Colon Drug Delivery Systems Based on Swellable and Microbially Degradable High-Methoxyl Pectin: Coating Process and In Vitro Performance
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Manufacturing and Characterization of Tablet Cores
2.2.2. HM Pectin Coating
Spray-Coating
Powder-Layering
Kollicoat® MAE Coating
2.2.3. Characterization of Coated Systems
Physico-Technological Characterization
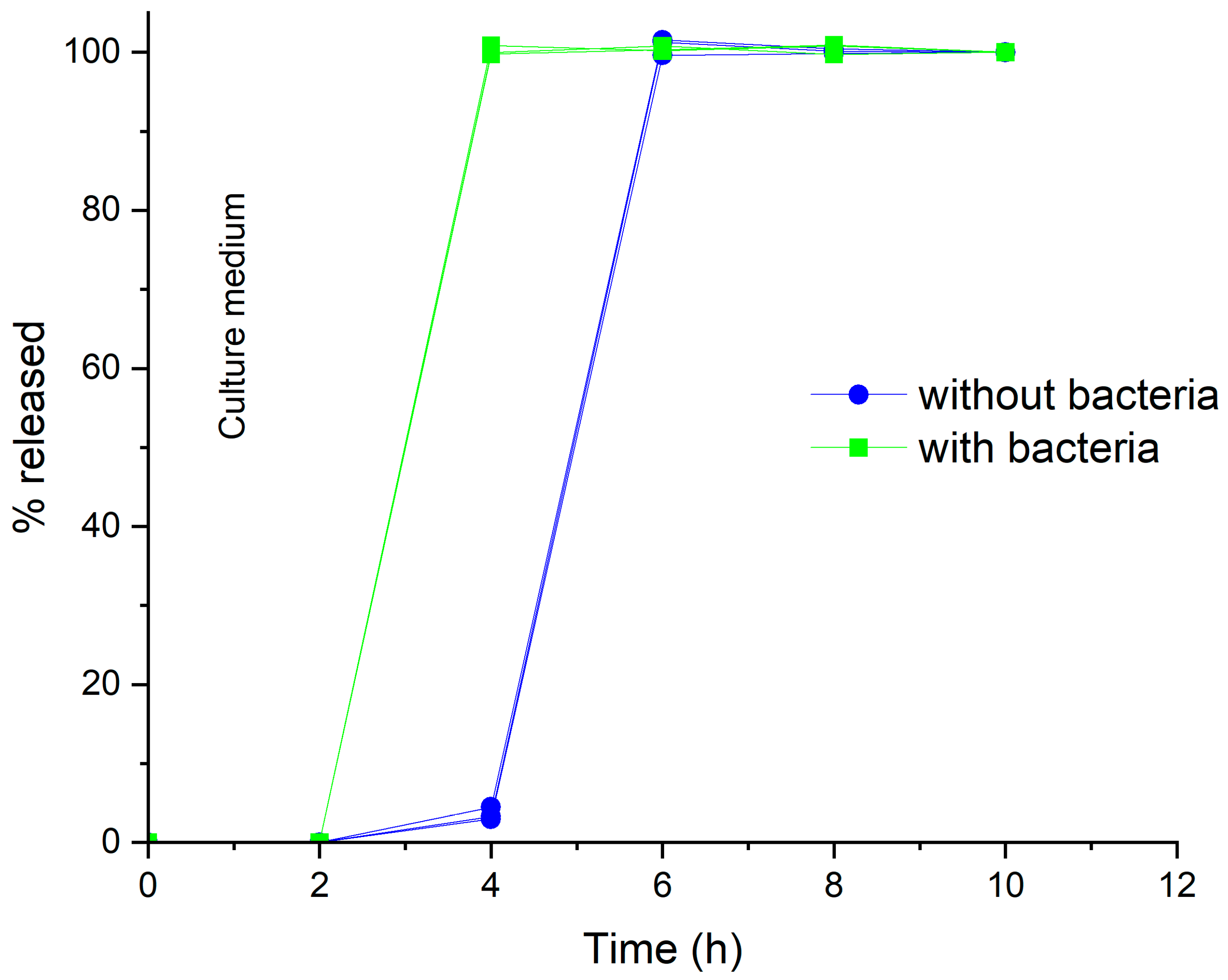
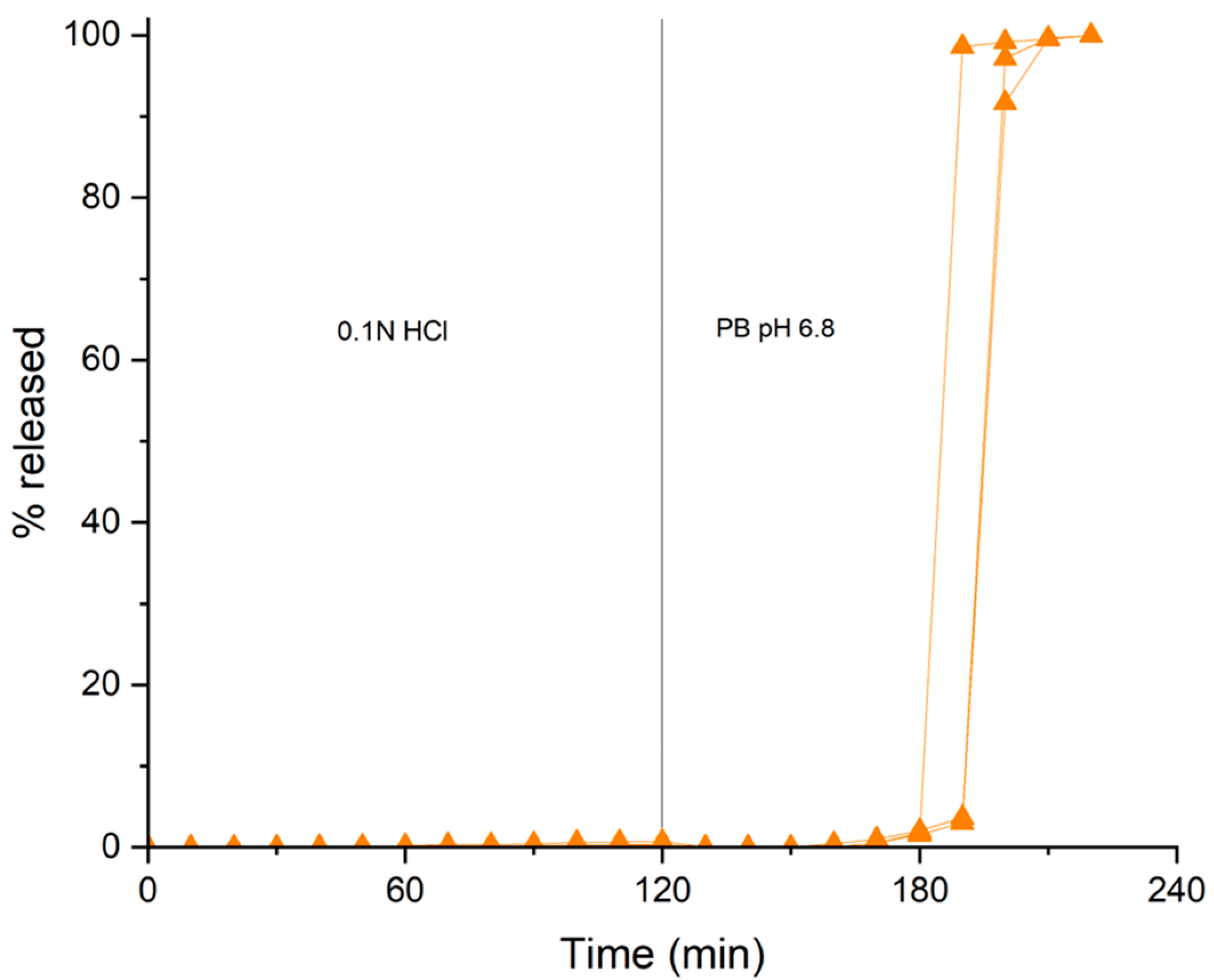
In Vitro Release Test
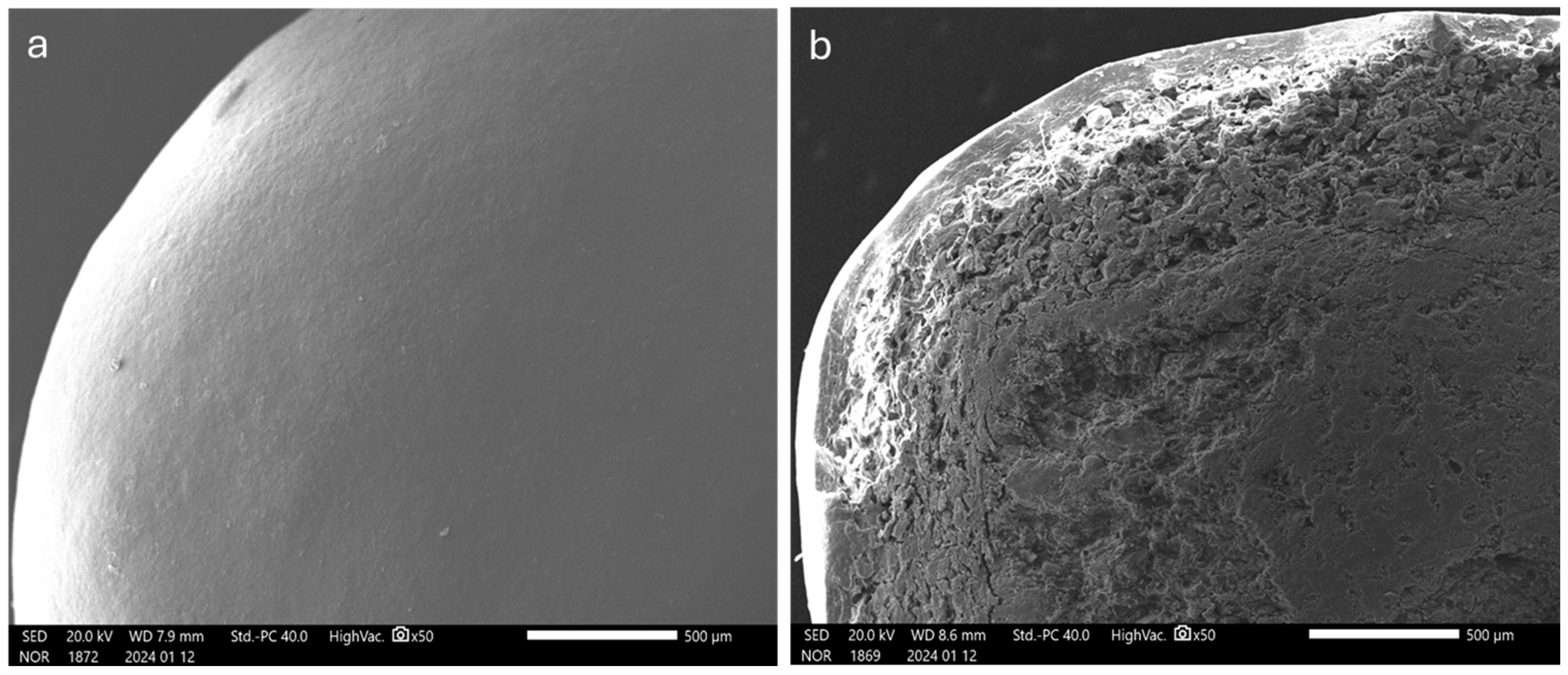
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- McConnell, E.L.; Liu, F.; Basit, A.W. Colonic treatments and targets: Issues and opportunities. J. Drug Target. 2009, 17, 335–363. [Google Scholar] [CrossRef] [PubMed]
- Gazzaniga, A.; Moutaharrik, S.; Filippin, I.; Foppoli, A.; Palugan, L.; Maroni, A.; Cerea, M. Time-Based Formulation Strategies for Colon Drug Delivery. Pharmaceutics 2022, 14, 2762. [Google Scholar] [CrossRef] [PubMed]
- McCoubrey, L.E.; Elbadawi, M.; Basit, A.W. Current clinical translation of microbiome medicines. Trends Pharmacol. Sci. 2022, 43, 281–292. [Google Scholar] [CrossRef] [PubMed]
- McCoubrey, L.E.; Favaron, A.; Awad, A.; Orlu, M.; Gaisford, S.; Basit, A.W. Colonic drug delivery: Formulating the next generation of colon-targeted therapeutics. J. Control. Release 2023, 353, 1107–1126. [Google Scholar] [CrossRef]
- Bosch, B.; Moutaharrik, S.; Gazzaniga, A.; Hiippala, K.; Santos, H.A.; Maroni, A.; Satokari, R. Development of a time-dependent oral colon delivery system of anaerobic Odoribacter splanchnicus for bacteriotherapy. Eur. J. Pharm. Biopharm. 2023, 190, 73–80. [Google Scholar] [CrossRef] [PubMed]
- Bourgeois, S.; Harvey, R.; Fattal, E. Polymer Colon Drug Delivery Systems and their Application to Peptides, Proteins, and Nucleic Acids. Am. J. Drug Deliv. 2005, 3, 171–204. [Google Scholar] [CrossRef]
