Improving the Antimicrobial Potency of Berberine for Endodontic Canal Irrigation Using Polymeric Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of Berberine-Loaded PLGA Nanoparticles
2.3. Characterization of Berberine-Loaded Nanoparticles
2.3.1. Size, Polydispersity Index and Zeta Potential
2.3.2. Encapsulation Efficiency and Drug Loading Determination
2.3.3. Morphology Assessment
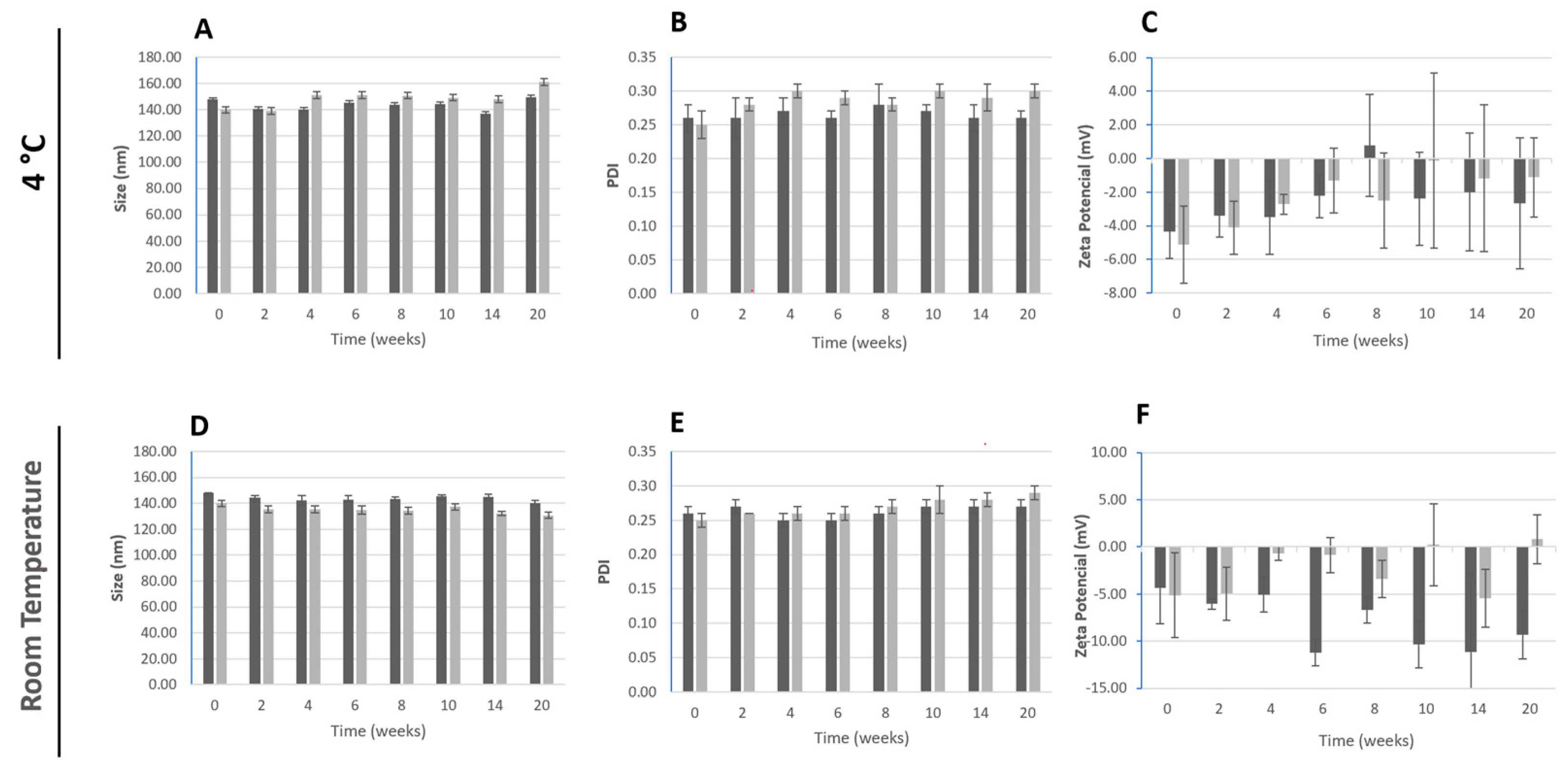
2.3.4. Storage Stability
2.3.5. Fourier Transform Infrared Spectroscopy
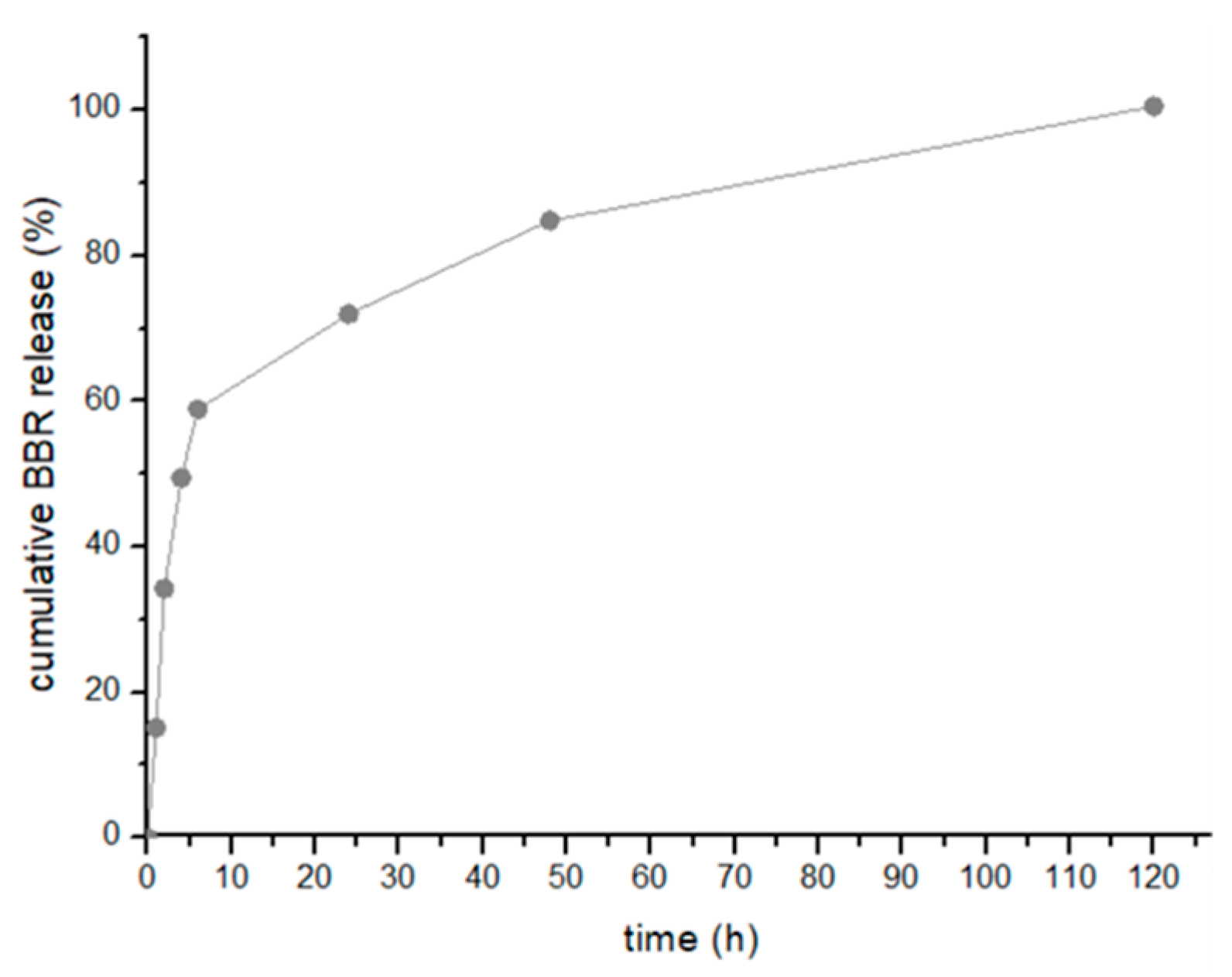
2.3.6. In Vitro Drug Release Assay
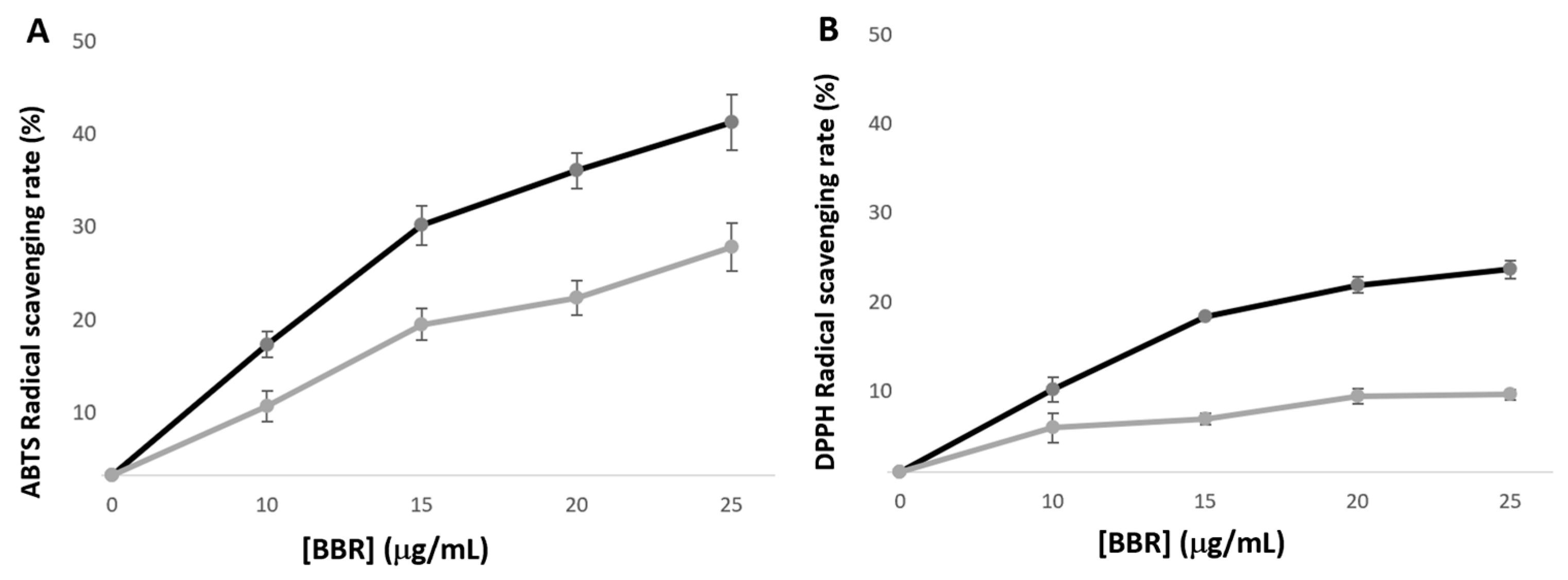
2.3.7. Antioxidant Activity
2.4. Antimicrobial Activity of Berberine-Loaded PLGA Nanoparticles
2.4.1. Microorganisms and Culture Conditions
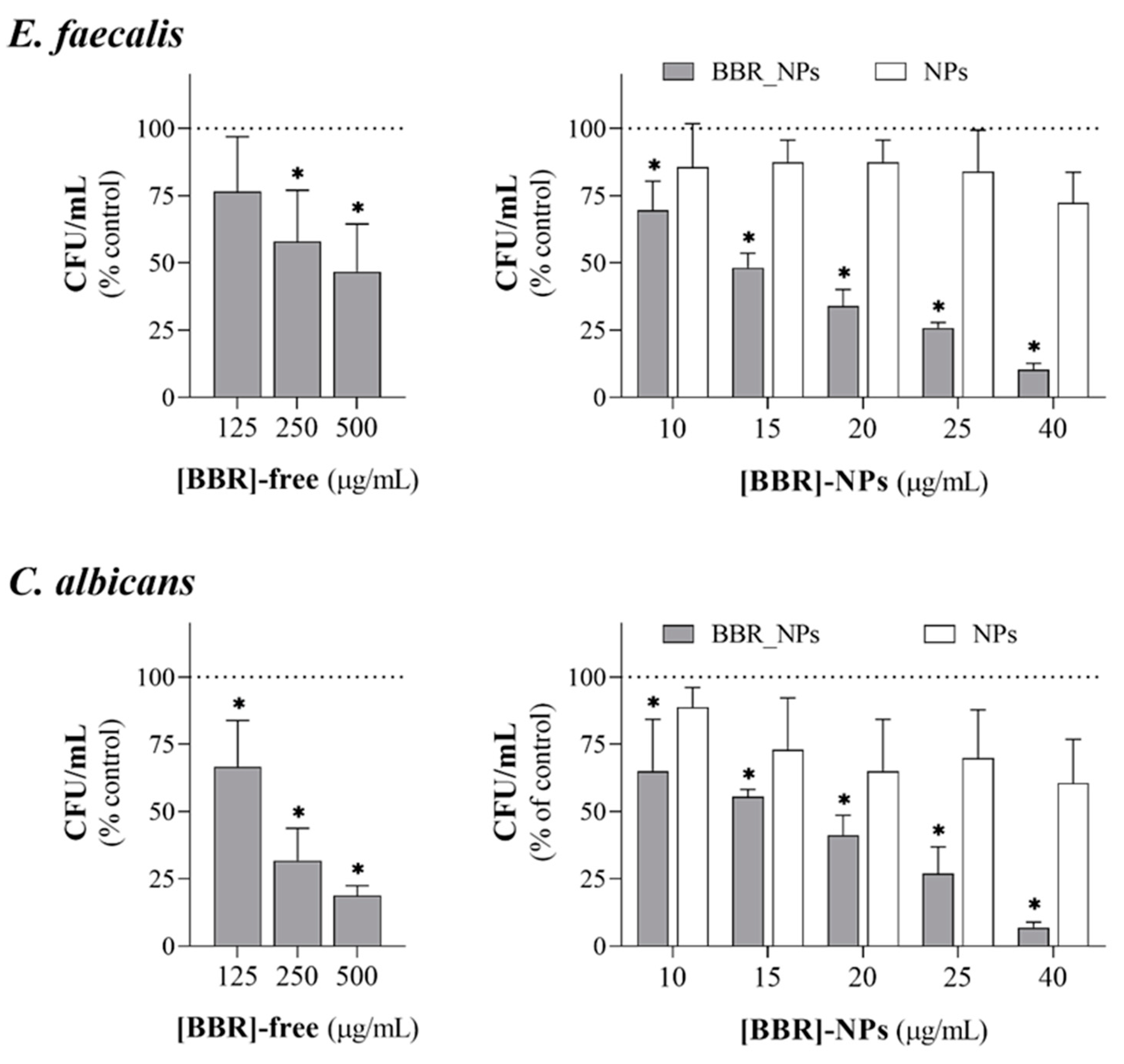
2.4.2. Antimicrobial Activity of Berberine-Loaded PLGA Nanoparticles
2.5. Cytocompatibility of Berberine-Loaded PLGA Nanoparticles
2.6. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Polymeric Nanoparticles Containing Berberine
3.2. Evaluation of the In Vitro Berberine Release
3.3. Berberine Antioxidant Activity
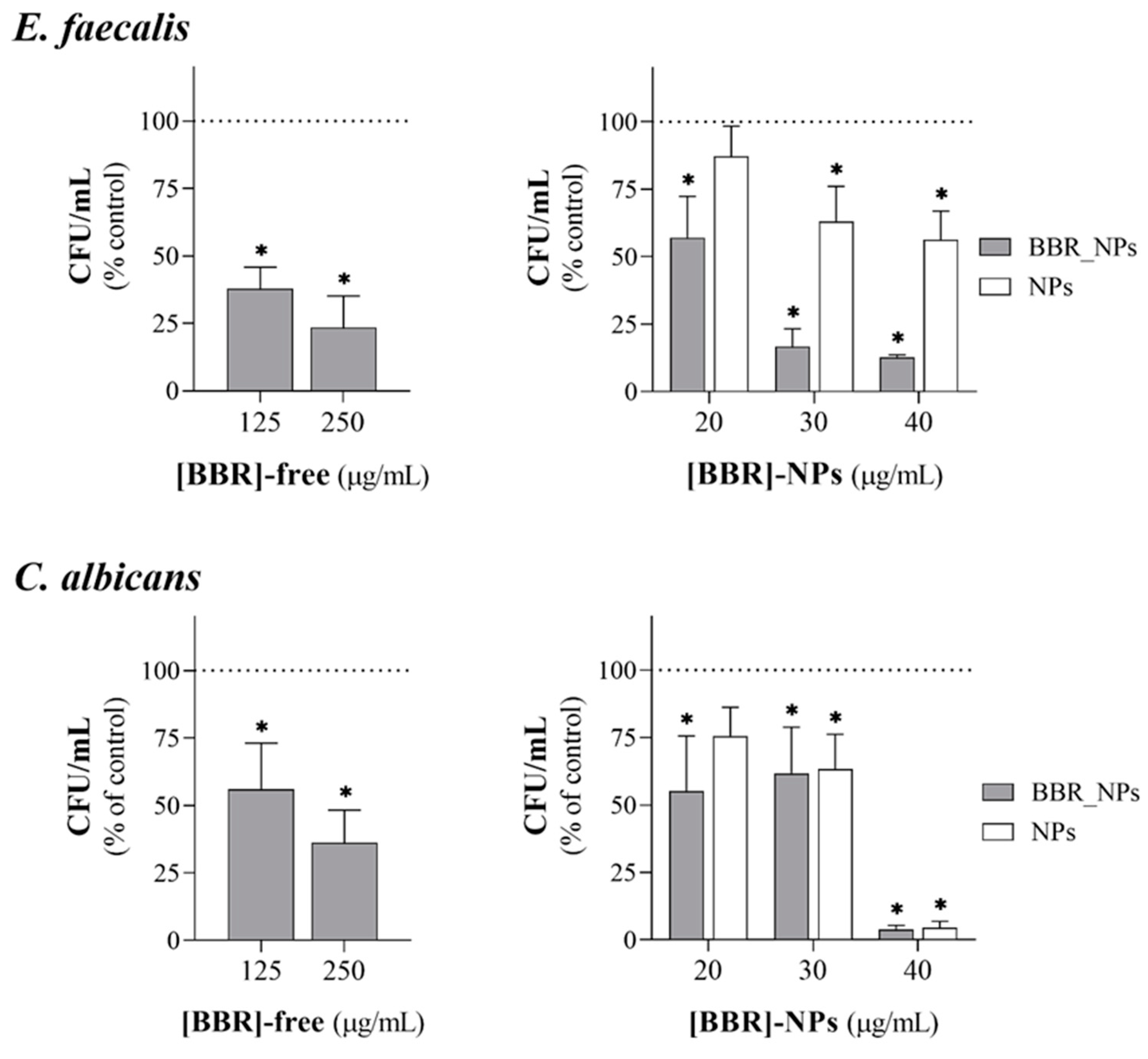
3.4. Antimicrobial Activity
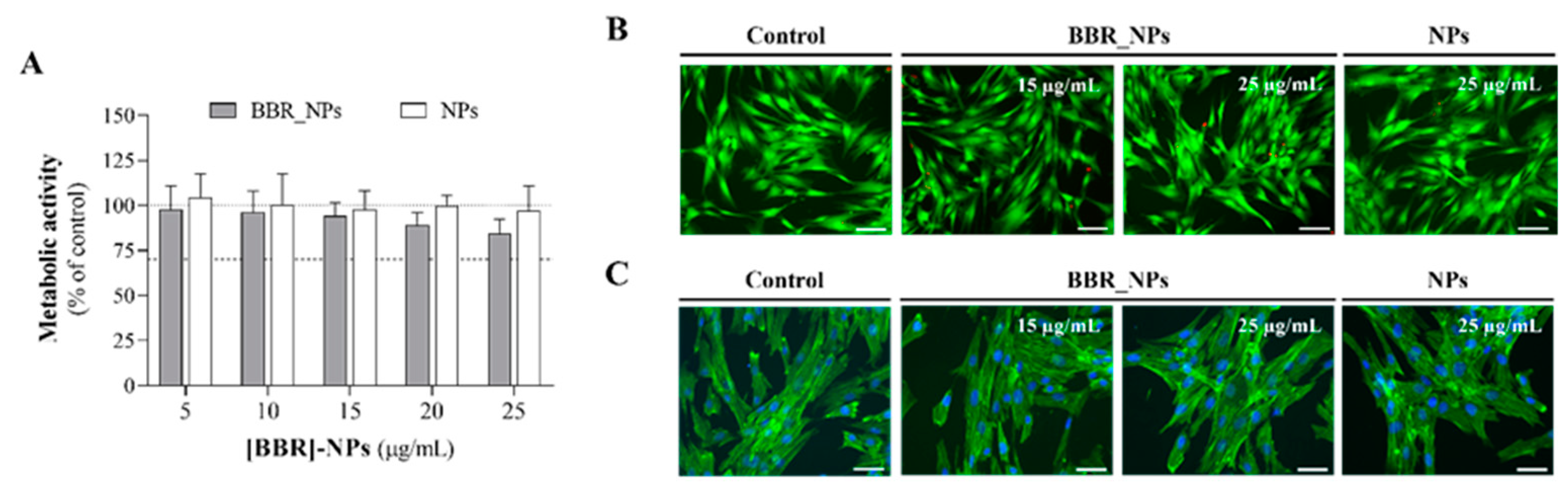
3.5. Cytocompatibility Assays
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nair, P.N.R. Pathogenesis of apical periodontitis and the causes of endodontic failures. Crit. Rev. Oral Biol. Med. 2004, 15, 348–381. [Google Scholar] [CrossRef] [PubMed]
- Prada, I.; Mico-Munoz, P.; Giner-Lluesma, T.; Mico-Martinez, P.; Collado-Castellano, N.; Manzano-Saiz, A. Influence of microbiology on endodontic failure. Literature review. Med. Oral 2019, 24, e364–e372. [Google Scholar] [CrossRef] [PubMed]
- Jhajharia, K.; Parolia, A.; Shetty, K.; Mehta, L. Biofilm in endodontics: A review. J. Int. Soc. Prev. Communit Dent. 2015, 5, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, P.; Romero, M.; Vera, J.; Daood, U.; Khan, A.U.; Yan, A.; Cheung, G.S.P. Biofilms in Endodontics—Current Status and Future Directions. Int. J. Mol. Sci. 2017, 18, 1748. [Google Scholar] [CrossRef] [PubMed]
