The Controlled Preparation of a Carrier-Free Nanoparticulate Formulation Composed of Curcumin and Piperine Using High-Gravity Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
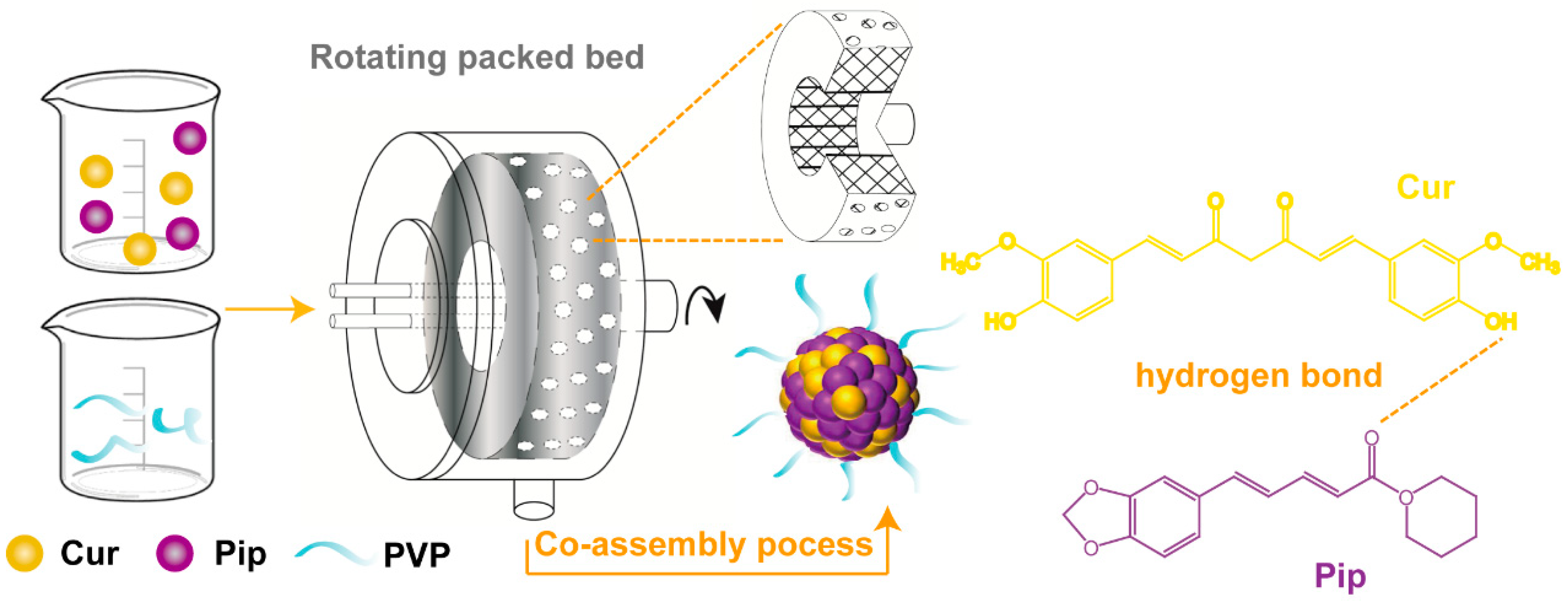
2.2. Preparation of the Cur-Pip CNF
2.3. Characterization
2.4. In Vitro Dissolution Study
2.5. In Vitro Cytotoxicity Study
2.6. In Vivo Pharmacokinetics Study
2.6.1. Preparation of Samples for Analysis
2.6.2. Determination of Pharmacokinetic Parameters
2.7. Statistical Analysis
3. Results and Discussion
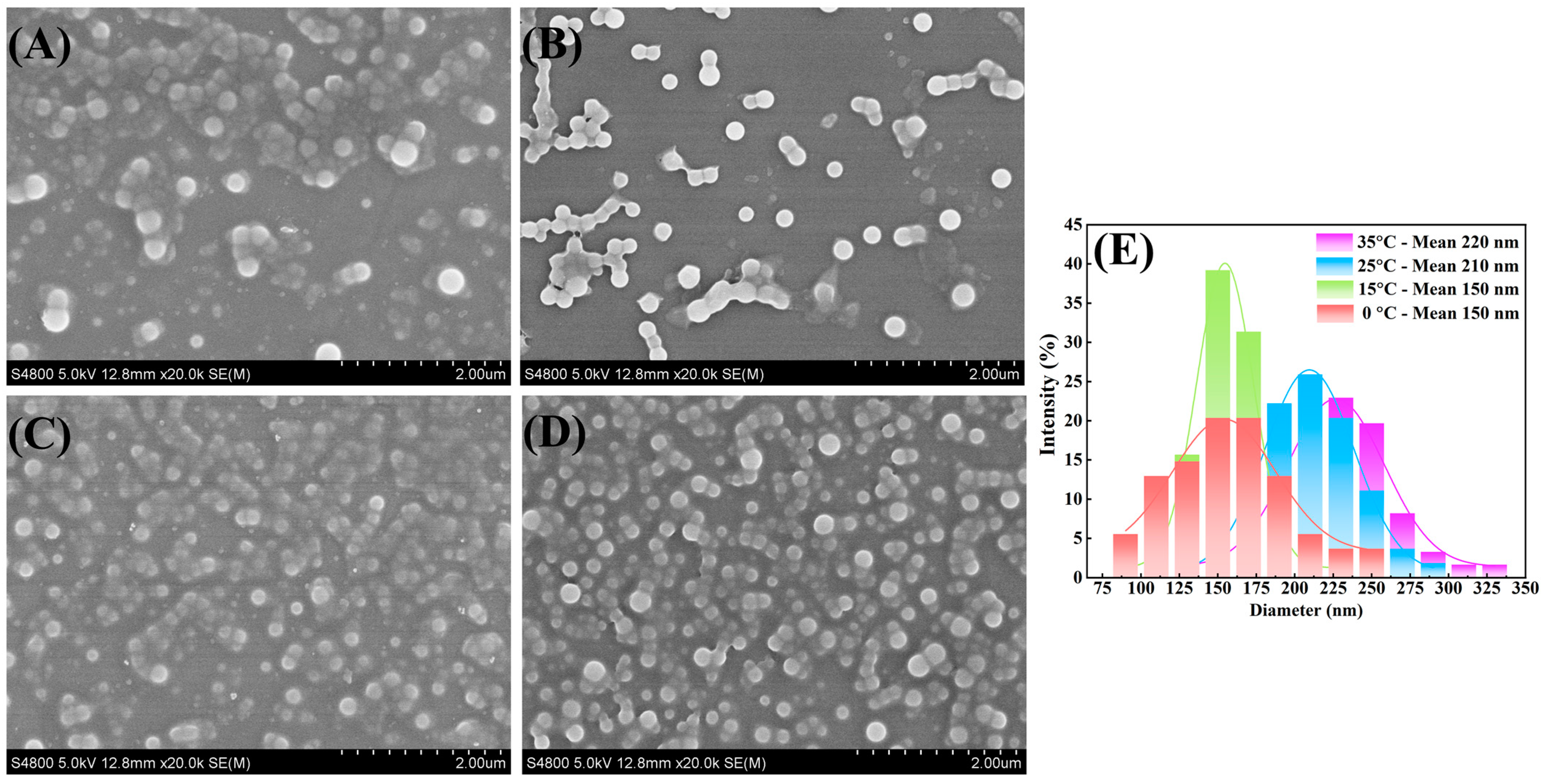
3.1. Influence of Temperature on Particle Size
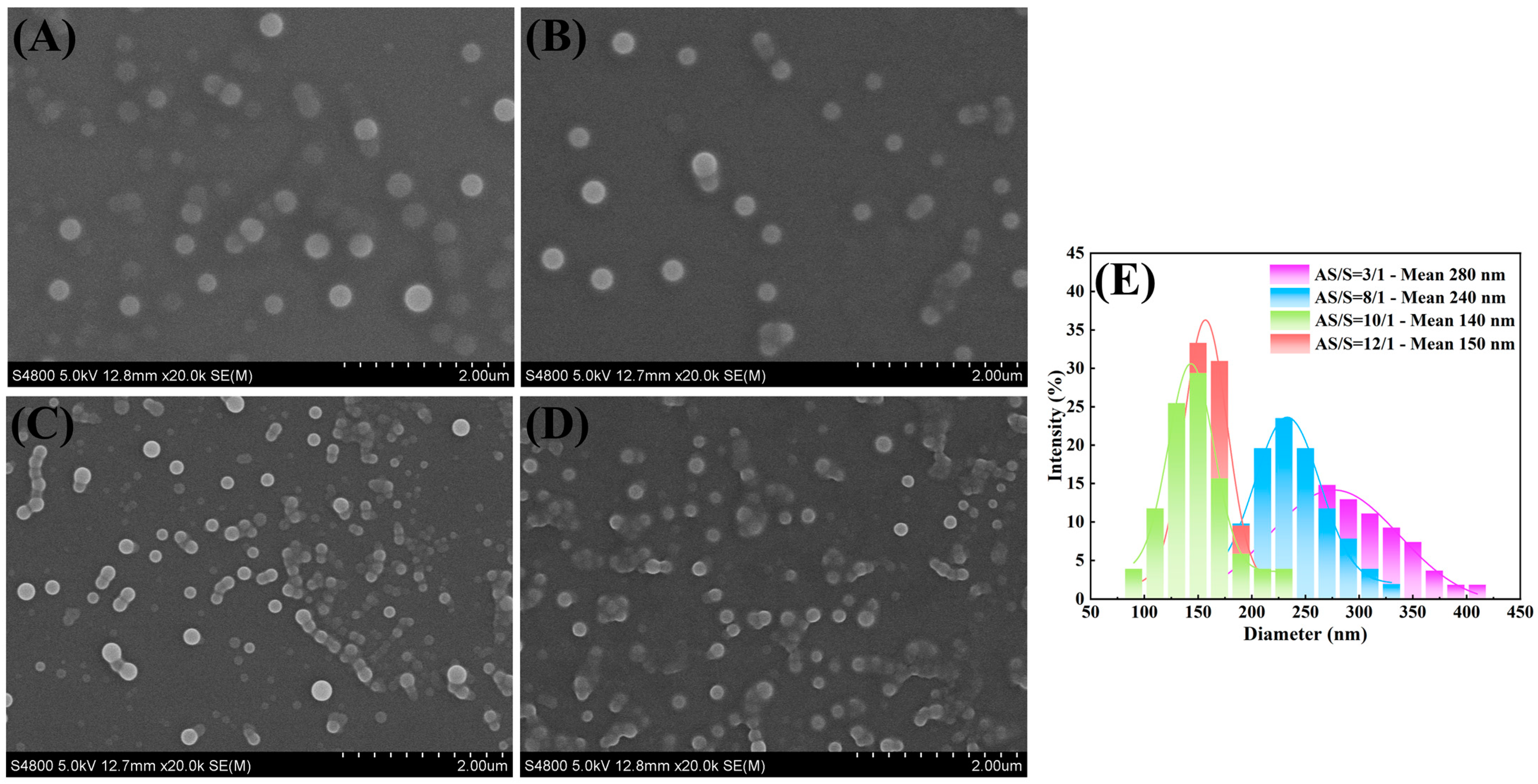
3.2. Influence of Antisolvent/Solvent Ratio on Particle Size
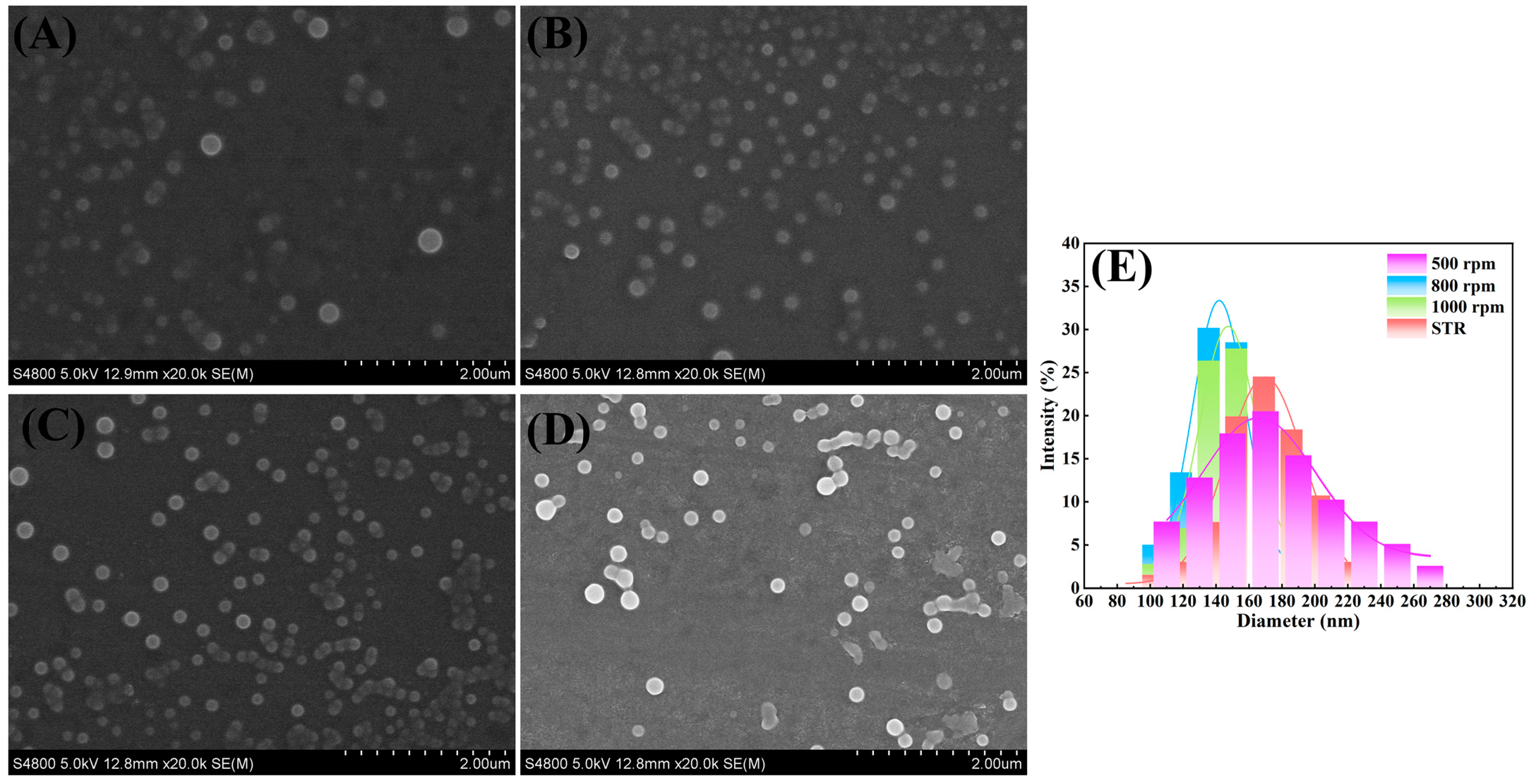
3.3. Influence of RPB Rotating Speed on Particle Size
3.4. Physicochemical Characterization of Cur-Pip CNF
3.5. In Vitro Dissolution Study
3.6. Cytotoxicity Study
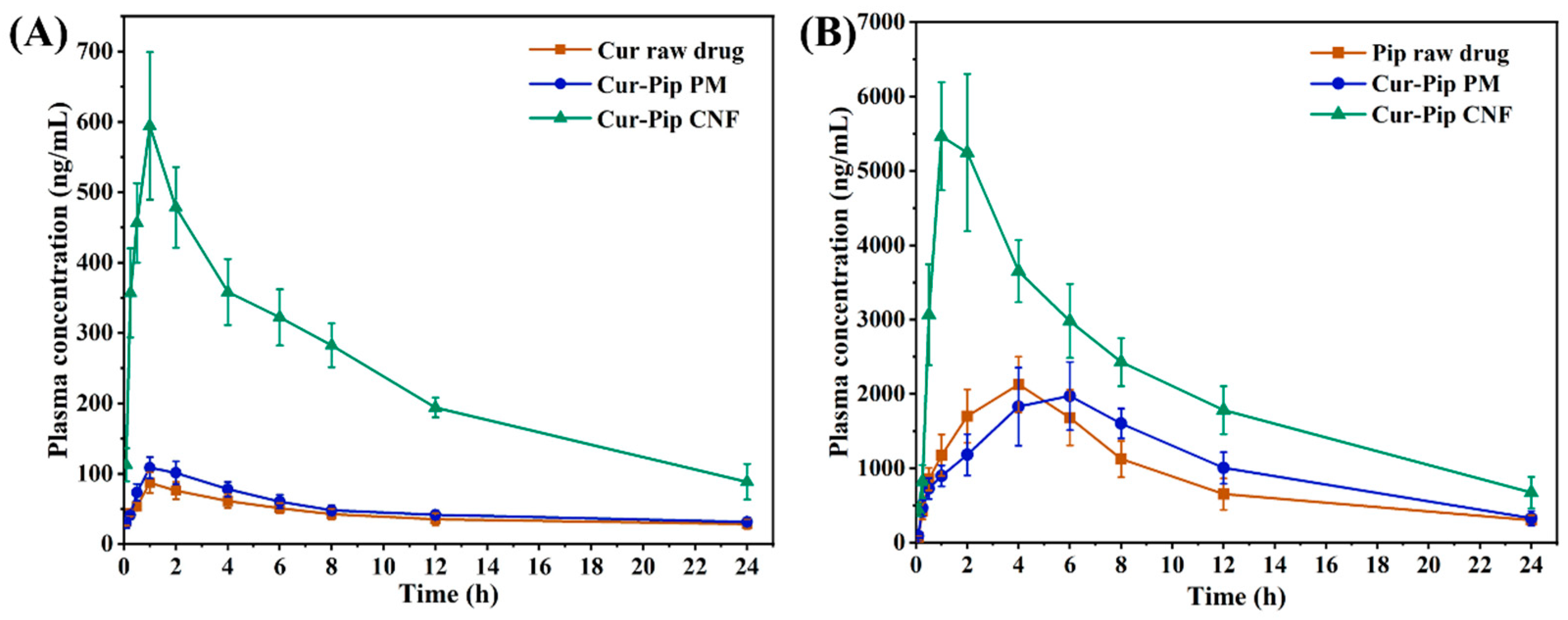
3.7. Pharmacokinetic Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ghadi, R.; Dand, N. BCS class IV drugs: Highly notorious candidates for formulation development. J. Control. Release 2017, 248, 71–95. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, S.; Higashino, H.; Sato, Y.; Minami, K.; Kataoka, M.; Yamashita, S.; Harashima, H. Maximizing the oral bioavailability of poorly water-soluble drugs using novel oil-like materials in lipid-based formulations. Mol. Pharm. 2021, 18, 3281–3289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, A.L.; Li, J.B.; Wang, S.S.; Xu, Y.; Li, Q.; Wu, Z.; Wang, C.; Meng, H.; Zhang, J. Rapid and improved oral absorption of N-butylphthalide by sodium cholate-appended liposomes for efficient ischemic stroke therapy. Drug. Deliv. 