Benznidazole-Loaded Polymeric Nanoparticles for Oral Chemotherapeutic Treatment of Chagas Disease
Abstract
:1. Introduction
2. Experimental Section
2.1. Development of Particulate Systems Containing Benznidazole
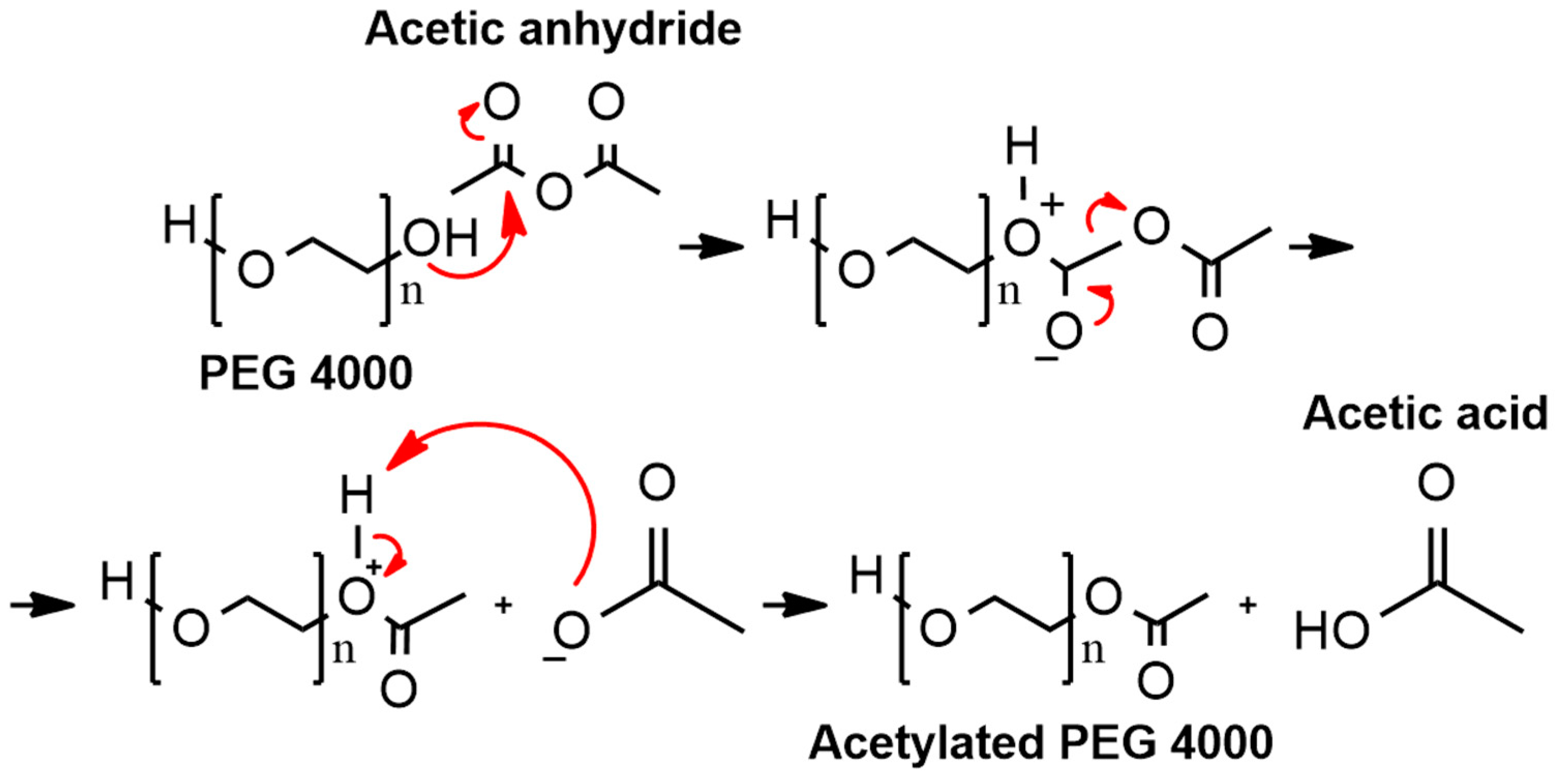
2.1.1. Synthesis of Acetylated PEG 4000
2.1.2. Preparation of Particulate Systems by Double Emulsification and Freeze Drying
2.2. Physicochemical Characterization
2.3. Morphological Characterization
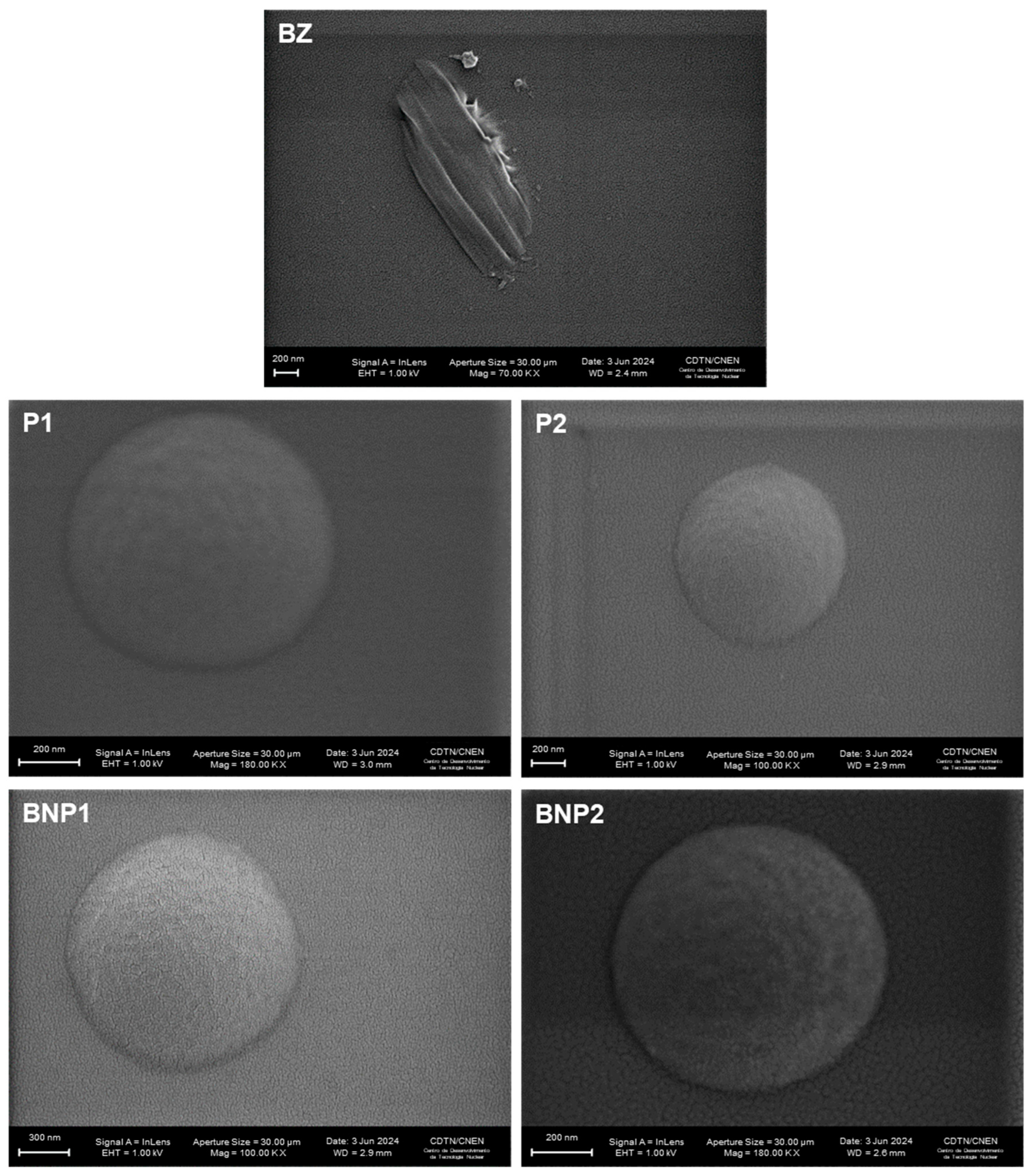
2.3.1. Scanning Electron Microscopy (SEM)
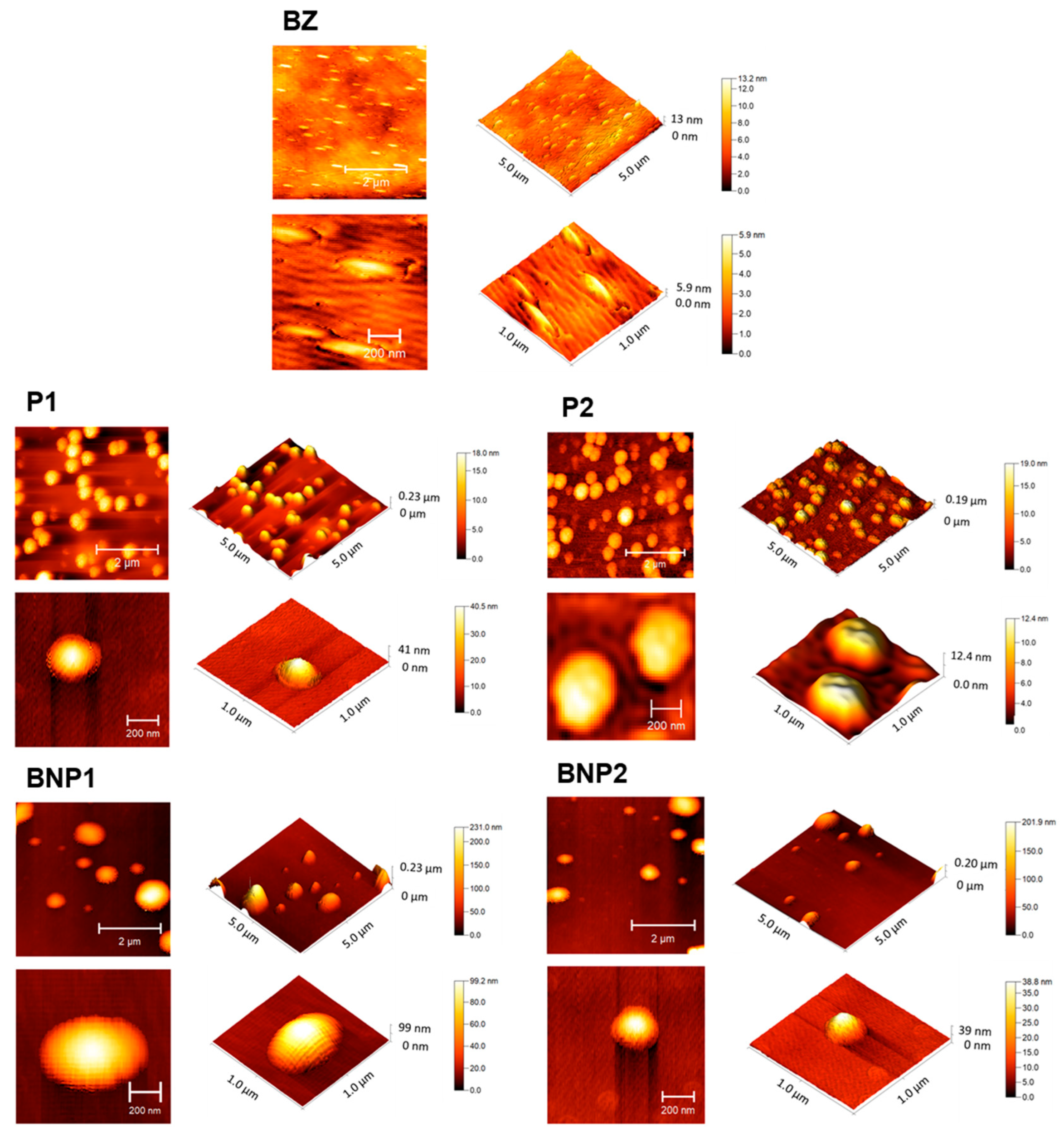
2.3.2. Atomic Force Microscopy (AFM)
2.4. Total Benznidazole Content and Encapsulation Efficiency
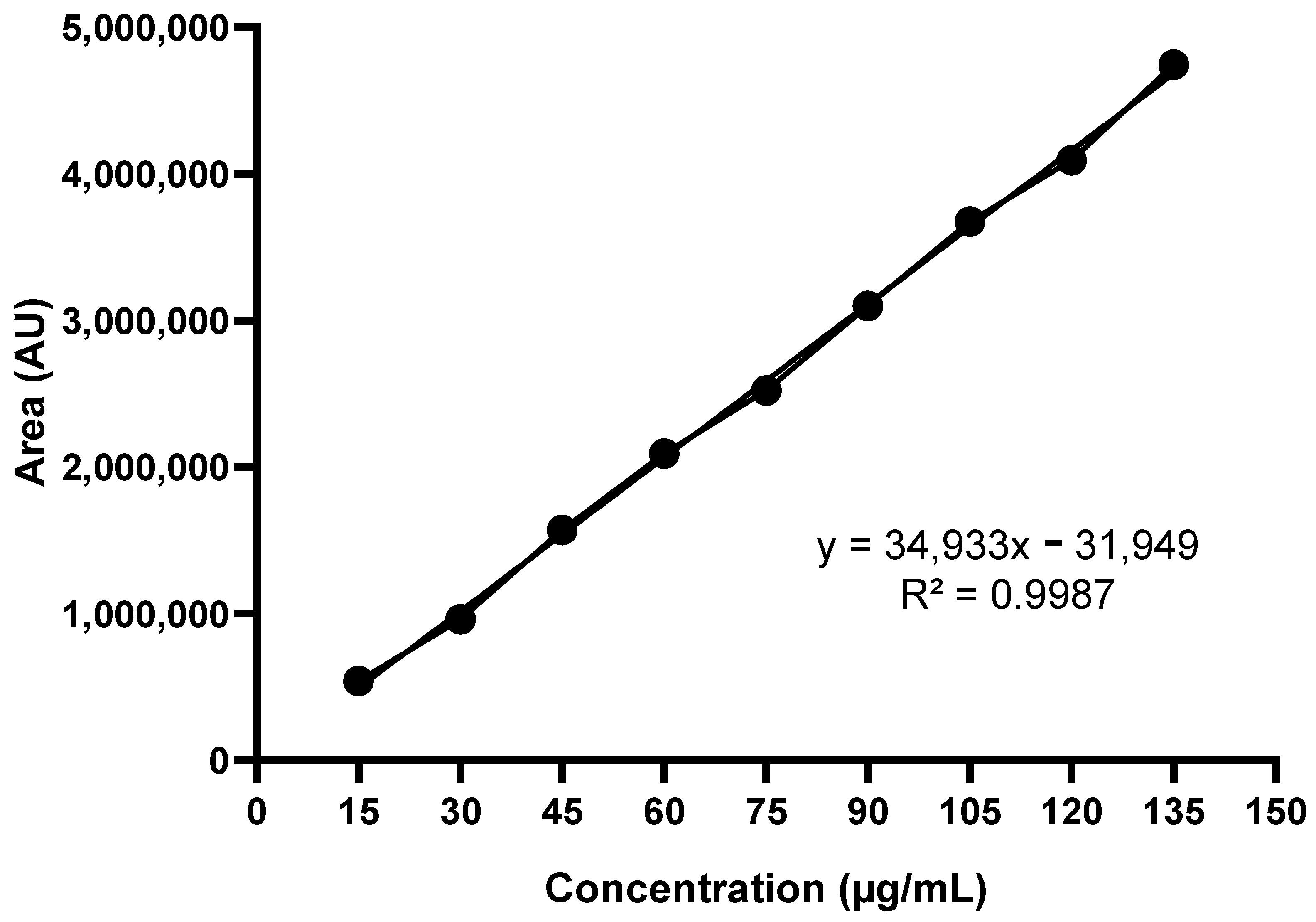
2.4.1. Standard Analytical Curve
2.4.2. Total Benznidazole Content
2.4.3. Encapsulation Efficiency
2.5. Cell Viability Using H9C2 Cardiomyocytes and RAW264.7 Macrophages
2.6. In Vitro Trypanocidal Activity against Amastigote Forms of the Y Strain of T. cruzi
2.7. Nitric Oxide Measurement in RAW 264.7 Macrophage Supernatants
2.8. Statistical Analyses
3. Results and Discussion
3.1. Obtainment and Percentage Yield of Acetylated PEG 4000 and of the Formulations
3.2. Particle Diameter and Polydispersity Index
3.3. Zeta Potential
3.4. Scanning Electron Microscopy (SEM)
3.5. Atomic Force Microscopy (AFM)
3.6. Total BZ Content and Encapsulation Efficiency
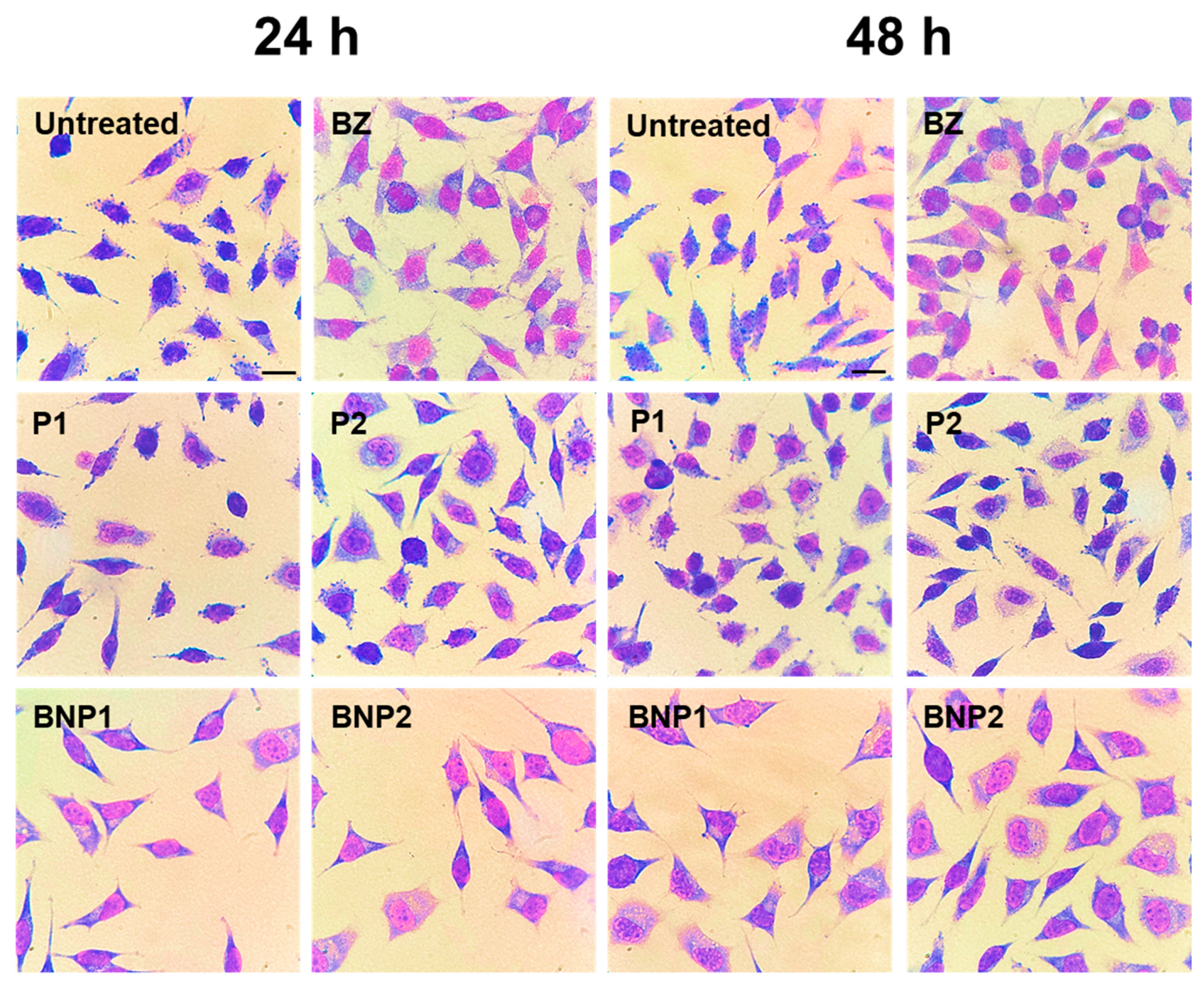
3.7. Cell Viability Assay
3.8. In Vitro Trypanocidal Assay
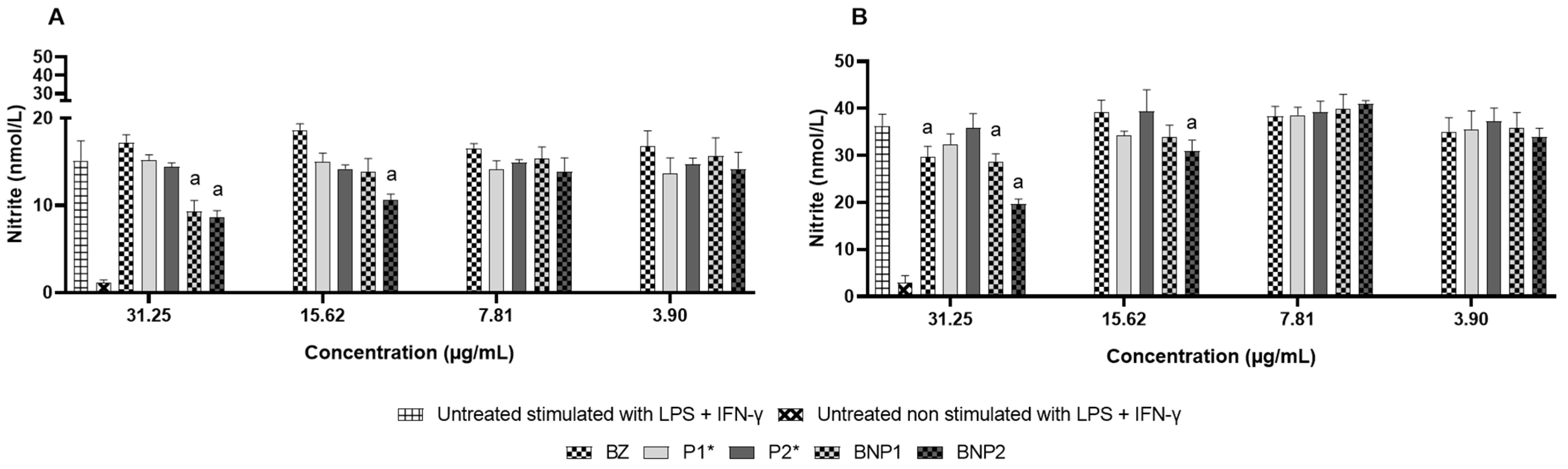
3.9. Nitric Oxide Measurement
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| FBS | Fetal bovine serum |
| BZ | Benznidazole |
| BNP1 | PEG 4000, PCL and PVA nanospheres-loaded with BZ |
| BNP2 | Acetylated PEG 4000, PCL and PVA nanospheres-loaded with BZ |
| CD | Chagas disease |
| DMSO | Dimethyl sulfoxide |
| EE | Encapsulation efficiency |
| HPLC | High performance liquid chromatography |
| IFN-γ | Interferon-gamma |
| ISO | International Organization for Standardization |
| LPS | Lipopolysaccharide |
| MTT | 3-(4,5-Dimethyl-2-thiazolyl)-2,5-diphenyl-2H-tetrazolium bromide |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| P1 | PEG 4000, PCL and PVA nanospheres |
| P2 | Acetylated PEG 4000, PCL and PVA nanospheres |
| PDI | Polydispersity index |
| PCL | Polycaprolactone |
| PEG | Polyethylene glycol |
| PVA | Polyvinyl alcohol |
| RPMI-1640 | Roswell Park Memorial Institute 1640 medium |
| SEM | Scanning electron microscopy |
| AFM | Atomic force microscopy |
References
- American Public Health Association. Factsheet: Chagas Disease in the Americas for Public Health Workers; American Public Health Association: Washington, DC, USA, 2022.
- World Health Organization. Sustaining the Drive to Overcome the Global Impact of Neglected Tropical Diseases: Second WHO Report on Neglected Diseases; World Health Organization: Geneva, Switzerland, 2013.
- World Health Organization. Menos de 10% das Pessoas com Doença de Chagas Recebem um Diagnóstico; World Health Organization: Geneva, Switzerland, 2023.