- Dioguardi, M.; Gioia, G.D.; Illuzzi, G.; Laneve, E.; Cocco, A.; Troiano, G. Endodontic irrigants: Different methods to improve efficacy and related problems. Eur. J. Dent. 2018, 12, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Tonini, R.; Salvadori, M.; Audino, E.; Sauro, S.; Garo, M.L.; Salgarello, S. Irrigating Solutions and Activation Methods Used in Clinical Endodontics: A Systematic Review. Front. Oral Health 2022, 3, 838043. [Google Scholar] [CrossRef]
- Boutsioukis, C.; Arias-Moliz, M.T. Present status and future directions—Irrigants and irrigation methods. Int. Endod. J. 2022, 55, 588–612. [Google Scholar] [CrossRef] [PubMed]
- Wong, J.; Manoil, D.; Näsman, P.; Belibasakis, G.N.; Neelakantan, P. Microbiological Aspects of Root Canal Infections and Disinfection Strategies: An Update Review on the Current Knowledge and Challenges. Front. Oral Health. 2021, 2, 672887. [Google Scholar] [CrossRef] [PubMed]
- Haapasalo, M.; Shen, Y.; Wang, Z.; Gao, Y. Irrigation in endodontics. Br. Dent. J. 2014, 216, 299–303. [Google Scholar] [CrossRef]
- Farook, S.A.; Shah, V.; Lenouvel, D.; Sheikh, O.; Sadiq, Z.; Cascarini, L. Guidelines for management of sodium hypochlorite extrusion injuries. Br. Dent. J. 2014, 217, 679–684. [Google Scholar] [CrossRef]
- Wang, Z.; Shen, Y.; Haapasalo, M. Effectiveness of Endodontic Disinfecting Solutions against Young and Old Enterococcus faecalis Biofilms in Dentin Canals. J. Endod. 2012, 38, 1376–1379. [Google Scholar] [CrossRef] [PubMed]
- Ruksakiet, K.; Hanák, L.; Farkas, N.; Hegyi, P.; Sadaeng, W.; Czumbel, L.M.; Sang-Ngoen, T.; Garami, A.; Mikó, A.; Varga, G.; et al. Antimicrobial Efficacy of Chlorhexidine and Sodium Hypochlorite in Root Canal Disinfection: A Systematic Review and Meta-analysis of Randomized Controlled Trials. J. Endod. 2020, 46, 1032–1041.e7. [Google Scholar] [CrossRef] [PubMed]