2021, 28, 2469–2479. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.L.; Li, Z.P.; Zhao, Z.H.; Li, Y.P.; Liu, Z.Y.; Sun, S.G. Mesoporous carbon in biomedicine: Modification strategies and biocompatibility. Carbon 2023, 212, 118121. [Google Scholar] [CrossRef]
- Almeida, A.; Castro, F.; Resende, C.; Lucio, M.; Schwartz, S.; Sarmento, B. Oral delivery of camptothecin-loaded multifunctional chitosan-based micelles is effective in reduce colorectal cancer. J. Control. Release 2022, 349, 731–743. [Google Scholar] [CrossRef] [PubMed]
- Alqahtani, M.S.; Kazi, M.; Alsenaidy, M.A.; Ahmad, M.Z. Advances in oral drug delivery. Front. Pharmacol. 2021, 12, 618411. [Google Scholar] [CrossRef] [PubMed]
- Jinno, J.; Kamada, N.; Miyake, M.; Yamada, K.; Mukai, T.; Odomi, M.; Toguchi, H.; Liversidge, G.G.; Higaki, K.; Kimura, T. Effect of particle size reduction on dissolution and oral absorption of a poorly water-soluble drug, cilostazol, in beagle dogs. J. Control. Release 2006, 111, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Tian, Z.H.; Ma, Y.P.; Meng, T.T.; Ma, S.J.; Gou, G.J.; Yang, J.H. Nanocrystals for improving oral bioavailability of drugs: Intestinal transport mechanisms and influencing factors. AAPS Pharmscitech. 2021, 22, 179. [Google Scholar] [CrossRef]
- Li, J.; Zhou, Y.P.; Aisha, M.; Wu, J.; Wang, H.; Huang, F.; Sun, M. Preparation of loratadine nanocrystal tablets to improve the solubility and dissolution for enhanced oral bioavailability. J. Pharm. Pharmacol. 2021, 73, 937–946. [Google Scholar] [CrossRef]
