Lymph Node-on-Chip Technology: Cutting-Edge Advances in Immune Microenvironment Simulation
Abstract
:1. Introduction
2. Integrating Lymph Node Models with Organ-on-a-Chip
2.1. Design and Fabrication of Lymph Node-on-Chip Technology
2.1.1. Lymph Node-on-Chip Scaffold Material
2.1.2. Simulation of ECM

2.1.3. Unique Cell Source of Lymph Node-on-Chip
2.1.4. Replication of the Spatial Configuration of the Lymph Node
2.2. Research Insights Derived from Lymph Node-on-Chip Technology
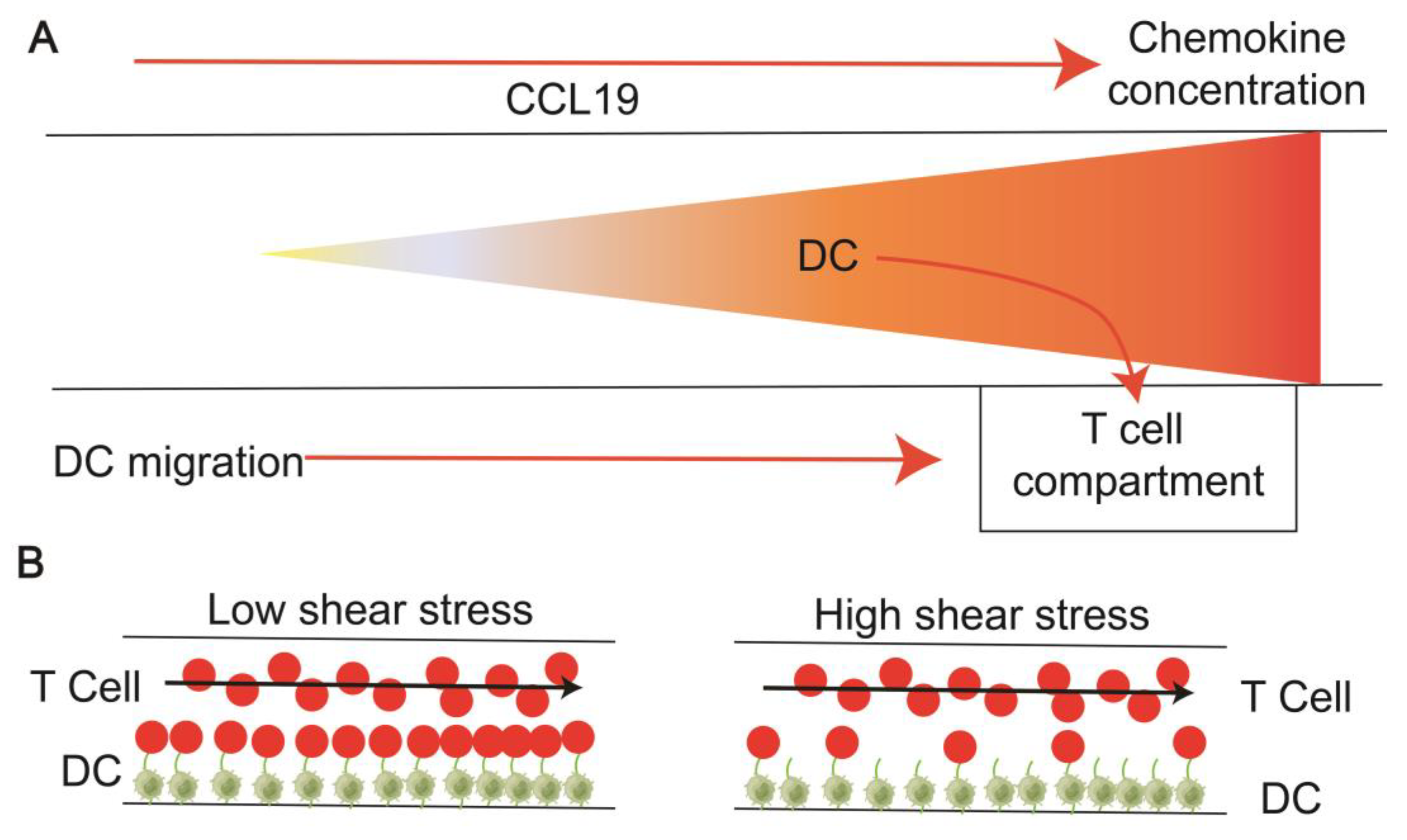
2.2.1. Chemokines Facilitate the Migration and Homing of Immune Cells
2.2.2. Interaction between T Cells and APCs in the Paracortical Region
2.2.3. B Cell Follicular and Germinal Center Model
3. Applications of Lymph Node on-a-Chip
3.1. Immune Response of Lymph Node Models to Vaccines, Pathogens, and Drugs In Vitro
3.2. Application of Lymph Node Model In Vitro in Disease and Cancer
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Krishnamurty, A.T.; Turley, S.J. Lymph node stromal cells: Cartographers of the immune system. Nat. Immunol. 2020, 21, 369–380. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Wiechers, C.; Huehn, J. Lymph node stromal cell subsets-Emerging specialists for tailored tissue-specific immune responses. Int. J. Med. Microbiol. 2021, 311, 151492. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, A.L.; Acton, S.E.; Knoblich, K. Lymph node fibroblastic reticular cells in health and disease. Nat. Rev. Immunol. 2015, 15, 350–361. [Google Scholar] [CrossRef] [PubMed]
- Chang, J.E.; Turley, S.J. Stromal infrastructure of the lymph node and coordination of immunity. Trends Immunol. 2015, 36, 30–39. [Google Scholar] [CrossRef]
- Yuan, X.H.; Jin, Z.H. Paracrine regulation of melanogenesis. Br. J. Dermatol. 2018, 178, 632–639. [Google Scholar] [CrossRef] [PubMed]
- Mehta, S.; Campbell, H.; Drummond, C.J.; Li, K.; Murray, K.; Slatter, T.; Bourdon, J.C.; Braithwaite, A.W. Adaptive homeostasis and the p53 isoform network. EMBO Rep. 2021, 22, e53085. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.M.; Lou, M.; Yao, L.; Germain, R.N.; Radtke, A.J. The lymph node at a glance—How spatial organization optimizes the immune response. J. Cell Sci. 2020, 133, jcs241828. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.Y.; Lyons-Cohen, M.R.; Gerner, M.Y. Information flow in the spatiotemporal organization of immune responses. Immunol. Rev. 2022, 306, 93–107. [Google Scholar] [CrossRef]
- Shou, Y.; Johnson, S.C.; Quek, Y.J.; Li, X.; Tay, A. Integrative lymph node-mimicking models created with biomaterials and computational tools to study the immune system. Mater. Today Bio 2022, 14, 100269. [Google Scholar] [CrossRef] [PubMed]
- Fu, S.; Wang, Y.; Bin, E.; Huang, H.; Wang, F.; Tang, N. c-JUN-mediated transcriptional responses in lymphatic endothelial cells are required for lung fluid clearance at birth. Proc. Natl. Acad. Sci. USA 2023, 120, e2215449120. [Google Scholar] [CrossRef] [PubMed]
- Geva-Zatorsky, N.; Alvarez, D.; Hudak, J.E.; Reading, N.C.; Erturk-Hasdemir, D.; Dasgupta, S.; von Andrian, U.H.; Kasper, D.L. In vivo imaging and tracking of host-microbiota interactions via metabolic labeling of gut anaerobic bacteria. Nat. Med. 2015, 21, 1091–1100. [Google Scholar] [CrossRef] [PubMed]
- Ozawa, M.; Nakajima, S.; Kobayashi, D.; Tomii, K.; Li, N.J.; Watarai, T.; Suzuki, R.; Watanabe, S.; Kanda, Y.; Takeuchi, A.; et al. Micro- and Macro-Anatomical Frameworks of Lymph Nodes Indispensable for the Lymphatic System Filtering Function. Front. Cell Dev. Biol. 2022, 10, 902601. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Miao, J.; Zhu, P. Regulatory T cell heterogeneity and therapy in autoimmune diseases. Autoimmun. Rev. 2021, 20, 102715. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Wang, D.; Hashemi, E.; Terhune, S.S.; Malarkannan, S. Implications of a ‘Third Signal’ in NK Cells. Cells 2021, 10, 1955. [Google Scholar] [CrossRef] [PubMed]
- Chee, S.J.; Lopez, M.; Mellows, T.; Gankande, S.; Moutasim, K.A.; Harris, S.; Clarke, J.; Vijayanand, P.; Thomas, G.J.; Ottensmeier, C.H. Evaluating the effect of immune cells on the outcome of patients with mesothelioma. Br. J. Cancer 2017, 117, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Fourcade, J.; Kudela, P.; Sun, Z.; Shen, H.; Land, S.R.; Lenzner, D.; Guillaume, P.; Luescher, I.F.; Sander, C.; Ferrone, S.; et al. PD-1 is a regulator of NY-ESO-1-specific CD8+ T cell expansion in melanoma patients. J. Immunol. 2009, 182, 5240–5249. [Google Scholar] [CrossRef]
- Heidkamp, G.F.; Sander, J.; Lehmann, C.H.K.; Heger, L.; Eissing, N.; Baranska, A.; Lühr, J.J.; Hoffmann, A.; Reimer, K.C.; Lux, A.; et al. Human lymphoid organ dendritic cell identity is predominantly dictated by ontogeny, not tissue microenvironment. Sci. Immunol. 2016, 1, eaai7677. [Google Scholar] [CrossRef] [PubMed]
- Peng, H.; Ning, H.; Wang, Q.; Lai, J.; Wei, L.; Stumpo, D.J.; Blackshear, P.J.; Fu, M.; Hou, R.; Hoft, D.F.; et al. Tristetraprolin Regulates TH17 Cell Function and Ameliorates DSS-Induced Colitis in Mice. Front. Immunol. 2020, 11, 1952. [Google Scholar] [CrossRef] [PubMed]