- Subramani, R.; Narayanasamy, M.; Feussner, K.D. Plant-derived antimicrobials to fight against multi-drug-resistant human pathogens. 3 Biotech 2017, 7, 172. [Google Scholar] [CrossRef] [PubMed]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crişan, G. Berberine: Botanical Occurrence, Traditional Uses, Extraction Methods, and Relevance in Cardiovascular, Metabolic, Hepatic, and Renal Disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yi, H.; Wu, J.; Kuang, T.; Zhang, J.; Li, Q.; Du, H.; Xu, T.; Jiang, G.; Fan, G. Therapeutic effect of berberine on metabolic diseases: Both pharmacological data and clinical evidence. Biomed. Pharmacother. 2021, 133, 110984. [Google Scholar] [CrossRef] [PubMed]
- Pang, B.; Zhao, L.H.; Zhou, Q.; Zhao, T.Y.; Wang, H.; Gu, C.J.; Tong, X.L. Application of Berberine on Treating Type 2 Diabetes Mellitus. Int. J. Endocrinol. 2015, 2015, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xie, W.; Su, F.; Wang, G.; Peng, Z.; Xu, Y.; Zhang, Y.; Xu, N.; Hou, K.; Hu, Z.; Chen, Y.; et al. Glucose-lowering effect of berberine on type 2 diabetes: A systematic review and meta-analysis. Front. Pharmacol. 2022, 13, 1015045. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xiao, X.; Feng, K.; Wang, T.; Li, W.; Yuan, T.; Sun, Q.; Xiang, H.; Wang, H. Berberine Moderates Glucose and Lipid Metabolism through Multipathway Mechanism. Evid. Based Complement. Altern. Med. 2011, 2011, 924851. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Xiang, Y.; Shi, Y.; Tang, X.; Pan, L.; Gao, J.; Bi, R.; Lai, X. Pharmacokinetics and Pharmacological Activities of Berberine in Diabetes Mellitus Treatment. Evid. Based Complement. Altern. Med. 2021, 2021, 1–15. [Google Scholar] [CrossRef]
- Imenshahidi, M.; Hosseinzadeh, H. Berberine neuroprotection and antioxidant activity. In Oxidative Stress and Dietary Antioxidants in Neurological Diseases; Martin, C.R., Preedy, V.R., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 199–216. [Google Scholar]
- Tian, E.; Sharma, G.; Dai, C. Neuroprotective Properties of Berberine: Molecular Mechanisms and Clinical Implications. Antioxidants 2023, 12, 1883. [Google Scholar] [CrossRef]
