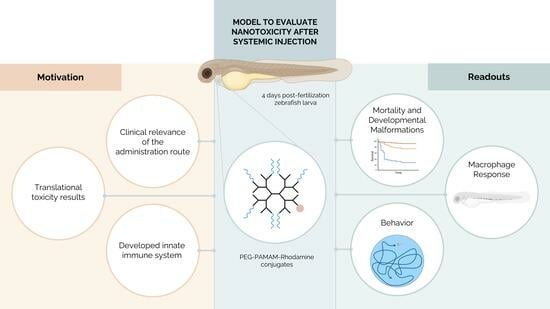
Biological Response Following the Systemic Injection of PEG–PAMAM–Rhodamine Conjugates in Zebrafish
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of PEG–PAMAM–Rho
2.2. Characterization of PEG–PAMAM–Rho
2.3. Zebrafish Models and Husbandry
2.4. Embryo and Larvae Collection and Maintenance
2.5. Nanosystem Administration
2.6. Survival Quantification
2.7. Imaging Acquisition and Processing
2.7.1. Rhodamine Intensity Quantification
2.7.2. Developmental Alteration Quantification
2.7.3. Macrophage Area Quantification
2.8. Behavioral Analysis
2.9. Statistical Analysis
3. Results
3.1. PEG–PAMAM–Rho Synthesis and Characterization
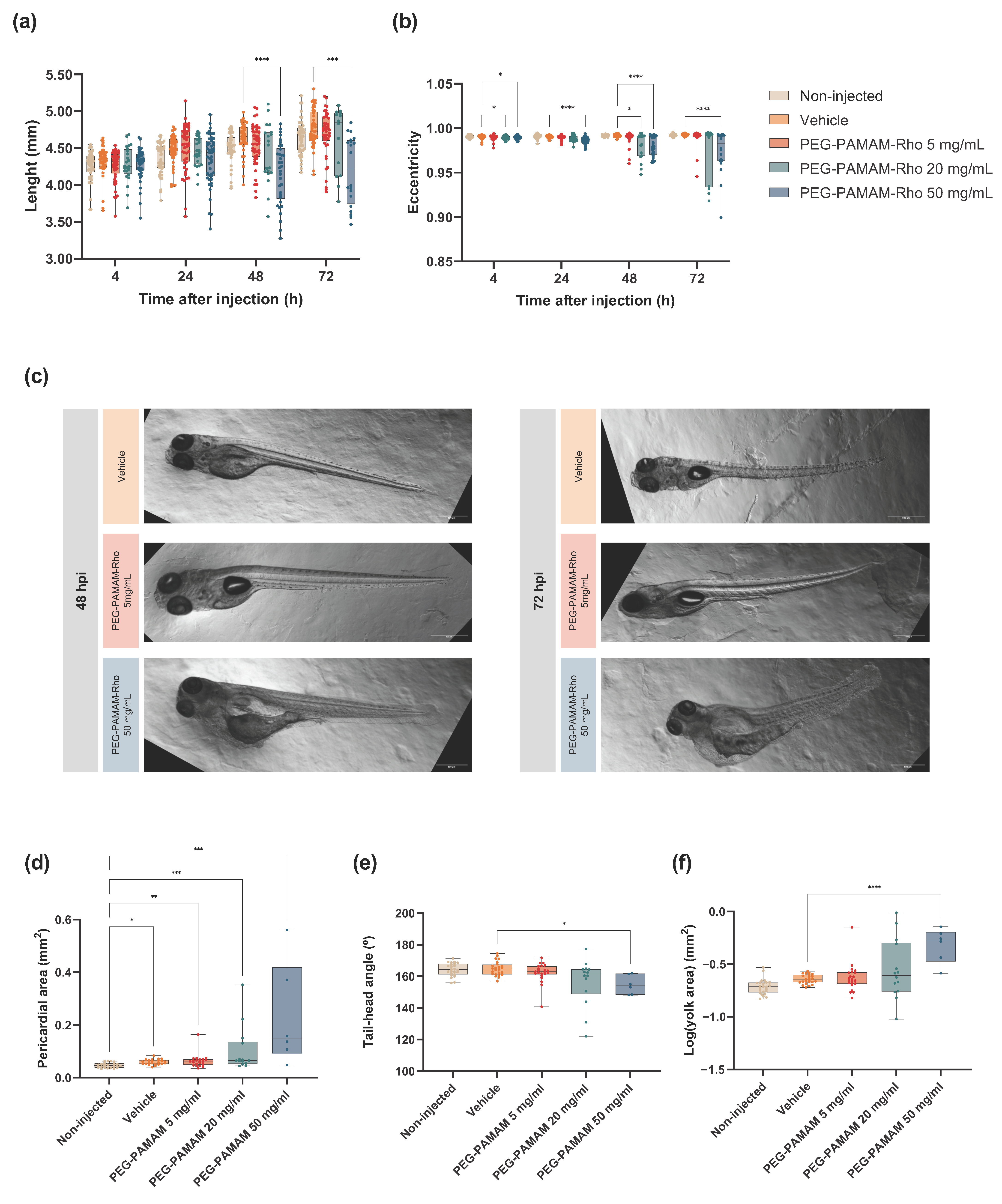
3.2. Highest Tested PEG–PAMAM–Rho Concentrations Impact the Development and Mortality after Systemic Injection in Zebrafish Larvae
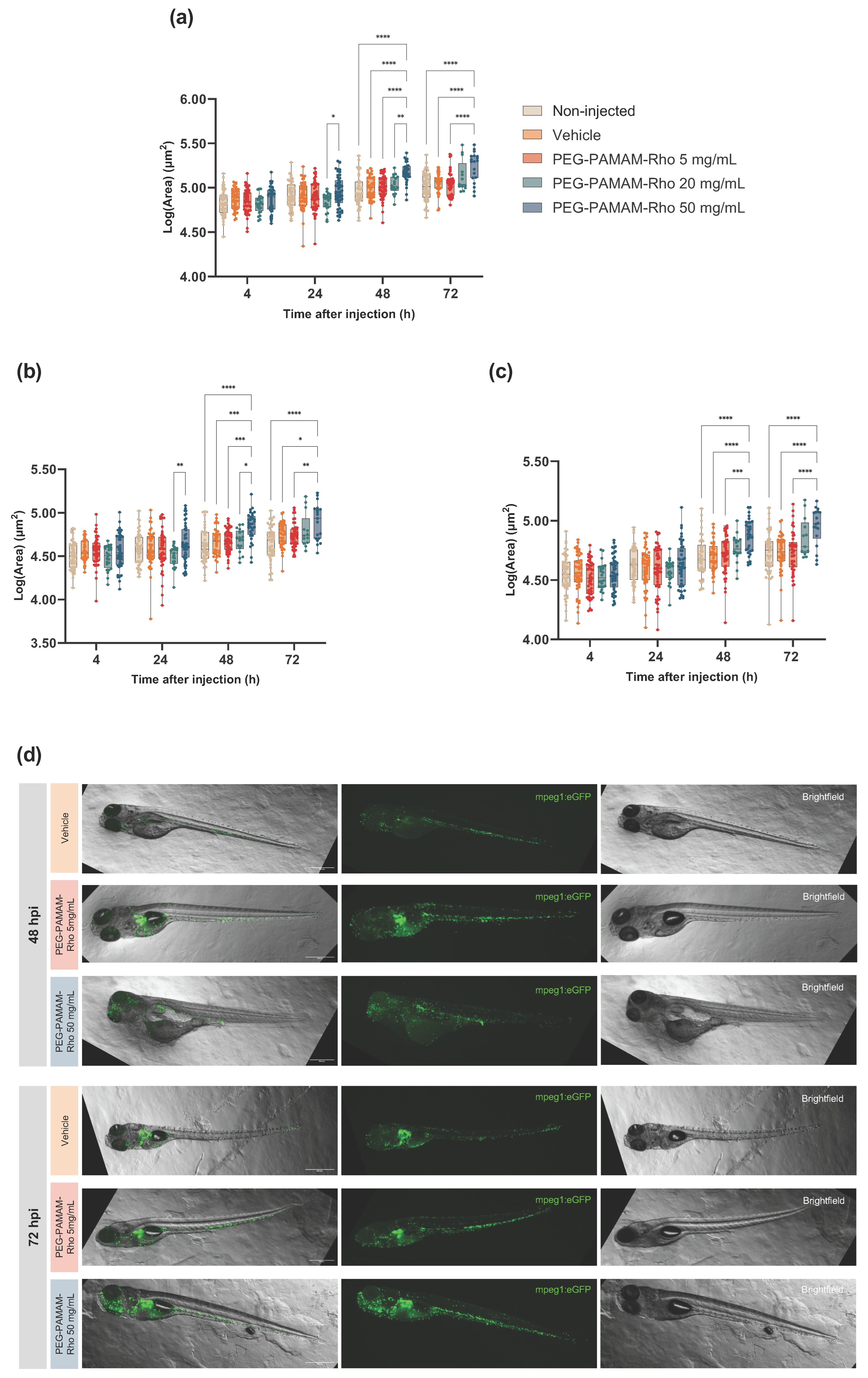
3.3. A Significant Inflammatory Response Is Observed When Higher PEG–PAMAM–Rho Dosages Are Administered
3.4. Behavior Is Not Altered for the Lowest PEG–PAMAM–Rho Formulation Tested
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gupta, D.; Gupta, S.V.; Yang, N. Understanding the Routes of Administration. In Handbook of Space Pharmaceuticals; Pathak, Y., Araújo dos Santos, M., Zea, L., Eds.; Springer International Publishing: Cham, Switzerland, 2018; pp. 1–25. [Google Scholar]
- Mitchell, M.J.; Billingsley, M.M.; Haley, R.M.; Wechsler, M.E.; Peppas, N.A.; Langer, R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021, 20, 101–124. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.P.; Torrado, M.; Custodio, B.; Silva-Reis, S.C.; Santos, S.D.; Leiro, V.; Pego, A.P. Breaking Barriers: Bioinspired Strategies for Targeted Neuronal Delivery to the Central Nervous System. Pharmaceutics 2020, 12, 192. [Google Scholar] [CrossRef] [PubMed]