- Lang, Y.; Lyu, Y.; Tan, Y.; Hu, Z. Progress in construction of mouse models to investigate the pathogenesis and immune therapy of human hematological malignancy. Front. Immunol. 2023, 14, 1195194. [Google Scholar] [CrossRef] [PubMed]
- Ozulumba, T.; Montalbine, A.N.; Ortiz-Cárdenas, J.E.; Pompano, R.R. New tools for immunologists: Models of lymph node function from cells to tissues. Front. Immunol. 2023, 14, 1183286. [Google Scholar] [CrossRef]
- Cirelli, K.M.; Carnathan, D.G.; Nogal, B.; Martin, J.T.; Rodriguez, O.L.; Upadhyay, A.A.; Enemuo, C.A.; Gebru, E.H.; Choe, Y.; Viviano, F.; et al. Slow Delivery Immunization Enhances HIV Neutralizing Antibody and Germinal Center Responses via Modulation of Immunodominance. Cell 2019, 177, 1153–1171.e1128. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Reese, T.A. Making Mouse Models That Reflect Human Immune Responses. Trends Immunol. 2017, 38, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.M.; Brehm, M.A.; Bridges, S.; Ferguson, S.; Kumar, P.; Mirochnitchenko, O.; Palucka, K.; Pelanda, R.; Sanders-Beer, B.; Shultz, L.D.; et al. Humanized immune system mouse models: Progress, challenges and opportunities. Nat. Immunol. 2019, 20, 770–774. [Google Scholar] [CrossRef]
- Tu, W.; Zheng, J. Application of Humanized Mice in Immunological Research. Methods Mol. Biol. 2016, 1371, 157–176. [Google Scholar] [CrossRef] [PubMed]
- Walsh, N.C.; Kenney, L.L.; Jangalwe, S.; Aryee, K.E.; Greiner, D.L.; Brehm, M.A.; Shultz, L.D. Humanized Mouse Models of Clinical Disease. Annu. Rev. Pathol. 2017, 12, 187–215. [Google Scholar] [CrossRef]
- McCune, J.M.; Namikawa, R.; Kaneshima, H.; Shultz, L.D.; Lieberman, M.; Weissman, I.L. The SCID-hu mouse: Murine model for the analysis of human hematolymphoid differentiation and function. Science 1988, 241, 1632–1639. [Google Scholar] [CrossRef] [PubMed]
- De La Rochere, P.; Guil-Luna, S.; Decaudin, D.; Azar, G.; Sidhu, S.S.; Piaggio, E. Humanized Mice for the Study of Immuno-Oncology. Trends Immunol. 2018, 39, 748–763. [Google Scholar] [CrossRef]
- Mionnet, C.; Mondor, I.; Jorquera, A.; Loosveld, M.; Maurizio, J.; Arcangeli, M.L.; Ruddle, N.H.; Nowak, J.; Aurrand-Lions, M.; Luche, H.; et al. Identification of a New Stromal Cell Type Involved in the Regulation of Inflamed B Cell Follicles. PLoS Biol. 2013, 11, e1001672. [Google Scholar] [CrossRef]
- Alotaibi, F.; Vincent, M.; Min, W.P.; Koropatnick, J. Reduced CD5 on CD8(+) T Cells in Tumors but Not Lymphoid Organs Is Associated With Increased Activation and Effector Function. Front. Immunol. 2020, 11, 584937. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Duval, K.; Grover, H.; Han, L.H.; Mou, Y.; Pegoraro, A.F.; Fredberg, J.; Chen, Z. Modeling Physiological Events in 2D vs. 3D Cell Culture. Physiology 2017, 32, 266–277. [Google Scholar] [CrossRef] [PubMed]
- German, S.V.; Abalymov, A.A.; Kurochkin, M.A.; Kan, Y.; Gorin, D.A.; Novoselova, M.V. Plug-and-Play Lymph Node-on-Chip: Secondary Tumor Modeling by the Combination of Cell Spheroid, Collagen Sponge and T-Cells. Int. J. Mol. Sci. 2023, 24, 3183. [Google Scholar] [CrossRef]
- Lin, H.J.; Wang, W.; Huang, Y.Y.; Liao, W.T.; Lin, T.Y.; Lin, S.Y.; Liu, D.Z. Decellularized Lymph Node Scaffolding as a Carrier for Dendritic Cells to Induce Antitumor Immunity. Pharmaceutics 2019, 11, 553. [Google Scholar] [CrossRef] [PubMed]
- Liao, Z.; Jiang, J.; Wu, W.; Shi, J.; Wang, Y.; Yao, Y.; Sheng, T.; Liu, F.; Liu, W.; Zhao, P.; et al. Lymph node-biomimetic scaffold boosts CAR-T therapy against solid tumor. Natl. Sci. Rev. 2024, 11, nwae018. [Google Scholar] [CrossRef]
- Braham, M.V.J.; van Binnendijk, R.S.; Buisman, A.M.; Mebius, R.E.; de Wit, J.; van Els, C. A synthetic human 3D in vitro lymphoid model enhancing B-cell survival and functional differentiation. iScience 2023, 26, 105741. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Korolj, A.; Lai, B.F.L.; Radisic, M. Advances in organ-on-a-chip engineering. Nat. Rev. Mater. 2018, 3, 257–278. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, Y.; Pan, Y.; Zhou, D.; Liu, Y.; Yin, Y.; Yang, J.; Wang, Y.; Song, Y. Emerging trends in organ-on-a-chip systems for drug screening. Acta Pharm. Sin. B 2023, 13, 2483–2509. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Lv, X.; Li, X.; Rcheulishvili, N.; Chen, Y.; Li, Z.; Deng, Y. Microfluidic Actuated and Controlled Systems and Application for Lab-on-Chip in Space Life Science. Space Sci. Technol. 2023, 3, 8. [Google Scholar] [CrossRef]
- Kravchenko, S.V.; Myasnikova, V.V.; Sakhnov, S.N. Application of the organ-on-a-chip technology in experimental ophthalmology. Vestn. Oftalmol. 2023, 139, 114–120. [Google Scholar] [CrossRef]
- Kim, J.; Lee, H.; Selimović, Š.; Gauvin, R.; Bae, H. Organ-on-a-chip: Development and clinical prospects toward toxicity assessment with an emphasis on bone marrow. Drug Saf. 2015, 38, 409–418. [Google Scholar] [CrossRef] [PubMed]
