Nanoarchitectonics-Based Materials as a Promising Strategy in the Treatment of Endodontic Infections
Abstract
1. Introduction
2. Endodontic Pathogenic Species
2.1. Enterococcus faecalis (E. faecalis)
2.2. Porphyromans gingivalis (P. gingivalis)
2.3. Fusobacterium nucleatum (F. nucleatum)
2.4. Candida albicans (C. albicans)
2.5. Endodontic Microorganisms and Systemic Diseases
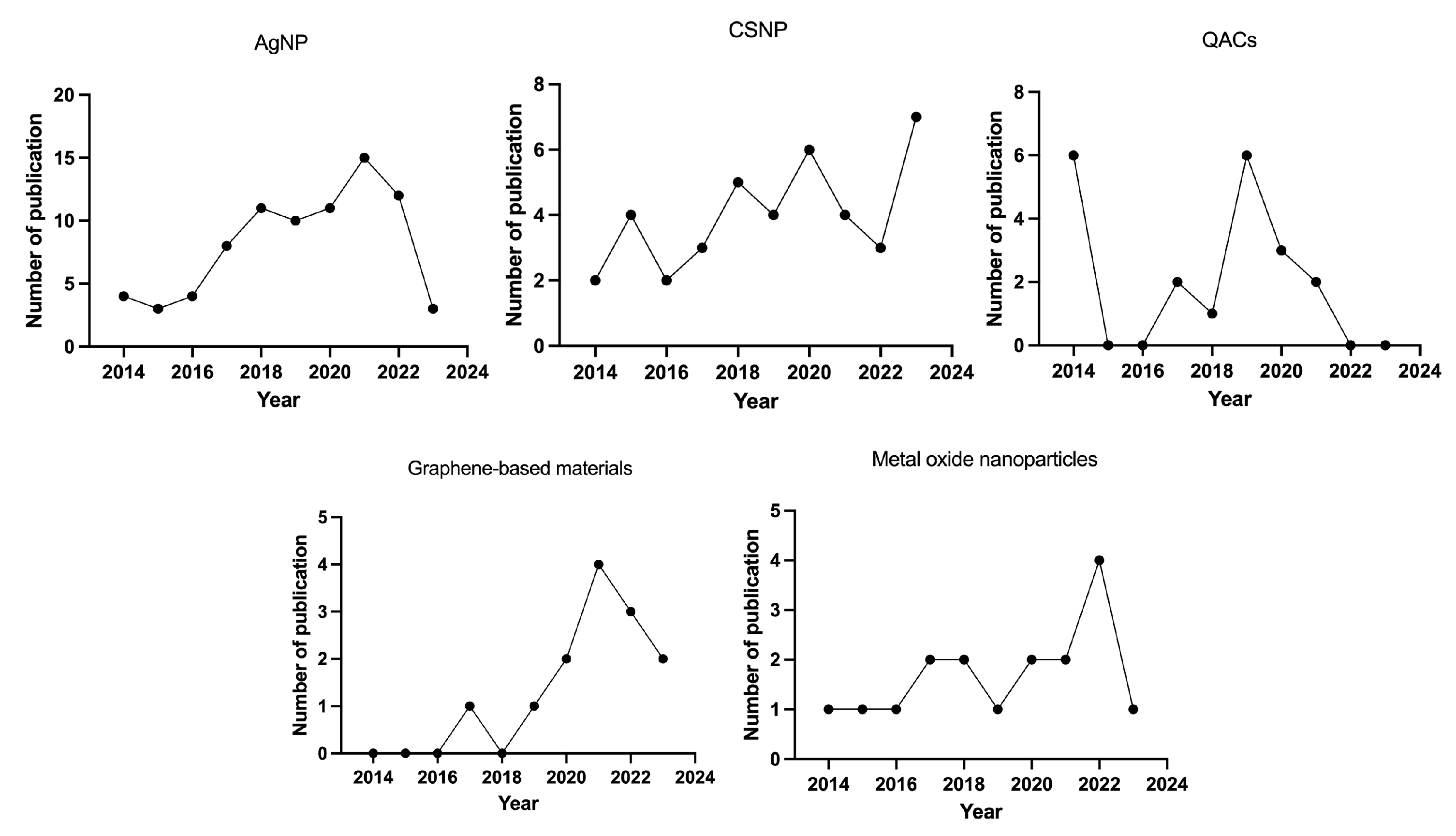
3. Nanomaterials Used in Endodontics and Their Antimicrobial Mechanisms
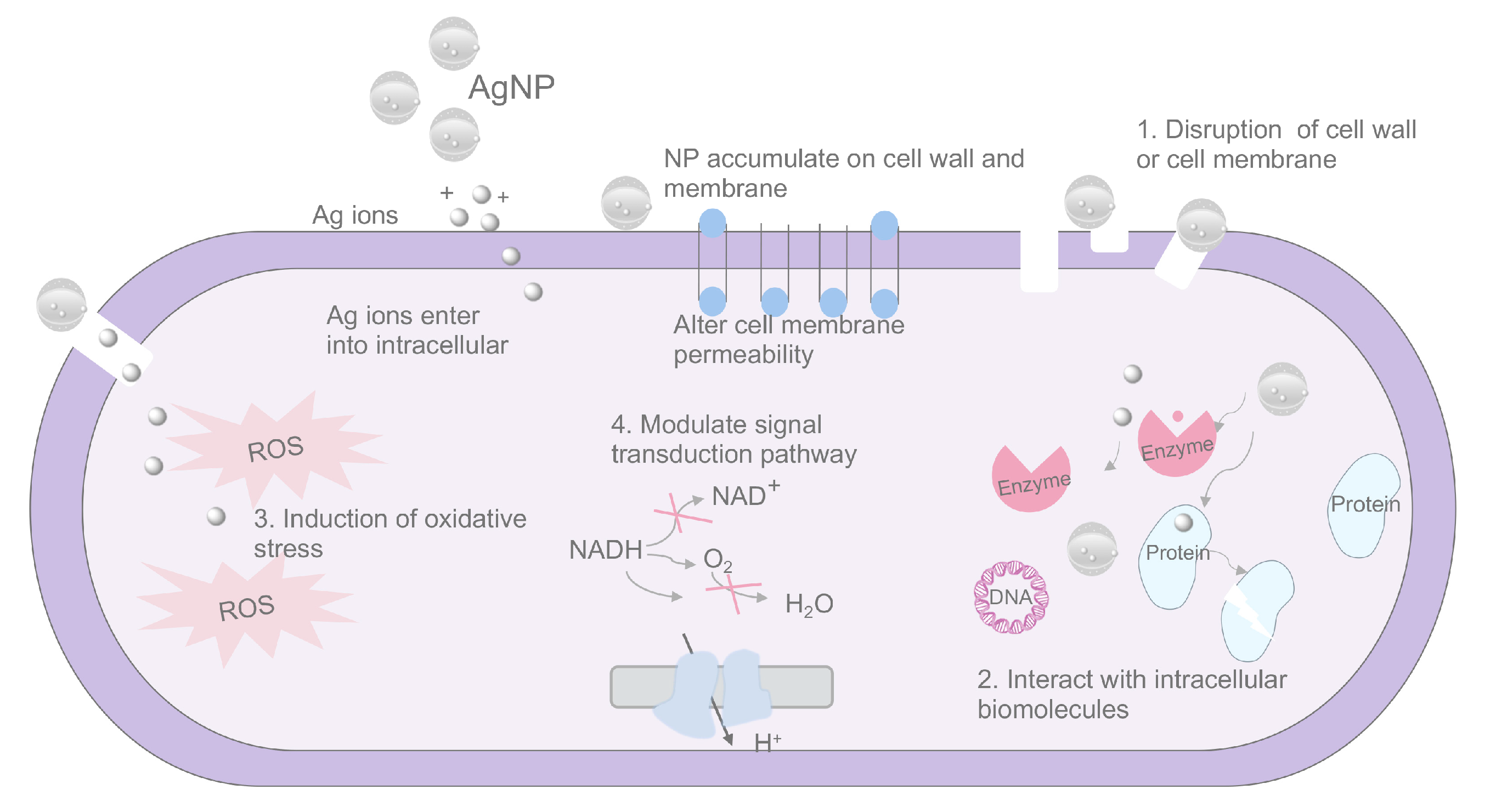
3.1. Silver Nanoparticles (AgNPs)
3.2. Chitosan Nanoparticles
3.3. Quaternary Ammonium Compounds (QACs)
3.4. Graphene-Based Materials
3.5. Metal Oxide Nanoparticles
4. The Application of Nanomaterials Used in Endodontics
4.1. Nanomaterials Act as Irrigants
| NP | Group | Substrate | Microbe | Detection Method | Other Detection | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| AgNPs | G 1: 94 ppm AgNP solution G 2: 2.5% NaOCl G 3: 2% CHX | Bovine dentin blocks | E. faecalis | Live/dead technique | NA | AgNP irrigant was not as effective against E. faecalis as solutions commonly used in root canal treatment. NaOCl is appropriate as an irrigant because it is effective in disrupting biofilm formation and eliminating bacteria in biofilms and dentinal tubules. | [137] |
| AgNPs | G 1: 0.1% AgNP solution G 2: 2% NaOCl G 3: sterile saline | Dentin sections | E. faecalis | Live/dead technique | NA | The findings from this study suggest that the antibiofilm efficacy of AgNPs depends on the mode of application. AgNPs as a medicament and not as an irrigant showed potential to eliminate residual bacterial biofilms during root canal disinfection. | [138] |
| AgNPs | G 1: AgNP solution G 2: AgNPs + ICG-aPDT G 3: AgNPs + PIPS G 4: AgNPs + MDA G 5: AgNPs + PUI G 6: 2.5% NaOCl G 7: no intervention | Human single-root teeth | E. faecalis | Counting of forming units | NA | Activation with PUI and PIPS enhanced the efficacy of the AgNP irrigating solution for the elimination of E. faecalis from the root canal system. | [139] |
| G 1: AgNPs + graphene G 2: 3% NaOCl G 3: saline | Mandibular and premolar | E. faecalis | Counting of forming units | NA | Within the confines of this study, graphene–silver composite nanoparticles demonstrated maximum antimicrobial effectiveness against E. Faecalis bacteria. | [140] |
| G 1: 0.1% ZnONPs G 2: AgNPs G 3: Im-AgNPs G 4: 2.5%NaOCl G 5: normal saline | Mandibular and premolar | NA | NA | Dentin microhardness | The irrigants containing Im-AgNPs and ZnONPs significantly enhanced the root dentin microhardness. However, the use of AgNPs resulted in decreased microhardness. | [141] |
| G 1: normal saline G 2: 2% CHX G 3: 17% EDTA + 2.5% NaOCl G 4: 17% EDTA + 0.1% AgNPs G 5: 17% EDTA + 0.1% TiO2 G 6: 17% EDTA + 0.1% ZNO-NPs | Single-root premolar teeth | NA | NA | Fracture resistance | The final irrigation of root canals with nanoparticles enhanced the fracture resistance of the endodontically treated roots. The lowest fracture resistance value was observed for NaOCl. | [142] |
| AgNPs | G 1: distilled water G 2: 5.25% NaClO G 3: 25% polyacrylic acid G 4: 2% CHX G 5: 23 ppm AgNPs | Single-root human teeth | NA | NA | Hardness and elastic modulus | Silver nanoparticle application is a viable option for the irrigation of the intraradicular dentin previously achieved through the cementation process of glass-fiber posts. | [143] |
| CSNPs | G 1: 0.2% CSNPs solution G 2: 17% EDTA G 3: solution | Mandibular and premolar | NA | NA | Dislocation resistance | This study provides the first evidence that chitosan irrigation improves the dislocation resistance of an MTA–resin hybrid root canal sealer, compared to EDTA and saline irrigation. | [147] |
| EDTA Chitosan CSNPs | G 1: 0.2% CSNPs solution G 2: 17% EDTA G 3: solution | Dentin | NA | NA |
| Proteins can be released from dentin via EDTA, CS, and CSnp conditioning. Raman spectra revealed changes in the inorganic content of the root dentin after chelation. Furthermore, the use of CSnps facilitated the preservation of the organic content. | [148] |
| QIS | G 1: saline G 2: 6% NaOCl G 3: 6% NaOCl + 2% CHX G 4: 2% CHX, G 5:0.5% k21/E G 6: 1% k21/E | Single-root teeth | E. faecalis | Live/dead staining |
| The current investigation showed that a new concentration of a 0.5% k21/E irrigant has the ability to reduce and disrupt E. faecalis biofilm formation, which can subsequently improve root canal treatment outcomes. | [149] |
| QIS | G 1: 6% NaOCl + 2% CHX G 2: 3.5% QIS G 3: 2% QIS G 4: sterile saline | Single-root teeth | E. faecalis |
|
| The novel QIS root canal irrigant achieved optimum antimicrobial protection inside the root canals compared to 6% NaOCl alone and 6% NaOCl + 2% CHX. | [150] |
| QIS | G 1: saline G 2: 0.5% K21 G 3: 1% K21 | Dentin slabs | E. faecalis |
|
| Altogether, our study reports the antimicrobial and reparative efficacy of a k21 disinfectant which is a proof of concept for enhanced killing of bacteria across the root dentin. | [151] |
4.2. Nanomaterial-Based Antimicrobial Photodynamic Therapy for Root Canal Disinfection
4.3. Nanomaterials Act as Intracanal Medicaments
| NP | Mode | Group | Microbe | Placement Duration | Other Detection | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| NCH Nanochitosan | Alone | G 1: calcium hydroxide G 2: NCH G 3: chitosan G 4: nanochitosan | E. faecalis | 1 month |
| Calcium hydroxide showed a limited depth of penetration and it significantly reduced the fracture resistance of teeth. Chitosan showed greater fracture resistance compared to all other groups, but had a limited depth of penetration. Both nanoforms showed superior penetration into the dentinal tubules and appreciable antibacterial efficacy. | [174] |
| AgNPs | Alone | G 1: PL alone G 2: Ca(OH)2 paste G 3: 16 μg/mL AgNPs -PL G 4: 32 μg/mL AgNPs PL | E. faecalis | 9 days |
| The prepared gels exhibited advantages of sustained release of Ag+ and strong antibiofilm effects against E. faecalis for 9 days. | [173] |
| AgNPs | Alone | G 1: Ca(OH)2 G 2: AgNPs G 3: AgNPs + Ca(OH)2 G 4: positive control G 5: negative control | E. faecalis | 7 days 14 days | NA | It was concluded that the antibacterial effect of AgNPs was lower than that of Ca(OH)2 or of the combination of both materials. | [172] |
| AgNPs | Alone | G 1: no treatment G 2: 0.01% AgNPs G 3: 0.015% AgNPs G 4: 0.02% AgNPs G 5: Ca(OH)2 | NA | NA |
| The cytotoxicity and proliferation of dental pulp stem cells in response to the AgNP gel were comparable to those resulting from calcium hydroxide. | [170] |
| Chitosan AgNPs | Mixed | G 1: Ca(OH)2 G 2: Ca(OH)2 + 2% CHX gel G 3: Ca(OH)2 + 2% chitosan gel G 4: Ca(OH)2 + 0.02% AgNP gel G 5: Ca(OH)2 + bioactive glass | E. faecalis | 7 days | NA | The combination with calcium hydroxide showed higher antibacterial efficacy than when used alone. Calcium hydroxide with bioactive glass showed better antimicrobial efficiency against E. faecalis when compared to other combinations of calcium hydroxide. | [176] |
| AgNPs | Mixed | G 1: saline solution G 2: Ca(OH)2 G 3: Ca(OH)2 + AgNPs G 4: 2% CHX gel G 5: Ca(OH)2 + 2% CHX gel | Multispecies biofilm | 7 days | NA | The present study put forth the potential use of AgNPs mixed with Ca(OH)2 or CHX on multispecies (Enterococcus faecalis, Streptococcus mutans, Lactobacillus acidophilus, and Actinomyces naeslundii) biofilms over 1- and 7-day application periods. | [177] |
| AgNPs | Mixed | G 1: sterile water G 2: Ca(OH)2 G 3: Ca(OH)2 + AgNPs | E. faecalis | 7 days | NA | This study highlighted the potential advantage of using a mixture of Ca(OH)2 and nanosilver as an intracanal medicament. | [169] |
| AgNPs | Mixed | G 1: cells alone G 2: Ca(OH)2 G 3: 0.04% AgNPs G 4: 0.06% AgNPs G 5: Ca(OH)2 + 0.03% AgNPs G 6: Ca(OH)2 + 0.04% AgNPs G 7: Ca(OH)2 + 0.06% AgNPs G 8: triple antibiotic paste | NA | NA |
| These results highlight the potential application of AgNPs combined with Ca(OH)2 or alone as a biomaterial for various clinical applications, such as intracanal medicaments in root canal treatments and endodontic regeneration. | [171] |
| AgNPs | Mixed | G 1: triple antibiotic paste (TAP) G 2: Ca(OH)2 G 3: AgNPs G 4: Ca(OH)2 + AgNPs | E. faecalis | 2 and 4 weeks | NA | The mixture of Ca(OH)2 + AgNPs showed a high antibiofilm effect that was not significantly different from 1 mg/mL TAP. Furthermore, long-term contact between intracanal medicaments and bacterial cells achieved significant antibiofilm efficacy. | [178] |