- Yin, Y.J.; Deng, H.L.; Wu, K.; He, B.; Dai, W.; Zhang, H.; Fu, J.; Le, Y.; Wang, X.; Zhang, Q. A multiaspect study on transcytosis mechanism of sorafenib nanogranules engineered by high-gravity antisolvent precipitation. J. Control. Release 2020, 323, 600–612. [Google Scholar] [CrossRef]
- Gamboa, J.M.; Leong, K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Deliver. Rev. 2013, 65, 800–810. [Google Scholar] [CrossRef] [PubMed]
- Na, Y.G.; Pham, T.M.A.; Byeon, J.J.; Kim, M.K.; Han, M.G.; Baek, J.S.; Lee, H.K.; Cho, C.W. Development and evaluation of TPGS/PVA-based nanosuspension for enhancing dissolution and oral bioavailability of ticagrelor. Int. J. Pharm. 2020, 581, 119287. [Google Scholar] [CrossRef] [PubMed]
- Sinha, B.; Muller, R.H.; Moschwitzer, J.P. Bottom-up approaches for preparing drug nanocrystals: Formulations and factors affecting particle size. Int. J. Pharmaceut. 2013, 453, 126–141. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Yu, X.X.; Yin, H.P. Study of top-down and bottom-up approaches using design of experiment (DoE) to produce meloxicam nanocrystal capsules. AAPS Pharmscitech. 2020, 21, 79. [Google Scholar] [CrossRef] [PubMed]
- Hennart, S.L.A.; van Hee, P.; Drouet, V.; Domingues, M.C.; Wildeboer, W.J.; Meesters, G.M.H. Characterization and modeling of a sub-micron milling process limited by agglomeration phenomena. Chem. Eng. Sci. 2012, 71, 484–495. [Google Scholar] [CrossRef]