- Coura, J.R.; Borges-Pereira, J. Chagas disease: 100 years after its discovery. A systemic review. Acta Trop. 2010, 115, 5–13. [Google Scholar] [CrossRef]
- Rodriques Coura, J.; de Castro, S.L. A critical review on Chagas disease chemotherapy. Mem. Inst. Oswaldo Cruz 2002, 97, 3–24. [Google Scholar] [CrossRef]
- Pereiro, A.C. Guidelines for the diagnosis and treatment of Chagas disease. Lancet 2019, 393, 1486–1487. [Google Scholar] [CrossRef]
- Pérez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef]
- Lascano, F.; García Bournissen, F.; Altcheh, J. Review of pharmacological options for the treatment of Chagas disease. Br. J. Clin. Pharmacol. 2022, 88, 383–402. [Google Scholar] [CrossRef] [PubMed]
- Filardi, L.S.; Brener, Z. Susceptibility and natural resistance of Trypanosoma cruzi strains to drugs used clinically in Chagas disease. Trans. R. Soc. Trop. Med. Hyg. 1987, 81, 755–759. [Google Scholar] [CrossRef]
- Perin, L.; Moreira da Silva, R.; Fonseca, K.D.; Cardoso, J.M.; Mathias, F.A.; Reis, L.E.; Molina, I.; Correa-Oliveira, R.; De Abreu Vieira, P.M.; Carneiro, C.M. Pharmacokinetics and Tissue Distribution of Benznidazole after Oral Administration in Mice. Antimicrob. Agents Chemother. 2017, 61, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- De Moura Ferraz, L.R.; Tabosa, A.G.; da Silva Nascimento, D.D.S.; Ferreira, A.S.; de Albuquerque Wanderley Sales, V.; Silva, J.Y.R.; Alves Júnior, S.; Rolim, L.A.; De Souza Pereira, J.J.; Rolim-Neto, P.J. ZIF-8 as a promising drug delivery system for benznidazole: Development, characterization, in vitro dialysis release and cytotoxicity. Sci. Rep. 2020, 10, 16815. [Google Scholar] [CrossRef] [PubMed]
- Sousa, L.R.D.; Azevedo, M.L.S.; Rocha, D.F.; Andrade, A.L.; Amparo, T.R.; dos Santos, O.D.H.; Seibert, J.B.; Pereira, L.R.; De Abreu Vieira, P.M.; Carneiro, C.M.; et al. Trypanocidal Activity and Increased Solubility of Benznidazole Incorporated in PEG 4000 and Its Derivatives. J. Braz. Chem. Soc. 2021, 32, 1162–1172. [Google Scholar] [CrossRef]
- Perin, L.; Pinto, L.; Nardotto, G.H.B.; Fonseca, K.D.S.; Paiva, B.O.; Mendes, T.F.R.B.; Molina, I.; Correa-Oliveira, R.; Vieira, P.M.D.A.; Carneiro, C.M. Population pharmacokinetics and biodistribution of benznidazole in mice. J. Antimicrob. Chemother. 2020, 75, 2213–2221. [Google Scholar] [CrossRef]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in Oral Drug Delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Ingersoll, K.S.; Cohen, J. The impact of medication regimen factors on adherence to chronic treatment: A review of literature. J. Behav. Med. 2008, 31, 213–224. [Google Scholar] [CrossRef] [PubMed]
- Pokharkar, V.; Sajith, M.; Vallet, T.; Akshantal, S.; Shah, R.; Ruiz, F.; Salunke, S. Acceptability of different oral dosage forms in paediatric patients in hospital setting. Arch. Dis. Child. 2022, 107, 796–801. [Google Scholar] [CrossRef] [PubMed]
- Date, T.; Paul, K.; Singh, N.; Jain, S. Drug-Lipid Conjugates for Enhanced Oral Drug Delivery. AAPS PharmSciTech 2019, 20, 41. [Google Scholar] [CrossRef] [PubMed]
