Challenges in Optimizing Nanoplatforms Used for Local and Systemic Delivery in the Oral Cavity
Abstract
:1. Introduction
Principles of Release of Active Substances into the Oral Cavity
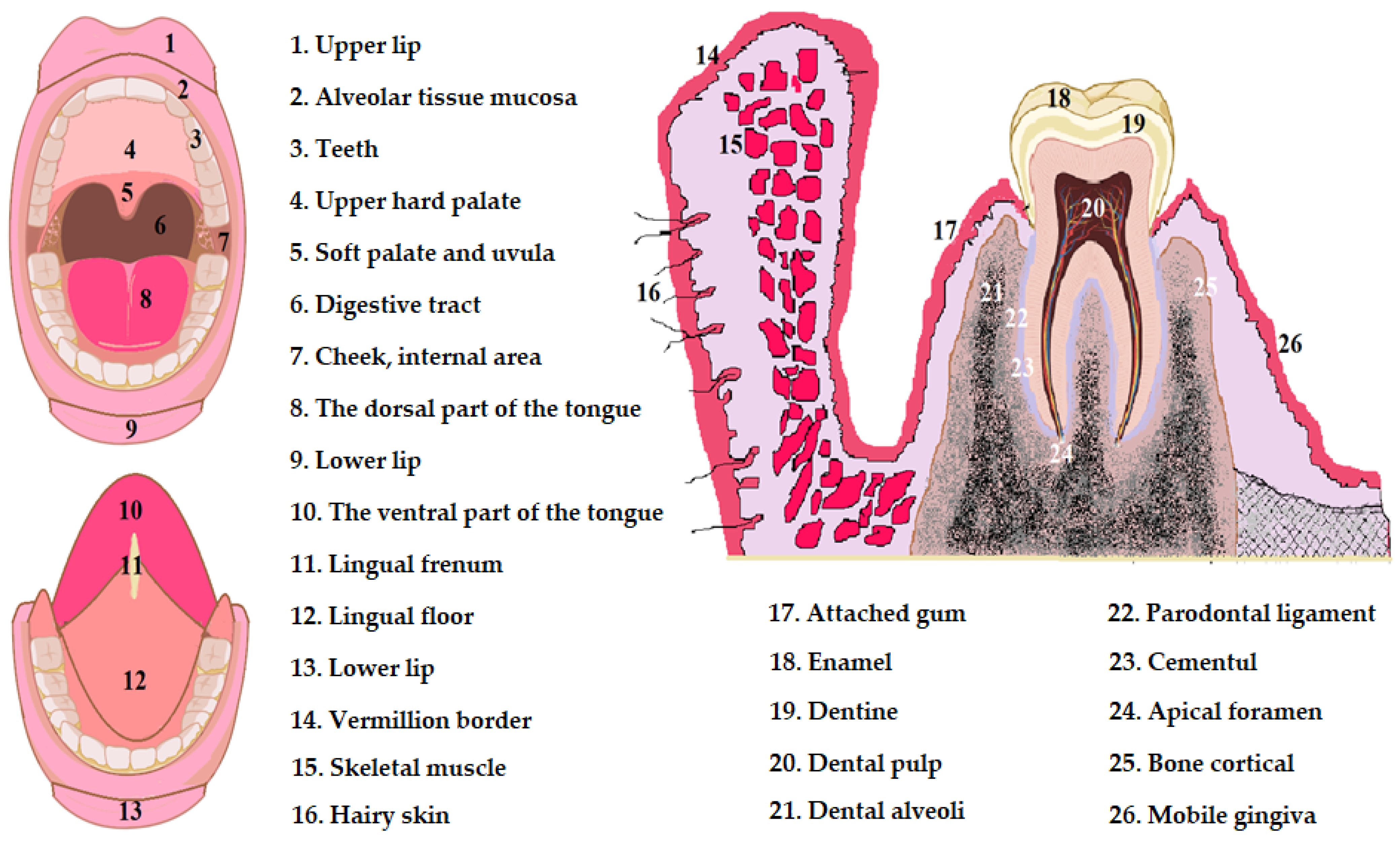
2. Physiology of Oral Cavity Pathology
- -
- The stratum basale, also called the stratum germinativum, is the deepest and is arranged on the basement membrane. This layer consists of two tall rows of cells with cuboidal or prismatic shape. These cells have intensely colored voluminous nuclei located in the basal third of the cells. Basal cells exhibit intense metabolism and frequent mitoses that provide flaking turnovers [35,36,37,38];
- -
- The stratum spinosum is arranged above the basal layer and presents 7–8 rows of well-defined polyhedral cells with visible intercellular spaces; between the cells of the stratum spinosum there are fine cytoplasmic filaments arranged in the form of thorns. The nuclei of the cells of the spinosum stratum stain less intensely than the nuclei of the cells of the stratum basale, so that the stratum spinosum is well delineated from the stratum basale. The cells in the deep areas of the stratum spinosum show mitosis and together with the basal cells form the germinative zone of the epithelium, and towards the surface, the cells of the stratum spinosum become flatter [35,36,37,38];
- -
- -
- The stratum corneum, also called the keratinized layer, is not always present in the mucosa and has an unstructured appearance composed of overlapping layers of keratin. The cells of the stratum corneum are flattened and degenerated with nuclear pycnosis, or without nuclei, they are weakly joined to each other. The epithelium is separated from the dermis by the basement membrane, which presents itself as a condensation of the fundamental substance of the underlying connective tissue [35,36,37,38].
