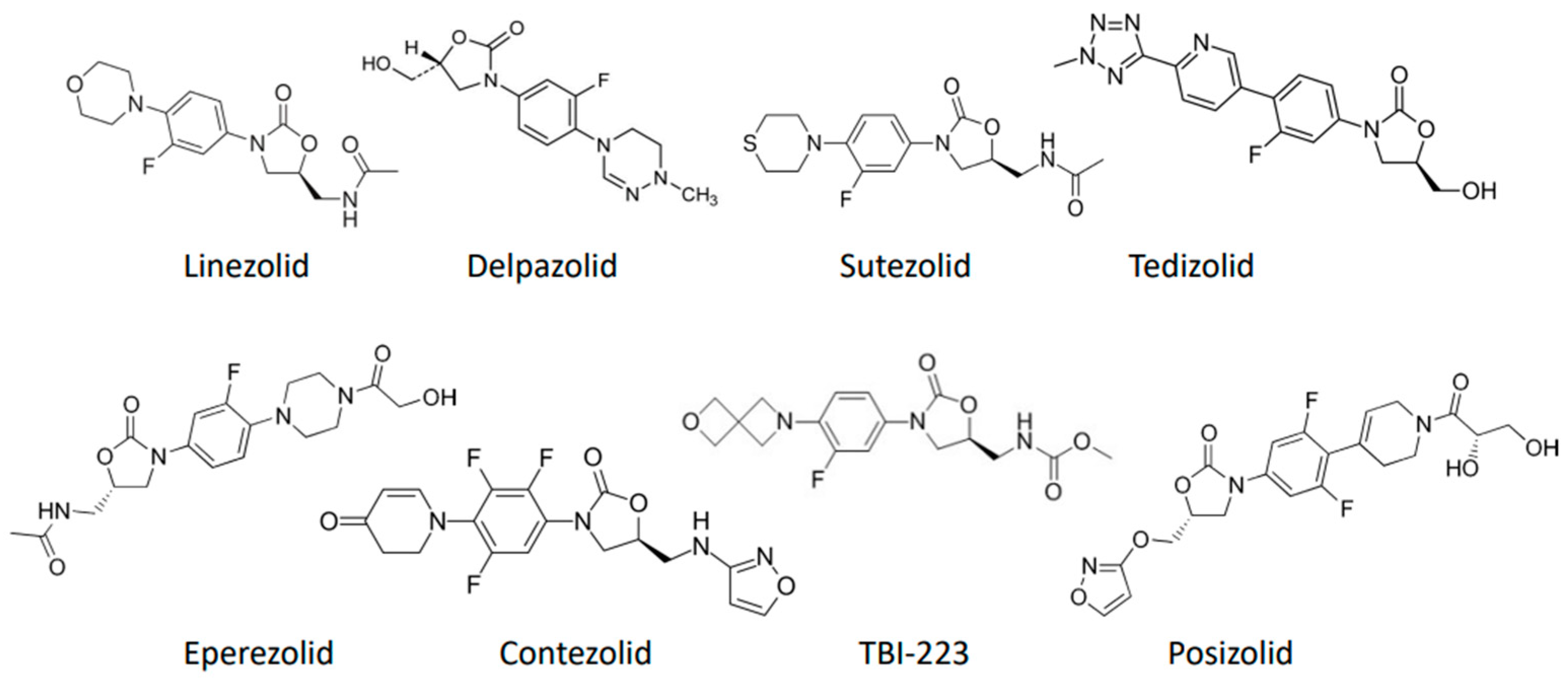
New Oxazolidinones for Tuberculosis: Are Novel Treatments on the Horizon?
Abstract
:1. Introduction
2. Materials and Methods
3. Results
3.1. Sutezolid
3.1.1. In Vitro Studies
3.1.2. In Vivo Studies
3.1.3. Clinical Studies
3.2. Tedizolid
3.2.1. In Vitro Studies
3.2.2. In Vivo Studies
3.2.3. Clinical Studies
3.3. Delpazolid
3.3.1. In Vitro Studies
3.3.2. In Vivo Studies
3.3.3. Clinical Studies
3.4. Eperezolid
3.4.1. In Vitro Studies
3.4.2. In Vivo Studies
3.4.3. Clinical Studies
3.5. Contezolid
3.5.1. In Vitro Studies
3.5.2. In Vivo Studies
3.5.3. Clinical Studies
3.6. Posizolid
3.6.1. In Vitro Studies
3.6.2. In Vivo Studies
3.6.3. Clinical Studies
3.7. TBI-223
3.7.1. In Vitro Studies
3.7.2. In Vivo Studies
3.7.3. Clinical Studies
| First Author | Drug Name | Model Type | TB Strain (n) | Bacterial Load (log10 CFU/mL) | Dose (mg/kg/d) | Intervention Duration (weeks) | CFU Reduction Outcome (log10 CFU/mL/d) | AUC (μg·h/mL) | MIC (μg/mL) | AUC/MIC |
|---|---|---|---|---|---|---|---|---|---|---|
| Ruiz [39] | Tedizolid | Bactec MGIT 960 | PS-TB (36) | N/A | <0.5 a | N/A | N/A | N/A | MIC50 0.25 (0.125–0.5) b MIC90 0.5 (0.125–0.5) b | N/A |
| SDR-TB (59) | MIC50 0.25 (0.06–0.5) b MIC90 0.5 (0.06–0.5) b | |||||||||
| MDR-TB (25) | MIC50 0.25 (0.125–0.5) b MIC90 0.5 (0.125–0.5) b | |||||||||
| Wang [43] | Tedizolid | MABA | H37Rv | N/A | 0.0625–64 a,b | N/A | N/A | N/A | 0.25 | N/A |
| DS-TB (17), MDR-TB (52) | MIC50 0.125 MIC90 0.25 ECOFF 0.25 | |||||||||
| Deshpande [40] | Tedizolid | Bactec MGIT 960 | H37Ra, H37Rv, CDC 1551, HN 878 | N/A | N/A | N/A | N/A | N/A | 0.25 | N/A |
| Bactec MGIT 960 | SS18b | N/A | N/A | N/A | N/A | N/A | 0.125 | N/A | ||
| Hollow fiber | H37Ra | 5.36 | 0–8 a,b | 4 | 0.14 c | 0–139.41 b | 0.5 | EC80 188.7 | ||
| Srivastava [41] | Tedizolid | Bactec MGIT 960 | H37Rv | N/A | N/A | N/A | N/A | N/A | 0.25 | N/A |
| Hollow fiber | 200 d | 6 | 0.17 | 31.0 ± 6.6 | 0.25 | EC80 200 | ||||
| Srivastava [44] | Tedizolid | Hollow fiber | H37Rv (log-phase growth) | 3.0 | TZD 200 mg d,e | 21 | 0.28 ± 0.12 | N/A | 0.25 | N/A |
| H37Rv (slowly replicating bacilli at pH 5.8) | TZD 200 mg d,e | 42 | 0.17 ± 0.027 | N/A | 0.25 | N/A | ||||
| SS18b (nonreplicating persisters) | TZD 200 mg d,e,f | 56 | 0.53 ± 0.09 | N/A | 0.25 | N/A | ||||
| Aono [42] | Tedizolid | Bactec MGIT 960 | MDR-TB (54) strains lineage 2.2.1 (Beijing) | 5.0 | 0.015–16 a,b | N/A | N/A | N/A | MIC50 0.25 (0.125–0.50) b MIC90 0.5 (0.125–0.50) b | N/A |
| H37Rv (7) strains resistant to LZD | N/A | N/A | N/A | N/A | N/A | 1.0 -16.0 b | N/A | |||
| Vera-Cabrera [37] | Tedizolid | MABA | SDR-TB (9), 25 MDR-TB (25), PS-TB (61) | 5.0–5.7 | N/A | N/A | N/A | N/A | MIC50 0.25 (0.125–0.5) b MIC90 0.5 (0.125–0.5) b | N/A |
| Molina-Torres [38] | Tedizolid | Middlebrook 7H10 Agar | H37Rv | 5.09 | 1 a (1 × MIC) | 72 h | 1.2 c | N/A | 1 | N/A |
| 16 a (16 × MIC) | N/A | 1.3 c | N/A | 1 | N/A | |||||
| Zurenko [62] | Eperezolid | Middlebrook 7H10 Agar | DS-TB (5) | N/A | 0.03–2 a,b | 3 | N/A | N/A | 0.125–0.5 b | N/A |
| MDR-TB (5) | N/A | N/A | 0.5–2 b | N/A | ||||||
| Brickner [60] | Eperezolid | Middlebrook 7H10 Agar | H37Rv | N/A | 0.03–2 a,b | 3 | N/A | N/A | ≤0.125 | N/A |
| Zong [52] | Delpazolid | MABA | MDR-TB (120) | N/A | N/A | N/A | N/A | N/A | ECOFF 2.0 MIC50 0.5 (<0.016–4) b MIC90 0.5 (<0.016–4) b | N/A |
| XDR-TB (120) | ECOFF 2.0 MIC50 0.5 (<0.016–>16) MIC90 1.0 (<0.016–>16) | |||||||||
| Shoen [69] | Contezolid | Middlebrook 7H10 Agar | H37Rv, ATCC 35,801 (strain Erdman) | 5 | N/A | N/A | N/A | N/A | MIC50 0.5 MIC90 1.0 | N/A |
| Wallis [24] | Sutezolid | Bactec MGIT 960 | H37Rv | N/A | 0.1 a | N/A | 0.442 | N/A | N/A | N/A |
| 1 a | 0.664 | |||||||||
| 2 a | 0.642 |
| First Author | Drug Name | Model Type | TB Strain | Inoculation Method | Bacterial Load (log10 CFU/Lung ± SD) | Route of Administration | Dose (mg) | Dose Frequency (h) | Treatment Duration (weeks) | PK data | Absolute CFU Reduction (log10 CFU/mL) | CFU Reduction per Week | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cmax (μg/mL) | AUC (μg·h/mL) | ||||||||||||
| Flanagan [46] | Tedizolid | Healthy subjects | N/A | N/A | N/A | PO | 200 | 24 | Single dose | 2.0 ± 0.4 | 25.4 ± 4.6 | N/A | N/A |
| 400 | 3.8 ± 1.0 | 56.1 ± 13.2 | |||||||||||
