Abstract
In recent years, there has been a notable surge in the utilization of stabilized bile acid liposomes, chemical conjugates, complexes, mixed micelles, and other drug delivery systems derived from bile acids, often referred to as bilosomes. The molecular structure and interactions of these amphiphilic compounds provide a distinctive and captivating subject for investigation. The enhanced stability of new generation bilosomes inside the gastrointestinal system results in the prevention of drug degradation and an improvement in mucosal penetration. These characteristics render bilosomes to be a prospective nanocarrier for pharmaceutical administration, prompting researchers to investigate their potential in other domains. This review paper discusses bilosomes that have emerged as a viable modality in the realm of drug delivery and have significant promise for use across several domains. Moreover, this underscores the need for additional investigation and advancement in order to comprehensively comprehend the prospective uses of bilosomes and their effectiveness in the field of pharmaceutical administration. This review study explores the current scholarly attention on bilosomes as prospective carriers for drug delivery. Therapeutic areas where bilosomes have shown outstanding performance in terms of drug delivery are outlined in the graphical abstract.
1. Introduction
New approaches in drug development have resulted in the introduction of several compounds with subpar bioavailability profiles. Lipophilicity is a problem for many therapeutic compounds because it reduces their solubility in gastrointestinal (GI) fluids and hence their bioavailability [1]. Most biological molecules (proteins and peptides) and vaccines do not work well when taken orally because they degrade extensively in the digestive tract. Numerous strategies have been implemented to address the shortcomings of conventional delivery methods. Vesicular carriers like liposomes and niosomes, which are part of the innovative drug delivery system, can protect the encapsulated components from degradation to some degree. Bilosomes are the novel modified form of liposomes and niosomes with the advantage of more flexibility, absorption, and permeation due to the presence of bile salts. Bilosomes are bile salt-containing nano vesicular systems used for the administration of highly lipophilic and poorly permeable therapeutics [2,3]. The role of bile acids in drug delivery as permeation enhancers is well explained in the literature [4,5,6]. Oral drug administration as an alternative to injectable therapy is made possible by bilosomes that protect therapeutic agents from being broken down in the stomach. Due to their stability in the gut, bilosomes improve the biocompatibility of medications and boost their therapeutic effectiveness [7]. Bilosomes are reported to improve the GI tract stability and permeability of hydrophilic molecules like proteins, peptides, and antigens across the cell membrane [8,9]. Due to their amphiphilic nature, bile salts incorporated within bilosomes, such as sodium glycocholate, sodium deoxycholate, and sodium taurocholate, enhance the penetration properties of the system, further improving the oral bioavailability and biodistribution of drugs with limited aqueous solubility and permeability [6,10].
Comparisons between conventional liposomes and niosomes formulations with advanced bilosomal formulations have demonstrated enhanced bioavailability and the permeation properties of bilosomes over other vesicular systems [11,12,13,14]. Liposomes and niosomes are both types of vesicular drug delivery systems, but they differ in their composition, structure, and properties. Liposomes are composed of phospholipids, which are natural or synthetic amphiphilic molecules with a hydrophilic head and hydrophobic tail. These phospholipids self-assemble into bilayer structures in aqueous environments, forming vesicles with aqueous cores enclosed by lipid bilayers. Niosomes, on the other hand, are composed of non-ionic surfactants, which are amphiphilic molecules similar to phospholipids but lack the phosphate group. Non-ionic surfactants typically consist of a hydrophilic head (such as polyethylene glycol) and a hydrophobic tail (such as alkyl chains). Niosomes also form vesicular structures similar to liposomes, with aqueous cores enclosed by surfactant bilayers. Liposomes formed by phospholipids tend to be more stable due to the presence of natural phospholipids with well-defined structures and packing arrangements. However, they may be susceptible to degradation by enzymes in biological fluids. Niosomes are generally less stable compared to liposomes, primarily because non-ionic surfactants may undergo phase transitions or degradation under certain conditions.
The therapeutic application of bilosomes as drug delivery carriers is well established for phytoconstituent drug delivery, anticancer drugs, CNS drugs, protein/peptide, and vaccine drug delivery [1,15,16,17]. In this review, the structural aspects and therapeutic application of bilosomes as nanovesicular drug delivery carriers are discussed. Moreover, a brief overview of bilosomes-based drug delivery systems for treating various disorders through different routes mainly oral, ocular, and transdermal are also discussed.
2. Methodology
In this review, we have summarized the advances in the application of bilosomes as a nanocarrier for drug delivery mainly for oral vaccines, proteins and peptides, and drugs used to treat CNS disorders, ocular disorders, and cancer. A literature search of relevant publications published in the last 10 years was performed using several scientific databases such as PubMed, Scopus, Elsevier, and ScienceDirect. The main keywords used to search the literature are bile salts, bilosomes, permeation enhancers, modified liposomes or niosomes, and ultra-deformable nanovesicles. Only English-language publications were included in this review.
3. Bilosomes: Structural Aspects
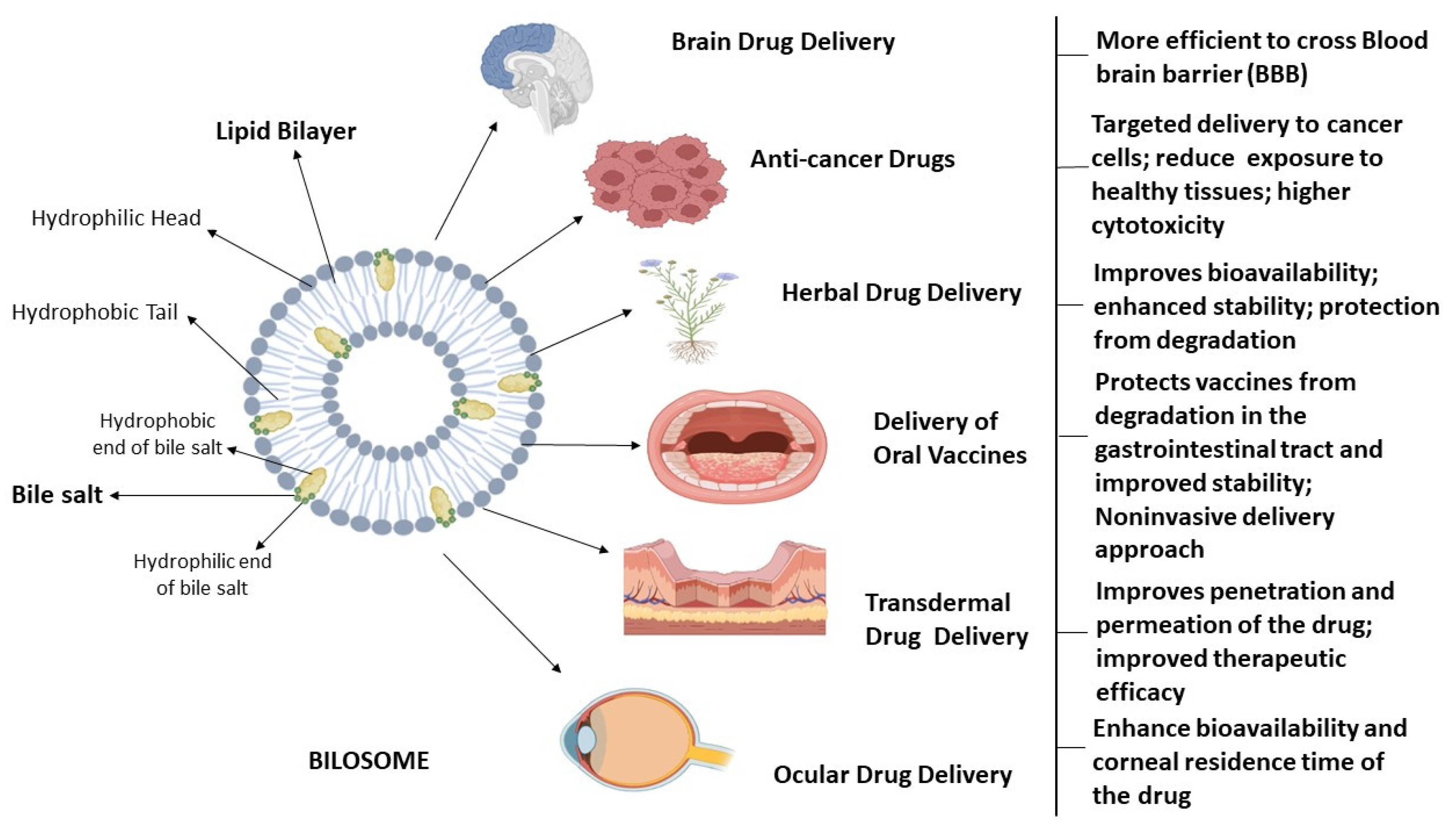
Conacher et al. were the first to describe bilosomes, which are closed bilayer structures of non-ionic amphiphiles that are structurally similar to non-ionic surfactant vesicles (niosomes) but additionally contain bile salts [10]. Bile salts shield the bilosomes and their contents from the acidic and alkaline conditions of the GI tract. Additionally, the inclusion of bile salts in bilosomal encapsulation has been shown to boost intestinal permeability, leading to the increased bioavailability of orally administered medications. Non-ionic surfactants, such as those found in the Span family (Span 40, Span 60, Span 80), are often used in the formulation of bilosomes, usually in conjunction with cholesterol and bile salts [1,3,18]. The components used in the formulation of bilosomes are widely acknowledged as safe and have been shown to exhibit perfect biocompatibility and biodegradability. Bilosomes, as a synthetic nano-sized vesicular carrier system, are a promising avenue for enhancing the efficacy of oral medication delivery across a diverse spectrum of pharmaceutical compounds. These vesicular nanostructures were analyzed for their ability to enclose hydrophilic and hydrophobic substances in their aqueous core and phospholipid bilayer, respectively. Bile salts, non-ionic surfactants, and lipids are principal vesicular components in the synthesis of bilosomes [19]. The bilosomes have two layers, with the innermost layer containing hydrophilic medicines and antigens and the outermost layer containing bile salts and hydrophobic medications. The bile salts are encased in lipid layers within the bilosomal structure, giving it a characteristic closed shape. The hydrophilic end of bile acid molecule is faced towards the hydrophilic part in the lipid bilayer whereas the hydrophobic end of bile acid is embedded in the hydrophobic part of lipid bilayer in a bilosome vesicle [2,20,21]. The structure of bilosomes and their utility in administering different types of drugs through different routes is illustrated in Figure 1.
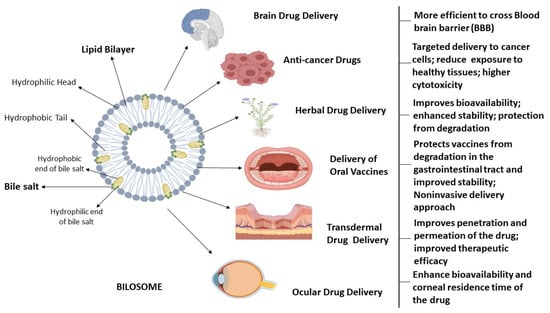
Figure 1.
Bilosomes in therapeutic drug delivery. This is a schematic representation of the architectural characteristics of bilosomes, which are enclosed double-layered structures composed of non-ionic amphiphiles that consist of hydrophilic head and hydrophobic tails, as well as bile salts. Bilosomes serve as highly efficient nanocarriers for delivering medications to address a range of brain illnesses, targeted drug delivery for cancer treatment, ophthalmic ailments, and improving the absorption of orally and transdermally administered pharmaceuticals.
3.1. Lipids as a Component of Bilosomes
Mostly, phospholipids and cholesterol are employed in the manufacturing of bilosomes. Phospholipids have a high degree of biocompatibility with biological membranes. The amphiphilic characteristic of phospholipids gives them the ability to self-assemble into concentric bilayers, resulting in wetting and emulsification [6]. The phospholipids often used in the formulation of bilosomes are dicetyl phosphate, distearoyl phosphatidylglycerol, soybean phosphatidylcholine, and monopalmitoyl glycerol [22]. Cholesterol is introduced into the cellular membrane as an amphiphilic molecule, with the hydroxyl groups towards the aqueous surface and the aliphatic chains aligned perpendicular to the acyl chains in the bilayer’s core. The effect of cholesterol on the bilosome membrane is dependent on the phase state of the lipid bilayer, which can be determined by the lipid composition. Cholesterol causes phase separations in multi-component lipid mixtures, selectively partitions between distinct coexisting lipid phases, and drives integral membrane proteins to change their conformation or to redistribute in the membrane [23]. Therefore, the rigidity of bilosomes is enhanced by adding cholesterol [24,25,26].