- Wei, W.; Yao, J.X.; Zhang, T.T.; Wen, J.Y.; Zhang, Z.; Luo, Y.M.; Cao, Y.; Li, H. Network pharmacology reveals that Berberine may function against Alzheimer’s disease via the AKT signaling pathway. Front. Neurosci. 2023, 17, 1059496. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Abu-Izneid, T.; Khalil, A.A.; Imran, M.; Shah, Z.A.; Emran, T.B.; Mitra, S.; Khan, Z.; Alhumaydhi, F.A.; Aljohani, A.S.M.; et al. Berberine as a Potential Anticancer Agent: A Comprehensive Review. Molecules 2021, 26, 7368. [Google Scholar] [CrossRef] [PubMed]
- Warowicka, A.; Nawrot, R.; Goździcka-Józefiak, A. Antiviral activity of berberine. Arch. Virol. 2020, 165, 1935–1945. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudvand, H.; Ayatollahi Mousavi, S.A.; Sepahvand, A.; Sharififar, F.; Ezatpour, B.; Gorohi, F.; Saedi Dezaki, E.; Jahanbakhsh, S. Antifungal, Antileishmanial, and Cytotoxicity Activities of Various Extracts of Berberis vulgaris (Berberidaceae) and Its Active Principle Berberine. ISRN Pharmacol. 2014, 2014, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Liu, X.; Zhou, P. In vitro antifungal effects of Berberine against Candida spp. in planktonic and biofilm conditions. Drug Des. Dev. Ther. 2020, 14, 87–101. [Google Scholar] [CrossRef] [PubMed]
- Tan, J.; Wang, J.; Yang, C.; Zhu, C.; Guo, G.; Tang, J.; Shen, H. Antimicrobial characteristics of Berberine against prosthetic joint infection-related Staphylococcus aureus of different multi-locus sequence types. BMC Complement. Altern. Med. 2019, 19, 218. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Huang, X.; Ma, G. Antimicrobial activities and mechanisms of extract and components of herbs in East Asia. RSC Adv. 2022, 12, 29197–29213. [Google Scholar] [CrossRef] [PubMed]
- Kamrani Rad, S.Z.; Rameshrad, M.; Hosseinzadeh, H. Toxicology effects of Berberis vulgaris (barberry) and its active constituent, berberine: A review. Iran. J. Basic. Med. Sci. 2017, 20, 5. [Google Scholar]
- Liu, C.S.; Zheng, Y.R.; Zhang, Y.F.; Long, X.Y. Research progress on berberine with a special focus on its oral bioavailability. Fitoterapia 2016, 109, 274–282. [Google Scholar] [CrossRef]