- Panhwar, M.H.; Czerwinski, F.; Dabbiru, V.A.S.; Komaragiri, Y.; Fregin, B.; Biedenweg, D.; Nestler, P.; Pires, R.H.; Otto, O. High-throughput cell and spheroid mechanics in virtual fluidic channels. Nat. Commun. 2020, 11, 2190. [Google Scholar] [CrossRef] [PubMed]
- Żuchowska, A.; Baranowska, P.; Flont, M.; Brzózka, Z.; Jastrzębska, E. Review: 3D cell models for organ-on-a-chip applications. Anal. Chim. Acta 2024, 1301, 342413. [Google Scholar] [CrossRef]
- Chramiec, A.; Teles, D.; Yeager, K.; Marturano-Kruik, A.; Pak, J.; Chen, T.; Hao, L.; Wang, M.; Lock, R.; Tavakol, D.N.; et al. Integrated human organ-on-a-chip model for predictive studies of antitumor drug efficacy and cardiac safety. Lab Chip 2020, 20, 4357–4372. [Google Scholar] [CrossRef] [PubMed]
- Shanti, A.; Hallfors, N.; Petroianu, G.A.; Planelles, L.; Stefanini, C. Lymph Nodes-On-Chip: Promising Immune Platforms for Pharmacological and Toxicological Applications. Front. Pharmacol. 2021, 12, 711307. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.; Hyung, S.; Heo, Y.J.; Jung, S.; Kim, S.T.; Park, S.H.; Hong, J.Y.; Lim, S.H.; Kim, K.M.; Yoo, S.; et al. Patient-derived tumor spheroid-induced angiogenesis preclinical platform for exploring therapeutic vulnerabilities in cancer. Biomaterials 2024, 306, 122504. [Google Scholar] [CrossRef] [PubMed]
- Tabibzadeh, N.; Morizane, R. Advancements in therapeutic development: Kidney organoids and organs on a chip. Kidney Int. 2024, 105, 702–708. [Google Scholar] [CrossRef] [PubMed]
- Min, S.; Kim, S.; Sim, W.S.; Choi, Y.S.; Joo, H.; Park, J.H.; Lee, S.J.; Kim, H.; Lee, M.J.; Jeong, I.; et al. Versatile human cardiac tissues engineered with perfusable heart extracellular microenvironment for biomedical applications. Nat. Commun. 2024, 15, 2564. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Xiao, Y.; Liu, H.; Fan, Y.; Dao, M. Patient-Specific Organoid and Organ-on-a-Chip: 3D Cell-Culture Meets 3D Printing and Numerical Simulation. Adv. Biol. 2021, 5, e2000024. [Google Scholar] [CrossRef] [PubMed]
- Cheon, H.; Gelvosa, M.N.; Kim, S.A.; Song, H.Y.; Jeon, J.Y. Lymphatic channel sheet of polydimethylsiloxane for preventing secondary lymphedema in the rat upper limb model. Bioeng. Transl. Med. 2023, 8, e10371. [Google Scholar] [CrossRef]
- Belanger, M.C.; Anbaei, P.; Dunn, A.F.; Kinman, A.W.L.; Pompano, R.R. Spatially Resolved Analytical Chemistry in Intact, Living Tissues. Anal. Chem. 2020, 92, 15255–15262. [Google Scholar] [CrossRef] [PubMed]
- Haessler, U.; Pisano, M.; Wu, M.; Swartz, M.A. Dendritic cell chemotaxis in 3D under defined chemokine gradients reveals differential response to ligands CCL21 and CCL19. Proc. Natl. Acad. Sci. USA 2011, 108, 5614–5619. [Google Scholar] [CrossRef] [PubMed]
- Haessler, U.; Kalinin, Y.; Swartz, M.A.; Wu, M. An agarose-based microfluidic platform with a gradient buffer for 3D chemotaxis studies. Biomed. Microdevices 2009, 11, 827–835. [Google Scholar] [CrossRef] [PubMed]
- Ricart, B.G.; John, B.; Lee, D.; Hunter, C.A.; Hammer, D.A. Dendritic cells distinguish individual chemokine signals through CCR7 and CXCR4. J. Immunol. 2011, 186, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Moura Rosa, P.; Gopalakrishnan, N.; Ibrahim, H.; Haug, M.; Halaas, Ø. The intercell dynamics of T cells and dendritic cells in a lymph node-on-a-chip flow device. Lab Chip 2016, 16, 3728–3740. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.; Butcher, E.C. T cell chemotaxis in a simple microfluidic device. Lab Chip 2006, 6, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Mitra, B.; Jindal, R.; Lee, S.; Xu Dong, D.; Li, L.; Sharma, N.; Maguire, T.; Schloss, R.; Yarmush, M.L. Microdevice integrating innate and adaptive immune responses associated with antigen presentation by dendritic cells. RSC Adv. 2013, 3, 16002–16010. [Google Scholar] [CrossRef] [PubMed]
- Sonmez, U.M.; Wood, A.; Justus, K.; Jiang, W.; Syed-Picard, F.; LeDuc, P.R.; Kalinski, P.; Davidson, L.A. Chemotactic Responses of Jurkat Cells in Microfluidic Flow-Free Gradient Chambers. Micromachines 2020, 11, 384. [Google Scholar] [CrossRef]
- Shanti, A.; Samara, B.; Abdullah, A.; Hallfors, N.; Accoto, D.; Sapudom, J.; Alatoom, A.; Teo, J.; Danti, S.; Stefanini, C. Multi-Compartment 3D-Cultured Organ-on-a-Chip: Towards a Biomimetic Lymph Node for Drug Development. Pharmaceutics 2020, 12, 464. [Google Scholar] [CrossRef]
- Hallfors, N.; Shanti, A.; Sapudom, J.; Teo, J.; Petroianu, G.; Lee, S.; Planelles, L.; Stefanini, C. Multi-Compartment Lymph-Node-on-a-Chip Enables Measurement of Immune Cell Motility in Response to Drugs. Bioengineering 2021, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Goyal, G.; Prabhala, P.; Mahajan, G.; Bausk, B.; Gilboa, T.; Xie, L.; Zhai, Y.; Lazarovits, R.; Mansour, A.; Kim, M.S.; et al. Ectopic Lymphoid Follicle Formation and Human Seasonal Influenza Vaccination Responses Recapitulated in an Organ-on-a-Chip. Adv. Sci. 2022, 9, e2103241. [Google Scholar] [CrossRef] [PubMed]