3. Controlled Releasing Systems Used in the Oral Cavity
3.1. Fast-Dissolving Oral Films (FDOF)
3.2. Intraoral Mucoadhesive Systems
| Failure System | Place of Action | Active Substance | Release Principle of the Active Substance | Local (LE)/Systemic (SE) Effect | References |
|---|---|---|---|---|---|
| Iontophoretic patches | Oral cavity | Lidocaine, prilocaine, chlorhexidine dexamethasone | Transmucosal absorption from patch having 3 layers (mucoadhesive, wrapping, and release) | LE | do Coutoet al., 2021 [12], Ren et al., 2016 [3] |
| Microneedles | Oral cavity | Human insulin | Macromolecule releasing | SE | González-Moles et al., 2021 [88] |
| Microneedles | Oral cavity | Human growth hormone | Macromolecule releasing | SE | González-Moles et al., 2021 [88] |
| Microcapsules | Oral cavity | Antibiotics, ciprofloxacin | Disperse in auxiliary substance | LE/SE | Drucker et al., 2020 [71] |
| Microcapsules | Root canal | Minocycline | Microcapsules (ionic gelling) having as polymers alginate or chitosan | LE | Duque et al., 2019 [72] |
| Microcapsules | Periodontal regeneration defects | Tetracycline + lovastatin | Microcapsules (based on chitosan) | LE/SE | Lee et al., 2016 [26] |
| Microcapsules | Post-surgical dental pain | Naproxen | Capsules with submicron particles (phase 2 study) | LE | Weisman et al., 2021 [97] |
| Microparticles with semisolid formulation | Subgingival in paradontal pouch | Propolis | Spray drying (yielding in about 7 days), polymer used gelatin | LE | Sahu et al., 2023 [98] |
| Mucoadhesive gels | Oral mucosa | Curcumin | Mucoadhesive polymer platforms useful in precancerous lesions | LE | Agarwal et al., 2015 [99] |
| Mucoadhesive patches with botanical extracts named Perio-patch | Oral mucosa | Herbal extract | Transmucosal absorption | LE | Neagu et al., 2023 [100] |
| IntelliDrugs | Teeth | Naltrexone, codeine, diazepam | Drug reservoir | LE | Handler et al., 2019 [101] |
| Mucoadhesives | Whole oral cavity | Domperidone maleate | Gum | SE | Lopes et al., 2015 [93] |
4. Potential Optimizations of Nanoplatforms with Intra- and Extraoral Use
4.1. Optimization of Transdermal Systems in Neuropathic/Neuralgic Pain
4.2. Optimization of Transdermal Systems in Temporomandibular Joint Osteoarthritis
4.3. Optimization of Transmucosal Controlled Failure Systems in Oral Cancer
4.4. Optimization of Transmucosal Controlled Release Systems in Periodontal Pockets
4.5. Optimization of Transmucosal Controlled Release Systems in Pericoronitis
4.6. Optimization of Copolymer Membranes Loaded with Fluorine Nanoparticles and Essential Oils in Maintaining Oral Health (Dental Caries, Lesions in the Oral Cavity)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tewabe, A.; Abate, A.; Tamrie, M.; Seyfu, A.; Abdela Siraj, E. Targeted Drug Delivery—From Magic Bullet to Nanomedicine: Principles, Challenges, and Future Perspectives. J. Multidiscip. Healthc. 2021, 14, 1711–1724. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Traynor, K. Targeted drug therapy remains a challenge. Am. J. Health-Syst. Pharm. 2011, 68, 2320–2324. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Baig, A.; White, D.J.; Li, S.K. Characterization of cornified oral mucosa for iontophoretically enhanced delivery of chlorhexidine. Eur. J. Pharm. Biopharm. 2016, 99, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Menzel, C.; Bonengel, S.; Pereira de Sousa, I.; Laffleur, F.; Prüfert, F.; Bernkop-Schnürch, A. Preactivated thiolated nanoparticles: A novel mucoadhesive dosage form. Int. J. Pharm. 2016, 497, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Jamaledin, R.; Yiu, C.K.Y.; Zare, E.N.; Niu, L.N.; Vecchione, R.; Chen, G.; Gu, Z.; Tay, F.R.; Makvandi, P. Advances in Antimicrobial Microneedle Patches for Combating Infections. Adv. Mater. 2020, 32, e2002129. [Google Scholar] [CrossRef] [PubMed]
- Grimaudo, M.A.; Concheiro, A.; Alvarez-Lorenzo, C. Nanogels for regenerative medicine. J. Control Release 2019, 313, 148–160. [Google Scholar] [CrossRef] [PubMed]
- Su, H.; Wang, Y.; Liu, S.; Wang, Y.; Liu, Q.; Liu, G.; Chen, Q. Emerging transporter-targeted nanoparticulate drug delivery systems. Acta Pharm. Sin. B 2019, 9, 49–58. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Tay, F.R.; Niu, L.N.; Chen, J.H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral. Sci. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Liang, J.; Peng, X.; Zhou, X.; Zou, J.; Cheng, L. Emerging Applications of Drug Delivery Systems in Oral Infectious Diseases Prevention and Treatment. Molecules 2020, 25, 516. [Google Scholar] [CrossRef] [PubMed]
- Günzel, D. Claudins: Vital partners in transcellular and paracellular transport coupling. Pflugers Arch. 2017, 469, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Barbara, G.; Barbaro, M.R.; Fuschi, D.; Palombo, M.; Falangone, F.; Cremon, C.; Marasco, G.; Stanghellini, V. Inflammatory and Microbiota-Related Regulation of the Intestinal Epithelial Barrier. Front. Nutr. 2021, 8, 718356. [Google Scholar] [CrossRef]
- do Couto, R.O.; Cubayachi, C.; Duarte, M.P.F.; Lopez, R.F.V.; Pedrazzi, V.; De Gaitani, C.M.; de Freitas, O. Towards the advance of a novel iontophoretic patch for needle-free buccal anesthesia. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 122, 111778. [Google Scholar] [CrossRef]
- Kováčik, A.; Kopečná, M.; Vávrová, K. Permeation enhancers in transdermal drug delivery: Benefits and limitations. Expert. Opin. Drug Deliv. 2020, 17, 145–155. [Google Scholar] [CrossRef]
- He, M.; Zhu, L.; Yang, N.; Li, H.; Yang, Q. Recent advances of ora film as platform for drug delivery. Int. J. Pharm. 2021, 604, 120759. [Google Scholar] [CrossRef]
- Chen, J.; Jiang, Q.-D.; Wu, Y.-M.; Liu, P.; Yao, J.-H.; Lu, Q.; Zhang, H.; Duan, J.-A. Potential of Essential Oils as Penetration Enhancers for Transdermal Administration of Ibuprofen to Treat Dysmenorrhoea. Molecules 2015, 20, 18219–18236. [Google Scholar] [CrossRef]
