Influence of Mechanical Loading on the Process of Tribochemical Action on Physicochemical and Biopharmaceutical Properties of Substances, Using Lacosamide as an Example: From Micronisation to Mechanical Activation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Powdery Material
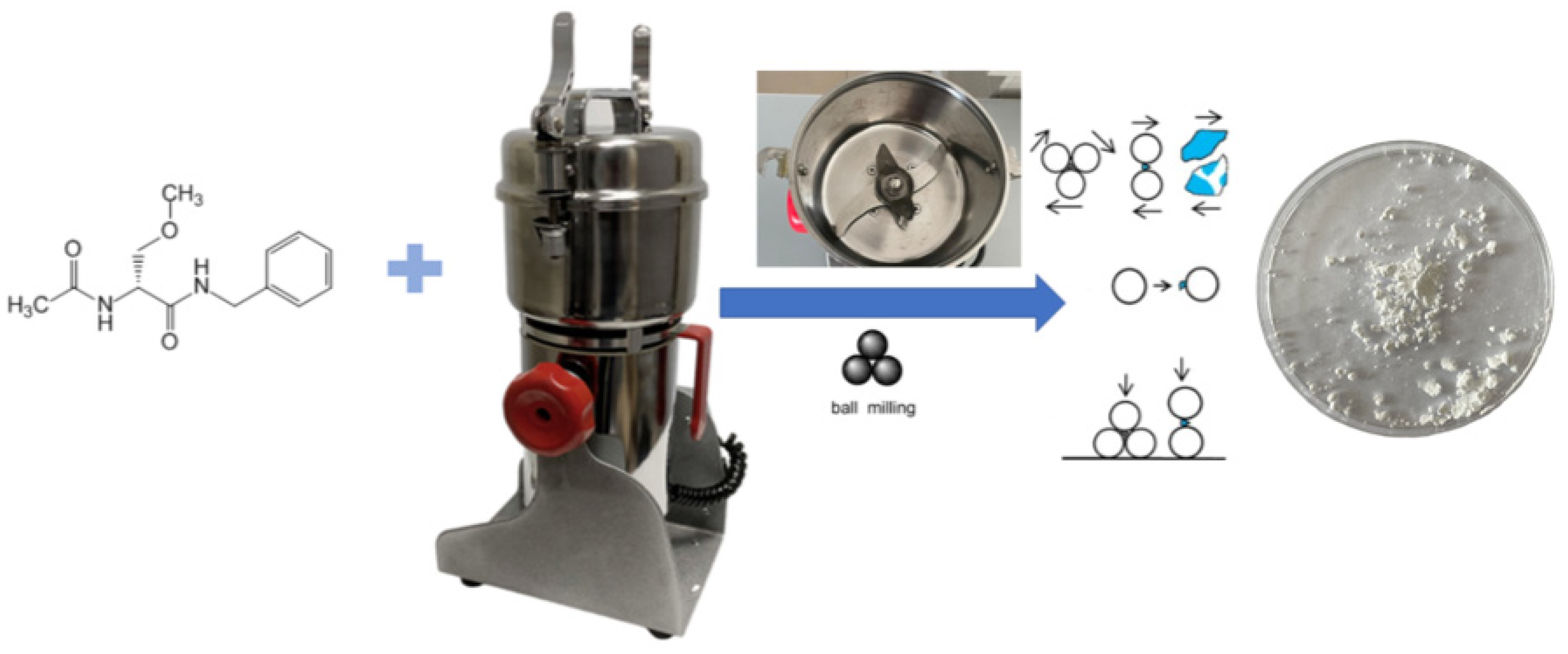
2.2. Tribochemical Equipment
Study Design
2.3. Optical Microscopy (OM) Method
2.4. Scanning Electron Microscopy (SEM)
2.5. Fourier-Transform IR Spectroscopy
2.6. Dynamic Light Scattering (DLS)
2.7. Static Light Scattering (SLS)
2.8. Spirotox-Method Study Design
2.9. Dissolution Rate Kinetics
2.10. Comparative Dissolution Kinetics Test (CDKT)
2.11. Statistical Analysis
3. Results and Discussion
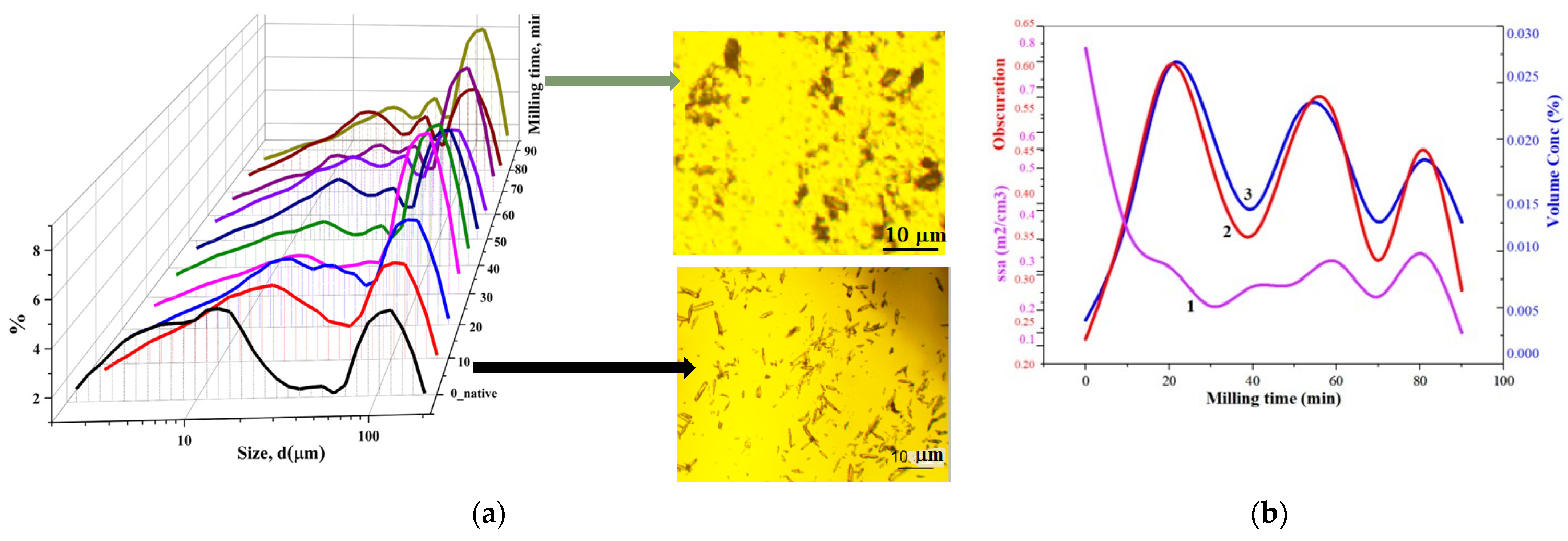
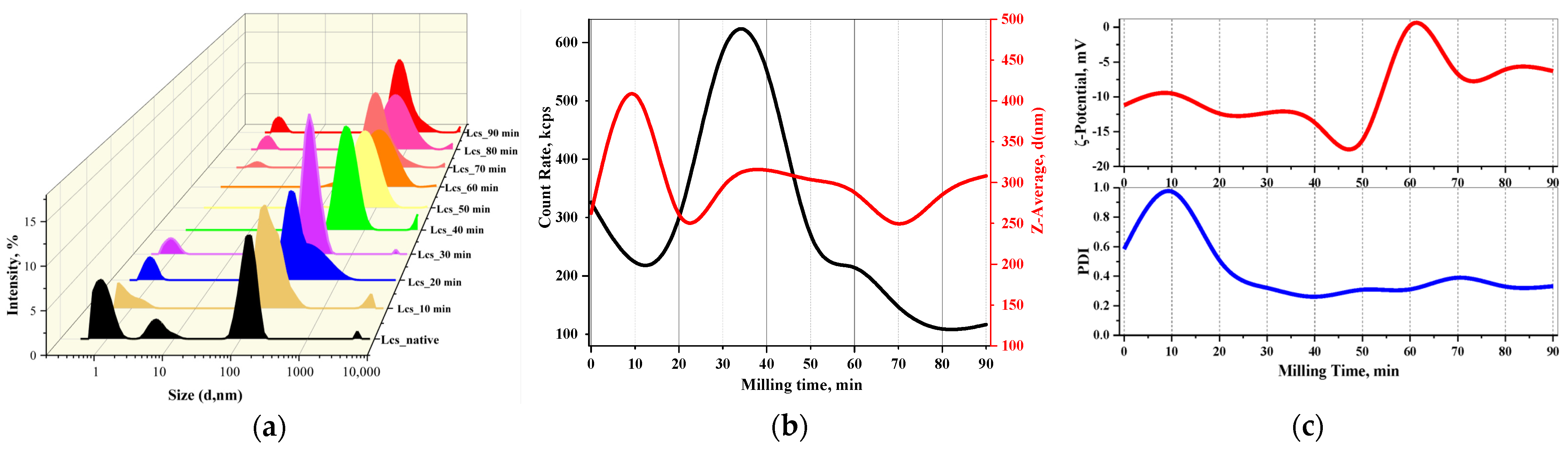
3.1. Particle Size Distributions (PSDs)
3.1.1. Static Light Scattering
3.1.2. Dynamic Light Scattering
3.2. Fourier Transform Infrared (FT-IR)
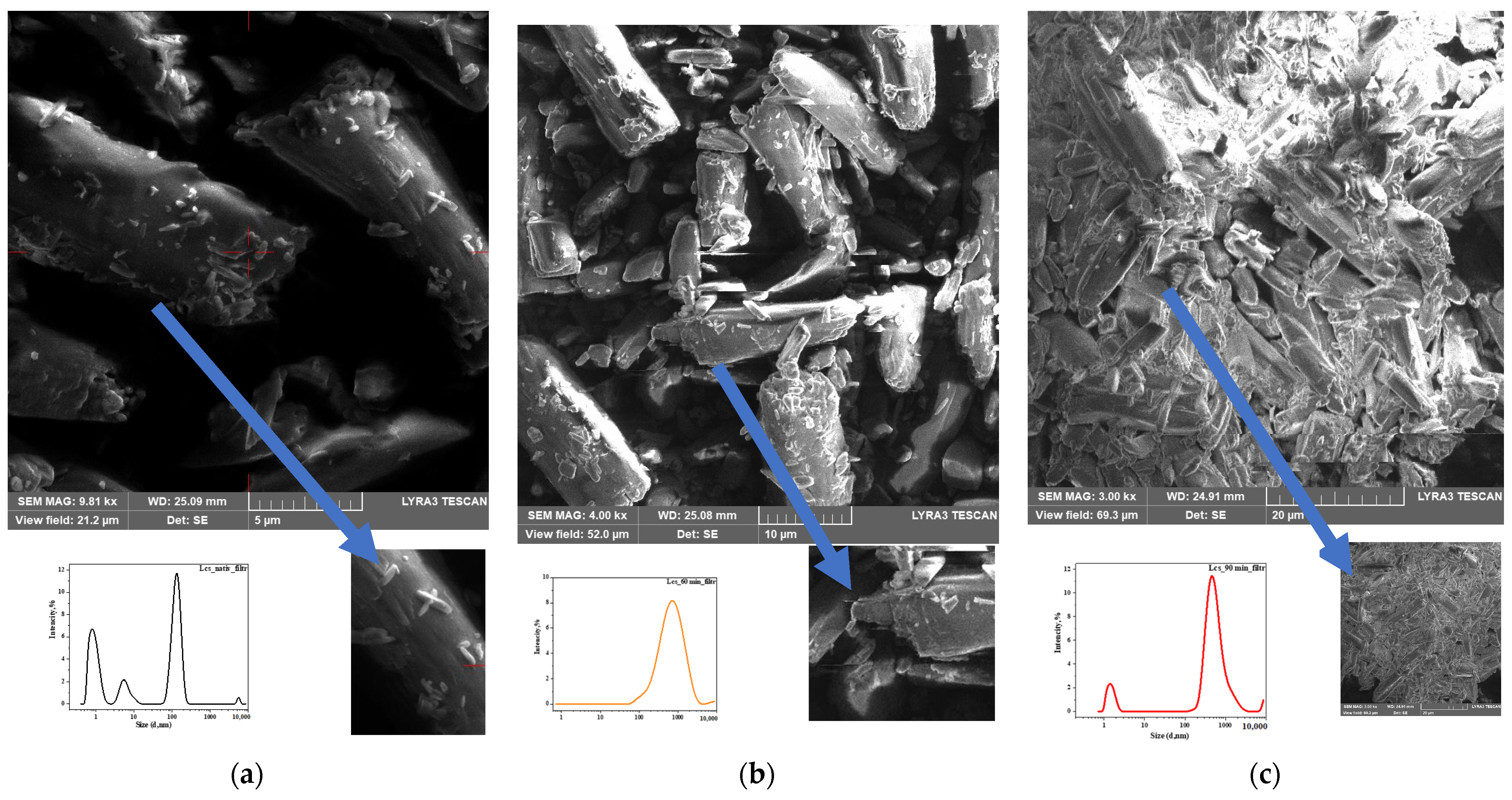
3.3. Surface and Near-Surface Morphology
Scanning Electron Microscopy (SEM)
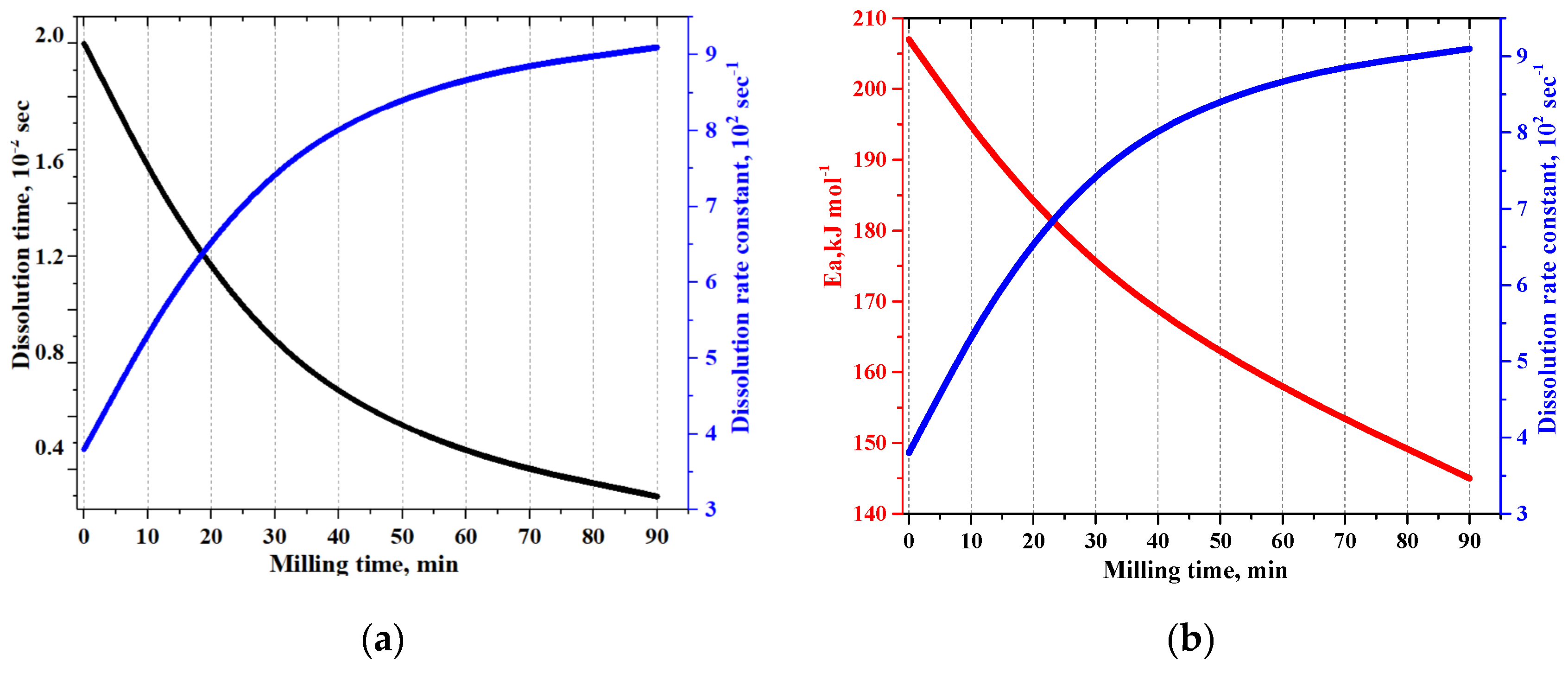
3.4. Lacosamide Dissolution Studies
3.4.1. Dissolution Kinetics by LALLS
3.4.2. In Vitro Equivalence Dissolution Test (CDKT)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| in situ | the original (primary, without movement) location of experiments |
| CRMP-2 | collapsin response mediator protein-2 |
| DLS | dynamic light scattering |
| DPh | dispersity phenomenon |
| DSA | dynamic strain aging |
| DRE | drug resistant epilepsy |
| EMA | The European Medicines Agency |
| Ea | activation energy |
