Impact of Time on Parameters for Assessing the Microstructure Equivalence of Topical Products: Diclofenac 1% Emulsion as a Case Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Rheological Analysis
2.1.1. Hysteresis Loops
2.1.2. Flow Curves
2.1.3. Small-Amplitude Oscillation Sweep Tests
2.2. Droplet Size Measurement
2.3. In Vitro Release Test (IVRT)
2.4. Drug Quantification
2.5. Statistical Analysis and Data Comparison
3. Results and Discussion
3.1. Parameter Evolution during Shelf Life
3.2. Impact of Parameters Evolution on Equivalence Contrast
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Food and Drug Administration. Draft Guidance on Acyclovir. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_018604.pdf (accessed on 5 May 2024).
- Food and Drug Administration Draft Guidance on Dapsone. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/PSG_207154.pdf (accessed on 5 May 2024).
- Food and Drug Administration. Draft Guidance on Silver Sulfadiazine. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Silver%20sulfadiazine_topical%20cream_NDA%20017381_RC05-17.pdf (accessed on 5 May 2024).
- Food and Drug Administration. Draft Guidance on Bexarotene. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Bexarotene%20topical%20gel%20NDA%20021056%20RC%202019.pdf (accessed on 5 May 2024).
- Food and Drug Administration. Draft Guidance on Benzyl Alcohol. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/psg/Benzyl_alcohol_lot_022129_RC12-14.pdf (accessed on 5 May 2024).
- Committee for Medicinal Products for Human Use (CHMP). Draft Guideline on Quality and Equivalence of Topical Product. CHMP/QWP/708282/2018. Available online: https://www.ema.europa.eu/en/quality-equivalence-topical-products (accessed on 4 May 2024).
- Miranda, M.; Cova, T.; Augusto, C.; Pais, A.A.C.C.; Cardoso, C.; Vitorino, C. Diving into Batch-to-Batch Variability of Topical Products-a Regulatory Bottleneck. Pharm. Res. 2020, 37, 218. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Cardoso, C.; Pais, A.A.C.C.; Brown, M.; Vitorino, C. Drilling down the Bioequivalence Assessment of Topical Antifungal Products: Microstructure and Release. Eur. J. Pharm. Biopharm. 2023, 185, 94–106. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.; Veloso, C.; Brown, M.; Pais, A.A.C.C.; Cardoso, C.; Vitorino, C. Topical Bioequivalence: Experimental and Regulatory Considerations Following Formulation Complexity. Int. J. Pharm. 2022, 620, 121705. [Google Scholar] [CrossRef] [PubMed]
- Navarro-Pujol, F.; Bulut, S.; Hessman, C.; Karabelas, K.; Nieto, C.; Fernandez-Campos, F. Pilot Equivalence Study Comparing Different Batches of Topical 0.025% Capsaicin Emulsion: Product Microstructure, Release, and Permeation Evaluation. Pharmaceutics 2021, 13, 2083. [Google Scholar] [CrossRef] [PubMed]
- Mangas-Sanjuán, V.; Pleguezuelos-Villa, M.; Merino-Sanjuán, M.; Hernández, M.J.; Nácher, A.; García-Arieta, A.; Peris, D.; Hidalgo, I.; Soler, L.; Sallan, M.; et al. Assessment of the Inter-Batch Variability of Microstructure Parameters in Topical Semisolids and Impact on the Demonstration of Equivalence. Pharmaceutics 2019, 11, 503, Erratum in Pharmaceutics 2020, 12, 436. [Google Scholar] [CrossRef] [PubMed]
- Pleguezuelos-Villa, M.; Merino-Sanjuán, M.; Hernández, M.J.; Nácher, A.; Peris, D.; Hidalgo, I.; Soler, L.; Sallan, M.; Merino, V. Relationship between Rheological Properties, In Vitro Release and In Vivo Equivalency of Topical Formulations of Diclofenac. Int. J. Pharm. 2019, 572, 118755. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Mangas-Sanjuán, V.; Merino-Sanjuán, M.; Merino, V.; García-Arieta, A. Influence of Inter- and Intra-Batch Variability on the Sample Size Required for Demonstration of Equivalent Microstructure of Semisolid Dosage Forms. Pharmaceutics 2020, 12, 1159. [Google Scholar] [CrossRef] [PubMed]
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use. ICH Harmonised Tripartite Guideline, Pharmaceutical Development—Q8(R2). Available online: https://database.ich.org/sites/default/files/Q8%28R2%29%20Guideline.pdf (accessed on 4 May 2024).