- Bak, A.; Ashford, M.; Brayden, D. Local delivery of macromolecules to treat diseases associated with the colon. Adv. Drug Deliv. Rev. 2018, 136–137, 2–27. [Google Scholar] [CrossRef] [PubMed]
- Awad, A.; Madla, C.M.; McCoubrey, L.E.; Ferraro, F.; Gavins, F.K.; Buanz, A.; Gaisford, S.; Orlu, M.; Siepmann, F.; Siepmann, J.; et al. Clinical translation of advanced colonic drug delivery technologies. Adv. Drug Deliv. Rev. 2022, 181, 114076. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Sangfuang, N.; McCoubrey, L.E.; Yadav, V.; Elbadawi, M.; Orlu, M.; Gaisford, S.; Basit, A.W. Advancing oral delivery of biologics: Machine learning predicts peptide stability in the gastrointestinal tract. Int. J. Pharm. 2023, 634, 122643. [Google Scholar] [CrossRef]
- Sinha, V.; Kumria, R. Microbially triggered drug delivery to the colon. Eur. J. Pharm. Sci. 2003, 18, 3–18. [Google Scholar] [CrossRef]
- Chourasia, M.K.; Jain, S.K. Polysaccharides for Colon Targeted Drug Delivery. Drug Deliv. 2004, 11, 129–148. [Google Scholar] [CrossRef]
- Thompson, R.P.H.; Bloor, J.R.; Ede, R.J.; Hawkey, C.; Hawthorne, B.; Muller, F.A.; Palmer, R.M. Preserved endogenous cortisol levels during treatment of ulcerative colitis with COLAL-PREDTM, a novel oral system consistently delivering prednisolone metasulphobenzoate to the colon. Gastroenterology 2002, 122, A433. [Google Scholar]
- Ibekwe, V.C.; Khela, M.K.; Evans, D.F.; Basit, A.W. A new concept in colonic drug targeting: A combined pH-responsive and bacterially-triggered drug delivery technology. Aliment. Pharmacol. Ther. 2008, 28, 911–916. [Google Scholar] [CrossRef]
- Karrout, Y.; Neut, C.; Wils, D.; Siepmann, F.; Deremaux, L.; Flament, M.P.; Dubreuil, L.; Desreumaux, P.; Siepmann, J. Peas starch-based film coatings for site-specific drug delivery to the colon. J. Appl. Polym. Sci. 2011, 119, 1176–1184. [Google Scholar] [CrossRef]