- Harikumar, R.S.L.; Aggarwal, G. Formulation Tactics for the Delivery of Poorly Soluble Drugs. Int. J. Pharmtech Res. 2012, 4, 914–923. [Google Scholar]
- Hartig, S.M.; Greene, R.R.; Dikov, M.M.; Prokop, A.; Davidson, J.M. Multifunctional nanoparticulate polyelectrolyte complexes. Pharm. Res. 2007, 24, 2353–2369. [Google Scholar] [CrossRef] [PubMed]
- McCall, R.L.; Sirianni, R.W. PLGA nanoparticles formed by single-or double-emulsion with vitamin E-TPGS. JoVE J. Vis. Exp. 2013, 82, e51015. [Google Scholar]
- Fruijtier-Pölloth, C. Safety assessment on polyethylene glycols (PEGs) and their derivatives as used in cosmetic products. Toxicology 2005, 214, 1–38. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.C.; Oh, J.T.; Jang, M.H.; Chung, S.I. Quantitative analysis of polyvinyl alcohol on the surface of poly(D,L-lactide-co-glycolide) microparticles prepared by solvent evaporation method: Effect of particle size and PVA concentration. J. Control. Release 1999, 59, 123–132. [Google Scholar] [CrossRef]
- Hoang Thi, T.T.; Pilkington, E.H.; Nguyen, D.H.; Lee, J.S.; Park, K.D.; Truong, N.P. The importance of poly (ethylene glycol) alternatives for overcoming PEG immunogenicity in drug delivery and bioconjugation. Polymers 2020, 12, 298. [Google Scholar] [CrossRef]
- Knop, K.; Hoogenboom, R.; Fischer, D.; Schubert, U.S. Poly(ethylene glycol) in drug delivery: Pros and cons as well as potential alternatives. Angew. Chem. Int. Ed. 2010, 49, 6288–6308. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Cai, X.; Zhang, J.; Xu, C. Particle-size measurements in a micro-channel with image dynamic light scattering method. Procedia Eng. 2015, 102, 904–910. [Google Scholar] [CrossRef]
- Sinha, V.R.; Bansal, K.; Kaushik, R.; Kumria, R.; Trehan, A. Poly-ϵ-caprolactone microspheres and nanospheres: An overview. Int. J. Pharm. 2004, 278, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhao, L.; Qi, L.; Li, Z.; Luan, Y. Polymer assembly: Promising carriers as co-delivery systems for cancer therapy. Prog. Polym. Sci. 2016, 58, 1–26. [Google Scholar] [CrossRef]
- Gagliardi, A.; Giuliano, E.; Venkateswararao, E.; Fresta, M.; Bulotta, S.; Awasthi, V.; Cosco, D. Biodegradable polymeric nanoparticles for drug delivery to solid tumors. Front. Pharmacol. 2021, 12, 601626. [Google Scholar] [CrossRef] [PubMed]
- Blatsios, G.; Tzimas, A.S.; Mattheolabakis, G.; Panagi, Z.; Avgoustakis, K.; Gartaganis, S.P. Development of biodegradable controlled release scleral systems of triamcinolone acetonide. Curr. Eye Res. 2010, 35, 916–924. [Google Scholar] [CrossRef] [PubMed]
- Abou-ElNour, M.; Ishak, R.A.H.; Tiboni, M.; Bonacucina, G.; Cespi, M.; Casettari, L.; Soliman, M.E.; Geneidi, A.S. Triamcinolone acetonide-loaded PLA/PEG-PDL microparticles for effective intra-articular delivery: Synthesis, optimization, in vitro and in vivo evaluation. J. Control. Release 2019, 309, 125–144. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.; Kristensen, K. Evaluation of methods for sizing of colloidal radiopharmaceuticals. Eur. J. Nucl. Med. 1981, 6, 521–526. [Google Scholar] [CrossRef]