| FDA | Food and Drug Administration |
| FT-IR | Fourier transform IR spectroscopy |
| kcps | kilo counts per second |
| Lcs | lacosamide |
| LALLS | low-angle laser light scattering |
| MCh | mechanochemistry |
| MAct | mechanoactivation |
| ML | mechanical loading |
| OM | optical microscopy |
| PDI | polydispersion index |
| SnCh | sound chemistry (sonochemia) |
| SEM | scanning electron microscopy |
| SB | solid body |
| TrbCh | tribochemical |
| CDKT | Comparative Dissolution Kinetics Test (In vitro equivalence dissolution test) |
| WHO | World Health Organization |
References
- Boldyrev, V.V.; Gerhard, H. Reaktionsursachen in der Tribochemie. Z. Phys. Chem. 1979, 19, 353–362. [Google Scholar] [CrossRef]
- Lapshin, O.V.; Boldyreva, E.V.; Boldyrev, V.V. Role of Mixing and Milling in Mechanochemical Synthesis. Russ. J. Inorg. Chem. 2021, 66, 433. [Google Scholar] [CrossRef]
- McNaught, A.D.; Wilkinson, A. Mechano-chemical Reaction. IUPAC Compend. Chem. Terminol. 2008, 889, 7141. [Google Scholar]
- Heinicke, G. Tribochemistry; Carl Hanser Verlag: Munich, Germany, 1985; p. 495. [Google Scholar]
- Chatel, G. How sonochemistry contributes to green chemistry? Ultrason. Sonochem 2018, 40, 117–122. [Google Scholar] [CrossRef]
- Martini, A.; Eder, S.J.; Dörr, N. Tribochemistry: A Review of Reactive Molecular Dynamics Simulations. Lubricants 2020, 8, 44. [Google Scholar] [CrossRef]
- Kharissova, O.V.; Kharisov, B.I.; Oliva González, C.M.; Méndez, Y.P.; López, I. Greener synthesis of chemical compounds and materials. R. Soc. Open Sci. 2019, 6, 191378. [Google Scholar] [CrossRef]