- Li, X.J.; Li, Y.; Meng, Y.; Pu, X.Q.; Qin, J.W.; Xie, R.; Wang, W.; Liu, Z.; Jiang, L.; Ju, X.J.; et al. Composite dissolvable microneedle patch for therapy of oral mucosal diseases. Biomater. Adv. 2022, 139, 213001. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Min, H.S.; Jang, M.; Kang, G.; Gong, S.; Lee, C.; Song, Y.W.; Jung, U.W.; Lee, S.; Ryu, H.Y.; et al. Lidocaine-loaded dissolving microneedle for safe local anesthesia on oral mucosa for dental procedure. Expert. Opin. Drug Deliv. 2023, 20, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Bilal, M.; Mehmood, S.; Raza, A.; Hayat, U.; Rasheed, T.; Iqbal, H.M.N. Microneedles in Smart Drug Delivery. Adv. Wound Care 2021, 10, 204–219. [Google Scholar] [CrossRef]
- Chen, W.; Cai, B.; Geng, Z.; Chen, F.; Wang, Z.; Wang, L.; Chen, X. Reducing False Negatives in COVID-19 Testing by Using Microneedle-Based Oropharyngeal Swabs. Matter 2020, 3, 1589–1600. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pham, M.N.; Van Vo, T.; Tran, V.T.; Tran, P.H.; Tran, T.T. Microemulsion-Based Mucoadhesive Buccal Wafers: Wafer Formation, In Vitro Release, and Ex Vivo Evaluation. AAPS PharmSciTech 2017, 18, 2727–2736. [Google Scholar] [CrossRef] [PubMed]
- Banakar, M.; Moayedi, S.; Shamsoddin, E.; Vahedi, Z.; Banakar, M.H.; Mousavi, S.M.; Rokaya, D.; Bagheri Lankarani, K. Chewing Gums as a Drug Delivery Approach for Oral Health. Int. J. Dent. 2022, 2022, 9430988. [Google Scholar] [CrossRef]
- Pickering, G.; Lucchini, C. Topical Treatment of Localized Neuropathic Pain in the Elderly. Drugs Aging 2020, 37, 83–89. [Google Scholar] [CrossRef] [PubMed]
- Tsai, J.H.; Liu, I.T.; Su, P.F.; Huang, Y.T.; Chiu, G.L.; Chen, Y.Y.; Lai, W.S.; Lin, P.C. Lidocaine transdermal patches reduced pain intensity in neuropathic cancer patients already receiving opioid treatment. BMC Palliat. Care 2023, 22, 4. [Google Scholar] [CrossRef]
- Zhao, C.; Shrestha, N.; Liu, H.; Shen, Y.; Meng, L.; Fan, B.; Luo, F. The PATCH trial: Efficacy and safety of 5% lidocaine-medicated plaster for the treatment of patients with trigeminal neuralgia: A study protocol for a multicentric, double-blind, enriched enrolment randomised withdrawal, vehicle-controlled study. BMJ Open 2021, 11, 045493. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Pilloni, A.; Zeza, B.; Kuis, D.; Vrazic, D.; Domic, T.; Olszewska-Czyz, I.; Popova, C.; Kotsilkov, K.; Firkova, E.; Dermendzieva, Y.; et al. Treatment of Residual Periodontal Pockets Using a Hyaluronic Acid-Based Gel: A 12 Month Multicenter Randomized Triple-Blinded Clinical Trial. Antibiotics 2021, 10, 924. [Google Scholar] [CrossRef]
- Lee, B.S.; Lee, C.C.; Wang, Y.P.; Chen, H.J.; Lai, C.H.; Hsieh, W.L.; Chen, Y.W. Controlled-release of tetracycline and lovastatin by poly(D,L-lactide-co-glycolide acid)-chitosan nanoparticles enhances periodontal regeneration in dogs. Int. J. Nanomed. 2016, 11, 285–297. [Google Scholar] [CrossRef]
- Goldberg, M.; Manzi, A.; Birdi, A.; Conway, P.; Cantin, S.; Mishra, V.; Singh, A.; Pearson, A.; Goldberg, S.; Flaum, B.; et al. A nanoengineered topical transmucosal cisplatin delivery system induces anti-tumor response in animal models and patients with oral cancer. Nat. Commun. 2022, 13, 4829. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sato, T.; Kakei, Y.; Hasegawa, T.; Kashin, M.; Teraoka, S.; Yamaguchi, A.; Sasaki, R.; Akashi, M. Gold Nanoparticles Enhance the Tumor Growth-Suppressing Effects of Cetuximab and Radiotherapy in Head and Neck Cancer In Vitro and In Vivo. Cancers 2023, 15, 5697. [Google Scholar] [CrossRef]
- Dobešová, L.; Gier, T.; Kopečná, O.; Pagáčová, E.; Vičar, T.; Bestvater, F.; Toufar, J.; Bačíková, A.; Kopel, P.; Fedr, R.; et al. Incorporation of Low Concentrations of Gold Nanoparticles: Complex Effects on Radiation Response and Fate of Cancer Cells. Pharmaceutics 2022, 14, 166. [Google Scholar] [CrossRef]
- Kali, G.; Fürst, A.; Efiana, N.A.; Dizdarević, A.; Bernkop-Schnürch, A. Intraoral Drug Delivery: Highly Thiolated κ-Carrageenan as Mucoadhesive Excipient. Pharmaceutics 2023, 15, 1993. [Google Scholar] [CrossRef]
- Derwich, M.; Lassmann, L.; Machut, K.; Zoltowska, A.; Pawlowska, E. General Characteristics, Biomedical and Dental Application, and Usage of Chitosan in the Treatment of Temporomandibular Joint Disorders: A Narrative Review. Pharmaceutics 2022, 14, 305. [Google Scholar] [CrossRef]
- Li, H.; Guo, H.; Lei, C.; Liu, L.; Xu, L.; Feng, Y.; Ke, J.; Fang, W.; Song, H.; Xu, C.; et al. Nanotherapy in Joints: Increasing Endogenous Hyaluronan Production by Delivering Hyaluronan Synthase 2. Adv. Mater. 2019, 31, 1904535. [Google Scholar] [CrossRef]
- Dumitriu Buzia, O.; Păduraru, A.M.; Stefan, C.S.; Dinu, M.; Cocoș, D.I.; Nwabudike, L.C.; Tatu, A.L. Strategies for Improving Transdermal Administration: New Approaches to Controlled Drug Release. Pharmaceutics 2023, 15, 1183. [Google Scholar] [CrossRef]
- van Alem, C.M.A.; Metselaar, J.M.; van Kooten, C.; Rotmans, J.I. Recent Advances in Liposomal-Based Anti-Inflammatory Therapy. Pharmaceutics 2021, 13, 1004. [Google Scholar] [CrossRef]
- Ciano, J.; Beatty, B.L. Regional Quantitative Histological Variations in Human Oral Mucosa. Anat. Rec. 2014, 298, 562–578. [Google Scholar] [CrossRef]
- Brizuela, M.; Winters, R. Histology, Oral Mucosa. In StatPearls [Internet]; StatPearls Publishing: Treasure Island, FL, USA, 2023. [Google Scholar] [PubMed]
- Bud, E.; Vlasa, A.; Pacurar, M.; Matei, A.; Bud, A.; Szoke, A.-R.; Minervini, G. A Retrospective Histological Study on Palatal and Gingival Mucosa Changes during a Rapid Palatal Expansion Procedure. Biomedicines 2023, 11, 3246. [Google Scholar] [CrossRef]
- Confer, M.P.; Falahkheirkhah, K.; Surendran, S.; Sunny, S.P.; Yeh, K.; Liu, Y.-T.; Sharma, I.; Orr, A.C.; Lebovic, I.; Magner, W.J.; et al. Rapid and Label-Free Histopathology of Oral Lesions Using Deep Learning Applied to Optical and Infrared Spectroscopic Imaging Data. J. Pers. Med. 2024, 14, 304. [Google Scholar] [CrossRef]
- Famuyide, A.; Massoud, T.F.; Moonis, G. Oral Cavity and Salivary Glands Anatomy. Neuroimaging Clin. N. Am. 2022, 32, 777–790. [Google Scholar] [CrossRef]
