Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Cutaneous T-Cell Lymphoma: A First-in-Human Phase I/II Study †
Abstract
1. Introduction
2. Material and Methods
2.1. Design
2.2. Inclusion and Exclusion
2.3. Procedures
2.4. Safety and Tolerability
2.5. Response Assessments
2.6. Statistical Methods
3. Results
3.1. Patient and Treatments
3.2. Safety and Tolerability
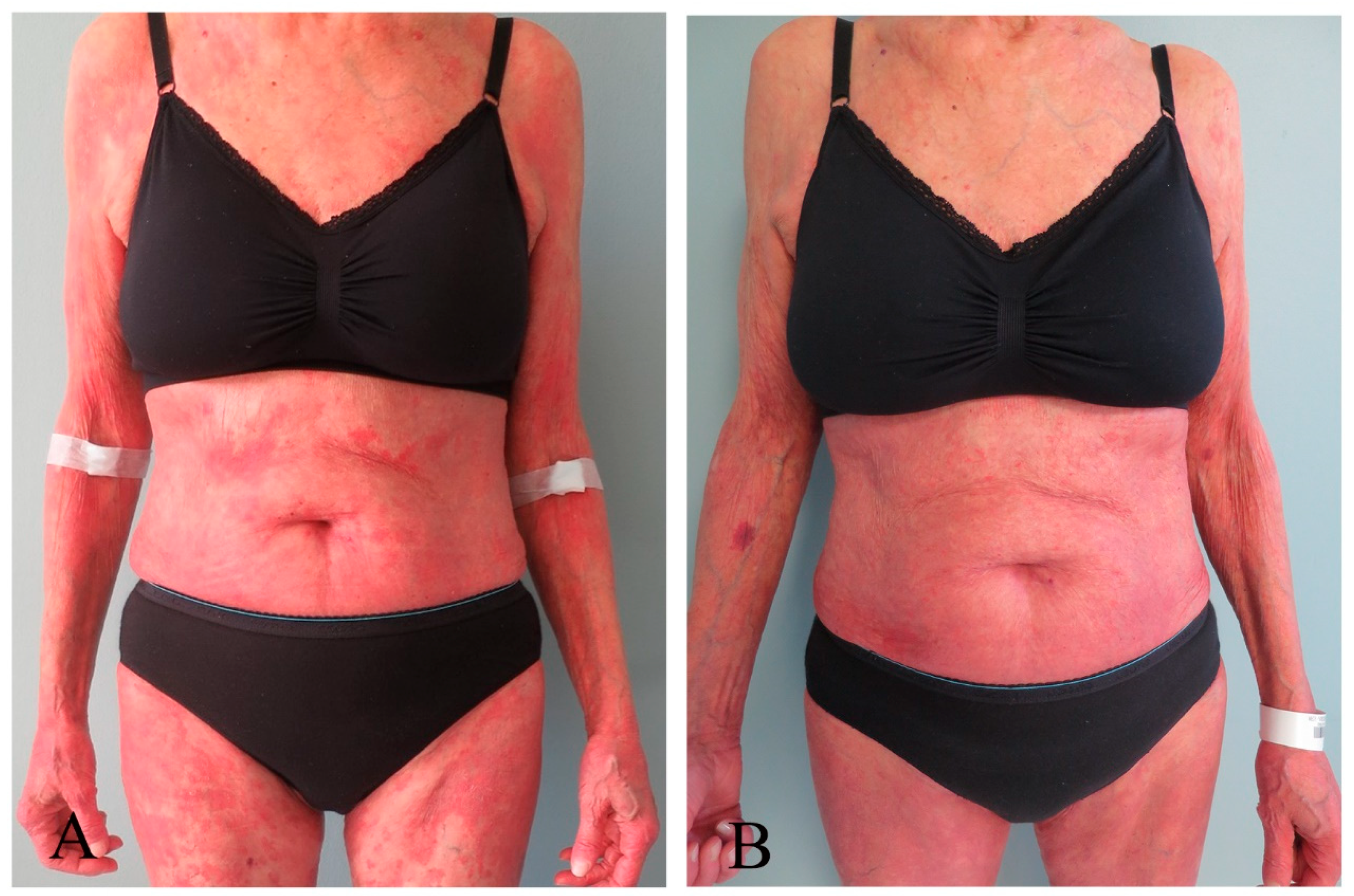
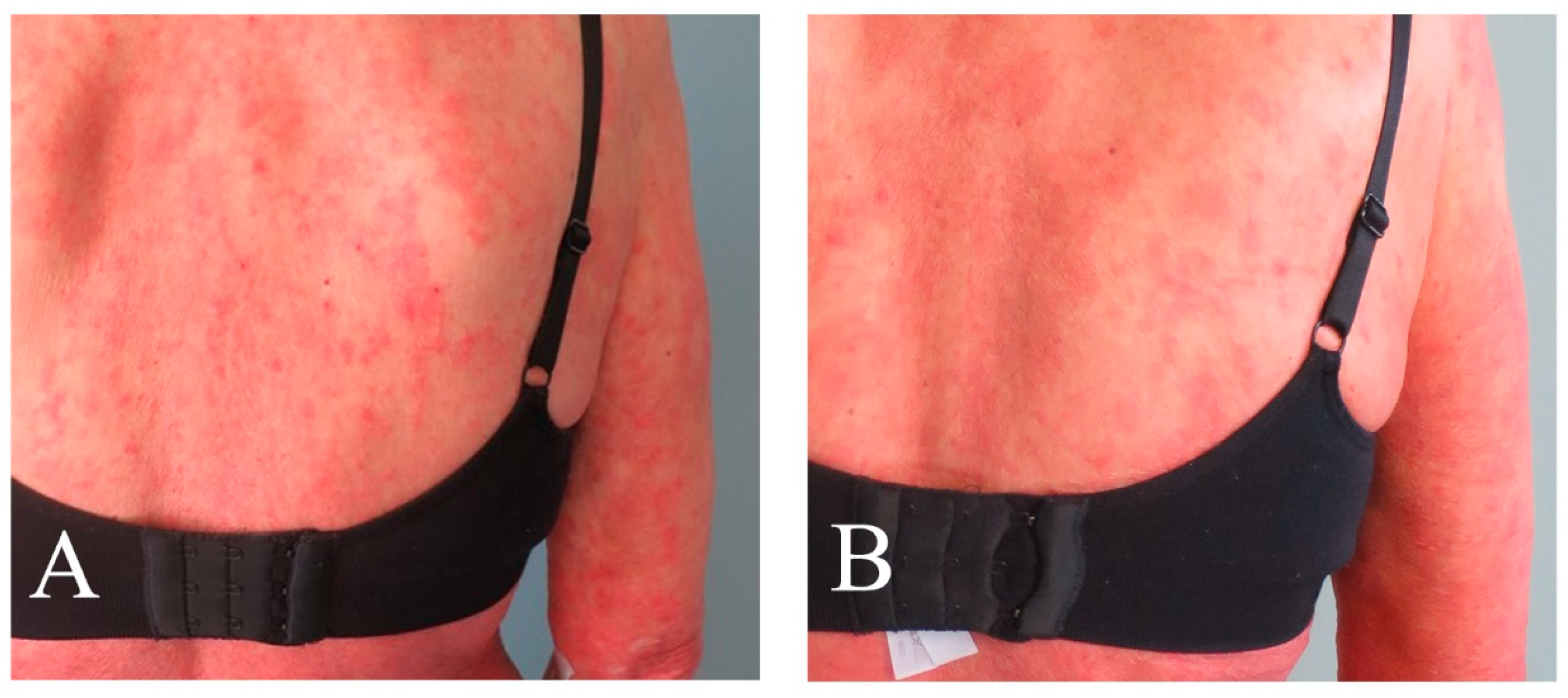
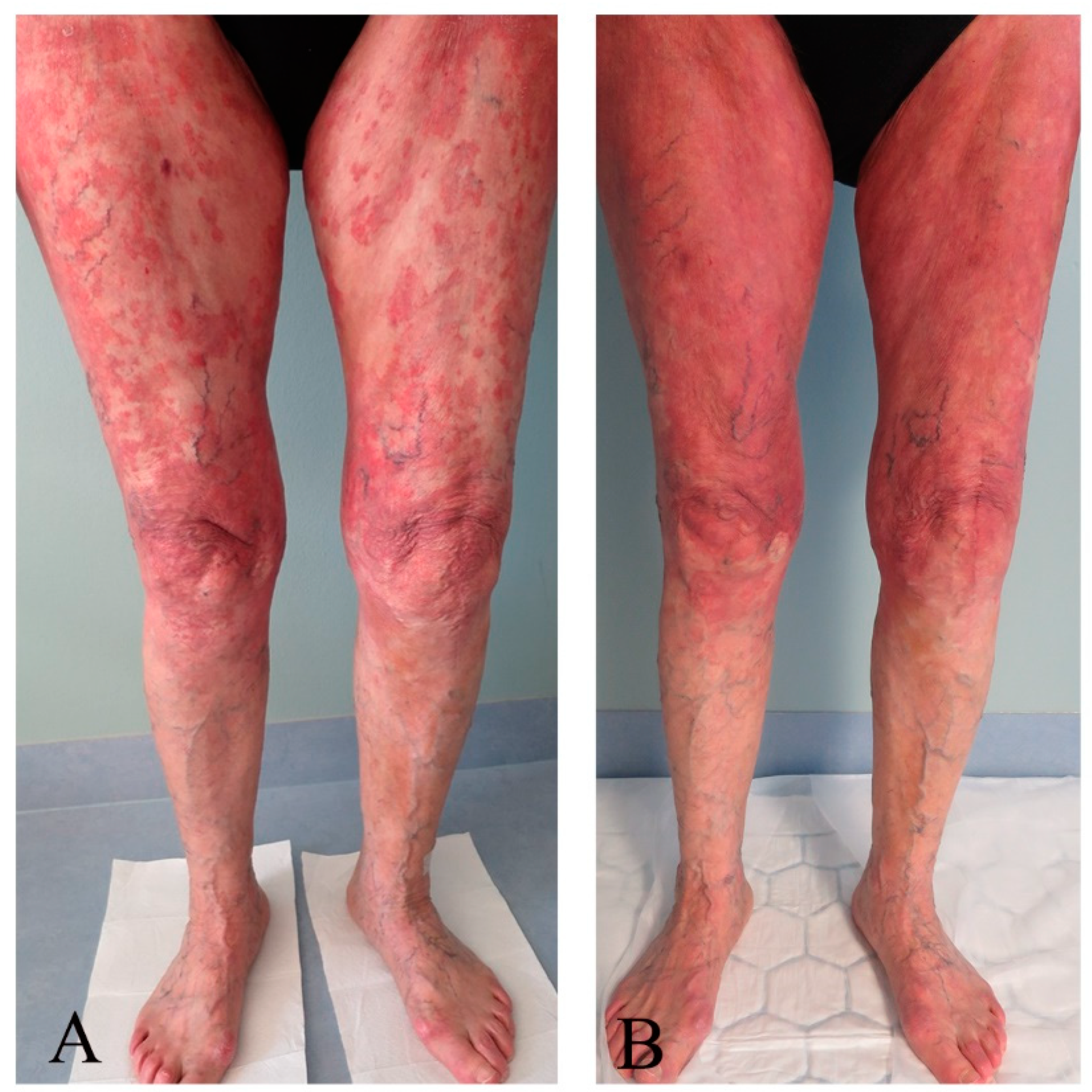
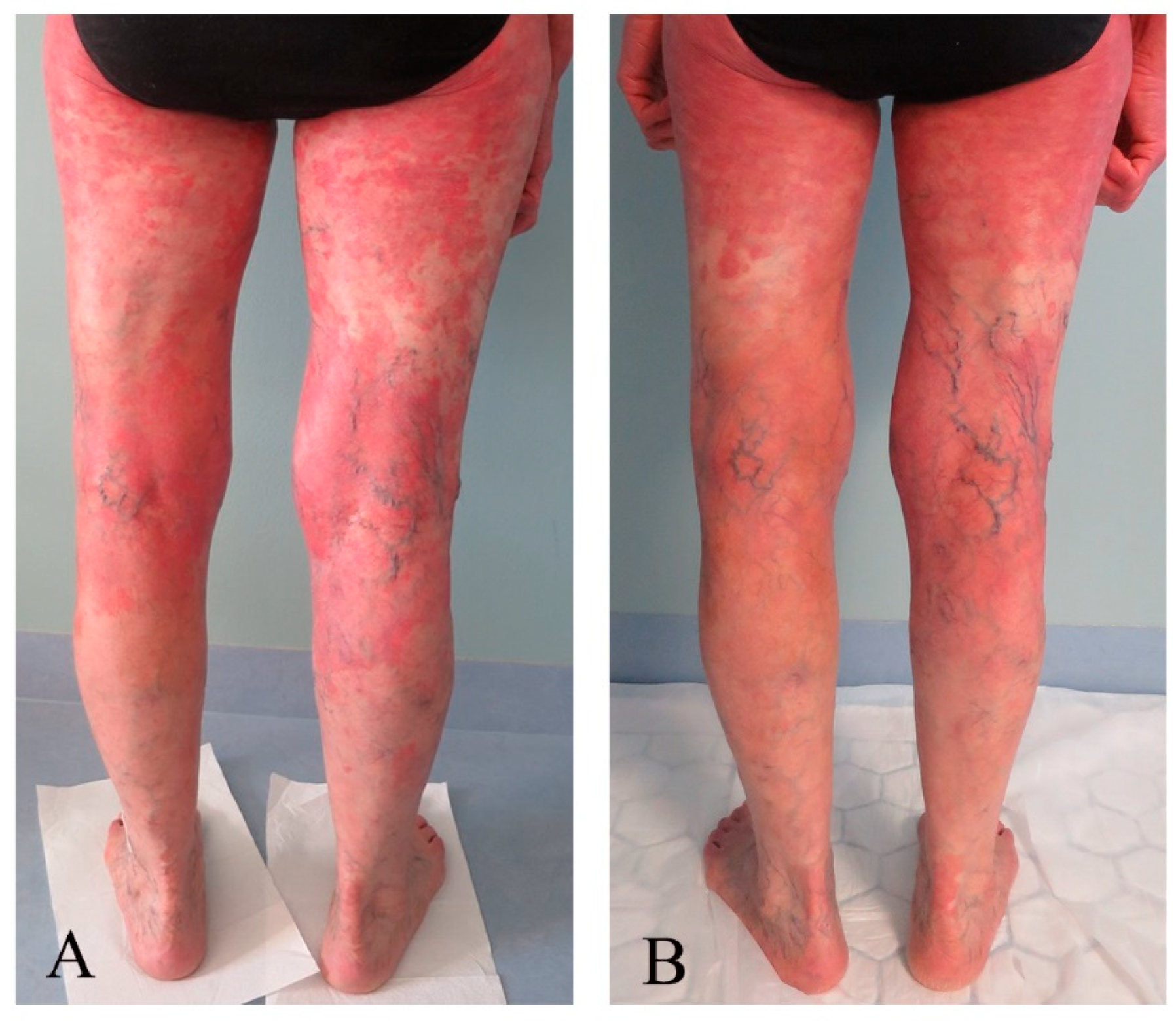
3.3. Response Assessments
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Edelson, R.; Berger, C.; Gasparro, F.; Jegasothy, B.; Heald, P.; Wintroub, B.; Vonderheid, E.; Knobler, R.; Wolff, K.; Plewig, G.; et al. Treatment of cutaneous T-cell lymphoma by extracorporeal photochemotherapy. Preliminary results. N. Engl. J. Med. 1987, 316, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Knobler, R.; Arenberger, P.; Arun, A.; Assaf, C.; Bagot, M.; Berlin, G.; Bohbot, A.; Calzavara-Pinton, P.; Child, F.; Cho, A.; et al. European dermatology forum–updated guidelines on the use of extracorporeal photopheresis 2020—Part 1. J. Eur. Acad. Dermatol. Venereol. 2020, 34, 2693–2716. [Google Scholar] [CrossRef] [PubMed]
- Mehta-Shah, N.; Horwitz, S.M.; Ansell, S.; Ai, W.Z.; Barnes, J.; Barta, S.K.; Clemens, M.W.; Dogan, A.; Fisher, K.; Goodman, A.M.; et al. NCCN Guidelines Insights: Primary Cutaneous Lymphomas, Version 2.2020. J. Natl. Compr. Cancer Netw. 2020, 18, 522–536. [Google Scholar] [CrossRef] [PubMed]
- Cho, A.; Jantschitsch, C.; Knobler, R. Extracorporeal Photopheresis—An Overview. Front. Med. 2018, 5, 236. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Warloe, T.; Berg, K.; Moan, J.; Kongshaug, M.; Giercksky, K.E.; Nesland, J.M. 5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges. Cancer 1997, 79, 2282–2308. [Google Scholar] [CrossRef]