- Birmingham, K.G.; O’Melia, M.J.; Bordy, S.; Reyes Aguilar, D.; El-Reyas, B.; Lesinski, G.; Thomas, S.N. Lymph Node Subcapsular Sinus Microenvironment-On-A-Chip Modeling Shear Flow Relevant to Lymphatic Metastasis and Immune Cell Homing. iScience 2020, 23, 101751. [Google Scholar] [CrossRef]
- Caldwell, D.J.; Rao, R.R.; Stegemann, J.P. Assembly of discrete collagen-chitosan microenvironments into multiphase tissue constructs. Adv. Healthc. Mater. 2013, 2, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Huang, J.; Liu, M.; Qiu, Y.; Chen, Q.; Zhao, T.; Xiao, Z.; Yang, Y.; Jiang, Y.; Huang, Q.; et al. Emerging Sonodynamic Therapy-Based Nanomedicines for Cancer Immunotherapy. Adv. Sci. 2023, 10, e2204365. [Google Scholar] [CrossRef] [PubMed]
- Qi, X.; Su, T.; Tong, X.; Xiong, W.; Zeng, Q.; Qian, Y.; Zhou, Z.; Wu, X.; Li, Z.; Shen, L.; et al. Facile formation of salecan/agarose hydrogels with tunable structural properties for cell culture. Carbohydr. Polym. 2019, 224, 115208. [Google Scholar] [CrossRef] [PubMed]
- Yun, E.J.; Yu, S.; Park, N.J.; Cho, Y.; Han, N.R.; Jin, Y.S.; Kim, K.H. Metabolic and enzymatic elucidation of cooperative degradation of red seaweed agarose by two human gut bacteria. Sci. Rep. 2021, 11, 13955. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Souza, A.; Sousa, P.; Ribeiro, J.; Castanheira, E.M.S.; Lima, R.; Minas, G. Properties and Applications of PDMS for Biomedical Engineering: A Review. J. Funct. Biomater. 2021, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, F.; Liu, J.; Zhang, Q.; Fan, Y. Rapid-release reversible bonding of PMMA-based microfluidic devices with PBMA coating. Biomed. Microdevices 2023, 26, 6. [Google Scholar] [CrossRef] [PubMed]
- Akther, F.; Little, P.; Li, Z.; Nguyen, N.T.; Ta, H.T. Hydrogels as artificial matrices for cell seeding in microfluidic devices. RSC Adv. 2020, 10, 43682–43703. [Google Scholar] [CrossRef] [PubMed]
- Wiig, H.; Keskin, D.; Kalluri, R. Interaction between the extracellular matrix and lymphatics: Consequences for lymphangiogenesis and lymphatic function. Matrix Biol. 2010, 29, 645–656. [Google Scholar] [CrossRef] [PubMed]
- Martino, M.M.; Hubbell, J.A. The 12th-14th type III repeats of fibronectin function as a highly promiscuous growth factor-binding domain. Faseb J. 2010, 24, 4711–4721. [Google Scholar] [CrossRef] [PubMed]
- Doherty, E.L.; Krohn, G.; Warren, E.C.; Patton, A.; Whitworth, C.P.; Rathod, M.; Biehl, A.; Aw, W.Y.; Freytes, D.O.; Polacheck, W.J. Human Cell-derived Matrix Composite Hydrogels with Diverse Composition for Use in Vasculature-on-chip Models. Adv. Healthc. Mater. 2024, e2400192. [Google Scholar] [CrossRef] [PubMed]
- Shao, Y.; Jia, H.; Cao, T.; Liu, D. Supramolecular Hydrogels Based on DNA Self-Assembly. Acc. Chem. Res. 2017, 50, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Laner-Plamberger, S.; Oeller, M.; Poupardin, R.; Krisch, L.; Hochmann, S.; Kalathur, R.; Pachler, K.; Kreutzer, C.; Erdmann, G.; Rohde, E.; et al. Heparin Differentially Impacts Gene Expression of Stromal Cells from Various Tissues. Sci. Rep. 2019, 9, 7258. [Google Scholar] [CrossRef] [PubMed]
- Black, C.; Kanczler, J.M.; de Andrés, M.C.; White, L.J.; Savi, F.M.; Bas, O.; Saifzadeh, S.; Henkel, J.; Zannettino, A.; Gronthos, S.; et al. Characterization and evaluation of the regenerative capacity of Stro-4+ enriched bone marrow mesenchymal stromal cells using bovine extracellular matrix hydrogel and a novel biocompatible melt electro-written medical-grade polycaprolactone scaffold. Biomaterials 2020, 247, 119998. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Wu, B.; Niedzielski, S.M.; Hill, M.T.; Coleman, R.M.; Ono, A.; Shikanov, A. Characterizing natural hydrogel for reconstruction of three-dimensional lymphoid stromal network to model T-cell interactions. J. Biomed. Mater. Res. A 2015, 103, 2701–2710. [Google Scholar] [CrossRef] [PubMed]
- Morrison, A.I.; Mikula, A.M.; Spiekstra, S.W.; de Kok, M.; Affandi, A.J.; Roest, H.P.; van der Laan, L.J.W.; de Winde, C.M.; Koning, J.J.; Gibbs, S.; et al. An Organotypic Human Lymph Node Model Reveals the Importance of Fibroblastic Reticular Cells for Dendritic Cell Function. Tissue Eng. Regen. Med. 2023, 21, 455–471. [Google Scholar] [CrossRef]
- Pérez Del Río, E.; Santos, F.; Rodriguez Rodriguez, X.; Martínez-Miguel, M.; Roca-Pinilla, R.; Arís, A.; Garcia-Fruitós, E.; Veciana, J.; Spatz, J.P.; Ratera, I.; et al. CCL21-loaded 3D hydrogels for T cell expansion and differentiation. Biomaterials 2020, 259, 120313. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Fauzi, F.A.M.; Hasnizam, S.B.; Azhari, A.S.; Lim, J.E.A.; Hao, L.Q.; Maarof, M.; Motta, A.; Fauzi, M.B. Engineered-Skin of Single Dermal Layer Containing Printed Hybrid Gelatin-Polyvinyl Alcohol Bioink via 3D-Bioprinting: In Vitro Assessment under Submerged vs. Air-Lifting Models. Pharmaceuticals 2022, 15, 1328. [Google Scholar] [CrossRef] [PubMed]
- Wassif, R.K.; Elkayal, M.; Shamma, R.N.; Elkheshen, S.A. Recent advances in the local antibiotics delivery systems for management of osteomyelitis. Drug Deliv. 2021, 28, 2392–2414. [Google Scholar] [CrossRef] [PubMed]