- Staudinger, H.; Heuer, W. Highly Polymerized Compounds XCIII—The Breaking Up of the Molecular Fibers of the Polystyrenes. Ber. Dtsch. Chem. Ges. B 1934, 67, 1159–1164. [Google Scholar] [CrossRef]
- Takacs, L.M. Carey Lea, the father of mechanochemistry. Bull. Hist. Chem. 2003, 28, 26–34. [Google Scholar]
- Khan, S.; Muhammad, Z.; Mudassir, U.R.; Gul, Z. Cocrystals; basic concepts, properties and formation strategies. Z. Phys. Chem. 2023, 237, 273–332. [Google Scholar] [CrossRef]
- Xu, Z.; Zheng, S.; Gao, X.; Hong, Y.; Cai, Y.; Zhang, Q.; Xiang, J.; Xie, D.; Song, F.; Zhang, H.; et al. Mechanochemical preparation of chrysomycin A self-micelle solid dispersion with improved solubility and enhanced oral bioavailability. J. Nanobiotechnol. 2021, 19, 164. [Google Scholar] [CrossRef]
- Colombo, I.; Grassi, G.; Grassi, M. Drug mechanochemical activation. J. Pharm. Sci. 2009, 98, 3961–3986. [Google Scholar] [CrossRef] [PubMed]
- Mu, Q.; Hu, J. Polymer mechanochemistry: From single molecule to bulk material. Phys. Chem. Chem. Phys. 2024, 26, 679–694. [Google Scholar] [CrossRef] [PubMed]
- Deneke, N.; Rencheck, M.L.; Davis, C.S. An engineer’s introduction to mechanophores. Soft Matter 2020, 16, 6230–6252. [Google Scholar] [CrossRef]
- Versaw, B.A.; McFadden, M.E.; Husic, C.C.; Robb, M.J. Designing naphthopyran mechanophores with tunable mechanochromic behavior. Chem. Sci. 2020, 11, 4525–4530. [Google Scholar] [CrossRef] [PubMed]
- Taghipour, S.; Yeung, K.-L.; Ataie-Ashtiani, B. Efficiency of Mechanochemical Ball Milling Technique in the Prepa-ration of Fe/TiO2 Photocatalysts. ChemEngineering 2022, 6, 77. [Google Scholar] [CrossRef]