- Merisko-Liversidge, E.; Liversidge, G.G. Nanosizing for oral and parenteral drug delivery: A perspective on formulating poorly-water soluble compounds using wet media milling technology. Adv. Drug. Deliver. Rev. 2011, 63, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Mohammad, I.S.; Hu, H.; Yin, L.; He, W. Drug nanocrystals: Fabrication methods and promising therapeutic applications. Int. J. Pharm. 2019, 562, 187–202. [Google Scholar] [CrossRef]
- Davey, R.J.; Schroeder, S.L.M.; ter Horst, J.H. Nucleation of organic crystals–A molecular perspective. Angew. Chem. Int. Edit. 2013, 52, 2166–2179. [Google Scholar] [CrossRef]
- Yang, H.; Kim, H.; Jung, S.; Seo, H.; Nida, S.K.; Yoo, S.Y.; Lee, J. Pharmaceutical strategies for stabilizing drug nanocrystals. Curr. Pharm. Des. 2018, 24, 2362–2374. [Google Scholar] [CrossRef]
- Malamatari, M.; Taylor, K.M.G.; Malamataris, S.; Douroumis, D.; Kachrimanis, K. Pharmaceutical nanocrystals: Production by wet milling and applications. Drug. Discov. Today 2018, 23, 534–547. [Google Scholar] [CrossRef]
- Grill, A.E.; Koniar, B.; Panyam, J. Co-delivery of natural metabolic inhibitors in a self-microemulsifying drug delivery system for improved oral bioavailability of curcumin. Drug. Deliv. Trans. Res. 2014, 4, 344–352. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.; Wang, G.C.; Wang, Z.H.; Zhang, C.; Wang, F.; Cui, X.; Guo, S.; Huang, W.; Zhang, R.; Yan, D. Floxuridine-chlorambucil conjugate nanodrugs for ovarian cancer combination chemotherapy. Colloid. Surf. B 2020, 194, 111164. [Google Scholar] [CrossRef]
- Li, T.; Wang, P.L.; Guo, W.B.; Huang, X.; Tian, X.; Wu, G.; Xu, B.; Li, F.; Yan, C.; Liang, X.J.; et al. Natural berberine-based Chinese herb medicine assembled nanostructures with modified antibacterial application. ACS Nano 2019, 13, 6770–6781. [Google Scholar] [CrossRef] [PubMed]
