Selenium Nanoparticle Activity against S. mutans Biofilms as a Potential Treatment Alternative for Periodontitis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. SeNP Synthesis
2.3. Characterisation of SeNPs
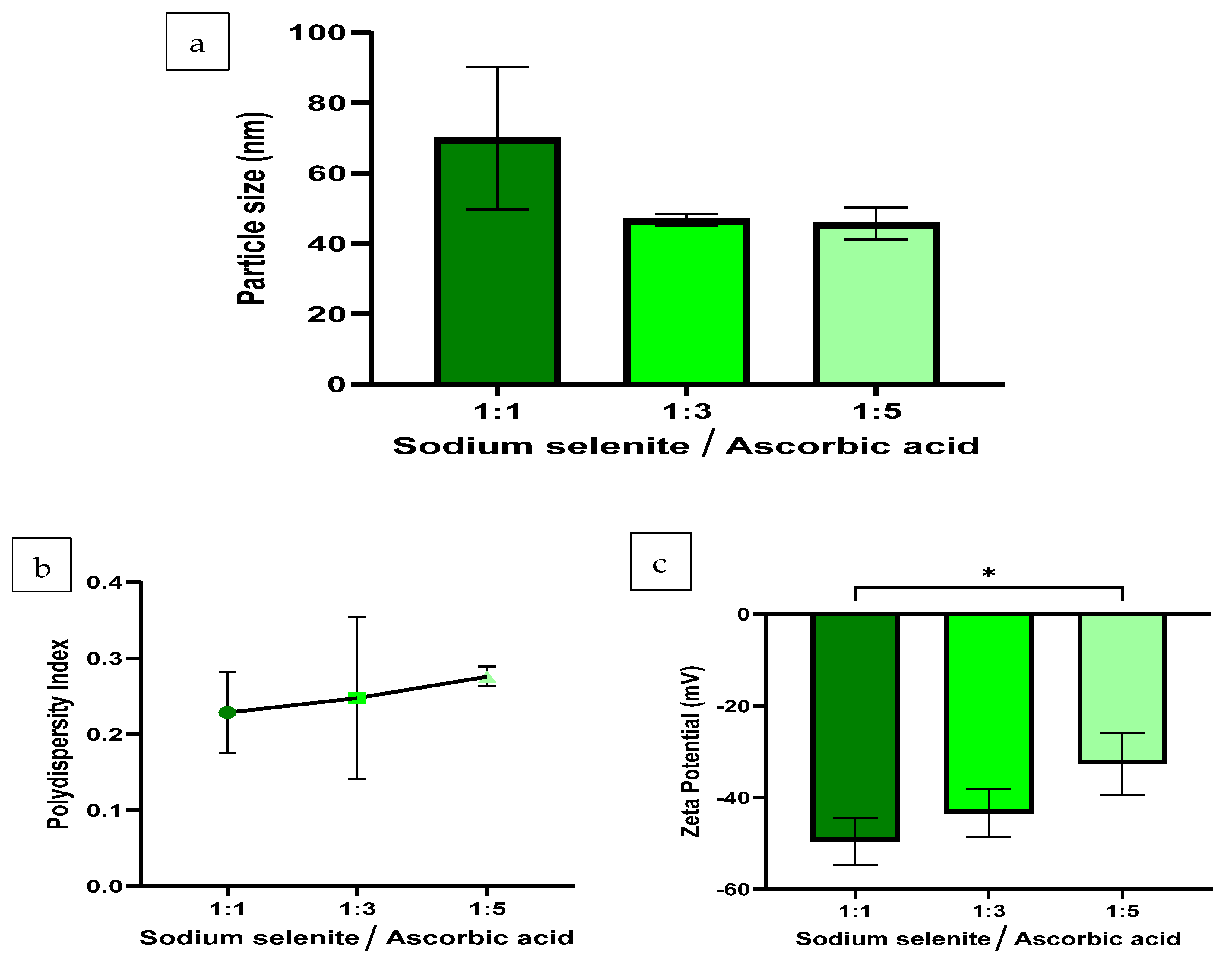
2.3.1. Hydrodynamic Size, Polydispersity Index and Zeta Potential Analysis
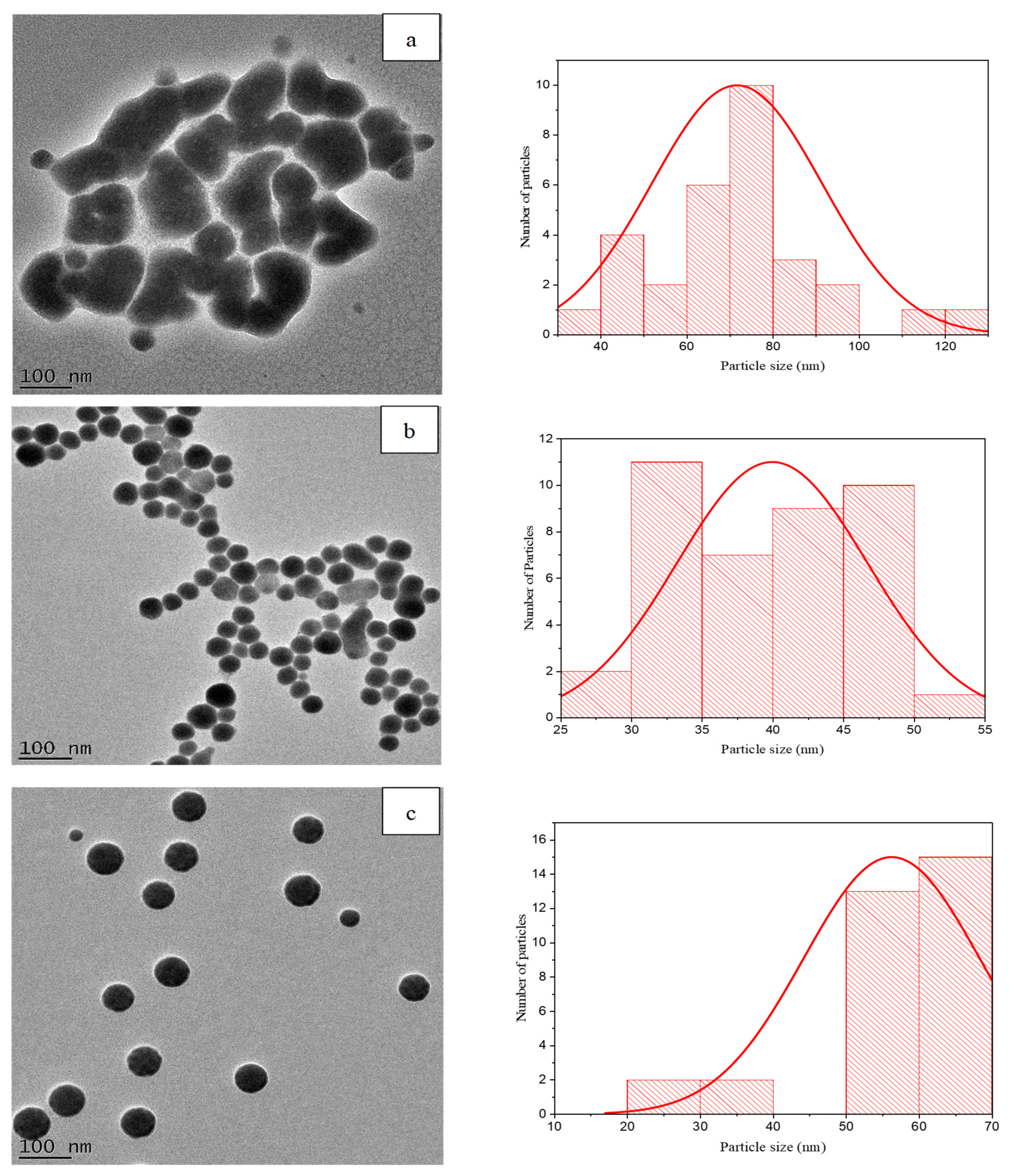