| 600 | 5.2 ± 0.7 | 79.3 ± 31.3 | |||||||||||
| 800 | 5.5 ± 1.2 | 91.8 ± 12.9 | |||||||||||
| 1200 | 9.5 ± 1.9 | 123.1 ± 31.2 | |||||||||||
| 200 | 24 | Day 15 | 1.8 ± 0.4 | 22.5 ± 6.5 | |||||||||
| 300 | Day 21 | 2.7 ± 0.5 | 31.2 ± 6.6 | ||||||||||
| 400 | Day 21 | 4.7 ± 0.5 | 52.0 ± 5.1 | ||||||||||
| Flanagan [47] | Tedizolid | Healthy subjects | N/A | N/A | N/A | IV | 100 | N/A | Single dose | 1.2 ± 0.2 | 17.4 ± 1.8 | N/A | N/A |
| 200 | 2.6 ± 0.6 | 32.6 ± 8.3 | |||||||||||
| 300 | 4.5 ± 1.1 | 51.9 ± 11.2 | |||||||||||
| 400 | 5.1 ± 0.8 | 58.7 ± 11.6 | |||||||||||
| Kim [27] | Tedizolid | Non-human primates | N/A | N/A | N/A | PO | 3 d | 24 | 8 days | 1.46 ± 0.51 | 24.9 ± 7.7 | N/A | N/A |
| Tasneen [28] | Tedizolid | BALB/c mice | N/A | N/A | N/A | PO | 10 d | N/A | Single dose | 6.4 ± 0.6 | 40.4 ± 2.9 | N/A | N/A |
| 20 d | 11.4 ± 1.6 | 77.7 ± 6.1 | |||||||||||
| Tasneen [28] | Tedizolid | BALB/c mice | H37Rv | Inhalation exposure system | 6.17 ± 0.27 | PO | 10 d,e | 24 l | 8 | N/A | N/A | Wk4: 1.97 ± 0.13 | 0.493 |
| Wk8: 4.50 ± 0.41 | 0.562 | ||||||||||||
| Kim [89] | Tedizolid | Healthy subjects | N/A | N/A | N/A | PO | 200 | N/A | Single dose | 2.7 ± 0.5 | 30.0 ± 6.6 | N/A | N/A |
| 400 | 5.1 ± 1.9 | 59.8 ± 12.8 | |||||||||||
| 600 | 7.0 ± 1.9 | 78.9 ± 17.5 | |||||||||||
| IV | 200 | 3.1 ± 0.5 | 31.5 ± 6.8 | ||||||||||
| Chen [90] | Tedizolid | Healthy subjects | N/A | N/A | N/A | PO | 200 | N/A | Single dose | 2.25 (1.66 –3.23) b | 26.1 (18.2–33.1) b | N/A | N/A |
| 200 | 24 | 1 | 2.36 (1.72–3.42) b | 25.1 (18.8–30.6) b | |||||||||
| IV | 200 | Single dose | 3.02 (1.86–3.89) b | 30.5 (22.9–40.2) b | |||||||||
| 200 | 3 days | 3.49 (2.95–4.41) b | N/A | ||||||||||
| Cho [54] | Delpazolid | Healthy subjects | N/A | N/A | N/A | PO | 400 | 12 | Single dose | 5.62 ± 2.39 | 7.79 ± 2.96 m | N/A | N/A |
| 1 | 5.11 ± 1.98 | 8.25 ± 2.77 m | |||||||||||
| 800 | Single dose | 11.15 ± 5.88 | 19.48 ± 4.43 m | ||||||||||
| 1 | 14.17 ± 4.69 | 28.08 ± 7.77 m | |||||||||||
| 1200 | Single dose | 13.83 ± 2.07 | 38.11 ± 14.19 m | ||||||||||
| 1 | 20.25 ± 7.15 | 41.12 ± 12.76 m | |||||||||||
| 1600 | Single dose | 26.75 ± 13.78 | 73.44 ± 34.95 m | ||||||||||
| Cho [56] | Delpazolid | Healthy subjects | N/A | N/A | N/A | IV | 200 mg/60 min | N/A | Single dose | 2.92 ± 0.46 | 5.63 ± 1.0 | N/A | N/A |
| 400 mg/60 min | 5.25 ± 0.96 | 9.42 ±1.46 | |||||||||||
| 800 mg/60 min | 12.16 ± 2.49 | 25.81 ± 8.12 | |||||||||||
| 800 mg/30 min | 16.69 ± 4.05 | 23.16 ± 5.21 | |||||||||||
| 1200 mg/30 min | 20.31 ± 3.22 | 28.82 ± 2.67 | |||||||||||
| PO | 800 mg | 8.20 ± 3.47 | 18.85 ± 4.98 | ||||||||||
| Zurenko [64] | Eperezolid | N/A | N/A | N/A | N/A | PO | 1000 | 6 | 14.25 days | 7840 | 26600 | N/A | N/A |
| Cynamon [5] | Eperezolid | CD1 Mice | ATCC 35,801 (strain Erdman) | Intravenous through caudal vein | 6.85 a | PO | 100 d | 24 l | 4 | N/A | N/A | 0.92 p ± 0.11 | 0.23 p |
| Kim [57] | Delpazolid | SSPTB (+) patients | N/A | N/A | 1 | PO | 800 | 24 | 2 | N/A | N/A | 0.044 ± 0.016 | 0.022 |
| 400 | 12 | 0.053 ± 0.017 | 0.027 | ||||||||||
| 800 | 12 | 0.043 ± 0.016 | 0.022 | ||||||||||
| 1200 | 24 | 0.019 ± 0.017 | 0.001 | ||||||||||
| Li [88] | TBI-223 | BALB/c mice | H37Rv | Inhalation exposure system | 8.85 ± 0.15 | PO | 100 d,j | 24 | 4 | N/A | 179 | 3.39 ± 0.40 | 0.848 |
| 8 | 7.23 ± 0.54 | 0.904 | |||||||||||
| 7.94 ± 0.27 | 100 d,k | 4 | 6.10 ± 0.63 | 1.53 | |||||||||
| Zhang [29] | Posizolid | BALB/c mice | H37Rv, SS18b | Inhalation exposure system | 7.0 a | PO | 125 d | 24 | 4 | N/A | N/A | N/A | N/A |
| Tasneen [28] | Posizolid | BALB/c mice | N/A | N/A | N/A | PO | 50 d | N/A | Single dose | 20.43 ± 2.08 | 74.76 ± 6.14 | N/A | N/A |
| 200 d | N/A | Single dose | 31.37 ± 1.27 | 220.25 ± 34.95 | N/A | N/A | |||||||
| Alsultan [79] | Posizolid | Patients with DS-TB | N/A | N/A | N/A | PO | 500 | 24 | N/A | N/A | N/A | N/A | N/A |
| 1200 | 24 | N/A | |||||||||||
| 500 | 12 | 126 | |||||||||||
| 800 | 12 | 201 | |||||||||||
| Furin [80] | Posizolid | Patients with DS-TB | N/A | N/A | 6.10 (5.59–6.61) c | PO | 500 | 24 | 2 | 5.56 (4.58–7.00) n | 43.97 (39.81–50.71) n | 0.04 p | 0.02 p |
| 5.77 (5.09–6.45) c | 1200 | 24 | 2 | 8.40 (7.82–10.05) n | 73.89 (56.88–83.12) n | 0.07 | 0.035 | ||||||
| 6.31 (5.67–6.96) c | 500 | 12 | 2 | 7.69 (7.32–9.17) n | 64.75 (54.12–70.32) n | 0.54 | 0.27 | ||||||