- Xiong, Z.; Zhong, L.; Wang, H.; Li, X. Structural Defects, Mechanical Behaviors, and Properties of Two-Dimensional Materials. Materials 2021, 14, 1192. [Google Scholar] [CrossRef] [PubMed]
- Ping, Y.; Smart, T.J. Computational design of quantum defects in two-dimensional materials. Nat. Comput. Sci. 2021, 1, 646–654. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, R.; Baba, S.; Mizuno, N.; Hasegawa, K.; Koizumi, H.; Kojima, K.; Kumasaka, T.; Tachibana, M. Radiation-induced defects in protein crystals observed by X-ray topography. Acta Crystallogr. D Struct. Biol. 2022, 78, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Alkemade, R.M.; Jager, M.; Meer, B.; Smallenburg, F.; Filion, L. Point defects in crystals of charged colloids. J. Chem. Phys. 2021, 154, 164905. [Google Scholar] [CrossRef]
- Zhukova, A.I.; Asabina, E.A.; Kharlanov, A.N.; Osaulenko, D.A.; Chuklina, S.G.; Zhukov, D.Y.; Pet’kov, V.I.; Deyneko, D.V. Novel Complex Titanium NASICON-Type Phosphates as Acidic Catalysts for Ethanol Dehydration. Catalysts 2023, 13, 185. [Google Scholar] [CrossRef]
- Syroeshkin, A.V.; Uspenskaya, E.V.; Pleteneva, T.V.; Morozova, M.A.; Zlatskiy, I.A.; Koldina, A.M.; Nikiforova, M.V. Mechanical Transformation of Compounds Leading to Physical, Chemical, and Biological Changes in Pharmaceutical Substances. Sci. World J. 2018, 2018, 8905471. [Google Scholar] [CrossRef] [PubMed]
- Brodka-Pfeiffer, K.; Langguth, P.; Graβ, P.; Häusler, H. Influence of mechanical activation on the physical stability of salbutamol sulphate. Eur. J. Pharm. Biopharm. 2003, 56, 393–400. [Google Scholar] [CrossRef]