| AgNPs | Mixed | G 1: non-infected dentin samples G 2: infected dentin samples G 3: Ca(OH)2 G 4: 2% CHX G 5: AgNPs + Ca(OH)2 | C. albicans | 7 days | NA | It can be concluded that a combination of Ca(OH)2 and 0.04% AgNPs showed the most effective antibiofilm activity against C. albicans biofilms. | [179] |
| AgNPs | Mixed | G 1: TAP G 2: Ca(OH)2 G 3: 0.04% AgNPs G 4: 0.06% AgNPs G 5: Ca(OH)2 + 0.03% AgNPs G 6: Ca(OH)2 + 0.04% AgNPs G 7: Ca(OH)2 + 0.06% AgNPs | F. nucleatum | 7 days 14 days | NA | The results of this study suggest a promising alternative of AgNPs as an intracanal medicament, especially in combination with Ca(OH)2. | [180] |
| CSNPs EPE | Mixed | G 1: distilled water G 2: Ca(OH)2 G 3: Ca(OH)2 + CSNPs G 4: Ca(OH)2 + EPE | E. faecalis Intraoral biofilm | 7 days 14 days | NA | Incorporating CSNPs into a Ca(OH)2-based paste has the potential to increase its antibacterial activity and inhibit bacterial recolonization on root canal dentin after endodontic therapy. The Ca(OH)2/EPE paste was not able to maintain its antibacterial efficacy over time. | [175] |
| PLGA | Carrier | G 1: Ca(OH)2 G 2: Ca(OH)2 -PLGA | NA | NA | Depth and area of penetration | Ca(OH)2-loaded PLGA NPs were successfully optimized and characterized. The NPs exhibited a prolonged drug release profile and superior penetration inside dentinal tubules of extracted teeth when compared to Ca(OH)2. | [181] |
| PLGA | Carrier | G 1: saline solution G 2: chlorhexidine G 3: Ca(OH)2 G 4: Ca(OH)2-PLGA | Multispecies biofilm | 7 days | NA | The CH-loaded PLGA NPs possessed a greater antimicrobial property against the multispecies biofilms of E. faecalis, S. gordonii, and C. albicans compared to conventional Ca(OH)2. | [182] |
| PLGA | Carrier | G 1: control medium G 2: Ca(OH)2-PLGA G 3: Ca(OH)2 | NA | NA |
| Ca(OH)2-loaded PLGA nanoparticles have the potential to inhibit osteoclast differentiation more effectively compared to Ca(OH)2 nanoparticles. | [183] |
4.4. Modification of Sealers by Nanomaterials
| NP | Group | Sealer Type | Microorganism | Detection Method | Other Detection | Conclusion | Reference |
|---|---|---|---|---|---|---|---|
| QPEI | G 1: RCS G 2: 1.5% QPEI + RCS | RCS | E. faecalis | Counting of forming units |
| As shown in the present study, affixation of polycationic antimicrobial nanoparticles in an endodontic sealer revealed long-lasting antimicrobial potency, providing an effective antimicrobial alternative. | [187] |
| QAS | G 1: AH plus G 2: 2% QAS + AH plus G 3: 4% QAS + AH plus G 4: 8% QAS + AH plus | AH plus | E. faecalis | DCT Live/dead staining | NA | The incorporation of QAES into epoxy resin-based AH plus may be a promising approach for controlling endodontic infection at the time of canal filling and preventing subsequent reinfection. | [192] |
| QPEI | G 1: control G 2: AH plus G 3: 1% QPEI + AH plus G 4: 2% QPEI + AH plus G 5: PCS G 6: 1% QPEI + PCS G 7: 2% QPEI + PCS | AH plus Pulp canal sealer (PCS) | E. faecalis | DCT |
| The incorporation of QPEI nanoparticles can improve the long-term antibacterial activity of pulp canal sealer EWT without relevant changes in physicochemical and mechanical properties. | [190] |
| QPEI | G 1: control G 2: 1% QPEI + AH plus/PCS G 3: 2% QPEI + AH plus/PCS G 4: 5% QPEI + AH plus/PCS G 5: 10% QPEI + AH plus/PCS | AH plus PCS | NA | NA |
| QPEI nanoparticles, at 2%, did not affect cell behavior. However, the incorporation of 2% QPEI particles into AH plus and PCS modulated the proliferation and differentiation of bone cells, depending on the sealer and the cell type, without increasing the sealers’ cytotoxicity. | [189] |
| QPEI | G 1: AH plus G 2: 2% QPEI + AH plus G 3: PCS G 4: 2% QPEI + PCS | AH plus PCS | E. faecalis | DCT CV staining | NA | The addition of QPEI nanoparticles improved the killing ability of PCS against biofilms of both E. faecalis strains and the effects of AH plus on the biomass of biofilms from the ATCC strain. | [188] |
| G 1: AH plus G 2: BTH + 40% glass + DAMHDM + Ag + NACP G 3: BTH + 30% glass + DAMHDM + Ag + NACP G 4: BTH + 20% glass + DAMHDM + Ag + NACP G 5: BTH + 10% glass + DAMHDM + Ag + NACP | BTH | E. faecalis | Live/dead staining |
| This new sealer with highly desirable antibacterial and remineralization properties showed promise in increasing the success rate of endodontic therapy and strengthening the tooth root structures. | [191] |
| AgNPs | G 1: AH plus G 2: AgNPs + AH plus | AH plus | Saliva | NA | NA | Silver nanoparticles used in tested concentrations did not improve the bacterial leakage resistance of the AH plus sealer. | [193] |
| ZnO nanoparticles | G 1: Grossman sealer G 2: 25% ZNO-NPs + Grossman sealer G 3: 50% ZNO-NPs + Grossman sealer G 4: 75% ZNO-NPs + Grossman sealer G 5: 100% ZNO- NPs + Grossman sealer | Grossman sealer | NA | NA |
| ZnO-Nps decreased the setting time and dimensional changes characteristic of Grossman sealer. | [194] |
| DMADMM MNPs | G 1: EndoREZ G 2: iRoot SP G 3: EndoREZ + 2.5% DMADDM and 1% MNPs | EndoREZ | E. faecalis | CFU Live/dead staining |
| Overall, the current study found that compared with iRoot SP, the modified root canal sealer had good sealing performance, penetration, and long-term antibacterial property in the single-cone technique. | [195] |
4.5. Hydrogel-Based Materials in Endodontic Treatment
5. Conclusions and Future Perspectives
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Aminoshariae, A.; Kulild, J.C.; Mickel, A.; Fouad, A.F. Association between Systemic Diseases and Endodontic Outcome: A Systematic Review. J. Endod. 2017, 43, 514–519. [Google Scholar] [CrossRef] [PubMed]
- Cotti, E.; Mercuro, G. Apical periodontitis and cardiovascular diseases: Previous findings and ongoing research. Int. Endod. J. 2015, 48, 926–932. [Google Scholar] [CrossRef] [PubMed]
- Aminoshariae, A.; Kulild, J.C.; Fouad, A.F. The Impact of Endodontic Infections on the Pathogenesis of Cardiovascular Disease(s): A Systematic Review with Meta-analysis Using GRADE. J. Endod. 2018, 44, 1361–1366.e3. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Cheng, C.Q.; Mohanraj, R.; Sriraman, P.; Subbarao, C.; Sharma, S. Antibiofilm activity of three irrigation protocols activated by ultrasonic, diode laser or Er:YAG laser in vitro. Int. Endod. J. 2015, 48, 602–610. [Google Scholar] [CrossRef] [PubMed]
- Caliskan, M.K.; Turkun, M.; Alper, S. Allergy to sodium hypochlorite during root canal therapy: A case report. Int. Endod. J. 1994, 27, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Carpenter, G.H.; Pramanik, R.; Proctor, G.B. An in vitro model of chlorhexidine-induced tooth staining. J. Periodontal. Res. 2005, 40, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Fonzar, F.; Fonzar, A.; Buttolo, P.; Worthington, H.V.; Esposito, M. The prognosis of root canal therapy: A 10-year retrospective cohort study on 411 patients with 1175 endodontically treated teeth. Eur. J. Oral Implantol. 2009, 2, 201–208. [Google Scholar] [PubMed]
- Ng, Y.L.; Mann, V.; Gulabivala, K. A prospective study of the factors affecting outcomes of nonsurgical root canal treatment: Part 1: Periapical health. Int. Endod. J. 2011, 44, 583–609. [Google Scholar] [CrossRef]
- Lopez-Valverde, I.; Vignoletti, F.; Vignoletti, G.; Martin, C.; Sanz, M. Long-term tooth survival and success following primary root canal treatment: A 5- to 37-year retrospective observation. Clin. Oral Investig. 2023, 27, 3233–3244. [Google Scholar] [CrossRef]
- Eyuboglu, T.F.; Olcay, K.; Ozcan, M. A clinical study on single-visit root canal retreatments on consecutive 173 patients: Frequency of periapical complications and clinical success rate. Clin. Oral Investig. 2017, 21, 1761–1768. [Google Scholar] [CrossRef]
- Caliskan, M.K. Nonsurgical retreatment of teeth with periapical lesions previously managed by either endodontic or surgical intervention. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2005, 100, 242–248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Y.; Li, C.; Xue, Z.; Wu, H.; Li, J.; Ou, H.; Shen, J.; Ding, D. Root Canal Disinfection Using Highly Effective Aggregation-Induced Emission Photosensitizer. ACS Appl. Bio Mater. 2021, 4, 3796–3804. [Google Scholar] [CrossRef]