- Lamy, E.; Capela-Silva, F.; Tvarijonaviciute, A. Research on Saliva Secretion and Composition. Biomed. Res. Int. 2018, 2018, 7406312. [Google Scholar] [CrossRef]
- Ichigaya, N.; Kawanishi, N.; Adachi, T.; Sugimoto, M.; Kimoto, K.; Hoshi, N. Effects of Denture Treatment on Salivary Metabolites: A Pilot Study. Int. J. Mol. Sci. 2023, 24, 13959. [Google Scholar] [CrossRef] [PubMed]
- Rowińska, I.; Szyperska-Ślaska, A.; Zariczny, P.; Pasławski, R.; Kramkowski, K.; Kowalczyk, P. The Influence of Diet on Oxidative Stress and Inflammation Induced by Bacterial Biofilms in the Human Oral Cavity. Materials 2021, 14, 1444. [Google Scholar] [CrossRef]
- Tadin, A.; Poljak Guberina, R.; Domazet, J.; Gavic, L. Oral Hygiene Practices and Oral Health Knowledge among Students in Split, Croatia. Healthcare 2022, 10, 406. [Google Scholar] [CrossRef]
- Petersen, P.E.; Baez, R.J.; Ogawa, H. Global application of oral disease prevention and health promotion as measured 10 years after the 2007 World Health Assembly statement on oral health. Commun. Dent. Oral Epidemiol. 2020, 48, 338–348. [Google Scholar] [CrossRef]
- Peres, M.A.; Macpherson, L.M.D.; Weyant, R.J.; Daly, B.; Venturelli, R.; Mathur, M.R.; Listl, S.; Celeste, R.K.; Guarnizo-Herreno, C.C.; Kearns, C.; et al. Oral diseases: A global public health challenge. Lancet 2019, 394, 249–260. [Google Scholar] [CrossRef]
- Vergnes, J.N.; Mazevet, M. Oral diseases: A global public health challenge. Lancet 2020, 395, 186. [Google Scholar] [CrossRef] [PubMed]
- Sischo, L.; Broder, H.L. Oral health-related quality of life: What, why, how, and future implications. J. Dent. Res. 2011, 90, 1264–1270. [Google Scholar] [CrossRef]
- Cavalli, E.; Mammana, S.; Nicoletti, F.; Bramanti, P.; Mazzon, E. The neuropathic pain: An overview of the current treatment and future therapeutic approaches. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419838383. [Google Scholar] [CrossRef] [PubMed]
- Yadav, Y.R.; Nishtha, Y.; Sonjjay, P.; Vijay, P.; Shailendra, R.; Yatin, K. Trigeminal Neuralgia. Asian J. Neurosurg. 2017, 12, 585–597. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Latorre, G.; González-García, N.; García-Ull, J.; González-Oria, C.; Porta-Etessam, J.; Molina, F.J.; Guerrero-Peral, A.L.; Belvís, R.; Rodríguez, R.; Bescós, A.; et al. Diagnosis and treatment of trigeminal neuralgia: Consensus statement from the Spanish Society of Neurology’s Headache Study Group. Neurología 2023, 38, S37–S52. [Google Scholar] [CrossRef]
- Cardoneanu, A.; Macovei, L.A.; Burlui, A.M.; Mihai, I.R.; Bratoiu, I.; Rezus, I.I.; Richter, P.; Tamba, B.-I.; Rezus, E. Temporomandibular Joint Osteoarthritis: Pathogenic Mechanisms Involving the Cartilage and Subchondral Bone, and Potential Therapeutic Strategies for Joint Regeneration. Int. J. Mol. Sci. 2023, 24, 171. [Google Scholar] [CrossRef]
- Derwich, M.; Mitus-Kenig, M.; Pawlowska, E. Interdisciplinary Approach to the Temporomandibular Joint Osteoarthritis—Review of the Literature. Medicina 2020, 56, 225. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, M.M.H.; Kubra, K.; Kanwar, R.K.; Kanwar, J.R. Chapter 13—Nanoparticles Advancing Cancer Immunotherapy. In Biomedical Applications of Graphene and 2D Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 283–304. [Google Scholar] [CrossRef]
- Badwelan, M.; Muaddi, H.; Ahmed, A.; Lee, K.T.; Tran, S.D. Oral Squamous Cell Carcinoma and Concomitant Primary Tumors, What Do We Know? A Review of the Literature. Curr. Oncol. 2023, 30, 3721–3734. [Google Scholar] [CrossRef]
- Caruntu, A.; Caruntu, C. Recent Advances in Oral Squamous Cell Carcinoma. J. Clin. Med. 2022, 11, 6406. [Google Scholar] [CrossRef]
- Checherita, L.E.; Antohe, M.E.; Stamatin, O.; Rudnic, I.; Lupu, I.C.; Croitoru, I.; Surdu, A.; Cioloca, D.; Gradinaru, I.; Francu, L.; et al. Periodontal Disease Diagnosis in the Context of Oral Rehabilitation Approaches. Appl. Sci. 2022, 12, 9067. [Google Scholar] [CrossRef]
- Wehr, C.; Cruz, G.; Young, S.; Fakhouri, W.D. An Insight into Acute Pericoronitis and the Need for an Evidence-Based Standard of Care. Dent. J. 2019, 7, 88. [Google Scholar] [CrossRef]
- Schmidt, J.; Kunderova, M.; Pilbauerova, N.; Kapitan, M. A Review of Evidence-Based Recommendations for Pericoronitis Management and a Systematic Review of Antibiotic Prescribing for Pericoronitis among Dentists: Inappropriate Pericoronitis Treatment Is a Critical Factor of Antibiotic Overuse in Dentistry. Int. J. Environ. Res. Public. Health 2021, 18, 6796. [Google Scholar] [CrossRef]
- Cianetti, S.; Valenti, C.; Orso, M.; Lomurno, G.; Nardone, M.; Lomurno, A.P.; Pagano, S.; Lombardo, G. Systematic Review of the Literature on Dental Caries and Periodontal Disease in Socio-Economically Disadvantaged Individuals. Int. J. Environ. Res. Public. Health 2021, 18, 12360. [Google Scholar] [CrossRef]
- Pitts, N.B.; Zero, D.T.; Marsh, P.D.; Ekstrand, K.; Weintraub, J.A.; Ramos-Gomez, F.; Tagami, J.; Twetman, S.; Tsakos, G.; Ismail, A. Dental caries. Nat. Rev. Dis. Primers 2017, 3, 17030. [Google Scholar] [CrossRef] [PubMed]
- Bala, R.; Pawar, P.; Khanna, S.; Arora, S. Orally dissolving strips: A new approach to oral drug delivery system. Int. J. Pharm. Investig. 2013, 3, 67–76. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef]
- Saxena, A.; Taruna, S. Oral dissolving films: A comprehensive review on recent perspectives and current approach to effective drug delivery. J. Drug Deliv. Ther. 2022, 12, 139–147. [Google Scholar] [CrossRef]
- Taufik, A.S.H.M.; Muharram, S.H.; Rahman, F.A.F.A.; Ramlee, A.M.F.A.; David, S.R.; Rajabalaya, R. Formulation and Evaluation of Chlorhexidine Oral Fast-Dissolving Films. J. Pharm. Negat. Results 2023, 14, 4. [Google Scholar] [CrossRef]