- Lee, D.; Kim, M.J.; Park, S.N.; Lim, Y.K.; Min, J.B. Antimicrobial activity of berberine against oral bacteria related to endodontic infections. Int. J. Oral Biol. 2013, 38, 141–147. [Google Scholar] [CrossRef]
- Xie, Q.; Johnson, B.R.; Wenckus, C.S.; Fayad, M.I.; Wu, C.D. Efficacy of Berberine, an antimicrobial plant alkaloid, as an endodontic irrigant against a nixed-culture biofilm in an in vitro tooth model. J. Endod. 2012, 38, 1114–1117. [Google Scholar] [CrossRef] [PubMed]
- Siqueira, J.F.; Rôças, I.N. Present status and future directions: Microbiology of endodontic infections. Int. Endod. J. 2022, 55, 512–530. [Google Scholar] [CrossRef] [PubMed]
- Yoo, Y.J.; Kim, A.R.; Perinpanayagam, H.; Han, S.H.; Kum, K.Y. Candida albicans Virulence Factors and Pathogenicity for Endodontic Infections. Microorganisms 2020, 8, 1300. [Google Scholar] [CrossRef] [PubMed]
- Kumari, L.; Choudhari, Y.; Patel, P.; Gupta, G.D.; Singh, D.; Rosenholm, J.M.; Bansal, K.K.; Kurmi, B.D. Advancement in Solubilization Approaches: A Step towards Bioavailability Enhancement of Poorly Soluble Drugs. Life 2023, 13, 1099. [Google Scholar] [CrossRef] [PubMed]
- Jacob, S.; Nair, A.B.; Shah, J. Emerging role of nanosuspensions in drug delivery systems. Biomater. Res. 2020, 24, 3. [Google Scholar] [CrossRef] [PubMed]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; Rodriguez-Torres, M.D.P.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S.; et al. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [PubMed]
- Samiei, M.; Farjami, A.; Dizaj, S.M.; Lotfipour, F. Nanoparticles for antimicrobial purposes in Endodontics: A systematic review of in vitro studies. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 58, 1269–1278. [Google Scholar] [CrossRef] [PubMed]
- Marques, C.; Fernandes, M.H.; Lima, S.A.C. Elucidating Berberine’s Therapeutic and Photosensitizer Potential through Nanomedicine Tools. Pharmaceutics 2023, 15, 2282. [Google Scholar] [CrossRef] [PubMed]
- Lakshminarayanan, R.; Ye, E.; Young, D.J.; Li, Z.; Loh, X.J. Recent Advances in the Development of Antimicrobial Nanoparticles for Combating Resistant Pathogens. Adv. Healthc. Mater. 2018, 7, 1701400. [Google Scholar] [CrossRef]