- Miranda, T.C.; Andrade, J.F.M.; Gelfuso, G.M.; Cunha-Filho, M.; Oliveira, L.A.; Gratieri, T. Novel technologies to improve the treatment of endodontic microbial infections: Inputs from a drug delivery perspective. Int. J. Pharm. 2023, 635, 122794. [Google Scholar] [CrossRef] [PubMed]
- Ndayishimiye, J.; Kumeria, T.; Popat, A.; Falconer, J.R.; Blaskovich, M.A.T. Nanomaterials: The New Antimicrobial Magic Bullet. ACS Infect. Dis. 2022, 8, 693–712. [Google Scholar] [CrossRef] [PubMed]
- Rubey, K.M.; Brenner, J.S. Nanomedicine to fight infectious disease. Adv. Drug Deliv. Rev. 2021, 179, 113996. [Google Scholar] [CrossRef] [PubMed]
- Khorsandi, K.; Hosseinzadeh, R.; Sadat Esfahani, H.; Keyvani-Ghamsari, S.; Ur Rahman, S. Nanomaterials as drug delivery systems with antibacterial properties: Current trends and future priorities. Expert Rev. Anti-Infect. Ther. 2021, 19, 1299–1323. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Ramos, M.A.; Da Silva, P.B.; Sposito, L.; De Toledo, L.G.; Bonifacio, B.V.; Rodero, C.F.; Dos Santos, K.C.; Chorilli, M.; Bauab, T.M. Nanotechnology-based drug delivery systems for control of microbial biofilms: A review. Int. J. Nanomed. 2018, 13, 1179–1213. [Google Scholar] [CrossRef]
- Sun, G.; Huang, S.; Wang, S.; Li, Y. Nanomaterial-based drug-delivery system as an aid to antimicrobial photodynamic therapy in treating oral biofilm. Future Microbiol. 2024. [Google Scholar] [CrossRef]
- Ricucci, D.; Siqueira, J.F., Jr. Biofilms and apical periodontitis: Study of prevalence and association with clinical and histopathologic findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rocas, I.N. Microbiology and treatment of acute apical abscesses. Clin. Microbiol. Rev. 2013, 26, 255–273. [Google Scholar] [CrossRef]
- Chavez de Paz, L.E. Redefining the persistent infection in root canals: Possible role of biofilm communities. J. Endod. 2007, 33, 652–662. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 2022, 55 (Suppl. S3), 512–530. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Wu, Z.; Niu, K.; Xie, Y.; Hu, X.; Fu, J.; Tian, D.; Fu, K.; Zhao, B.; Kong, W.; et al. Microbiome of Deep Dentinal Caries from Reversible Pulpitis to Irreversible Pulpitis. J. Endod. 2019, 45, 302–309.e1. [Google Scholar] [CrossRef] [PubMed]
- Zahran, S.; Witherden, E.; Mannocci, F.; Koller, G. Characterization of Root Canal Microbiota in Teeth Diagnosed with Irreversible Pulpitis. J. Endod. 2021, 47, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Rocas, I.N.; Lima, K.C.; Assuncao, I.V.; Gomes, P.N.; Bracks, I.V.; Siqueira, J.F., Jr. Advanced Caries Microbiota in Teeth with Irreversible Pulpitis. J. Endod. 2015, 41, 1450–1455. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.S.; Martinho, F.C.; Cardoso, F.G.; Nascimento, G.G.; Carvalho, C.A.; Valera, M.C. Microbiological profile resistant to different intracanal medications in primary endodontic infections. J. Endod. 2015, 41, 824–830. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Rocas, I.N. Diversity of endodontic microbiota revisited. J. Dent. Res. 2009, 88, 969–981. [Google Scholar] [CrossRef] [PubMed]
- Pinto, K.P.; Barbosa, A.F.A.; Silva, E.; Santos, A.P.P.; Sassone, L.M. What Is the Microbial Profile in Persistent Endodontic Infections? A Scoping Review. J. Endod. 2023, 49, 786–798.e7. [Google Scholar] [CrossRef] [PubMed]
- Barbosa-Ribeiro, M.; Arruda-Vasconcelos, R.; Louzada, L.M.; Dos Santos, D.G.; Andreote, F.D.; Gomes, B. Microbiological analysis of endodontically treated teeth with apical periodontitis before and after endodontic retreatment. Clin. Oral Investig. 2021, 25, 2017–2027. [Google Scholar] [CrossRef]
- Deng, Z.; Lin, B.; Liu, F.; Zhao, W. Role of Enterococcus faecalis in refractory apical periodontitis: From pathogenicity to host cell response. J. Oral Microbiol. 2023, 15, 2184924. [Google Scholar] [CrossRef]
- Stuart, C.H.; Schwartz, S.A.; Beeson, T.J.; Owatz, C.B. Enterococcus faecalis: Its role in root canal treatment failure and current concepts in retreatment. J. Endod. 2006, 32, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Delboni, M.G.; Gomes, B.P.; Francisco, P.A.; Teixeira, F.B.; Drake, D. Diversity of Enterococcus faecalis Genotypes from Multiple Oral Sites Associated with Endodontic Failure Using Repetitive Sequence-based Polymerase Chain Reaction and Arbitrarily Primed Polymerase Chain Reaction. J. Endod. 2017, 43, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Rocas, I.N.; Siqueira, J.F., Jr.; Santos, K.R. Association of Enterococcus faecalis with different forms of periradicular diseases. J. Endod. 2004, 30, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Gaeta, C.; Marruganti, C.; Ali, I.A.A.; Fabbro, A.; Pinzauti, D.; Santoro, F.; Neelakantan, P.; Pozzi, G.; Grandini, S. The presence of Enterococcus faecalis in saliva as a risk factor for endodontic infection. Front. Cell. Infect. Microbiol. 2023, 13, 1061645. [Google Scholar] [CrossRef] [PubMed]
- Figdor, D.; Davies, J.K.; Sundqvist, G. Starvation survival, growth and recovery of Enterococcus faecalis in human serum. Oral Microbiol. Immunol. 2003, 18, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wei, X.; Ling, J.; Wang, W.; Huang, X. Biofilm formation capability of Enterococcus faecalis cells in starvation phase and its susceptibility to sodium hypochlorite. J. Endod. 2010, 36, 630–635. [Google Scholar] [CrossRef] [PubMed]
- George, S.; Kishen, A.; Song, K.P. The role of environmental changes on monospecies biofilm formation on root canal wall by Enterococcus faecalis. J. Endod. 2005, 31, 867–872. [Google Scholar] [CrossRef]
- Ran, S.; He, Z.; Liang, J. Survival of Enterococcus faecalis during alkaline stress: Changes in morphology, ultrastructure, physiochemical properties of the cell wall and specific gene transcripts. Arch. Oral Biol. 2013, 58, 1667–1676. [Google Scholar] [CrossRef]
- Vatkar, N.A.; Hegde, V.; Sathe, S. Vitality of Enterococcus faecalis inside dentinal tubules after five root canal disinfection methods. J. Conserv. Dent. 2016, 19, 445–449. [Google Scholar] [CrossRef]
- Kayaoglu, G.; Orstavik, D. Virulence factors of Enterococcus faecalis: Relationship to endodontic disease. Crit. Rev. Oral Biol. Med. 2004, 15, 308–320. [Google Scholar] [CrossRef]
- Sedgley, C.M.; Lee, E.H.; Martin, M.J.; Flannagan, S.E. Antibiotic resistance gene transfer between Streptococcus gordonii and Enterococcus faecalis in root canals of teeth ex vivo. J. Endod. 2008, 34, 570–574. [Google Scholar] [CrossRef]
- Lin, D.; Gao, Y.; Zhao, L.; Chen, Y.; An, S.; Peng, Z. Enterococcus faecalis lipoteichoic acid regulates macrophages autophagy via PI3K/Akt/mTOR pathway. Biochem. Biophys. Res. Commun. 2018, 498, 1028–1036. [Google Scholar] [CrossRef]
- Lee, P.; Tan, K.S. Effects of Epigallocatechin gallate against Enterococcus faecalis biofilm and virulence. Arch. Oral Biol. 2015, 60, 393–399. [Google Scholar] [CrossRef]
- Rams, T.E.; Feik, D.; Mortensen, J.E.; Degener, J.E.; van Winkelhoff, A.J. Antibiotic susceptibility of periodontal Enterococcus faecalis. J. Periodontol. 2013, 84, 1026–1033. [Google Scholar] [CrossRef]
- Lins, R.X.; de Oliveira Andrade, A.; Hirata Junior, R.; Wilson, M.J.; Lewis, M.A.; Williams, D.W.; Fidel, R.A. Antimicrobial resistance and virulence traits of Enterococcus faecalis from primary endodontic infections. J. Dent. 2013, 41, 779–786. [Google Scholar] [CrossRef]
- Francisco, P.A.; Fagundes, P.; Lemes-Junior, J.C.; Lima, A.R.; Passini, M.R.Z.; Gomes, B. Pathogenic potential of Enterococcus faecalis strains isolated from root canals after unsuccessful endodontic treatment. Clin. Oral Investig. 2021, 25, 5171–5179. [Google Scholar] [CrossRef]
- Anderson, A.C.; Jonas, D.; Huber, I.; Karygianni, L.; Wolber, J.; Hellwig, E.; Arweiler, N.; Vach, K.; Wittmer, A.; Al-Ahmad, A. Enterococcus faecalis from Food, Clinical Specimens, and Oral Sites: Prevalence of Virulence Factors in Association with Biofilm Formation. Front. Microbiol. 2015, 6, 1534. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Ferrer-Luque, C.M.; Espigares-Garcia, M.; Baca, P. Enterococcus faecalis biofilms eradication by root canal irrigants. J. Endod. 2009, 35, 711–714. [Google Scholar] [CrossRef]
- Radcliffe, C.E.; Potouridou, L.; Qureshi, R.; Habahbeh, N.; Qualtrough, A.; Worthington, H.; Drucker, D.B. Antimicrobial activity of varying concentrations of sodium hypochlorite on the endodontic microorganisms Actinomyces israelii, A. naeslundii, Candida albicans and Enterococcus faecalis. Int. Endod. J. 2004, 37, 438–446. [Google Scholar] [CrossRef]