- Noodt, B.B.; Berg, K.; Stokke, T.; Peng, Q.; Nesland, J.M. Apoptosis and necrosis induced with light and 5-aminolaevulinic acid-derived protoporphyrin IX. Br. J. Cancer 1996, 74, 22–29. [Google Scholar] [CrossRef] [PubMed]
- Darvekar, S.; Juzenas, P.; Oksvold, M.; Kleinauskas, A.; Holien, T.; Christensen, E.; Stokke, T.; Sioud, M.; Peng, Q. Selective Killing of Activated T Cells by 5-Aminolevulinic Acid Mediated Photodynamic Effect: Potential Improvement of Extracorporeal Photopheresis. Cancers 2020, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Warloe, T.; Kroon, S.; Funk, J.; Helsing, P.; Soler, A.M.; Stang, H.J.; Vatne, O.; Mork, C.; Norwegian Photodynamic Therapy, G. Guidelines for practical use of MAL-PDT in non-melanoma skin cancer. J. Eur. Acad. Dermatol. Venereol. 2010, 24, 505–512. [Google Scholar] [CrossRef]
- Stummer, W.; Pichlmeier, U.; Meinel, T.; Wiestler, O.D.; Zanella, F.; Reulen, H.J.; Group, A.L.-G.S. Fluorescence-guided surgery with 5-aminolevulinic acid for resection of malignant glioma: A randomised controlled multicentre phase III trial. Lancet Oncol. 2006, 7, 392–401. [Google Scholar] [CrossRef]
- Dunn, J.; Lovat, L. Photodynamic therapy using 5-aminolaevulinic acid for the treatment of dysplasia in Barrett’s oesophagus. Expert. Opin. Pharmacother. 2008, 9, 851–858. [Google Scholar] [CrossRef]