- Elshafeey, A.H.; El-Dahmy, R.M. Formulation and Development of Oral Fast-Dissolving Films Loaded with Nanosuspension to Augment Paroxetine Bioavailability: In Vitro Characterization, Ex Vivo Permeation, and Pharmacokinetic Evaluation in Healthy Human Volunteers. Pharmaceutics 2021, 13, 1869. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, I.; Hanna, P.A.; Gad, S.; Abd-Allah, F.I.; El-Say, K.M. Enhancing Pharmacokinetics and Pharmacodynamics of Rosuvastatin Calcium through the Development and Optimization of Fast-Dissolving Films. Pharmaceutics 2023, 15, 2640. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho, A.C.W.; Paiva, N.F.; Demonari, I.K.; Duarte, M.P.F.; do Couto, R.O.; de Freitas, O.; Vicentini, F.T.M.d.C. The Potential of Films as Transmucosal Drug Delivery Systems. Pharmaceutics 2023, 15, 2583. [Google Scholar] [CrossRef]
- Surendranath, M.; Rekha, M.R.; Parameswaran, R. Recent advances in functionally modified polymers for mucoadhesive drug delivery. J. Mater. Chem. B 2022, 31, 5913–5924. [Google Scholar] [CrossRef] [PubMed]
- Leichner, C.; Jelkmann, M.; Bernkop-Schnürch, A. Thiolated polymers: Bioinspired polymers utilizing one of the most important bridging structures in nature. Adv. Drug Deliv. Rev. 2019, 151–152, 191–221. [Google Scholar] [CrossRef]
- Tejada, G.; Piccirilli, G.N.; Sortino, M.; Salomón, C.J.; Lamas, M.C.; Leonardi, D. Formulation and in-vitro efficacy of antifungal mucoadhesive polymeric matrices for the delivery of miconazole nitrate. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 79, 140–150. [Google Scholar] [CrossRef]
- Drucker, D.J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2020, 19, 277–289. [Google Scholar] [CrossRef] [PubMed]
- Duque, T.M.; Prado, M.; Herrera, D.R. Gomes BPFA. Periodontal and endodontic infectious/inflammatory profile in primary periodontal lesions with secondary endodontic involvement after a calcium hydroxide-based intracanal medication. Clin. Oral. Investig. 2019, 23, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Pacheco-Quito, E.-M.; Ruiz-Caro, R.; Veiga, M.-D. Carrageenan: Drug Delivery Systems and Other Biomedical Applications. Mar. Drugs. 2020, 18, 583. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Yuan, S.; Wang, J.; Shen, Y.; Deng, S.; Xie, L.; Yang, Q. The Formation Mechanism of Hydrogels. Curr. Stem Cell Res. Ther. 2018, 13, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Khan, F.; Atif, M.; Haseen, M.; Kamal, S.; Khan, M.S.; Shahid, S.; Nam, S.A. Synthesis, classification and properties of hydrogels: Their applications in drug delivery and agriculture. J. Mater. Chem. B 2022, 10, 170–203. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Wang, Y.; Li, Q.; Yu, C.; Chu, W. Natural Polymer-based Stimuli-responsive Hydrogels. Curr. Med. Chem. 2020, 27, 2631–2657. [Google Scholar] [CrossRef] [PubMed]
- Zhao, C.; Pan, B.; Wang, T.; Yang, H.; Vance, D.; Li, X.; Zhao, H.; Hu, X.; Yang, T.; Chen, Z.; et al. Advances in NIR-Responsive Natural Macromolecular Hydrogel Assembly Drugs for Cancer Treatment. Pharmaceutics 2023, 15, 2729. [Google Scholar] [CrossRef] [PubMed]
- Abruzzo, A.; Vitali, B.; Lombardi, F.; Guerrini, L.; Cinque, B.; Parolin, C.; Bigucci, F.; Cerchiara, T.; Arbizzani, C.; Gallucci, M.C.; et al. Mucoadhesive Buccal Films for Local Delivery of Lactobacillus brevis. Pharmaceutics 2020, 12, 241. [Google Scholar] [CrossRef] [PubMed]
- Al-Nemrawi, N.K.; Alsharif, S.S.M.; Alzoubi, K.H.; Alkhatib, R.Q. Preparation and characterization of insulin chitosan-nanoparticles loaded in buccal films. Pharm. Dev. Technol. 2019, 24, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Jamshidnejad-Tosaramandani, T.; Kashanian, S.; Karimi, I.; Schiöth, H.B. Synthesis of a Rivastigmine and Insulin Combinational Mucoadhesive Nanoparticle for Intranasal Delivery. Polymers 2024, 16, 510. [Google Scholar] [CrossRef]
- Pichayakorn, W.; Monton, C.; Sampaopan, Y.; Panrat, K.; Suksaeree, J. Fabrication and Characterization of Buccal Film Loaded Self-emulsifying Drug Delivery System containing Lysiphyllum strychnifolium Stem Extracts. AAPS PharmSciTech 2022, 23, 194. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Duan, H.; Pan, H.; Yang, X.; Pan, W. Two types of core/shell fibers based on carboxymethyl chitosan and Sodium carboxymethyl cellulose with self-assembled liposome for buccal delivery of carvedilol across TR146 cell culture and porcine buccal mucosa. Int. J. Biol. Macromol. 2019, 128, 700–709. [Google Scholar] [CrossRef]
- Mazzinelli, E.; Favuzzi, I.; Arcovito, A.; Castagnola, R.; Fratocchi, G.; Mordente, A.; Nocca, G. Oral Mucosa Models to Evaluate Drug Permeability. Pharmaceutics 2023, 15, 1559. [Google Scholar] [CrossRef] [PubMed]
- Talianu, M.-T.; Dinu-Pîrvu, C.-E.; Ghica, M.V.; Anuţa, V.; Prisada, R.M.; Popa, L. Development and Characterization of New Miconazole-Based Microemulsions for Buccal Delivery by Implementing a Full Factorial Design Modeling. Pharmaceutics 2024, 16, 271. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.-S.; Abu Lila, A.S.; Sallam, N.M.; Sanad, R.A.-B.; Ahmed, M.M.; Ghorab, M.M.; Alotaibi, H.F.; Alalaiwe, A.; Aldawsari, M.F.; Alshahrani, S.M.; et al. Preparation and Characterization of a Novel Mucoadhesive Carvedilol Nanosponge: A Promising Platform for Buccal Anti-Hypertensive Delivery. Gels 2022, 8, 235. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, N.; Aggarwal, G.; Gaurav, K.J.; Gaurav, M. Multi-drug loaded microneedles for emergency treatment of snakebite envenomation. Med. Hypotheses 2022, 165, 110908. [Google Scholar] [CrossRef]
- Birk, S.E.; Mosgaard, M.D.; Kjeldsen, R.B.; Boisen, A.; Meyer, R.L.; Nielsen, L.H. Management of oral biofilms by nisin delivery in adhesive microdevices. Eur. J. Pharm. Biopharm. 2021, 167, 83–88. [Google Scholar] [CrossRef] [PubMed]
- González-Moles, M.Á.; Ramos-García, P. State of Evidence on Oral Health Problems in Diabetic Patients: A Critical Review of the Literature. J. Clin. Med. 2021, 10, 5383. [Google Scholar] [CrossRef] [PubMed]
- Birk, S.E.; Haagensen, J.A.J.; Johansen, H.K.; Molin, S.; Nielsen, L.H.; Boisen, A. Microcontainer Delivery of Antibiotic Improves Treatment of Pseudomonas aeruginosa Biofilms. Adv. Healthc. Mater. 2020, 9, 1901779. [Google Scholar] [CrossRef]