- Ozdemir, H.O.; Buzoglu, H.D.; Calt, S.; Stabholz, A.; Steinberg, D. Effect of ethylenediaminetetraacetic acid and sodium hypochlorite irrigation on Enterococcus faecalis biofilm colonization in young and old human root canal dentin: In vitro study. J. Endod. 2010, 36, 842–846. [Google Scholar] [CrossRef]
- Abou-Rass, M.; Bogen, G. Microorganisms in closed periapical lesions. Int. Endod. J. 1998, 31, 39–47. [Google Scholar] [CrossRef]
- Gomes, B.P.; Montagner, F.; Jacinto, R.C.; Zaia, A.A.; Ferraz, C.C.; Souza-Filho, F.J. Polymerase chain reaction of Porphyromonas gingivalis, Treponema denticola, and Tannerella forsythia in primary endodontic infections. J. Endod. 2007, 33, 1049–1052. [Google Scholar] [CrossRef]
- Ozbek, S.M.; Ozbek, A. Real-time polymerase chain reaction of “red complex” (Porphyromonas gingivalis, Tannerella forsythia, and Treponema denticola) in periradicular abscesses. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 110, 670–674. [Google Scholar] [CrossRef]
- Cao, H.; Qi, Z.; Jiang, H.; Zhao, J.; Liu, Z.; Tang, Z. Detection of Porphyromonas endodontalis, Porphyromonas gingivalis and Prevotella intermedia in primary endodontic infections in a Chinese population. Int. Endod. J. 2012, 45, 773–781. [Google Scholar] [CrossRef]
- Rocas, I.N.; Alves, F.R.; Santos, A.L.; Rosado, A.S.; Siqueira, J.F., Jr. Apical root canal microbiota as determined by reverse-capture checkerboard analysis of cryogenically ground root samples from teeth with apical periodontitis. J. Endod. 2010, 36, 1617–1621. [Google Scholar] [CrossRef]
- Siqueira, J.F., Jr.; Rocas, I.N. The microbiota of acute apical abscesses. J. Dent. Res. 2009, 88, 61–65. [Google Scholar] [CrossRef]
- Amano, A.; Nakagawa, I.; Okahashi, N.; Hamada, N. Variations of Porphyromonas gingivalis fimbriae in relation to microbial pathogenesis. J. Periodontal. Res. 2004, 39, 136–142. [Google Scholar] [CrossRef]
- Rocas, I.N.; Siqueira, J.F., Jr. Distribution of Porphyromonas gingivalis fimA genotypes in primary endodontic infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2010, 109, 474–478. [Google Scholar] [CrossRef]
- Veloso, P.; Fernandez, A.; Astorga, J.; Gonzalez-Quintanilla, D.; Castro, A.; Escobar, A.; Hoare, A.; Hernandez, M. Lipopolysaccharide from Porphyromonas gingivalis, but Not from Porphyromonas endodontalis, Induces Macrophage M1 Profile. Int. J. Mol. Sci. 2022, 23, 10011. [Google Scholar] [CrossRef]
- Sena, N.T.; Gomes, B.P.; Vianna, M.E.; Berber, V.B.; Zaia, A.A.; Ferraz, C.C.; Souza-Filho, F.J. In vitro antimicrobial activity of sodium hypochlorite and chlorhexidine against selected single-species biofilms. Int. Endod. J. 2006, 39, 878–885. [Google Scholar] [CrossRef]
- Yamaguchi, M.; Noiri, Y.; Kuboniwa, M.; Yamamoto, R.; Asahi, Y.; Maezono, H.; Hayashi, M.; Ebisu, S. Porphyromonas gingivalis biofilms persist after chlorhexidine treatment. Eur. J. Oral Sci. 2013, 121, 162–168. [Google Scholar] [CrossRef]
- Gomes, B.; Bronzato, J.D.; Almeida-Gomes, R.F.; Pinheiro, E.T.; Sousa, E.L.R.; Jacinto, R.C. Identification of Fusobacterium nucleatum in primary and secondary endodontic infections and its association with clinical features by using two different methods. Clin. Oral Investig. 2021, 25, 6249–6258. [Google Scholar] [CrossRef]
- Arias-Moliz, M.T.; Perez-Carrasco, V.; Uroz-Torres, D.; Santana Ramos, J.D.; Garcia-Salcedo, J.A.; Soriano, M. Identification of keystone taxa in root canals and periapical lesions of post-treatment endodontic infections: Next generation microbiome research. Int. Endod. J. 2024. [Google Scholar] [CrossRef]
- Gomes, B.P.; Pinheiro, E.T.; Gade-Neto, C.R.; Sousa, E.L.; Ferraz, C.C.; Zaia, A.A.; Teixeira, F.B.; Souza-Filho, F.J. Microbiological examination of infected dental root canals. Oral Microbiol. Immunol. 2004, 19, 71–76. [Google Scholar] [CrossRef]
- Ganesh, A.; Veronica, A.K.; Ashok, R.; Varadan, P.; Deivanayagam, K. Quantification of Fusobacterium nucleatum at Depths of Root Dentinal Tubules in the Tooth Using Real-time Polymerase Chain Reaction: An In Vitro Study. Cureus 2019, 11, e4711. [Google Scholar] [CrossRef]
- Kolenbrander, P.E.; Andersen, R.N.; Blehert, D.S.; Egland, P.G.; Foster, J.S.; Palmer, R.J., Jr. Communication among oral bacteria. Microbiol. Mol. Biol. Rev. 2002, 66, 486–505. [Google Scholar] [CrossRef]
- Chavez de Paz Villanueva, L.E. Fusobacterium nucleatum in endodontic flare-ups. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2002, 93, 179–183. [Google Scholar] [CrossRef]
- Martinho, F.C.; Leite, F.R.; Nobrega, L.M.; Endo, M.S.; Nascimento, G.G.; Darveau, R.P.; Gomes, B.P. Comparison of Fusobacterium nucleatum and Porphyromonas gingivalis Lipopolysaccharides Clinically Isolated from Root Canal Infection in the Induction of Pro-Inflammatory Cytokines Secretion. Braz. Dent. J. 2016, 27, 202–207. [Google Scholar] [CrossRef]
- Ran, S.; Liu, B.; Gu, S.; Sun, Z.; Liang, J. Analysis of the expression of NLRP3 and AIM2 in periapical lesions with apical periodontitis and microbial analysis outside the apical segment of teeth. Arch. Oral Biol. 2017, 78, 39–47. [Google Scholar] [CrossRef]
- Koca-Unsal, R.B.; Sehirli, A.O.; Sayiner, S.; Aksoy, U. Relationship of NLRP3 inflammasome with periodontal, endodontic and related systemic diseases. Mol. Biol. Rep. 2022, 49, 11123–11132. [Google Scholar] [CrossRef]
- Ozok, A.R.; Wu, M.K.; Luppens, S.B.; Wesselink, P.R. Comparison of growth and susceptibility to sodium hypochlorite of mono- and dual-species biofilms of Fusobacterium nucleatum and Peptostreptococcus (micromonas) micros. J. Endod. 2007, 33, 819–822. [Google Scholar] [CrossRef]
- Sassone, L.M.; Fidel, R.A.; Murad, C.F.; Fidel, S.R.; Hirata, R., Jr. Antimicrobial activity of sodium hypochlorite and chlorhexidine by two different tests. Aust. Endod. J. 2008, 34, 19–24. [Google Scholar] [CrossRef]
- Okamoto, A.C.; Gaetti-Jardim, E.; Arana-Chavez, V.E.; Avila-Campos, M.J. Influence of subinhibitory concentrations of antimicrobials on hydrophobicity, adherence and ultra-structure of Fusobacterium nucleatum. Braz. J. Microbiol. 2002, 33, 178–184. [Google Scholar] [CrossRef]
- Rocas, I.N.; Hulsmann, M.; Siqueira, J.F., Jr. Microorganisms in root canal-treated teeth from a German population. J. Endod. 2008, 34, 926–931. [Google Scholar] [CrossRef]
- Peciuliene, V.; Reynaud, A.H.; Balciuniene, I.; Haapasalo, M. Isolation of yeasts and enteric bacteria in root-filled teeth with chronic apical periodontitis. Int. Endod. J. 2001, 34, 429–434. [Google Scholar] [CrossRef]
- Senges, C.; Wrbas, K.T.; Altenburger, M.; Follo, M.; Spitzmuller, B.; Wittmer, A.; Hellwig, E.; Al-Ahmad, A. Bacterial and Candida albicans adhesion on different root canal filling materials and sealers. J. Endod. 2011, 37, 1247–1252. [Google Scholar] [CrossRef]
- Sevilla, M.J.; Odds, F.C. Development of Candida albicans hyphae in different growth media–variations in growth rates, cell dimensions and timing of morphogenetic events. J. Gen. Microbiol. 1986, 132, 3083–3088. [Google Scholar] [CrossRef]
- Alshanta, O.A.; Shaban, S.; Nile, C.J.; McLean, W.; Ramage, G. Candida albicans Biofilm Heterogeneity and Tolerance of Clinical Isolates: Implications for Secondary Endodontic Infections. Antibiotics 2019, 8, 204. [Google Scholar] [CrossRef]
- Souza, J.G.S.; Bertolini, M.; Thompson, A.; Mansfield, J.M.; Grassmann, A.A.; Maas, K.; Caimano, M.J.; Barao, V.A.R.; Vickerman, M.M.; Dongari-Bagtzoglou, A. Role of glucosyltransferase R in biofilm interactions between Streptococcus oralis and Candida albicans. ISME J. 2020, 14, 1207–1222. [Google Scholar] [CrossRef]
- Montelongo-Jauregui, D.; Saville, S.P.; Lopez-Ribot, J.L. Contributions of Candida albicans Dimorphism, Adhesive Interactions, and Extracellular Matrix to the Formation of Dual-Species Biofilms with Streptococcus gordonii. mBio 2019, 10, e01179-19. [Google Scholar] [CrossRef] [PubMed]
- Diogo, P.; Fernandes, C.; Caramelo, F.; Mota, M.; Miranda, I.M.; Faustino, M.A.F.; Neves, M.; Uliana, M.P.; de Oliveira, K.T.; Santos, J.M.; et al. Antimicrobial Photodynamic Therapy against Endodontic Enterococcus faecalis and Candida albicans Mono and Mixed Biofilms in the Presence of Photosensitizers: A Comparative Study with Classical Endodontic Irrigants. Front. Microbiol. 2017, 8, 498. [Google Scholar] [CrossRef] [PubMed]