3.2. Non-Ionic Surfactant as a Component of Bilosomes
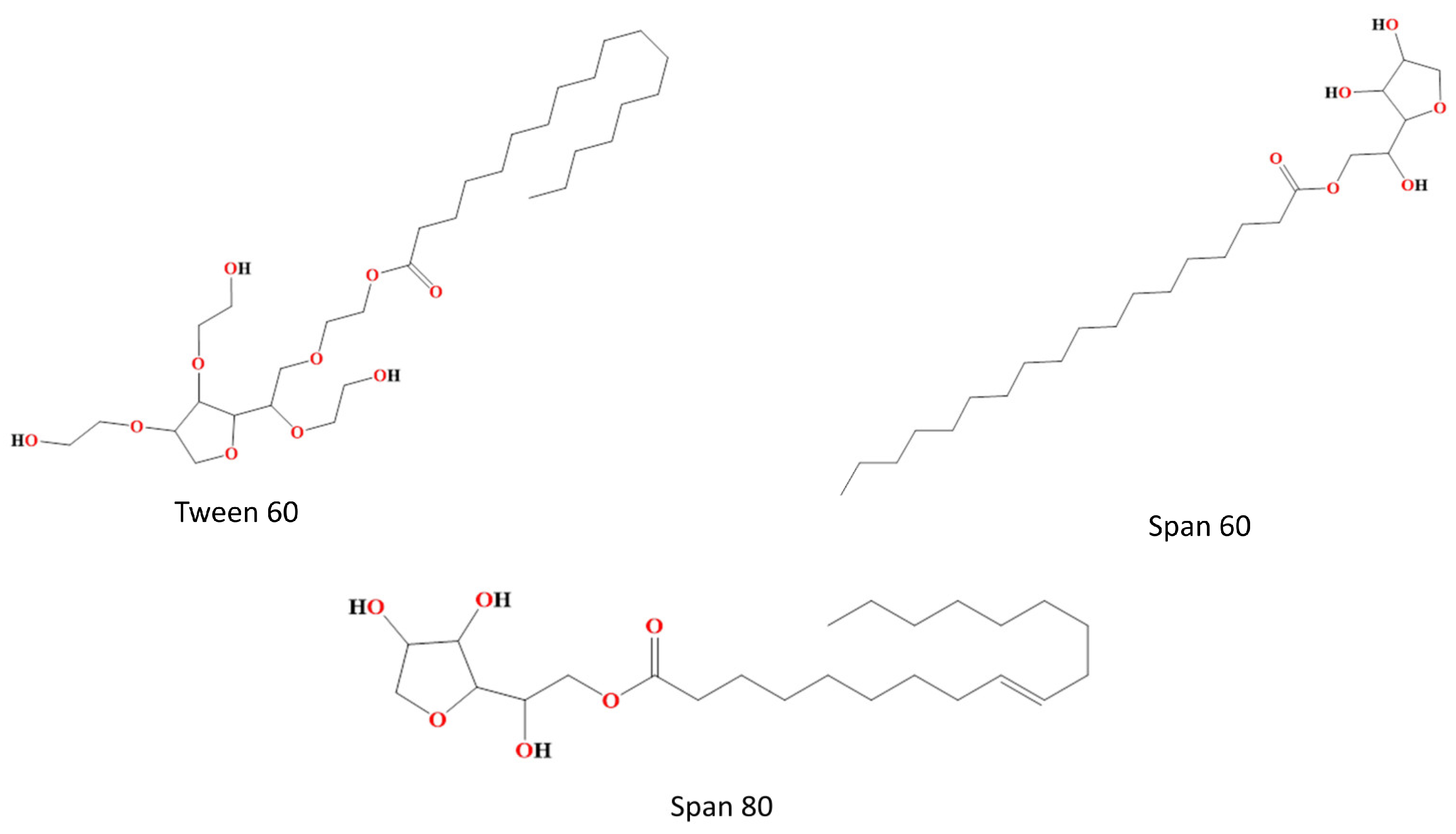
Non-ionic surfactants are often utilized in the formulation of bilosomes due to their superior stability and compatibility as compared to anionic, cationic, and amphoteric surfactants. Non-ionic surfactants are less hemolytic and more conducive in preserving the solution’s pH around its physiological range. These substances serve as solubilizers, wetting agents, emulsifiers, and permeability enhancers [27]. Additionally, non-ionic surfactants serve as potent inhibitors of P-glycoprotein, preventing drug efflux from cells. They facilitate the absorption of drugs and enable targeted delivery to certain tissues. Non-ionic surfactants consist of both polar and non-polar regions, hence exhibiting significant interfacial activity. The entrapment effectiveness of the medication is influenced by the chain length and size of the hydrophilic head groups of non-ionic surfactants. The entrapment efficiency of non-ionic surfactants, namely those containing stearyl (C18) chains, is greater compared to those containing lauryl (C12) chains [28,29]. To achieve the enhanced entrapment efficiency of water-soluble medications, a mixture of Tweens including elongated alkyl chains and substantial hydrophilic components, combined with cholesterol in a 1:1 ratio, can be used [30]. The hydrophilic–lipophilic balance (HLB) value of surfactants is a significant factor in the regulation of drug entrapment inside vesicles. Surfactants with an HLB within the range of 14–17 have been deemed unsuitable for the production of bilosomes vesicles. Conversely, an HLB value of 8.6 has been shown to provide maximum effectiveness in terms of entrapment. The entrapment efficiency decreases when the HLB value decreases from 8.6 to 1.7. The non-ionic surfactants that are often used for the purpose of vesicle production are alkyl esters, alkyl glyceryl ethers and sorbitan fatty acid esters [30]. Non-ionic surfactants which are mainly used in bilosome formulation are shown in Figure 2.
Figure 2.
Non-ionic surfactants used in bilosome formulation, e.g., Tween 60, Span 60 and Span 80.
3.3. Bile Salts as a Component of Bilosomes
Bile salts are endogenous biosurfactants that are found inside the lumen of the gastrointestinal tract. They serve a crucial function in facilitating the digestion and absorption of lipids. Increased bile production results in the absorption of more physiologically active chemicals. This has led to the development of several mixed micelle systems to enhance the solubility of extremely lipophilic pharmaceuticals [31]. Bile salts have the additional effect of improving the flexibility of bilosomes in simulated fluids and the same is reported by Mondal et al. through the use of microscopy and fluorescence techniques [32]. This is achieved through the induction of repulsion between the bile salts contained inside the bilosomes and the external bile salts found in the gut lumen. The most common bile salts used in the manufacturing of bilosomes are sodium glycocholate (SGC), sodium deoxycholate (SDC), and sodium taurocholate (STC). The selection of sodium glycocholate (SGC), sodium deoxycholate (SDC), and sodium taurocholate (STC) as the main bile salts in bilosomes is primarily due to their unique properties and advantages in the drug delivery system [29,30]. These bile salts possess excellent solubilization properties, particularly for lipophilic compounds. By forming mixed micelles with lipids, they can encapsulate hydrophobic drugs effectively, enhancing their solubility in aqueous environments. This is crucial for drug delivery systems like bilosomes, where the efficient solubilization of drugs is essential for their bioavailability and therapeutic efficacy. SGC, SDC, and STC are naturally occurring bile salts found in the bile of mammals, including humans. They are biocompatible and well-tolerated by the body, which is essential for pharmaceutical applications. Their use minimizes the risk of adverse effects, making them suitable for incorporation into drug delivery systems intended for medical use. These salts can interact with cell membranes, facilitating the absorption of encapsulated drugs. This property is advantageous for enhancing the delivery of therapeutic agents to target cells or tissues. By incorporating bile salts with a lipid membrane, the bioavailability and therapeutic outcomes of the encapsulated drugs in bilosomes can be improved. These salts contribute to the stability of bilosomes by participating in the formation of bilayer structures alongside phospholipids. This helps to prevent the aggregation or fusion of bilosomes, ensuring their integrity during storage and administration. Overall, the selection of SGC, SDC, and STC as the primary bile salts in bilosomes is based on their solubilization ability, biocompatibility, interaction with cell membranes, and contribution to structural stability, all of which are crucial for the successful formulation and performance of bilosomes as drug delivery system [20].
4. Bilosomes as Nanocarrier Drug Delivery System for Treating Various Disorders
Bilosomes are a novel and promising lipid-based nanocarrier system used in pharmaceutical drug delivery. These nanocarriers have gained attention due to their potential to enhance the delivery and bioavailability of drugs, particularly for both hydrophilic and lipophilic compounds. Bilosomes as a nanocarrier drug delivery system for treating various disorders are discussed below.
4.1. Bilosomes as Drug Delivery System for Brain Disorders
The blood–brain barrier (BBB) is the primary stumbling block in the way of novel therapies for entering the central nervous system [33,34]. The BBB is composed of three biological components of the brain microvasculature: endothelial cells, astrocyte end-feet, and pericytes [35]. Since bile acids are orally bioavailable and may cross the microvascular endothelial cells in the human brain, studying their role in neurodegenerative diseases is an exciting prospect [36,37]. CNS drugs with limited therapeutic efficacy can be encapsulated into bilosomes for brain targeting. The bioavailability of such formulations could be enhanced by administering them through an oral or intranasal route.
For instance, Abbas et al. [38] developed resveratrol (RES)-loaded bilosomes for the management of Alzheimer’s disease (AD) through the oral route of administration. Resveratrol (RES), a well-known bioactive polyphenol compound, is used to treat AD but its clinical applicability is limited due to poor water solubility [39]. In vitro drug release showed 97% of the RES released from RES-loaded bilosomes as compared to the 25% of the drug released from RES suspension after 2 h [38]. In the mice model of AD, RES-loaded bilosomes outperformed the standard drug suspension in improving mice memory using the Y-maze and Morri’s water maze tests. In another study by the same group, the brain targeting of RES bilosomes through the intranasal route was investigated. In this study, the formulation of RES-loaded and chitosan (CS)-coated bilosomes was developed, which was compared with SPION (superparamagnetic iron oxide nanoparticles)-loaded CS-coated bilosomes to allow bilosomes guidance and retention in the brain by the use of an external magnetic field [40]. An in vivo evaluation in a lipopolysaccharide-induced AD mice model demonstrated that the CS-coated SPION bilosomes outperformed in reducing neuroinflammatory markers and improving cognitive and memory functions as compared to the CS-coated bilosomes and RES suspension. Conclusively, in vitro and in vivo data have suggested that the therapeutic efficacy of the poorly bioavailable neuroprotective drug RES can be improved by encapsulating it in bilosomes and this could be a viable medication delivery approach for the treatment of AD [38,40].
Another drug, zolmitriptan, belongs to the third class of the biopharmaceutics classification system (BCS) due to its high solubility and low permeability. Taweel et al. [41] developed zolmitriptan-loaded bilosomes and encased them in a mucoadhesive in situ gel with a delayed nasal mucociliary transit time so that the zolmitriptan could be delivered intranasally to the brain. With a sol–gel temperature of 34.03 °C, the bilosomal formulation of the mucoadhesive in situ gel enhanced the mucociliary transit time by 22.36 min. The intranasal distribution through the gelling system resulted in greater brain bioavailability than bilosomal dispersion [41]. Elsheikh et al. [42] developed intranasally administered luteolin-loaded bilosomes for use in preserving both short- and long-term spatial memory in AD patients. Luteolin is a medicine of dubious efficacy due to its poor solubility. Formulation evaluation results revealed an entrapment efficiency of 70.4% and a maximum in vitro drug release of up to 98.7 ± 4.20% from luteolin-loaded bilosomes as compared to 9.75 ± 1.01% from a pure drug suspension. In vivo findings in a sporadic AD mouse model demonstrated that luteolin-loaded bilosomes boosted both short- and long-term spatial memory by suppressing amyloid β aggregation and hyperphosphorylated Tau protein levels in the hippocampus [42]. In a recent study, an herbal extract of Bacopa monnieri, which is reported to possess strong neuroprotective activities, was encapsulated into liposomes and bilosomes to improve its oral bioavailability. In vitro and in vivo evaluation results for both formulations showed the better potential of B. monnieri-loaded bilosomes in enhancing bioavailability as compared to B. monnieri-loaded liposomes [43].
Numerous methods have been investigated and different drug formulations have been proposed in an attempt to enable greater drug penetration into the brain despite the BBB continuing to be a considerable barrier for pharmaceuticals to pass. Since bilosomes are permeation-modifying agents and drug absorption enhancers, they merit more research for application in brain drug delivery in the future.
4.2. Bilosomes as an Ocular Drug Delivery System
Novel therapeutics for the treatment of ocular disease have been developed as a result of recent advancements in drug delivery techniques and materials sciences. Currently, only topical application, redistribution into the eye after systemic administration, or targeted intraocular/periocular injections can deliver drugs to the eye [44]. Drug transport to the targeted ocular tissues is impeded by a number of precorneal, dynamic, and static ocular obstacles. The conventional system for ocular drug delivery suffers with the problem of poor bioavailability due to low corneal residence time and the fast elimination of the administered dose from the nasolacrimal drainage system [45]. Moreover, the target tissues do not retain therapeutic medication levels for a prolonged period of time. As the eye is a sensitive and delicate human organ, the adverse effect is a major concern while designing the drug delivery system for ocular diseases [46]. To overcome these limitations and to improve ocular delivery including delivery to the anterior segment, topical gelling systems are being explored. The incorporation of drug-loaded bilosomes into an in situ gelling system is reported to be advantageous over conventional modes of drug delivery for treating ocular disorders in terms of increased transcorneal flux, improved ocular residence time, controlled drug release, increased bioavailability, and ocular permeability.
Abdelbary et al. [47] employed ultra-deformable bilosomes to encapsulate the water-insoluble antifungal medication terconazole for ocular delivery. In ex vivo studies, drug flux through a rabbit cornea was found to be enhanced by the optimal ultra-deformable bilosomes formulation compared to that of conventional bilosomes, niosomes, and drug suspension [47]. In another study, Janga et al. [48] tested the ocular delivery of bilosomes loaded with the ion-sensitive in situ gel of natamycin, an antifungal agent. The transcorneal flux of the natamycin-loaded bilosomes (NB) increased six to nine times, indicating that bilosomes were effectively absorbed by the eyes. Corneal histology and in vitro cytotoxicity tests showed that the natamycin-loaded bilosomes were safe and cytocompatible for ocular application. The results demonstrated that ion-sensitive in situ hydrogels loaded with bilosomes provided an efficient platform for ocular medication that lasted longer and normalised natamycin levels in rabbit eye tissues [48].