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline, Quality Risk Management—Q9(R1). Available online: https://database.ich.org/sites/default/files/ICH_Q9%28R1%29_Guideline_Step4_2023_0126_0.pdf (accessed on 4 May 2024).
- International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use ICH Harmonised Tripartite Guideline, Pharmaceutical Quality Systems—Q10. Available online: https://database.ich.org/sites/default/files/Q10%20Guideline.pdf (accessed on 4 May 2024).
- Alves, T.; Arranca, D.; Martins, A.; Ribeiro, H.; Raposo, S.; Marto, J. Complying with the Guideline for Quality and Equivalence for Topical Semisolid Products: The Case of Clotrimazole Cream. Pharmaceutics 2021, 13, 555. [Google Scholar] [CrossRef] [PubMed]
- Chiarentin, L.; Cardoso, C.; Miranda, M.; Vitorino, C. Rheology of Complex Topical Formulations: An Analytical Quality by Design Approach to Method Optimization and Validation. Pharmaceutics 2023, 15, 1810. [Google Scholar] [CrossRef] [PubMed]
- Agencia Española de Medicamentos y Productos Sanitarios Technical Data Sheet Diclofenaco Kern Pharma 11.6 Mg/g Gel. Available online: https://cima.aemps.es/cima/pdfs/es/ft/74119/74119_ft.pdf (accessed on 4 May 2024).
- Dolz, M.; Hernández, M.J.; Pellicer, J.; Delegido, J. Shear Stress Synergism Index and Relative Thixotropic Area. J. Pharm. Sci. 1995, 84, 728–732. [Google Scholar] [CrossRef] [PubMed]
- Mezger, T.G. The Rheology Handbook: For Users of Rotational and Oscillatory Rheometers, 4th ed.; Vincentz Network: Hannover, Germany, 2014; ISBN 978-3-87870-174-3. [Google Scholar]
- Food and Drug Administration. SUPAC-SS: Nonsterile Semisolid Dosage Forms; Scale-Up and Post-Approval Changes: Chemistry, Manufacturing and Controls; In Vitro Release Testing and In Vivo Bioequivalence Documentation. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/supac-ss-nonsterile-semisolid-dosage-forms-scale-and-post-approval-changes-chemistry-manufacturing (accessed on 4 May 2024).
- International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use ICH Harmonised Guideline, Validation of Analytical Procedures—Q2(R2). Available online: https://database.ich.org/sites/default/files/ICH_Q2%28R2%29_Guideline_2023_1130.pdf (accessed on 5 May 2024).