- Keum, C.; Hong, J.Y.; Kim, D.; Lee, S.Y.; Kim, H. Lysosome-instructed self-assembly of amino-acid-functionalized perylene diimide for multidrug-resistant cancer cells. ACS Appl. Mater. Interfaces 2021, 13, 14866–14874. [Google Scholar] [CrossRef]
- Yue, X.J.; Luo, Y.; Chen, Q.Y.; Chu, G.W.; Luo, J.Z.; Zhang, L.L.; Chen, J.F. Investigation of micromixing and precipitation process in a rotating packed bed reactor with PTFE. Chem. Eng. Process. 2018, 125, 227–233. [Google Scholar] [CrossRef]
- Xu, J.T.; Liu, C.S.; Wang, M.; Shao, L.; Deng, L.; Nie, K.; Wang, F. Rotating packed bed reactor for enzymatic synthesis of biodiesel. Bioresour. Technol. 2017, 224, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.K.; Lu, Z.; Liu, W.; Shi, C.; Xiao, H.; Jiang, S. Precipitation of homogeneous nanoscale HfRe2 in NiAl by strain-induced precipitation. Mater. Charact. 2022, 193, 112280. [Google Scholar] [CrossRef]
- Wu, K.; Wu, H.R.; Dai, T.C.; Liu, X.Z.; Chen, J.F.; Le, Y. Controlling nucleation and fabricating nanoparticulate formulation of sorafenib using a high-gravity rotating packed bed. Ind. Eng. Chem. Res. 2018, 57, 1903–1911. [Google Scholar] [CrossRef]
- Zhang, Z.L.; Ji, J.B. Large-scale preparation of stable irbesartan nanoparticles by high-gravity liquid antisolvent precipitation technique. Powder Technol. 2017, 305, 546–552. [Google Scholar] [CrossRef]
- Wang, C.Q.; Wang, M.T.; Chen, P.; Wang, J.X.; Le, Y. Dasatinib nanoemulsion and nanocrystal for enhanced oral drug delivery. Pharmaceutics 2022, 14, 197. [Google Scholar] [CrossRef]