| 6.28 (5.62–6.94) c | 800 | 12 | 2 | 11.54 (10.13–12.05) n | 93.19 (79.81–105.36) n | 0.23 | 0.115 | ||||||
| Kim [57] | Sutezolid | Non-human primates | N/A | N/A | N/A | PO | 20 d | 24 | 4 days | 7.71 ± 4.95 | 28.28 ± 18.04 | N/A | N/A |
| 40 d | 24 | 4 days | 1.2 ± 1.24 | 2.28 ± 1.81 | N/A | N/A | |||||||
| 40 d | 12 | 3 days | N/A | N/A | N/A | N/A | |||||||
| Tasneen [28] | Sutezolid | BALB/c mice | N/A | N/A | N/A | PO | 50 d | N/A | Single dose | 1.29 ± 7.07 | 3.36 ± 4.99 | N/A | N/A |
| Lanoix [91] | Sutezolid | C3HeB/FeJ mice | H37Rv | Inhalation exposure system | 6.7–7.7 a,b | PO | 50 d | 24 | 4 | N/A | N/A | 0 | N/A |
| BALB/c mice | H37Rv | Inhalation exposure system | 6.2–6.8 a,b | PO | 50 d | 24 | 4 | N/A | N/A | 2.13 | 0.533 | ||
| Zhang [29] | Sutezolid | BALB/c mice | H37Rv, SS18b | Inhalation exposure system | 7.0 a | PO | 100 d | 24 | 4 | N/A | N/A | 0.3 | 0.075 |
| Lanoix [30] | Sutezolid | BALB/c mice | H37Rv | Inhalation exposure system | 4.23 ± 0.05 | PO | 50 d | 24 | 8 | N/A | N/A | Wk4: 0.9 ± 0.22 | 0.225 |
| Wk8: 1.64 ± 0.52 | 0.205 | ||||||||||||
| C3HeB/FeJ mice | H37Rv | 4.32 ± 0.41 | PO | 50 d | 24 | 8 | N/A | N/A | Wk4: 0.85 ± 0.40 | 0.213 | |||
| Wk8: 1.72 ± 0.43 | 0.215 | ||||||||||||
| Williams [92] | Sutezolid | BALB/c mice | H37Rv | Inhalation exposure system | 7.27 ± 0.44 | PO | 50 d,f | 24 l | 8 | N/A | N/A | Wk4: 3.79 ± 0.57 | 0.948 |
| Wk8: 6.9 ± 0.75 | 0.863 | ||||||||||||
| 50 d,g | Wk4: 3.9 ± 0.74 | 0.975 | |||||||||||
| Wk8: 7.27 | 0.909 | ||||||||||||
| 50 d,h | Wk4: 3.28 ± 0.89 | 0.82 | |||||||||||
| Wk8: 6.3 ± 1.18 | 0.788 | ||||||||||||
| Williams [26] | Sutezolid | BALB/c mice | H37Rv | Inhalation exposure system | 7.92 ± 0.15 | PO | 160 d,i | 24 l | 8 | N/A | N/A | 7.21 | 0.901 |
| Williams [25] | Sutezolid | BALB/c mice | H37Rv | Inhalation exposure system | 7.49 ± 0.11 | PO | 50 d | 24 l | 4 | D1: 6.07 D24: 4.32 | D1: 7.33 D24: 8.74 | 2.19 ± 0.53 | 0.548 |
| 100 d | 24 l | 2.4 ± 0.14 | 0.6 | ||||||||||
| 25 d | 12 l | 1.96 ± 0.14 | 0.49 | ||||||||||
| 50 d | 12 l | 2.58 ± 0.27 | 0.645 | ||||||||||
| Shoen [93] | Sutezolid | C57BL/6 mice | ATCC 35,801 (strain Erdman) | Inhalation exposure system | 6.00 a | PO | 100 d | 24 | 3 days | N/A | N/A | 0.81 ± 0.15 | N/A |
| Cynamon [5] | Sutezolid | CD1 Mice | ATCC 35,801 (strain Erdman) | Intravenous through caudal vein | 6.85 a | PO | 100 d | 24 l | 4 | N/A | N/A | 3.26 ± 0.33 | 0.815 |
| Wallis [31] | Sutezolid | SSPTB (+) patients | N/A | N/A | 6.88 ± 1.11 | PO | 600 | 12 | N/A | 0.986 (36) o | 6.494 (35) o | N/A | 0.176 ± 0.126 |
| 6.92 ± 1.20 | 1200 | 24 | 1.972 (50) o | 7.127 (36) o | 0.221 ± 0.080 | ||||||||
| Zhu [16] | Sutezolid | SSPTB (+) patients | N/A | N/A | N/A | PO | 600 | 12 | N/A | N/A | N/A | N/A | 0.269 (0.237–0.293) |
| 1200 | 24 | 0.186 (0.160–0.208) | |||||||||||
| Wallis [94] | Sutezolid | Healthy subjects | N/A | N/A | N/A | PO | 100 | 12 | 2 | 0.2524 (43) o | 0.8455 (43) o | N/A | N/A |
| 300 | 12 | 0.4587 (45) o | 2.133 (23) o | ||||||||||
| 600 | 12 | 0.9427 (20) o | 4.294 (23) o | ||||||||||
| 1200 | 24 | 2.016 (50) o | 10.10 (30) o | ||||||||||
| 600 | 12 | 4 | N/A | N/A | |||||||||
| First Author | Drug Name | Trial Phase | Study Design | Patients (n) | Type of TB | Treatment Regimen | Dose (mg) | Duration (weeks) | CFU Reduction (Log10 CFU/Ml) per Day | Mean Change in Time to Detection (MGIT) per Day | AUC (mg⋅h/L) | Cmax (mg/L) | MIC (mg/L) | AUC/MIC |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Kim [57] | Delpazolid | Phase 2 | RCT | 15 | SSPTB (+) | PO QD | 800 | 2 | 0.044 (22.9%) a | 2.69 | N/A | N/A | N/A | N/A |
| 16 | PO BD | 400 | 0.053 (27.6%) a | 0.30 | ||||||||||
| 16 | PO QID | 800 | 0.043 (22.4%) a | 2.33 | ||||||||||
| 16 | PO QD | 1200 | 0.019 (9.9%) a | 0.68 | ||||||||||
| Wallis [95] | Sutezolid | Phase 1 | RCT | 19 | Healthy subjects | PO QD | 1500 | N/A | N/A | N/A | N/A | 659 ± 165 | N/A | N/A |
| 1000 | 0.37 ± 0.06 | 839 ± 386 | ||||||||||||
| 600 | 0.06 | N/A | ||||||||||||
| 300 | 0.08 ± 0.07 | N/A |
| First Author | Drug Name | Dose (mg) | Vd/F (L) | CL/F (L/h) | Ka (h−1) | F |
|---|---|---|---|---|---|---|
| Flanagan [46] | Tedizolid | 200 | 95.7 (23.5) | 6.08 (1.08) | N/A | 0.91 (0.87–0.96) l |
| 400 | 87.0 (18.0) | 5.58 (1.23) | ||||
| 600 | 101 (23.5) | 6.58 (3.00) | ||||