- de la Zerda, A.; Kratochvil, M.J.; Suhar, N.A.; Heilshorn, S.C. Review: Bioengineering strategies to probe T cell mechanobiology. APL Bioeng. 2018, 2, 021501. [Google Scholar] [CrossRef]
- Khalili, A.A.; Ahmad, M.R. A Review of Cell Adhesion Studies for Biomedical and Biological Applications. Int. J. Mol. Sci. 2015, 16, 18149–18184. [Google Scholar] [CrossRef] [PubMed]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef] [PubMed]
- Lamberti, G.; Prabhakarpandian, B.; Garson, C.; Smith, A.; Pant, K.; Wang, B.; Kiani, M.F. Bioinspired microfluidic assay for in vitro modeling of leukocyte-endothelium interactions. Anal. Chem. 2014, 86, 8344–8351. [Google Scholar] [CrossRef] [PubMed]
- Hammel, J.H.; Zatorski, J.M.; Cook, S.R.; Pompano, R.R.; Munson, J.M. Engineering in vitro immune-competent tissue models for testing and evaluation of therapeutics. Adv. Drug Deliv. Rev. 2022, 182, 114111. [Google Scholar] [CrossRef] [PubMed]
- Mittelbrunn, M.; Gutiérrez-Vázquez, C.; Villarroya-Beltri, C.; González, S.; Sánchez-Cabo, F.; González, M.; Bernad, A.; Sánchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Lallemand, C.; Liang, F.; Staub, F.; Simansour, M.; Vallette, B.; Huang, L.; Ferrando-Miguel, R.; Tovey, M.G. A Novel System for the Quantification of the ADCC Activity of Therapeutic Antibodies. J. Immunol. Res. 2017, 2017, 3908289. [Google Scholar] [CrossRef]
- Shim, S.; Belanger, M.C.; Harris, A.R.; Munson, J.M.; Pompano, R.R. Two-way communication between ex vivo tissues on a microfluidic chip: Application to tumor-lymph node interaction. Lab Chip 2019, 19, 1013–1026. [Google Scholar] [CrossRef] [PubMed]
- Belanger, M.C.; Ball, A.G.; Catterton, M.A.; Kinman, A.W.L.; Anbaei, P.; Groff, B.D.; Melchor, S.J.; Lukens, J.R.; Ross, A.E.; Pompano, R.R. Acute Lymph Node Slices Are a Functional Model System to Study Immunity Ex Vivo. ACS Pharmacol. Transl. Sci. 2021, 4, 128–142. [Google Scholar] [CrossRef] [PubMed]
- Farh, K.K.; Marson, A.; Zhu, J.; Kleinewietfeld, M.; Housley, W.J.; Beik, S.; Shoresh, N.; Whitton, H.; Ryan, R.J.; Shishkin, A.A.; et al. Genetic and epigenetic fine mapping of causal autoimmune disease variants. Nature 2015, 518, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Pape, K.A.; Catron, D.M.; Itano, A.A.; Jenkins, M.K. The humoral immune response is initiated in lymph nodes by B cells that acquire soluble antigen directly in the follicles. Immunity 2007, 26, 491–502. [Google Scholar] [CrossRef] [PubMed]
- Chtanova, T.; Han, S.J.; Schaeffer, M.; van Dooren, G.G.; Herzmark, P.; Striepen, B.; Robey, E.A. Dynamics of T cell, antigen-presenting cell, and pathogen interactions during recall responses in the lymph node. Immunity 2009, 31, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Comerford, I.; Harata-Lee, Y.; Bunting, M.D.; Gregor, C.; Kara, E.E.; McColl, S.R. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor. Rev. 2013, 24, 269–283. [Google Scholar] [CrossRef] [PubMed]
- Park, S.M.; Brooks, A.E.; Chen, C.J.; Sheppard, H.M.; Loef, E.J.; McIntosh, J.D.; Angel, C.E.; Mansell, C.J.; Bartlett, A.; Cebon, J.; et al. Migratory cues controlling B-lymphocyte trafficking in human lymph nodes. Immunol. Cell Biol. 2021, 99, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Cosgrove, J.; Novkovic, M.; Albrecht, S.; Pikor, N.B.; Zhou, Z.; Onder, L.; Mörbe, U.; Cupovic, J.; Miller, H.; Alden, K.; et al. B cell zone reticular cell microenvironments shape CXCL13 gradient formation. Nat. Commun. 2020, 11, 3677. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Asokan, S.B.; Bear, J.E.; Haugh, J.M. Quantitative analysis of B-lymphocyte migration directed by CXCL13. Integr. Biol. 2016, 8, 894–903. [Google Scholar] [CrossRef] [PubMed]
- Calvo, V.; Izquierdo, M. Inducible Polarized Secretion of Exosomes in T and B Lymphocytes. Int. J. Mol. Sci. 2020, 21, 2631. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liu, H.; Komai-Koma, M. Direct and indirect role of Toll-like receptors in T cell mediated immunity. Cell Mol. Immunol. 2004, 1, 239–246. [Google Scholar] [PubMed]
- Rastogi, I.; Jeon, D.; Moseman, J.E.; Muralidhar, A.; Potluri, H.K.; McNeel, D.G. Role of B cells as antigen presenting cells. Front. Immunol. 2022, 13, 954936. [Google Scholar] [CrossRef] [PubMed]
- Ung, T.; Rutledge, N.S.; Weiss, A.M.; Esser-Kahn, A.P.; Deak, P. Cell-targeted vaccines: Implications for adaptive immunity. Front. Immunol. 2023, 14, 1221008. [Google Scholar] [CrossRef] [PubMed]
- Purwada, A.; Shah, S.B.; Béguelin, W.; August, A.; Melnick, A.M.; Singh, A. Ex vivo synthetic immune tissues with T cell signals for differentiating antigen-specific, high affinity germinal center B cells. Biomaterials 2019, 198, 27–36. [Google Scholar] [CrossRef] [PubMed]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef] [PubMed]
- Ji, H.; Hu, C.; Yang, X.; Liu, Y.; Ji, G.; Ge, S.; Wang, X.; Wang, M. Lymph node metastasis in cancer progression: Molecular mechanisms, clinical significance and therapeutic interventions. Signal Transduct. Target. Ther. 2023, 8, 367. [Google Scholar] [CrossRef] [PubMed]