- Liew, K.B.; Janakiraman, A.K.; Sundarapandian, R.; Khalid, S.H.; Razzaq, F.A.; Ming, L.C.; Khan, A.; Kalusalingam, A.; Ng, P.W. A review and revisit of nanoparticles for antimicrobial drug delivery. J. Med. Life 2022, 15, 328–335. [Google Scholar] [CrossRef]
- Shrestha, A.; Kishen, A. Antibacterial Nanoparticles in Endodontics: A Review. J. Endod. 2016, 42, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, D.N.; Bhatia, A.; Kaur, R.; Sharma, R.; Kaur, G.; Dhawan, S. PLGA: A unique polymer for drug delivery. Ther. Deliv. 2015, 6, 41–58. [Google Scholar] [CrossRef] [PubMed]
- Oncu, A.; Huang, Y.; Amasya, G.; Sevimay, F.S.; Orhan, K.; Celikten, B. Silver nanoparticles in endodontics: Recent developments and applications. Restor. Dent. Endod. 2021, 46, e38. [Google Scholar] [CrossRef] [PubMed]
- Mammari, N.; Lamouroux, E.; Boudier, A.; Duval, R.E. Current Knowledge on the Oxidative-Stress-Mediated Antimicrobial Properties of Metal-Based Nanoparticles. Microorganisms 2022, 10, 437. [Google Scholar] [CrossRef] [PubMed]
- Min, Y.; Suminda, G.G.D.; Heo, Y.; Kim, M.; Ghosh, M.; Son, Y.O. Metal-Based Nanoparticles and Their Relevant Consequences on Cytotoxicity Cascade and Induced Oxidative Stress. Antioxidants 2023, 12, 703. [Google Scholar] [CrossRef] [PubMed]
- Govender, T. PLGA nanoparticles prepared by nanoprecipitation: Drug loading and release studies of a water soluble drug. J. Control Release 1999, 57, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Battu, S.K.; Repka, M.A.; Maddineni, S.; Chittiboyina, A.G.; Avery, M.A.; Majumdar, S. Physicochemical Characterization of Berberine Chloride: A Perspective in the Development of a Solution Dosage Form for Oral Delivery. AAPS PharmSciTech 2010, 11, 1466–1475. [Google Scholar] [CrossRef]
- Barzegar-Jalali, M. Kinetic Analysis of Drug Release from Nanoparticles. J. Pharm. Pharm. Sci. 2008, 11, 167. [Google Scholar] [CrossRef]