- Savage, D.T.; Hilt, J.Z.; Dziubla, T.D. In Vitro Methods for Assessing Nanoparticle Toxicity. Methods Mol. Biol. 2019, 1894, 1–29. [Google Scholar] [CrossRef] [PubMed]
- Khabib, M.N.H.; Sivasanku, Y.; Lee, H.B.; Kumar, S.; Kue, C.S. Alternative animal models in predictive toxicology. Toxicology 2022, 465, 153053. [Google Scholar] [CrossRef]
- Jia, H.-R.; Zhu, Y.-X.; Duan, Q.-Y.; Chen, Z.; Wu, F.-G. Nanomaterials meet zebrafish: Toxicity evaluation and drug delivery applications. J. Control. Release 2019, 311–312, 301–318. [Google Scholar] [CrossRef] [PubMed]
- Bauer, B.; Mally, A.; Liedtke, D. Zebrafish Embryos and Larvae as Alternative Animal Models for Toxicity Testing. Int. J. Mol. Sci. 2021, 22, 13417. [Google Scholar] [CrossRef] [PubMed]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD Publishing: Paris, France, 2013. [Google Scholar] [CrossRef]
- Rothenbucher, T.S.P.; Ledin, J.; Gibbs, D.; Engqvist, H.; Persson, C.; Hulsart-Billstrom, G. Zebrafish embryo as a replacement model for initial biocompatibility studies of biomaterials and drug delivery systems. Acta Biomater. 2019, 100, 235–243. [Google Scholar] [CrossRef]
- Sipes, N.S.; Padilla, S.; Knudsen, T.B. Zebrafish—As an integrative model for twenty-first century toxicity testing. Birth Defects Res. Part C Embryo Today Rev. 2011, 93, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Cassar, S.; Adatto, I.; Freeman, J.L.; Gamse, J.T.; Iturria, I.; Lawrence, C.; Muriana, A.; Peterson, R.T.; Van Cruchten, S.; Zon, L.I. Use of Zebrafish in Drug Discovery Toxicology. Chem. Res. Toxicol. 2020, 33, 95–118. [Google Scholar] [CrossRef]