- Du, Q.; Yuan, S.; Zhao, S.; Fu, D.; Chen, Y.; Zhou, Y.; Cao, Y.; Gao, Y.; Xu, X.; Zhou, X.; et al. Coexistence of Candida albicans and Enterococcus faecalis increases biofilm virulence and periapical lesions in rats. Biofouling 2021, 37, 964–974. [Google Scholar] [CrossRef]
- Ferguson, J.W.; Hatton, J.F.; Gillespie, M.J. Effectiveness of intracanal irrigants and medications against the yeast Candida albicans. J. Endod. 2002, 28, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, Z.; Giardino, L.; Palazzi, F. Evaluation of the antifungal activity of four solutions used as a final rinse in vitro. Aust. Endod. J. 2013, 39, 31–34. [Google Scholar] [CrossRef] [PubMed]
- Segura-Egea, J.J.; Martin-Gonzalez, J.; Castellanos-Cosano, L. Endodontic medicine: Connections between apical periodontitis and systemic diseases. Int. Endod. J. 2015, 48, 933–951. [Google Scholar] [CrossRef] [PubMed]
- Mei, F.; Xie, M.; Huang, X.; Long, Y.; Lu, X.; Wang, X.; Chen, L. Porphyromonas gingivalis and Its Systemic Impact: Current Status. Pathogens 2020, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.; Patoine, A.; Wu, T.T.; Castillo, D.A.; Xiao, J. Oral microflora and pregnancy: A systematic review and meta-analysis. Sci. Rep. 2021, 11, 16870. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, D.; Liu, S.; Zhang, S.; Pan, Y. The Role of Porphyromonas gingivalis Outer Membrane Vesicles in Periodontal Disease and Related Systemic Diseases. Front. Cell. Infect. Microbiol. 2020, 10, 585917. [Google Scholar] [CrossRef] [PubMed]
- Fan, Z.; Tang, P.; Li, C.; Yang, Q.; Xu, Y.; Su, C.; Li, L. Fusobacterium nucleatum and its associated systemic diseases: Epidemiologic studies and possible mechanisms. J. Oral Microbiol. 2023, 15, 2145729. [Google Scholar] [CrossRef] [PubMed]
- Hajishengallis, G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat. Rev. Immunol. 2015, 15, 30–44. [Google Scholar] [CrossRef]
- Sanz, M.; Kornman, K.; Working Group 3 of the Joint EFP/AAP Workshop. Periodontitis and adverse pregnancy outcomes: Consensus report of the Joint EFP/AAP Workshop on Periodontitis and Systemic Diseases. J. Periodontol. 2013, 84, S164–S169. [Google Scholar] [CrossRef] [PubMed]
- Fardini, Y.; Wang, X.; Temoin, S.; Nithianantham, S.; Lee, D.; Shoham, M.; Han, Y.W. Fusobacterium nucleatum adhesin FadA binds vascular endothelial cadherin and alters endothelial integrity. Mol. Microbiol. 2011, 82, 1468–1480. [Google Scholar] [CrossRef] [PubMed]
- Parhi, L.; Alon-Maimon, T.; Sol, A.; Nejman, D.; Shhadeh, A.; Fainsod-Levi, T.; Yajuk, O.; Isaacson, B.; Abed, J.; Maalouf, N.; et al. Breast cancer colonization by Fusobacterium nucleatum accelerates tumor growth and metastatic progression. Nat. Commun. 2020, 11, 3259. [Google Scholar] [CrossRef] [PubMed]
- Fattah, B.; Arif, H.; Hamzah, H. Antimicrobial and Antibiofilm Activity of Biosynthesized Silver Nanoparticles against Beta-lactamase-Resistant Enterococcus faecalis. Appl. Biochem. Biotechnol. 2022, 194, 2036–2046. [Google Scholar] [CrossRef] [PubMed]
- Kirmanidou, Y.; Sidira, M.; Bakopoulou, A.; Tsouknidas, A.; Prymak, O.; Papi, R.; Choli-Papadopoulou, T.; Epple, M.; Michailidis, N.; Koidis, P.; et al. Assessment of cytotoxicity and antibacterial effects of silver nanoparticle-doped titanium alloy surfaces. Dent. Mater. 2019, 35, e220–e233. [Google Scholar] [CrossRef] [PubMed]
- Adam, R.Z.; Khan, S.B. Antimicrobial Efficacy of Silver Nanoparticles against Candida albicans. Materials 2022, 15, 5666. [Google Scholar] [CrossRef] [PubMed]
- Duran, N.; Duran, M.; de Jesus, M.B.; Seabra, A.B.; Favaro, W.J.; Nakazato, G. Silver nanoparticles: A new view on mechanistic aspects on antimicrobial activity. Nanomedicine 2016, 12, 789–799. [Google Scholar] [CrossRef] [PubMed]
- Yin, I.X.; Zhang, J.; Zhao, I.S.; Mei, M.L.; Li, Q.; Chu, C.H. The Antibacterial Mechanism of Silver Nanoparticles and Its Application in Dentistry. Int. J. Nanomed. 2020, 15, 2555–2562. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.; Zheng, J. Antibacterial Activity of Silver Nanoparticles: Structural Effects. Adv. Healthc. Mater. 2018, 7, e1701503. [Google Scholar] [CrossRef]
- Anaya, N.M.; Faghihzadeh, F.; Ganji, N.; Bothun, G.; Oyanedel-Craver, V. Comparative study between chemostat and batch reactors to quantify membrane permeability changes on bacteria exposed to silver nanoparticles. Sci. Total Environ. 2016, 565, 841–848. [Google Scholar] [CrossRef]
- Hetrick, E.M.; Shin, J.H.; Paul, H.S.; Schoenfisch, M.H. Anti-biofilm efficacy of nitric oxide-releasing silica nanoparticles. Biomaterials 2009, 30, 2782–2789. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.R.; Singh, B.N.; Singh, A.; Khan, W.; Naqvi, A.H.; Singh, H.B. Mycofabricated biosilver nanoparticles interrupt Pseudomonas aeruginosa quorum sensing systems. Sci. Rep. 2015, 5, 13719. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Gaikwad, S.; Nagar, S.; Kulshrestha, S.; Vaidya, V.; Nawani, N.; Pawar, S. Biofilm inhibition and anti-quorum sensing activity of phytosynthesized silver nanoparticles against the nosocomial pathogen Pseudomonas aeruginosa. Biofouling 2019, 35, 34–49. [Google Scholar] [CrossRef] [PubMed]
- Hou, X.; Fu, H.; Han, Y.; Xue, Y.; Li, C. Analysis of Transcriptome in Enterococcus faecalis Treated with Silver Nanoparticles. J. Nanosci. Nanotechnol. 2020, 20, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Lo, E.C.M. Compare the physicochemical and biological properties of engineered polymer-functionalized silver nanoparticles against Porphyromonas gingivalis. Front. Microbiol. 2022, 13, 985708. [Google Scholar] [CrossRef] [PubMed]
- Takamiya, A.S.; Monteiro, D.R.; Gorup, L.F.; Silva, E.A.; de Camargo, E.R.; Gomes-Filho, J.E.; de Oliveira, S.H.P.; Barbosa, D.B. Biocompatible silver nanoparticles incorporated in acrylic resin for dental application inhibit Candida albicans biofilm. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111341. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cao, H.; Qiao, S.; Wang, M.; Gu, Y.; Luo, H.; Meng, F.; Liu, X.; Lai, H. Hierarchical micro/nanostructured titanium with balanced actions to bacterial and mammalian cells for dental implants. Int. J. Nanomed. 2015, 10, 6659–6674. [Google Scholar] [CrossRef] [PubMed]
- Oncu, A.; Celikten, B.; Aydin, B.; Amasya, G.; Acik, L.; Sevimay, F.S. Comparative evaluation of the antifungal efficacy of sodium hypochlorite, chlorhexidine, and silver nanoparticles against Candida albicans. Microsc. Res. Tech. 2022, 85, 3755–3760. [Google Scholar] [CrossRef]
- Panpaliya, N.P.; Dahake, P.T.; Kale, Y.J.; Dadpe, M.V.; Kendre, S.B.; Siddiqi, A.G.; Maggavi, U.R. In vitro evaluation of antimicrobial property of silver nanoparticles and chlorhexidine against five different oral pathogenic bacteria. Saudi Dent. J. 2019, 31, 76–83. [Google Scholar] [CrossRef]
- Landriscina, A.; Rosen, J.; Friedman, A.J. Biodegradable chitosan nanoparticles in drug delivery for infectious disease. Nanomedicine 2015, 10, 1609–1619. [Google Scholar] [CrossRef]
- Kong, M.; Chen, X.G.; Xing, K.; Park, H.J. Antimicrobial properties of chitosan and mode of action: A state of the art review. Int. J. Food Microbiol. 2010, 144, 51–63. [Google Scholar] [CrossRef] [PubMed]
- Kou, S.G.; Peters, L.; Mucalo, M. Chitosan: A review of molecular structure, bioactivities and interactions with the human body and micro-organisms. Carbohydr. Polym. 2022, 282, 119132. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Shi, Z.; Neoh, K.G.; Kishen, A. Nanoparticulates for antibiofilm treatment and effect of aging on its antibacterial activity. J. Endod. 2010, 36, 1030–1035. [Google Scholar] [CrossRef] [PubMed]
- Upadya, M.; Shrestha, A.; Kishen, A. Role of efflux pump inhibitors on the antibiofilm efficacy of calcium hydroxide, chitosan nanoparticles, and light-activated disinfection. J. Endod. 2011, 37, 1422–1426. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Li, B.; Guo, X.; Wang, H.; Cheng, L. Applications of quaternary ammonium compounds in the prevention and treatment of oral diseases: State-of-the-art and future directions. J. Dent. 2023, 137, 104678. [Google Scholar] [CrossRef] [PubMed]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef] [PubMed]
- Kumar Tiwari, S.; Guo, X.; Huang, Y.; Zhou, X.; Xu, H.H.K.; Ren, B.; Peng, X.; Weir, M.D.; Li, M.; Cheng, L. The inhibitory effect of quaternary ammonium salt on bacteria in root canal. Sci. Rep. 2019, 9, 12463. [Google Scholar] [CrossRef] [PubMed]