- Holien, T.; Gederaas, O.A.; Darvekar, S.R.; Christensen, E.; Peng, Q. Comparison between 8-methoxypsoralen and 5-aminolevulinic acid in killing T cells of photopheresis patients ex vivo. Lasers Surg. Med. 2018, 50, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Christensen, E.; Foss, O.A.; Quist-Paulsen, P.; Staur, I.; Pettersen, F.; Holien, T.; Juzenas, P.; Peng, Q. Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Patients with Chronic Graft-versus-Host Disease: A First-in-Human Study. Pharmaceutics 2021, 13, 1558. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.; Moan, J.; Warloe, T.; Nesland, J.M.; Rimington, C. Distribution and photosensitizing efficiency of porphyrins induced by application of exogenous 5-aminolevulinic acid in mice bearing mammary carcinoma. Int. J. Cancer 1992, 52, 433–443. [Google Scholar] [CrossRef] [PubMed]
- Gomer, C.J.; Jester, J.V.; Razum, N.J.; Szirth, B.C.; Murphree, A.L. Photodynamic therapy of intraocular tumors: Examination of hematoporphyrin derivative distribution and long-term damage in rabbit ocular tissue. Cancer Res. 1985, 45, 3718–3725. [Google Scholar] [PubMed]
- Stevens, S.R.; Ke, M.S.; Parry, E.J.; Mark, J.; Cooper, K.D. Quantifying skin disease burden in mycosis fungoides-type cutaneous T-cell lymphomas: The severity-weighted assessment tool (SWAT). Arch. Dermatol. 2002, 138, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Chren, M.M. The Skindex instruments to measure the effects of skin disease on quality of life. Dermatol. Clin. 2012, 30, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Luckett, T.; King, M.T.; Butow, P.N.; Oguchi, M.; Rankin, N.; Price, M.A.; Hackl, N.A.; Heading, G. Choosing between the EORTC QLQ-C30 and FACT-G for measuring health-related quality of life in cancer clinical research: Issues, evidence and recommendations. Ann. Oncol. 2011, 22, 2179–2190. [Google Scholar] [CrossRef] [PubMed]
