhaskar, K. Interleukin-10 deficiency exacerbates inflammation-induced tau pathology. J. Neuroinflamm. 2021, 18, 161. [Google Scholar] [CrossRef]
- Xie, Z.H.; Liu, Z.; Zhang, X.R.; Yang, H.; Wei, L.F.; Wang, Y.; Xu, S.L.; Sun, L.; Lai, C.; Bi, J.Z.; et al. Wharton’s Jelly-derived mesenchymal stem cells alleviate memory deficits and reduce amyloid-β deposition in an APP/PS1 transgenic mouse model. Clin. Exp. Med. 2016, 16, 89–98. [Google Scholar] [CrossRef]
- Yokokawa, K.; Iwahara, N.; Hisahara, S.; Emoto, M.C.; Saito, T.; Suzuki, H.; Manabe, T.; Matsumura, A.; Matsushita, T.; Suzuki, S.; et al. Transplantation of Mesenchymal Stem Cells Improves Amyloid-β Pathology by Modifying Microglial Function and Suppressing Oxidative Stress. J. Alzheimers Dis. 2019, 72, 867–884. [Google Scholar] [CrossRef]
- Nakano, M.; Kubota, K.; Kobayashi, E.; Chikenji, T.S.; Saito, Y.; Konari, N.; Fujimiya, M. Bone marrow-derived mesenchymal stem cells improve cognitive impairment in an Alzheimer’s disease model by increasing the expression of microRNA-146a in hippocampus. Sci. Rep. 2020, 10, 10772. [Google Scholar] [CrossRef]
- Murphy, S.J.; Werring, D.J. Stroke: Causes and clinical features. Medicine 2020, 48, 561–566. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Chen, X.; Wei, Y. Neuronal injuries in cerebral infarction and ischemic stroke: From mechanisms to treatment (Review). Int. J. Mol. Med. 2022, 49, 15. [Google Scholar] [CrossRef] [PubMed]
- Magid-Bernstein, J.; Girard, R.; Polster, S.; Srinath, A.; Romanos, S.; Awad, I.A.; Sansing, L.H. Cerebral Hemorrhage: Pathophysiology, Treatment, and Future Directions. Circ. Res. 2022, 130, 1204–1229. [Google Scholar] [CrossRef] [PubMed]
- Neifert, S.N.; Chapman, E.K.; Martini, M.L.; Shuman, W.H.; Schupper, A.J.; Oermann, E.K.; Mocco, J.; Macdonald, R.L. Aneurysmal Subarachnoid Hemorrhage: The Last Decade. Transl. Stroke Res. 2021, 12, 428–446. [Google Scholar] [CrossRef] [PubMed]
- Rodrigo, R.; Fernández-Gajardo, R.; Gutiérrez, R.; Matamala, J.M.; Carrasco, R.; Miranda-Merchak, A.; Feuerhake, W. Oxidative stress and pathophysiology of ischemic stroke: Novel therapeutic opportunities. CNS Neurol. Disord. Drug Targets 2013, 12, 698–714. [Google Scholar] [CrossRef]
- Babenko, V.A.; Silachev, D.N.; Popkov, V.A.; Zorova, L.D.; Pevzner, I.B.; Plotnikov, E.Y.; Sukhikh, G.T.; Zorov, D.B. Miro1 Enhances Mitochondria Transfer from Multipotent Mesenchymal Stem Cells (MMSC) to Neural Cells and Improves the Efficacy of Cell Recovery. Molecules 2018, 23, 687. [Google Scholar] [CrossRef]
- Lin, S.L.; Lee, W.; Liu, S.P.; Chang, Y.W.; Jeng, L.B.; Shyu, W.C. Novel Programmed Death Ligand 1-AKT-engineered Mesenchymal Stem Cells Promote Neuroplasticity to Target Stroke Therapy. Mol. Neurobiol. 2023. [Google Scholar] [CrossRef]