2.3.2. High-Resolution Transmission Electron Microscopy (HR-TEM) Analysis and Size Distribution
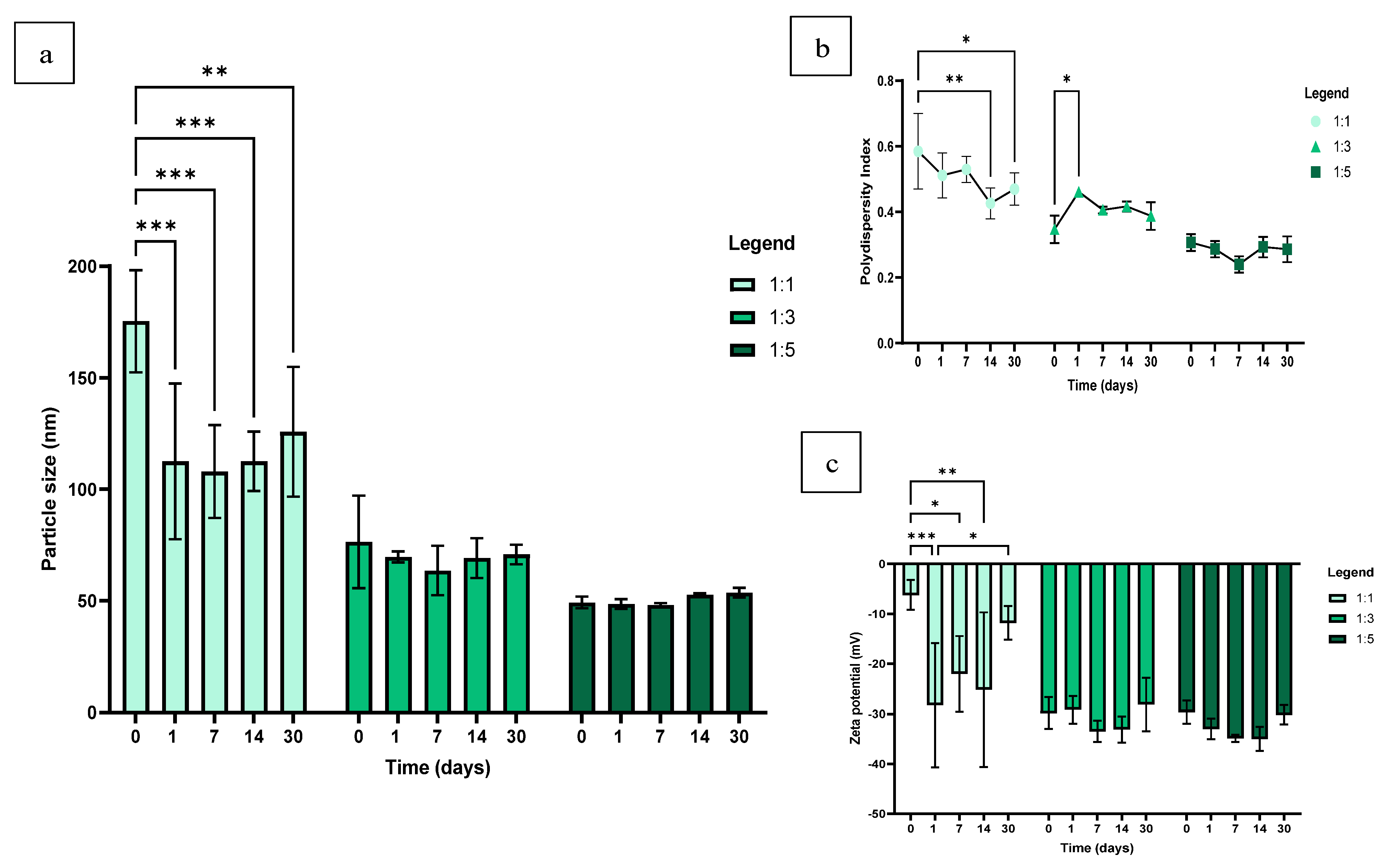
2.3.3. Stability of SeNPs
2.3.4. S. mutans Culture Preparation
2.3.5. Crystal Violet Biofilm Assay
2.3.6. Antibiofilm Assay
2.3.7. Statistical Methods
3. Results and Discussion
3.1. Hydrodynamic Size, PDI and ZP
3.2. HR-TEM Analysis and Size Distribution of SeNPs
3.3. Stability of SeNPs in dH2O
3.4. Stability of SeNPs in TSB + 2.5% Sucrose
3.5. Biofilm Forming Ability of S. mutans
3.6. Effect of Size and Concentration on Antibiofilm Activity of SeNPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organisation. Oral Health. Available online: https://www.who.int/news-room/fact-sheets/detail/oral-health (accessed on 27 August 2023).
