Recent Progress of Multifunctional Molecular Probes for Triple-Negative Breast Cancer Theranostics
Abstract
:1. Introduction
2. Design of Multifunctional Molecular Probes
2.1. Targeting Groups for Multifunctional Molecular Probes
2.2. Carriers of Multifunctional Molecular Probes
2.3. Imaging Modalities for Multifunctional Molecular Probes
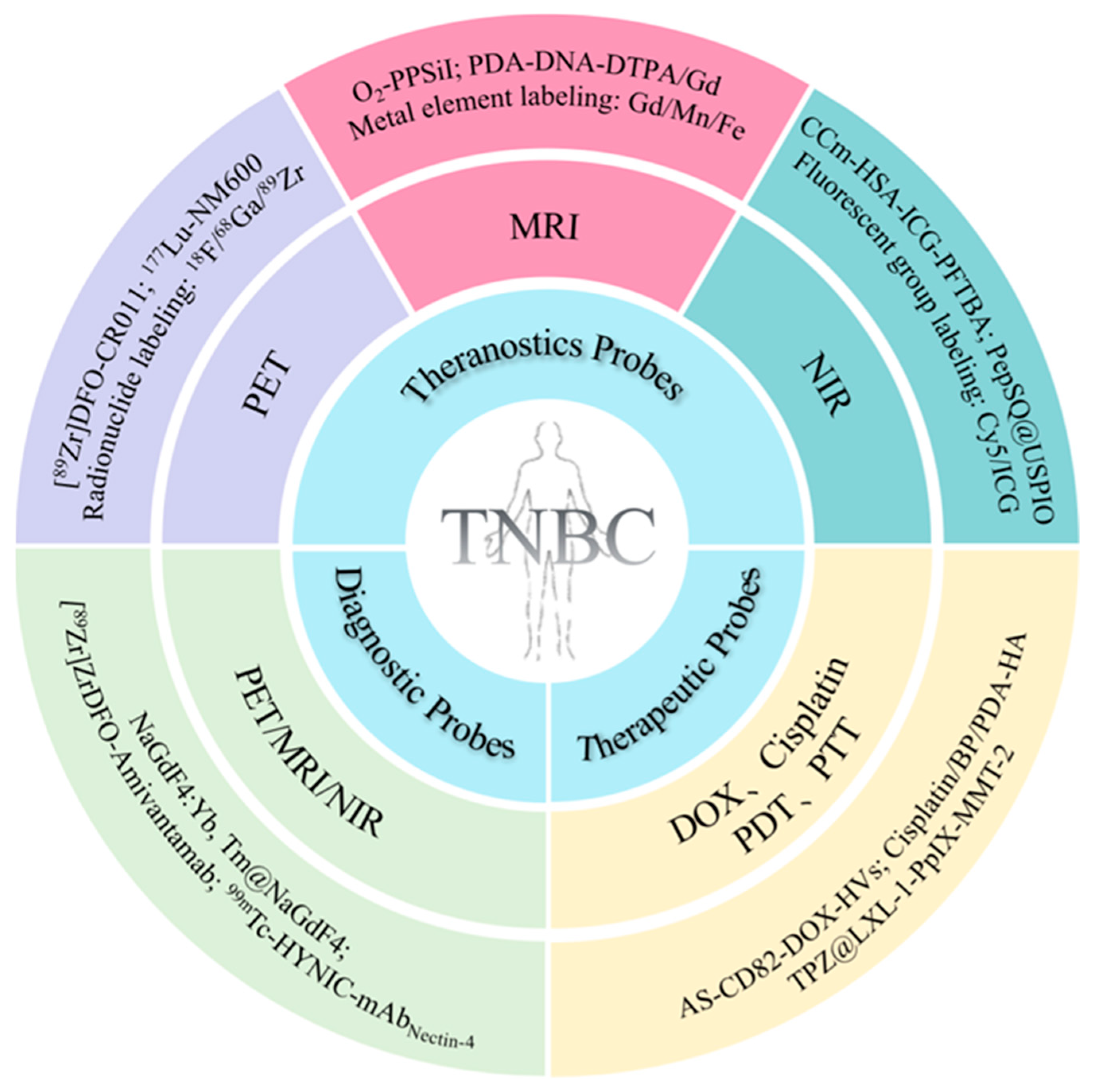
3. Application of Multifunctional Molecular Probes in TNBC
3.1. MMPs for the Targeted Diagnosis of TNBC
3.2. MMPs for the Targeted Therapy of TNBC
3.3. MMPs for the Theranostics of TNBC
3.3.1. Theranostic Probes Based on MRI
3.3.2. Theranostic Probes Based on NIR Fluorescence Imaging
3.3.3. Theranostic Probes Based on PET
4. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| BP | Black phosphorus |
| DFO | Desferrioxamine |
| DOX | Doxorubicin |
| EGFR | Epidermal growth factor receptor |
| EPR | Enhanced permeability and retention |
| EPS | Epalrestat |
| ER | Estrogen receptor |
| FDA | Food and Drug Administration |
| gpNMB | Glycoprotein non-metastatic B |
| HA | Hyaluronic acid |
| HER2 | Human epidermal growth factor receptor 2 |
| ICAM1 | Intercellular adhesion molecule-1 |
| IHC | Immunohistochemistry |
| LET | Linear energy transfer |
| mAb | Monoclonal antibody |
| MELK | Maternal embryonic leucine zipper kinase |
| MMAE | Monomethyl auristatin E |
| MMP | Multifunctional molecular probe |
| MR | Magnetic resonance |
| MRI | Magnetic resonance imaging |
| Nectin-4 | Nectin cell adhesion molecule 4 |
| NIR | Near-infrared |
| NIRF | Near-infrared fluorescence |
| NPs | Nanoparticles |
| PD-1 | Programmed cell death protein-1 |
| PD-L1 | Programmed death-ligand 1 |
| PDA | Polydopamine |
| PDT | Photodynamic therapy |
| PEG | Polyethylene glycol |
| PET | Positron emission tomography |
| PFH | Perfluorohexane |
| PLGA | Poly (lactic-co-glycolic acid) |
| PpIX | Protoporphyrin IX |
| PR | Progesterone receptor |
| PTT | Photothermal therapy |
| PTX | Paclitaxel |
| RES | Reticuloendothelial system |
| ROS | Reactive oxygen species |
| SPECT | Single photon emission computed tomography |
| TNBC | Triple-negative breast cancer |
| TPZ | Tirapazamine |
| UCL | Upconversion luminescence |
| UCNPs | Upconversion nanoparticles |
| uPA | Urokinase plasminogen activator |
| uPAR | Urokinase plasminogen activator receptor |
References
- Siegel, R.L.; Wagle, N.S.; Cercek, A.; Smith, R.A.; Jemal, A. Colorectal cancer statistics, 2023. CA Cancer J. Clin. 2023, 73, 233–254. [Google Scholar] [CrossRef] [PubMed]
- Denkert, C.; Liedtke, C.; Tutt, A.; von Minckwitz, G. Molecular alterations in triple-negative breast cancer-the road to new treatment strategies. Lancet 2017, 389, 2430–2442. [Google Scholar] [CrossRef] [PubMed]
- Zagami, P.; Carey, L.A. Triple negative breast cancer: Pitfalls and progress. NPJ Breast Cancer 2022, 8, 95. [Google Scholar] [CrossRef]
- Liedtke, C.; Mazouni, C.; Hess, K.R.; André, F.; Tordai, A.; Mejia, J.A.; Symmans, W.F.; Gonzalez-Angulo, A.M.; Hennessy, B.; Green, M.; et al. Response to neoadjuvant therapy and long-term survival in patients with triple-negative breast cancer. J. Clin. Oncol. Off. J. Am. Soc. Clin. Oncol. 2008, 26, 1275–1281. [Google Scholar] [CrossRef] [PubMed]
- Tan, D.S.; Marchió, C.; Jones, R.L.; Savage, K.; Smith, I.E.; Dowsett, M.; Reis-Filho, J.S. Triple negative breast cancer: Molecular profiling and prognostic impact in adjuvant anthracycline-treated patients. Breast Cancer Res. Treat. 2008, 111, 27–44. [Google Scholar] [CrossRef]