- Martínez, L.M.; Cruz-Angeles, J.; Vázquez-Dávila, M.; Martínez, E.; Cabada, P.; Navarrete-Bernal, C.; Cortez, F. Mechanical Activation by Ball Milling as a Strategy to Prepare Highly Soluble Pharmaceutical Formulations in the Form of Co-Amorphous, Co-Crystals, or Polymorphs. Pharmaceutics 2022, 14, 2003. [Google Scholar] [CrossRef]
- Boldyrev, V.V. Mechanochemical modification and synthesis of drugs. J. Mater. Sci. 2004, 39, 5117–5120. [Google Scholar] [CrossRef]
- Huo, S.; Zhao, P.; Shi, Z.; Zou, M.; Yang, X.; Warszawik, E.; Loznik, M.; Göstl, R.; Herrmann, A. Mechanochemical bond scission for the activation of drugs. Nat. Chem. 2021, 13, 131–139. [Google Scholar] [CrossRef]
- David, J.W.; Grant, P.Y. Entropy of processing: A new quantity for comparing the solid-state disorder of pharmaceutical materials. Int. J. Pharm. 1986, 30, 161–180. [Google Scholar]
- Perucca, E.; Yasothan, U.; Clincke, G.; Kirkpatrick, P. Lacosamide. Nat. Rev. Drug Discov. 2008, 7, 973–974. [Google Scholar] [CrossRef] [PubMed]
- Bamgbose, O.; Boyle, F.; Kean, A.C. Tolerability and Safety of Lacosamide in Neonatal Population. J. Child. Neurol. 2023, 38, 137–141. [Google Scholar] [CrossRef]
- Cawello, W. Clinical pharmacokinetic and pharmacodynamic profile of lacosamide. Clin. Pharmacokinet. 2015, 54, 901–914. [Google Scholar] [CrossRef]
- Department of Computational and Biological Sciences DrugBank Online (Version 5.1.12, Released 14 March 2024). Available online: https://go.drugbank.com/drugs/DB06218 (accessed on 4 June 2024).