- Vos, T.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; Aboyans, V.; et al. Global, Regional, and National Incidence, Prevalence, and Years Lived with Disability for 328 Diseases and Injuries for 195 Countries, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet 2017, 390, 1211–1259. [Google Scholar] [CrossRef]
- Tonetti, M.S.; Jepsen, S.; Jin, L.; Otomo-Corgel, J. Impact of the Global Burden of Periodontal Diseases on Health, Nutrition and Wellbeing of Mankind: A Call for Global Action. J. Clin. Periodontol. 2017, 44, 456–462. [Google Scholar] [CrossRef]
- Botelho, J.; Machado, V.; Leira, Y.; Proença, L.; Chambrone, L.; Mendes, J.J. Economic Burden of Periodontitis in the United States and Europe: An Updated Estimation. J. Periodontol. 2022, 93, 373–379. [Google Scholar] [CrossRef]
- Frédéric, L.J.; Michel, B.; Selena, T. Oral Microbes, Biofilms and their Role in Periodontal and Peri-Implant Diseases. Materials 2018, 11, 1802. [Google Scholar] [CrossRef]
- Song, W.S.; Lee, J.K.; Park, S.H.; Um, H.S.; Lee, S.Y.; Chang, B.S. Comparison of Periodontitis-Associated Oral Biofilm Formation Under Dynamic and Static Conditions. J. Periodontal Implant Sci. 2017, 47, 219–230. [Google Scholar] [CrossRef]
- Vieira Colombo, A.P.; Magalhães, C.B.; Hartenbach, F.A.R.R.; Martins do Souto, R.; Maciel da Silva-Boghossian, C. Periodontal-Disease-Associated Biofilm: A Reservoir for Pathogens of Medical Importance. Microb. Pathog. 2015, 94, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Loesche, W.J. Role of Streptococcus mutans in Human Dental Decay. Microbiol. Rev. 1986, 50, 353–380. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H. Do We Need to be Concerned about Dental Caries in the Coming Millennium? Crit. Rev. Oral Biol. Med. 2002, 13, 126–131. [Google Scholar] [CrossRef] [PubMed]
- Lemos, J.A.; Palmer, S.R.; Zeng, L.; Wen, Z.T.; Kajfasz, J.K.; Freires, I.A.; Abranches, J.; Brady, L.J. The Biology of Streptococcus mutans. Microbiol. Spectr. 2019, 7, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- American Academy of Periodontology. Guidelines for Periodontal Therapy. J. Periodontol. 2001, 72, 1624–1628. [Google Scholar] [CrossRef]
- Lertpimonchai, A.; Rattanasiri, S.; Arj-Ong Vallibhakara, S.; Attia, J.; Thakkinstian, A. The Association Between Oral Hygiene and Periodontitis: A Systematic Review and Meta-Analysis. Int. Dent. J. 2017, 67, 332–343. [Google Scholar] [CrossRef]
- Rams, T.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic Resistance in Human Chronic Periodontitis Microbiota. J. Periodontol. 2014, 85, 160–169. [Google Scholar] [CrossRef]
- Joshi, D.; Garg, T.; Goyal, A.K.; Rath, G. Advanced Drug Delivery Approaches Against Periodontitis. Drug Deliv. 2016, 23, 363–377. [Google Scholar] [CrossRef]
- Aeran, H.; Kumar, V.; Uniyal, S.; Tanwer, P. Nanodentistry: Is just a Fiction or Future. J. Oral Biol. Craniofac. Res. 2015, 5, 207–211. [Google Scholar] [CrossRef]
- Ramburrun, P.; Pringle, N.A.; Dube, A.; Adam, R.Z.; D’Souza, S.; Aucamp, M. Recent Advances in the Development of Antimicrobial and Antifouling Biocompatible Materials for Dental Applications. Materials 2021, 14, 3167. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Ge, S. Application of Antimicrobial Nanoparticles in Dentistry. Molecules 2019, 24, 1033. [Google Scholar] [CrossRef] [PubMed]
- Khan, S.T.; Ahamed, M.; Musarrat, J.; Al-Khedhairy, A.A. Anti-Biofilm and Antibacterial Activities of Zinc Oxide Nanoparticles Against the Oral Opportunistic Pathogens Rothia Dentocariosa and Rothia Mucilaginosa. Eur. J. Oral Sci. 2014, 122, 397–403. [Google Scholar] [CrossRef]
- Hemeg, H.A. Nanomaterials for Alternative Antibacterial Therapy. Int. J. Nanomed. 2017, 12, 8211–8225. [Google Scholar] [CrossRef] [PubMed]