- Karrout, Y.; Dubuquoy, L.; Piveteau, C.; Siepmann, F.; Moussa, E.; Wils, D.; Beghyn, T.; Neut, C.; Flament, M.-P.; Guerin-Deremaux, L.; et al. In vivo efficacy of microbiota-sensitive coatings for colon targeting: A promising tool for IBD therapy. J. Control. Release 2015, 197, 121–130. [Google Scholar] [CrossRef]
- Varum, F.; Cristina Freire, A.; Fadda, H.M.; Bravo, R.; Basit, A.W. A dual pH and microbiota-triggered coating (Phloral™) for fail-safe colonic drug release. Int. J. Pharm. 2020, 583, 119379. [Google Scholar] [CrossRef]
- Varum, F.; Cristina Freire, A.; Bravo, R.; Basit, A.W. OPTICORE, an innovative and accurate colonic targeting technology. Int. J. Pharm. 2020, 583, 119372. [Google Scholar] [CrossRef]
- Moutaharrik, S.; Maroni, A.; Melocchi, A.; Zema, L.; Foppoli, A.; Cerea, M.; Palugan, L.; Neut, C.; Siepmann, F.; Siepmann, J.; et al. Oral colon delivery platform based on a novel combination approach: Design concept and preliminary evaluation. J. Drug Deliv. Sci. Technol. 2021, 66, 102919. [Google Scholar] [CrossRef]
- Moutaharrik, S.; Maroni, A.; Neut, C.; Dubuquoy, C.; Dubuquoy, L.; Foppoli, A.; Cerea, M.; Palugan, L.; Siepmann, F.; Siepmann, J.; et al. In vitro and in vivo evaluation of a pH-, microbiota- and time-based oral delivery platform for colonic release. Eur. J. Pharm. Biopharm. 2023, 183, 13–23. [Google Scholar] [CrossRef]
- Moutaharrik, S.; Meroni, G.; Soggiu, A.; Foppoli, A.; Cerea, M.; Palugan, L.; Caloni, F.; Martino, P.A.; Gazzaniga, A.; Maroni, A. Guar gum as a microbially degradable component for an oral colon delivery system based on a combination strategy: Formulation and in vitro evaluation. Drug Deliv. Transl. Res. 2024, 14, 826–838. [Google Scholar] [CrossRef]
- Ji, C.; Xu, H.; Wu, W. In vitro evaluation and pharmacokinetics in dogs of guar gum and Eudragit FS30D-coated colon-targeted pellets of indomethacin. J. Drug Target. 2007, 15, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Ji, C.; Xu, H.; Wu, W. Guar Gum as Potential Film Coating Material for Colon-specific Delivery of Fluorouracil. J. Biomater. Appl. 2009, 23, 311–329. [Google Scholar] [CrossRef] [PubMed]