- Ruffell, B.; Coussens, L.M. Macrophages and therapeutic resistance in cancer. Cancer Cell 2015, 27, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Pan, S.T.; Li, Z.L.; He, Z.X.; Qiu, J.X.; Zhou, S.F. Molecular mechanisms for tumour resistance to chemotherapy. Clin. Exp. Pharmacol. Physiol. 2016, 43, 723–737. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, H.; Merkher, Y.; Chen, L.; Liu, N.; Leonov, S.; Chen, Y. Recent advances in therapeutic strategies for triple-negative breast cancer. J. Hematol. Oncol. 2022, 15, 121. [Google Scholar] [CrossRef]
- Andreopoulou, E.; Schweber, S.J.; Sparano, J.A.; McDaid, H.M. Therapies for triple negative breast cancer. Expert Opin. Pharmacother. 2015, 16, 983–998. [Google Scholar] [CrossRef]
- Oualla, K.; El-Zawahry, H.M.; Arun, B.; Reuben, J.M.; Woodward, W.A.; Gamal El-Din, H.; Lim, B.; Mellas, N.; Ueno, N.T.; Fouad, T.M. Novel therapeutic strategies in the treatment of triple-negative breast cancer. Ther. Adv. Med. Oncol. 2017, 9, 493–511. [Google Scholar] [CrossRef]
- Choi, H.; Kim, K. Theranostics for Triple-Negative Breast Cancer. Diagnostics 2023, 13, 272. [Google Scholar] [CrossRef] [PubMed]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar] [CrossRef]
- Zhang, X.; Xiong, J.; Wang, K.; Yu, H.; Sun, B.; Ye, H.; Zhao, Z.; Wang, N.; Wang, Y.; Zhang, S.; et al. Erythrocyte membrane-camouflaged carrier-free nanoassembly of FRET photosensitizer pairs with high therapeutic efficiency and high security for programmed cancer synergistic phototherapy. Bioact. Mater. 2021, 6, 2291–2302. [Google Scholar] [CrossRef] [PubMed]
- Thakur, V.; Kutty, R.V. Recent advances in nanotheranostics for triple negative breast cancer treatment. J. Exp. Clin. Cancer Res. CR 2019, 38, 430. [Google Scholar] [CrossRef] [PubMed]
- Zinn, S.; Vazquez-Lombardi, R.; Zimmermann, C.; Sapra, P.; Jermutus, L.; Christ, D. Advances in antibody-based therapy in oncology. Nat. Cancer 2023, 4, 165–180. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Lee, J.; Yang, L.; Hilton, M.B.; Morris, K.; Seaman, S.; Edupuganti, V.; Hsu, K.S.; Dower, C.; Yu, G.; et al. Engineering CD276/B7-H3-targeted antibody-drug conjugates with enhanced cancer-eradicating capability. Cell Rep. 2023, 42, 113503. [Google Scholar] [CrossRef] [PubMed]
- Guo, P.; Huang, J.; Zhu, B.; Huang, A.C.; Jiang, L.; Fang, J.; Moses, M.A. A rationally designed ICAM1 antibody drug conjugate eradicates late-stage and refractory triple-negative breast tumors in vivo. Sci. Adv. 2023, 9, eabq7866. [Google Scholar] [CrossRef] [PubMed]
- Shao, F.; Pan, Z.; Long, Y.; Zhu, Z.; Wang, K.; Ji, H.; Zhu, K.; Song, W.; Song, Y.; Song, X.; et al. Nectin-4-targeted immunoSPECT/CT imaging and photothermal therapy of triple-negative breast cancer. J. Nanobiotechnol. 2022, 20, 243. [Google Scholar] [CrossRef] [PubMed]
- Schmid, P.; Adams, S.; Rugo, H.S.; Schneeweiss, A.; Barrios, C.H.; Iwata, H.; Diéras, V.; Hegg, R.; Im, S.A.; Shaw Wright, G.; et al. Atezolizumab and Nab-Paclitaxel in Advanced Triple-Negative Breast Cancer. N. Engl. J. Med. 2018, 379, 2108–2121. [Google Scholar] [CrossRef]
- Chen, Z.; Kankala, R.K.; Yang, Z.; Li, W.; Xie, S.; Li, H.; Chen, A.Z.; Zou, L. Antibody-based drug delivery systems for cancer therapy: Mechanisms, challenges, and prospects. Theranostics 2022, 12, 3719–3746. [Google Scholar] [CrossRef]
- Scodeller, P.; Asciutto, E.K. Targeting Tumors Using Peptides. Molecules 2020, 25, 808. [Google Scholar] [CrossRef] [PubMed]
- O’Reilly, D.; Sendi, M.A.; Kelly, C.M. Overview of recent advances in metastatic triple negative breast cancer. World J. Clin. Oncol. 2021, 12, 164–182. [Google Scholar] [CrossRef]