- Guery, D.; Rheims, S. Clinical Management of Drug Resistant Epilepsy: A Review on Current Strategies. Neuropsychiatr. Dis. Treat. 2021, 17, 2229–2242. [Google Scholar] [CrossRef]
- Ali, A.; Zhang, N.; Santos, R.M. Mineral Characterization Using Scanning Electron Microscopy (SEM): A review of the fundamentals, advancements, and research directions. Appl. Sci. 2023, 13, 12600. [Google Scholar] [CrossRef]
- Grant, H.; Joseph, B. Particle Size Distributions of Inert Spheres and Pelletized Pharmaceutical Products by Image Analysis. Pharm. Dev. Technol. 2005, 4, 359–367. [Google Scholar]
- Uspenskaya, E.V.; Pleteneva, T.V.; Pham My, H.; Kazimova, I.V. Assessment of biology activity of the peeling substances by the physicochemical approaches on the Spirostomum Ambiguum cell model. Int. J. Pharm. Pharm. Sci. 2021, 13, 82–86. [Google Scholar]
- Uspenskaya, E.; Pleteneva, T.; Kazimova, I.; Syroeshkin, A. Evaluation of Poorly Soluble Drugs’ Dissolution Rate by Laser Scattering in Different Water Isotopologues. Molecules 2021, 26, 601. [Google Scholar] [CrossRef] [PubMed]
- База Данных Метoдик Теста Раствoрение» FDA. Available online: http://www.accessdata.fda.gov/scripts/cder/ (accessed on 10 June 2016).
- Ivanov, K.V.; Razorenov, S.V.; Garkushin, G.V. Investigation of structure and mechanical properties under quasi-static and planar impact loading of aluminum composite reinforced with Al2O3 nanoparticles of different shape. Mater. Today Commun. 2021, 29, 102942. [Google Scholar] [CrossRef]
- Xinmin, L.; Linfa, P.; Peng, H.; Shuhuai, L.; Jun, N. Material behavior modelling in micro/meso-scale forming process with considering size/scale effects. Comput. Mater. Sci. 2008, 43, 1003–1009. [Google Scholar]
- Glema, A. Physical and numerical aspects of dispersion phenomenon in solids under impact loading. CEE 2006, 7, 127–146. [Google Scholar]
- Harold, P. Dispersion phenomena in high viscosity immiscible fluid systems and application of static mixers as dispersion devices in such systems. Chem. Eng. Commun. 1982, 14, 225–277. [Google Scholar]
- Ponomarenko, A.T.; Tameev, A.R.; Shevchenko, V.G. Action of Mechanical Forces on Polymerization and Polymers. Polymers 2022, 14, 604. [Google Scholar] [CrossRef]
- Michalis, A.N.; Kalarakis, E.D.; Skouras, V.N. Burganos, Mesoscopic modeling of flow and dispersion phenomena in fractured solids. Comput. Math. Appl. 2008, 55, 1525–1540. [Google Scholar] [CrossRef]
- Uspenskaya, E.; Simutina, A.; Kuzmina, E.; Sukhanova, V.; Garaev, T.; Pleteneva, T.; Koldina, A.; Kolyabina, E.; Petrov, G.; Syroeshkin, A. Exploring the Effects of Cramped-Impact-Type Mechanical Action on Active Pharmaceutical Ingredient (Levofloxacin)—Prospects for Pharmaceutical Applications. Powders 2023, 2, 464–483. [Google Scholar] [CrossRef]
- Senturk Parreidt, T.; Schmid, M.; Hauser, C. Validation of a Novel Technique and Evaluation of the Surface Free Energy of Food. Foods 2017, 6, 31. [Google Scholar] [CrossRef] [PubMed]
- Bakkara, A.; Sadykov, B.; Zhapekova, A.; Oserov, T.; Batkal, A.; Khairullina, A.; Mofa, N. Efficiency and Prospects of the Use of Mechanochemical Treatment to Obtain Innovative Composite Systems. Chem. Eng. 2022, 6, 90. [Google Scholar] [CrossRef]
- Engblom, J.; Hyde, S. On the swelling of bicontinuous lyotropic mesophases. J. Phys. II 1995, 5, 171–190. [Google Scholar] [CrossRef]