- Yajing, W.; Jin, S.; Tianze, L.; Zhenhai, D.; Shanshan, G. Photon correlation spectroscopy of the small amount of data based on auto-regressive power spectrum. Opt. Commun. 2014, 325, 71–77. [Google Scholar] [CrossRef]
- Felix, C.; Jao, T.; Pasupathi, S.; Pollet, B.G. Optimisation of electrophoretic deposition parameters for gas diffusion electrodes in high temperature polymer electrolyte membrane fuel cells. J. Power Sources 2013, 243, 40–47. [Google Scholar] [CrossRef]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Green, L.C.; Wagner, D.A.; Glogowski, J.; Skipper, P.L.; Wishnok, J.S.; Tannenbaum, S.R. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal. Biochem. 1982, 126, 131–138. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wu, F.; Liao, C.; Gu, Z.; Zhang, S. Terminal acetylated/acrylated poly(ethylene glycol) fabricated drug carriers: Design, synthesis, and biological evaluation. Biomacromolecules 2017, 18, 1956–1964. [Google Scholar] [CrossRef] [PubMed]
- Bilati, U.; Allémann, E.; Doelker, E. Sonication parameters for the preparation of biodegradable nanocapsules of controlled size by the double emulsion method. Pharm. Dev. Technol. 2003, 8, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Zambaux, M.F.; Bonneaux, F.; Gref, R.; Maincent, P.; Dellacherie, E.; Alonso, M.J.; Labrude, P.; Vigneron, C. Influence of experimental parameters on the characteristics of poly(lactic acid) nanoparticles prepared by a double emulsion method. J. Control. Release 1998, 50, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Manchanda, R.; Fernandez-Fernandez, A.; Nagesetti, A.; McGoron, A.J. Preparation and characterization of a polymeric (PLGA) nanoparticulate drug delivery system with simultaneous incorporation of chemotherapeutic and thermo-optical agents. Colloids Surf. B. Biointerfaces 2010, 75, 260–267. [Google Scholar] [CrossRef] [PubMed]
- Schaffazick, S.R.; Guterres, S.S.; Freitas, L.L.; Pohlmann, A.R. Caracterização e estabilidade físico-química de sistemas poliméricos nanoparticulados para administração de fármacos. Quim. Nova 2003, 26, 726–737. [Google Scholar] [CrossRef]
- Schärtl, W. Light Scattering from Polymer Solutions and Nanoparticle Dispersions; Springer Science Business Media: Berlin/Heidelberg, Germany, 2007. [Google Scholar]
- Franceschinis, E.; Roverso, M.; Gabbia, D.; De Martin, S.; Brusegan, M.; Vaccarin, C.; Bogialli, S.; Chilin, A. Self-Emulsifying Formulations to Increase the Oral Bioavailability of 4,6,4’-Trimethylangelicin as a Possible Treatment for Cystic Fibrosis. Pharmaceutics 2022, 14, 1806. [Google Scholar] [CrossRef]
- Da Silva Gündel, S.; de Souza, M.E.; Quatrin, P.M.; Klein, B.; Wagner, R.; Gündel, A.; de Almeida Vaucher, R.; Santos, R.C.V.; Ourique, A.F. Nanoemulsions containing Cymbopogon flexuosus essential oil: Development, characterization, stability study and evaluation of antimicrobial and antibiofilm activities. Microb. Pathog. 2018, 118, 268–276. [Google Scholar] [CrossRef]