- Doggwiler, V.; Lanz, M.; Paredes, V.; Lipps, G.; Imanidis, G. Tablet formulation with dual control concept for efficient colonic drug delivery. Int. J. Pharm. 2023, 631, 122499. [Google Scholar] [CrossRef] [PubMed]
- Davis, S. The design and evaluation of controlled release systems for the gastrointestinal tract. J. Control. Release 1985, 2, 27–38. [Google Scholar] [CrossRef]
- Davis, S.S.; Hardy, J.G.; Fara, J.W. Transit of pharmaceutical dosage forms through the small intestine. Gut 1986, 27, 886–892. [Google Scholar] [CrossRef] [PubMed]
- Foppoli, A.; Maroni, A.; Palugan, L.; Zema, L.; Moutaharrik, S.; Melocchi, A.; Cerea, M.; Gazzaniga, A. Erodible coatings based on HPMC and cellulase for oral time-controlled release of drugs. Int. J. Pharm. 2020, 585, 119425. [Google Scholar] [CrossRef]
- Li, J.; Peng, C.; Mao, A.; Zhong, M.; Hu, Z. An overview of microbial enzymatic approaches for pectin degradation. Int. J. Biol. Macromol. 2024, 254, 127804. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Fishman, M.L.; Kost, J.; Hicks, K.B. Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 2003, 24, 3333–3343. [Google Scholar] [CrossRef]
- Sriamornsak, P. Application of pectin in oral drug delivery. Expert Opin. Drug Deliv. 2011, 8, 1009–1023. [Google Scholar] [CrossRef]
- Tung, N.-T.; Pham, T.-M.; Nguyen, T.-H.; Pham, T.-T.; Nguyen, T.-Q. Pectin/HPMC dry powder coating formulations for colon specific targeting tablets of metronidazole. J. Drug Deliv. Sci. Technol. 2016, 33, 19–27. [Google Scholar] [CrossRef]
- Tung, N.T.; Nguyen, C.H.; Nguyen, V.D.; Nguyen, T.H.; Tran, C.S.; Pham, T.M. Formulation and in vivo imaging evaluation of colonic targeting tablets prepared by a simple dry powder coating technique. J. Pharm. Investig. 2020, 50, 383–398. [Google Scholar] [CrossRef]