- Regula, J.; MacRobert, A.J.; Gorchein, A.; Buonaccorsi, G.A.; Thorpe, S.M.; Spencer, G.M.; Hatfield, A.R.; Bown, S.G. Photosensitisation and photodynamic therapy of oesophageal, duodenal, and colorectal tumours using 5 aminolaevulinic acid induced protoporphyrin IX—A pilot study. Gut 1995, 36, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Yoon, H.K.; Lee, H.C.; Park, H.P.; Park, C.K.; Dho, Y.S.; Hwang, J.W. Preoperative 5-aminolevulinic acid administration for brain tumor surgery is associated with an increase in postoperative liver enzymes: A retrospective cohort study. Acta Neurochir. 2019, 161, 2289–2298. [Google Scholar] [CrossRef]
- Scarisbrick, J.J.; Taylor, P.; Holtick, U.; Makar, Y.; Douglas, K.; Berlin, G.; Juvonen, E.; Marshall, S.; Photopheresis Expert, G.U.K. consensus statement on the use of extracorporeal photopheresis for treatment of cutaneous T-cell lymphoma and chronic graft-versus-host disease. Br. J. Dermatol. 2008, 158, 659–678. [Google Scholar] [CrossRef]
- Scarisbrick, J. Extracorporeal photopheresis: What is it and when should it be used? Clin. Exp. Dermatol. 2009, 34, 757–760. [Google Scholar] [CrossRef] [PubMed]
- Quaglino, P.; Knobler, R.; Fierro, M.T.; Savoia, P.; Marra, E.; Fava, P.; Bernengo, M.G. Extracorporeal photopheresis for the treatment of erythrodermic cutaneous T-cell lymphoma: A single center clinical experience with long-term follow-up data and a brief overview of the literature. Int. J. Dermatol. 2013, 52, 1308–1318. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Jiang, W.; Andraos, T.Y.; Reddy, J.P.; Yehia, Z.A.; Lloyd, S.; Duvic, M.; D’Souza, N.M.; Milgrom, S.A.; Pinnix, C.C.; et al. Multi-institutional Investigation: Circulating CD4:CD8 ratio is a prognosticator of response to total skin electron beam radiation in mycosis fungoides. Radiother. Oncol. 