- Kadioglu, O.; Klauck, S.M.; Fleischer, E.; Shan, L.; Efferth, T. Selection of safe artemisinin derivatives using a machine learning-based cardiotoxicity platform and in vitro and in vivo validation. Arch. Toxicol. 2021, 95, 2485–2495. [Google Scholar] [CrossRef]
- Rizzo, L.Y.; Golombek, S.K.; Mertens, M.E.; Pan, Y.; Laaf, D.; Broda, J.; Jayapaul, J.; Mockel, D.; Subr, V.; Hennink, W.E.; et al. In Vivo Nanotoxicity Testing Using the Zebrafish Embryo Assay. J. Mater. Chem. B 2013, 1, 3918–3925. [Google Scholar] [CrossRef] [PubMed]
- Patton, E.E.; Zon, L.I.; Langenau, D.M. Zebrafish disease models in drug discovery: From preclinical modelling to clinical trials. Nat. Rev. Drug Discov. 2021, 20, 611–628. [Google Scholar] [CrossRef] [PubMed]
- Kislyuk, S.; Kroonen, J.; Adams, E.; Augustijns, P.; de Witte, P.; Cabooter, D. Development of a sensitive and quantitative UHPLC-MS/MS method to study the whole-body uptake of pharmaceuticals in zebrafish. Talanta 2017, 174, 780–788. [Google Scholar] [CrossRef]
- Santos, S.D.; Xavier, M.; Leite, D.M.; Moreira, D.A.; Custodio, B.; Torrado, M.; Castro, R.; Leiro, V.; Rodrigues, J.; Tomas, H.; et al. PAMAM dendrimers: Blood-brain barrier transport and neuronal uptake after focal brain ischemia. J. Control. Release 2018, 291, 65–79. [Google Scholar] [CrossRef] [PubMed]
- Leiro, V.; Spencer, A.P.; Magalhaes, N.; Pego, A.P. Versatile fully biodegradable dendritic nanotherapeutics. Biomaterials 2022, 281, 121356. [Google Scholar] [CrossRef] [PubMed]
- Spencer, A.P.; Leiro, V.; Pego, A.P. Unravelling the interactions of biodegradable dendritic nucleic acid carriers and neural cells. Biomater. Sci. 2023, 11, 1499–1516. [Google Scholar] [CrossRef] [PubMed]
- Leiro, V.; Santos, S.D.; Lopes, C.D.F.; Pêgo, A.P. Dendrimers as Powerful Building Blocks in Central Nervous System Disease: Headed for Successful Nanomedicine. Adv. Funct. Mater. 2018, 28, 1700313. [Google Scholar] [CrossRef]