- Shen, Y.D.; Li, X.Y.; Le, Y. Amorphous nanoparticulate formulation of sirolimus and its tablets. Pharmaceutics 2018, 10, 155. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.B.; Xie, M.L.; Kuang, Y.Y.; Wang, J.X.; Le, Y.; Zeng, X.F.; Chen, J.F. Preparation of amorphous drug nanoparticles by high-gravity reactive precipitation technique. Chem. Eng. Process. 2016, 104, 253–261. [Google Scholar] [CrossRef]
- Giordano, A.; Tommonaro, G. Curcumin and cancer. Nutrients 2019, 11, 2376. [Google Scholar] [CrossRef] [PubMed]
- Mathew, D.; Hsu, W.L. Antiviral potential of curcumin. J. Funct. Foods 2018, 40, 692–699. [Google Scholar] [CrossRef]
- Hasanzadeh, S.; Read, M.I.; Bland, A.R. Curcumin: An inflammasome silencer. Pharmacol. Res. 2020, 159, 104921. [Google Scholar] [CrossRef] [PubMed]
- Oskouie, M.N.; Moghaddam, N.S.A.; Butler, A.E.; Zamani, P.; Sahebkar, A. Therapeutic use of curcumin-encapsulated and curcumin-primed exosomes. J. Cell. Physiol. 2019, 234, 8182–8191. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.C.; Hsu, W.H.; Huang, Z.Y.; Chuang, K.L.; Lin, K.T.; Tseng, C.L.; Tsai, T.H.; Dao, A.H.; Su, C.L.; Huang, C.Y.F. Bioactivity evaluation of a novel formulated curcumin. Nutrients 2019, 11, 2982. [Google Scholar] [CrossRef] [PubMed]
- Servida, S.; Panzeri, E.; Tomaino, L.; Marfia, G.; Garzia, E.; Appiani, G.C.; Moroncini, G.; Colonna, V.D.G.; La Vecchia, C.; Vigna, L. Overview of curcumin and piperine effects on glucose metabolism: The case of an insulinoma patient’s loss of consciousness. Int. J. Mol. Sci. 2023, 24, 6621. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Han, J.W.; Jiang, A.; Huang, R.; Fu, T.; Wang, L.; Zheng, Q.; Li, W.; Li, J. Involvement of metabolism-permeability in enhancing the oral bioavailability of curcumin in excipient-free solid dispersions co-formed with piperine. Int. J. Pharm. 2019, 561, 9–18. [Google Scholar] [CrossRef]
- Li, Q.P.; Zhai, W.W.; Jiang, Q.L.; Huang, R.; Liu, L.; Dai, J.; Gong, W.; Du, S.; Wu, Q. Curcumin-piperine mixtures in self-microemulsifying drug delivery system for ulcerative colitis therapy. Int. J. Pharm. 2015, 490, 22–31. [Google Scholar] [CrossRef]
- Ren, T.J.; Hu, M.Y.; Cheng, Y.; Shek, T.L.; Xiao, M.; Ho, N.J.; Zhang, C.; Leung, S.S.Y.; Zuo, Z. Piperine-loaded nanoparticles with enhanced dissolution and oral bioavailability for epilepsy control. Eur. J. Pharm. Sci. 2019, 137, 104988. [Google Scholar] [CrossRef] [PubMed]
- Zafar, A.; Iman, S.S.; Alruwaili, N.K.; Alsaidan, O.A.; Elkomy, M.H.; Ghoneim, M.M.; Alshehri, S.; Ali, A.M.A.; Alharbi, K.S.; Yasir, M.; et al. Development of piperine-loaded solid self-nanoemulsifying drug delivery system: Optimization, in-vitro, ex-vivo, and in-vivo evaluation. Nanomaterials 2021, 11, 2920. [Google Scholar] [CrossRef] [PubMed]
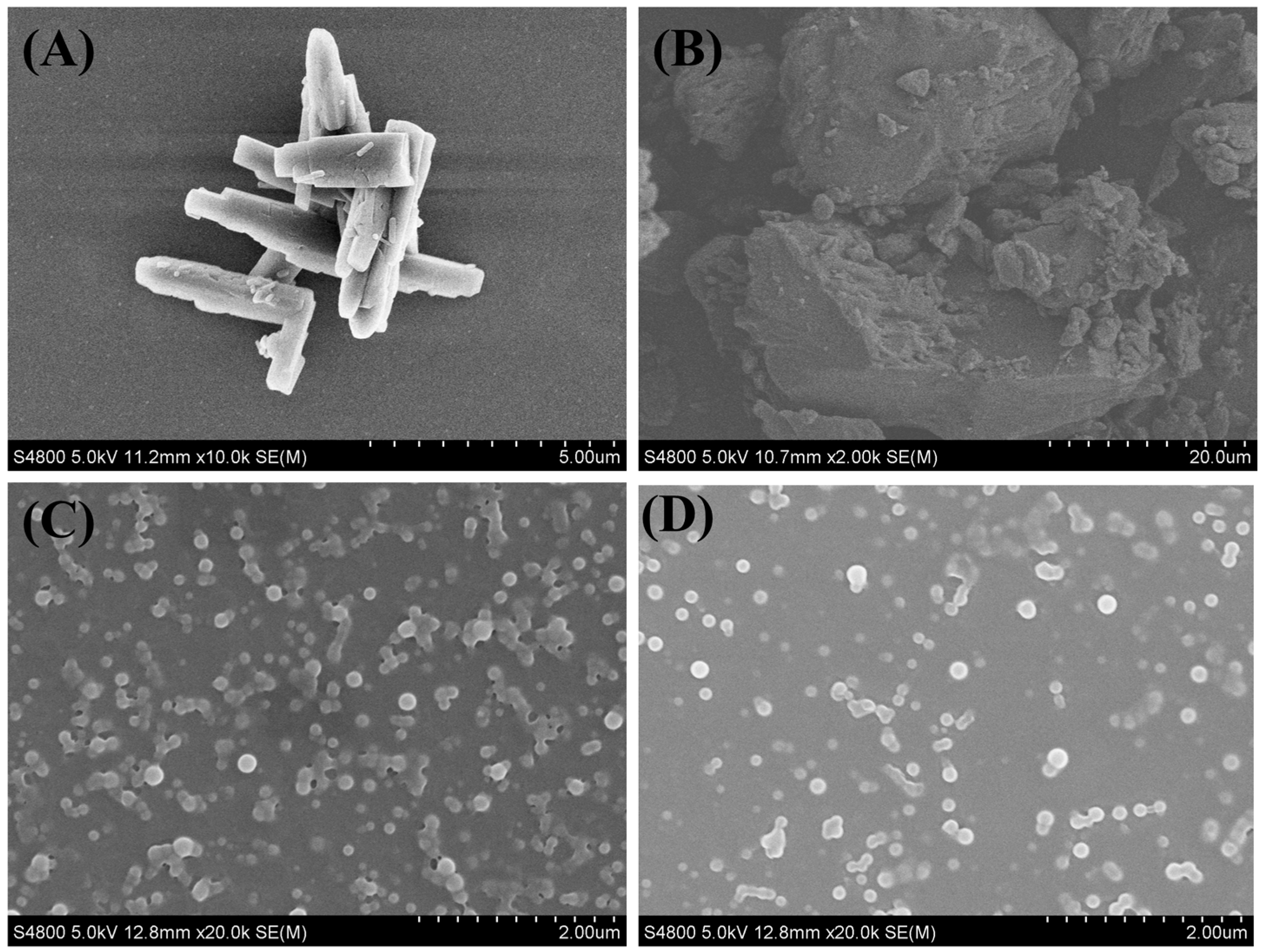
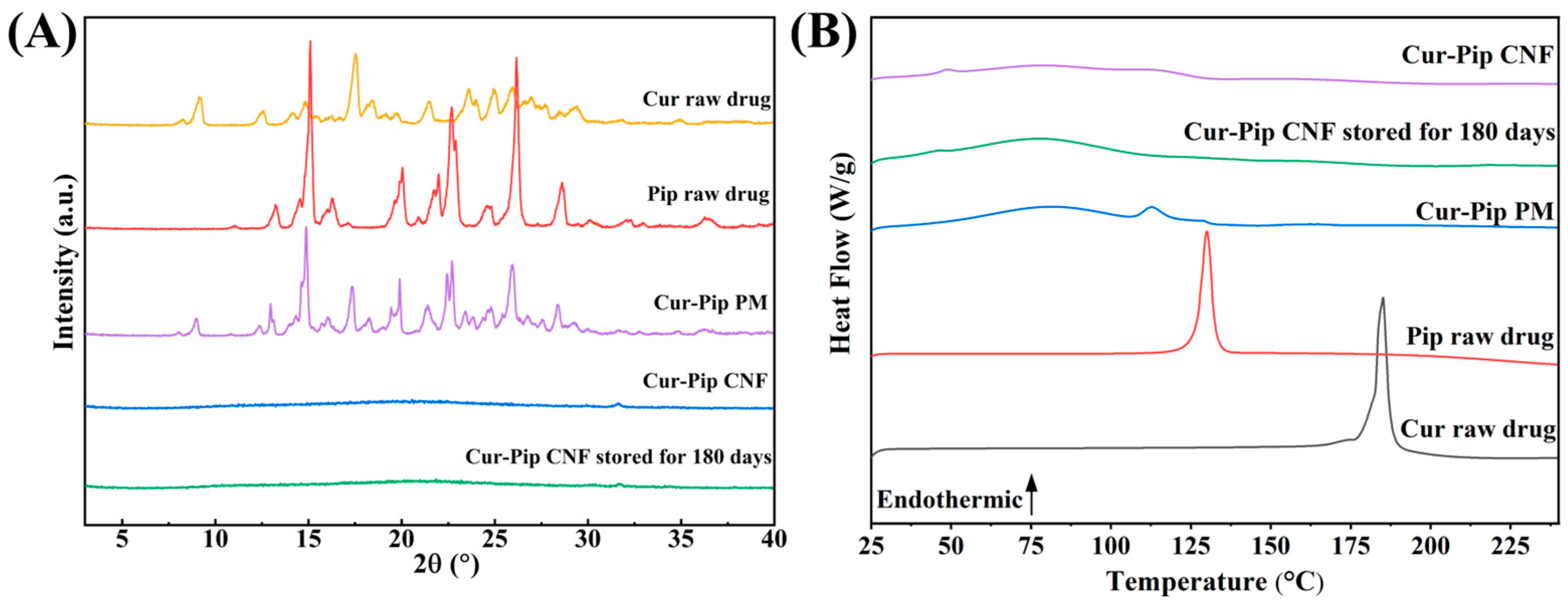

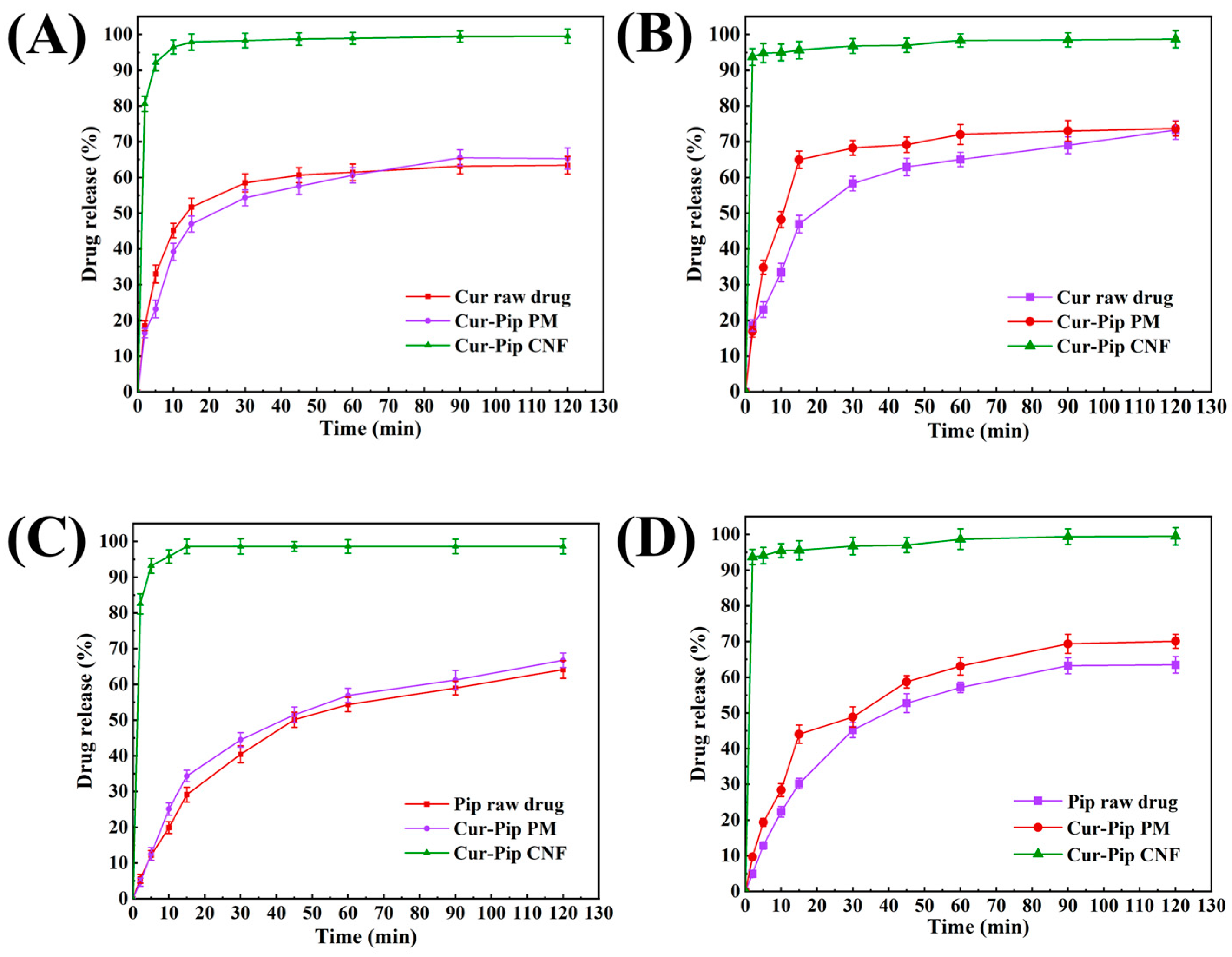
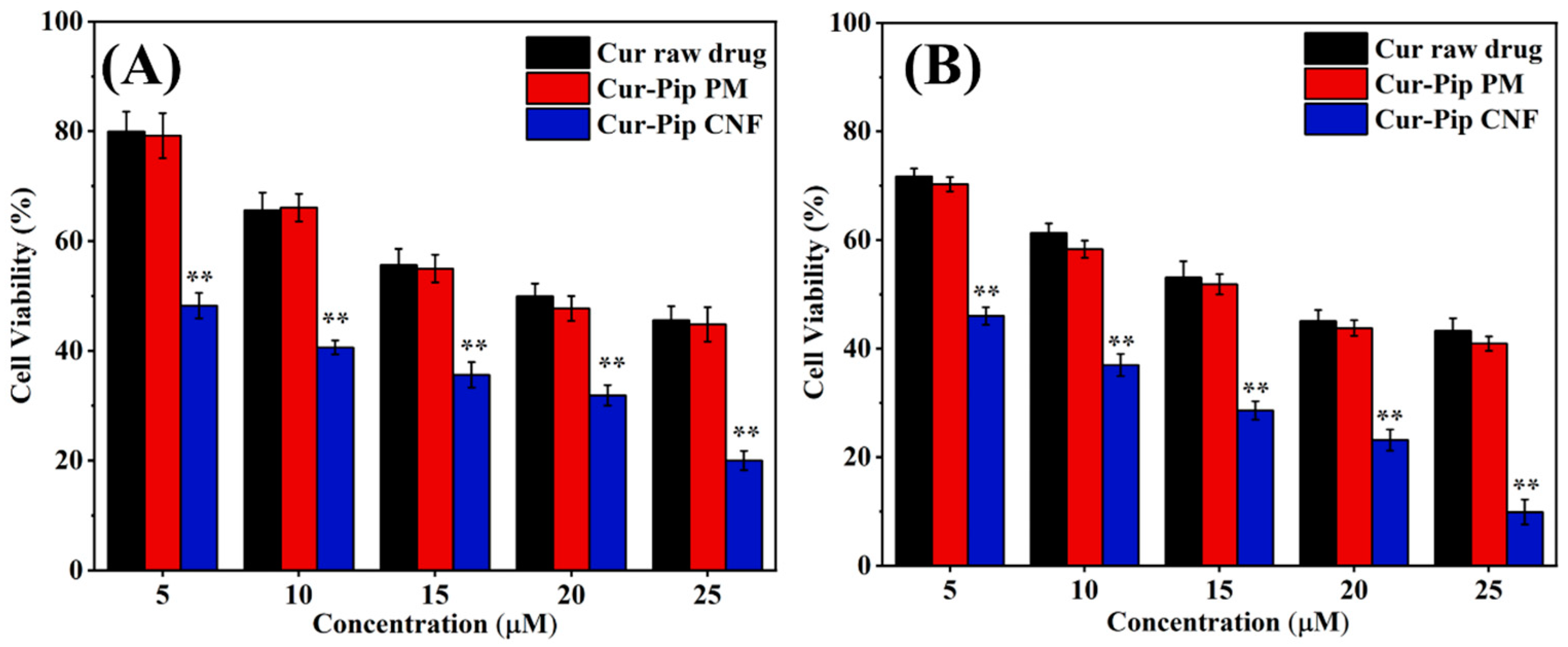
| Process Parameters of High-Gravity Technology | Particle Size of Cur-Pip Nanoparticles (nm) | ||
|---|---|---|---|
| Temperature (°C) | AS/S Ratio | Rotating Speed of RPB (rpm) | |
| 35 | 11/1 | 600 | 220 |
| 25 | 11/1 | 600 | 210 |
| 15 | 11/1 | 600 | 150 |
| 0 | 11/1 | 600 | 150 |