2019, 131, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Cunderlikova, B.; Vasovic, V.; Sieber, F.; Furre, T.; Borgen, E.; Nesland, J.M.; Peng, Q. Hexaminolevulinate-mediated photodynamic purging of marrow grafts with murine breast carcinoma. Bone Marrow Transplant. 2011, 46, 1118–1127. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Espeland, K.; Kleinauskas, A.; Juzenas, P.; Brech, A.; Darvekar, S.; Vasovic, V.; Warloe, T.; Christensen, E.; Jahnsen, J.; Peng, Q. Photodynamic Effects with 5-Aminolevulinic Acid on Cytokines and Exosomes in Human Peripheral Blood Mononuclear Cells. Biomedicines 2022, 10, 232. [Google Scholar] [CrossRef]
| Treatment Day 1 | Treatment Day 2 | |||
|---|---|---|---|---|
| Test | Before | After | Before | After |
| Systolic BP, mm Hg | 123 (99–159) | 119 (99–142) | 123 (93–145) | 124 (102–144) |
| Diastolic BP, mm Hg | 68 (53–86) | 64 (52–89) | 62 (47–80) | 66 (52–80) |
| Pulse, beats/min | 61 (51–73) | 64 (59–71) | 63 (54–73) | 65 (55–76) |
| Temperature, °C | 35.9 (35.6–36.2) | 36.0 (35.8–36.3) | 35.9 (35.5–36.2) | 36.1 (36.0–36.3) |
| AST | 30 (26–34) | n.a. | n.a | 28 (25–38) |
| ALT | 26 (24–32) | n.a | n.a | 24 (20–27) |
| Bilirubin | 11 (9–12) | n.a | n.a | 9 (8–10) |
| PT-INR | 1.0 (1.0–1.3) | n.a. | n.a. | 1.2 (1.1–1.2) |
| Test [Reference Values] | Baseline | Mean (Min.–Max.) |
|---|---|---|
| Haemoglobin g/dL [11.7–15.3] | 12.8 | 12.2 (11.4–13.1) |
| White blood cells 109/L [39–73] | 4.8 | 4.4 (3.7–5.6) |
| Diff. neutrophils % [39–73] | 57 | 61 (51–75) |
| Diff. lymphocytes % [18–48] | 24 | 19 (14–22) |
| Diff. monocytes % [5–13] | 15 | 15 (7–21) |
| Diff. eosinophils % [0–8] | 4 | 4 (1–5) |