Several medications used for the treatment of glaucoma have been formulated in bilosomes to test their effectiveness through ocular delivery. Acetazolamide (ACZ), which is used to treat glaucoma, suffers from poor corneal penetration due to low solubility and permeability. Mohsen et al. [49] encapsulated acetazolamide in bilosomes to test its ocular delivery through the cornea. In vitro data for ACZ-loaded bilosomes showed the highest entrapment efficiency of 74.24% and biphasic drug release pattern starting in 30 min followed by a slow and prolonged release for up to 8 h. In vivo pharmacokinetic and pharmacodynamic studies in male albino rabbits revealed the highest bioavailability from the bilosomal formulation as compared to ACZ niosomes and marketed ACZ formulations, including plain ACZ, ACZ oral tablets, and dorzolamide eye drops. Moreover, the ACZ-loaded vesicular formulations demonstrated a slower decrease in intraocular pressure which lasted for 1 h. Lastly, the Draize irritancy test confirmed that the ACZ bilosomal formulation was safe after topical application to the eye. Therefore, the bilosomal formulation is thought to be a potentially effective nanocarrier for the administration of acetazolamide to the eyes in order to treat increased intraocular pressure [49]. Nemr et al. [50] developed AGO-loaded hyaluronic acid-bilosomes using D-optimal design to enhance its bioavailability in ocular tissues. An in vitro drug release study revealed a slow and controlled drug release from all bilosomal formulations which lasted for 24 h as compared to the AGO solution which released most of the drug within the first 2 h. Consequently, the AGO bilosomal formulation significantly reduced the intraocular pressure in male albino rabbits when compared to its solution. Histopathological evaluation showed no eye irritation or obscured vision with AGO bilosomal formulation, confirming its safety for topical application to the eye [50]. Sakr et al. [51] encapsulated betaxolol hydrochloride, a poorly bioavailable drug, into highly permeable bilosomes for ocular delivery for the treatment of glaucoma. In vitro drug release studies exhibited a biphasic and sustained release of most of the drug over 24 h period. An ex vivo permeation study showed a 2.2-fold increase in drug permeation compared to the conventional marketed dosage form. A preclinical assessment in male albino rabbits displayed 57.81% reduction in intraocular pressure compared to 35.27% in the case of the marketed dosage form, confirming improved transcorneal permeation [51].
Bilosomes loaded with antibiotics have been tested for the management of bacterial eye infections. Alsaidan et al. [52] developed and evaluated ciprofloxacin-loaded bilosomal in situ gel (CIP-BLO) for the management of bacterial conjunctivitis. The optimized CIP-BLO formulation showed sustained CIP release with superior entrapment efficiency. This formulation also exhibited > 2-fold permeability than pure CIP. Evidently, no sign of toxicity was displayed by the CIP-BLO formulation in a corneal hydration test (76.15%), histology, and the hen’s egg-chorioallantoic membrane (HET-CAM) test (zero scores). The antimicrobial activity against P. aeruginosa and S. aureus was also found to be significantly higher for CIP-BLO formulation than pure CIP. Conclusively, CIP-BLO was found to be an effective strategy for enhancing corneal residence time and the pharmacological activity of CIP [52]. Similarly, moxifloxacin (MX), another antibiotic used to treat conjunctivitis, was loaded into bilosomal in situ gel to improve ocular residence time. The bilosomal formulation of this antibiotic showed 2.8-fold higher permeation compared to pure MX solution [53].
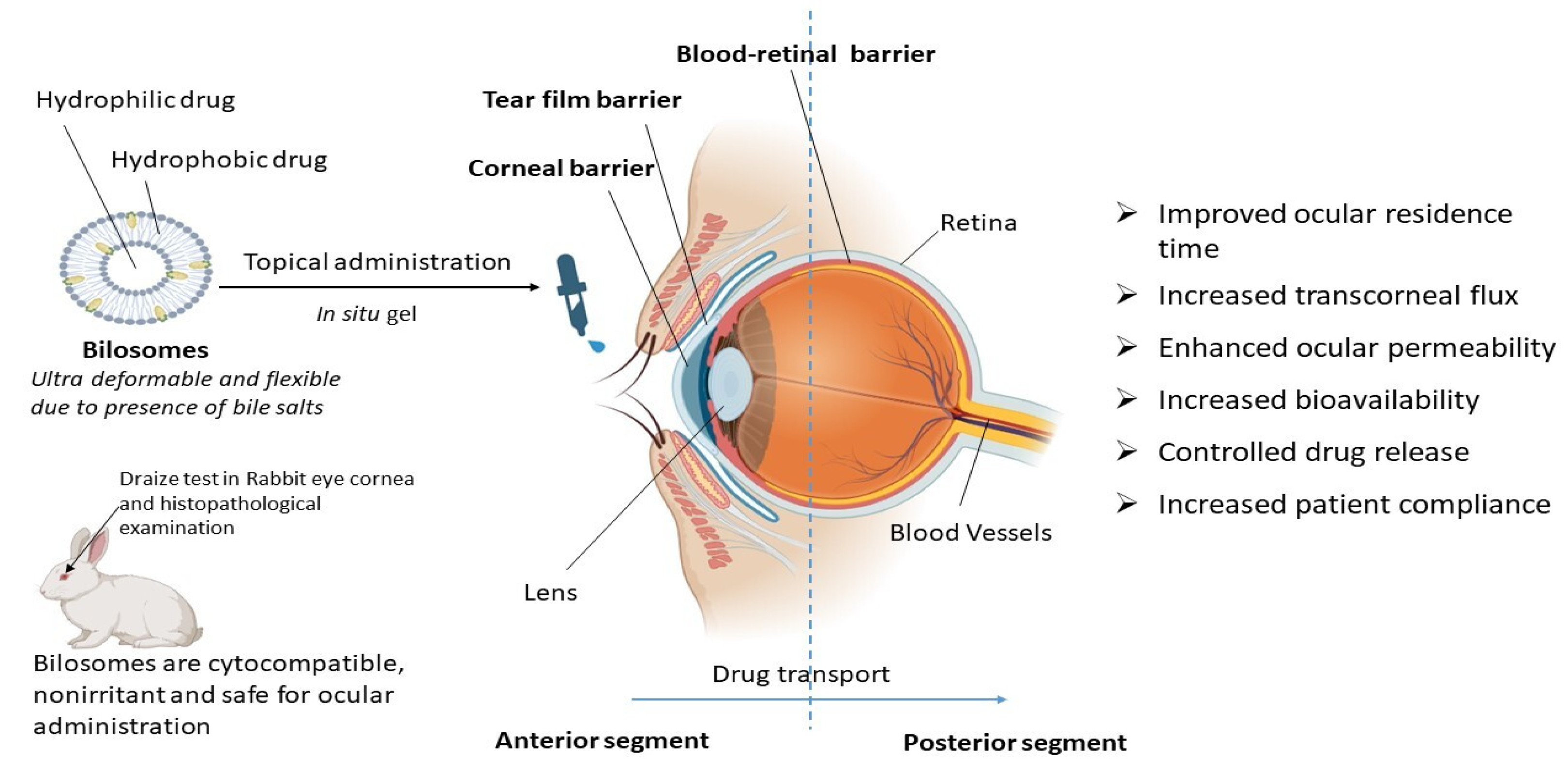
From all these reported studies it can be concluded that bilosomes are efficient in ocular drug delivery due to increased transcorneal flux, prolonged corneal contact time, controlled ocular delivery, and improved bioavailability due to the ultra-deformable and flexible properties of bilosomes, as depicted in Figure 3. Bilosomal formulations are also reported to be safe and non-irritant for ocular administration [49,50]. Using the traditional ocular delivery system, the distribution of the drug in the target tissue is limited and its therapeutic levels are rapidly lost due to several precorneal, dynamic, and static barriers. In this regard, bilosomal formulations open up a huge possibility as a means of improving and extending eye care treatment.
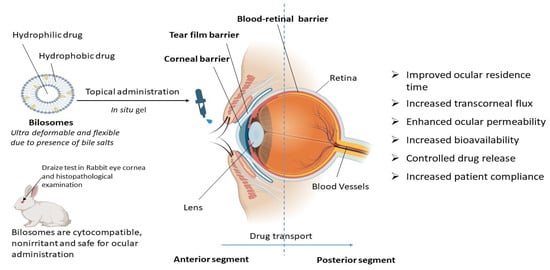
Figure 3.
Therapeutic advantages of bilosomes in ocular drug delivery. Bilosomes are ultra-deformable novel vesicular carriers that are also used for ocular drug delivery. They are advantageous over conventional drug delivery systems for topical ocular administration in terms of controlled drug release, increased bioavailability, an improvement in ocular residence time, transcorneal flux, ocular permeability, and patient compliance.
4.3. Bilosomes for Delivery of Oral Vaccines
Oral dosing is the gold standard for patient compliance when it comes to the administration of vaccines. To elicit an immune response, the vaccine antigen is taken up by gut-associated lymphoid tissue consisting of Peyer’s patches overlying M cells. Antigens are then trafficked to dendritic cells, where they undergo a series of events that lead to the generation of mucosal and systemic immune responses. Although highly preferred, the oral route is not the recommended route of administration for most vaccines and biologicals due to poor absorption. Orally administered vaccines are entrapped in the mucus gel of GI tract epithelia and become diluted, denatured by enzymes, and excluded by an epithelial barrier, which leads to a weaker immune response. There is an unmet need for a delivery system that can withstand the degradation in the stomach and intestine. In this respect, bilosomes were found to be much more stable in the stomach’s acid and enzymes [2,54]. Moreover, due to the presence of bile salts, bilosomes can destabilize the membranes and were found to be an effective formulation for oral therapeutic delivery [1].
Conacher et al. [10] studied the efficacy of a bilosomes-encapsulating influenza subunit vaccine in mice and found a comparable antibody response to a parenterally administered vaccine using the same quantity of antigen. Moreover, the Th1/Th2 response was found to be similar following immunization by both routes of administration [10]. In another study, the conjugation of a cholera toxin B subunit (CTB) to hepatitis B surface antigen (HBsAg)-loaded bilosomes facilitated the binding of bilosomes to a Glucomannan-modified (GM)1 ganglioside receptor on M cells. The results showed the generation of comparable anti-HBsAg IgG antibody titer in mice when administered via an oral and intramuscular route [55]. However, the dose required via oral route is twice the dose required in intramuscular administration. Premanand et al. [13] investigated the efficacy of orally administered recombinant baculovirus (Bac-VP1) and bilosomes loaded with Bac-VP1 in a murine model for developing an antiviral vaccine against human enterovirus 71 (HEV71). It was found that Bac-VP1, associated with bilosomes, elicited significantly higher immune responses (both systemic and mucosal) compared to bilosomes administered alone. Interestingly, it was found that mice immunized subcutaneously with live Bac-VP1 had considerably greater VP1 specific blood IgG levels and stronger neutralizing antibody titers than mice inoculated orally with live Bac-VP1 alone or in combination with bilosomes [13]. Similarly, Wilkhu et al. [30] investigated the targeting efficacy of bilosomes containing the influenza vaccine to Peyer’s patches in mice. Their study results showed increased bilosomes in Peyer’s patches relative to a free antigen. Further studies were performed to evaluate the efficacy by challenging the mice with empty bilosomes and bilosomes containing the influenza antigen. Their study findings revealed a decrease in median body temperature and reduced viral load in the nasal secretion in a group of mice that received bilosomes containing influenza antigen compared to empty bilosomes [30]. In another study, the augmentation of immune response by targeting the M cells was investigated by Jain et al. [56] in which GM bilosomes loaded with tetanus toxoid (TT) undergo enhanced uptake through the mannosyl receptors of dendritic cells. The presence of abundant mannose molecules on GM-modified bilosomes facilitated the interaction with mannose receptors on dendritic cells, leading to enhanced cellular uptake. This resulted in an increased systemic immune response relative to conventional liposomes and additionally, mucosal immune response was also obtained via oral route [56].
Limited studies have been reported on delivering vaccines in conjugation with bilosomes through the oral route. Bilosomes could be useful in delivering vaccines through the oral route as they are noninvasive, painless, and effective in stimulating the systemic immune response against an antigen in the body. In this context, more research is needed to explore the potential of a bilosomes drug delivery system for the delivery of vaccines through the oral route of administration.
4.4. Bilosomes for Delivery of Proteins and Peptides
Proteins and peptides provide a potent therapeutic alternative to conventional small molecules for several diseases. Despite the superior therapeutic efficacy of large molecules when administered via the parenteral route, they have the limitations of a short biological half-life, poor patient compliance, and a need for technical skills for their administration. Oral administration of protein and peptide with a molecular weight above 700 Da showed poor stability in the GIT environment and had low permeation through the intestinal barrier, leading to a poor bioavailability of less than 10%. The limitation in the delivery of protein and peptides can be addressed using a vesicular nanocarrier, i.e., liposomes or niosomes, that can deliver both hydrophilic and hydrophobic molecules. However, there are reports of instability, leakage, and poor permeability of vesicular nanocarrier in the GI environment. Furthermore, gastric leakage exposes the peptides to GI enzymes, causing their denaturation [57]. Thus, the need for an alternative dosage form that can mitigate the issues associated with vesicular nanocarriers led to the development of a modified vesicular system called bilosomes. In bilosomes, bile salts are added to the bilayer thus stabilizing the vesicular nanocarrier and preventing leakage in the gastric milieu. The addition of bile salts in the bilayer also leads to its increased fluidity and thus improves permeability across GI membrane [5].