- Ashford, M.; Fell, J.; Attwood, D.; Sharma, H.; Woodhead, P. Studies on pectin formulations for colonic drug delivery. J. Control. Release 1994, 30, 225–232. [Google Scholar] [CrossRef]
- Chan, S.Y.; Choo, W.S.; Young, D.J.; Loh, X.J. Pectin as a rheology modifier: Origin, structure, commercial production and rheology. Carbohydr. Polym. 2017, 161, 118–139. [Google Scholar] [CrossRef]
- Ashford, M.; Fell, J.; Attwood, D.; Sharma, H.; Woodhead, P. An evaluation of pectin as a carrier for drug targeting to the colon. J. Control. Release 1993, 26, 213–220. [Google Scholar] [CrossRef]
- Rubinstein, A.; Radai, R. In vitro and in vivo analysis of colon specificity of calcium pectinate formulations. Eur. J. Pharm. Biopharm. 1995, 41, 291–295. [Google Scholar]
- Wakerly, Z.; Fell, J.T.; Attwood, D.; Parkins, D.A. In vitro evaluation of pectin-based colonic drug delivery systems. Int. J. Pharm. 1996, 129, 73–77. [Google Scholar] [CrossRef]
- Ugurlu, T.; Turkoglu, M.; Gurer, U.S.; Akarsu, B.G. Colonic delivery of compression coated nisin tablets using pectin/HPMC polymer mixture. Eur. J. Pharm. Biopharm. 2007, 67, 202–210. [Google Scholar] [CrossRef] [PubMed]
- Yehia, S.A.; Elshafeey, A.H.; Sayed, I.; Shehata, A.H. Optimization of Budesonide Compression-Coated Tablets for Colonic Delivery. AAPS PharmSciTech 2009, 10, 147–157. [Google Scholar] [CrossRef]
- Hodges, L.; Connolly, S.; Band, J.; O’mahony, B.; Ugurlu, T.; Turkoglu, M.; Wilson, C.; Stevens, H. Scintigraphic evaluation of colon targeting pectin–HPMC tablets in healthy volunteers. Int. J. Pharm. 2009, 370, 144–150. [Google Scholar] [CrossRef]
- Wanasawas, P.; Sinchaipanid, N.; Fell, J.; Mitrevej, A. Influence of pectin and calcium pectinate films on in vitro drug release from coated theophylline pellets. J. Drug Deliv. Sci. Technol. 2013, 23, 465–470. [Google Scholar] [CrossRef]