- Li, Y.; Dong, Y.; Ran, Y.; Zhang, Y.; Wu, B.; Xie, J.; Cao, Y.; Mo, M.; Li, S.; Deng, H.; et al. Three-dimensional cultured mesenchymal stem cells enhance repair of ischemic stroke through inhibition of microglia. Stem Cell Res. Ther. 2021, 12, 358. [Google Scholar] [CrossRef]
- Ghajar, J. Traumatic brain injury. Lancet 2000, 356, 923–929. [Google Scholar] [CrossRef]
- Maiti, P.; Peruzzaro, S.; Kolli, N.; Andrews, M.; Al-Gharaibeh, A.; Rossignol, J.; Dunbar, G.L. Transplantation of mesenchymal stem cells overexpressing interleukin-10 induces autophagy response and promotes neuroprotection in a rat model of TBI. J. Cell. Mol. Med. 2019, 23, 5211–5224. [Google Scholar] [CrossRef]
- Choi, B.Y.; Hong, D.K.; Kang, B.S.; Lee, S.H.; Choi, S.; Kim, H.-J.; Lee, S.M.; Suh, S.W. Engineered Mesenchymal Stem Cells Over-Expressing BDNF Protect the Brain from Traumatic Brain Injury-Induced Neuronal Death, Neurological Deficits, and Cognitive Impairments. Pharmaceuticals 2023, 16, 436. [Google Scholar] [CrossRef] [PubMed]
- Shahror, R.A.; Linares, G.R.; Wang, Y.; Hsueh, S.C.; Wu, C.C.; Chuang, D.M.; Chiang, Y.H.; Chen, K.Y. Transplantation of Mesenchymal Stem Cells Overexpressing Fibroblast Growth Factor 21 Facilitates Cognitive Recovery and Enhances Neurogenesis in a Mouse Model of Traumatic Brain Injury. J. Neurotraum. 2020, 37, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Feldman, E.L.; Goutman, S.A.; Petri, S.; Mazzini, L.; Savelieff, M.G.; Shaw, P.J.; Sobue, G. Amyotrophic lateral sclerosis. Lancet 2022, 400, 1363–1380. [Google Scholar] [CrossRef]
- Morata-Tarifa, C.; Azkona, G.; Glass, J.; Mazzini, L.; Sanchez-Pernaute, R. Looking backward to move forward: A meta-analysis of stem cell therapy in amyotrophic lateral sclerosis. NPJ Regen. Med. 2021, 6, 20. [Google Scholar] [CrossRef]
- Blair, H.A. Tofersen: First Approval. Drugs 2023, 83, 1039–1043. [Google Scholar] [CrossRef]
- Dobson, R.; Giovannoni, G. Multiple sclerosis–a review. Eur. J. Neurol. 2019, 26, 27–40. [Google Scholar] [CrossRef] [PubMed]
- Tabansky, I.; Messina, M.D.; Bangeranye, C.; Goldstein, J.; Blitz-Shabbir, K.M.; Machado, S.; Jeganathan, V.; Wright, P.; Najjar, S.; Cao, Y.; et al. Advancing drug delivery systems for the treatment of multiple sclerosis. Immunol. Res. 2015, 63, 58–69. [Google Scholar] [CrossRef] [PubMed]
- Muid, R.E.; Dale, M.M.; Davis, P.D.; Elliott, L.H.; Hill, C.H.; Kumar, H.; Lawton, G.; Twomey, B.M.; Wadsworth, J.; Wilkinson, S.E.; et al. A novel conformationally restricted protein kinase C inhibitor, Ro 31-8425, inhibits human neutrophil superoxide generation by soluble, particulate and post-receptor stimuli. FEBS Lett. 1991, 293, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Levy, O.; Rothhammer, V.; Mascanfroni, I.; Tong, Z.; Kuai, R.; De Biasio, M.; Wang, Q.; Majid, T.; Perrault, C.; Yeste, A.; et al. A cell-based drug delivery platform for treating central nervous system inflammation. J. Mol. Med. 2021, 99, 663–671. [Google Scholar] [CrossRef] [PubMed]