- Wang, J.; Li, B.; Qiu, L.; Qiao, X.; Yang, H. Dendrimer-based drug delivery systems: History, challenges, and latest developments. J. Biol. Eng. 2022, 16, 18. [Google Scholar] [CrossRef]
- Tomalia, D.A.; Baker, H.; Dewald, J.; Hall, M.; Kallos, G.; Martin, S.; Roeck, J.; Ryder, J.; Smith, P. A New Class of Polymers—Starburst-Dendritic Macromolecules. Polym. J. 1985, 17, 117–132. [Google Scholar] [CrossRef]
- Araujo, R.V.; Santos, S.D.S.; Ferreira, E.I.; Giarolla, J. New Advances in General Biomedical Applications of PAMAM Dendrimers. Molecules 2018, 23, 2849. [Google Scholar] [CrossRef]
- Duncan, R.; Izzo, L. Dendrimer biocompatibility and toxicity. Adv. Drug Deliv. Rev. 2005, 57, 2215–2237. [Google Scholar] [CrossRef] [PubMed]
- Naha, P.C.; Mukherjee, S.P.; Byrne, H.J. Toxicology of Engineered Nanoparticles: Focus on Poly(amidoamine) Dendrimers. Int. J. Environ. Res. Public Health 2018, 15, 338. [Google Scholar] [CrossRef] [PubMed]
- Jones, C.F.; Campbell, R.A.; Brooks, A.E.; Assemi, S.; Tadjiki, S.; Thiagarajan, G.; Mulcock, C.; Weyrich, A.S.; Brooks, B.D.; Ghandehari, H.; et al. Cationic PAMAM dendrimers aggressively initiate blood clot formation. ACS Nano 2012, 6, 9900–9910. [Google Scholar] [CrossRef] [PubMed]
- Owen, J.P.; Kelsh, R.N. A suitable anaesthetic protocol for metamorphic zebrafish. PLoS ONE 2021, 16, e0246504. [Google Scholar] [CrossRef] [PubMed]
- Raghunath, A.; Perumal, E. Analysis of Lethality and Malformations During Zebrafish (Danio rerio) Development. Methods Mol. Biol. 2018, 1797, 337–363. [Google Scholar] [CrossRef] [PubMed]
- CellProfiler Manual. Measurement. Available online: https://cellprofiler-manual.s3.amazonaws.com/CellProfiler-3.0.0/modules/measurement.html (accessed on 22 March 2024).