- Krishnaiah, Y.; Satyanarayana, S.; Prasad, Y.R.; Rao, S.N. Evaluation of guar gum as a compression coat for drug targeting to colon. Int. J. Pharm. 1998, 171, 137–146. [Google Scholar] [CrossRef]
- Rosiaux, Y.; Muschert, S.; Chokshi, R.; Leclercq, B.; Siepmann, F.; Siepmann, J. Ethanol-resistant polymeric film coatings for controlled drug delivery. J. Control. Release 2013, 169, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Rosiaux, Y.; Velghe, C.; Muschert, S.; Chokshi, R.; Leclercq, B.; Siepmann, F.; Siepmann, J. Ethanol-resistant ethylcellulose/guar gum coatings—Importance of formulation parameters. Eur. J. Pharm. Biopharm. 2013, 85, 1250–1258. [Google Scholar] [CrossRef] [PubMed]
- Foppoli, A.; Cerea, M.; Palugan, L.; Zema, L.; Melocchi, A.; Maroni, A.; Gazzaniga, A. Evaluation of powder-layering vs. spray-coating techniques in the manufacturing of a swellable/erodible pulsatile delivery system. Drug Dev. Ind. Pharm. 2020, 46, 1230–1237. [Google Scholar] [CrossRef] [PubMed]
- Cerea, M.; Foppoli, A.; Palugan, L.; Melocchi, A.; Zema, L.; Maroni, A.; Gazzaniga, A. Non-uniform drug distribution matrix system (NUDDMat) for zero-order release of drugs with different solubility. Int. J. Pharm. 2020, 581, 119217. [Google Scholar] [CrossRef] [PubMed]
- Sangalli, M.E.; Maroni, A.; Zema, L.; Cerea, M.; Gazzaniga, A. ChronotopicTM Technology. In Chronopharmaceutics; Wiley: Hoboken, NJ, USA, 2009; pp. 145–163. [Google Scholar] [CrossRef]
- Quaranta, G.; Fancello, G.; Ianiro, G.; Graffeo, R.; Gasbarrini, A.; Cammarota, G.; Sanguinetti, M.; Masucci, L. Laboratory handling practice for faecal microbiota transplantation. J. Appl. Microbiol. 2020, 128, 893–898. [Google Scholar] [CrossRef]
- Ibekwe, V.C.; Fadda, H.M.; Parsons, G.E.; Basit, A.W. A comparative in vitro assessment of the drug release performance of pH-responsive polymers for ileo-colonic delivery. Int. J. Pharm. 2006, 308, 52–60. [Google Scholar] [CrossRef]




| % w/w | |
|---|---|