- Liu, Y.; Long, S.; Zhang, S.; Tan, Y.; Wang, T.; Wu, Y.; Jiang, T.; Liu, X.; Peng, D.; Liu, Z. Synthesis and antioxidant activities of berberine 9-O-benzoic acid derivatives. RSC Adv. 2021, 11, 17611–17621. [Google Scholar] [CrossRef]
- Yu, F.; Ao, M.; Zheng, X.; Li, N.; Xia, J.; Li, Y.; Li, D.; Hou, Z.; Qi, Z.; Chen, X.D. PEG–lipid–PLGA hybrid nanoparticles loaded with berberine–phospholipid complex to facilitate the oral delivery efficiency. Drug Deliv. 2017, 24, 825–833. [Google Scholar] [CrossRef]
- Danaei, M.; Dehghankhold, M.; Ataei, S.; Hasanzadeh Davarani, F.; Javanmard, R.; Dokhani, A.; Khorasani, S.; Mozafari, M.R. Impact of Particle Size and Polydispersity Index on the Clinical Applications of Lipidic Nanocarrier Systems. Pharmaceutics 2018, 10, 57. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Hillaireau, H.; Nicolas, J.; Le Droumaguet, B.; Gueutin, C.; Zanna, S.; Tsapis, N.; Fattal, E. Influence of surface charge on the potential toxicity of PLGA nanoparticles towards Calu-3 cells. Int. J. Nanomed. 2011, 6, 2591–2605. [Google Scholar]
- Ayala, V.; Herrera, A.P.; Latorre-Esteves, M.; Torres-Lugo, M.; Rinaldi, C. Effect of surface charge on the colloidal stability and in vitro uptake of carboxymethyl dextran-coated iron oxide nanoparticles. J. Nanopart. Res. 2013, 15, 1874. [Google Scholar] [CrossRef] [PubMed]
- Costa Lima, S.A.; Reis, S. Temperature-responsive polymeric nanospheres containing methotrexate and gold nanoparticles: A multi-drug system for theranostic in rheumatoid arthritis. Colloids Surf. B Biointerfaces 2015, 133, 378–387. [Google Scholar] [CrossRef] [PubMed]
- Sahibzada, M.U.K.; Sadiq, A.; Faidah, H.S.; Khurram, M.; Amin, M.U.; Haseeb, A.; Kakar, M. Berberine nanoparticles with enhanced in vitro bioavailability: Characterization and antimicrobial activity. Drug Des. Dev. Ther. 2018, 12, 303–312. [Google Scholar] [CrossRef] [PubMed]
- Yurtdaş Kırımlıoğlu, G. Drug Loading Methods and Drug Release Mechanisms of PLGA Nanoparticles; Elsiever: Amsterdam, The Netherlands, 2023; pp. 55–86. Available online: https://linkinghub.elsiever.com/retrieve/pii/B9780323912150000054 (accessed on 14 January 2024).