- Igartua, D.E.; Martinez, C.S.; Temprana, C.F.; Alonso, S.D.; Prieto, M.J. PAMAM dendrimers as a carbamazepine delivery system for neurodegenerative diseases: A biophysical and nanotoxicological characterization. Int. J. Pharm. 2018, 544, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Bodewein, L.; Schmelter, F.; Di Fiore, S.; Hollert, H.; Fischer, R.; Fenske, M. Differences in toxicity of anionic and cationic PAMAM and PPI dendrimers in zebrafish embryos and cancer cell lines. Toxicol. Appl. Pharmacol. 2016, 305, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Nasr, G.; Daakour, S.; Holl, M.M.B.; Attallah, C.; Hamade, A.; Greige-Gerges, H. p-Coumaric acid in poly(amidoamine) G4 dendrimer: Characterization and toxicity evaluation on zebrafish model. J. Drug Deliv. Sci. Technol. 2023, 79, 104039. [Google Scholar] [CrossRef]
- Igartua, D.E.; Martinez, C.S.; Alonso, S.D.; Chiaramoni, N.S.; Prieto, M.J. Toxicity assessment of free and dendrimer-complexed curcumin in zebrafish larvae. Pharmanutrition 2020, 13, 100201. [Google Scholar] [CrossRef]
- Crecente-Campo, J.; Guerra-Varela, J.; Peleteiro, M.; Gutiérrez-Lovera, C.; Fernández-Mariño, I.; Diéguez-Docampo, A.; González-Fernández, Á.; Sánchez, L.; Alonso, M.J. The size and composition of polymeric nanocapsules dictate their interaction with macrophages and biodistribution in zebrafish. J. Control. Release 2019, 308, 98–108. [Google Scholar] [CrossRef]
- Lam, S.H.; Chua, H.L.; Gong, Z.; Lam, T.J.; Sin, Y.M. Development and maturation of the immune system in zebrafish, Danio rerio: A gene expression profiling, in situ hybridization and immunological study. Dev. Comp. Immunol. 2004, 28, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.Y.; Jang, G.H.; Byun, C.H.; Jeun, M.; Searson, P.C.; Lee, K.H. Zebrafish models for functional and toxicological screening of nanoscale drug delivery systems: Promoting preclinical applications. Biosci. Rep. 2017, 37, BSR20170199. [Google Scholar] [CrossRef] [PubMed]
- Valentim, A.M. Behavioral Profiling of Zebrafish (Danio rerio) Larvae: Activity, Anxiety, Avoidance, and Startle Response. Methods Mol. Biol. 2024, 2753, 421–446. [Google Scholar] [CrossRef] [PubMed]
- Basnet, R.M.; Zizioli, D.; Taweedet, S.; Finazzi, D.; Memo, M. Zebrafish Larvae as a Behavioral Model in Neuropharmacology. Biomedicines 2019, 7, 23. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Muhammad, K.; Waheed, Y. Emerging Applications of Nanotechnology in Healthcare and Medicine. Molecules 2023, 28, 6624. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, U.; Taccone, M.S.; Kumar, S.A.; Okura, H.; Krumholtz, S.; Ishida, J.; Mine, C.; Gouveia, K.; Edgar, J.; Smith, C.; et al. Modeling human brain tumors in flies, worms, and zebrafish: From proof of principle to novel therapeutic targets. Neuro Oncol. 2021, 23, 718–731. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Grossen, P.; Bussmann, J.; Campbell, F.; Kros, A.; Witzigmann, D.; Huwyler, J. Zebrafish as a preclinical in vivo screening model for nanomedicines. Adv. Drug Deliv. Rev. 2019, 151, 152–168. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Lee, S.S. Zebrafish: A complete animal model to enumerate the nanoparticle toxicity. J. Nanobiotechnol. 2016, 14, 65. [Google Scholar] [CrossRef] [PubMed]