- Mulero, P.; Midaglia, L.; Montalban, X. Ocrelizumab: A new milestone in multiple sclerosis therapy. Ther. Adv. Neurol. Disord. 2018, 11, 1756286418773025. [Google Scholar] [CrossRef]
- Fukuta, T. Development of functional microparticles capable of binding to neutrophils to overcome the blood-brain barrier for the treatment of ischemic stroke. Pharm. Tech. Jpn. 2023, 39, 81–83. [Google Scholar]
- Uno, N.; Takata, S.; Komoto, S.; Miyamoto, H.; Nakayama, Y.; Osaki, M.; Mayuzumi, R.; Miyazaki, N.; Hando, C.; Abe, S.; et al. Panel of human cell lines with human/mouse artificial chromosomes. Sci. Rep. 2022, 12, 3009. [Google Scholar] [CrossRef]


| # | Name | MSC Type | Diseases | Status | References |
|---|---|---|---|---|---|
| 1 | Sutéramikku | Autologous bone marrow-derived human MSCs | Spinal cord injury | Launched | [14,15,16] |
| 2 | Temcell HS | Allogeneic bone marrow-derived human MSCs | Acute graft-versus-host disease | Launched | [18] |
| 3 | Alofisel (darvadstrocel) | Allogeneic bone marrow-derived human MSCs | Complex perianal fistula in adults with Crohn’s disease | Launched | [19] |
| 4 | SB623 (vandefitemcel) | Allogeneic bone marrow-derived human MSCs | Traumatic brain injury | Launched | [20] |
| 5 | FF-31501 | Autologous bone marrow-derived human MSCs | Meniscal injury | Phase 3 clinical trial | - |
| 6 | CYP-004 | iPSC-derived human MSCs | Osteoarthritis | Phase 3 clinical trial (ACTRN12620000870954) | - |
| 7 | MutiStem (HLCM051) | Allogeneic bone marrow-derived human MSCs | Ischemic cerebral infarction | Phase 3 clinical trial (MASTERS-2 trial, NCT03545607), finished in2023 | - |
| 8 | Rexlemestrocel-L | Allogeneic bone marrow-derived human MSCs | Lower back pain | Phase 3 clinical trial (MSB-DR003 trial, NCT02412735), finished in 2021 | - |
| 9 | Remestemcel-L | Allogeneic bone marrow-derived human MSCs | Acute respiratory distress syndrome | Phase 3 clinical trial, finished | - |
| 10 | gMSC1 | Allogeneic synovial membrane-derived human MSCs | Knee cartilage damage | Phase 3 clinical trial | - |
| # | Formulation | Diseases | Cargo | Status | References |
|---|---|---|---|---|---|
| 1 | MSCs containing silica nanorattle encapsulating doxorubicin | Glioma | Doxorubicin | Basic research | [40] |
| 2 | PTX-encapsulated hyaluronic acid-poly (D,L-lactide-co-glycolide) polymeric micelles (PTX/HA-PLGA micelles) | Glioma | PTX | Basic research | [41] |