- Pal, R. Effect of Droplet Size on the Rheology of Emulsions. AIChE J. 1996, 42, 3181–3190. [Google Scholar] [CrossRef]
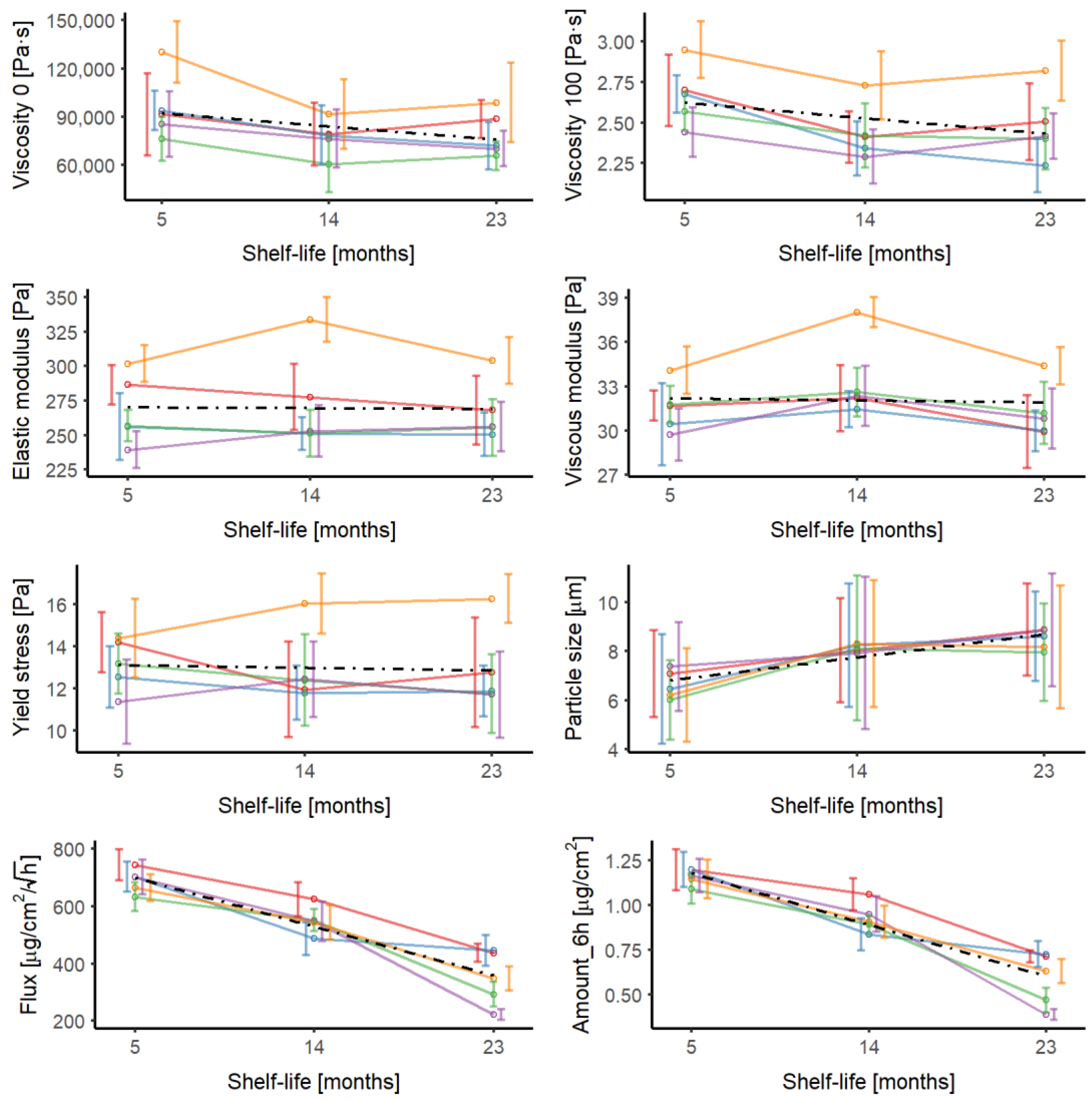
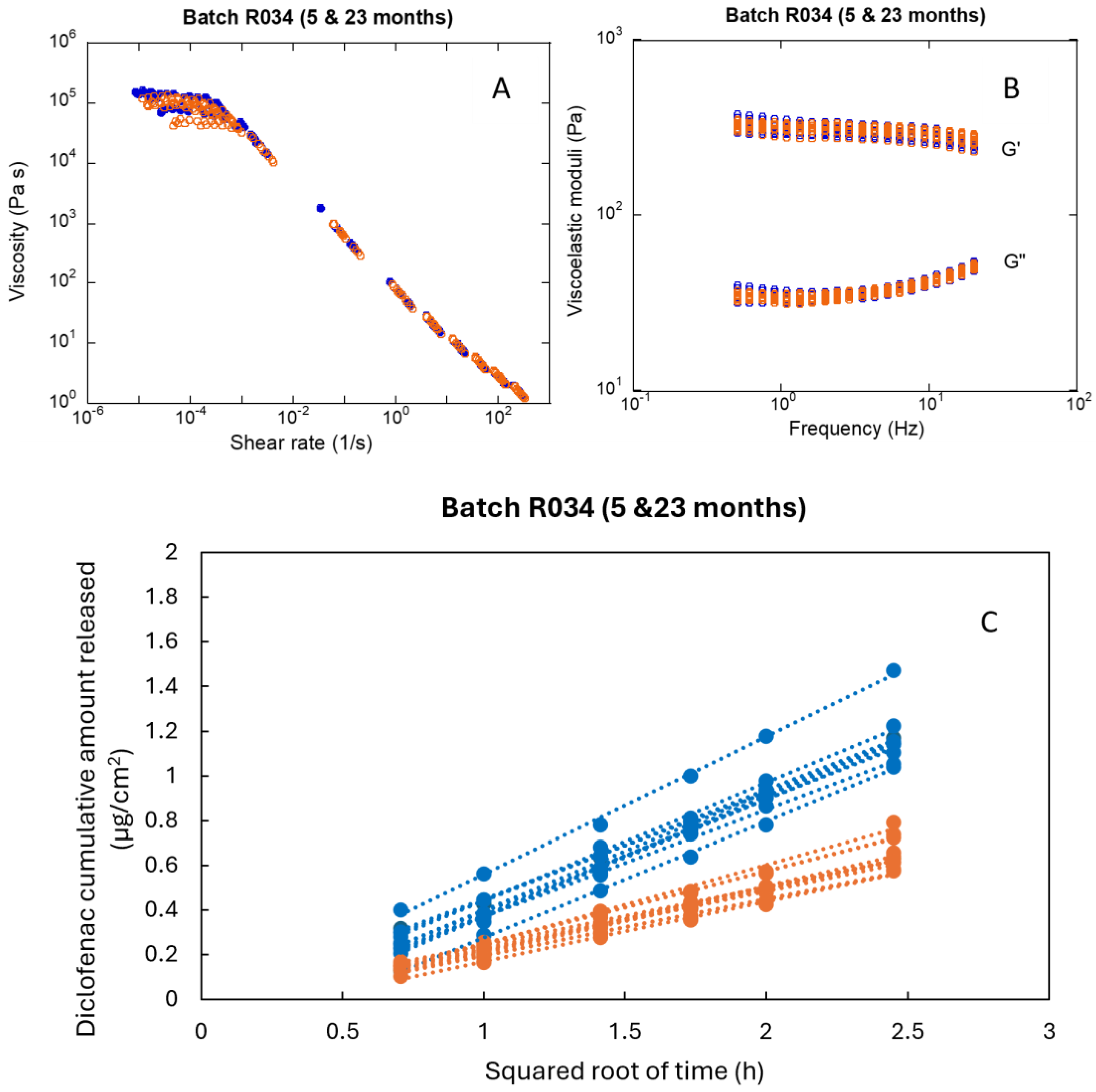
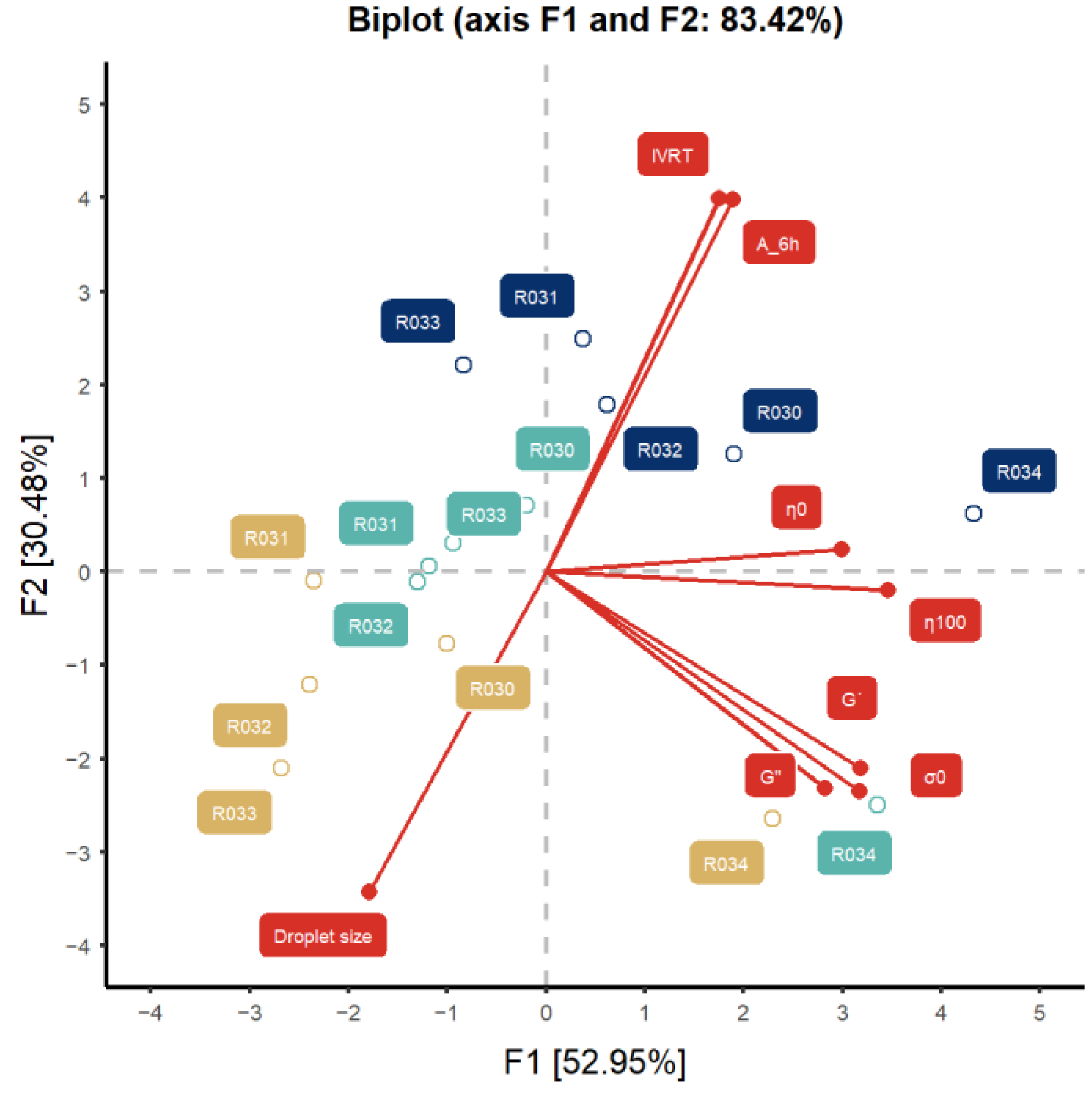

| Parameter | Time (Months) | CV % of the Parameter | ||||||
|---|---|---|---|---|---|---|---|---|
| R030 | R031 | R032 | R033 | R034 | Inter-Batch | Total | ||
| η0 | 5 | 30.4 | 13.5 | 17.9 | 25.8 | 14.7 | 23.5 | 29.0 |
| 14 | 23.8 | 22.5 | 28.1 | 25.1 | 23.8 | 13.9 | 26.8 | |
| 23 | 12.9 | 20.4 | 13.8 | 15.8 | 25.8 | 17.6 | 24.6 | |
| η100 | 5 | 7.8 | 4.3 | 4.6 | 6.1 | 5.8 | 7.0 | 8.6 |
| 14 | 6.5 | 7.1 | 8.2 | 7.1 | 7.6 | 7.0 | 9.5 | |
| 23 | 9.4 | 7.2 | 8.0 | 5.7 | 6.5 | 8.9 | 10.8 | |
| G′ | 5 | 4.9 | 9.9 | 4.4 | 5.5 | 4.4 | 10.3 | 11.0 |
| 14 | 8.7 | 4.6 | 6.8 | 7.4 | 4.8 | 12.5 | 13.0 | |
| 23 | 9.4 | 6.4 | 8.2 | 7.0 | 5.5 | 9.0 | 10.8 | |
| G″ | 5 | 3.2 | 9.4 | 3.9 | 5.7 | 4.7 | 6.1 | 7.7 |
| 14 | 7.1 | 3.8 | 5.1 | 6.4 | 2.6 | 8.4 | 9.0 | |
| 23 | 8.4 | 4.6 | 6.8 | 6.6 | 3.7 | 6.6 | 8.4 | |