A study by Hu et al. [58] compared conventional liposomes and liposomes containing bile salts (sodium glycocholate) in their ability to enclose insulin and prevent gastric leakage. The testing was performed using simulated and ex vivo GI fluids. The results of the study demonstrated that liposomes made with bile salts conferred better protection and less gastric leakage relative to conventional liposomes [58]. A study by Niu et al. [17] investigated the bioavailability of insulin administered via oral route using liposomes containing bile salts in rats. In this study, the performance of conventional liposomes was compared with liposomes prepared using different bile salts (sodium deoxycholate (SDC), sodium taurocholate (STC), and sodium glycocholate (SGC). The study findings revealed that SGC liposomes showed higher bioavailability than SDC and STC liposomes. Insulin protection against gastric enzymes (pepsin, trypsin, and α-chymotrypsin) was also evaluated in vitro and the results obtained showed the superior protection of insulin against enzymatic degradation by liposomes containing bile salts compared to conventional liposomes [17]. Another study by the same research group investigated the ability of liposomes containing bile salts (BS liposomes) in enhancing the GI stability of insulin by using fluorescent imaging tools. They also studied the interaction of BS liposomes with the biomembrane using Caco-2 cell lines as a surrogate. The study results found that BS liposomes showed prolonged GI residence time and enhanced permeation compared to conventional liposomes. Visualization using confocal laser scanning microscopy further revealed trans-enterocytic internalization as a proposed mechanism of transport for BS liposomes across the biomembrane [59]. In another study, Nallamothu et al. [60] investigated the effect of the charge and chain length of bile salts in bilosomes on the oral bioavailability of insulin. Deoxycholic acid bile salts conjugated to amino acid were used and uptake studies were performed using the Caco-2 cell line. The study results revealed that anionic bilosomes were 4-fold more efficiently taken up relative to cationic liposomes (1.3-fold). The study results also demonstrated a 1.6-fold increase in AUC with bilosomes administered via the oral route relative to the subcutaneous route [60].
4.5. Bilosomes as Transdermal Drug Delivery System
Outer skin is the primary obstacle between the organism’s body and its outer environment. Stratum corneum, the epidermis’s outermost layer, is responsible for the skin’s excellent barrier characteristics [61]. Technological advances have made it feasible to transdermally distribute a broader spectrum of drugs, typically from hydrophobic small-molecule pharmaceuticals to hydrophilic medications and macromolecules [62]. Among novel drug delivery approaches for transdermal drug delivery, liposomes and niosomes are well explored. Later, the permeation characteristics of liposomes were improved by incorporating bile salts in them. Bile salts aid in the transportation of drugs across biological membranes either by enhancing drug dissolution or modifying cellular membrane permeability [20]. Bile salts also increase the flexibility of these nanovesicles which also leads to high transdermal permeability. In the literature, many reports are available showing the enhanced transdermal permeability of poorly soluble and bioavailable drugs utilizing bilosomes as a novel carrier for topical delivery, as shown in Table 1. Aziz et al. [11] altered the composition of traditional niosomes by adding various bile salts and then tested their efficacy in the transdermal distribution of diacerein (DCN). DCN is a poorly bioavailable (35–56%) osteoarthritic drug which has GI side effects when administered orally [63]. To establish its safety for topical usage, bilosomes were developed using factorial design optimization techniques. Optimized bilosome formulation displayed nanosized vesicles (301.65 ± 17.32 nm) and 100.00 ± 0.00% entrapment efficiency. Ex vivo permeation and in vivo studies demonstrated a higher permeation and an improved drug retention capacity of bilosomal formulation compared to the drug suspension and niosomal formulation. These findings corroborated the hypothesis that bilosomes are superior than niosomes for enhancing DCN permeation through the skin [11].
Another drug, olmesartan medoxomil (OLM), which is used as an antihypertensive agent, has a poor oral bioavailability of 26% which limits its therapeutic efficacy. So, to enhance its bioavailability, Albash et al. [24] developed and characterized olmesartan medoxomil-loaded PEGylated bilosomes for transdermal delivery. The higher deposition of OLM in rat skin was observed from bilosomal formulation compared to transethosomes and OLM suspension as per in vivo skin deposition studies. The optimized bilosomal formulation showed a higher relative bioavailability of 235.04% in the pharmacokinetic study performed in male albino rabbits, which is significantly higher than oral tablets [24]. Tizanidine hydrochloride (TZN), a muscle relaxant with poor oral bioavailability, was also formulated into bilosomes for topical delivery. Preclinical studies reported an improved transdermal permeation of the drug through bilosomal nanovesicles compared to the plain drug [64]. Similarly, Terbutaline (TBN) sulfate, which is used for the treatment of asthma and suffers poor oral bioavailability due to hepatic first-pass metabolism, was loaded into bilosomal gel formulation [65]. TBN chitosan-coated bilosomes (TBN-CTS-BLS) were optimized using a central composite design. A pharmacokinetic study in the rat model showed that the TBN bioavailability was increased by approximately 2.33-fold and t1/2 was increased to 6.21 ± 0.24 h when using the optimized TBN-CTS-BLS formulation compared to the oral TBN solution. This research revealed that BLS may be used as an effective and secure transdermal carrier for TBN to overcome hepatic first-pass metabolism [65]. Lornoxicam (LOR) was also encapsulated in bilosomes for enhanced transdermal permeability. The in vivo pharmacodynamic activity in male rats and a mice model demonstrated enhanced anti-inflammatory and antinociceptive activity compared to that of the oral marketed product [66]. Recently, magnetic-targeted superparamagnetic iron oxide nanoparticles (SPIONS) loaded with LOR-bilosomes were developed for local intramuscular administration in osteoarthritis [67]. El-Nabarawi et al. [68] prepared and characterized dapsone (DPS)-loaded bilosomes for the topical treatment of acne, in which the optimized DPS-loaded bilosomes showed 170.57 ± 55.12 µg/mL DPS retention compared to 120.24 ± 10.7 µg/mL from DPS alcoholic solution in an ex vivo deposition study.
Abdelalim et al. [69] formulated nanobilosomes integrated with the sodium tauroglycocholate (STGC) of poorly bioavailable sildenafil citrate (SC) for topical delivery to treat erectile dysfunction. An ex vivo permeability study using human skin showed improved permeation and about 39% of the drug permeated within 15 min. An in vivo study in Sprague Dawley rats also revealed a 2-fold increase in intromission frequency and intromission ratio compared to the untreated group. The study findings concluded that there was a fast onset of action using bilosomes as a topical drug delivery system for treatment of impotency at low dose, which is about 20% of the conventional dose [69]. In another study, Mosallam et al. [70] successfully transformed the antifungal medication terconazole (TCZ) into highly deformable bilosomes (HBs) for topical administration, despite its limited permeability. HBs significantly reduced the Candida albicans growth compared to TCZ suspension and were evaluated using XTT (2,3-bis(2-methyloxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide) reduction assay. In vivo studies showed an enhanced improvement in TCZ skin deposition compared to traditional bilosomal formulation and TCZ suspension [70]. Mahmoud et al. [71] developed topical diclofenac sodium (DNa)-loaded bilosomal gel for treating inflammation. The oral administration of DNa is associated with GI side effects. The increased efficacy of bilosomal formulation was confirmed by performing ex vivo permeation studies showing 2.5 times higher skin permeability than the DNa solution. In vivo studies in the paw edema rat model also revealed a significant suppression of inflammatory mediators and a reduction in paw edema, thereby demonstrating the greater efficacy of the bilosomal system in transdermal drug delivery [71]. Salem et al. [72] investigated the possible use of bilosomes as a carrier for the active targeted transdermal distribution of metformin hydrochloride, with the aim of mitigating its adverse effects. The release profile of the optimized formulation within 24 h was found to be 75.28% and permeation flux was found to be enhanced (198.79–431.91 ng cm−2 h−1) in comparison to the plain drug (154.26 ng cm−2 h−1). These findings indicate that the incorporation of metformin hydrochloride, an antidiabetic drug, into bilosomes resulted in enhanced permeation and provided a novel nanocarrier approach for effective transdermal administration [72]. Another antifungal drug miconazole nitrate (MN), which is poorly water-soluble, was loaded into chitosan–carbopol bilosomal gel to improve its therapeutic efficacy through a transdermal route [73]. Khafagy et al. [74] developed and evaluated topical novel simvastatin (SMV)-loaded bilosomal gel for treating inflammation. A 3-fold enhancement in SMV transdermal flux was shown by SMV-loaded bilosomal gel compared to the suspension of the pure drug [74]. Another poor orally bioavailable drug, dronedarone hydrochloride (DRN), was also formulated into bilosomal nanogel to enhance transdermal permeability and bioavailability. The optimized bilosomal gel formula demonstrated an in vitro drug release of 57.0 ± 8.68% relative to 13.3 ± 1.2% from drug suspension after 12 h. The ex vivo permeation study findings showed an enhanced skin permeation of 21.23 ± 1.54 µg/cm2 h across rat skin and collectively showed the superior efficacy of bilosomal gel compared to free drug suspension [75].
The process of delivering drugs through the skin is challenging because of several barriers that restrict permeation. This necessitates the use of external agents or carrier systems to facilitate successful penetration, which has led to the emergence of bilosome vesicular systems as a novel approach to therapy. The use of bilosomes for the topical or transdermal delivery of drugs is beneficial as it can avoid the first-pass metabolism and can lead to increased bioavailability. Moreover, as per currently reported studies, the histological examination of bilosomes also ensures the safety and tolerability of this vesicular system. Because of this, formulation scientists are now exploring the topical and transdermal delivery of active pharmaceutical components for their superior efficacy as an alternative to the oral administration of medications.

Table 1.
Current perspectives on bilosomes as transdermal drug delivery system.
Table 1.
Current perspectives on bilosomes as transdermal drug delivery system.
| No. | Drug | Formulation | Therapeutic Problem | Study Outcomes | Ref. |
|---|---|---|---|---|---|
| 1 | Diacerin (DCN) | Bilosomes | Poorly bioavailable (35–56%) osteoarthritic drug with gastrointestinal side effects on oral administration | Bilosomes were found to be superior than niosomes and pure suspension for enhancing DCN flow through the skin | [11] |
| 2 | Olmesartan medoxomil (OLM) | PEGylated bilosomes | Poor oral bioavailability of 26% which limits its therapeutic efficacy | Higher OLM deposition in rat’s skin from bilosomal formulation compared to transethosomes and OLM suspension was found as per in vivo skin deposition studies | [24] |
| 3 | Tizanidine HCL (TZN) | Bilosomes | Poor bioavailability | Enhanced transdermal permeation compared to plain drug | [64] |
| 4 | Terbutaline sulfate (TBN) | TBN chitosan-coated bilosomes (TBN-CTS-BLS) | Poor oral bioavailability due to hepatic first-pass metabolism | 2.33-fold increase in TBN bioavailability and t1/2 was increased to 6.21 ± 0.24 h when using the optimized TBN-CTS-BLS formulation compared to the oral TBN solution in the rat model | [65] |
| 5 | Lornoxicam | Bilosomes | Poor aqueous solubility and rapid clearance, GIT toxicity | In vivo pharmacodynamic activity in male rats and mice model demonstrated enhanced anti-inflammatory and antinociceptive activity compared to that of the oral marketed product | [66] |
| 6 | Dapsone (DPS) | Bilosomes | Low solubility and toxicity through oral administration | Ex vivo deposition study of optimized DPS-loaded bilosomes showed 170.57 ± 55.12 μg/mL DPS retention compared to 120.24 ± 10.7 μg/mL from DPS alcoholic solution | [68] |
| 7 | Sildenafil citrate (SC) | Nanobilosomes | Poor bioavailability | In vivo study in Sprague Dawley rats revealed 2-folds increase in intromission frequency and intromission ratio compared to untreated group | [69] |
| 8 | Teroconazole (TCZ) | Highly deformable bilosomes (HBs) | Poor permeability | In vivo studies showed improvement in terconazole skin deposition compared to typical bilosomal formulation and TCZ suspension | [70] |
| 9 | Diclofenac sodium (DNa) | Bilosomal gel | Gastrointestinal side effects on oral administration | Ex vivo permeation studies of optimized formulation showed a 2.5-fold increase in skin permeability than DNa solution, In vivo studies in paw edema rat model also revealed significant suppression of inflammatory mediators and reduction in paw edema | [71] |
| 10 | Metformin HCL | Bilosomes | Adverse effects | Permeation flux was found to be enhanced (198.79–431.91 ng cm −2 h−1) in comparison to the plain drug (154.26 ng cm −2 h−1). | [72] |
| 11 | Miconaole nitrate (MN) | Chitosan–carbopol bilosomal gel | Poor water solubility | Enhanced antifungal activity compared to pure drug against Candida albicans and Aspergillus niger | [73] |
| 12 | Simvastatin (SMV) | Bilosomal gel | Poor bioavailability due to water insolubility and hepatic first-pass effect. | 3-fold increase in SMV transdermal flux from SMV-loaded bilosomal gel compared to plain drug suspension. Bioavailability of SMV-BS gel was also found to be ~2-fold and ~3-fold higher than those of oral SMV suspension and SMV gel, respectively | [74] |
| 13 | Dronedarone hydrochloride (DRN) | Bilosomal nanogel | Poor bioavailability | Bilosomal gel demonstrated enhanced release (57.0 ± 8.68% of DRN compared to only 13.3 ± 1.2% released from drug suspension after 12 h) and enhanced skin permeation | [75] |
4.6. Bilosomes as Cancer Drug Delivery System
The global rise in cancer mortality rates has prompted an intensified pursuit of innovative treatment interventions. Bilosomes have numerous advantages over other lipid-based vesicular systems for the delivery of anticancer drugs such as GIT stability, superior intestinal permeation, being more deformable, and possessing increased drug loading. Consequently, a range of vesicular delivery methods are being used for the targeted administration of medicines to tumor cells. There is ongoing research on the potential of bilosomes for delivering drugs for cancer treatment [76].