- Jawahar, N.; Meyyanathan, S.N.; Reddy, G.; Sood, S. Solid lipid Nanoparticles for Oral delivery of Poorly Soluble Drugs. J. Pharm. Sci. Res. 2012, 4, 1848–1855. [Google Scholar] [CrossRef]
- Wang, J.J.; Sung, K.C.; Hu, O.Y.P.; Yeh, C.H.; Fang, J.Y. Submicron lipid emulsion as a drug delivery system for nalbuphine and its prodrugs. J. Control. Release 2006, 115, 140–149. [Google Scholar] [CrossRef] [PubMed]
- Date, P.V.; Samad, A.; Devarajan, P.V. Freeze thaw: A simple approach for prediction of optimal cryoprotectant for freeze drying. Aaps Pharmscitech 2010, 11, 304–313. [Google Scholar] [CrossRef]
- Dos Santos-Silva, A.M.; de Caland, L.B.; de S L Oliveira, A.L.C.; de Araújo-Júnior, R.F.; Fernandes-Pedrosa, M.F.; Cornélio, A.M.; Da Silva-Júnior, A.A. Designing structural features of novel benznidazole-loaded cationic nanoparticles for inducing slow drug release and improvement of biological efficacy. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 978–987. [Google Scholar] [CrossRef] [PubMed]
- Streck, L.; Sarmento, V.H.; Machado, P.R.; Farias, K.J.; Fernandes-Pedrosa, M.F.; da Silva-Júnior, A.A. Phase Transitions of Isotropic to Anisotropic Biocompatible Lipid-Based Drug Delivery Systems Overcoming Insoluble Benznidazole Loading. Int. J. Mol. Sci. 2016, 17, 981. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahed, W.; Degobert, G.; Fessi, H. A pilot study of freeze drying of poly(epsilon-caprolactone) nanocapsules stabilized by poly(vinyl alcohol): Formulation and process optimization. Int. J. Pharm. 2006, 309, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Konan, Y.N.; Gurny, R.; Allémann, E. Preparation and characterization of sterile and freeze-dried sub-200 nm nanoparticles. Int. J. Pharm. 2002, 233, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, K.S.; Soliman, M.E.; Casettari, L.; Bonacucina, G.; Cespi, M.; Palmieri, G.F.; Sammour, O.A.; El Shamy, A.A. Determination of factors controlling the particle size and entrapment efficiency of noscapine in PEG/PLA nanoparticles using artificial neural networks. Int. J. Nanomed. 2014, 9, 4953–4964. [Google Scholar] [CrossRef] [PubMed]
- Behl, A.; Solanki, S.; Paswan, S.K.; Datta, T.K.; Saini, A.K.; Saini, R.V.; Parmar, V.S.; Thakur, V.K.; Malhotra, S.; Chhillar, A.K. Biodegradable PEG-PCL Nanoparticles for Co-delivery of MUC1 Inhibitor and Doxorubicin for the Confinement of Triple-Negative Breast Cancer. J. Polym. Environ. 2023, 31, 999–1018. [Google Scholar] [CrossRef] [PubMed]
- Surender, V.; Deepika, M. Solid lipid nanoparticles: A comprehensive review. J. Chem. Pharm. Res. 2016, 8, 102–114. [Google Scholar]
- Capek, I. Sterically and electrosterically stabilized emulsion polymerization. Kinetics and preparation. Adv. Colloid Interface Sci. 2002, 99, 77–162. [Google Scholar] [CrossRef]
- Forgiarini, A.; Esquena, J.; González, C.; Solans, C. Formation of Nano-emulsions by Low-Energy Emulsification Methods at Constant Temperature. Langmuir 2001, 17, 2076–2083. [Google Scholar] [CrossRef]