| σ0 | 5 | 10.5 | 11.9 | 10.7 | 18.2 | 13.0 | 10.2 | 15.4 |
| 14 | 17.6 | 10.6 | 17.5 | 14.0 | 8.9 | 11.9 | 17.1 | |
| 23 | 20.5 | 10.2 | 15.8 | 18.2 | 7.2 | 15.1 | 19.6 | |
| droplet size | 5 | 23.9 | 32.3 | 25.6 | 23.6 | 28.1 | 7.8 | 27.6 |
| 14 | 25.3 | 29.6 | 33.9 | 38.2 | 29.8 | 2.8 | 31.6 | |
| 23 | 21.3 | 21.2 | 24.6 | 25.2 | 30.1 | 5.0 | 24.9 | |
| IVRT Flux | 5 | 7.4 | 7.4 | 7.8 | 8.4 | 4.3 | 5.9 | 8.9 |
| 14 | 9.3 | 11.3 | 7.0 | 12.2 | 11.1 | 8.6 | 12.6 | |
| 23 | 7.45 | 11.7 | 14.7 | 8.2 | 11.45 | 26.9 | 26.5 | |
| A6h | 5 | 9.34 | 8.21 | 7.51 | 7.80 | 4.56 | 4.0 | 8.3 |
| 14 | 8.25 | 10.59 | 5.98 | 10.43 | 10.02 | 9.9 | 12.6 | |
| 23 | 4.79 | 9.73 | 14.39 | 7.84 | 10.58 | 14.4 | 24.8 | |
| Parameter | Compared Groups | ANOVA | EMA CI 90% | Status |
|---|---|---|---|---|
| η0 | 5 vs. 14 | * | 85.5 (77.5–94.3) | NC |
| 14 vs. 23 | NS | 102.0 (94.0–110.0) | C | |
| 5 vs. 23 | * | 87.2 (80.2–94.9) | NC | |
| η100 | 5 vs. 14 | * | 90.1 (89.2–92.9) | NC |
| 14 vs. 23 | NS | 100.7 (98.3–103.3) | C | |
| 5 vs. 23 | * | 91.7 (89.2–94.3) | NC | |
| G’ | 5 vs. 14 | NS | 101.6 (99.3–104.0) | C |