| 800 | 101 (14.0) | 6.65 (0.94) | ||||
| 1200 | 116 (29.2) | 7.77 (2.24) | ||||
| 200 b | 117 (21.9) | 7.48 (2.12) | ||||
| 200 e | 108 (38.1) | 7.16 (1.99) | ||||
| 300 b | 116 (27.8) | 7.98 (1.80) | ||||
| 300 f | 127 (22.0) | 7.51 (1.60) | ||||
| 400 b | 64.8 (9.96) | 5.66 (0.835) | ||||
| 400 f | 108 (20.4) | 5.82 (0.607) | ||||
| Flanagan [47] | Tedizolid | 100 h | 74.5 (9.4) | 4.8 (0.5) | N/A | 0.915 |
| 200 h | 67.1 (15.3) | 5.4 (1.8) | ||||
| 300 h | 61.2 (15.2) | 4.9 (0.9) | ||||
| 400 h | 67.5 (12.2) | 5.8 (1.1) | ||||
| 200 h,i | 71.5 (12.7) | 5.9 (1.5) | ||||
| 200 i | 100.1 (17.7) | 6.5 (1.9) | ||||
| Kim [89] | Tedizolid | 200 | 89.7 | 5.7 (1.3) | N/A | 0.952 (0.927–0.978) l |
| 200 h | 92.1 | 5.5 (1.2) | ||||
| 400 | 91.3 | 5.7 (1.1) | ||||
| 600 | 99.0 | 6.6 (1.7) | ||||
| Chen [90] | Tedizolid | 200 | 92.4 (66.8–119) m | 6.31 (4.98–9.06) m | N/A | 0.855 (69.3–105) m |
| 200 h | 78.3 (59.7–105) m | 5.39 (4.09–7.19) m | ||||
| 200 c | 98.9 (86.5–114) m | 6.56 (5.37–8.75) m | ||||
| Cho [54] | Delpazolid | 400 c | 158.84 (115.53) | 53.14 (17.53) | N/A | N/A |
| 800 c | 72.26 (22.81) | 31.12 (11.65) | ||||
| 1200 c | 81.24 (27.38) | 32.88 (14.82) | ||||
| 1600 b | 76.7 (30.47) | 24.76 (7.98) | ||||
| Choi [55] | Delpazolid | 800 | 108.2 | 44.1 (18.3) | N/A | N/A |
| 800 a | 86.4 | 39.9 (14.2) | ||||
| 1200 a | 65.1 | 30.1 (7.5) | ||||
| Cho [53] | Delpazolid | 50 | 121.18 (10.45) | 57.47 (4.42) | N/A | N/A |
| 100 | 123.93 (31.3) | 62.28 (20.31) | ||||
| 200 | 136.36 (16.93) | 65.12 (11.02) | ||||
| 400 | 122.46 (20.10) | 55.16 (11.83) | ||||
| 800 | 97.21 (20.81) | 41.84 (7.80) | ||||
| 1600 | 94.96 (42.28) | 31.58 (8.10) | ||||
| 2400 | 153.79 (79.01) | 30.29 (9.72) | ||||
| 3200 | 85.57 (39.01) | 24.95 (6.02) | ||||
| Cho [56] | Delpazolidh | 200 mg/60 min | 87.48 (11.89) | 36.43 (6.23) | N/A | 0.998 (0.206) |
| 400 mg/60 min | 92.48 (13.57) | 43.31 (6.5) | ||||
| 800 mg/60 min | 76.39 (9.93) | 33.17 (8.47) | ||||
| 800 mg/30 min | 83.1 (13.67) | 35.84 (7.03) | ||||
| 1200 mg/30 min | 96.5 (18.06) | 41.96 (3.9) | ||||
| Bulitta [96] | Contezolid | 800 | 17.1 | 16 | N/A | 0.640 |
| Yang [97] | Contezolid | 1000 h | 67.77 | 3.05 (0.89) | N/A | N/A |
| 1500 h | 43.09 | 2.24 (0.62) | ||||
| 2000 h | 57.58 | 2.83 (1.03) | ||||
| 1500 | 2.97 | 0.13 (0.03) | ||||
| 1500 a | N/A | 0.16 (0.04) | ||||
| Li [98] | Contezolid | 600 | 0.53 | 0.16 (0.04) | 1.48 (0.19) | N/A |
| 800 | 0.66 | 0.18 (0.04) | 1.52 (0.31) | |||
| Eckburg [99] | Contezolid | 400 k | 61.0 (35.1) | 28.3 (12.1) | N/A | N/A |
| 400 j | 29.0 (5.5) | 16.3 (3.5) | ||||
| 800 k | 86.1 (32.5) | 35.2 (13.7) | ||||
| 800 j | 31.2 (7.7) | 16.7 (5.1) | ||||
| 1200 k | 150 (102) | 36.5 (10.0) | ||||
| 1200 j | 45.1 (9.9) | 20.6 (5.1) | ||||
| 800 a | 23.4 (4.5) | 13.9 (2.6) | ||||
| 800 a,d | 32.4 (17.1) | 13.2 (1.6) | ||||
| 800 a | 24.7 (7.5) | 14.6 (4.3) | ||||
| 800 a,g | 35.3 (16.5) | 14.3 (6.1) | ||||
| Wu [100] | Contezolid | 800 | 24.5 (13.1) | 8.83 (2.29) | 1.51 (0.70) | N/A |
| 1200 | 22.8 (15.1) | 6.19 (1.62) | 2.19 (0.38) | |||
| 1600 | 26.9 (10.7) | 6.98 (1.57) | 2.54 (0.89) | |||
| Bruinenberg [15] | Sutezolid | 300 | 990 (335) | 167 (38.2) | N/A | N/A |
| 600 | 1360 (436) | 161 (27.1) | ||||
| 1200 | 2060 (898) | 167 (54.8) | ||||
| 1800 | 2040 (382) | 145 (55.4) |
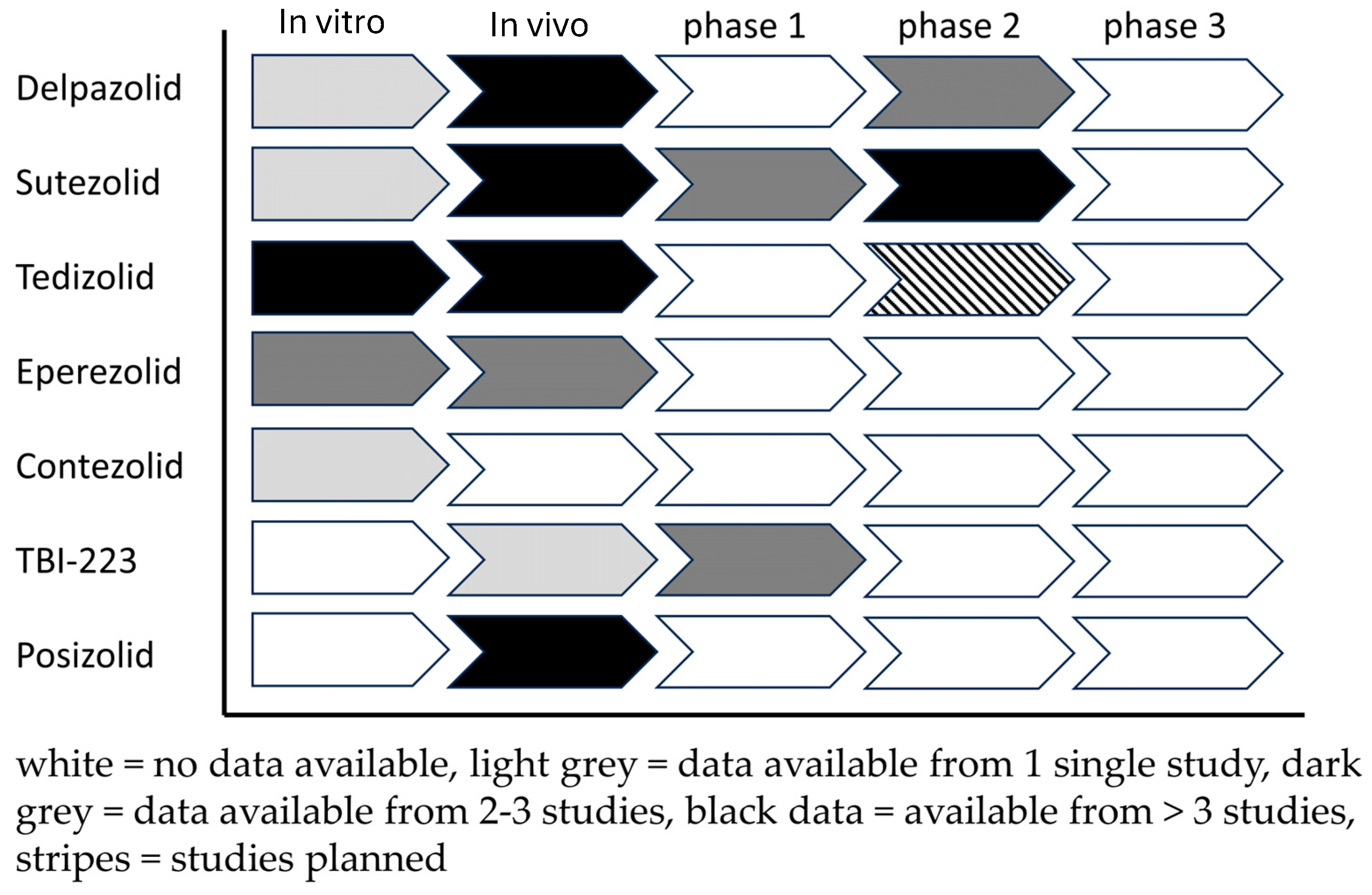
3.8. Landscape Analysis
4. Discussion
4.1. Limitations
4.2. Future Perspectives
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Dheda, K.; Mirzayev, F.; Cirillo, D.M.; Udwadia, Z.; Dooley, K.E.; Chang, K.-C.; Omar, S.V.; Reuter, A.; Perumal, T.; Horsburgh, C.R.; et al. Multidrug-resistant tuberculosis. Nat. Rev. Dis. Primers 2024, 10, 22. [Google Scholar] [CrossRef] [PubMed]
- Alcalá, L.; Ruiz-Serrano, M.J.; Pérez-Fernández Turégano, C.; García De Viedma, D.; Díaz-Infantes, M.; Marín-Arriaza, M.; Bouza, E. In Vitro Activities of Linezolid against Clinical Isolates of Mycobacterium tuberculosis That Are Susceptible or Resistant to First-Line Antituberculous Drugs. Antimicrob. Agents Chemother. 2003, 47, 416–417. [Google Scholar] [CrossRef] [PubMed]
- Erturan, Z.; Uzun, M. In vitro activity of linezolid against multidrug-resistant Mycobacterium tuberculosis isolates. Int. J. Antimicrob. Agents 2005, 26, 78–80. [Google Scholar] [CrossRef] [PubMed]