- Weng, J.; Tong, H.H.Y.; Chow, S.F. In Vitro Release Study of the Polymeric Drug Nanoparticles: Development and Validation of a Novel Method. Pharmaceutics 2020, 12, 732. [Google Scholar] [CrossRef] [PubMed]
- Magalhães, L.M.; Segundo, M.A.; Reis, S.; Lima, J.L.F.C. Methodological aspects about in vitro evaluation of antioxidant properties. Anal. Chim. Acta 2008, 613, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Staško, A.; Brezová, V.; Biskupič, S.; Mišík, V. The potential pitfalls of using 1, 1-diphenyl-2-picrylhydrazyl to characterize antioxidants in mixed water solvents. Free Radic. Res. 2007, 41, 379–390. [Google Scholar] [CrossRef]
- Younis, F.A.; Saleh, S.R.; El-Rahman, S.S.A.; Newairy, A.S.A.; El-Demellawy, M.A.; Ghareeb, D.A. Preparation, physicochemical characterization, and bioactivity evaluation of berberine-entrapped albumin nanoparticles. Sci. Rep. 2022, 12, 17431. [Google Scholar] [CrossRef]
- Mir, M.A.; Akhter, M.H.; Afzal, O.; Rab, S.O.; Altamimi, A.S.A.; Alossaimi, M.A.; Nasar Mir Najib Ullah, S.; Jaremko, M.; Emwas, A.H.; Ahmad, S.; et al. Design-of-Experiment-Assisted Fabrication of Biodegradable Polymeric Nanoparticles: In Vitro Characterization, Biological Activity, and In Vivo Assessment. ACS Omega 2023, 8, 38806–38821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Sun, X.; Wu, J.; Wu, Y.; Wang, Y.; Hu, X.; Wang, X. Berberine Damages the Cell Surface of Methicillin-Resistant Staphylococcus aureus. Front. Microbiol. 2020, 11, 621. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Siqueira, J.F. Biofilms and Apical Periodontitis: Study of Prevalence and Association with Clinical and Histopathologic Findings. J. Endod. 2010, 36, 1277–1288. [Google Scholar] [CrossRef] [PubMed]
- Bjarnsholt, T. The role of bacterial biofilms in chronic infections. APMIS Suppl. 2013, 121, 1–58. [Google Scholar] [CrossRef] [PubMed]
- Ricucci, D.; Loghin, S.; Siqueira, J.F. Exuberant Biofilm Infection in a Lateral Canal as the Cause of Short-term Endodontic Treatment Failure: Report of a Case. J. Endod. 2013, 39, 712–718. [Google Scholar] [CrossRef] [PubMed]
- Karaosmanoglu, K.; Sayar, N.A.; Kurnaz, I.A.; Akbulut, B.S. Assessment of berberine as a multi-target antimicrobial: A multi-omics study for drug discovery and repositioning. OMICS 2014, 8, 42–53. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, O.; Hazar, E.; Koçak, S.; Sağlam, B.; Koçak, M. The frequency of sodium hypochlorite extrusion during root canal treatment: An observational clinical study. Aust. Dent. J. 2022, 67, S57–S64. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Aranda, M.L.; Canalda-Sahli, C.; Figueiredo, R.; Gay-Escoda, C. Complications following an accidental sodium hypochlorite extrusion: A report of two cases. J. Clin. Exp. Dent. 2012, 4, e194–e198. [Google Scholar] [CrossRef] [PubMed]
- Onay, E.O.; Ungor, M.; Yazici, A.C. The evaluation of endodontic flare-ups and their relationship to various risk factors. BMC Oral Health 2015, 15, 142. [Google Scholar] [CrossRef]
- Kadzik, R.S.; Homa, K.E.; Kovar, D.R. F-Actin Cytoskeleton Network Self-Organization Through Competition and Cooperation. Annu. Rev. Cell Dev. Biol. 2020, 36, 35–60. [Google Scholar] [CrossRef]
- Ispanixtlahuatl-Meráz, O.; Schins, R.P.F.; Chirino, Y.I. Cell type specific cytoskeleton disruption induced by engineered nanoparticles. Environ. Sci. Nano 2018, 5, 228–245. [Google Scholar] [CrossRef]
- Danhier, F.; Ansorena, E.; Silva, J.M.; Coco, R.; Le Breton, A.; Préat, V. PLGA-based nanoparticles: An overview of biomedical applications. J. Control. Release 2012, 161, 505–522. [Google Scholar] [CrossRef] [PubMed]
| Formulation | Size (nm) | PDI | ζ-Potential (mV) | EE (%) | DL (%) |
|---|---|---|---|---|---|
| PLGA | 148 ± 2 | 0.26 ± 0.01 | −6 ± 1 | ||
| BBR-loaded PLGA | 140 ± 2 | 0.25 ± 0.01 | −4 ± 1 | 67 ± 4 | 7 ± 1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marques, C.; Grenho, L.; Fernandes, M.H.; Costa Lima, S.A. Improving the Antimicrobial Potency of Berberine for Endodontic Canal Irrigation Using Polymeric Nanoparticles. Pharmaceutics 2024, 16, 786. https://doi.org/10.3390/pharmaceutics16060786
Marques C, Grenho L, Fernandes MH, Costa Lima SA. Improving the Antimicrobial Potency of Berberine for Endodontic Canal Irrigation Using Polymeric Nanoparticles. Pharmaceutics. 2024; 16(6):786. https://doi.org/10.3390/pharmaceutics16060786
Chicago/Turabian StyleMarques, Célia, Liliana Grenho, Maria Helena Fernandes, and Sofia A. Costa Lima. 2024. "Improving the Antimicrobial Potency of Berberine for Endodontic Canal Irrigation Using Polymeric Nanoparticles" Pharmaceutics 16, no. 6: 786. https://doi.org/10.3390/pharmaceutics16060786