- Harper, B.; Thomas, D.; Chikkagoudar, S.; Baker, N.; Tang, K.; Heredia-Langner, A.; Lins, R.; Harper, S. Comparative hazard analysis and toxicological modeling of diverse nanomaterials using the embryonic zebrafish (EZ) metric of toxicity. J. Nanopart Res. 2015, 17, 250. [Google Scholar] [CrossRef]
- Martinez, C.S.; Igartúa, D.E.; Calienni, M.N.; Feas, D.A.; Siri, M.; Montanari, J.; Chiaramoni, N.S.; Alonso, S.d.V.; Prieto, M.J. Relation between biophysical properties of nanostructures and their toxicity on zebrafish. Biophys. Rev. 2017, 9, 775–791. [Google Scholar] [CrossRef]
- Luong, D.; Kesharwani, P.; Deshmukh, R.; Amin, M.C.I.M.; Gupta, U.; Greish, K.; Iyer, A.K. PEGylated PAMAM dendrimers: Enhancing efficacy and mitigating toxicity for effective anticancer drug and gene delivery. Acta Biomater. 2016, 43, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Johnston, B.M.; Grodzinsky, A.J.; Hammond, P.T. Charge shielding effects of PEG bound to NH-terminated PAMAM dendrimers—An experimental approach. Soft Matter 2023, 19, 3033–3046. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.; Zhang, J.; Zhao, M.; Tang, S.; Cheng, X.; Zhang, W.; Li, W.; Liu, X.; Peng, H.; Wang, Q. Effects of polyethylene glycol on the surface of nanoparticles for targeted drug delivery. Nanoscale 2021, 13, 10748–10764. [Google Scholar] [CrossRef] [PubMed]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Fleming, A.; Diekmann, H.; Goldsmith, P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS ONE 2013, 8, e77548. [Google Scholar] [CrossRef] [PubMed]
- Paatero, I.; Casals, E.; Niemi, R.; Özliseli, E.; Rosenholm, J.M.; Sahlgren, C. Analyses in zebrafish embryos reveal that nanotoxicity profiles are dependent on surface-functionalization controlled penetrance of biological membranes. Sci. Rep. 2017, 7, 8423. [Google Scholar] [CrossRef] [PubMed]
- Calienni, M.N.; Feas, D.A.; Igartúa, D.E.; Chiaramoni, N.S.; Alonso, S.d.V.; Prieto, M.J. Nanotoxicological and teratogenic effects: A linkage between dendrimer surface charge and zebrafish developmental stages. Toxicol. Appl. Pharm. 2017, 337, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Heiden, T.C.; Dengler, E.; Kao, W.J.; Heideman, W.; Peterson, R.E. Developmental toxicity of low generation PAMAM dendrimers in zebrafish. Toxicol. Appl. Pharmacol. 2007, 225, 70–79. [Google Scholar] [CrossRef] [PubMed]
- Mathias, J.R.; Dodd, M.E.; Walters, K.B.; Yoo, S.K.; Ranheim, E.A.; Huttenlocher, A. Characterization of zebrafish larval inflammatory macrophages. Dev. Comp. Immunol. 2009, 33, 1212–1217. [Google Scholar] [CrossRef]
- Renshaw, S.A.; Trede, N.S. A model 450 million years in the making: Zebrafish and vertebrate immunity. Dis. Model. Mech. 2012, 5, 38–47. [Google Scholar] [CrossRef]
- Campbell, F.; Bos, F.L.; Sieber, S.; Arias-Alpizar, G.; Koch, B.E.; Huwyler, J.; Kros, A.; Bussmann, J. Directing Nanoparticle Biodistribution through Evasion and Exploitation of Stab2-Dependent Nanoparticle Uptake. ACS Nano 2018, 12, 2138–2150. [Google Scholar] [CrossRef] [PubMed]