- Vlieghe, P.; Lisowski, V.; Martinez, J.; Khrestchatisky, M. Synthetic therapeutic peptides: Science and market. Drug Discov. Today 2010, 15, 40–56. [Google Scholar] [CrossRef]
- Huang, Y.; Zeng, A.; Song, L. Facts and prospects of peptide in targeted therapy and immune regulation against triple-negative breast cancer. Front. Immunol. 2023, 14, 1255820. [Google Scholar] [CrossRef]
- Demeule, M.; Charfi, C.; Currie, J.C.; Larocque, A.; Zgheib, A.; Kozelko, S.; Béliveau, R.; Marsolais, C.; Annabi, B. TH1902, a new docetaxel-peptide conjugate for the treatment of sortilin-positive triple-negative breast cancer. Cancer Sci. 2021, 112, 4317–4334. [Google Scholar] [CrossRef] [PubMed]
- Bressler, E.M.; Kim, J.; Shmueli, R.B.; Mirando, A.C.; Bazzazi, H.; Lee, E.; Popel, A.S.; Pandey, N.B.; Green, J.J. Biomimetic peptide display from a polymeric nanoparticle surface for targeting and antitumor activity to human triple-negative breast cancer cells. J. Biomed. Mater. Res. Part A 2018, 106, 1753–1764. [Google Scholar] [CrossRef] [PubMed]
- Saghaeidehkordi, A.; Chen, S.; Yang, S.; Kaur, K. Evaluation of a Keratin 1 Targeting Peptide-Doxorubicin Conjugate in a Mouse Model of Triple-Negative Breast Cancer. Pharmaceutics 2021, 13, 661. [Google Scholar] [CrossRef]
- Yu, B.; Su, H.; Zhao, L.; Yang, J.; Zhu, M.; Zhao, J. (99m)Tc-labeled iRGD for single-positron emission computed tomography imaging of triple-negative breast cancer. Front. Bioeng. Biotechnol. 2022, 10, 1001899. [Google Scholar] [CrossRef]
- Keenan, T.E.; Tolaney, S.M. Role of Immunotherapy in Triple-Negative Breast Cancer. J. Natl. Compr. Cancer Netw. 2020, 18, 479–489. [Google Scholar] [CrossRef]
- Bing, T.; Zhang, N.; Shangguan, D. Cell-SELEX, an Effective Way to the Discovery of Biomarkers and Unexpected Molecular Events. Adv. Biosyst. 2019, 3, e1900193. [Google Scholar] [CrossRef]
- Kumar, P.; Salve, R.; Paknikar, K.M.; Gajbhiye, V. Nucleolin aptamer conjugated MSNPs-PLR-PEG multifunctional nanoconstructs for targeted co-delivery of anticancer drug and siRNA to counter drug resistance in TNBC. Int. J. Biol. Macromol. 2023, 229, 600–614. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Peng, T.; Peng, Y.; Ai, L.; Deng, Z.; Wang, X.Q.; Tan, W. Molecularly Engineering Triptolide with Aptamers for High Specificity and Cytotoxicity for Triple-Negative Breast Cancer. J. Am. Chem. Soc. 2020, 142, 2699–2703. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Zhao, H.; He, K.; Du, W.; Kong, Y.; Wang, Z.; Li, M.; Shen, Q.; Sun, P.; Fan, Q. NIR-II Excitation Phototheranostic Nanomedicine for Fluorescence/Photoacoustic Tumor Imaging and Targeted Photothermal-Photonic Thermodynamic Therapy. Small 2021, 17, e2102527. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Tang, S.; Wang, M.; Li, L.; Li, J.; Wang, D.; Mi, X.; Zhang, Y.; Tan, X.; Yue, S. “Triple-Punch” Strategy Exosome-Mimetic Nanovesicles for Triple Negative Breast Cancer Therapy. ACS Nano 2024, 18, 5470–5482. [Google Scholar] [CrossRef] [PubMed]
- Kang, Z.; Yang, M.; Feng, X.; Liao, H.; Zhang, Z.; Du, Y. Multifunctional Theranostic Nanoparticles for Enhanced Tumor Targeted Imaging and Synergistic FUS/Chemotherapy on Murine 4T1 Breast Cancer Cell. Int. J. Nanomed. 2022, 17, 2165–2187. [Google Scholar] [CrossRef] [PubMed]
- Duan, T.; Xu, Z.; Sun, F.; Wang, Y.; Zhang, J.; Luo, C.; Wang, M. HPA aptamer functionalized paclitaxel-loaded PLGA nanoparticles for enhanced anticancer therapy through targeted effects and microenvironment modulation. Biomed. Pharmacother. 2019, 117, 109121. [Google Scholar] [CrossRef] [PubMed]