- Tato, M.; De La Pedrosa, E.G.-G.; Cantón, R.; Gómez-García, I.; Fortún, J.; Martín-Davila, P.; Baquero, F.; Gomez-Mampaso, E. In vitro activity of linezolid against Mycobacterium tuberculosis complex, including multidrug-resistant Mycobacterium bovis isolates. Int. J. Antimicrob. Agents 2006, 28, 75–78. [Google Scholar] [CrossRef]
- Cynamon, M.H.; Klemens, S.P.; Sharpe, C.A.; Chase, S. Activities of Several Novel Oxazolidinones against Mycobacterium tuberculosis in a Murine Model. Antimicrob. Agents Chemother. 1999, 43, 1189–1191. [Google Scholar] [CrossRef]
- Zhao, W.; Guo, Z.; Zheng, M.; Zhang, J.; Wang, B.; Li, P.; Fu, L.; Liu, S. Activity of linezolid-containing regimens against multidrug-resistant tuberculosis in mice. Int. J. Antimicrob. Agents 2014, 43, 148–153. [Google Scholar] [CrossRef]
- Soriano, A.; Miró, O.; Mensa, J. Mitochondrial Toxicity Associated with Linezolid. N. Engl. J. Med. 2005, 353, 2305–2306. [Google Scholar] [CrossRef]
- Sotgiu, G.; Centis, R.; D’Ambrosio, L.; Alffenaar, J.-W.C.; Anger, H.A.; Caminero, J.A.; Castiglia, P.; De Lorenzo, S.; Ferrara, G.; Koh, W.-J.; et al. Efficacy, safety and tolerability of linezolid containing regimens in treating MDR-TB and XDR-TB: Systematic review and meta-analysis. Eur. Respir. J. 2012, 40, 1430–1442. [Google Scholar] [CrossRef]
- World Health Organisation (WHO). WHO Consolidated Guidelines on Tuberculosis: Module 4: Treatment: Drug-Resistant Tuberculosis Treatment; WHO: Geneva, Switzerland, 2022. [Google Scholar]
- Negatu, D.A.; Aragaw, W.W.; Cangialosi, J.; Dartois, V.; Dick, T. Side-by-Side Profiling of Oxazolidinones to Estimate the Therapeutic Window against Mycobacterial Infections. Antimicrob. Agents Chemother. 2023, 67, e0165522. [Google Scholar] [CrossRef]
- Alffenaar, J.-W.C.; De Steenwinkel, J.E.M.; Diacon, A.H.; Simonsson, U.S.H.; Srivastava, S.; Wicha, S.G. Pharmacokinetics and pharmacodynamics of anti-tuberculosis drugs: An evaluation of in vitro, in vivo methodologies and human studies. Front. Pharmacol. 2022, 13, 1063453. [Google Scholar] [CrossRef]
- Romero, K.; Clay, R.; Hanna, D. Strategic Regulatory Evaluation and Endorsement of the Hollow Fiber Tuberculosis System as a Novel Drug Development Tool: Figure 1. Clin. Infect. Dis. 2015, 61, S5–S9. [Google Scholar] [CrossRef] [PubMed]
- Gumbo, T.; Pasipanodya, J.G.; Romero, K.; Hanna, D.; Nuermberger, E. Forecasting Accuracy of the Hollow Fiber Model of Tuberculosis for Clinical Therapeutic Outcomes. Clin. Infect. Dis. 2015, 61, S25–S31. [Google Scholar] [CrossRef] [PubMed]
- Jadhavar, P.S.; Vaja, M.D.; Dhameliya, T.M.; Chakraborti, A.K. Oxazolidinones as Anti-tubercular Agents: Discovery, Development and Future Perspectives. Curr. Med. Chem. 2015, 22, 4379–4397. [Google Scholar] [CrossRef] [PubMed]
- Bruinenberg, P.; Nedelman, J.; Yang, T.J.; Pappas, F.; Everitt, D. Single Ascending-Dose Study To Evaluate the Safety, Tolerability, and Pharmacokinetics of Sutezolid in Healthy Adult Subjects. Antimicrob. Agents Chemother. 2022, 66, e0210821. [Google Scholar] [CrossRef] [PubMed]
- Zhu, T.; Friedrich, S.O.; Diacon, A.; Wallis, R.S. Population Pharmacokinetic/Pharmacodynamic Analysis of the Bactericidal Activities of Sutezolid (PNU-100480) and Its Major Metabolite against Intracellular Mycobacterium tuberculosis in Ex Vivo Whole-Blood Cultures of Patients with Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 3306–3311. [Google Scholar] [CrossRef] [PubMed]
- Alffenaar, J.W.C.; Van Der Laan, T.; Simons, S.; Van Der Werf, T.S.; Van De Kasteele, P.J.; De Neeling, H.; Van Soolingen, D. Susceptibility of Clinical Mycobacterium tuberculosis Isolates to a Potentially Less Toxic Derivate of Linezolid, PNU-100480. Antimicrob. Agents Chemother. 2011, 55, 1287–1289. [Google Scholar] [CrossRef]
- Louie, A.; Brown, D.; Swift, M.; Files, K.; Drusano, G. Clinical Doses of PNU[1]100480 (U, Sutezolid) Plus Rifampin (R) Synergistically Kills Mycobacterium tuberculosis (Mtb) while Linezolid (L) Plus R Does Not. In Proceedings of the 52nd Inter-Science Conference on Anti-Microbials and Chemotherapy, San Francisco, CA, USA, 9–12 December 2012. [Google Scholar]