- Grinyaev, Y.V.; Chertova, N.V.; Shilko, E.V.; Psakhie, S.G. The Continuum Approach to the Description of Semi-Crystalline Polymers Deformation Regimes: The Role of Dynamic and Translational Defects. Polymers 2018, 10, 1155. [Google Scholar] [CrossRef]
- Cloete, J.; Tarleton, E.; Hofmann, F. Computation of Burgers vectors from elastic strain and lattice rotation data. Proc. Math. Phys. Eng. Sci. 2022, 478, 20210909. [Google Scholar] [CrossRef]
- Chertova, N.V. Wave processes in elastic-viscoplastic media with dislocations. Phys. Mesomech. 2013, 16, 34–41. [Google Scholar] [CrossRef]
- Bianchi, O.; Pereira, P.B.; Ferreira, C.A. Mechanochemical Treatment in High-Shear Thermokinetic Mixer as an Alternative for Tire Recycling. Polymers 2022, 14, 4419. [Google Scholar] [CrossRef] [PubMed]
- Deev, I.S.; Kobets, L.P. Study of the microstructure and specifics of destruction of epoxide matrices. Polym. Sci. Ser. 2014, 7, 49–56. [Google Scholar] [CrossRef]
- Ives, B. On kink kinetics in crystal dissolution. J. Phys. Chem. Solids 1963, 24, 275–281. [Google Scholar] [CrossRef]
- Jayant, I.; Michael, B.; Dattatray, M.; Amrit, P. Role of Crystal Disorder and Mechanoactivation in Solid-State Stability of Pharmaceuticals. J. Pharm. Sci. 2023, 112, 1539–1565. [Google Scholar]
- Keisuke, U.; Lynne, S.T. Polymer Type Impacts Amorphous Solubility and Drug-Rich Phase Colloidal Stability: A Mechanistic Study Using Nuclear Magnetic Resonance SpectroscopyMol. Pharmaceutics 2020, 17, 1352–1362. [Google Scholar]
- Burt, H.M.; Mitchell, A.G. Crystal defects and dissolution. Int. J. Pharm. 1981, 9, 137–152. [Google Scholar] [CrossRef]
- Zhu, G.; Legg, B.; Sassi, M.; Liang, X.; Zong, M.; Rosso, K.M.; De Yoreo, J.J. Crystal dissolution by particle detachment. Nat. Commun. 2023, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Chiradeep, G.; Mahadev, S. Dynamic strain aging during wire drawing and its effect on electrochemical behaviour. Ironmak. Steelmak. 2017, 44, 789–795. [Google Scholar]
- Shen, F.; Xiong, X.; Fu, J.; Yang, J.; Qiu, M.; Qi, X.; Tsang, D.C. Recent advances in mecha-nochemical production of chemicals and carbon materials from sustainable biomass resources. Renew. Sustain. Energy Rev. 2020, 130, 109944. [Google Scholar] [CrossRef]
- Kostewicz, E.S.; Vertzoni, M.H.; Benson, A.E.; Michael, S.R. Oral Drug Delivery for Modified Release Formulations Chapter 24 Regulatory Assessment; Wiley: New York, NY, USA, 2022; p. 496. [Google Scholar]
- Hermans, A.; Milsmann, J.; Li, H. Challenges and Strategies for Solubility Measurements and Dissolution Method Development for Amorphous Solid Dispersion Formulations. AAPS J. 2023, 25, 11. [Google Scholar] [CrossRef] [PubMed]
- Silaeva, S.Y.; Belenova, A.S.; Slivkin, A.I.; Chupandina, E.E.; Naryshkin, S.R.; Krasnyuk, I.I., Jr.; Krasnyuk, I.I. Use of Solid Dispersion Systems in Pharmacy. Condens. Matter 2020, 22, 173–181. [Google Scholar]
- Qin, S.; Fang, L.; Stacy, Y.; Yanan, W.; Junbo, X. Physical stability of amorphous pharmaceutical solids: Nucleation, crystal growth, phase separation and effects of the polymers. Int. J. Pharm. 2020, 590, 119925. [Google Scholar]
- Johari, G.P.; Ram, S.; Astl, G.; Mayer, E. Characterizing amorphous and microcrystalline solids by calorimetry. J. Non-Cryst. Solids 1990, 116, 282–285. [Google Scholar] [CrossRef]








| Absorption *, % per os | Time, h for Cmax in Plasma | Volume of Distribution, L/kg | Bound to Plasma Proteins, % | Half-Life, h | Metabolism, % in the Urine | Reaction Type, the Major Compound | Toxicity **, per os in Rats, mg/kg |