- Chou, Y.T.; Lin, C.Y.; Wen, J.W.; Hung, L.C.; Chang, Y.F.; Yang, C.M.; Wu, L.C.; Ho, J.A. Targeting triple-negative breast cancer with an aptamer-functionalized nanoformulation: A synergistic treatment that combines photodynamic and bioreductive therapies. J. Nanobiotechnology 2021, 19, 89. [Google Scholar] [CrossRef] [PubMed]
- Agnello, L.; Tortorella, S.; d’Argenio, A.; Carbone, C.; Camorani, S.; Locatelli, E.; Auletta, L.; Sorrentino, D.; Fedele, M.; Zannetti, A.; et al. Optimizing cisplatin delivery to triple-negative breast cancer through novel EGFR aptamer-conjugated polymeric nanovectors. J. Exp. Clin. Cancer Res. 2021, 40, 239. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Cheng, Z.; He, J.; Yao, X.; Liu, Y.; Cai, K.; Li, M.; Hu, Y.; Luo, Z. Inhibition of glycolysis-driven immunosuppression with a nano-assembly enhances response to immune checkpoint blockade therapy in triple negative breast cancer. Nat. Commun. 2023, 14, 7021. [Google Scholar] [CrossRef]
- Camorani, S.; Tortorella, S.; Agnello, L.; Spanu, C.; d’Argenio, A.; Nilo, R.; Zannetti, A.; Locatelli, E.; Fedele, M.; Comes Franchini, M.; et al. Aptamer-Functionalized Nanoparticles Mediate PD-L1 siRNA Delivery for Effective Gene Silencing in Triple-Negative Breast Cancer Cells. Pharmaceutics 2022, 14, 2225. [Google Scholar] [CrossRef]
- Kim, M.W.; Jeong, H.Y.; Kang, S.J.; Jeong, I.H.; Choi, M.J.; You, Y.M.; Im, C.S.; Song, I.H.; Lee, T.S.; Lee, J.S.; et al. Anti-EGF Receptor Aptamer-Guided Co-Delivery of Anti-Cancer siRNAs and Quantum Dots for Theranostics of Triple-Negative Breast Cancer. Theranostics 2019, 9, 837–852. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Passariello, M.; Agnello, L.; Esposito, S.; Collina, F.; Cantile, M.; Di Bonito, M.; Ulasov, I.V.; Fedele, M.; Zannetti, A.; et al. Aptamer targeted therapy potentiates immune checkpoint blockade in triple-negative breast cancer. J. Exp. Clin. Cancer Res. CR 2020, 39, 180. [Google Scholar] [CrossRef] [PubMed]
- Camorani, S.; Crescenzi, E.; Gramanzini, M.; Fedele, M.; Zannetti, A.; Cerchia, L. Aptamer-mediated impairment of EGFR-integrin αvβ3 complex inhibits vasculogenic mimicry and growth of triple-negative breast cancers. Sci. Rep. 2017, 7, 46659. [Google Scholar] [CrossRef] [PubMed]
- Soundararajan, S.; Chen, W.; Spicer, E.K.; Courtenay-Luck, N.; Fernandes, D.J. The nucleolin targeting aptamer AS1411 destabilizes Bcl-2 messenger RNA in human breast cancer cells. Cancer Res. 2008, 68, 2358–2365. [Google Scholar] [CrossRef] [PubMed]
- Odeh, F.; Nsairat, H.; Alshaer, W.; Ismail, M.A.; Esawi, E.; Qaqish, B.; Bawab, A.A.; Ismail, S.I. Aptamers Chemistry: Chemical Modifications and Conjugation Strategies. Molecules 2019, 25, 3. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Song, Y.; Li, Q.; Fan, C.; Lan, X.; Jiang, D. Advances in aptamer-based nuclear imaging. Eur. J. Nucl. Med. Mol. Imaging 2022, 49, 2544–2559. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Ma, Y.; Sun, X.L. Recent developments in carbohydrate-decorated targeted drug/gene delivery. Med. Res. Rev. 2010, 30, 270–289. [Google Scholar] [CrossRef] [PubMed]
- Wang, N.; Zhang, Q.; Wang, Z.; Liu, Y.; Yang, S.; Zhao, X.; Peng, J. A chemo/chemodynamic nanoparticle based on hyaluronic acid induces ferroptosis and apoptosis for triple-negative breast cancer therapy. Carbohydr. Polym. 2024, 329, 121795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Hu, H.; Tang, W.; Zhang, Q.; Li, M.; Jin, H.; Huang, Z.; Cui, Z.; Xu, J.; Wang, K.; et al. A multifunctional magnetic nanosystem based on “two strikes” effect for synergistic anticancer therapy in triple-negative breast cancer. J. Control. Release 2020, 322, 401–415. [Google Scholar] [CrossRef]