- Oblak, E.; Futoma-Koloch, B.; Wieczynska, A. Biological activity of quaternary ammonium salts and resistance of microorganisms to these compounds. World J. Microbiol. Biotechnol. 2021, 37, 22. [Google Scholar] [CrossRef] [PubMed]
- van de Lagemaat, M.; Grotenhuis, A.; van de Belt-Gritter, B.; Roest, S.; Loontjens, T.J.A.; Busscher, H.J.; van der Mei, H.C.; Ren, Y. Comparison of methods to evaluate bacterial contact-killing materials. Acta Biomater. 2017, 59, 139–147. [Google Scholar] [CrossRef]
- Sun, N.; Du, R.L.; Zheng, Y.Y.; Huang, B.H.; Guo, Q.; Zhang, R.F.; Wong, K.Y.; Lu, Y.J. Antibacterial activity of N-methylbenzofuro[3,2-b]quinoline and N-methylbenzoindolo[3,2-b]-quinoline derivatives and study of their mode of action. Eur. J. Med. Chem. 2017, 135, 1–11. [Google Scholar] [CrossRef]
- Dan, W.; Gao, J.; Qi, X.; Wang, J.; Dai, J. Antibacterial quaternary ammonium agents: Chemical diversity and biological mechanism. Eur. J. Med. Chem. 2022, 243, 114765. [Google Scholar] [CrossRef]
- Kwasniewska, D.; Chen, Y.L.; Wieczorek, D. Biological Activity of Quaternary Ammonium Salts and Their Derivatives. Pathogens 2020, 9, 459. [Google Scholar] [CrossRef] [PubMed]
- Karahan, H.E.; Wiraja, C.; Xu, C.; Wei, J.; Wang, Y.; Wang, L.; Liu, F.; Chen, Y. Graphene Materials in Antimicrobial Nanomedicine: Current Status and Future Perspectives. Adv. Healthc. Mater. 2018, 7, e1701406. [Google Scholar] [CrossRef] [PubMed]
- Cao, G.; Yan, J.; Ning, X.; Zhang, Q.; Wu, Q.; Bi, L.; Zhang, Y.; Han, Y.; Guo, J. Antibacterial and antibiofilm properties of graphene and its derivatives. Colloids Surf. B Biointerfaces 2021, 200, 111588. [Google Scholar] [CrossRef] [PubMed]
- Pandit, S.; Cao, Z.; Mokkapati, V.R.S.S.; Celauro, E.; Yurgens, A.; Lovmar, M.; Westerlund, F.; Sun, J.; Mijakovic, I. Vertically Aligned Graphene Coating is Bactericidal and Prevents the Formation of Bacterial Biofilms. Adv. Mater. Interfaces 2018, 5, 1701331. [Google Scholar] [CrossRef]
- Zheng, H.; Ji, Z.; Roy, K.R.; Gao, M.; Pan, Y.; Cai, X.; Wang, L.; Li, W.; Chang, C.H.; Kaweeteerawat, C.; et al. Engineered Graphene Oxide Nanocomposite Capable of Preventing the Evolution of Antimicrobial Resistance. ACS Nano 2019, 13, 11488–11499. [Google Scholar] [CrossRef] [PubMed]
- Shankar, K.; Agarwal, S.; Mishra, S.; Bhatnagar, P.; Siddiqui, S.; Abrar, I. A review on antimicrobial mechanism and applications of graphene-based materials. Biomater. Adv. 2023, 150, 213440. [Google Scholar] [CrossRef]
- Mohammed, H.; Kumar, A.; Bekyarova, E.; Al-Hadeethi, Y.; Zhang, X.; Chen, M.; Ansari, M.S.; Cochis, A.; Rimondini, L. Antimicrobial Mechanisms and Effectiveness of Graphene and Graphene-Functionalized Biomaterials. A Scope Review. Front. Bioeng. Biotechnol. 2020, 8, 465. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the Antimicrobial Activities of Graphene Materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef]
- Li, J.; Wang, G.; Zhu, H.; Zhang, M.; Zheng, X.; Di, Z.; Liu, X.; Wang, X. Antibacterial activity of large-area monolayer graphene film manipulated by charge transfer. Sci. Rep. 2014, 4, 4359. [Google Scholar] [CrossRef]
- Dizaj, S.M.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.H.; Adibkia, K. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 44, 278–284. [Google Scholar] [CrossRef]
- Mansoor, A.; Khan, M.T.; Mehmood, M.; Khurshid, Z.; Ali, M.I.; Jamal, A. Synthesis and Characterization of Titanium Oxide Nanoparticles with a Novel Biogenic Process for Dental Application. Nanomaterials 2022, 12, 1078. [Google Scholar] [CrossRef] [PubMed]
- Javidi, M.; Zarei, M.; Naghavi, N.; Mortazavi, M.; Nejat, A.H. Zinc oxide nano-particles as sealer in endodontics and its sealing ability. Contemp. Clin. Dent. 2014, 5, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Azam, A.; Ahmed, A.S.; Oves, M.; Khan, M.S.; Habib, S.S.; Memic, A. Antimicrobial activity of metal oxide nanoparticles against Gram-positive and Gram-negative bacteria: A comparative study. Int. J. Nanomed. 2012, 7, 6003–6009. [Google Scholar] [CrossRef]
- Djearamane, S.; Loh, Z.C.; Lee, J.J.; Wong, L.S.; Rajamani, R.; Luque, P.A.; Gupta, P.K.; Liang, S.X.T. Remedial Aspect of Zinc Oxide Nanoparticles Against Serratia Marcescens and Enterococcus faecalis. Front. Pharmacol. 2022, 13, 891304. [Google Scholar] [CrossRef] [PubMed]
- Rojas, B.; Soto, N.; Villalba, M.; Bello-Toledo, H.; Melendrez-Castro, M.; Sanchez-Sanhueza, G. Antibacterial Activity of Copper Nanoparticles (CuNPs) against a Resistant Calcium Hydroxide Multispecies Endodontic Biofilm. Nanomaterials 2021, 11, 2254. [Google Scholar] [CrossRef]
- Rodrigues, C.T.; de Andrade, F.B.; de Vasconcelos, L.; Midena, R.Z.; Pereira, T.C.; Kuga, M.C.; Duarte, M.A.H.; Bernardineli, N. Antibacterial properties of silver nanoparticles as a root canal irrigant against Enterococcus faecalis biofilm and infected dentinal tubules. Int. Endod. J. 2018, 51, 901–911. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Fan, W.; Kishen, A.; Gutmann, J.L.; Fan, B. Evaluation of the antibacterial efficacy of silver nanoparticles against Enterococcus faecalis biofilm. J. Endod. 2014, 40, 285–290. [Google Scholar] [CrossRef]
- Afkhami, F.; Ahmadi, P.; Chiniforush, N.; Sooratgar, A. Effect of different activations of silver nanoparticle irrigants on the elimination of Enterococcus faecalis. Clin. Oral Investig. 2021, 25, 6893–6899. [Google Scholar] [CrossRef]
- Sharma, D.K.; Bhat, M.; Kumar, V.; Mazumder, D.; Singh, S.V.; Bansal, M. Evaluation of Antimicrobial Efficacy of Graphene Silver Composite Nanoparticles against E. faecalis as Root Canal Irrigant: An ex-vivo study. Int. J. Pharm. Med. Res. 2015, 3, 267–272. [Google Scholar]
- Sahebi, S.; Mofidi, H.; Abbaszadegan, A.; Gholami, A.; Eskandari, F. The effect of nanobased irrigants on the root canal dentin microhardness: An ex-vivo study. BMC Oral Health 2023, 23, 581. [Google Scholar] [CrossRef]
- Jowkar, Z.; Hamidi, S.A.; Shafiei, F.; Ghahramani, Y. The Effect of Silver, Zinc Oxide, and Titanium Dioxide Nanoparticles Used as Final Irrigation Solutions on the Fracture Resistance of Root-Filled Teeth. Clin. Cosmet. Investig. Dent. 2020, 12, 141–148. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.Y.U.; Gallego, J.; Assuncao, W.G.; Briso, A.L.F.; Dos Santos, P.H. Influence of silver nanoparticle solution on the mechanical properties of resin cements and intrarradicular dentin. PLoS ONE 2019, 14, e0217750. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.V.; Guedes, D.F.; Nakadi, F.V.; Pecora, J.D.; Cruz-Filho, A.M. Chitosan: A new solution for removal of smear layer after root canal instrumentation. Int. Endod. J. 2013, 46, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Shenoi, P.R.; Morey, E.S.; Makade, C.S.; Gunwal, M.K.; Khode, R.T.; Wanmali, S.S. In vitro evaluation of the antimicrobial efficacy of chitosan and other endodontic irrigants against Enterococcus faecalis. Gen. Dent. 2016, 64, 60–63. [Google Scholar] [PubMed]
- Pascale, C.; Geaman, J.; Mendoza, C.; Gao, F.; Kaminski, A.; Cuevas-Nunez, M.; Darvishan, B.; Mitchell, J.C.; Carrilho, M.R.; Sigar, I. In vitro assessment of antimicrobial potential of low molecular weight chitosan and its ability to mechanically reinforce and control endogenous proteolytic activity of dentine. Int. Endod. J. 2023, 56, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Ozlek, E.; Rath, P.P.; Kishen, A.; Neelakantan, P. A chitosan-based irrigant improves the dislocation resistance of a mineral trioxide aggregate-resin hybrid root canal sealer. Clin. Oral Investig. 2020, 24, 151–156. [Google Scholar] [CrossRef] [PubMed]
- Quijano-Guauque, S.; Bernal-Cepeda, L.J.; Delgado, F.G.; Castellanos, J.E.; Garcia-Guerrero, C. Effect of chitosan irrigant solutions on the release of bioactive proteins from root dentin. Clin. Oral Investig. 2023, 27, 691–703. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Bapat, R.A.; Sidhu, P.; Ilyas, M.S.; Khan, A.S.; Mak, K.K.; Pichika, M.R.; Nagendrababu, V.; Peters, O.A. Antibacterial and antibiofilm efficacy of k21-E in root canal disinfection. Dent. Mater. 2021, 37, 1511–1528. [Google Scholar] [CrossRef]
- Daood, U.; Parolia, A.; Matinlinna, J.; Yiu, C.; Ahmed, H.M.A.; Fawzy, A. Properties of a modified quaternary ammonium silane formulation as a potential root canal irrigant in endodontics. Dent. Mater. 2020, 36, e386–e402. [Google Scholar] [CrossRef]