- Korkmaz, E.; Friedrich, E.E.; Ramadan, M.H.; Erdos, G.; Mathers, A.R.; Burak Ozdoganlar, O.; Washburn, N.R.; Falo, L.D., Jr. Therapeutic intradermal delivery of tumor necrosis factor-alpha antibodies using tip-loaded dissolvable microneedle arrays. Acta Biomater. 2015, 24, 96–105. [Google Scholar] [CrossRef] [PubMed]
- Restifo, N.P.; Dudley, M.E.; Rosenberg, S.A. Adoptive immunotherapy for cancer: Harnessing the T cell response. Nat. Rev. Immunol. 2012, 12, 269–281. [Google Scholar] [CrossRef] [PubMed]
- Gillot, L.; Baudin, L.; Rouaud, L.; Kridelka, F.; Noël, A. The pre-metastatic niche in lymph nodes: Formation and characteristics. Cell. Mol. Life Sci. 2021, 78, 5987–6002. [Google Scholar] [CrossRef] [PubMed]
- Akkaya, M.; Kwak, K.; Pierce, S.K. B cell memory: Building two walls of protection against pathogens. Nat. Rev. Immunol. 2020, 20, 229–238. [Google Scholar] [CrossRef] [PubMed]
- Giese, C.; Lubitz, A.; Demmler, C.D.; Reuschel, J.; Bergner, K.; Marx, U. Immunological substance testing on human lymphatic micro-organoids in vitro. J. Biotechnol. 2010, 148, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Kraus, T.; Lubitz, A.; Schließer, U.; Giese, C.; Reuschel, J.; Brecht, R.; Engert, J.; Winter, G. Evaluation of a 3D Human Artificial Lymph Node as Test Model for the Assessment of Immunogenicity of Protein Aggregates. J. Pharm. Sci. 2019, 108, 2358–2366. [Google Scholar] [CrossRef] [PubMed]
- Radke, L.; Sandig, G.; Lubitz, A.; Schließer, U.; von Horsten, H.H.; Blanchard, V.; Keil, K.; Sandig, V.; Giese, C.; Hummel, M.; et al. In Vitro Evaluation of Glycoengineered RSV-F in the Human Artificial Lymph Node Reactor. Bioengineering 2017, 4, 70. [Google Scholar] [CrossRef] [PubMed]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef] [PubMed]
- GeurtsvanKessel, C.H.; Willart, M.A.; Bergen, I.M.; van Rijt, L.S.; Muskens, F.; Elewaut, D.; Osterhaus, A.D.; Hendriks, R.; Rimmelzwaan, G.F.; Lambrecht, B.N. Dendritic cells are crucial for maintenance of tertiary lymphoid structures in the lung of influenza virus-infected mice. J. Exp. Med. 2009, 206, 2339–2349. [Google Scholar] [CrossRef] [PubMed]
- Epeldegui, M.; Thapa, D.R.; De la Cruz, J.; Kitchen, S.; Zack, J.A.; Martínez-Maza, O. CD40 ligand (CD154) incorporated into HIV virions induces activation-induced cytidine deaminase (AID) expression in human B lymphocytes. PLoS ONE 2010, 5, e11448. [Google Scholar] [CrossRef] [PubMed]
- Muramatsu, M.; Kinoshita, K.; Fagarasan, S.; Yamada, S.; Shinkai, Y.; Honjo, T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 2000, 102, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Havenar-Daughton, C.; Lindqvist, M.; Heit, A.; Wu, J.E.; Reiss, S.M.; Kendric, K.; Belanger, S.; Kasturi, S.P.; Landais, E.; Akondy, R.S.; et al. CXCL13 is a plasma biomarker of germinal center activity. Proc. Natl. Acad. Sci. USA 2016, 113, 2702–2707. [Google Scholar] [CrossRef]
- Jackson, D.J.; Johnston, S.L. The role of viruses in acute exacerbations of asthma. J. Allergy Clin. Immunol. 2010, 125, 1178–1187, quiz 1188–1179. [Google Scholar] [CrossRef] [PubMed]
- Ai, J.; Guo, J.; Zhang, H.; Zhang, Y.; Yang, H.; Lin, K.; Song, J.; Fu, Z.; Fan, M.; Zhang, Q.; et al. Cellular basis of enhanced humoral immunity to SARS-CoV-2 upon homologous or heterologous booster vaccination analyzed by single-cell immune profiling. Cell Discov. 2022, 8, 114. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.E.; Belanger, M.C.; Woodroof, J.F.; Pompano, R.R. Spatially resolved microfluidic stimulation of lymphoid tissue ex vivo. Analyst 2017, 142, 649–659. [Google Scholar] [CrossRef]
- Zhao, J.; Wei, C.; Wang, S.; Zhang, Y.; Wang, W.; Zhao, D.; Wang, Z.; Zhou, Z.; Bai, J.; Zhang, W.; et al. The intrinsic defects of T cells impact the efficacy of CAR-T therapy in patients with diffuse large B-cell lymphoma. Blood Cancer J. 2023, 13, 186. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Zou, Z.; Pu, Z.; Wu, M.; Zhang, Y. Application of microfluidic technologies on COVID-19 diagnosis and drug discovery. Acta Pharm. Sin. B 2023, 13, 2877–2896. [Google Scholar] [CrossRef] [PubMed]
- Ruggiu, M.; Guérin, M.V.; Corre, B.; Bardou, M.; Alonso, R.; Russo, E.; Garcia, Z.; Feldmann, L.; Lemaître, F.; Dusseaux, M.; et al. Anti-PD-1 therapy triggers Tfh cell-dependent IL-4 release to boost CD8 T cell responses in tumor-draining lymph nodes. J. Exp. Med. 2024, 221, e20232104. [Google Scholar] [CrossRef] [PubMed]
- Kwee, B.J.; Li, X.; Nguyen, X.X.; Campagna, C.; Lam, J.; Sung, K.E. Modeling immunity in microphysiological systems. Exp. Biol. Med. 2023, 248, 2001–2019. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Peluzzi, V.; Raniolo, S.; Roscilli, G.; Trombetta, M.; Mozetic, P.; Rainer, A. On-chip recapitulation of the tumor microenvironment: A decade of progress. Biomaterials 2024, 306, 122482. [Google Scholar] [CrossRef] [PubMed]