- Li, Y.; Xiong, J.; Guo, W.; Jin, Y.; Miao, W.; Wang, C.; Zhang, H.; Hu, Y.; Huang, H. Decomposable black phosphorus nano-assembly for controlled delivery of cisplatin and inhibition of breast cancer metastasis. J. Control. Release 2021, 335, 59–74. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, X.; Li, Z.; Pan, D.; Zhu, H.; Gu, Z.; Chen, J.; Zhang, H.; Gong, Q.; Luo, K. Dendronized hyaluronic acid-docetaxel conjugate as a stimuli-responsive nano-agent for breast cancer therapy. Carbohydr. Polym. 2021, 267, 118160. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Yang, R.; Yu, W.; Hu, C.; Zhang, Z.; Liu, D.; An, Y.; Wang, X.; He, C.; Liu, P.; et al. Chitosan oligosaccharide decorated liposomes combined with TH302 for photodynamic therapy in triple negative breast cancer. J. Nanobiotechnology 2021, 19, 147. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Song, Y.; Yu, L.; Xue, X.; Pang, M.; Li, Y.; Luo, X.; Hua, Z.; Lu, C.; Lu, A.; et al. Co-Delivery of Hesperetin and Cisplatin via Hyaluronic Acid-Modified Liposome for Targeted Inhibition of Aggression and Metastasis of Triple-Negative Breast Cancer. ACS Appl. Mater. Interfaces 2023, 15, 34360–34377. [Google Scholar] [CrossRef] [PubMed]
- Dong, S.; Bi, Y.; Sun, X.; Zhao, Y.; Sun, R.; Hao, F.; Sun, Y.; Wang, Y.; Li, X.; Deng, W.; et al. Dual-Loaded Liposomes Tagged with Hyaluronic Acid Have Synergistic Effects in Triple-Negative Breast Cancer. Small 2022, 18, e2107690. [Google Scholar] [CrossRef] [PubMed]
- Mi, P.; Cabral, H.; Kataoka, K. Ligand-Installed Nanocarriers toward Precision Therapy. Adv. Mater. 2020, 32, e1902604. [Google Scholar] [CrossRef] [PubMed]
- Jain, K.K. Role of Nanobiotechnology in Drug Delivery. Methods Mol. Biol. 2020, 2059, 55–73. [Google Scholar] [CrossRef] [PubMed]
- Dai, W.; Yang, F.; Ma, L.; Fan, Y.; He, B.; He, Q.; Wang, X.; Zhang, H.; Zhang, Q. Combined mTOR inhibitor rapamycin and doxorubicin-loaded cyclic octapeptide modified liposomes for targeting integrin α3 in triple-negative breast cancer. Biomaterials 2014, 35, 5347–5358. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zou, W.; Bian, S.; Huang, Y.; Tan, Y.; Liang, J.; Fan, Y.; Zhang, X. Bioreducible PAA-g-PEG graft micelles with high doxorubicin loading for targeted antitumor effect against mouse breast carcinoma. Biomaterials 2013, 34, 6818–6828. [Google Scholar] [CrossRef] [PubMed]
- Zeng, W.; Luo, Y.; Gan, D.; Zhang, Y.; Deng, H.; Liu, G. Advances in Doxorubicin-based nano-drug delivery system in triple negative breast cancer. Front. Bioeng. Biotechnol. 2023, 11, 1271420. [Google Scholar] [CrossRef]
- Tang, F.; Li, L.; Chen, D. Mesoporous silica nanoparticles: Synthesis, biocompatibility and drug delivery. Adv. Mater. 2012, 24, 1504–1534. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, Q.; Han, N.; Bai, L.; Li, J.; Liu, J.; Che, E.; Hu, L.; Zhang, Q.; Jiang, T.; et al. Mesoporous silica nanoparticles in drug delivery and biomedical applications. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 313–327. [Google Scholar] [CrossRef]
- He, Z.; Zhang, H.; Li, H.; Wang, Y.; Qian, J.; Cai, X.; Sun, L.; Huang, J. Preparation, Biosafety, and Cytotoxicity Studies of a Newly Tumor-Microenvironment-Responsive Biodegradable Mesoporous Silica Nanosystem Based on Multimodal and Synergistic Treatment. Oxid. Med. Cell. Longev. 2020, 2020, 7152173. [Google Scholar] [CrossRef] [PubMed]