- Daood, U.; Ilyas, M.S.; Ashraf, M.; Akbar, M.; Bapat, R.A.; Khan, A.S.; Pichika, M.R.; Parolia, A.; Seow, L.L.; Khoo, S.P.; et al. Biochemical changes and macrophage polarization of a silane-based endodontic irrigant in an animal model. Sci. Rep. 2022, 12, 6354. [Google Scholar] [CrossRef] [PubMed]
- Daood, U.; Matinlinna, J.P.; Pichika, M.R.; Mak, K.K.; Nagendrababu, V.; Fawzy, A.S. A quaternary ammonium silane antimicrobial triggers bacterial membrane and biofilm destruction. Sci. Rep. 2020, 10, 10970. [Google Scholar] [CrossRef] [PubMed]
- Bapat, R.A.; Libat, R.; Yuin, O.S.; Parolia, A.; Ilyas, M.S.; Khan, A.S.; Kay, M.K.; Pichika, M.R.; Saxena, K.; Seow, L.L.; et al. Antimicrobial FiteBac(R) K21 promotes antimicrobial Potency and wound healing. Heliyon 2023, 9, e19282. [Google Scholar] [CrossRef] [PubMed]
- Umer, D.; Yiu, C.K.; Burrow, M.F.; Niu, L.N.; Tay, F.R. Effect of a novel quaternary ammonium silane on dentin protease activities. J. Dent. 2017, 58, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Correr, G.M.; Alonso, R.C.; Grando, M.F.; Borges, A.F.; Puppin-Rontani, R.M. Effect of sodium hypochlorite on primary dentin--a scanning electron microscopy (SEM) evaluation. J. Dent. 2006, 34, 454–459. [Google Scholar] [CrossRef] [PubMed]
- Oyarzun, A.; Cordero, A.M.; Whittle, M. Immunohistochemical evaluation of the effects of sodium hypochlorite on dentin collagen and glycosaminoglycans. J. Endod. 2002, 28, 152–156. [Google Scholar] [CrossRef]
- Cieplik, F.; Deng, D.; Crielaard, W.; Buchalla, W.; Hellwig, E.; Al-Ahmad, A.; Maisch, T. Antimicrobial photodynamic therapy—What we know and what we don’t. Crit. Rev. Microbiol. 2018, 44, 571–589. [Google Scholar] [CrossRef]
- Plotino, G.; Grande, N.M.; Mercade, M. Photodynamic therapy in endodontics. Int. Endod. J. 2019, 52, 760–774. [Google Scholar] [CrossRef]
- Li, Y.; Sun, G.; Xie, J.; Xiao, S.; Lin, C. Antimicrobial photodynamic therapy against oral biofilm: Influencing factors, mechanisms, and combined actions with other strategies. Front. Microbiol. 2023, 14, 1192955. [Google Scholar] [CrossRef]
- Cieplik, F.; Tabenski, L.; Buchalla, W.; Maisch, T. Antimicrobial photodynamic therapy for inactivation of biofilms formed by oral key pathogens. Front. Microbiol. 2014, 5, 405. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial efficacy of photosensitizer functionalized biopolymeric nanoparticles in the presence of tissue inhibitors in root canal. J. Endod. 2014, 40, 566–570. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Cordova, M.; Kishen, A. Photoactivated polycationic bioactive chitosan nanoparticles inactivate bacterial endotoxins. J. Endod. 2015, 41, 686–691. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, A.; Kishen, A. Antibiofilm efficacy of photosensitizer-functionalized bioactive nanoparticles on multispecies biofilm. J. Endod. 2014, 40, 1604–1610. [Google Scholar] [CrossRef]
- Zong, B.; Li, X.; Xu, Q.; Wang, D.; Gao, P.; Zhou, Q. Enhanced Eradication of Enterococcus faecalis Biofilms by Quaternized Chitosan-Coated Upconversion Nanoparticles for Photodynamic Therapy in Persistent Endodontic Infections. Front. Microbiol. 2022, 13, 909492. [Google Scholar] [CrossRef] [PubMed]
- McGurkin-Smith, R.; Trope, M.; Caplan, D.; Sigurdsson, A. Reduction of intracanal bacteria using GT rotary instrumentation, 5.25% NaOCl, EDTA, and Ca(OH)2. J. Endod. 2005, 31, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Magalhaes, K.M.; Rocas, I.N. Bacterial reduction in infected root canals treated with 2.5% NaOCl as an irrigant and calcium hydroxide/camphorated paramonochlorophenol paste as an intracanal dressing. J. Endod. 2007, 33, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F., Jr.; Guimaraes-Pinto, T.; Rocas, I.N. Effects of chemomechanical preparation with 2.5% sodium hypochlorite and intracanal medication with calcium hydroxide on cultivable bacteria in infected root canals. J. Endod. 2007, 33, 800–805. [Google Scholar] [CrossRef] [PubMed]
- Zand, V.; Mokhtari, H.; Hasani, A.; Jabbari, G. Comparison of the Penetration Depth of Conventional and Nano-Particle Calcium Hydroxide into Dentinal Tubules. Iran Endod. J. 2017, 12, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Javidi, M.; Afkhami, F.; Zarei, M.; Ghazvini, K.; Rajabi, O. Efficacy of a combined nanoparticulate/calcium hydroxide root canal medication on elimination of Enterococcus faecalis. Aust. Endod. J. 2014, 40, 61–65. [Google Scholar] [CrossRef]
- Mahmoud, A.; Moussa, S.; El Backly, R.; El-Gendy, R. Investigating the residual effect of silver nanoparticles gel as an intra-canal medicament on dental pulp stromal cells. BMC Oral Health 2022, 22, 545. [Google Scholar] [CrossRef]
- Algazlan, A.S.; Almuraikhi, N.; Muthurangan, M.; Balto, H.; Alsalleeh, F. Silver Nanoparticles Alone or in Combination with Calcium Hydroxide Modulate the Viability, Attachment, Migration, and Osteogenic Differentiation of Human Mesenchymal Stem Cells. Int. J. Mol. Sci. 2022, 24, 702. [Google Scholar] [CrossRef] [PubMed]
- Saad, A.; Alabdulmohsen, Z. Antibacterial effect of silver nanoparticles against Enterococcus faecalis. Saudi Endod. J. 2017, 7, 29–35. [Google Scholar] [CrossRef]
- Liu, T.; Aman, A.; Ainiwaer, M.; Ding, L.; Zhang, F.; Hu, Q.; Song, Y.; Ni, Y.; Tang, X. Evaluation of the anti-biofilm effect of poloxamer-based thermoreversible gel of silver nanoparticles as a potential medication for root canal therapy. Sci. Rep. 2021, 11, 12577. [Google Scholar] [CrossRef] [PubMed]
- Sireesha, A.; Jayasree, R.; Vidhya, S.; Mahalaxmi, S.; Sujatha, V.; Kumar, T.S.S. Comparative evaluation of micron- and nano-sized intracanal medicaments on penetration and fracture resistance of root dentin—An in vitro study. Int. J. Biol. Macromol. 2017, 104, 1866–1873. [Google Scholar] [CrossRef] [PubMed]
- Del Carpio-Perochena, A.; Kishen, A.; Felitti, R.; Bhagirath, A.Y.; Medapati, M.R.; Lai, C.; Cunha, R.S. Antibacterial Properties of Chitosan Nanoparticles and Propolis Associated with Calcium Hydroxide against Single- and Multispecies Biofilms: An In Vitro and In Situ Study. J. Endod. 2017, 43, 1332–1336. [Google Scholar] [CrossRef] [PubMed]
- Teja, K.V.; Janani, K.; Srivastava, K.C.; Shrivastava, D.; Natoli, V.; Di Blasio, M.; Cicciu, M.; Minervini, G. Comparative evaluation of antimicrobial efficacy of different combinations of calcium hydroxide against Enterococcus faecalis. BMC Oral Health 2023, 23, 849. [Google Scholar] [CrossRef]
- Tulu, G.; Kaya, B.U.; Cetin, E.S.; Kole, M. Antibacterial effect of silver nanoparticles mixed with calcium hydroxide or chlorhexidine on multispecies biofilms. Odontology 2021, 109, 802–811. [Google Scholar] [CrossRef] [PubMed]
- Balto, H.; Bukhary, S.; Al-Omran, O.; BaHammam, A.; Al-Mutairi, B. Combined Effect of a Mixture of Silver Nanoparticles and Calcium Hydroxide against Enterococcus faecalis Biofilm. J. Endod. 2020, 46, 1689–1694. [Google Scholar] [CrossRef] [PubMed]
- Alghofaily, M.; Alfraih, J.; Alsaud, A.; Almazrua, N.; Sumague, T.S.; Auda, S.H.; Alsalleeh, F. The Effectiveness of Silver Nanoparticles Mixed with Calcium Hydroxide against Candida albicans: An Ex Vivo Analysis. Microorganisms 2024, 12, 289. [Google Scholar] [CrossRef]
- AlGazlan, A.S.; Auda, S.H.; Balto, H.; Alsalleeh, F. Antibiofilm Efficacy of Silver Nanoparticles Alone or Mixed with Calcium Hydroxide as Intracanal Medicaments: An Ex-Vivo Analysis. J. Endod. 2022, 48, 1294–1300. [Google Scholar] [CrossRef]
- Elmsmari, F.; Gonzalez Sanchez, J.A.; Duran-Sindreu, F.; Belkadi, R.; Espina, M.; Garcia, M.L.; Sanchez-Lopez, E. Calcium hydroxide-loaded PLGA biodegradable nanoparticles as an intracanal medicament. Int. Endod. J. 2021, 54, 2086–2098. [Google Scholar] [CrossRef] [PubMed]
- Leelapornpisid, W.; Wanwatanakul, P.; Mahatnirunkul, T. Efficacy of calcium hydroxide-loaded poly(lactic-co-glycolic acid) biodegradable nanoparticles as an intracanal medicament against endodontopathogenic microorganisms in a multi-species biofilm model. Aust. Endod. J. 2023, 50, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Promta, P.; Chaiyosang, P.; Panya, A.; Laorodphun, P.; Leelapornpisid, W.; Imerb, N. The Evaluation of Anti-Osteoclastic Activity of the Novel Calcium Hydroxide Biodegradable Nanoparticles as an Intracanal Medicament. J. Endod. 2024, 50, 667–673. [Google Scholar] [CrossRef] [PubMed]