- Filipović, N.; Ušjak, D.; Milenković, M.T.; Zheng, K.; Liverani, L.; Boccaccini, A.R.; Stevanović, M.M. Comparative Study of the Antimicrobial Activity of Selenium Nanoparticles with Different Surface Chemistry and Structure. Front. Bioeng. Biotechnol. 2021, 8, 624621. [Google Scholar] [CrossRef] [PubMed]
- Hosnedlova, B.; Kepinska, M.; Skalickova, S.; Fernandez, C.; Ruttkay-Nedecky, B.; Peng, Q.; Baron, M.; Melcova, M.; Opatrilova, R.; Zidkova, J. Nano-Selenium and its Nanomedicine Applications: A Critical Review. Int. J. Nanomed. 2018, 13, 2107–2128. [Google Scholar] [CrossRef] [PubMed]
- Wadhwani, S.A.; Shedbalkar, U.U.; Singh, R.; Chopade, B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016, 100, 2555–2566. [Google Scholar] [CrossRef]
- Alpaslan, E.; Geilich, B.M.; Yazici, H.; Webster, T.J. PH-Controlled Cerium Oxide Nanoparticle Inhibition of Both Gram-Positive and Gram-Negative Bacteria Growth. Sci. Rep. 2017, 7, 45859. [Google Scholar] [CrossRef] [PubMed]
- Vahdati, M.; Tohidi Moghadam, T. Synthesis and Characterization of Selenium Nanoparticles-Lysozyme Nanohybrid System with Synergistic Antibacterial Properties. Sci. Rep. 2020, 10, 510. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.H.; Lin, F.; Wang, C.R. Observation in the Growth of Selenium Nanoparticles. J. Chin. Chem. Soc. 2004, 51, 239–242. [Google Scholar] [CrossRef]
- Malhotra, S.; Jha, N.; Desai, K. A Superficial Synthesis of Selenium Nanospheres Using Wet Chemical Approach. Int. J. Nanotechnol. Appl. 2014, 3, 7–14. [Google Scholar]
- Christensen, G.D.; Simpson, W.A.; Younger, J.J.; Baddour, L.M.; Barrett, F.F.; Melton, D.M.; Beachey, E.H. Adherence of Coagulase-Negative Staphylococci to Plastic Tissue Culture Plates: A Quantitative Model for the Adherence of Staphylococci to Medical Devices. J. Clin. Microbiol. 1985, 649, 38163. [Google Scholar] [CrossRef] [PubMed]
- Shinde, S.; Lee, L.H.; Chu, T. Inhibition of Biofilm Formation by the Synergistic Action of EGCG-S and Antibiotics. Antibiotics 2021, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Adeyemo, R.O.; Famuyide, I.M.; Dzoyem, J.P.; Lyndy Joy, M. Anti-Biofilm, Antibacterial, and Anti-Quorum Sensing Activities of Selected South African Plants Traditionally Used to Treat Diarrhoea. Evid. Based Complement. Altern. Med. 2022, 2022, 1307801. [Google Scholar] [CrossRef]
- Li, Q.; Chen, T.; Yang, F.; Liu, J.; Zheng, W. Facile and Controllable One-Step Fabrication of Selenium Nanoparticles Assisted by L-Cysteine. Mater. Lett. 2010, 64, 614–617. [Google Scholar] [CrossRef]
- Chung, S.; Zhou, R.; Webster, T.J. Green Synthesized BSA-Coated Selenium Nanoparticles Inhibit Bacterial Growth While Promoting Mammalian Cell Growth. Int. J. Nanomed. 2020, 15, 115–124. [Google Scholar] [CrossRef]
- Hei, H.; Wang, R.; Liu, X.; He, L.; Zhang, G. Controlled Synthesis and Characterization of Nobel Metal Nanoparticles. Soft Nanosci. Lett. 2012, 2, 34–40. [Google Scholar] [CrossRef]
- Menamo, D.S.; Ayele, D.W.; Ali, M.T. Green Synthesis, Characterization and Antibacterial Activity of Copper Nanoparticles Using L-Ascorbic Acid as a Reducing Agent. Ethiop. J. Sci. Technol. 2017, 10, 209. [Google Scholar] [CrossRef]
- Yu, B.; Zhang, Y.; Zheng, W.; Fan, C.; Chen, T. Positive Surface Charge Enhances Selective Cellular Uptake and Anticancer Efficacy of Selenium Nanoparticles. Inorg. Chem. 2012, 51, 8956–8963. [Google Scholar] [CrossRef] [PubMed]
- Bai, K.; Hong, B.; He, J.; Hong, Z.; Tan, R. Preparation and Antioxidant Properties of Selenium Nanoparticles-Loaded Chitosan Microspheres. Int. J. Nanomed. 2017, 12, 4527–4539. [Google Scholar] [CrossRef]