| 15 | 3/1 | 600 | 280 |
| 15 | 8/1 | 600 | 240 |
| 15 | 10/1 | 600 | 140 |
| 15 | 12/1 | 600 | 150 |
| 15 | 10/1 | 500 | 170 |
| 15 | 10/1 | 800 | 130 |
| 15 | 10/1 | 1000 | 140 |
| Model Drug | Parameters | Raw Drug | Cur-Pip PM | Cur-Pip CNF |
|---|---|---|---|---|
| Cur | Cmax (ng/mL) | 90.40 ± 12.80 | 108.69 ± 15.20 * | 601.27 ± 96.19 ** |
| Tmax (h) | 1.33 ± 0.52 | 1 ± 0 | 1.17 ± 0.41 | |
| AUC0–24 h (ng·h/mL) | 1019.76 ± 168.33 | 1217.19 ± 128.59 | 5713.5 ± 307.84 ** | |
| Pip | Cmax (ng/mL) | 2203.86 ± 248.33 | 2141.58 ± 459.51 | 6092.44 ± 402.59 ## |
| Tmax (h) | 3.67 ± 0.82 | 5 ± 1.10 | 1.33 ± 0.52 ## | |
| AUC0–24 h (ng·h/mL) | 21,894.12 ± 2790.69 | 25,259.18 ± 4006.03 | 52,162.70 ± 4812.74 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, N.; Liu, Y.; Liu, X.; Li, P.; Lu, Y.; Du, S.; Wu, K. The Controlled Preparation of a Carrier-Free Nanoparticulate Formulation Composed of Curcumin and Piperine Using High-Gravity Technology. Pharmaceutics 2024, 16, 808. https://doi.org/10.3390/pharmaceutics16060808
Han N, Liu Y, Liu X, Li P, Lu Y, Du S, Wu K. The Controlled Preparation of a Carrier-Free Nanoparticulate Formulation Composed of Curcumin and Piperine Using High-Gravity Technology. Pharmaceutics. 2024; 16(6):808. https://doi.org/10.3390/pharmaceutics16060808
Chicago/Turabian StyleHan, Ning, Yue Liu, Xin Liu, Pengyue Li, Yang Lu, Shouying Du, and Kai Wu. 2024. "The Controlled Preparation of a Carrier-Free Nanoparticulate Formulation Composed of Curcumin and Piperine Using High-Gravity Technology" Pharmaceutics 16, no. 6: 808. https://doi.org/10.3390/pharmaceutics16060808