| 3 | Human umbilical cord blood MSCs loaded with the novel oncolytic adenovirus carrying interleukin (IL)-24 and/or endostatin | Glioma | IL-24 | Basic research | [45] |
| 4 | Oncolytic virus, CRAd.S.pK7, encapsulated within MSCs | Glioma | CRAd.S.pK7 | Basic research | [48] |
| 5 | MSCs encoding three critical genes for dopamine synthesis | Parkinson’s disease | Dopamine | Basic research | [54] |
| 6 | MSC priming with α-synuclein | Parkinson’s disease | miR 376-3p | Basic research | [55] |
| 7 | MSCs naturally possessing matrix metalloproteinase-2 (MMP-2) | Parkinson’s disease | MMP-2 | Basic research | [56] |
| 8 | Transplantation of MSCs | Alzheimer’s disease | IL-10 | Basic research | [65] |
| 9 | Bone marrow-derived MSCs | Alzheimer’s disease | Th2 cytokines | Basic research | [66] |
| 10 | Bone marrow-derived MSCs | Alzheimer’s disease | miR-146a | Basic research | [67] |
| 11 | Mitochondrial Rho-GTPase 1 (Miro1)-overexpressed multipotent MSCs | Stroke | Mitochondria | Basic research | [73] |
| 12 | Programmed cell death-ligand 1 (PD-L1) and AKT-modified umbilical cord-derived MSCs | Stroke | PD-L1 and AKT | Basic research | [74] |
| 13 | Three-dimensional (3D) spheroid cultured MSCs | Stroke | Unknown | Basic research | [75] |
| 14 | Genetically engineered MSCs overexpressing IL-10 | Traumatic brain injury | IL-10 | Basic research | [77] |
| 15 | Engineered MSCs overexpressing BDNF | Traumatic brain injury | BDNF | Basic research | [78] |
| 16 | MSCs overexpressing fibroblast growth factor 21 (FGF21) | Traumatic brain injury | FGF21 | Basic research | [79] |
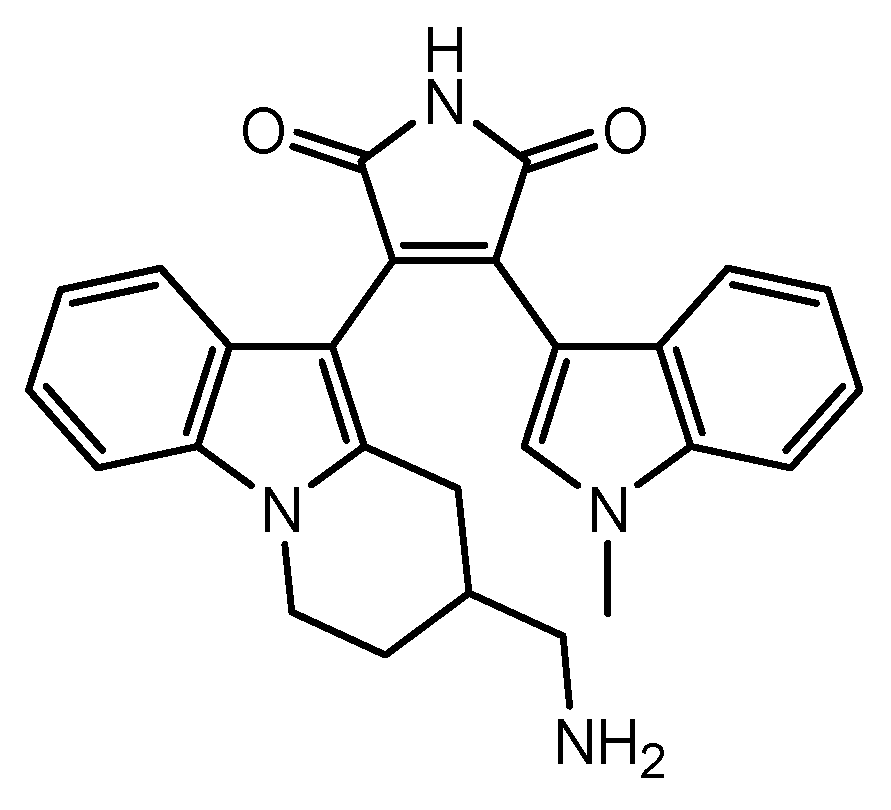
| 17 | Ro-31-8425-loaded MSCs | Multiple sclerosis | Ro-31-8425 | Basic research | [85] |
| 18 | MSCs as a carrier of nanoparticles containing biologically active substances | CNS disease | Arbitrary substances | Under analysis in Tashima lab | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tashima, T. Mesenchymal Stem Cell (MSC)-Based Drug Delivery into the Brain across the Blood–Brain Barrier. Pharmaceutics 2024, 16, 289. https://doi.org/10.3390/pharmaceutics16020289
Tashima T. Mesenchymal Stem Cell (MSC)-Based Drug Delivery into the Brain across the Blood–Brain Barrier. Pharmaceutics. 2024; 16(2):289. https://doi.org/10.3390/pharmaceutics16020289
Chicago/Turabian StyleTashima, Toshihiko. 2024. "Mesenchymal Stem Cell (MSC)-Based Drug Delivery into the Brain across the Blood–Brain Barrier" Pharmaceutics 16, no. 2: 289. https://doi.org/10.3390/pharmaceutics16020289
APA StyleTashima, T. (2024). Mesenchymal Stem Cell (MSC)-Based Drug Delivery into the Brain across the Blood–Brain Barrier. Pharmaceutics, 16(2), 289. https://doi.org/10.3390/pharmaceutics16020289