| 14 vs. 23 | NS | 97.2 (94.3–100.2) | C | |
| 5 vs. 23 | NS | 99.2 (96.7–101.7) | C | |
| G” | 5 vs. 14 | * | 104.2 (102.2–106.0) | C |
| 14 vs. 23 | * | 93.6 (91.2–96.2) | C | |
| 5 vs. 23 | NS | 97.9 (95.5–100.4) | C | |
| σ0 | 5 vs. 14 | NS | 101.5 (97.7–105.5) | C |
| 14 vs. 23 | NS | 96.8 (91.4–102.5) | C | |
| 5 vs. 23 | NS | 98.9 (94.1–103.8) | C | |
| Droplet size | 5 vs. 14 | * | 118.94 (115.8–122.2) | NC |
| 14 vs. 23 | NS | 103.1 (100.4–106.0) | C | |
| 5 vs. 23 | * | 122.7 (119.7–125.7) | NC | |
| IVRT Flux | 5 vs. 14 | * | 80.7 (78.7–82.9) | NC |
| 14 vs. 23 | * | 61.3 (57.5–65.5) | NC | |
| 5 vs. 23 | * | 49.6 (46.5–52.8) | NC | |
| A6h | 5 vs. 14 | * | 79.29 (76.77–81.89) | NC |
| 14 vs. 23 | * | 61.95 (58.17–65.97) | NC | |
| 5 vs. 23 | * | 49.12 (46.26–52.15) | NC |
| INTERVAL | η0 | η100 | G′ | G″ | σ0 | Droplet Size | IVRT Flux | A6h | ||
|---|---|---|---|---|---|---|---|---|---|---|
| 5 months | 90–111 | n | 0/18 | 18/18 | 11/18 | 18/18 | 5/18 | 7/18 | 18/18 | 18/18 |
| % | 0 | 100 | 61.11 | 100 | 27.77 | 38.88 | 100 | 100 | ||