- Dogan, B.E.; Turnbull, L.W. Imaging of triple-negative breast cancer. Ann. Oncol. 2012, 23 (Suppl. 6), vi23–vi29. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Liu, M.; Yang, T.; Luo, J.; Wei, C.; Shen, J.; Song, X.; Ke, H.; Sun, P.; Guo, M.; et al. Self-activated arsenic manganite nanohybrids for visible and synergistic thermo/immuno-arsenotherapy. J. Control. Release 2022, 350, 761–776. [Google Scholar] [CrossRef] [PubMed]
- Sun, W.; Wang, Z.; Liu, J.; Jiang, C.; Chen, W.; Yu, B.; Wang, W.; Lu, L. On-demand degradable magnetic resonance imaging nanoprobes. Sci. Bull. 2021, 66, 676–684. [Google Scholar] [CrossRef] [PubMed]
- Orsini, F.; Lorenzoni, A.; Puta, E.; Mariani, G. Positron-Emitting Radiopharmaceuticals for Diagnostic Applications in Oncology. In Nuclear Oncology: From Pathophysiology to Clinical Applications; Volterrani, D., Erba, P.A., Strauss, H.W., Mariani, G., Larson, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2020; pp. 1–19. [Google Scholar] [CrossRef]
- Orsini, F.; Mazzarri, S.; Puta, E.; Guidoccio, F.; Lorenzoni, A.; Mariani, G. Radiopharmaceuticals for Therapy. In Nuclear Oncology: From Pathophysiology to Clinical Applications; Volterrani, D., Erba, P.A., Strauss, H.W., Mariani, G., Larson, S.M., Eds.; Springer International Publishing: Cham, Switzerland, 2022; pp. 133–149. [Google Scholar] [CrossRef]
- Yang, R.; Lu, M.; Ming, L.; Chen, Y.; Cheng, K.; Zhou, J.; Jiang, S.; Lin, Z.; Chen, D. (89)Zr-Labeled Multifunctional Liposomes Conjugate Chitosan for PET-Trackable Triple-Negative Breast Cancer Stem Cell Targeted Therapy. Int. J. Nanomed. 2020, 15, 9061–9074. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Yang, Z.; Li, F.; Zhao, H.; Li, C.; Yu, N.; Hamilton, D.J.; Li, Z. (64)Cu/(177)Lu-DOTA-diZD, a Small-Molecule-Based Theranostic Pair for Triple-Negative Breast Cancer. J. Med. Chem. 2021, 64, 2705–2713. [Google Scholar] [CrossRef] [PubMed]
- Fernández, A.; Vendrell, M. Smart fluorescent probes for imaging macrophage activity. Chem. Soc. Rev. 2016, 45, 1182–1196. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Pu, K. Recent Advances of Activatable Molecular Probes Based on Semiconducting Polymer Nanoparticles in Sensing and Imaging. Adv. Sci. 2017, 4, 1600481. [Google Scholar] [CrossRef]
- Luo, S.; Zhang, E.; Su, Y.; Cheng, T.; Shi, C. A review of NIR dyes in cancer targeting and imaging. Biomaterials 2011, 32, 7127–7138. [Google Scholar] [CrossRef]
- Potara, M.; Nagy-Simon, T.; Focsan, M.; Licarete, E.; Soritau, O.; Vulpoi, A.; Astilean, S. Folate-targeted Pluronic-chitosan nanocapsules loaded with IR780 for near-infrared fluorescence imaging and photothermal-photodynamic therapy of ovarian cancer. Colloids Surf. B Biointerfaces 2021, 203, 111755. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Liu, H.; Li, L.; Guo, Z.; Song, J.; Yang, X.; Wan, G.; Li, R.; Wang, Y. Leukocyte/platelet hybrid membrane-camouflaged dendritic large pore mesoporous silica nanoparticles co-loaded with photo/chemotherapeutic agents for triple negative breast cancer combination treatment. Bioact. Mater. 2021, 6, 3865–3878. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Gao, Z.; Zeng, J.; Hou, Y.; Fang, F.; Li, Y.; Qiao, R.; Shen, L.; Lei, H.; Yang, W.; et al. Magnetic/upconversion fluorescent NaGdF4:Yb,Er nanoparticle-based dual-modal molecular probes for imaging tiny tumors in vivo. ACS Nano 2013, 7, 7227–7240. [Google Scholar] [CrossRef] [PubMed]
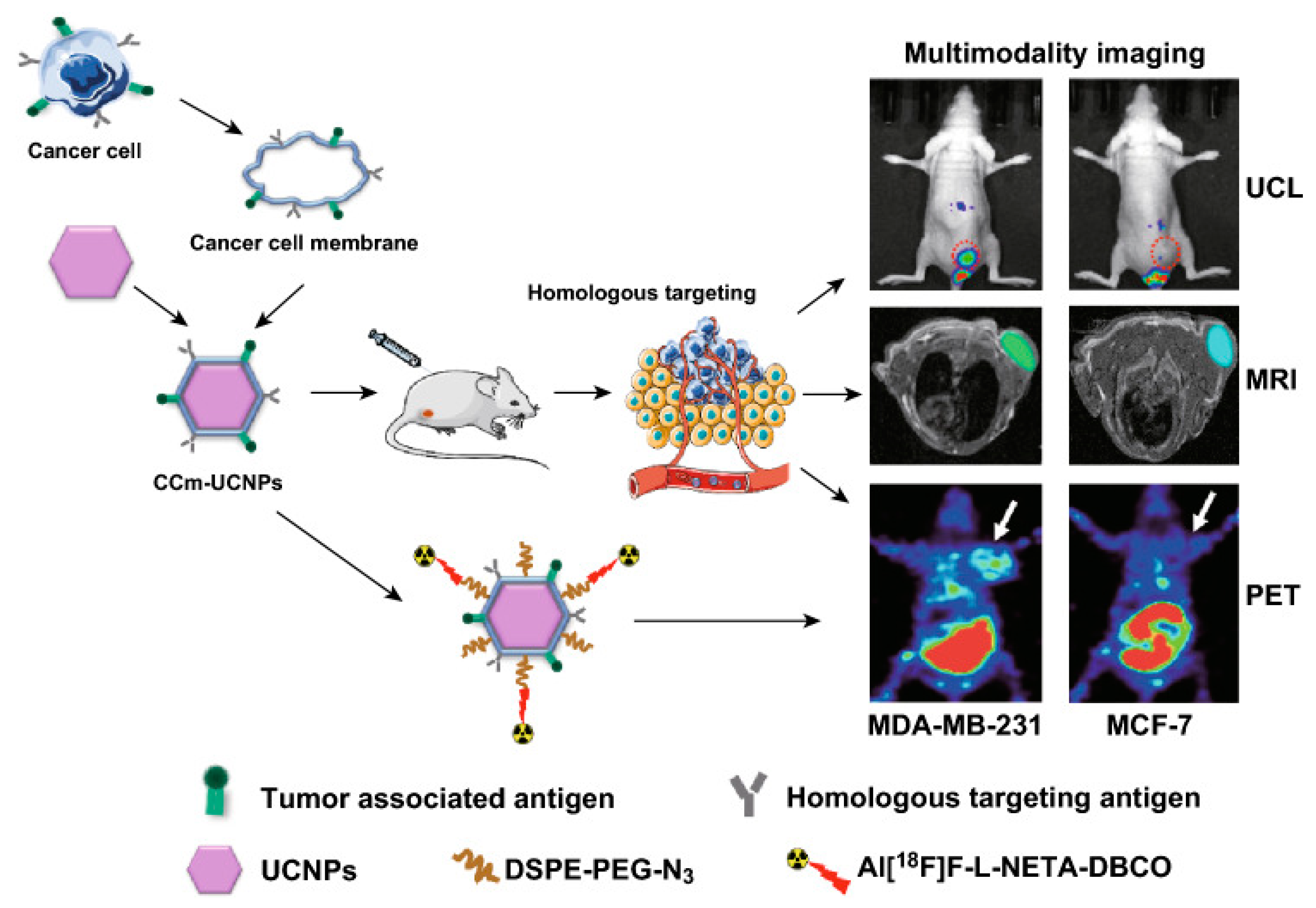
- Fang, H.; Li, M.; Liu, Q.; Gai, Y.; Yuan, L.; Wang, S.; Zhang, X.; Ye, M.; Zhang, Y.; Gao, M.; et al. Ultra-sensitive Nanoprobe Modified with Tumor Cell Membrane for UCL/MRI/PET Multimodality Precise Imaging of Triple-Negative Breast Cancer. Nano-Micro Lett. 2020, 12, 62. [Google Scholar] [CrossRef] [PubMed]