| Paracetamol DC | 80.0 |
| Avicel® PH-101 | 12.5 |
| Explotab® CLV | 4.5 |
| Kollidon® VA 64 | 2.0 |
| Aerosil® 200 | 0.5 |
| Magnesium stearate | 0.5 |
| Weight (mg) | 40.8 ± 1.2 |
| Height (mm) | 3.20 ± 0.05 |
| Diameter (mm) | 4.00 ± 0.01 |
| Crushing strength (N) | 47.1 ± 7.3 |
| Friability (%) | 0.23 |
| Disintegration time (min) | 4.10 ± 0.36 |
| HPMC Seal-Coating | Pectin Spray-Coating | Pectin Powder-Layering | Kollicoat® MAE Spray-Coating | |
|---|---|---|---|---|
| Equipment | Tangential-spray fluid bed | Bottom-spray fluid bed | Tangential-spray fluid bed | Bottom-spray fluid bed |
| Inlet air temperature (°C) | 60 | 60 | 60 | 60 |
| Outlet air temperature (°C) | 30–34 | 40–42 | 30–32 | - |
| Product temperature (°C) | 32–34 | 38–42 | 30–32 | 37–39 |
| Disk rotation speed (rpm) | 400 | - | 400 | - |
| Nebulization air pressure (bar) | 2 | 2 | 2 | 1.0 |
| Nozzle port diameter (mm) | 1.2 | 0.8 | 1.2 | 0.5 |
| Inlet air volume (m3/h) | 60 | 60 | 60 | 34–40 |
| Spray rate (g/min) | 11.2–11.9 | 8.5 | 5.1–13.5 | 1–1.3 |
| Powder feeding rate (g/min) | - | - | 2 | - |
| % w/w | |
|---|---|
| HM pectin | 1.74 |
| Glycerol | 0.35 |
| GMS | 0.17 |
| Tween® 80 | 0.07 |
| Deionized water | 97.67 |
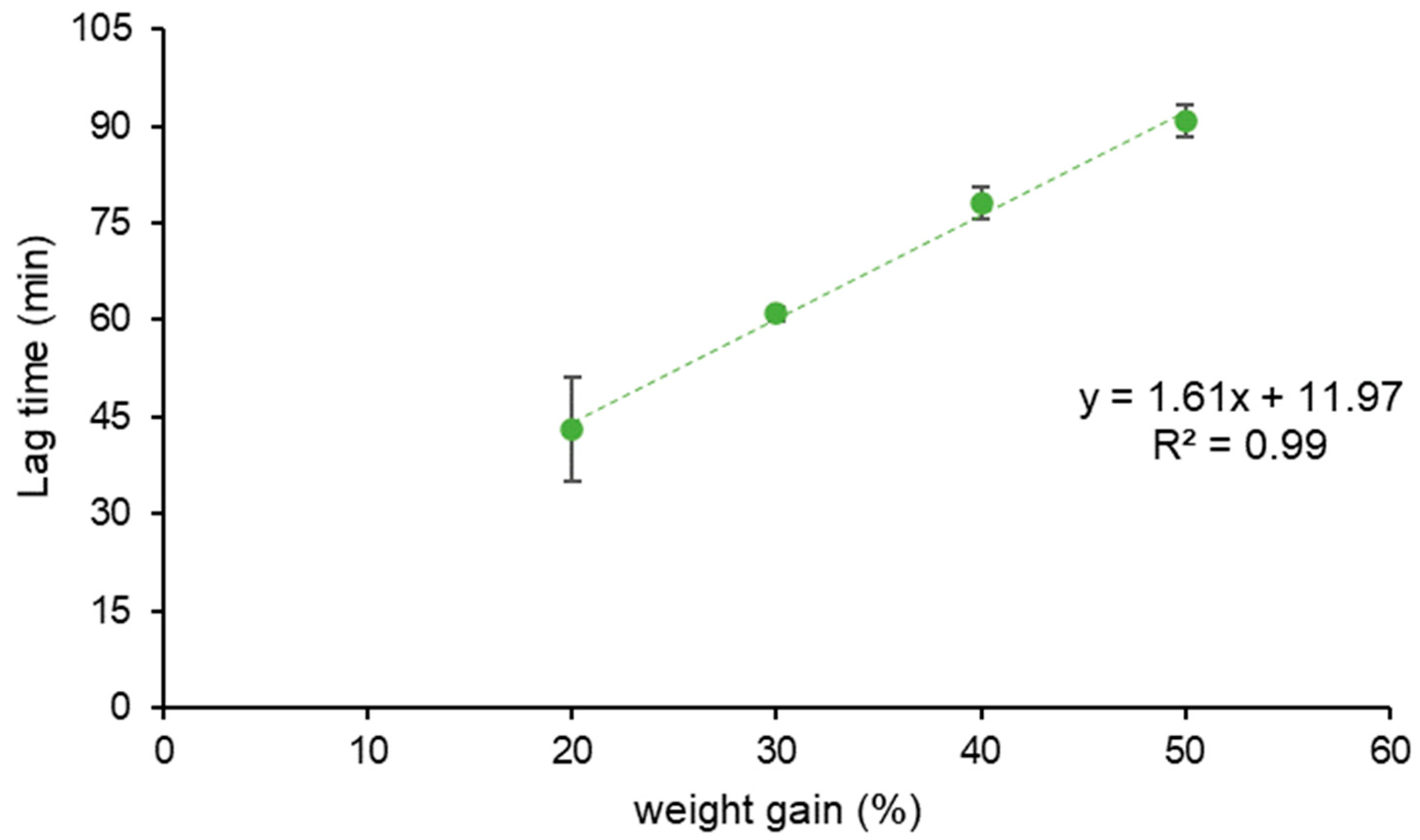
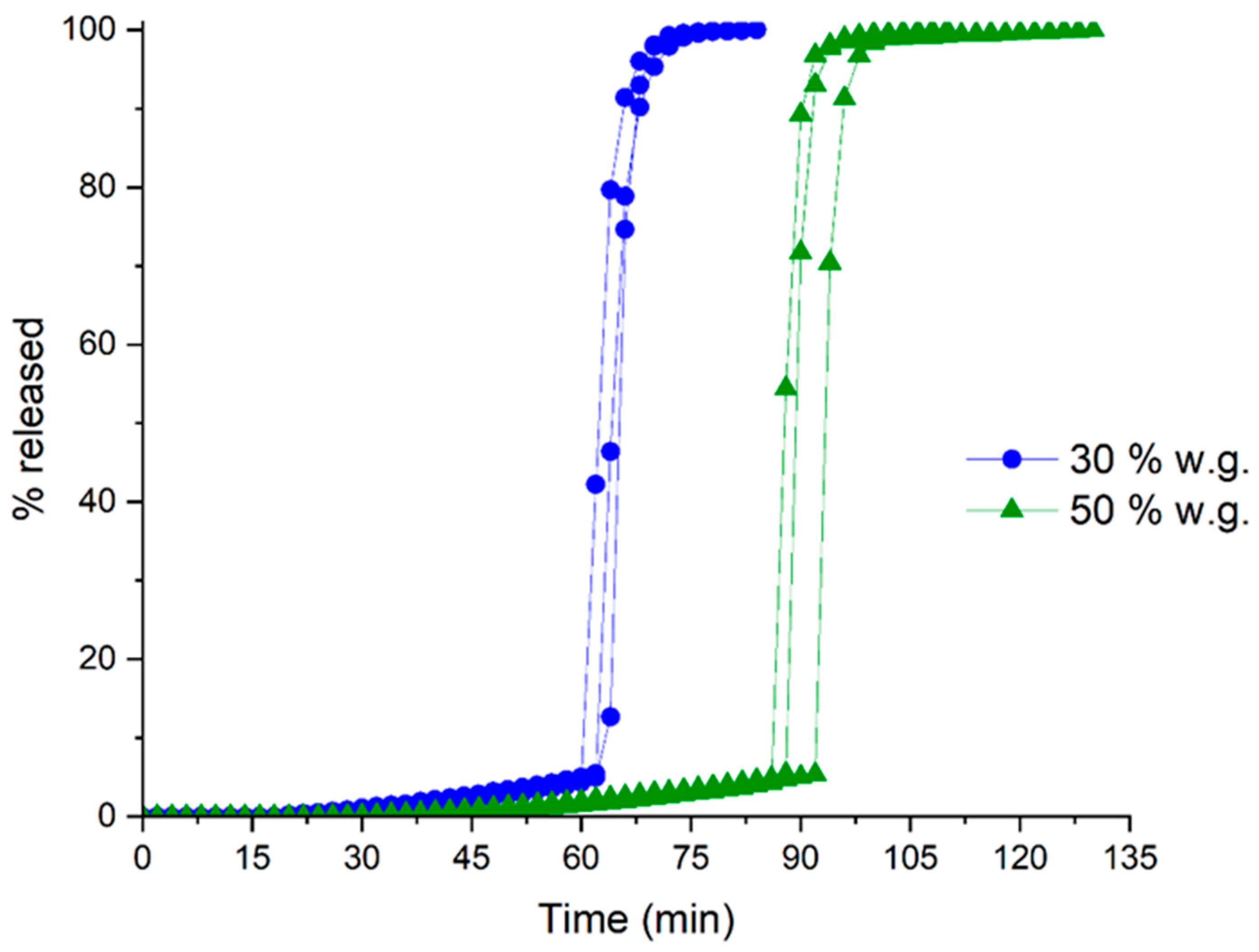
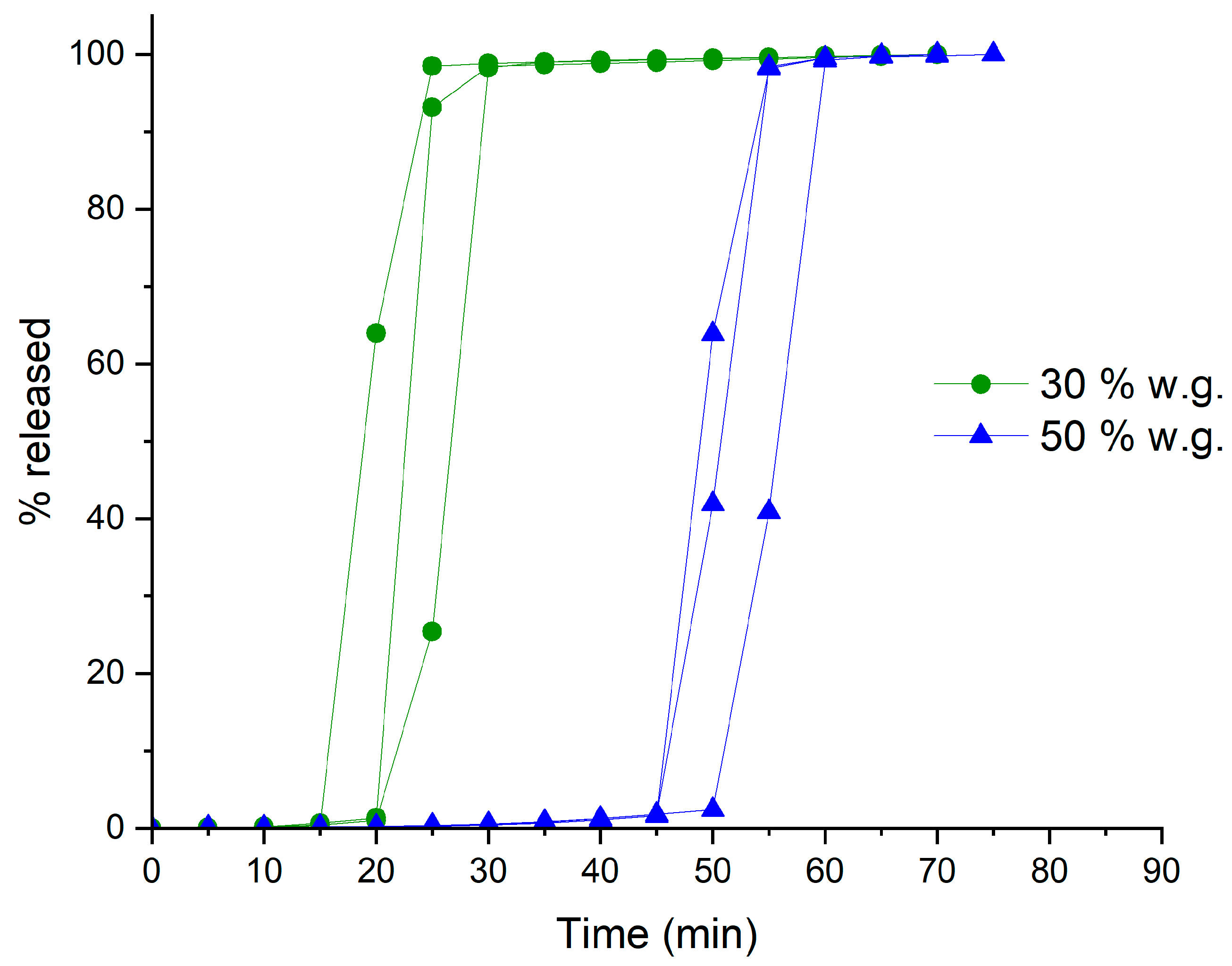
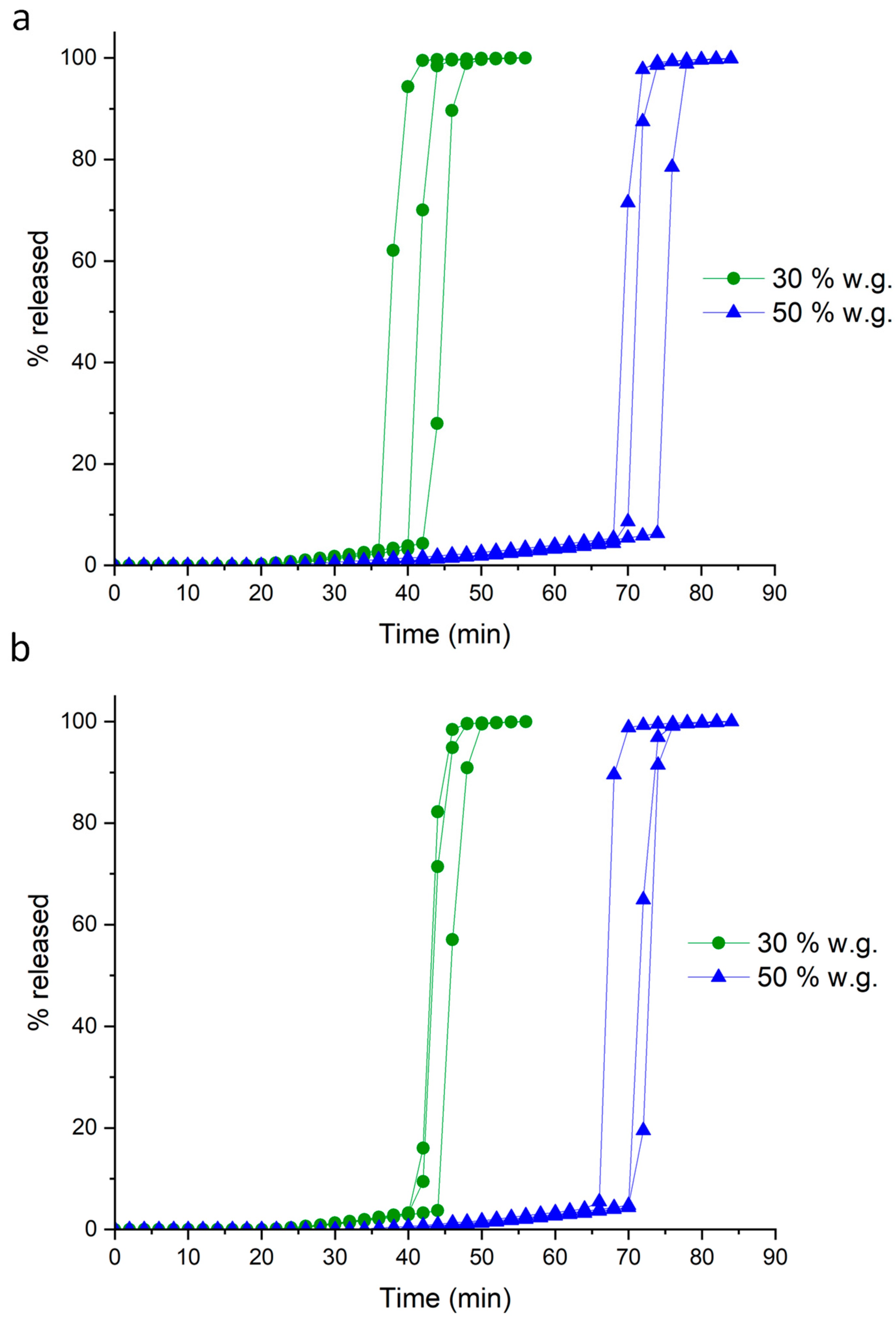
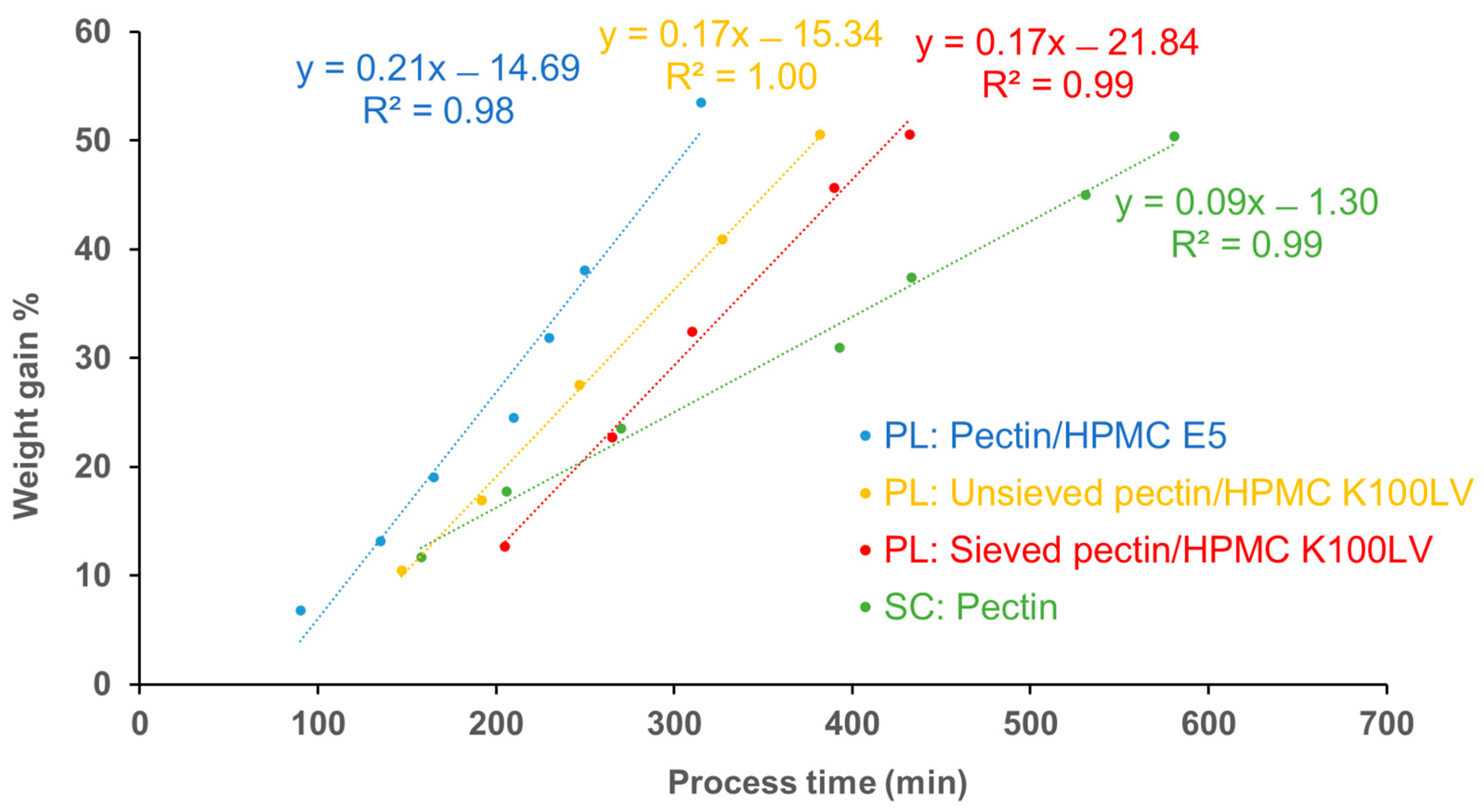
| Coating Technique | Composition | Weight Gain (%) | Amount of Coating Material Applied (mg/cm2) | Coating Thickness (µm) | t10% Mean ± SD (min) | Process Time (min) | TEAP mg/cm2·min | Coating Density g/mL | TEPP |
|---|---|---|---|---|---|---|---|---|---|
| Spray-coating | HM pectin | 28.3 | 23.6 | 148.9 | 60.9 ± 1.0 | 393 | 0.39 | 1.46 | 6.45 |
| 47.0 | 39.1 | 242.0 | 90.8 ± 2.4 | 581 | 0.43 | 1.42 | 6.40 | ||
| Powder-layering | HM pectin–MethocelTM E5 | 31.4 | 24.9 | 279.5 | 19.4 ± 3.2 | 230 | 1.28 | 0.77 | 11.80 |