- Xue, B.; Okusanya, O.O.; Zhu, T.; van der Graaf, P.H.; Buli, C.C.; Bhavnani, S.M.; Ambrose, P.G.; Forrest, A. Pharmacokinetic-Pharmacodynamic (PK-PD) Analysis Evaluating the Effects of Rifampin (RIF), Linezolid (LZD), PNU-100480 (Sutezolid, U-480), and Its Metabolite PNU-101603 (U-603), as Single Agents, Against Mycobacterium tuberculosis (Mtb) in Log-Phase Growth Using Data from a Hollow Fiber Infection Model (HFIM). In Proceedings of the 52nd Inter-Science Conference on Anti-Microbials and Chemotherapy, San Francisco, CA, USA, 9–12 December 2012. [Google Scholar]
- Okusanya, O.O.; Xue, B.; Zhu, T.; van der Graaf, P.H.; Bulik, C.C.; Bhavnani, S.M.; Ambrose, P.G.; Forrest, A. Pharmacokinetic-Pharmacodynamic (PK-PD) Analysis Evaluating the Effects of Rifampin (RIF), PNU-100480 (Sutezolid, U-480), and Its Metabolite PNU-101603 (U-603), Alone and In Combination, Against Mycobacterium tuberculosis (Mtb) in the Non-Replicating Persister (NRP) State Using Data from a Hollow-Fiber Infection Model (HFIM). In Proceedings of the 52nd Inter-Science Conference on Anti-Microbials and Chemotherapy, San Francisco, CA, USA, 9–12 December 2012. [Google Scholar]
- Louie, A.E.K.; Files, K.; Swift, M.; Bahniuk, N.; Brown, D.; Drusano, G.L. Activities of PNU-100480 (PNU 480) alone, PNU 480 plus its major metabolite PNU-101603 (PNU 1603) and PNU 480 plus PNU 1603 in combination with rifampin (RIF) against Mycobacterium tuberculosis. In Proceedings of the 51st Inter-Science Conference on Anti-Microbials and Chemotherapy, Washington, DC, USA, 17–20 September 2011. [Google Scholar]
- Louie, A.B.D.; Files, K.; Swift, M.; Fikes, S.; Drusano, G. Pharmacodynamics of PNU-100480 (U, Sutezolid), A New Oxazolidinone, In Combination with Its Active Metabolite In The Killing of Mycobacterium tuberculosis (Mtb) in An in vitro Hollow Fiber Infection Model (HFIM). In Proceedings of the 52nd Inter-Science Conference on Anti-Microbials and Chemotherapy, San Francisco, CA, USA, 9–12 December 2012. [Google Scholar]
- Srivastava, S.; Magombedze, G.; Koeuth, T.; Sherman, C.; Pasipanodya, J.G.; Raj, P.; Wakeland, E.; Deshpande, D.; Gumbo, T. Linezolid Dose That Maximizes Sterilizing Effect While Minimizing Toxicity and Resistance Emergence for Tuberculosis. Antimicrob. Agents Chemother. 2017, 61, e00751-17. [Google Scholar] [CrossRef]
- Wallis, R.S.; Jakubiec, W.; Mitton-Fry, M.; Ladutko, L.; Campbell, S.; Paige, D.; Silvia, A.; Miller, P.F. Rapid Evaluation in Whole Blood Culture of Regimens for XDR-TB Containing PNU-100480 (Sutezolid), TMC207, PA-824, SQ109, and Pyrazinamide. PLoS ONE 2012, 7, e30479. [Google Scholar] [CrossRef]
- Williams, K.N.; Stover, C.K.; Zhu, T.; Tasneen, R.; Tyagi, S.; Grosset, J.H.; Nuermberger, E. Promising Antituberculosis Activity of the Oxazolidinone PNU-100480 Relative to That of Linezolid in a Murine Model. Antimicrob. Agents Chemother. 2009, 53, 1314–1319. [Google Scholar] [CrossRef]
- Williams, K.N.; Brickner, S.J.; Stover, C.K.; Zhu, T.; Ogden, A.; Tasneen, R.; Tyagi, S.; Grosset, J.H.; Nuermberger, E.L. Addition of PNU-100480 to First-Line Drugs Shortens the Time Needed to Cure Murine Tuberculosis. Am. J. Respir. Crit. Care Med. 2009, 180, 371–376. [Google Scholar] [CrossRef]
- Kim, S.; Scanga, C.A.; Miranda Silva, C.D.; Zimmerman, M.; Causgrove, C.; Stein, B.; Dartois, V.; Peloquin, C.A.; Graham, E.; Louie, A.; et al. Pharmacokinetics of tedizolid, sutezolid, and sutezolid-M1 in non-human primates. Eur. J. Pharm. Sci. 2020, 151, 105421. [Google Scholar] [CrossRef] [PubMed]
- Tasneen, R.; Betoudji, F.; Tyagi, S.; Li, S.-Y.; Williams, K.; Converse, P.J.; Dartois, V.; Yang, T.; Mendel, C.M.; Mdluli, K.E.; et al. Contribution of Oxazolidinones to the Efficacy of Novel Regimens Containing Bedaquiline and Pretomanid in a Mouse Model of Tuberculosis. Antimicrob. Agents Chemother. 2016, 60, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Sala, C.; Dhar, N.; Vocat, A.; Sambandamurthy, V.K.; Sharma, S.; Marriner, G.; Balasubramanian, V.; Cole, S.T. In Vitro and In Vivo Activities of Three Oxazolidinones against Nonreplicating Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 3217–3223. [Google Scholar] [CrossRef] [PubMed]
- Lanoix, J.-P.; Betoudji, F.; Nuermberger, E. Novel Regimens Identified in Mice for Treatment of Latent Tuberculosis Infection in Contacts of Patients with Multidrug-Resistant Tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 2316–2321. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Dawson, R.; Friedrich, S.O.; Venter, A.; Paige, D.; Zhu, T.; Silvia, A.; Gobey, J.; Ellery, C.; Zhang, Y.; et al. Mycobactericidal Activity of Sutezolid (PNU-100480) in Sputum (EBA) and Blood (WBA) of Patients with Pulmonary Tuberculosis. PLoS ONE 2014, 9, e94462. [Google Scholar] [CrossRef]