- Pavesi, A.; Tan, A.T.; Koh, S.; Chia, A.; Colombo, M.; Antonecchia, E.; Miccolis, C.; Ceccarello, E.; Adriani, G.; Raimondi, M.T.; et al. A 3D microfluidic model for preclinical evaluation of TCR-engineered T cells against solid tumors. JCI Insight 2017, 2, e89762. [Google Scholar] [CrossRef] [PubMed]
- Ke, Y.; Xin, K.; Tao, Y.; Li, L.; Chen, A.; Shao, J.; Zhu, J.; Zhang, D.; Cen, L.; Chu, Y.; et al. A Thermosensitive Bi-Adjuvant Hydrogel Triggers Epitope Spreading to Promote the Antitumor Efficacy of Frameshift Neoantigens. Adv. Sci. 2024, 11, e2306889. [Google Scholar] [CrossRef] [PubMed]
- Park, J.Y.; Jang, J.; Kang, H.-W. 3D Bioprinting and its application to organ-on-a-chip. Microelectron. Eng. 2018, 200, 1–11. v. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Yue, K.; Aleman, J.; Moghaddam, K.M.; Bakht, S.M.; Yang, J.; Jia, W.; Dell’Erba, V.; Assawes, P.; Shin, S.R.; et al. 3D Bioprinting for Tissue and Organ Fabrication. Ann. Biomed. Eng. 2017, 45, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Yin, Y.; Zhou, H.; Fan, Y.; Yang, Y.; Gao, Q.; Li, P.; Gao, G.; Li, J. Recent Advances in Electrospinning Techniques for Precise Medicine. Cyborg Bionic Syst. 2024, in press. [CrossRef]
- Valverde, M.G.; Mille, L.S.; Figler, K.P.; Cervantes, E.; Li, V.Y.; Bonventre, J.V.; Masereeuw, R.; Zhang, Y.S. Biomimetic models of the glomerulus. Nat. Rev. Nephrol. 2022, 18, 241–257. [Google Scholar] [CrossRef] [PubMed]
- Mazzaglia, C.; Munir, H.; Le, I.M.; Gerigk, M.; Huang, Y.Y.S.; Shields, J.D. Modelling Structural Elements and Functional Responses to Lymphatic-Delivered Cues in a Murine Lymph Node on a Chip. Adv. Healthc. Mater. 2024, e2303720. [Google Scholar] [CrossRef] [PubMed]
- Yin, D.E.; Palin, A.C.; Lombo, T.B.; Mahon, R.N.; Poon, B.; Wu, D.Y.; Atala, A.; Brooks, K.M.; Chen, S.; Coyne, C.B.; et al. 3D human tissue models and microphysiological systems for HIV and related comorbidities. Trends Biotechnol. 2023, 42, 526–543. [Google Scholar] [CrossRef] [PubMed]
- Nawroth, J.C.; Barrile, R.; Conegliano, D.; van Riet, S.; Hiemstra, P.S.; Villenave, R. Stem cell-based Lung-on-Chips: The best of both worlds? Adv. Drug Deliv. Rev. 2019, 140, 12–32. [Google Scholar] [CrossRef] [PubMed]
- Alderfer, L.; Hall, E.; Hanjaya-Putra, D. Harnessing biomaterials for lymphatic system modulation. Acta Biomater. 2021, 133, 34–45. [Google Scholar] [CrossRef]
- Solari, E.; Marcozzi, C.; Negrini, D.; Moriondo, A. Lymphatic Vessels and Their Surroundings: How Local Physical Factors Affect Lymph Flow. Biology 2020, 9, 463. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.; Zhao, G.; Chen, R.; Li, Y.; Huang, J.; Kuang, L.; Zhang, D.; Li, Z.; Xu, H.; Xiang, W.; et al. Lymphatic vessels: Roles and potential therapeutic intervention in rheumatoid arthritis and osteoarthritis. Theranostics 2024, 14, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Johnson, L.A.; Clasper, S.; Holt, A.P.; Lalor, P.F.; Baban, D.; Jackson, D.G. An inflammation-induced mechanism for leukocyte transmigration across lymphatic vessel endothelium. J. Exp. Med. 2006, 203, 2763–2777. [Google Scholar] [CrossRef] [PubMed]
- Henderson, A.R.; Ilan, I.S.; Lee, E. A bioengineered lymphatic vessel model for studying lymphatic endothelial cell-cell junction and barrier function. Microcirculation 2021, 28, e12730. [Google Scholar] [CrossRef] [PubMed]
- Van Os, L.; Engelhardt, B.; Guenat, O.T. Integration of immune cells in organs-on-chips: A tutorial. Front. Bioeng. Biotechnol. 2023, 11, 1191104. [Google Scholar] [CrossRef] [PubMed]
- Hammel, J.H.; Cook, S.R.; Belanger, M.C.; Munson, J.M.; Pompano, R.R. Modeling Immunity In Vitro: Slices, Chips, and Engineered Tissues. Annu. Rev. Biomed. Eng. 2021, 23, 461–491. [Google Scholar] [CrossRef] [PubMed]



| Reference Article | Spatial Configuration | Lymph Node Scaffolding Material | ECM Material | Dynamic Condition | Fluid Flow Rate | Cellular Component | CELL Source | Cell Culture Time | Validated Immune System Function | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Haessler et al. (2011) [52] | Immune cells in the middle channel and chemokine channels of different concentrations on either side. | Agarose | Hydrogel (Type I collagen+ Matrigel) | Active pumping | 1 μL/min (chemokine) | Dendritic cell (primary) | Mouse | 120 min | Establish stable and well-defined gradients in advance. Visualize live-cell migration. The DC migrated more efficiently to the higher gradient of CCL21. |
| 2 | Haessler et al. (2009) [53] | 1.0 mm thick agarose membrane patterned with four sets of three-channel units, each unit containing a cell-ECM channel and two flow channels. | Agarose | Hydrogel (Type I collagen) | Active pumping | 5 μL/min (chemokine) | Dendritic cell | Mouse bone marrow | Quantified the chemotactic response of murine DC to a gradient of CCL19 | |
| 3 | Ricart et al. (2011) [54] | Three different cytokine input mix regions (forms smooth gradient). | PDMS | Fibronectin | Syringe pump | 9 μL/min (chemokine) | Dendritic cell | Mouse bone marrow | 60 min | A single chemokine gradient and a competitive chemokine gradient were presented in the controlled microenvironment (CCL21, CCL19, CXCL12). |