- Farhadian, N.; Godiny, M.; Moradi, S.; Hemati Azandaryani, A.; Shahlaei, M. Chitosan/gelatin as a new nano-carrier system for calcium hydroxide delivery in endodontic applications: Development, characterization and process optimization. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 92, 540–546. [Google Scholar] [CrossRef] [PubMed]
- Dultra, F.; Barroso, J.M.; Carrasco, L.D.; Capelli, A.; Guerisoli, D.M.; Pecora, J.D. Evaluation of apical microleakage of teeth sealed with four different root canal sealers. J. Appl. Oral Sci. 2006, 14, 341–345. [Google Scholar] [CrossRef] [PubMed]
- Grossman; Louis, I.; Grossman, L.I.; Oliet, S.; Rio, C.E.D. Endodontic Practice; Wolters Kluwer Health: Rhine, The Netherlands, 1988; Chapter 15; p. 367. [Google Scholar]
- Beyth, N.; Kesler Shvero, D.; Zaltsman, N.; Houri-Haddad, Y.; Abramovitz, I.; Davidi, M.P.; Weiss, E.I. Rapid kill-novel endodontic sealer and Enterococcus faecalis. PLoS ONE 2013, 8, e78586. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Silva, M.G.; Rocas, I.N.; Goncalves, L.S.; Alves, F.F.; Lopes, M.A.; Pina-Vaz, I.; Siqueira, J.F., Jr. Antibiofilm effects of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. J. Endod. 2014, 40, 1167–1171. [Google Scholar] [CrossRef]
- Barros, J.; Costa-Rodrigues, J.; Lopes, M.A.; Pina-Vaz, I.; Fernandes, M.H. Response of human osteoblastic and osteoclastic cells to AH plus and pulp canal sealer containing quaternary ammonium polyethylenimine nanoparticles. J. Endod. 2014, 40, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Barros, J.; Silva, M.G.; Rodrigues, M.A.; Alves, F.R.; Lopes, M.A.; Pina-Vaz, I.; Siqueira, J.F., Jr. Antibacterial, physicochemical and mechanical properties of endodontic sealers containing quaternary ammonium polyethylenimine nanoparticles. Int. Endod. J. 2014, 47, 725–734. [Google Scholar] [CrossRef]
- Baras, B.H.; Sun, J.; Melo, M.A.S.; Tay, F.R.; Oates, T.W.; Zhang, K.; Weir, M.D.; Xu, H.H.K. Novel root canal sealer with dimethylaminohexadecyl methacrylate, nano-silver and nano-calcium phosphate to kill bacteria inside root dentin and increase dentin hardness. Dent. Mater. 2019, 35, 1479–1489. [Google Scholar] [CrossRef]
- Gong, S.Q.; Huang, Z.B.; Shi, W.; Ma, B.; Tay, F.R.; Zhou, B. In vitro evaluation of antibacterial effect of AH Plus incorporated with quaternary ammonium epoxy silicate against Enterococcus faecalis. J. Endod. 2014, 40, 1611–1615. [Google Scholar] [CrossRef] [PubMed]
- Afkhami, F.; Nasri, S.; Valizadeh, S. Bacterial leakage assessment in root canals sealed with AH Plus sealer modified with silver nanoparticles. BMC Oral Health 2021, 21, 577. [Google Scholar] [CrossRef] [PubMed]
- Versiani, M.A.; Abi Rached-Junior, F.J.; Kishen, A.; Pecora, J.D.; Silva-Sousa, Y.T.; de Sousa-Neto, M.D. Zinc Oxide Nanoparticles Enhance Physicochemical Characteristics of Grossman Sealer. J. Endod. 2016, 42, 1804–1810. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, Z.; Sun, Y.; Guo, X.; Wang, H.; Xu, H.H.K.; Wang, S.; Zhou, X.; Li, B.; Cheng, L. Effect of the Modified Methacrylate-Based Root Canal Sealer in Single-Cone Technique. Nanomaterials 2022, 12, 3722. [Google Scholar] [CrossRef] [PubMed]
- Rucker, V.B.; Balbinot, G.S.; Collares, F.M.; de Araujo Neto, V.G.; Giannini, M.; Leitune, V.C.B. Synthesis of silver core-shell nanoparticles and their influence on an experimental resin endodontic sealer: An in vitro analysis. Int. Endod. J. 2023, 56, 289–303. [Google Scholar] [CrossRef] [PubMed]
- Khalil, I.; Naaman, A.; Camilleri, J. Properties of Tricalcium Silicate Sealers. J. Endod. 2016, 42, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Kapralos, V.; Koutroulis, A.; Orstavik, D.; Sunde, P.T.; Rukke, H.V. Antibacterial Activity of Endodontic Sealers against Planktonic Bacteria and Bacteria in Biofilms. J. Endod. 2018, 44, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Bukhari, S.; Karabucak, B. The Antimicrobial Effect of Bioceramic Sealer on an 8-week Matured Enterococcus faecalis Biofilm Attached to Root Canal Dentinal Surface. J. Endod. 2019, 45, 1047–1052. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.N.; Hong, J.U.; Kim, S.M.; Jang, J.H.; Chang, H.S.; Hwang, Y.C.; Hwang, I.N.; Oh, W.M. Anti-inflammatory and Osteogenic Effects of Calcium Silicate-based Root Canal Sealers. J. Endod. 2019, 45, 73–78. [Google Scholar] [CrossRef]
- Glynis, A.; Foschi, F.; Kefalou, I.; Koletsi, D.; Tzanetakis, G.N. Regenerative Endodontic Procedures for the Treatment of Necrotic Mature Teeth with Apical Periodontitis: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2021, 47, 873–882. [Google Scholar] [CrossRef]
- Leveque, M.; Bekhouche, M.; Farges, J.C.; Aussel, A.; Sy, K.; Richert, R.; Ducret, M. Bioactive Endodontic Hydrogels: From Parameters to Personalized Medicine. Int. J. Mol. Sci. 2023, 24, 14056. [Google Scholar] [CrossRef] [PubMed]
- Astudillo-Ortiz, E.; Babo, P.S.; Reis, R.L.; Gomes, M.E. Evaluation of Injectable Hyaluronic Acid-Based Hydrogels for Endodontic Tissue Regeneration. Materials 2021, 14, 7325. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, J.S.; Sanz, C.K.; Munchow, E.A.; Kalra, N.; Dubey, N.; Suarez, C.E.C.; Fenno, J.C.; Lund, R.G.; Bottino, M.C. Photocrosslinkable methacrylated gelatin hydrogel as a cell-friendly injectable delivery system for chlorhexidine in regenerative endodontics. Dent. Mater. 2022, 38, 1507–1517. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Zhou, P.; Lu, H.; Guan, Y.; Ma, M.; Wang, J.; Shang, G.; Jiang, B. Potential apply of hydrogel-carried chlorhexidine and metronidazole in root canal disinfection. Dent. Mater. J. 2021, 40, 986–993. [Google Scholar] [CrossRef] [PubMed]
- McNeilly, O.; Mann, R.; Hamidian, M.; Gunawan, C. Emerging Concern for Silver Nanoparticle Resistance in Acinetobacter baumannii and Other Bacteria. Front. Microbiol. 2021, 12, 652863. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Filho, J.E.; Silva, F.O.; Watanabe, S.; Cintra, L.T.; Tendoro, K.V.; Dalto, L.G.; Pacanaro, S.V.; Lodi, C.S.; de Melo, F.F. Tissue reaction to silver nanoparticles dispersion as an alternative irrigating solution. J. Endod. 2010, 36, 1698–1702. [Google Scholar] [CrossRef] [PubMed]
- Abbaszadegan, A.; Nabavizadeh, M.; Gholami, A.; Aleyasin, Z.S.; Dorostkar, S.; Saliminasab, M.; Ghasemi, Y.; Hemmateenejad, B.; Sharghi, H. Positively charged imidazolium-based ionic liquid-protected silver nanoparticles: A promising disinfectant in root canal treatment. Int. Endod. J. 2015, 48, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zhou, G.; Chen, C.; Yu, H.; Wang, T.; Ma, Y.; Jia, G.; Gao, Y.; Li, B.; Sun, J.; et al. Acute toxicity and biodistribution of different sized titanium dioxide particles in mice after oral administration. Toxicol. Lett. 2007, 168, 176–185. [Google Scholar] [CrossRef]
- Shrivastava, R.; Kushwaha, P.; Bhutia, Y.C.; Flora, S. Oxidative stress following exposure to silver and gold nanoparticles in mice. Toxicol. Ind. Health 2016, 32, 1391–1404. [Google Scholar] [CrossRef]
- van der Zande, M.; Vandebriel, R.J.; Van Doren, E.; Kramer, E.; Herrera Rivera, Z.; Serrano-Rojero, C.S.; Gremmer, E.R.; Mast, J.; Peters, R.J.; Hollman, P.C.; et al. Distribution, elimination, and toxicity of silver nanoparticles and silver ions in rats after 28-day oral exposure. ACS Nano 2012, 6, 7427–7442. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, S.; Sun, G.; Huang, S.; Lin, C.; Li, Y. Nanoarchitectonics-Based Materials as a Promising Strategy in the Treatment of Endodontic Infections. Pharmaceutics 2024, 16, 759. https://doi.org/10.3390/pharmaceutics16060759
Xiao S, Sun G, Huang S, Lin C, Li Y. Nanoarchitectonics-Based Materials as a Promising Strategy in the Treatment of Endodontic Infections. Pharmaceutics. 2024; 16(6):759. https://doi.org/10.3390/pharmaceutics16060759
Chicago/Turabian StyleXiao, Suli, Guanwen Sun, Shan Huang, Chen Lin, and Yijun Li. 2024. "Nanoarchitectonics-Based Materials as a Promising Strategy in the Treatment of Endodontic Infections" Pharmaceutics 16, no. 6: 759. https://doi.org/10.3390/pharmaceutics16060759
APA StyleXiao, S., Sun, G., Huang, S., Lin, C., & Li, Y. (2024). Nanoarchitectonics-Based Materials as a Promising Strategy in the Treatment of Endodontic Infections. Pharmaceutics, 16(6), 759. https://doi.org/10.3390/pharmaceutics16060759