|---|---|---|---|---|---|---|---|
| 100 | 0.5–4 | 0.6 | 15 | 13 | 95 | O-demethylation | 253 |
| Molecular Weight | Chemical Formula | Water Solubility, mg/mL * | log Po/w | pKa | pKBH+ | T, melting (°C) | T, Boiling (°C) |
|---|---|---|---|---|---|---|---|
| 250.3 | C13H18N2O3 | 0.465 * | 0.728 ** | 12.5 * | −2 * | 140–146 ** | 536.5 ** |
| Frequency Range, cm−1 | Group | Compound Class | Appearance/ Comments |
|---|---|---|---|
| 3650–3200 | O-H stretching | alcohol | strong, broad/ intermolecular bonded |
| 3350–3310 | N-H stretching | secondary amine | - |
| 3300–2500 | O-H stretching | carboxylic acid | strong/usually centred on 3000 cm−1 |
| 3100–3000 | C-H stretching | alkene | medium |
| 2250–1800 | amino acid residues, CO2 | ||
| 1698 | C=O stretching | secondary amide | strong/free associated |
| 1690–1640 | C=N stretching | imine (tautomer) | medium |
| 1650–1580 | N-H bending | amine | strong |
| 1465 | C-H bending | alkane/methylene group | medium |
| 1450–1375 | C-H bending | alkane/methyl group | medium |
| 1420–1330 | O-H bending | alcohol (tautomer) | medium |
| 1250–1020 | C-N stretching | amine | medium |
| 1210–1163 | C-O stretching | ester | strong |
| 750 ± 20 700 ± 20 | C-H bending | monosubstituted benzene derivative | strong |
| Mechanical Stress Time, Min | Dissolution Time, s | Dissolution Rate Constant, k·102, s−1 ± SD |
|---|---|---|
| 0 | 200 | 3.80 ± 0.001 |
| 30 | 60 | 8.40 ± 0.001 |
| 90 | 30 | 9.10 ± 0.017 |
| Time, min | Lacosamide (Unloaded) tML = 0 min | Lacosamide (Loaded) tML = 90 min | ||
|---|---|---|---|---|
| C, % | RSD, % | C, % | RSD, % | |
| 5 | 73.0 | 1.1 | 71.2 | 5.5 |
| 10 | 85.2 | 0.2 | 91.9 | 2.2 |
| 15 | 93.3 | 1.6 | 95.0 | 1.4 |
| 20 | 94.7 | 2.0 | 95.9 | 0.5 |
| 30 | 96.3 | 1.3 | 95.5 | 0.4 |
| 40 | 96.7 | 1.6 | 96.3 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uspenskaya, E.V.; Kuzmina, E.; Quynh, H.T.N.; Komkova, M.A.; Kazimova, I.V.; Timofeev, A.A. Influence of Mechanical Loading on the Process of Tribochemical Action on Physicochemical and Biopharmaceutical Properties of Substances, Using Lacosamide as an Example: From Micronisation to Mechanical Activation. Pharmaceutics 2024, 16, 798. https://doi.org/10.3390/pharmaceutics16060798
Uspenskaya EV, Kuzmina E, Quynh HTN, Komkova MA, Kazimova IV, Timofeev AA. Influence of Mechanical Loading on the Process of Tribochemical Action on Physicochemical and Biopharmaceutical Properties of Substances, Using Lacosamide as an Example: From Micronisation to Mechanical Activation. Pharmaceutics. 2024; 16(6):798. https://doi.org/10.3390/pharmaceutics16060798
Chicago/Turabian StyleUspenskaya, Elena V., Ekaterina Kuzmina, Hoang Thi Ngoc Quynh, Maria A. Komkova, Ilaha V. Kazimova, and Aleksey A. Timofeev. 2024. "Influence of Mechanical Loading on the Process of Tribochemical Action on Physicochemical and Biopharmaceutical Properties of Substances, Using Lacosamide as an Example: From Micronisation to Mechanical Activation" Pharmaceutics 16, no. 6: 798. https://doi.org/10.3390/pharmaceutics16060798
APA StyleUspenskaya, E. V., Kuzmina, E., Quynh, H. T. N., Komkova, M. A., Kazimova, I. V., & Timofeev, A. A. (2024). Influence of Mechanical Loading on the Process of Tribochemical Action on Physicochemical and Biopharmaceutical Properties of Substances, Using Lacosamide as an Example: From Micronisation to Mechanical Activation. Pharmaceutics, 16(6), 798. https://doi.org/10.3390/pharmaceutics16060798