- Guo, F.; Guo, D.; Zhang, W.; Yan, Q.; Yang, Y.; Hong, W.; Yang, G. Preparation of curcumin-loaded PCL-PEG-PCL triblock copolymeric nanoparticles by a microchannel technology. Eur. J. Pharm. Sci. 2017, 99, 328–336. [Google Scholar] [CrossRef] [PubMed]
- Palmeiro-Roldán, R.; Fonseca-Berzal, C.; Gómez-Barrio, A.; Arán, V.J.; Escario, J.A.; Torrado-Durán, S.; Torrado-Durán, S. Development of novel benznidazole formulations: Physicochemical characterization and in vivo evaluation on parasitemia reduction in Chagas disease. Int. J. Pharm. 2014, 472, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, D.; Bombardiere, M.E.; Salomon, C.J. Effects of benznidazole:cyclodextrin complexes on the drug bioavailability upon oral administration to rats. Int. J. Biol. Macromol. 2013, 62, 543–548. [Google Scholar] [CrossRef] [PubMed]
- de Souza, A.; Castro-Borges, W.; de Andrade, M.H.G.; de Paiva Maia, Y.C.; Goulart, L.R.; Lanna, E.G.; de Brito, A.C.F.; Barboza, A.P.M.; Mosqueira, V.C.F.; Rubio, K.T.S. PLA-PEG nanospheres decorated with phage display selected peptides as biomarkers for detection of human colorectal adenocarcinoma. Drug. Deliv. Transl. Res. 2020, 10, 1771–1787. [Google Scholar] [CrossRef] [PubMed]
- Abriata, J.P.; Eloy, J.O.; Riul, T.B.; Campos, P.M.; Baruffi, M.D.; Marchetti, J.M. Poly-epsilon-caprolactone nanoparticles enhance ursolic acid in vivo efficacy against Trypanosoma cruzi infection. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 77, 1196–1203. [Google Scholar] [CrossRef] [PubMed]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-loaded PLGA nanoparticles: Systematic study of particle size and drug content. Int. J. Pharm. 2007, 336, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Lee, J.; Choi, S.; Jang, Y.; Kim, J. Preparation of PLGA nanoparticles containing estrogen by emulsification–diffusion method. Colloids Surf. A Physicochem. Eng. Asp. 2001, 182, 123–130. [Google Scholar] [CrossRef]
- Mobarak, D.H.; Salah, S.; Elkheshen, S.A. Formulation of ciprofloxacin hydrochloride loaded biodegradable nanoparticles: Optimization of technique and process variables. Pharm. Dev. Technol. 2014, 19, 891–900. [Google Scholar] [CrossRef]
- Oberdörster, G.; Maynard, A.; Donaldson, K.; Castranova, V.; Fitzpatrick, J.; Ausman, K.; Carter, J.; Karn, B.; Kreyling, W.; Lai, D.; et al. Principles for characterizing the potential human health effects from exposure to nanomaterials: Elements of a screening strategy. Part. Fibre Toxicol. 2005, 2, 8. [Google Scholar] [CrossRef]
- Sousa, L.R.D.; Amparo, T.R.; Souza, G.H.B.; Ferraz, A.T.; Fonseca, K.D.S.; Azevedo, A.S.; Nascimento, A.M.D.; Andrade, L.; Seibert, J.B.; Valverde, T.M.; et al. Anti-Trypanosoma cruzi Potential of Vestitol Isolated from Lyophilized Red Propolis. Molecules 2023, 28, 7812. [Google Scholar] [CrossRef] [PubMed]
- ISO 10993-5; Biological Evaluation of Medical Devices-Part 5: Test dor In Vitro Cytotoxicity. International Standard Organization: Geneva, Switzerland, 2009; pp. 1–11.