The delivery potential of many anticancer drugs using bilosomes as a nanocarrier was investigated by numerous scientists as shown in Table 2. Cytostatic bile acid derivatives, Bamet-R2 [cis-diamminechloro cholylglycinate platinum(II)] and Bamet-UD2 (cis-diammine bis-ursodeoxycholate platinum(II) have been studied for their potential to inhibit cell proliferation in a variety of hepatocellular cancer cell lines, including LS174T/R (human colon adenocarcinoma), WIF-B9/R (rat hepatoma–human fibroblast hybrid), and Hepa 1-6/R (mouse hepatoma). The absorption and cytostatic activity of Bamets were much greater than the poorly water-soluble anticancer drug cisplatin in their wild-type counterparts. Bamets were effective in penetrating all cell lines that had developed resistance to cisplatin. One study reported the incorporation of these Bamets in the liposomes and demonstrated their potential for overcoming chemotherapy resistance, especially in cases with enterohepatic circuit tumors [77]. Parashar et al. [78] developed bilosomes that were modified with dextrose (DEX) and loaded with silymarin (DEX-SYL-BL) for peroral and targeted drug delivery for treating hepatic carcinoma. The therapeutic efficacy of silymarin (SYL) is limited due to its inadequate bioavailability and low solubility. Cytotoxicity studies performed in Hep-G2 cell lines showed a greater reduction in cell viability using DEX-SYL-BL when compared with pure SYL and SYL-containing bilosomes (SYL-BL). In vivo results evaluated in a mice model of diethyl nitrosamine (DEN)-induced hepatic carcinoma showed that DEX-SYL-BL treated mice exhibited an improved lifespan and a reduced tumor burden, indicating a greater therapeutic potential of the bilosomes in anticancer drug delivery [78]. Zaki et al. [79] synthesized acrylamide derivatives and evaluated their cytotoxicity against human breast cancer MCF-7 cells. Compound 4e, which was found to have more cytotoxicity potential against MCF-7 cells lines, was further loaded into PEGylated bilosomal nanovesicles in order to enhance the suboptimal oral bioavailability. A remarkable improvement in the cytotoxic activity of the compound 4e from PEGylated nanovesicle (IC50 = 0.75 ± 0.03 µM) was found compared to the pure form (IC50 = 2.11 ± 0.19 µM) [79]. In another study, Alhakamy et al. [80] developed and evaluated icariin-loaded bilosomes-melittin (ICA-BM) in an attempt to increase the efficacy of these natural biomolecules against pancreatic cancer cells, PNAC1. The evaluation of pro-apoptotic protein levels, cell cycle analysis, and annexin V staining in PNAC1 cells demonstrated that the optimized ICA-BM formulation significantly augmented icariin’s efficacy in combating malignant pancreatic cells [80]. Another study by the same research group evaluated piceatannol (PIC), an herbal polyphenolic compound with anti-cancercytotoxic activity, which was loaded into zein-modified bilosomes for treating lung cancer. In this study, the cytotoxicity evaluation of the optimized formulation revealed smaller IC50 against A549 cells compared to the pure drug [81]. Zafar et al. [82] developed luteolin-loaded PEGylated bilosomes (LL-BLs) for oral administration. Human breast cancer cell lines, MDA-MB-231 and MCF-7, were used in the cytotoxicity study. Luteolin-loaded pegylated bilosomes showed a greater cell viability than pure drug on both cell lines. It was found that the IC50 values for MCF-7 and MDA-MB-231 cancer cells were 390 M and 510 M, respectively. Therefore, PG-BLs may offer an effective nano-oral delivery system for specifically targeting a breast cancer cell line [82]. Matloub et al. [83] delivered sulfated polysaccharide–protein complexes that had a significant anticancer potential extracted from Enteromorpha intestinalis (EHEM), using bilosomes as a delivery carrier in order to treat hepatocellular carcinoma. Based on the in vivo results, an optimized bilosomal formulation can be employed as a prospective option for treating hepatocellular carcinoma due to its strong anti-cancer and anti-angiogenic action [83].
Curcumin (CUR), a naturally occurring polyphenolic phytoingredient, possesses strong anti-inflammatory, antioxidant, and anticancer properties, but its clinical efficacy is limited due to poor oral bioavailability and permeability [84]. Abbas et al. [85] developed nano-bilosomes for the delivery of the CUR analogue, 3,5-bis(4-bromobenzylidene)-1-propanoylpiperidin-4-one, in order to enhance its bioavailability. The antitumor selectivity index of CUR analogue-loaded bilosomes was reported to be significantly higher against Huh-7 liver cancer cells as compared to a CUR suspension or doxorubicin, another standard anticancer drug [85]. Hegazy et al. [86] also designed D-alpha-tocopheryl polyethylene glycol succinate (TPGS)-coated bilosomes for the oral delivery of CUR for enhanced antitumor activity. In a Caco-2 cell lines cellular uptake study, 61.9 ± 5.3% uptake of CUR was demonstrated from TPGS-CUR-bilosomes compared to 7.4 ± 2.12% from CUR suspension. Ex vivo permeation was also found to be increased by 6.6 folds from TPGS-CUR-bilosomes compared to pure suspension [86]. So, the therapeutic efficacy of CUR in terms of solubility, permeability, and stability is found to be improved significantly by using bilosomes as a nano-delivery carrier.
Imam et al. [87] loaded apigenin into chitosan-coated bilosomes to enhance its bioavailability. The antimicrobial and cytotoxicity analysis in two distinct cancer cell lines, MCF-7 breast cancer cells, and A549 lung cancer cells, showed better activity than pure apigenin. Pitavastatin (PIT) has been demonstrated to provide an anticancer effect against hepatocellular carcinoma (HCC) in addition to its well-known efficacy as an anti-hyperlipidemic drug. Kharouba et al. [88] developed and evaluated PIT-loaded bilosomes for oral delivery in treating HCC. In this study, the surface modification of bilosomes with lactoferrin was also evaluated and the results showed an improvement in the cytotoxicity of HepG2 spheroids, which was 44 times greater than that of PIT solution. Moreover, the apoptotic potential of PIT was found to be increased by two-fold, suggesting the potential application of bilosomes as a drug delivery system for treating cancer [88]. Another bioactive compound quercetin (QT), which is reported to possess antioxidant and chemoprotective activity, suffers from a poor bioavailability problem. To improve its therapeutic efficacy, Alruwaili et al. [89] developed surface-modified bilosomes loaded with QT. The results demonstrated 1.61-fold higher cytotoxicity of surface-modified chitosan-coated QT-bilosomes against MFC-7 and 1.44-fold higher cytotoxicity against MDA-MB-231 breast cancer cells than pure QT.
The oral bioavailability and antitumor activity of psoralidin (Ps), which also possess strong antimicrobial, antioxidant, antipsoriatic, anti-inflammatory, and antidepressant activities [90], is improved by developing its chitosan-coated bilosomes nanoformulation for oral delivery. The apoptotic and necrotic potential of the developed formulation was evaluated in MCF-7 human breast cancer cells and A549 human lung cancer cells A549, in which a significant increase in the percentage of the apoptotic and necrotic cells were found compared to the control and free Ps [91]. Doxorubicin (DOX) is another potent anticancer drug whose clinical applicability is limited due to dose-related toxicity. DOX was loaded into bilosomes for improved cytotoxic activity and systemic absorption [18]. The investigations of the optimized formulation showed an improved DOX cytotoxicity against MCF-7 breast cancer cells and a reduced IC50 value from 13.3 μM to 0.1 μM. A 4.5–6 and 1.8–2.5-fold increase in drug absorption was reported from the jejuno-ileum and colon, respectively [18].
Scientists have successfully tested diverse molecular and vesicular methodologies for the therapeutic delivery of several anticancer drugs. Bilosomes have a significant relevance in this context due to their capacity to enhance several aspects of cytotoxic efficacy against cancerous cells. The potential of stimuli-sensitive bilosomes as a vesicular carrier in cancer cell targeting still needs to be explored, as there is very limited literature available currently.

Table 2.
Reported bilosomal formulation for cancer drugs.
Table 2.
Reported bilosomal formulation for cancer drugs.
| No. | Drug | Formulation | Therapeutic Problem | Study Outcomes | Ref. |
|---|---|---|---|---|---|
| 1 | Cisplatin | Cytostatic bile acid incorporated liposomes | Cisplatin resistance in cancer cell lines | Enhanced ability of anticancer drug to be taken up by cancer cell due to the presence of cytostatic bile acids. | [77] |
| 2 | Silymarin | Dextrose-modified bilosomes | Poor bioavailability and low solubility | Dextrose bilosomes loaded with silymarin were tested in vivo for treating Diethyl nitrosamine (DEN)-induced hepatic malignancy. The mice had longer lifespans and less tumor load, suggesting more therapeutic potential. | [78] |
| 3 | Lead acrylamide molecules | Lead acrylamide molecules utilizing 4e-charged PEGylated bilosomes | Poor bioavailability and low solubility | Cytotoxicity testing showed that compounds 4e and 5d were effective against MCF-7 cells. After integration into the nano-PEGylated bilosomal system, the drug’s cytotoxic activity was increased. | [79] |
| 4 | Icariin and Melittin | Icariin-loaded bilosomes-melittin (ICA-BM) | Poor bioavailability and low solubility | ICA-BM showed a lower IC50 than blank-BM and ICA-pure. ICA-BM formulation increased icariin’s effectiveness against malignant pancreatic cells. | [80] |
| 5 | Luteolin | PEGylated bilosomes (LL-BLs) | Poor bioavailability and low solubility | LL-BLs showed greater cell viability than the pure drug on MDA-MB-231 and MCF-7 breast cancer cell lines. It was found that the IC50 values for MCF-7 and MDA-MB-231 cancer cells were 390 M and 510 M, respectively. | [82] |
| 6 | Sulfated polysaccharide–protein complexes | Bilosomes | Poor bioavailability and low solubility | Substantial decrease in serum α-fetoprotein, endoglin, lipocalin-2, and heat shock protein 70 levels. The photomicrographs of rat liver tissue slices showed a focal area of pleomorphic hepatocytes that had deteriorated, together with fine fibrosis emanating from the portal region. | [83] |
| 7 | Curcumin (CUR) analogue 3,5-bis(4-bromobenzylidene)-1-propanoylpiperidin-4-one | Nano-bilosomes | Poor bioavailability and permeability | Antitumor selectivity index of CUR analogue-loaded bilosomes recorded 420.55 against liver cancer cells when compared to a CUR suspension. | [85] |
| 8 | Apigenin | Chitosan-coated bilosomes | Poor bioavailability and low solubility | Antimicrobial and cell viability analyses demonstrated better outcomes with regard to inhibition and cell line assessment against two MCF-7 breast cancer and A549 lung cancer cell lines. | [87] |
| 9 | Pitvaststin | Bilosomes | Poor bioavailability | Improvement in the cytotoxicity of HepG2 spheroids, which was 44 times greater than that of pitavastatin (PIT) solution. | [88] |
| 10 | Quercetin | Chitosan-coated quercetin-bilosomes | Low bioavailability | 1.61-fold higher cytotoxicity of surface-modified chitosan-coated quercetin-bilosomes against MFC7 and 1.44-fold higher cytotoxicity against MDA-MB-231 than pure quercetin. | [89] |
| 11 | Psoralidin (Ps) | Chitosan-coated bilosomes | Water insoluble, dose-dependent toxicity | Apoptotic and necrotic potential of the developed formulation was evaluated in human breast cancer cell lines (MCF-7) and human lung adenocarcinoma cell lines (A549), in which a significant increase in the percentages of the apoptotic and necrotic cells was found compared to the control and free Ps. | [91] |
| 12 | Doxorubicin (DOX) | Bilosomes | High dose-related toxicity | Formulation showed improved DOX cytotoxicity against breast cancer cells (MCF-7) and the reduced IC50 value from 13.3 μM to 0.1 μM. A 4.5–6 and 1.8–2.5-fold increase in drug absorption from jejuno-ileum and colon was demonstrated. | [18] |
4.7. Bilosomes for Delivery of Herbal Drugs
Several alternative medicinal practices have been widely acknowledged and consistently used across the world, which include Ayurveda, Yoga, Unani, Siddha, Homeopathy, and Naturopathy. The herbal medicine industry has significant potential for expansion. The significant demand for herbal medications in both developed and developing nations may be attributed to their diversified biological activity, relatively cheaper cost, and superior safety margin compared to synthetic therapies [92]. It has been observed that bilosomes had the potential to serve as a viable vehicle for improving the pharmacokinetic profile of herbal medications, as shown in Table 3.