- Christfort, J.F.; Polhaus, C.J.M.; Bondegaard, P.W.; Chang, T.J.; Hwu, E.T.; Hagner Nielsen, L.; Zór, K.; Boisen, A. Open source anaerobic and temperature-controlled in vitro model enabling real-time release studies with live bacteria. HardwareX 2022, 11, 00275. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Weldrick, P.J.; Hardman, M.J.; Paunov, V.N. Enhanced Clearing of Wound-Related Pathogenic Bacterial Biofilms Using Protease-Functionalized Antibiotic Nanocarriers. ACS Appl. Mater. Interfaces 2019, 11, 43902–43919. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.N.H.; Nelson, S.M. Pharmacodynamics of ciprofloxacin against Pseudomonas aeruginosa planktonic and biofilm-derived cells. Lett. Appl. Microbiol. 2019, 68, 350–359. [Google Scholar] [CrossRef]
- Lopes, L.B.; Garcia, M.T.; Bentley, M.V. Chemical penetration enhancers. Ther. Deliv. 2015, 6, 1053–1061. [Google Scholar] [CrossRef] [PubMed]
- Alghanem, S.; Dziurkowska, E.; Ordyniec-Kwaśnica, I.; Sznitowska, M. Intraoral medical devices for sustained drug delivery. Clin. Oral. Investig. 2023, 27, 7157–7169. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Giannola, L.I.; Paderni, C.; De Caro, V.; Florena, A.M.; Wolff, A.; Campisi, G. New prospectives in the delivery of galantamine for elderly patients using the IntelliDrug intraoral device: In vivo animal studies. Curr. Pharm. Des. 2010, 16, 653–659. [Google Scholar] [CrossRef] [PubMed]
- Angellotti, G.; Di Prima, G.; D’Agostino, F.; Peri, E.; Tricoli, M.R.; Belfiore, E.; Allegra, M.; Cancemi, P.; De Caro, V. Multicomponent Antibiofilm Lipid Nanoparticles as Novel Platform to Ameliorate Resveratrol Properties: Preliminary Outcomes on Fibroblast Proliferation and Migration. Int. J. Mol. Sci. 2023, 24, 8382. [Google Scholar] [CrossRef]
- Weisman, S. Naproxen for Post-Operative Pain. J. Pharm. Pharm. Sci. 2021, 24, 62–70. [Google Scholar] [CrossRef] [PubMed]
- Sahu, S.A.; Panda, S.; Das, A.C.; Mishra, L.; Rath, S.; Sokolowski, K.; Kumar, M.; Mohanty, R.; Nayak, R.; Satpathy, A.; et al. Efficacy of Sub-Gingivally Delivered Propolis Nanoparticle in Non-Surgical Management of Periodontal Pocket: A Randomized Clinical Trial. Biomolecules 2023, 13, 1576. [Google Scholar] [CrossRef]
- Agarwal, S.; Aggarwal, S. Mucoadhesive platform for drug delivery. A comprehensive review. Curr. Drug Deliv. 2015, 12, 139–156. [Google Scholar] [CrossRef]
- Neagu, O.M.; Ghitea, T.; Marian, E.; Vlase, L.; Vlase, A.-M.; Ciavoi, G.; Fehér, P.; Pallag, A.; Bácskay, I.; Nemes, D.; et al. Formulation and Characterization of Mucoadhesive Polymeric Films Containing Extracts of Taraxaci Folium and Matricariae Flos. Molecules 2023, 28, 4002. [Google Scholar] [CrossRef]
- Handler, A.M.; Marxen, E.; Jacobsen, J.; Janfelt, C. Visualization of the penetration modifying mechanism of laurocapram by Mass Spectrometry Imaging in buccal drug delivery. Eur. J. Pharm. Sci. 2019, 127, 276–281. [Google Scholar] [CrossRef]
- Carmen, G.; Hancu, G. Antimicrobial and Antifungal Activity of Pelargonium roseum Essential Oils. Adv. Pharm. Bull. 2014, 4, 511–514. [Google Scholar] [CrossRef]
- Hosny, K.M.; Sindi, A.M.; Alkhalidi, H.M.; Kurakula, M.; Alruwaili, N.K.; Alhakamy, N.A.; Abualsunun, W.A.; Bakhaidar, R.B.; Bahmdan, R.H.; Rizg, W.Y.; et al. Oral gel loaded with penciclovir-lavender oil nanoemulsion to enhance bioavailability and alleviate pain associated with herpes labialis. Drug Deliv. 2021, 28, 1043–1054. [Google Scholar] [CrossRef]
- Muresan, S.M.C.; Dreanca, A.; Repciuc, C.; Dejescu, C.; Rotar, O.; Pop, R.A.; Pantea, S.; Pall, E.; Ciotlaus, I.; Sarosi, C.; et al. Dental Hydrogels with Essential Oils with Potential Activity in Periodontitis. Appl. Sci. 2023, 13, 1787. [Google Scholar] [CrossRef]
- Hou, T.; Sana, S.S.; Li, H.; Xing, Y.; Nanda, A.; Netala, V.R.; Zhang, Z. Essential oils and its antibacterial, antifungal and anti-oxidant activity applications: A review. Food Biosci. 2022, 47, 101716. [Google Scholar] [CrossRef]
- Cheshire, W.P. Trigeminal neuralgia: For one nerve a multitude of treatments. Expert. Rev. Neurother. 2007, 7, 1565–1579. [Google Scholar] [CrossRef]
- Scott, L.J. Fentanyl Iontophoretic Transdermal System: A Review in Acute Postoperative Pain. Clin. Drug Investig. 2016, 36, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Rivaz, M.; Rahpeima, M.; Khademian, Z.; Dabbaghmanesh, M.H. The effects of aromatherapy massage with lavender essential oil on neuropathic pain and quality of life in diabetic patients: A randomized clinical trial. Complement. Ther. Clin. Pract. 2021, 44, 101430. [Google Scholar] [CrossRef]
- Kern, K.U.; Nowack, W.; Poole, C. Treatment of neuropathic pain with the capsaicin 8% patch: Is pretreatment with lidocaine necessary? Pain Pract. 2014, 14, 42–50. [Google Scholar] [CrossRef]
- Ridouh, I.; Hackshaw, K.V. Essential Oils and Neuropathic Pain. Plants 2022, 11, 1797. [Google Scholar] [CrossRef]
- Wang, S.; Zhang, D.; Hu, J.; Jia, Q.; Xu, W.; Su, D.; Song, H.; Xu, Z.; Cui, J.; Zhou, M.; et al. A clinical and mechanistic study of topical borneol-induced analgesia. EMBO Mol. Med. 2017, 9, 802–815. [Google Scholar] [CrossRef]
- Cigerim, L.; Kaplan, V. Analgesic efficacy of naproxen-codeine, naproxen+dexamethasone, and naproxen on myofascial pain: A randomized double-blind controlled trial. CRANIO® 2020, 41, 119–125. [Google Scholar] [CrossRef]
- Ding, D.; Zhu, Q. Recent advances of PLGA micro/nanoparticles for the delivery of biomacromolecular therapeutics. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 1041–1060. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, H.; Xu, W.; Lai, W.; Song, L.; Deng, J. Hyaluronic Acid/Parecoxib-Loaded PLGA Microspheres for Therapy of Temporomandibular Disorders. Curr. Drug Deliv. 2021, 18, 234–245. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Feng, N. Mesoporous silica nanoparticles: Synthesis, classification, drug loading, pharmacokinetics, biocompatibility, and application in drug delivery. Expert. Opin. Drug Deliv. 2019, 16, 219–237. [Google Scholar] [CrossRef]