- Hescheler, J.; Meyer, R.; Plant, S.; Krautwurst, D.; Rosenthal, W.; Schultz, G. Morphological, biochemical, and electrophysiological characterization of a clonal cell (H9c2) line from rat heart. Circ. Res. 1991, 69, 1476–1486. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Chapa-Dubocq, X.R.; Tyurina, Y.Y.; Croix, C.M.S.; Kapralov, A.A.; Tyurin, V.A.; Bayır, H.; Kagan, V.E.; Javadov, S. Elucidating the contribution of mitochondrial glutathione to ferroptosis in cardiomyocytes. Redox Biol. 2021, 45, 102021. [Google Scholar] [CrossRef] [PubMed]
- Farani, P.S.G.; Ferreira, B.I.S.; Gibaldi, D.; Lannes-Vieira, J.; Moreira, O.C. Modulation of miR-145-5p and miR-146b-5p levels is linked to reduced parasite load in H9C2 Trypanosoma cruzi infected cardiomyoblasts. Sci. Rep. 2022, 12, 1436. [Google Scholar] [CrossRef] [PubMed]
- Lam, C.W.; Perretti, M.; Getting, S.J. Melanocortin receptor signaling in RAW264.7 macrophage cell line. Peptides 2006, 27, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Kröger, M.; Liu, W.K. Endocytosis of PEGylated nanoparticles accompanied by structural and free energy changes of the grafted polyethylene glycol. Biomaterials 2014, 35, 8467–8478. [Google Scholar] [CrossRef] [PubMed]
- Chattopadhyay, S.; Dash, S.K.; Kar Mahapatra, S.; Tripathy, S.; Ghosh, T.; Das, B.; Das, D.; Pramanik, P.; Roy, S. Chitosan-modified cobalt oxide nanoparticles stimulate TNF-α-mediated apoptosis in human leukemic cells. J. Biol. Inorg. Chem. 2014, 19, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Moghimi, S.M.; Szebeni, J. Stealth liposomes and long circulating nanoparticles: Critical issues in pharmacokinetics, opsonization and protein-binding properties. Prog. Lipid Res. 2003, 42, 463–478. [Google Scholar] [CrossRef]
- Aggarwal, P.; Hall, J.B.; McLeland, C.B.; Dobrovolskaia, M.A.; McNeil, S.E. Nanoparticle interaction with plasma proteins as it relates to particle biodistribution, biocompatibility and therapeutic efficacy. Adv. Drug Deliv. Rev. 2009, 61, 428–437. [Google Scholar] [CrossRef]
- Dobrovolskaia, M.A.; Aggarwal, P.; Hall, J.B.; McNeil, S.E. Preclinical studies to understand nanoparticle interaction with the immune system and its potential effects on nanoparticle biodistribution. Mol. Pharm. 2008, 5, 487–495. [Google Scholar] [CrossRef]
- Ahsan, F.; Rivas, I.P.; Khan, M.A.; Torres Suarez, A.I. Targeting to macrophages: Role of physicochemical properties of particulate carriers—Liposomes and microspheres--on the phagocytosis by macrophages. J. Control. Release 2002, 79, 29–40. [Google Scholar] [CrossRef] [PubMed]
- Thasneem, Y.M.; Sajeesh, S.; Sharma, C.P. Effect of thiol functionalization on the hemo-compatibility of PLGA nanoparticles. J. Biomed. Mater. Res. 2011, 99, 607–617. [Google Scholar] [CrossRef]
- Dhorm Pimentel de Moraes, A.R.; Tavares, G.D.; Soares Rocha, F.J.; de Paula, E.; Giorgio, S. Effects of nanoemulsions prepared with essential oils of copaiba- and andiroba against Leishmania infantum and Leishmania amazonensis infections. Exp. Parasitol. 2018, 187, 12–21. [Google Scholar] [CrossRef]
- Tian, H.; He, Z.; Sun, C.; Yang, C.; Zhao, P.; Liu, L.; Leong, K.W.; Mao, H.Q.; Liu, Z.; Chen, Y. Uniform Core-Shell Nanoparticles with Thiolated Hyaluronic Acid Coating to Enhance Oral Delivery of Insulin. Adv. Healthc. Mater. 2018, 7, 1800285. [Google Scholar] [CrossRef]
- Wong, C.Y.; Luna, G.; Martinez, J.; Al-Salami, H.; Dass, C.R. Bio-nanotechnological advancement of orally administered insulin nanoparticles: Comprehensive review of experimental design for physicochemical characterization. Int. J. Pharm. 2019, 572, 118720. [Google Scholar] [CrossRef]
- Mengarda, A.C.; Iles, B.; Longo, J.P.F.; de Moraes, J. Recent approaches in nanocarrier-based therapies for neglected tropical diseases. Wiley Interdisciplinary Reviews. Nanomed. Nanobiotechnology 2023, 15, e1852. [Google Scholar] [CrossRef]
- Nornoo, A.O.; Zheng, H.; Lopes, L.B.; Johnson-Restrepo, B.; Kannan, K.; Reed, R. Oral microemulsions of paclitaxel: In situ and pharmacokinetic studies. Eur. J. Pharm. Biopharm. 2009, 71, 310–317. [Google Scholar] [CrossRef] [PubMed]
- Mazzeti, A.L.; Gonçalves, K.R.; Boasquívis, P.F.; Barbosa, J.; Pereira, B.G.; Soeiro, M.N.C.; Mosqueira, V.C.F.; Bahia, M.T. Poly-ε-Caprolactone Implants for Benznidazole Prolonged Release: An Alternative to Chagas Disease Oral Treatment. Pharmaceutics 2023, 15, 1126. [Google Scholar] [CrossRef] [PubMed]
- Tessarolo, L.D.; de Menezes, R.R.P.P.; Mello, C.P.; Lima, D.B.; Magalhães, E.P.; Bezerra, E.M.; Sales, F.A.M.; Neto, I.L.B.N.; de Fátima Oliveira, M.; Dos Santos, R.P. Nanoencapsulation of benznidazole in calcium carbonate increases its selectivity to Trypanosoma cruzi. Parasitology 2018, 145, 1191–1198. [Google Scholar] [CrossRef]
- Patel, R.R.; Patel, J.K. Novel Technologies of Oral Controlled Release Drug Delivery. System. Syst. Rev. Pharm. 2010, 1, 128–132. [Google Scholar] [CrossRef]
- Yusuf, A.; Almotairy, A.R.Z.; Henidi, H.; Alshehri, O.Y.; Aldughaim, M.S. Nanoparticles as Drug Delivery Systems: A Review of the Implication of Nanoparticles’ Physicochemical Properties on Responses in Biological Systems. Polymers 2023, 15, 1596. [Google Scholar] [CrossRef] [PubMed]