| 4 | Moura et al. (2016) [55] | It has one main channel, two entrances, and two exits. Two distinct inlets pump CD4+ T cells and CD8+ T cells, respectively. | PDMS | Hydrogel (Collagen or Fibronectin) | Syringe pump | 10−4–1 mL/min (T cell) | Murine tumor DC MF2.2D, OVAII RF33.70/OVAI | Mouse | Dynamic interaction of flowing lymphocytes with adherent DC, Effects of low and high shear stress variations on adhesion | |
| 5 | Lin et al. (2006) [56] | A “Y” type fluidic channel. | PDMS | Fibronectin | Syringe pump | 0.2 mL/min (chemokine) | T cell (activation) | Human Blood | 20 min | Human T cells in response to single and competing gradients of chemokine CCL19 and CXCL12. |
| 6 | Mitra et al. (2013) [57] | Two layers of PDMS: the top layer contains the chemotaxis chamber, and the bottom layer includes the T cell compartment. | PDMS | Hydrogel | Syringe pump | 0.4–0.5 μL/min | MUTZ-3: Human dendritic cell line T cell | Human Human Blood | 2 h | Mature DCs are subject to a gradient effect by the chemokine CCL19 Mature DCs are collected in T cell compartments to induce T cell activation. |
| 7 | Sonmez et al. (2020) [58] | PC membrane filters separate two PDMS layers: the upper layer consists of flow channels, and the lower layer consists of flow-free chambers. | PDMS (0.4 μm PC membrane filter) | Fibronectin | Jurkat: Human T cell line | Human | 30 min | The chemotaxis of the Jurkat cells was also found to be governed by the CXCL12 gradient and the average CXCL12 concentration. | ||
| 8 | Shanti et al. (2020) [59] | A multi-chamber bioreactor, separated by circularly distributed microcolumns. The outermost region corresponds to the subcapsular sinus, the middle region corresponds to the reticular ductal structure, and the inner region is divided into upper and lower regions, corresponding to the B follicle and paracortex. | PDMS (Hydrogel microcolumns) | Hydrogel (Type I collagen) | micropump | EB1: Human B cell line THP-1:Human Dendritic cell line Jurkat: Human T cell line | Human | 72 h | Long-term culture and in situ viability testing of Sertoli cells Interactions between different cell types across chamber boundaries were observed. The flow pattern of lymphatic fluid was replicated. | |
| 9 | Hallfors et al. (2021) [60] | microfluidic pump S | 3 μL/min | Raji B: Human B cell line Jurkat: Human T cell line | Tested the effect of the immunomodulatory drug hydroxychloroquine (HCQ) on cells. | |||||
| 10 | Goyal et al. (2022) [61] | Two channels are divided by a porous membrane; the lower channel consists of T, B lymphocytes, and hydrogels, and the upper channel is continuously perfused with medium | PDMS | Hydrogel | peristaltic pumps or Automated Zoe Organ Chip instruments | 60 µL/h | T cell B cell | Human Blood | >9 d | Mimic germinal center formation, class switching, and Ab production. Antigen-specific Ab can be produced by the commercial Fluzone influenza vaccine for three different strains and the H5N1 pandemic influenza antigen inoculated LF chip formulated with the oil-in-water adjuvant SVE of squalene. |
| 11 | Birmingham et al. (2020) [62] | Constructed from a sheet of PDMS and a polystyrene tissue culture plate between which is a 125 μm adhesive gasket. | PDMS | No hydrogel | Active pumping | 2.5 μg/mL | Thp1 human monocyte line LS174T human colon cancer cell line PANC-1 human pancreatic cell line | Human | Effect of subcapsular sinus biophysical (flow and structure) and biochemical (adhesion molecule expression) remodeling on cellular adhesion. | |
| 12 | German et al. (2023) [33] | A central channel with an extension in the center of the main channel in which a collagen sponge is mounted, inside which cell spheroids are placed. | PDMS | Collagen sponge | Micro pumps | 0.65 mL/h | 4T1 breast cancer spheroids Jurkat cell | Human | To evaluate the effect of contrast/drug vehicle size on the penetration and accumulation of particles in 3D spheroids simulating secondary tumors with lymphadenopathy. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Q.; Yang, Y.; Chen, Z.; Li, B.; Niu, Y.; Li, X. Lymph Node-on-Chip Technology: Cutting-Edge Advances in Immune Microenvironment Simulation. Pharmaceutics 2024, 16, 666. https://doi.org/10.3390/pharmaceutics16050666
Wang Q, Yang Y, Chen Z, Li B, Niu Y, Li X. Lymph Node-on-Chip Technology: Cutting-Edge Advances in Immune Microenvironment Simulation. Pharmaceutics. 2024; 16(5):666. https://doi.org/10.3390/pharmaceutics16050666
Chicago/Turabian StyleWang, Qi, Yuanzhan Yang, Zixuan Chen, Bo Li, Yumeng Niu, and Xiaoqiong Li. 2024. "Lymph Node-on-Chip Technology: Cutting-Edge Advances in Immune Microenvironment Simulation" Pharmaceutics 16, no. 5: 666. https://doi.org/10.3390/pharmaceutics16050666
APA StyleWang, Q., Yang, Y., Chen, Z., Li, B., Niu, Y., & Li, X. (2024). Lymph Node-on-Chip Technology: Cutting-Edge Advances in Immune Microenvironment Simulation. Pharmaceutics, 16(5), 666. https://doi.org/10.3390/pharmaceutics16050666