- Zuo, X.; Gu, Y.; Wang, C.; Zhang, J.; Zhang, J.; Wang, G.; Wang, F. A Systematic Review of the Anti-Inflammatory and Immunomodulatory Properties of 16 Essential Oils of Herbs. Evid. Based Complement. Alternat. Med. 2020, 2020, 8878927. [Google Scholar] [CrossRef]
- Dinu, M.; Tatu, A.L.; Cocoș, D.I.; Nwabudike, L.C.; Chirilov, A.M.; Stefan, C.S.; Earar, K.; Dumitriu Buzia, O. Natural Sources of Therapeutic Agents Used in Skin Conditions. Life 2024, 14, 492. [Google Scholar] [CrossRef]
- Georgantopoulos, A.; Vougioukas, A.; Kalousi, F.D.; Tsialtas, I.; Psarra, A.-M.G. Comparative Studies on the Anti-Inflammatory and Apoptotic Activities of Four Greek Essential Oils: Involvement in the Regulation of NF-κΒ and Steroid Receptor Signaling. Life 2023, 13, 1534. [Google Scholar] [CrossRef]
- Arsić, I.; Tadić, V.; Vlaović, D.; Homšek, I.; Vesić, S.; Isailović, G.; Vuleta, G. Preparation of novel apigenin-enriched, liposomal and non-liposomal, antiinflammatory topical formulations as substitutes for corticosteroid therapy. Phytother. Res. 2011, 25, 228–233. [Google Scholar] [CrossRef]
- Peng, T.; Chen, Y.; Hu, W.; Huang, Y.; Zhang, M.; Lu, C.; Pan, X.; Wu, C. Microneedles for Enhanced Topical Treatment of Skin Disorders: Applications, Challenges, and Prospects. Engineering 2023, 30, 170–189. [Google Scholar] [CrossRef]
- Rubió-Casadevall, J.; Cirauqui Cirauqui, B.; Martinez Trufero, J.; Plana Serrahima, M.; García Castaño, A.; Carral Maseda, A.; Iglesias Docampo, L.; Pérez Segura, P.; Ceballos Lenza, I.; Gutiérrez Calderón, V.; et al. TTCC-2019-02: Real-world evidence of first-line cetuximab plus paclitaxel in recurrent or metastatic squamous cell carcinoma of the head and neck. Front. Oncol. 2023, 13, 1226939. [Google Scholar] [CrossRef]
- Alavi, S.E.; Muflih Al Harthi, S.; Ebrahimi Shahmabadi, H.; Akbarzadeh, A. Cisplatin-Loaded Polybutylcyanoacrylate Nanoparticles with Improved Properties as an Anticancer Agent. Int. J. Mol. Sci. 2019, 20, 1531. [Google Scholar] [CrossRef]
- Habib, L.; Alyan, M.; Ghantous, Y.; Shklover, J.; Shainsky, J.; Abu El-Naaj, I.; Bianco-Peled, H.; Schroeder, A. A mucoadhesive patch loaded with freeze-dried liposomes for the local treatment of oral tumors. Drug Deliv. Transl. Res. 2023, 13, 1228–1245. [Google Scholar] [CrossRef] [PubMed]
- Sohail, M.F.; Hussain, S.Z.; Saeed, H.; Javed, I.; Sarwar, H.S.; Nadhman, A.; Huma, Z.-E.; Rehman, M.; Jahan, S.; Hussain, I.; et al. Polymeric nanocapsules embedded with ultra-small silver nanoclusters for synergistic pharmacology and improved oral delivery of Docetaxel. Sci. Rep. 2018, 8, 13304. [Google Scholar] [CrossRef]
- Hanna, G.J.; Villa, A.; Nandi, S.P.; Shi, R.; Oneill, A.; Liu, M.; Quinn, C.T.; Treister, N.S.; Sroussi, H.Y.; Vacharotayangul, P.; et al. Nivolumab for Patients with High-Risk Oral Leukoplakia: A Nonrandomized Controlled Trial. JAMA Oncol. 2024, 10, 32–41. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, T.; Suita, Y.; Miriyala, S.; Dean, J.; Tapinos, N.; Shen, J. Advances in Lipid-Based Nanoparticles for Cancer Chemoimmunotherapy. Pharmaceutics 2021, 13, 520. [Google Scholar] [CrossRef] [PubMed]
- Sürer, Ş.İ.; Elçitepe, T.B.; Akçay, D.; Daşkın, E.; Çalıbaşı Kocal, G.; Arıcan Alıcıkuş, Z.; Eskiizmir, G.; Yapıcı, K.; Başbınar, Y. A Promising, Novel Radiosensitizer Nanodrug Complex for Oral Cavity Cancer: Cetuximab and Cisplatin-Conjugated Gold Nanoparticles. Balkan Med. J. 2021, 38, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Jeremic, B.; Aguerri, A.R.; Filipovic, N. Radiosensitization by gold nanoparticles. Clin. Transl. Oncol. 2013, 15, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Kashin, M.; Kakei, Y.; Teraoka, S.; Hasegawa, T.; Yamaguchi, A.; Fukuoka, T.; Sasaki, R.; Akashi, M. Gold Nanoparticles Enhance EGFR Inhibition and Irradiation Effects in Head and Neck Squamous Carcinoma Cells. Biomed. Res. Int. 2020, 2020, 1281645. [Google Scholar] [CrossRef]
- Park, S.Y.; Lee, H.U.; Lee, Y.C.; Kim, G.H.; Park, E.C.; Han, S.H.; Lee, J.G.; Choi, S.; Heo, N.S.; Kim, D.L.; et al. Wound healing potential of antibacterial microneedles loaded with green tea extracts. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 42, 757–762. [Google Scholar] [CrossRef]
- Yu, X.; Zhao, J.; Fan, D. The Progress in the Application of Dissolving Microneedles in Biomedicine. Polymers 2023, 15, 4059. [Google Scholar] [CrossRef]
- González García, L.E.; MacGregor, M.N.; Visalakshan, R.M.; Ninan, N.; Cavallaro, A.A.; Trinidad, A.D.; Zhao, Y.; Hayball, A.J.D.; Vasilev, K. Self-sterilizing antibacterial silver-loaded microneedles. Chem. Commun. 2018, 55, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Nasiri, K.; Masoumi, S.M.; Amini, S.; Goudarzi, M.; Tafreshi, S.M.; Bagheri, A.; Yasamineh, S.; Alwan, M.; Arellano, M.T.C.; Gholizadeh, O. Recent advances in metal nanoparticles to treat periodontitis. J. Nanobiotechnol. 2023, 21, 283. [Google Scholar] [CrossRef] [PubMed]
- Amato, M.; Santonocito, S.; Polizzi, A.; Tartaglia, G.M.; Ronsivalle, V.; Viglianisi, G.; Grippaudo, C.; Isola, G. Local Delivery and Controlled Release Drugs Systems: A New Approach for the Clinical Treatment of Periodontitis Therapy. Pharmaceutics 2023, 15, 1312. [Google Scholar] [CrossRef] [PubMed]
- Todorovic, V.S.; Vasovic, M.; Andric, M.; Todorovic, L.; Kokovic, V. Efficacy of fentanyl transdermal patch in pain control after lower third molar surgery: A preliminary study. Med. Oral Patol. Oral Cir. Bucal 2016, 21, 621–625. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Aljaafari, M.N.; AlAli, A.O.; Baqais, L.; Alqubaisy, M.; AlAli, M.; Molouki, A.; Ong-Abdullah, J.; Abushelaibi, A.; Lai, K.-S.; Lim, S.-H.E. An Overview of the Potential Therapeutic Applications of Essential Oils. Molecules 2021, 26, 628. [Google Scholar] [CrossRef] [PubMed]
- Kong, A.S.-Y.; Maran, S.; Yap, P.S.-X.; Lim, S.-H.E.; Yang, S.-K.; Cheng, W.-H.; Tan, Y.-H.; Lai, K.-S. Anti- and Pro-Oxidant Properties of Essential Oils against Antimicrobial Resistance. Antioxidants 2022, 11, 1819. [Google Scholar] [CrossRef] [PubMed]