Silymarin (SM), a polyphenolic flavonoid, is a strong hepatoprotective compound but its therapeutic use is limited due to poor water solubility and stability [93]. Mohsen et al. [12] developed and characterized SM-loaded bilosomes to enhance the drug’s hepatoprotective effects. Studies conducted in animals showed that SM-loaded bilosomes and liposomes had a stronger hepatoprotective effect than the drug alone. However, an ex vivo intestinal uptake study revealed the superiority of bilosomes compared to liposomes suggesting the potential application of bilosomes for delivering hepatoprotectants orally [12]. A combination of berberine (BER) CUR has been known for treating non-alcohol fatty liver disease (NAFLD). However, the synergistic effects of BER and CUR in combination are still a challenge to achieve due to their inconsistent bioavailability and biodistribution. To improve the synergistic ameliorative effects of these two natural compounds, Chen et al. [15] developed dextran-coated bilosomes-encapsulating BER and CUR for treating NAFLD. The in vivo pharmacokinetic evaluation of a developed bilosomal formulation in a mice model demonstrated improved oral absorption, synchronized biodistribution, and a prolonged circulation of both BER and CUR [15]. It can be stated that bilosomes are a promising drug delivery strategy for administrating herbal drug combinations orally with synchronized and enhanced efficacy against NAFLD. Moreover, stimuli-responsive self-assembled bilosomes loaded with CUR as a model compound and methylene blue dye were developed and investigated. One study reported a pH- and temperature-dependent release of the model compound from bilosomes [94]. In another study, for the treatment of diabetic (type-2) diabetes, Zafar et al. [95] developed bilosomes that were loaded with apigenin (AG), a natural flavone compound having anticancer and antidiabetic potential that suffers from poor aqueous solubility. In vivo permeation and pharmacokinetic studies showed a 4.49 times higher flux and a 4.67-fold higher AUC0–t for the bilosomal formulation compared to the free compound [95]. Similarly, to boost piperine’s water solubility and its therapeutic efficacy, Zakaria et al. [96] developed piperine-loaded bilosomes. An in vivo pharmacokinetic study showed a higher oral bioavailability of piperine-loaded bilosomes than piperine suspension. Additionally, the antiviral activity and safety margin of an optimized bilosomal formulation were much higher than those of the drug suspension [96].
BER, a naturally obtained isoquinoline alkaloid, is reported to have potential anti-inflammatory, antidiabetic, and anticancer activities [97]. Its therapeutic efficacy is still a challenge because of poor aqueous solubility and permeability [98]. Many attempts have been made to enhance its bioavailability and efficacy. Elkomy et al. [16] encapsulated BER, a phytoconstituent used to treat chronic inflammation in rheumatoid arthritis, into chitosan-coated bilosomal gel to improve its transdermal delivery. An in vivo evaluation was performed in a rat model of carrageenan-induced paw edema which demonstrated a 24.4% edema swelling reduction after 12 h treatment [16]. In another work, Elkomy et al. [99] developed BER-loaded bilosomes to improve the oral bioavailability and therapeutic efficacy of BER for treating diabetes. In comparison to the BER solution, the bilosomal formulation showed greater stability and better-sustained release of BER for up to 8 h. An in vivo evaluation study for the optimized formulation showed a significant reduction in blood sugar, with a maximum drop of 41%, compared to the BER solution with a 19% reduction in sugar level [99]. Conclusively, the therapeutical efficacy of BER can be significantly improved using bilosomes as a nanocarrier delivery system. Quercetin (QT), a flavonoid with antioxidant and chemo-preventive properties, was encapsulated in surface-modified bilosomes by Alruwaili et al. [89]. The surface modification was performed with chitosan and surface-modified bilosomes-encapsulating QT, which showed remarkable antioxidant properties compared to pure QT. Likewise, it showed a 1.44-fold higher cytotoxicity relative to pure QT when tested against the MDA-MB-231 cell line [89]. These findings revealed that the efficacy of QT can be enhanced by encapsulating them in surface-modified bilosomes. Resveratrol (RSV), a phenolic compound with poor bioavailability, was loaded into nanobilosomes for increased cell permeability and antiviral activity [100]. An in vitro drug release study demonstrated an 82.4 ± 3.8% extended drug release for over 12 h compared to 21.9 ± 1.9% from RSV suspension. In vitro studies on Caco-2 cells showed a 4.7-fold increase in cellular uptake as compared to RSV dispersion [100].
Conclusively, it can be stated that bilosomes are effective nanovesicles for delivering herbal compounds through an oral and transdermal route of administration. Bilosomes are reported to be a new generation of nanovesicles which are superior to liposomes and niosomes for the therapeutic delivery of natural drugs due to their stability, deformable flexibility, and permeability characteristics. The integration of Ayurveda, contemporary medicine, and scientific methodologies is a formidable catalyst for exploration, potentially yielding the development of enhanced therapeutic interventions that are both safer and more cost-effective while minimizing adverse effects. This is attributed to the ability of bilosomes to shield the pharmaceuticals from harsh GI conditions, hence impeding the detrimental impact of stomach acid and enzymes.

Table 3.
Current perspectives on bilosomes incorporating herbal drugs.
Table 3.
Current perspectives on bilosomes incorporating herbal drugs.
| No | Drug | Formulation | Therapeutic Problem | Outcome of Study | Ref. |
|---|---|---|---|---|---|
| 1 | Silymarin | Bilosomes | Poor water solubility and stability | Ex vivo intestinal uptake study revealed the superiority of bilosomes compared to liposomes for strong hepatoprotective effect | [12] |
| 2 | Berberine (BER) and curcumin (CUR) | Bilosomes | Poor bioavailability and biodistribution | In vivo pharmacokinetic evaluation of bilosomal formulation in mice model showed improved and synchronized oral bioabsorption of both BER and CUR | [15] |
| 3 | Apigenin (AG) | Bilosomes | Poor bioavailability and low solubility | In vivo permeation and pharmacokinetic studies showed that the free AG-dispersion had a 4.49 times higher flux and a 4.67 folds higher AUC0–t | [95] |
| 4 | Piperine | Bilosomes | Poor bioavailability and low solubility | Piperine-loaded bilosomes had higher oral bioavailability than piperine suspension. Additionally, the optimized formula’s antiviral activity and safety margin were much higher than those of the drug suspension | [96] |
| 5 | Berberine (BER) | Chitosan-coated bilosomal gel | Poor bioavailability and low solubility | In comparison to the BER solution, formulation showed greater stability and sustained release of BER. In vivo study, formulation significantly reduced blood sugar, with a maximum drop of 41%, compared to BER-blood SOL’s sugar reduction of only 19% | [16] |
| 6 | Resveratrol (RSV) | Nanobilosomes | Poor bioavailability | Caco-2 cell lines study showed 4.7 fold increase in cellular uptake compared to RSV dispersion | [100] |
5. Conclusions and Future Perspectives
Bilosomes can encapsulate proteins, peptides, anticancer compounds, phytoconstituents, and several other therapeutic compounds that exhibit poor water solubility and limited bioavailability. Based on the findings of the literature search, it has been observed that the bilosomes have the potential to enhance the therapeutic efficacy and bioavailability of certain substances as they are deformable nanovesicles with enhanced permeation properties due to the presence of bile salts. In addition, bilosomes that are engineered with surface modifications have shown the ability to selectively target the therapeutic agents to specific cells or organs through the attachment of ligands onto the bilosomal surface. Bile acids have garnered growing attention as valuable constituents for the development of novel medicines and drug delivery systems due to their convenient accessibility, cost-effectiveness, and straightforward derivatization procedures.
The current need is to direct research efforts toward investigating the targeted transportation of drugs to the intestinal lymphatic system, specifically at the cellular level, using bilosomes. The investigation of the potential use of bilosomes for conveying a diverse array of antigens with varying physicochemical properties and a susceptibility to degradation in the gastrointestinal tract is necessary. The use of an intelligent bilosomal drug delivery strategy is anticipated to enhance the efficacy against many life-threatening illnesses by targeting the lymphatic system. Additionally, there is a lack of research papers on stimuli-sensitive bilosomes for drug delivery. It is indeed an area that warrants further exploration and study. The development of stimuli-sensitive bilosomes holds great potential for enhancing drug delivery efficiency, especially in targeting specific tissues or cancer cells with controlled release properties. By exploring these unique characteristics, researchers can uncover new opportunities for improving drug delivery systems and addressing challenges in various fields. Consequently, it is anticipated that bilosomes will play a vital role in combating and ultimately eradicating severe and contagious illnesses. Presently, it is of paramount significance for clinical researchers to use the expertise surrounding bilosomes to conduct safe and efficacious studies involving human patients, thus elucidating the precise mechanism behind the administration of bilosomes.
Author Contributions
Conceptualization, H.K. and M.T.; methodology, H.K., M.T. and S.D.K.; software, H.K.; resources, H.K. and D.N.K.; data curation, H.K. and D.N.K.; writing—original draft preparation, H.K., M.T. and S.D.K.; writing—review and editing, H.K., A.B. and S.S.; supervision, H.K., A.B. and S.S.; project administration, H.K. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Acknowledgments
The authors would like to thank Shoolini University of Biotechnology and Management Sciences for constant support and guidance.
Conflicts of Interest
Author A.B. is employed by Perrigo Company plc. All authors declare that the manuscript was written in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Kecman, S.; Škrbić, R.; Badnjevic Cengic, A.; Mooranian, A.; Al-Salami, H.; Mikov, M.; Golocorbin-Kon, S. Potentials of human bile acids and their salts in pharmaceutical nano delivery and formulations adjuvants. Technol. Health Care 2020, 28, 325–335. [Google Scholar] [CrossRef]
- Aburahma, M.H. Bile salts-containing vesicles: Promising pharmaceutical carriers for oral delivery of poorly water-soluble drugs and peptide/protein-based therapeutics or vaccines. Drug Deliv. 2016, 23, 1847–1867. [Google Scholar] [CrossRef]
- Faustino, C.; Serafim, C.; Rijo, P.; Reis, C.P. Bile acids and bile acid derivatives: Use in drug delivery systems and as therapeutic agents. Expert Opin. Drug Deliv. 2016, 13, 1133–1148. [Google Scholar] [CrossRef]
- Moghimipour, E.; Ameri, A.; Handali, S. Absorption-Enhancing Effects of Bile Salts. Molecules 2015, 20, 14451–14473. [Google Scholar] [CrossRef]
- Pavlović, N.; Goločorbin-Kon, S.; Ðanić, M.; Stanimirov, B.; Al-Salami, H.; Stankov, K.; Mikov, M. Bile Acids and Their Derivatives as Potential Modifiers of Drug Release and Pharmacokinetic Profiles. Front. Pharmacol. 2018, 9, 1283. [Google Scholar] [CrossRef]
- Stojančević, M.; Pavlović, N.; Goločorbin-Kon, S.; Mikov, M. Application of bile acids in drug formulation and delivery. Front. Life Sci. 2013, 7, 112–122. [Google Scholar] [CrossRef]
- Ammar, H.O.; Mohamed, M.I.; Tadros, M.I.; Fouly, A.A. High frequency ultrasound mediated transdermal delivery of ondansetron hydrochloride employing bilosomal gel systems: Ex-vivo and in-vivo characterization studies. J. Pharm. Investig. 2020, 50, 613–624. [Google Scholar] [CrossRef]