- Li, M.; Huang, R.; Zhou, X.D.; Gregory, R. Role of Sortase in Streptococcus mutans Under the Effect of Nicotine. Int. J. Oral Sci. 2013, 5, 206–211. [Google Scholar] [CrossRef]
- Saeki, E.; Yamada, A.; Araujo, L.; Anversa, L.; Garcia, D.; Souza, R.; Martins, H.; Katsuko, R.; Kobayashi, T.; Nakazato, G.; et al. Subinhibitory Concentrations of Biogenic Silver Nanoparticles Affect Motility and Biofilm Formation in Pseudomonas aeruginosa. Front. Cell. Infect. Microbiol. 2021, 11, 656984. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hu, C.; Shao, L. The Antimicrobial Activity of Nanoparticles: Present Situation and Prospects for the Future. Int. J. Nanomed. 2017, 12, 1227–1249. [Google Scholar] [CrossRef] [PubMed]
- Su, L.J.; Zhang, J.H.; Gómez, H.; Murugan, R.; Hong, X.; Xu, D.; Jiang, F.; Peng, Z.Y. Reactive Oxygen Species-Induced Lipid Peroxidation in Apoptosis, Autophagy, and Ferroptosis. Oxid. Med. Cell. Longev. 2019, 2019, 5080843. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Alvarez, P.J.J. Sublethal Concentrations of Silver Nanoparticles Stimulate Biofilm Development. Environ. Sci. Technol. Lett. 2015, 2, 221–226. [Google Scholar] [CrossRef]
- Darroudi, M.; Rangrazi, A.; Ghazvini, K.; Bagheri, H.; Boruziniat, A. Antimicrobial Activity of Colloidal Selenium Nanoparticles in Chitosan Solution Against Streptococcus mutans, Lactobacillus acidophilus, and Candida albicans. Pesqui. Bras. Odontopediatria Clin. Integr. 2021, 21. [Google Scholar] [CrossRef]
- Shahmoradi, S.; Shariati, A.; Amini, S.M.; Zargar, N.; Yadegari, Z.; Darban-Sarokhalil, D. The Application of Selenium Nanoparticles for Enhancing the Efficacy of Photodynamic Inactivation of Planktonic Communities and the Biofilm of Streptococcus mutans. BMC Res. Notes 2022, 15, 84. [Google Scholar] [CrossRef] [PubMed]
- Kwasny, S.M.; Opperman, T.J. Static Biofilm Cultures of Gram-Positive Pathogens Grown in a Microtiter Format Used for Anti-Biofilm Drug Discovery. Curr. Protoc. Pharmacol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Zonaro, E.; Lampis, S.; Turner, R.J.; Qazi, S.J.S.; Vallini, G. Biogenic Selenium and Tellurium Nanoparticles Synthesized by Environmental Microbial Isolates Efficaciously Inhibit Bacterial Planktonic Cultures and Biofilms. Front. Microbiol. 2015, 6, 584. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Jiang, Y.; Ding, Y.; Povey, M.; York, D. Investigation into the Antibacterial Behaviour of Suspensions of ZnO Nanoparticles (ZnO Nanofluids). J. Nanoparticle Res. 2007, 9, 479–489. [Google Scholar] [CrossRef]



| OD Criteria | Biofilm Classification |
|---|---|
| OD ≤ 2 × OD (control) | Weakly adherent |
| 2 × OD (control) ≤ OD ≤ 4 × OD (control) | Moderately adherent |
| 4 × OD (control) ≤ OD | Strongly adherent |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamman, N.; Ramburrun, P.; Dube, A. Selenium Nanoparticle Activity against S. mutans Biofilms as a Potential Treatment Alternative for Periodontitis. Pharmaceutics 2024, 16, 450. https://doi.org/10.3390/pharmaceutics16040450
Hamman N, Ramburrun P, Dube A. Selenium Nanoparticle Activity against S. mutans Biofilms as a Potential Treatment Alternative for Periodontitis. Pharmaceutics. 2024; 16(4):450. https://doi.org/10.3390/pharmaceutics16040450
Chicago/Turabian StyleHamman, Naasika, Poornima Ramburrun, and Admire Dube. 2024. "Selenium Nanoparticle Activity against S. mutans Biofilms as a Potential Treatment Alternative for Periodontitis" Pharmaceutics 16, no. 4: 450. https://doi.org/10.3390/pharmaceutics16040450
APA StyleHamman, N., Ramburrun, P., & Dube, A. (2024). Selenium Nanoparticle Activity against S. mutans Biofilms as a Potential Treatment Alternative for Periodontitis. Pharmaceutics, 16(4), 450. https://doi.org/10.3390/pharmaceutics16040450