- Maximiano, F.P.; de Paula, L.M.; Figueiredo, V.P.; de Andrade, I.M.; Talvani, A.; Sá-Barreto, L.C.; Bahia, M.T.; Cunha-Filho Marcílio, S.S. Benznidazole microcrystal preparation by solvent change precipitation and in vivo evaluation in the treatment of Chagas disease. Eur. J. Pharm. Biopharm. 2011, 78, 377–384. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, D.; Salomón, C.J.; Lamas, M.C.; Olivieri, A.C. Development of novel formulations for Chagas’ disease: Optimization of benznidazole chitosan microparticles based on artificial neural networks. Int. J. Pharm. 2009, 367, 140–147. [Google Scholar] [CrossRef] [PubMed]
- Barrera, M.G.; Tejada, G.; Leonardi, D.; Lamas, M.C.; Salomón, C.J. A Novel Prototype Device for Microencapsulation of Benznidazole: In Vitro/In Vivo Studies. AAPS PharmSciTech 2020, 21, 112. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Bhatia, V.; Wen, J.J.; Wu, Y.; Huang, M.H.; Garg, N.J. Trypanosoma cruzi infection disturbs mitochondrial membrane potential and ROS production rate in cardiomyocytes. Free. Radic. Biol. Med. 2009, 47, 1414–1421. [Google Scholar] [CrossRef] [PubMed]
- Revelli, S.; Le Page, C.; Piaggio, E.; Wietzerbin, J.; Bottasso, O. Benznidazole, a drug employed in the treatment of Chagas’ disease, down-regulates the synthesis of nitrite and cytokines by murine stimulated macrophages. Clin. Exp. Immunol. 1999, 118, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Cevey, Á.; Mascolo, P.D.; Penas, F.N.; Pieralisi, A.V.; Sequeyra, A.S.; Mirkin, G.A.; Goren, N.B. Benznidazole Anti-Inflammatory Effects in Murine Cardiomyocytes and Macrophages Are Mediated by Class I PI3Kδ. Front. Immunol. 2021, 12, 782891. [Google Scholar] [CrossRef]
- Vespa, G.N.; Cunha, F.Q.; Silva, J.S. Nitric oxide is involved in control of Trypanosoma cruzi-induced parasitemia and directly kills the parasite in vitro. Infect. Immun. 1994, 62, 5177–5182. [Google Scholar] [CrossRef]
- Lieke, T.; Steeg, C.; Graefe, S.E.; Fleischer, B.; Jacobs, T. Interaction of natural killer cells with Trypanosoma cruzi-infected fibroblasts. Clin. Exp. Immunol. 2006, 145, 357–364. [Google Scholar] [CrossRef]
- Arantes, R.M.; Marche, H.H.; Bahia, M.T.; Cunha, F.Q.; Rossi, M.A.; Silva, J.S. Interferon-gamma-induced nitric oxide causes intrinsic intestinal denervation in Trypanosoma cruzi-infected mice. Am. J. Pathol. 2004, 164, 1361–1368. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, D.A.; Calvi, S.A.; Picka, M.M.; Persi, E.; de Carvalho, T.B.; Caetano, P.K.; Nagoshi, L.R.; Lima, C.R.G.; Machado, J.M.; Salvadori, D. DNA damage and nitric oxide synthesis in experimentally infected Balb/c mice with Trypanosoma cruzi. Exp. Parasitol. 2007, 116, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Hovsepian, E.; Mirkin, G.A.; Penas, F.; Manzano, A.; Bartrons, R.; Goren, N.B. Modulation of inflammatory response and parasitism by 15-Deoxy-Δ(12,14) prostaglandin J(2) in Trypanosoma cruzi-infected cardiomyocytes. Int. J. Parasitol. 2011, 41, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Machado, F.S.; Souto, J.T.; Rossi, M.A.; Esper, L.; Tanowitz, H.B.; Aliberti, J.; Silva, J.S. Nitric oxide synthase-2 modulates chemokine production by Trypanosoma cruzi-infected cardiac myocytes. Microbes Infect. 2008, 10, 1558–1566. [Google Scholar] [CrossRef]
- Gutierrez, F.R.; Guedes, P.M.; Gazzinelli, R.T.; Silva, J.S. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite Immunol. 2009, 31, 673–685. [Google Scholar] [CrossRef]



| Sample | Yield (%) |
|---|---|
| Acetylated PEG 4000 | 94.6 |
| BNP1 | 96.57 ± 0.35 |
| BNP2 | 98.17 ± 0.48 |
| Formulation | Zeta Potential (mV) | |
|---|---|---|
| Pre-Freeze Drying | Post-Freeze Drying | |
| BNP1 | −25.0 ± 4.12 a | −21.4 ± 3.30 |
| BNP2 | −15.1 ± 1.69 | −21.4 ± 4.62 |
| Formulation | Total BZ (%) | EE (%) |
|---|---|---|
| BNP1 | 91.83 ± 0.14 | 69.11 ± 3.46 |
| BNP2 | 95.08 ± 1.04 | 70.83 ± 1.05 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sousa, L.R.D.; Duarte, T.H.C.; Xavier, V.F.; das Mercês, A.C.; Vieira, G.M.; Martins, M.D.; Carneiro, C.M.; dos Santos, V.M.R.; dos Santos, O.D.H.; Vieira, P.M.d.A. Benznidazole-Loaded Polymeric Nanoparticles for Oral Chemotherapeutic Treatment of Chagas Disease. Pharmaceutics 2024, 16, 800. https://doi.org/10.3390/pharmaceutics16060800
Sousa LRD, Duarte THC, Xavier VF, das Mercês AC, Vieira GM, Martins MD, Carneiro CM, dos Santos VMR, dos Santos ODH, Vieira PMdA. Benznidazole-Loaded Polymeric Nanoparticles for Oral Chemotherapeutic Treatment of Chagas Disease. Pharmaceutics. 2024; 16(6):800. https://doi.org/10.3390/pharmaceutics16060800
Chicago/Turabian StyleSousa, Lucas Resende Dutra, Thays Helena Chaves Duarte, Viviane Flores Xavier, Aline Coelho das Mercês, Gabriel Maia Vieira, Maximiliano Delany Martins, Cláudia Martins Carneiro, Viviane Martins Rebello dos Santos, Orlando David Henrique dos Santos, and Paula Melo de Abreu Vieira. 2024. "Benznidazole-Loaded Polymeric Nanoparticles for Oral Chemotherapeutic Treatment of Chagas Disease" Pharmaceutics 16, no. 6: 800. https://doi.org/10.3390/pharmaceutics16060800