- Cavaliere, A.; Sun, S.; Lee, S.; Bodner, J.; Li, Z.; Huang, Y.; Moores, S.L.; Marquez-Nostra, B. Development of [(89)Zr]ZrDFO-amivantamab bispecific to EGFR and c-MET for PET imaging of triple-negative breast cancer. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 383–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; You, Y.; Xu, Y.; Cheng, Q.; Xiao, Z.; Chen, T.; Shi, C.; Luo, L. Facile synthesis of near-infrared responsive on-demand oxygen releasing nanoplatform for precise MRI-guided theranostics of hypoxia-induced tumor chemoresistance and metastasis in triple negative breast cancer. J. Nanobiotechnology 2022, 20, 104. [Google Scholar] [CrossRef] [PubMed]
- Fang, H.; Gai, Y.; Wang, S.; Liu, Q.; Zhang, X.; Ye, M.; Tan, J.; Long, Y.; Wang, K.; Zhang, Y.; et al. Biomimetic oxygen delivery nanoparticles for enhancing photodynamic therapy in triple-negative breast cancer. J. Nanobiotechnology 2021, 19, 81. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Jiang, L.; Zhang, Y.; Lu, Y.; Li, J.; Wang, H.; Yao, D.; Wang, D. Fibronectin-Targeting and Cathepsin B-Activatable Theranostic Nanoprobe for MR/Fluorescence Imaging and Enhanced Photodynamic Therapy for Triple Negative Breast Cancer. ACS Appl. Mater. Interfaces 2020, 12, 33564–33574. [Google Scholar] [CrossRef] [PubMed]
- Marquez-Nostra, B.V.; Lee, S.; Laforest, R.; Vitale, L.; Nie, X.; Hyrc, K.; Keler, T.; Hawthorne, T.; Hoog, J.; Li, S.; et al. Preclinical PET imaging of glycoprotein non-metastatic melanoma B in triple negative breast cancer: Feasibility of an antibody-based companion diagnostic agent. Oncotarget 2017, 8, 104303–104314. [Google Scholar] [CrossRef]
- Hernandez, R.; Grudzinski, J.J.; Aluicio-Sarduy, E.; Massey, C.F.; Pinchuk, A.N.; Bitton, A.N.; Patel, R.; Zhang, R.; Rao, A.V.; Iyer, G.; et al. (177)Lu-NM600 Targeted Radionuclide Therapy Extends Survival in Syngeneic Murine Models of Triple-Negative Breast Cancer. J. Nucl. Med. 2020, 61, 1187–1194. [Google Scholar] [CrossRef]
- Napier, T.S.; Hunter, C.L.; Song, P.N.; Larimer, B.M.; Sorace, A.G. Preclinical PET Imaging of Granzyme B Shows Promotion of Immunological Response Following Combination Paclitaxel and Immune Checkpoint Inhibition in Triple Negative Breast Cancer. Pharmaceutics 2022, 14, 440. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Gai, Y.; Li, K.; Hu, F.; Gong, C.; Wang, S.; Feng, F.; Altine, B.; Hu, J.; Lan, X. A novel carbon-11 radiolabeled maternal embryonic leucine zipper kinase inhibitor for PET imaging of triple-negative breast cancer. Bioorganic Chem. 2021, 107, 104609. [Google Scholar] [CrossRef] [PubMed]
- M-Rabet, M.; Cabaud, O.; Josselin, E.; Finetti, P.; Castellano, R.; Farina, A.; Agavnian-Couquiaud, E.; Saviane, G.; Collette, Y.; Viens, P.; et al. Nectin-4: A new prognostic biomarker for efficient therapeutic targeting of primary and metastatic triple-negative breast cancer. Ann. Oncol. 2017, 28, 769–776. [Google Scholar] [CrossRef] [PubMed]
- Mahata, M.K.; Bae, H.; Lee, K.T. Upconversion Luminescence Sensitized pH-Nanoprobes. Molecules 2017, 22, 2064. [Google Scholar] [CrossRef] [PubMed]
- Bi, H.; He, F.; Dai, Y.; Xu, J.; Dong, Y.; Yang, D.; Gai, S.; Li, L.; Li, C.; Yang, P. Quad-Model Imaging-Guided High-Efficiency Phototherapy Based on Upconversion Nanoparticles and ZnFe(2)O(4) Integrated Graphene Oxide. Inorg. Chem. 2018, 57, 9988–9998. [Google Scholar] [CrossRef] [PubMed]
- Hong, G.; Antaris, A.L.; Dai, H. Near-infrared fluorophores for biomedical imaging. Nat. Biomed. Eng. 2017, 1, 0010. [Google Scholar] [CrossRef]