- Tatsi, C.; Toumba, K.J. Effect of fluoride slow-release glass devices on salivary and gingival crevicular fluid levels of fluoride: A pilot study. Clin. Exp. Dent. Res. 2019, 5, 620–626. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque, P.M.; Azevedo, S.G.; de Andrade, C.P.; D’Ambros, N.C.d.S.; Pérez, M.T.M.; Manzato, L. Biotechnological Applications of Nanoencapsulated Essential Oils: A Review. Polymers 2022, 14, 5495. [Google Scholar] [CrossRef]
- Horky, P.; Skalickova, S.; Smerkova, K.; Skladanka, J. Essential Oils as Feed Additives: Pharmacokinetics and Potential Toxicity in Monogastric Animals. Animals 2019, 9, 352. [Google Scholar] [CrossRef]







| Nr crt. | Active Substance | Pharmacological Group | Yielding Systems/Carriers | Improvement | Local (LE)/Systemic (SE) Effect | References |
|---|---|---|---|---|---|---|
| 1. | Cetuximab | Monoclonal antibodies | Gold nanoparticles | Increased efficacy of the active substance | SE | Sato et al., 2023 [28] |
| Cetuximab + cisplatin | Monoclonal antibodies + chemotherapeutic agent | Gold nanoparticles | Used in aggressive forms of metastatic cancer | SE | Sürer et al., 2021 [127] | |
| 2. | Cisplatina | Chemotherapy agent | Nano-designed cisplatin patch | Used in aggressive forms of metastatic cancer | SE | Goldberg et al., 2022 [27] |
| Cisplatina | Polybutylcyanoacrylate (PEG) nanoparticles | Improve efficacy, reduce toxicity | SE | Alavi et al., 2019 [122] | ||
| 3. | Doxorubicin | Powerful anticancer agent | Mucoadhesive patches loaded with liposomes | Increased efficiency and reduced side effects | LE/SE | Habib et al., 2023 [123] |
| 4. | Docetaxel | Anticancer agent | Polymeric nanocapsules containing silver nanoclusters | Obtaining a much-increased bioavailability and half-life | LE/SE | Sohail et al., 2018 [124] |
| 5. | Nivolumab | Monoclonal antibodies | Polymer/lipid nanoparticles | May decrease immunosuppression and promote active substances at the site of action | SE | Hanna et al. 2024 [125], Wang et al., 2021 [126] |
| Nr crt. | Oral Pathologies | Proposed Yield Systems | Local (LE)/Systemic (SE) Effect | Optimization | References |
|---|---|---|---|---|---|
| 1. | Neuropathy/neuralgia | Transdermal patches with lidocaine, capsaicin, and lavender oil | LE | Lavender oil as penetration enhancer/synergistic action of three pharmacological principles with different mechanisms of action | Pickering et al., 2020 [22], Tsai et al., 2023 [23], Zhao et al., 2021 [24] |
| 2. A | Osteoarthritis of the temporomandibular joint | Plasters with micro-needle saws with alternating heights | LE | Saw-type needles and their alternation with penetration on different height levels | Derwich et al., 2022 [31], Cigerim et al., 2020 [112] |
| 2. B | Osteoarthritis of the temporomandibular joint | Liposomal cream based on non-steroidal, anti-inflammatory, and essential oils (thymus, geranium, eucalyptus, cloves) | LE | Combining nanoparticles loaded with non-steroidal anti-inflammatory drugs with nanoparticles loaded with essential oils for a synergistic effect | Chen et al., 2015 [15], Hou et al., 2022 [105] |
| 3. | Oral cancer | Nanoprojected mucoadhesive patches | LE/SE | Mucoadhesive patches are an excellent option in oral cancer and combining two therapeutic groups of anticancer agents and monoclonal antibodies gives us increased chances in the process of remission or even healing | Goldberg et al., 2022 [27], Sato et al., 2023 [28], Habib et al., 2023 [123] |
| 4. | Periodontal pockets | Nanocarriers associated with micro-ace systems | LE/Prevent the dissemination of the infectious process | 1. The use of needles in the saw taking into account the weak vascularized area 2. Conjugation of three drug groups: antibiotics, chemotherapy, and non-steroidal anti-inflammatory drugs | Bilal et al., 2021 [18], Yu et al., 2023 [131], Amato et al., 2023 [134] |
| 5. | Pericoronitis | Micro-needle saw system loaded with nanoparticles of essential oils, and we attach tanks with non-steroidal anti-inflammatory | LE/Role in maintaining oral health through the action of essential oils | Use of dual-role essential oils: 1. Amplifier agent in nanoformulation 2. By the anti-inflammatory action, antibacterial, and soothing role in maintaining oral health | Aljaafari et al., 2021 [136], Kong et al., 2022 [137] |
| 6. | Oral health/dental caries | Copolymer membranes loaded with fluorine nanoparticles and essential oils | LE/Role in maintaining oral health through the action of essential oils | Compared to the existing data, we propose this association of fluoride nanoparticles with essential oil nanoparticles with real oral health benefits | Jiao et al., 2019 [8], Liang et al., 2020 [9], Tatsi et al., 2019 [138], Albuquerque et al., 2022 [139] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cocoș, D.I.; Dumitriu Buzia, O.; Tatu, A.L.; Dinu, M.; Nwabudike, L.C.; Stefan, C.S.; Earar, K.; Galea, C. Challenges in Optimizing Nanoplatforms Used for Local and Systemic Delivery in the Oral Cavity. Pharmaceutics 2024, 16, 626. https://doi.org/10.3390/pharmaceutics16050626
Cocoș DI, Dumitriu Buzia O, Tatu AL, Dinu M, Nwabudike LC, Stefan CS, Earar K, Galea C. Challenges in Optimizing Nanoplatforms Used for Local and Systemic Delivery in the Oral Cavity. Pharmaceutics. 2024; 16(5):626. https://doi.org/10.3390/pharmaceutics16050626
Chicago/Turabian StyleCocoș, Dorin Ioan, Olimpia Dumitriu Buzia, Alin Laurențiu Tatu, Monica Dinu, Lawrence Chukwudi Nwabudike, Claudia Simona Stefan, Kamel Earar, and Carmen Galea. 2024. "Challenges in Optimizing Nanoplatforms Used for Local and Systemic Delivery in the Oral Cavity" Pharmaceutics 16, no. 5: 626. https://doi.org/10.3390/pharmaceutics16050626
APA StyleCocoș, D. I., Dumitriu Buzia, O., Tatu, A. L., Dinu, M., Nwabudike, L. C., Stefan, C. S., Earar, K., & Galea, C. (2024). Challenges in Optimizing Nanoplatforms Used for Local and Systemic Delivery in the Oral Cavity. Pharmaceutics, 16(5), 626. https://doi.org/10.3390/pharmaceutics16050626