- Gupta, D.K.; Ahad, A.; Waheed, A.; Aqil, M.; Al-Jenoobi, F.I.; Al-Mohizea, A.M. Bilosomes: A novel platform for drug delivery. In Systems of Nanovesicular Drug Delivery; Nayak, A.K., Hasnain, M.S., Aminabhavi, T.M., Torchilin, V.P., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 293–309. [Google Scholar]
- Palekar-Shanbhag, P.; Lande, S.; Chandra, R.; Rane, D. Bilosomes: Superior Vesicular Carriers. Curr. Drug Ther. 2020, 15, 312–320. [Google Scholar] [CrossRef]
- Conacher, M.; Alexander, J.; Brewer, J.M. Oral immunisation with peptide and protein antigens by formulation in lipid vesicles incorporating bile salts (bilosomes). Vaccine 2001, 19, 2965–2974. [Google Scholar] [CrossRef]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Investigating superiority of novel bilosomes over niosomes in the transdermal delivery of diacerein: In vitro characterization, ex vivo permeation and in vivo skin deposition study. J. Liposome Res. 2019, 29, 73–85. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Asfour, M.H.; Salama, A.A.A. Improved hepatoprotective activity of silymarin via encapsulation in the novel vesicular nanosystem bilosomes. Drug. Dev. Ind. Pharm. 2017, 43, 2043–2054. [Google Scholar] [CrossRef]
- Premanand, B.; Prabakaran, M.; Kiener, T.K.; Kwang, J. Recombinant baculovirus associated with bilosomes as an oral vaccine candidate against HEV71 infection in mice. PLoS ONE 2013, 8, e55536. [Google Scholar] [CrossRef]
- Wang, L.; Huang, X.; Jing, H.; Ma, C.; Wang, H. Bilosomes as effective delivery systems to improve the gastrointestinal stability and bioavailability of epigallocatechin gallate (EGCG). Food Res. Int. 2021, 149, 110631. [Google Scholar] [CrossRef]
- Chen, Y.; Jiang, Z.; Xu, J.; Zhang, J.; Sun, R.; Zhou, J.; Lu, Y.; Gong, Z.; Huang, J.; Shen, X.; et al. Improving the ameliorative effects of berberine and curcumin combination via dextran-coated bilosomes on non-alcohol fatty liver disease in mice. J. Nanobiotechnol. 2021, 19, 230. [Google Scholar] [CrossRef]
- Elkomy, M.H.; Alruwaili, N.K.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Ahmad, N.; Alsalahat, I.; Ghoneim, M.M.; Eissa, E.M.; Eid, H.M. Surface-Modified Bilosomes Nanogel Bearing a Natural Plant Alkaloid for Safe Management of Rheumatoid Arthritis Inflammation. Pharmaceutics 2022, 14, 563. [Google Scholar] [CrossRef]
- Niu, M.; Lu, Y.; Hovgaard, L.; Guan, P.; Tan, Y.; Lian, R.; Qi, J.; Wu, W. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: The effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 2012, 81, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Sultan, A.A.; Saad, G.A.; El Maghraby, G.M. Permeation enhancers loaded bilosomes for improved intestinal absorption and cytotoxic activity of doxorubicin. Int. J. Pharm. 2023, 630, 122427. [Google Scholar] [CrossRef]
- Waglewska, E.; Maliszewska, I.; Bazylińska, U. Antimicrobial phyto-photodynamic activity inducing by polyphenol-supported Methylene Blue co-loaded into multifunctional bilosomes: Advanced hybrid nanoplatform in the skin infections treatment? J. Photochem. Photobiol. B 2023, 240, 112650. [Google Scholar] [CrossRef]
- Abdel-moneum, R.; Abdel-Rashid, R.S. Bile salt stabilized nanovesicles as a promising drug delivery technology: A general overview and future perspectives. J. Drug Deliv. Sci. Technol. 2023, 79, 104057. [Google Scholar] [CrossRef]
- Zarenezhad, E.; Marzi, M.; Abdulabbas, H.T.; Jasim, S.A.; Kouhpayeh, S.A.; Barbaresi, S.; Ahmadi, S.; Ghasemian, A. Bilosomes as Nanocarriers for the Drug and Vaccine Delivery against Gastrointestinal Infections: Opportunities and Challenges. J. Funct. Biomater. 2023, 14, 453. [Google Scholar] [CrossRef]
- Mann, J.F.; Shakir, E.; Carter, K.C.; Mullen, A.B.; Alexander, J.; Ferro, V.A. Lipid vesicle size of an oral influenza vaccine delivery vehicle influences the Th1/Th2 bias in the immune response and protection against infection. Vaccine 2009, 27, 3643–3649. [Google Scholar] [CrossRef]
- Yang, S.T.; Kreutzberger, A.J.B.; Lee, J.; Kiessling, V.; Tamm, L.K. The role of cholesterol in membrane fusion. Chem. Phys. Lipids 2016, 199, 136–143. [Google Scholar] [CrossRef]
- Albash, R.; El-Nabarawi, M.A.; Refai, H.; Abdelbary, A.A. Tailoring of PEGylated bilosomes for promoting the transdermal delivery of olmesartan medoxomil: In-vitro characterization, ex-vivo permeation and in-vivo assessment. Int. J. Nanomed. 2019, 14, 6555–6574. [Google Scholar] [CrossRef]
- Nair, V.S.; Srivastava, V.; Bhavana, V.; Yadav, R.; Rajana, N.; Singh, S.B.; Mehra, N.K. Exploring Penetration Ability of Carbonic Anhydrase Inhibitor-Loaded Ultradeformable Bilosome for Effective Ocular Application. AAPS PharmSciTech 2023, 24, 157. [Google Scholar] [CrossRef]
- Saifi, Z.; Rizwanullah, M.; Mir, S.R.; Amin, S. Bilosomes nanocarriers for improved oral bioavailability of acyclovir: A complete characterization through in vitro, ex-vivo and in vivo assessment. J. Drug Deliv. Sci. Technol. 2020, 57, 101634. [Google Scholar] [CrossRef]
- Kumar, G.P.; Rajeshwarrao, P. Nonionic surfactant vesicular systems for effective drug delivery—An overview. Acta Pharm. Sin. B 2011, 1, 208–219. [Google Scholar] [CrossRef]
- Abdelbari, M.A.; Elshafeey, A.H.; Abdelbary, A.A.; Mosallam, S. Implementing Nanovesicles for Boosting the Skin Permeation of Non-steroidal Anti-inflammatory Drugs. AAPS PharmSciTech 2023, 24, 195. [Google Scholar] [CrossRef]
- Ge, X.; Wei, M.; He, S.; Yuan, W.E. Advances of Non-Ionic Surfactant Vesicles (Niosomes) and Their Application in Drug Delivery. Pharmaceutics 2019, 11, 55. [Google Scholar] [CrossRef]
- Wilkhu, J.S.; McNeil, S.E.; Anderson, D.E.; Perrie, Y. Characterization and optimization of bilosomes for oral vaccine delivery. J. Drug Target 2013, 21, 291–299. [Google Scholar] [CrossRef]
- Chilkwar, R.N.; Nanjwade, B.K.; Nwaji, M.S.; Idris, S.A.M.; Mohamied, A.S. Bilosomes Based Drug Delivery System. J. Chem. Appl. 2015, 2, 5. [Google Scholar] [CrossRef]
- Mondal, D.; Mandal, R.P.; De, S. Addressing the Superior Drug Delivery Performance of Bilosomes—A Microscopy and Fluorescence Study. ACS Appl. Bio Mater. 2022, 5, 3896–3911. [Google Scholar] [CrossRef]
- Kaurav, H.; Kapoor, D.N. Implantable systems for drug delivery to the brain. Ther. Deliv. 2017, 8, 1097–1107. [Google Scholar] [CrossRef]
- Pardridge, W.M. Blood-brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood-brain barrier: An overview: Structure, regulation, and clinical implications. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef]
- Kaurav, H.; Kapoor, D.N.; Upadhyay, N.K. Bilosomes in brain drug delivery. Ther. Deliv. 2023, 14, 473–475. [Google Scholar] [CrossRef]
- Palmela, I.; Correia, L.; Silva, R.F.; Sasaki, H.; Kim, K.S.; Brites, D.; Brito, M.A. Hydrophilic bile acids protect human blood-brain barrier endothelial cells from disruption by unconjugated bilirubin: An in vitro study. Front. Neurosci. 2015, 9, 80. [Google Scholar] [CrossRef]
- Abbas, H.; Gad, H.A.; Khattab, M.A.; Mansour, M. The Tragedy of Alzheimer’s Disease: Towards Better Management via Resveratrol-Loaded Oral Bilosomes. Pharmaceutics 2021, 13, 1635. [Google Scholar] [CrossRef]
- Salehi, B.; Mishra, A.P.; Nigam, M.; Sener, B.; Kilic, M.; Sharifi-Rad, M.; Fokou, P.V.T.; Martins, N.; Sharifi-Rad, J. Resveratrol: A Double-Edged Sword in Health Benefits. Biomedicines 2018, 6, 91. [Google Scholar] [CrossRef]
- Abbas, H.; Refai, H.; El Sayed, N.; Rashed, L.A.; Mousa, M.R.; Zewail, M. Superparamagnetic iron oxide loaded chitosan coated bilosomes for magnetic nose to brain targeting of resveratrol. Int. J. Pharm. 2021, 610, 121244. [Google Scholar] [CrossRef]
- El Taweel, M.M.; Aboul-Einien, M.H.; Kassem, M.A.; Elkasabgy, N.A. Intranasal Zolmitriptan-Loaded Bilosomes with Extended Nasal Mucociliary Transit Time for Direct Nose to Brain Delivery. Pharmaceutics 2021, 13, 1828. [Google Scholar] [CrossRef]
- Elsheikh, M.A.; El-Feky, Y.A.; Al-Sawahli, M.M.; Ali, M.E.; Fayez, A.M.; Abbas, H. A Brain-Targeted Approach to Ameliorate Memory Disorders in a Sporadic Alzheimer’s Disease Mouse Model via Intranasal Luteolin-Loaded Nanobilosomes. Pharmaceutics 2022, 14, 576. [Google Scholar] [CrossRef]
- Narayanan, V.A.; Sharma, A.; Rajesh, K.S.; Arunraj, T.R.; Gururaj, M.P.; Parasuraman, S.; John, A. Bilosomes as a Potential Carrier to Enhance Cognitive Effects of Bacopa monnieri Extract on Oral Administration. J. Health Allied Sci. NU 2022, 13, 421–430. [Google Scholar] [CrossRef]
- Sultana, Y.; Jain, R.; Aqil, M.; Ali, A. Review of ocular drug delivery. Curr. Drug Deliv. 2006, 3, 207–217. [Google Scholar] [CrossRef]
- Srivastava, V.; Singh, V.; Kumar Khatri, D.; Kumar Mehra, N. Recent trends and updates on ultradeformable and elastic vesicles in ocular drug delivery. Drug Discov. Today 2023, 28, 103647. [Google Scholar] [CrossRef] [PubMed]
- Gote, V.; Sikder, S.; Sicotte, J.; Pal, D. Ocular Drug Delivery: Present Innovations and Future Challenges. J. Pharmacol. Exp. Ther. 2019, 370, 602–624. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Abd-Elsalam, W.H.; Al-Mahallawi, A.M. Fabrication of novel ultradeformable bilosomes for enhanced ocular delivery of terconazole: In vitro characterization, ex vivo permeation and in vivo safety assessment. Int. J. Pharm. 2016, 513, 688–696. [Google Scholar] [CrossRef] [PubMed]
- Janga, K.Y.; Tatke, A.; Balguri, S.P.; Lamichanne, S.P.; Ibrahim, M.M.; Maria, D.N.; Jablonski, M.M.; Majumdar, S. Ion-sensitive in situ hydrogels of natamycin bilosomes for enhanced and prolonged ocular pharmacotherapy: In vitro permeability, cytotoxicity and in vivo evaluation. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1039–1050. [Google Scholar] [CrossRef]
- Mohsen, A.M.; Salama, A.; Kassem, A.A. Development of acetazolamide loaded bilosomes for improved ocular delivery: Preparation, characterization and in vivo evaluation. J. Drug Deliv. Sci. Technol. 2020, 59, 101910. [Google Scholar] [CrossRef]
- Nemr, A.A.; El-Mahrouk, G.M.; Badie, H.A. Hyaluronic acid-enriched bilosomes: An approach to enhance ocular delivery of agomelatine via D-optimal design: Formulation, in vitro characterization, and in vivo pharmacodynamic evaluation in rabbits. Drug Deliv. 2022, 29, 2343–2356. [Google Scholar] [CrossRef]
- Sakr, M.G.; El-Zahaby, S.A.; Al-Mahallawi, A.M.; Ghorab, D.M. Fabrication of betaxolol hydrochloride-loaded highly permeable ocular bilosomes (HPOBs) to combat glaucoma: In vitro, ex vivo & in vivo characterizations. J. Drug Deliv. Sci. Technol. 2023, 82, 104363. [Google Scholar] [CrossRef]
- Alsaidan, O.A.; Zafar, A.; Yasir, M.; Alzarea, S.I.; Alqinyah, M.; Khalid, M. Development of Ciprofloxacin-Loaded Bilosomes In-Situ Gel for Ocular Delivery: Optimization, In-Vitro Characterization, Ex-Vivo Permeation, and Antimicrobial Study. Gels 2022, 8, 687. [Google Scholar] [CrossRef]
- Zafar, A.; Alsaidan, O.A.; Imam, S.S.; Yasir, M.; Alharbi, K.S.; Khalid, M. Formulation and Evaluation of Moxifloxacin Loaded Bilosomes In-Situ Gel: Optimization to Antibacterial Evaluation. Gels 2022, 8, 418. [Google Scholar] [CrossRef]
- Shukla, A.; Mishra, V.; Kesharwani, P. Bilosomes in the context of oral immunization: Development, challenges and opportunities. Drug Discov. Today 2016, 21, 888–899. [Google Scholar] [CrossRef]
- Shukla, A.; Katare, O.P.; Singh, B.; Vyas, S.P. M-cell targeted delivery of recombinant hepatitis B surface antigen using cholera toxin B subunit conjugated bilosomes. Int. J. Pharm. 2010, 385, 47–52. [Google Scholar] [CrossRef]
- Jain, S.; Indulkar, A.; Harde, H.; Agrawal, A.K. Oral mucosal immunization using glucomannosylated bilosomes. J. Biomed. Nanotechnol. 2014, 10, 932–947. [Google Scholar] [CrossRef]
- Ahmad, J.; Singhal, M.; Amin, S.; Rizwanullah, M.; Akhter, S.; Kamal, M.A.; Haider, N.; Midoux, P.; Pichon, C. Bile Salt Stabilized Vesicles (Bilosomes): A Novel Nano-Pharmaceutical Design for Oral Delivery of Proteins and Peptides. Curr. Pharm. Des. 2017, 23, 1575–1588. [Google Scholar] [CrossRef]