| Diff. basophils % [0.2–1.3] | 1.0 | 1.0 (1.0–1.0) |
| Platelet count 109/L [164–370] | 276 | 264 (220–280) |
| Mean cell volume fL [81–95] | 94 | 91.7 (90.0–93.0) |
| Mean cell haemoglobin pg [27.1–32.6] | 30.6 | 29.3 (28.5–30.3) |
| PT-INR [0.9–1.2] | 1.3 | 1.0 (1.0–1.0) |
| Albumin g/L [36–45] | 38 | 37.2 (34–40) |
| Protein, total g/L [62–78] | 51 | 57 (51–62) |
| ALT U/L [10–45] | 26 | 26.9 (24–32) |
| Alkaline phosphatase U/L [35–105] | 79 | 70.8 (62–87) |
| AST U/L [15–35] | 26 | 30.6 (26–34) |
| Bilirubin µmol/L [5–25] | 12 | 10.2 (8–12) |
| Cholesterol mmol/L [3.9–7.8] | 3.8 | 4.5 (4.0–5.2) |
| C-reactive protein mg/L [<5] | 5 | 7 (5–24) |
| Glycated hemoglobin A1c mmol/mol [28–40] | 35 | 35.6 (35–37) |
| Gamma-glutamyltransferase U/L [10–75] | 35 | 24.6 (17–34) |
| Creatinine µmol/L [45–90] | 46 | 46.8 (42–51) |
| Lactate dehydrogenase U/L [105–205] | 212 | 219 (193–233) |
| Potassium mmol/L [3.6–4.6] | 3.2 | 3.4 (3.3–3.6) |
| Sodium mmol/L [136–145] | 141 | 141 (139–143) |
| Caicium cation mmol/L [2.15–2.51] | 2.22 | 2.29 (2.20–2.35) |
| Erythrocyte sedimentation rate mm/h [≤17] | 7 | (7–24) |
| Lymphocyte subset CD4/CD8 ratio | 15.9 | 8.6 (7.4–9.5) |
| Type of Conceivable Event | Grade 1, No | Grade 2, No | Relation to Study Medication |
|---|---|---|---|
| Headache | 2 | 0 | Not applicable |
| Photosensitivity | 0 | 1 | Not applicable |
| Chills | 2 | 1 | Not applicable |
| Type of adverse event | |||
| Soreness in skin | 1 | 0 | Possibly |
| Clotting in photo chamber | 1 | 0 | Possibly |
| Target | Scoring Tool | Scoring Scale | Baseline | Last Control |
|---|---|---|---|---|
| Skin | mSWAT | 0–100 (0 = no symptom) | 93 | 29 |
| Itch | Visual analog scale | 0–10 (0 = no symptom) | 8 | 4 |