| 53.5 | 42.3 | 438.5 | 47.6 ± 3.0 | 315 | 0.89 | 0.77 | 6.62 | ||
| HM pectinunsieved–MethocelTM K100LV | 30.5 | 24.3 | 312.9 | 39.6 ± 3.2 | 247 | 0.64 | 0.67 | 6.24 | |
| 50.5 | 40.3 | 457.2 | 70.8 ± 3.0 | 382 | 0.58 | 0.71 | 5.39 | ||
| HM pectinsieved–MethocelTM K100LV | 32.5 | 25.4 | 251.8 | 42.4 ± 1.6 | 272 | 0.57 | 0.89 | 6.41 | |
| 50.6 | 40.9 | 385.1 | 69.0 ± 2.5 | 432 | 0.58 | 0.87 | 6.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moutaharrik, S.; Palugan, L.; Cerea, M.; Meroni, G.; Casagni, E.; Roda, G.; Martino, P.A.; Gazzaniga, A.; Maroni, A.; Foppoli, A. Colon Drug Delivery Systems Based on Swellable and Microbially Degradable High-Methoxyl Pectin: Coating Process and In Vitro Performance. Pharmaceutics 2024, 16, 508. https://doi.org/10.3390/pharmaceutics16040508
Moutaharrik S, Palugan L, Cerea M, Meroni G, Casagni E, Roda G, Martino PA, Gazzaniga A, Maroni A, Foppoli A. Colon Drug Delivery Systems Based on Swellable and Microbially Degradable High-Methoxyl Pectin: Coating Process and In Vitro Performance. Pharmaceutics. 2024; 16(4):508. https://doi.org/10.3390/pharmaceutics16040508
Chicago/Turabian StyleMoutaharrik, Saliha, Luca Palugan, Matteo Cerea, Gabriele Meroni, Eleonora Casagni, Gabriella Roda, Piera Anna Martino, Andrea Gazzaniga, Alessandra Maroni, and Anastasia Foppoli. 2024. "Colon Drug Delivery Systems Based on Swellable and Microbially Degradable High-Methoxyl Pectin: Coating Process and In Vitro Performance" Pharmaceutics 16, no. 4: 508. https://doi.org/10.3390/pharmaceutics16040508
APA StyleMoutaharrik, S., Palugan, L., Cerea, M., Meroni, G., Casagni, E., Roda, G., Martino, P. A., Gazzaniga, A., Maroni, A., & Foppoli, A. (2024). Colon Drug Delivery Systems Based on Swellable and Microbially Degradable High-Methoxyl Pectin: Coating Process and In Vitro Performance. Pharmaceutics, 16(4), 508. https://doi.org/10.3390/pharmaceutics16040508