- Pensado-Lopez, A.; Fernandez-Rey, J.; Reimunde, P.; Crecente-Campo, J.; Sanchez, L.; Torres Andon, F. Zebrafish Models for the Safety and Therapeutic Testing of Nanoparticles with a Focus on Macrophages. Nanomaterials 2021, 11, 1784. [Google Scholar] [CrossRef] [PubMed]
- Herbomel, P.; Thisse, B.; Thisse, C. Ontogeny and behaviour of early macrophages in the zebrafish embryo. Development 1999, 126, 3735–3745. [Google Scholar] [CrossRef]
- Gore, A.V.; Pillay, L.M.; Venero Galanternik, M.; Weinstein, B.M. The zebrafish: A fintastic model for hematopoietic development and disease. Wiley Interdiscip. Rev. Dev. Biol. 2018, 7, e312. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yin, J.; Liang, Y.; Yuan, S.; Wang, F.; Song, M.; Wang, H. Oxidative stress and immunotoxicity induced by graphene oxide in zebrafish. Aquat. Toxicol. 2016, 174, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Sieber, S.; Grossen, P.; Uhl, P.; Detampel, P.; Mier, W.; Witzigmann, D.; Huwyler, J. Zebrafish as a predictive screening model to assess macrophage clearance of liposomes. Nanomed. Nanotechnol. 2019, 17, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Prieto, M.J.; del Rio Zabala, N.E.; Marotta, C.H.; Gutierrez, H.C.; Arevalo, R.A.; Chiaramoni, N.S.; del Valle Alonso, S. Optimization and in vivo toxicity evaluation of G4.5 PAMAM dendrimer-risperidone complexes. PLoS ONE 2014, 9, e90393. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, E.; Casado, M.; Faria, M.; Soares, A.M.; Navas, J.M.; Barata, C.; Pina, B. Transcriptomic response of zebrafish embryos to polyaminoamine (PAMAM) dendrimers. Nanotoxicology 2014, 8 (Suppl. S1), 92–99. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Inoue, A.; Sasagawa, S.; Koiwa, J.; Kawaguchi, K.; Kawase, R.; Maruyama, T.; Kim, S.; Tanaka, T. Using zebrafish in systems toxicology for developmental toxicity testing. Congenit. Anom. 2016, 56, 18–27. [Google Scholar] [CrossRef]
- Leiro, V.; Santos, S.D.; Pego, A.P. Delivering siRNA with Dendrimers: In Vivo Applications. Curr. Gene Ther. 2017, 17, 105–119. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Custódio, B.; Carneiro, P.; Marques, J.; Leiro, V.; Valentim, A.M.; Sousa, M.; Santos, S.D.; Bessa, J.; Pêgo, A.P. Biological Response Following the Systemic Injection of PEG–PAMAM–Rhodamine Conjugates in Zebrafish. Pharmaceutics 2024, 16, 608. https://doi.org/10.3390/pharmaceutics16050608
Custódio B, Carneiro P, Marques J, Leiro V, Valentim AM, Sousa M, Santos SD, Bessa J, Pêgo AP. Biological Response Following the Systemic Injection of PEG–PAMAM–Rhodamine Conjugates in Zebrafish. Pharmaceutics. 2024; 16(5):608. https://doi.org/10.3390/pharmaceutics16050608
Chicago/Turabian StyleCustódio, Beatriz, Patrícia Carneiro, Joana Marques, Victoria Leiro, Ana M. Valentim, Mafalda Sousa, Sofia D. Santos, José Bessa, and Ana P. Pêgo. 2024. "Biological Response Following the Systemic Injection of PEG–PAMAM–Rhodamine Conjugates in Zebrafish" Pharmaceutics 16, no. 5: 608. https://doi.org/10.3390/pharmaceutics16050608
APA StyleCustódio, B., Carneiro, P., Marques, J., Leiro, V., Valentim, A. M., Sousa, M., Santos, S. D., Bessa, J., & Pêgo, A. P. (2024). Biological Response Following the Systemic Injection of PEG–PAMAM–Rhodamine Conjugates in Zebrafish. Pharmaceutics, 16(5), 608. https://doi.org/10.3390/pharmaceutics16050608