| Skindex, emotions | Questionnaire | 0–100 (0 = no symptom) | 48 | 3 |
| Skindex, symptoms | Questionnaire | 0–100 (0 = no symptom) | 39 | 25 |
| Skindex, function | Questionnaire | 0–100 (0 = no symptom) | 2 | 4 |
| Skindex, single item | Questionnaire | 0–100 (0 = no symptom) | 50 | 0 |
| EORTC30, functional | Questionnaire | 0–100% (0 = low function) | 100 | 100 |
| EORTC30, symptoms | Questionnaire | 0–100% (0 = low level of symptoms) | 0 | 0 |
| EORTC30, global health status | Questionnaire | 0–100% (0 = low state) | 75 | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christensen, E.; Foss, O.A.; Holien, T.; Juzenas, P.; Peng, Q. Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Cutaneous T-Cell Lymphoma: A First-in-Human Phase I/II Study. Pharmaceutics 2024, 16, 815. https://doi.org/10.3390/pharmaceutics16060815
Christensen E, Foss OA, Holien T, Juzenas P, Peng Q. Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Cutaneous T-Cell Lymphoma: A First-in-Human Phase I/II Study. Pharmaceutics. 2024; 16(6):815. https://doi.org/10.3390/pharmaceutics16060815
Chicago/Turabian StyleChristensen, Eidi, Olav Andreas Foss, Toril Holien, Petras Juzenas, and Qian Peng. 2024. "Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Cutaneous T-Cell Lymphoma: A First-in-Human Phase I/II Study" Pharmaceutics 16, no. 6: 815. https://doi.org/10.3390/pharmaceutics16060815
APA StyleChristensen, E., Foss, O. A., Holien, T., Juzenas, P., & Peng, Q. (2024). Application of Photodynamic Therapy with 5-Aminolevulinic Acid to Extracorporeal Photopheresis in the Treatment of Cutaneous T-Cell Lymphoma: A First-in-Human Phase I/II Study. Pharmaceutics, 16(6), 815. https://doi.org/10.3390/pharmaceutics16060815