- Zhu, S.; Hu, Z.; Tian, R.; Yung, B.C.; Yang, Q.; Zhao, S.; Kiesewetter, D.O.; Niu, G.; Sun, H.; Antaris, A.L.; et al. Repurposing Cyanine NIR-I Dyes Accelerates Clinical Translation of Near-Infrared-II (NIR-II) Bioimaging. Adv. Mater. 2018, 30, e1802546. [Google Scholar] [CrossRef] [PubMed]
- Vagia, E.; Mahalingam, D.; Cristofanilli, M. The Landscape of Targeted Therapies in TNBC. Cancers 2020, 12, 916. [Google Scholar] [CrossRef]
- Deepak, K.G.K.; Vempati, R.; Nagaraju, G.P.; Dasari, V.R.; Nagini, S.; Rao, D.N.; Malla, R.R. Tumor microenvironment: Challenges and opportunities in targeting metastasis of triple negative breast cancer. Pharmacol. Res. 2020, 153, 104683. [Google Scholar] [CrossRef]






| MMPs | Targeting Group | Carriers | Imaging Group | Drug | Application | Cancer Type | Mouse Strain | Refs |
|---|---|---|---|---|---|---|---|---|
| AS-CD82-DOX-HVs | AS1411 | HVs | DOX/CD82 | MDA-MB-231 | Balb/C-nude | [34] | ||
| TPZ@LXL-1-PpIX-MMT-2 | LXL-1 | MMT-2 | PpIX | MDA-MB-231 | Balb/C-nude | [37] | ||
| Cis-Pt@PNPs-CL4 | CL4 | PNPs | Cy7 | Cisplatin | NIR | MDA-MB-231/BT-549 | Balb/C-nude | [38] |
| Cisplatin/BP/PDA-HA | HA | BP | Cisplatin | 4T1 | Balb/C | [50] | ||
| As/Mn-NHs | EPR | Albumin | Mn2+ | Arsenic trioxide (ATO) | MRI | 4T1/CT26/HT29 | Balb/C | [64] |
| PDA-DNA-DTPA/Gd | EPR | PDA | DTPA/Gd | PDA/DOX | MRI | 4T1 | Balb/C-nude | [65] |
| 177Lu-DOTA-diZD/64Cu-DOTA-diZD | diZD | 64Cu | 177Lu | PET | 4T1 | Balb/C | [69] | |
| LPHM@DDI NPs | LPHM | DLMSN | IR780 | DOX | NIR | 4T1 | Balb/C | [74] |
| NaGdF4:Yb, Er nanocrystals, | EPR | NaYF4 nanocrystals | Gd3+ | MRI/NIR | LS180 | Balb/C-nude | [75] | |
| cancer-cell membrane mimic Gd3+-doped upconversion nanoparticles (CCm-UCNPs) | Homologous targeting | Cancer-cell membrane | UCNPs | UCL/MRI/PET | MDA-MB-231/MCF-7 | Balb/C-nude | [76] | |
| 99mTc-HYNIC-mAbNectin-4/mAbNectin-4-ICG | mAbNectin-4 | 99mTc/ICG | SPECT/NIR | MDA-MB-468 | Balb/C-nude | [18] | ||
| [89Zr]ZrDFO-Amivantamab | Amivantamab | 89Zr | PET | MDA-MB-468/MDA-MB-231/MDA-MB-453 | Balb/C-nude | [77] | ||
| O2-PPSiI | RGD/uPA | PLGA | ICG/Gd-DTPA | PTX | NIR/MRI | MDA-MB-231 | Balb/C-nude | [78] |
| CCm-HSA-ICG-PFTBA | Homologous targeting | Cancer-cell membrane | ICG/18F | PFTBA | NIR/PET | 4T1 | Balb/C-nude | [79] |
| PepSQ@USPIO | Cys-Arg-Glu-Lys-Ala (CREKA) | USPIO | SQ/USPIO | SQ | NIR/MRI | MDA-MB-231/MCF-7 | Balb/C-nude | [80] |
| [89Zr]DFO-CR011 | CR011 | 89Zr | PET | MDA-MB-157/MDA-MB-468/MDA-MB-231 | Balb/C-nude | [81] | ||
| 177Lu-NM600/86Y-NM600 | NM600 | NM600 | 86Y | 177Lu | PET | 4T1/4T07 | Balb/C-nude | [82] |
| [68Ga]-NOTA-GZP | GZP | 68Ga | PTX/anti-PD-1/anti-CTLA4 | PET | 4T1/E0771 | Balb/C/C57Bl6 | [83] | |
| 11C-methoxy-OTSSP167 | OTSSP167 | 11C | PET | MCF-7/MDA-MB-231 | Balb/C-nude | [84] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, D.; Li, Z.; Ji, D.-K.; Xia, Q. Recent Progress of Multifunctional Molecular Probes for Triple-Negative Breast Cancer Theranostics. Pharmaceutics 2024, 16, 803. https://doi.org/10.3390/pharmaceutics16060803
Zhao D, Li Z, Ji D-K, Xia Q. Recent Progress of Multifunctional Molecular Probes for Triple-Negative Breast Cancer Theranostics. Pharmaceutics. 2024; 16(6):803. https://doi.org/10.3390/pharmaceutics16060803
Chicago/Turabian StyleZhao, Deyi, Zhe Li, Ding-Kun Ji, and Qian Xia. 2024. "Recent Progress of Multifunctional Molecular Probes for Triple-Negative Breast Cancer Theranostics" Pharmaceutics 16, no. 6: 803. https://doi.org/10.3390/pharmaceutics16060803