- Livermore, D.M.; Mushtaq, S.; Warner, M.; Woodford, N. Activity of oxazolidinone TR-700 against linezolid-susceptible and -resistant staphylococci and enterococci. J. Antimicrob. Chemother. 2009, 63, 713–715. [Google Scholar] [CrossRef]
- Locke, J.B.; Finn, J.; Hilgers, M.; Morales, G.; Rahawi, S.; Kedar, G.C.; Picazo, J.J.; Im, W.; Shaw, K.J.; Stein, J.L. Structure-Activity Relationships of Diverse Oxazolidinones for Linezolid-Resistant Staphylococcus aureus Strains Possessing the cfr Methyltransferase Gene or Ribosomal Mutations. Antimicrob. Agents Chemother. 2010, 54, 5337–5343. [Google Scholar] [CrossRef] [PubMed]
- Shaw, K.J.; Poppe, S.; Schaadt, R.; Brown-Driver, V.; Finn, J.; Pillar, C.M.; Shinabarger, D.; Zurenko, G. In Vitro Activity of TR-700, the Antibacterial Moiety of the Prodrug TR-701, against Linezolid-Resistant Strains. Antimicrob. Agents Chemother. 2008, 52, 4442–4447. [Google Scholar] [CrossRef] [PubMed]
- Rybak, J.M.; Marx, K.; Martin, C.A. Early Experience with Tedizolid: Clinical Efficacy, Pharmacodynamics, and Resistance. Pharmacotherapy 2014, 34, 1198–1208. [Google Scholar] [CrossRef]
- Burdette, S.D.; Trotman, R. Tedizolid: The First Once-Daily Oxazolidinone Class Antibiotic. Clin. Infect. Dis. 2015, 61, 1315–1321. [Google Scholar] [CrossRef]
- Vera-Cabrera, L.; Gonzalez, E.; Rendon, A.; Ocampo-Candiani, J.; Welsh, O.; Velazquez-Moreno, V.M.; Hak Choi, S.; Molina-Torres, C. In Vitro Activities of DA-7157 and DA-7218 against Mycobacterium tuberculosis and Nocardia brasiliensis. Antimicrob. Agents Chemother. 2006, 50, 3170–3172. [Google Scholar] [CrossRef] [PubMed]
- Molina-Torres, C.A.; Barba-Marines, A.; Valles-Guerra, O.; Ocampo-Candiani, J.; Cavazos-Rocha, N.; Pucci, M.J.; Castro-Garza, J.; Vera-Cabrera, L. Intracellular activity of tedizolid phosphate and ACH-702 versus Mycobacterium tuberculosis infected macrophages. Ann. Clin. Microbiol. Antimicrob. 2014, 13, 13. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Causse, M.; Vaquero, M.; Casal, M. In Vitro Activity of Tedizolid against Mycobacterium Tuberculosis. Antimicrob. Agents Chemother. 2019, 63, e01939-18. [Google Scholar] [CrossRef] [PubMed]
- Deshpande, D.; Srivastava, S.; Nuermberger, E.; Koeuth, T.; Martin, K.R.; Cirrincione, K.N.; Lee, P.S.; Gumbo, T. Multiparameter Responses to Tedizolid Monotherapy and Moxifloxacin Combination Therapy Models of Children with Intracellular Tuberculosis. Clin. Infect. Dis. 2018, 67, S342–S348. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Deshpande, D.; Nuermberger, E.; Lee, P.S.; Cirrincione, K.; Dheda, K.; Gumbo, T. The Sterilizing Effect of Intermittent Tedizolid for Pulmonary Tuberculosis. Clin. Infect. Dis. 2018, 67, S336–S341. [Google Scholar] [CrossRef] [PubMed]
- Aono, A.; Murase, Y.; Chikamatsu, K.; Igarashi, Y.; Shimomura, Y.; Hosoya, M.; Osugi, A.; Morishige, Y.; Takaki, A.; Yamada, H.; et al. In vitro activity of tedizolid and linezolid against multidrug-resistant Mycobacterium tuberculosis: A comparative study using microdilution broth assay and genomics. Diagn. Microbiol. Infect. Dis. 2022, 103, 115714. [Google Scholar] [CrossRef]
- Wang, C.; Wang, G.; Huo, F.; Xue, Y.; Jia, J.; Dong, L.; Zhao, L.; Wang, F.; Huang, H.; Duan, H. Novel oxazolidinones harbor potent in vitro activity against the clinical isolates of multidrug-resistant Mycobacterium tuberculosis in China. Front. Med. 2022, 9, 1067516. [Google Scholar] [CrossRef]
- Srivastava, S.; Cirrincione, K.N.; Deshpande, D.; Gumbo, T. Tedizolid, Faropenem, and Moxifloxacin Combination With Potential Activity against Nonreplicating Mycobacterium tuberculosis. Front. Pharmacol. 2021, 11, 616294. [Google Scholar] [CrossRef] [PubMed]
- Tessier, P.R.; Keel, R.A.; Hagihara, M.; Crandon, J.L.; Nicolau, D.P. Comparative In Vivo Efficacies of Epithelial Lining Fluid Exposures of Tedizolid, Linezolid, and Vancomycin for Methicillin-Resistant Staphylococcus aureus in a Mouse Pneumonia Model. Antimicrob. Agents Chemother. 2012, 56, 2342–2346. [Google Scholar] [CrossRef]