| 80–125 | n | 5/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | |
| % | 27.77 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 75–133 | n | 13/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | |
| % | 72.22 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 14 months | 90–111 | n | 0/18 | 18/18 | 3/18 | 18/18 | 4/18 | 0/18 | 10/18 | 11/18 |
| % | 0 | 100 | 16.66 | 100 | 22.22 | 0 | 55.55 | 61.11 | ||
| 80–125 | n | 11/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | |
| % | 61.11 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 75–133 | n | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | |
| % | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | ||
| 23 months | 90–111 | n | 0/18 | 12/18 | 11/18 | 18/18 | 1/18 | 4/18 | 0/18 | 5/18 |
| % | 0 | 66.66 | 61.11 | 100 | 5.55 | 22.22 | 0 | 27.77 | ||
| 80–125 | n | 11/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 7/18 | 17/18 | |
| % | 61.11 | 100 | 100 | 100 | 100 | 100 | 38.88 | 94.44 | ||
| 75–133 | n | 16/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | 18/18 | |
| % | 88.88 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mañez-Asensi, A.; Hernández, M.J.; Mangas-Sanjuán, V.; Salvador, A.; Merino-Sanjuán, M.; Merino, V. Impact of Time on Parameters for Assessing the Microstructure Equivalence of Topical Products: Diclofenac 1% Emulsion as a Case Study. Pharmaceutics 2024, 16, 749. https://doi.org/10.3390/pharmaceutics16060749
Mañez-Asensi A, Hernández MJ, Mangas-Sanjuán V, Salvador A, Merino-Sanjuán M, Merino V. Impact of Time on Parameters for Assessing the Microstructure Equivalence of Topical Products: Diclofenac 1% Emulsion as a Case Study. Pharmaceutics. 2024; 16(6):749. https://doi.org/10.3390/pharmaceutics16060749
Chicago/Turabian StyleMañez-Asensi, Andreu, Mª Jesús Hernández, Víctor Mangas-Sanjuán, Ana Salvador, Matilde Merino-Sanjuán, and Virginia Merino. 2024. "Impact of Time on Parameters for Assessing the Microstructure Equivalence of Topical Products: Diclofenac 1% Emulsion as a Case Study" Pharmaceutics 16, no. 6: 749. https://doi.org/10.3390/pharmaceutics16060749