- Hu, S.; Niu, M.; Hu, F.; Lu, Y.; Qi, J.; Yin, Z.; Wu, W. Integrity and stability of oral liposomes containing bile salts studied in simulated and ex vivo gastrointestinal media. Int. J. Pharm. 2013, 441, 693–700. [Google Scholar] [CrossRef]
- Niu, M.; Tan, Y.; Guan, P.; Hovgaard, L.; Lu, Y.; Qi, J.; Lian, R.; Li, X.; Wu, W. Enhanced oral absorption of insulin-loaded liposomes containing bile salts: A mechanistic study. Int. J. Pharm. 2014, 460, 119–130. [Google Scholar] [CrossRef]
- Nallamothu, B.; Kuche, K.; Ghadi, R.; Chaudhari, D.; Jain, S. Enhancing oral bioavailability of insulin through bilosomes: Implication of charge and chain length on apical sodium-dependent bile acid transporter (ASBT) uptake. Int. J. Biol. Macromol. 2023, 252, 126565. [Google Scholar] [CrossRef]
- Prausnitz, M.R.; Elias, P.M.; Franz, T.J.; Schmuth, M.; Tsai, J.-C.; Menon, G.K.; Holleran, W.M.; Feingold, K.R. Skin Barrier and Transdermal Drug Delivery. Dermatology 2012, 3, 2065–2073. [Google Scholar]
- Paudel, K.S.; Milewski, M.; Swadley, C.L.; Brogden, N.K.; Ghosh, P.; Stinchcomb, A.L. Challenges and opportunities in dermal/transdermal delivery. Ther. Deliv. 2010, 1, 109–131. [Google Scholar] [CrossRef]
- El-Laithy, H.M.; Basalious, E.B.; El-Hoseiny, B.M.; Adel, M.M. Novel self-nanoemulsifying self-nanosuspension (SNESNS) for enhancing oral bioavailability of diacerein: Simultaneous portal blood absorption and lymphatic delivery. Int. J. Pharm. 2015, 490, 146–154. [Google Scholar] [CrossRef]
- Khalil, R.M.; Abdelbary, A.; Kocova El-Arini, S.; Basha, M.; El-Hashemy, H.A. Evaluation of bilosomes as nanocarriers for transdermal delivery of tizanidine hydrochloride: In vitro and ex vivo optimization. J. Liposome Res. 2019, 29, 171–182. [Google Scholar] [CrossRef]
- El Menshawe, S.F.; Aboud, H.M.; Elkomy, M.H.; Kharshoum, R.M.; Abdeltwab, A.M. A novel nanogel loaded with chitosan decorated bilosomes for transdermal delivery of terbutaline sulfate: Artificial neural network optimization, in vitro characterization and in vivo evaluation. Drug Deliv. Transl. Res. 2020, 10, 471–485. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in vitro Optimization, ex vivo Permeation and in vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef]
- Ibrahiem, B.; Shamma, R.; Salama, A.; Refai, H. Magnetic targeting of lornoxicam/SPION bilosomes loaded in a thermosensitive in situ hydrogel system for the management of osteoarthritis: Optimization, in vitro, ex vivo, and in vivo studies in rat model via modulation of RANKL/OPG. Drug Deliv. Transl. Res. 2023. [Google Scholar] [CrossRef]
- El-Nabarawi, M.A.; Shamma, R.N.; Farouk, F.; Nasralla, S.M. Bilosomes as a novel carrier for the cutaneous delivery for dapsone as a potential treatment of acne: Preparation, characterization and in vivo skin deposition assay. J. Liposome Res. 2020, 30, 1–11. [Google Scholar] [CrossRef]
- Abdelalim, L.R.; Abdallah, O.Y.; Elnaggar, Y.S.R. High efficacy, rapid onset nanobiolosomes of sildenafil as a topical therapy for erectile dysfunction in aged rats. Int. J. Pharm. 2020, 591, 119978. [Google Scholar] [CrossRef]
- Mosallam, S.; Sheta, N.M.; Elshafeey, A.H.; Abdelbary, A.A. Fabrication of Highly Deformable Bilosomes for Enhancing the Topical Delivery of Terconazole: In Vitro Characterization, Microbiological Evaluation, and In Vivo Skin Deposition Study. AAPS PharmSciTech 2021, 22, 74. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, T.M.; Nafady, M.M.; Farouk, H.O.; Mahmoud, D.M.; Ahmed, Y.M.; Zaki, R.M.; Hamad, D.S. Novel Bile Salt Stabilized Vesicles-Mediated Effective Topical Delivery of Diclofenac Sodium: A New Therapeutic Approach for Pain and Inflammation. Pharmaceuticals 2022, 15, 1106. [Google Scholar] [CrossRef]
- Salem, H.F.; Nafady, M.M.; Ali, A.A.; Khalil, N.M.; Elsisi, A.A. Evaluation of Metformin Hydrochloride Tailoring Bilosomes as an Effective Transdermal Nanocarrier. Int. J. Nanomed. 2022, 17, 1185–1201. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Gilani, S.J.; Zafar, A.; Jumah, M.N.B.; Alshehri, S. Formulation of Miconazole-Loaded Chitosan-Carbopol Vesicular Gel: Optimization to In Vitro Characterization, Irritation, and Antifungal Assessment. Pharmaceutics 2023, 15, 581. [Google Scholar] [CrossRef] [PubMed]
- Khafagy, E.S.; Almutairy, B.K.; Abu Lila, A.S. Tailoring of Novel Bile Salt Stabilized Vesicles for Enhanced Transdermal Delivery of Simvastatin: A New Therapeutic Approach against Inflammation. Polymers 2023, 15, 677. [Google Scholar] [CrossRef] [PubMed]
- Teaima, M.H.; Alsofany, J.M.; El-Nabarawi, M.A. Clove Oil Endorsed Transdermal Flux of Dronedarone Hydrochloride Loaded Bilosomal Nanogel: Factorial Design, In vitro Evaluation and Ex vivo Permeation. AAPS PharmSciTech 2022, 23, 182. [Google Scholar] [CrossRef] [PubMed]
- Beg, S.; Alharbi, K.S.; Alruwaili, N.K.; Alotaibi, N.H.; Almalki, W.H.; Alenezi, S.K.; Altowayan, W.M.; Alshammari, M.S.; Rahman, M. Nanotherapeutic systems for delivering cancer vaccines: Recent advances. Nanomedicine 2020, 15, 1527–1537. [Google Scholar] [CrossRef] [PubMed]
- Briz, O.; Macias, R.I.; Vallejo, M.; Silva, A.; Serrano, M.A.; Marin, J.J. Usefulness of liposomes loaded with cytostatic bile acid derivatives to circumvent chemotherapy resistance of enterohepatic tumors. Mol. Pharmacol. 2003, 63, 742–750. [Google Scholar] [CrossRef] [PubMed]
- Parashar, P.; Rana, P.; Dwivedi, M.; Saraf, S.A. Dextrose modified bilosomes for peroral delivery: Improved therapeutic potential and stability of silymarin in diethylnitrosamine-induced hepatic carcinoma in rats. J. Liposome Res. 2019, 29, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Zaki, I.; Abou-Elkhair, R.A.; Abu Almaaty, A.H.; AAbu Ali, O.; Fayad, E.; Ahmed Gaafar, A.G.; Zakaria, M.Y. Design and Synthesis of Newly Synthesized Acrylamide Derivatives as Potential Chemotherapeutic Agents against MCF-7 Breast Cancer Cell Line Lodged on PEGylated Bilosomal Nano-Vesicles for Improving Cytotoxic Activity. Pharmaceuticals 2021, 14, 1021. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Badr-Eldin, S.M.; Alharbi, W.S.; Alfaleh, M.A.; Al-Hejaili, O.D.; Aldawsari, H.M.; Eid, B.G.; Bakhaidar, R.; Drago, F.; Caraci, F.; et al. Development of an Icariin-Loaded Bilosome-Melittin Formulation with Improved Anticancer Activity against Cancerous Pancreatic Cells. Pharmaceuticals 2021, 14, 1309. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Caruso, G.; Al-Rabia, M.W.; Badr-Eldin, S.M.; Aldawsari, H.M.; Asfour, H.Z.; Alshehri, S.; Alzaharani, S.H.; Alhamdan, M.M.; Rizg, W.Y.; et al. Piceatannol-Loaded Bilosome-Stabilized Zein Protein Exhibits Enhanced Cytostatic and Apoptotic Activities in Lung Cancer Cells. Pharmaceutics 2021, 13, 638. [Google Scholar] [CrossRef]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Alsaidan, O.A.; Yasir, M.; Ghoneim, M.M.; Alshehri, S.; Anwer, M.K.; Almurshedi, A.S.; Alanazi, A.S. Development and evaluation of luteolin loaded pegylated bilosome: Optimization, in vitro characterization, and cytotoxicity study. Drug Deliv. 2021, 28, 2562–2573. [Google Scholar] [CrossRef] [PubMed]
- Matloub, A.A.; Salama, A.H.; Aglan, H.A.; AbouSamra, M.M.; ElSouda, S.S.M.; Ahmed, H.H. Exploiting bilosomes for delivering bioactive polysaccharide isolated from Enteromorpha intestinalis for hacking hepatocellular carcinoma. Drug Dev. Ind. Pharm. 2018, 44, 523–534. [Google Scholar] [CrossRef] [PubMed]
- Sohn, S.I.; Priya, A.; Balasubramaniam, B.; Muthuramalingam, P.; Sivasankar, C.; Selvaraj, A.; Valliammai, A.; Jothi, R.; Pandian, S. Biomedical Applications and Bioavailability of Curcumin-An Updated Overview. Pharmaceutics 2021, 13, 2102. [Google Scholar] [CrossRef] [PubMed]
- Abbas, H.; El-Feky, Y.A.; Al-Sawahli, M.M.; El-Deeb, N.M.; El-Nassan, H.B.; Zewail, M. Development and optimization of curcumin analog nano-bilosomes using 2(1).3(1) full factorial design for anti-tumor profiles improvement in human hepatocellular carcinoma: In-vitro evaluation, in-vivo safety assay. Drug Deliv. 2022, 29, 714–727. [Google Scholar] [CrossRef] [PubMed]
- Hegazy, H.; Amin, M.M.; Fayad, W.; Zakaria, M.Y. TPGS surface modified bilosomes as boosting cytotoxic oral delivery systems of curcumin against doxorubicin resistant MCF-7 breast cancer cells. Int. J. Pharm. 2022, 619, 121717. [Google Scholar] [CrossRef] [PubMed]
- Imam, S.S.; Alshehri, S.; Altamimi, M.A.; Almalki, R.K.H.; Hussain, A.; Bukhari, S.I.; Mahdi, W.A.; Qamar, W. Formulation of Chitosan-Coated Apigenin Bilosomes: In Vitro Characterization, Antimicrobial and Cytotoxicity Assessment. Polymers 2022, 14, 921. [Google Scholar] [CrossRef] [PubMed]
- Kharouba, M.; El-Kamel, A.; Mehanna, R.; Thabet, E.; Heikal, L. Pitavastatin-loaded bilosomes for oral treatment of hepatocellular carcinoma: A repurposing approach. Drug Deliv. 2022, 29, 2925–2944. [Google Scholar] [CrossRef] [PubMed]
- Alruwaili, N.K.; Zafar, A.; Alsaidan, O.A.; Yasir, M.; Mostafa, E.M.; Alnomasy, S.F.; Rawaf, A.; Alquraini, A.; Alomar, F.A. Development of surface modified bilosomes for the oral delivery of quercetin: Optimization, characterization in-vitro antioxidant, antimicrobial, and cytotoxicity study. Drug Deliv. 2022, 29, 3035–3050. [Google Scholar] [CrossRef]
- Sharifi-Rad, J.; Kamiloglu, S.; Yeskaliyeva, B.; Beyatli, A.; Alfred, M.A.; Salehi, B.; Calina, D.; Docea, A.O.; Imran, M.; Anil Kumar, N.V.; et al. Pharmacological Activities of Psoralidin: A Comprehensive Review of the Molecular Mechanisms of Action. Front. Pharmacol. 2020, 11, 571459. [Google Scholar] [CrossRef] [PubMed]
- Youness, R.A.; Al-Mahallawi, A.M.; Mahmoud, F.H.; Atta, H.; Braoudaki, M.; Fahmy, S.A. Oral Delivery of Psoralidin by Mucoadhesive Surface-Modified Bilosomes Showed Boosted Apoptotic and Necrotic Effects against Breast and Lung Cancer Cells. Polymers 2023, 15, 1464. [Google Scholar] [CrossRef]
- Shrikumar, S.; Ravi, T. Approaches towards development and promotion of herbal drugs. Pharmacog. Rev. 2007, 1, 180–184. [Google Scholar]
- Abrol, S.; Trehan, A.; Katare, O.P. Comparative study of different silymarin formulations: Formulation, characterisation and in vitro/in vivo evaluation. Curr. Drug Deliv. 2005, 2, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Waglewska, E.; Pucek-Kaczmarek, A.; Bazylińska, U. Self-assembled bilosomes with stimuli-responsive properties as bioinspired dual-tunable nanoplatform for pH/temperature-triggered release of hybrid cargo. Colloids Surf. B Biointerfaces 2022, 215, 112524. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Alruwaili, N.K.; Imam, S.S.; Hadal Alotaibi, N.; Alharbi, K.S.; Afzal, M.; Ali, R.; Alshehri, S.; Alzarea, S.I.; Elmowafy, M.; et al. Bioactive Apigenin loaded oral nano bilosomes: Formulation optimization to preclinical assessment. Saudi Pharm. J. 2021, 29, 269–279. [Google Scholar] [CrossRef]
- Zakaria, M.Y.; Fayad, E.; Althobaiti, F.; Zaki, I.; Abu Almaaty, A.H. Statistical optimization of bile salt deployed nanovesicles as a potential platform for oral delivery of piperine: Accentuated antiviral and anti-inflammatory activity in MERS-CoV challenged mice. Drug Deliv. 2021, 28, 1150–1165. [Google Scholar] [CrossRef]
- Song, D.; Hao, J.; Fan, D. Biological properties and clinical applications of berberine. Front. Med. 2020, 14, 564–582. [Google Scholar] [CrossRef]
- Mirhadi, E.; Rezaee, M.; Malaekeh-Nikouei, B. Nano strategies for berberine delivery, a natural alkaloid of Berberis. Biomed. Pharmacother. 2018, 104, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Elkomy, M.H.; Eid, H.M.; Elmowafy, M.; Shalaby, K.; Zafar, A.; Abdelgawad, M.A.; Rateb, M.E.; Ali, M.R.A.; Alsalahat, I.; Abou-Taleb, H.A. Bilosomes as a promising nanoplatform for oral delivery of an alkaloid nutraceutical: Improved pharmacokinetic profile and snowballed hypoglycemic effect in diabetic rats. Drug Deliv. 2022, 29, 2694–2704. [Google Scholar] [CrossRef]
- Zakaria, M.Y.; Abd El-Halim, S.M.; Beshay, B.Y.; Zaki, I.; Abourehab, M.A.S. Poly phenolic phytoceutical loaded nano-bilosomes for enhanced caco-2 cell permeability and SARS-CoV-2 antiviral activity: In-vitro and insilico studies. Drug Deliv. 2023, 30, 2162157. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).