- Flanagan, S.D.; Bien, P.A.; Muñoz, K.A.; Minassian, S.L.; Prokocimer, P.G. Pharmacokinetics of Tedizolid Following Oral Administration: Single and Multiple Dose, Effect of Food, and Comparison of Two Solid Forms of the Prodrug. Pharmacotherapy 2014, 34, 240–250. [Google Scholar] [CrossRef]
- Flanagan, S.; Fang, E.; Muñoz, K.A.; Minassian, S.L.; Prokocimer, P.G. Single- and Multiple-Dose Pharmacokinetics and Absolute Bioavailability of Tedizolid. Pharmacotherapy 2014, 34, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Flanagan, S.; Passarell, J.; Lu, Q.; Fiedler-Kelly, J.; Ludwig, E.; Prokocimer, P. Tedizolid Population Pharmacokinetics, Exposure Response, and Target Attainment. Antimicrob. Agents Chemother. 2014, 58, 6462–6470. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.L.; Jang, J. Development of Delpazolid for the Treatment of Tuberculosis. Appl. Sci. 2020, 10, 2211. [Google Scholar] [CrossRef]
- Jeong, J.-W.; Jung, S.-J.; Lee, H.-H.; Kim, Y.-Z.; Park, T.-K.; Cho, Y.-L.; Chae, S.-E.; Baek, S.-Y.; Woo, S.-H.; Lee, H.-S.; et al. In Vitro and In Vivo Activities of LCB01-0371, a New Oxazolidinone. Antimicrob. Agents Chemother. 2010, 54, 5359–5362. [Google Scholar] [CrossRef] [PubMed]
- Sunwoo, J.; Kim, Y.K.; Choi, Y.; Yu, K.-S.; Nam, H.; Cho, Y.L.; Yoon, S.; Chung, J.-Y. Effect of food on the pharmacokinetic characteristics of a single oral dose of LCB01-0371, a novel oxazolidinone antibiotic. Drug Des. Dev. Ther. 2018, 12, 1707–1714. [Google Scholar] [CrossRef] [PubMed]
- Zong, Z.; Jing, W.; Shi, J.; Wen, S.a.; Zhang, T.; Huo, F.; Shang, Y.; Liang, Q.; Huang, H.; Pang, Y. Comparison of In Vitro Activity and MIC Distributions between the Novel Oxazolidinone Delpazolid and Linezolid against Multidrug-Resistant and Extensively Drug-Resistant Mycobacterium tuberculosis in China. Antimicrob. Agents Chemother. 2018, 62, e00165-18. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Lim, H.-S.; Lee, S.-H.; Cho, Y.L.; Nam, H.-S.; Bae, K.-S. Pharmacokinetics, Pharmacodynamics, and Tolerability of Single-Dose Oral LCB01-0371, a Novel Oxazolidinone with Broad-Spectrum Activity, in Healthy Volunteers. Antimicrob. Agents Chemother. 2018, 62, e00451-18. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Lim, H.-S.; Cho, Y.L.; Nam, H.-S.; Bae, K.-S. Multiple-dose Safety, Tolerability, Pharmacokinetics, and Pharmacodynamics of Oral LCB01-0371 in Healthy Male Volunteers. Clin. Ther. 2018, 40, 2050–2064. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.; Lee, S.W.; Kim, A.; Jang, K.; Nam, H.; Cho, Y.L.; Yu, K.-S.; Jang, I.-J.; Chung, J.-Y. Safety, tolerability and pharmacokinetics of 21 day multiple oral administration of a new oxazolidinone antibiotic, LCB01-0371, in healthy male subjects. J. Antimicrob. Chemother. 2018, 73, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.-S.; Lim, H.-S.; Han, S.; Yoon, S.K.; Kim, H.; Cho, Y.L.; Nam, H.-S.; Bae, K.-S. Single-dose Intravenous Safety, Tolerability, and Pharmacokinetics and Absolute Bioavailability of LCB01-0371. Clin. Ther. 2019, 41, 92–106. [Google Scholar] [CrossRef]
- Kim, J.S.; Kim, Y.-H.; Lee, S.H.; Kim, Y.H.; Kim, J.-W.; Kang, J.Y.; Kim, S.K.; Kim, S.J.; Kang, Y.-S.; Kim, T.-H.; et al. Early Bactericidal Activity of Delpazolid (LCB01-0371) in Patients with Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 2022, 66, e0168421. [Google Scholar] [CrossRef] [PubMed]
- Diacon, A.H.; De Jager, V.R.; Dawson, R.; Narunsky, K.; Vanker, N.; Burger, D.A.; Everitt, D.; Pappas, F.; Nedelman, J.; Mendel, C.M. Fourteen-Day Bactericidal Activity, Safety, and Pharmacokinetics of Linezolid in Adults with Drug-Sensitive Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 2020, 64, e02012-19. [Google Scholar] [CrossRef] [PubMed]
- Dierig, A.; Hoelscher, M.; Schultz, S.; Hoffmann, L.; Jarchow-MacDonald, A.; Svensson, E.; Te Brake, L.; Aarnoutse, R.; Boeree, M.; McHugh, T.; et al. A phase IIb, open-label, randomized controlled dose ranging multi-centre trial to evaluate the safety, tolerability, pharmacokinetics and exposure-response relationship of different doses of delpazolid in combination with bedaquiline delamanid moxifloxacin in adult subjects with newly diagnosed, uncomplicated, smear-positive, drug-sensitive pulmonary tuberculosis. Trials 2023, 24, 382. [Google Scholar] [CrossRef]
- Brickner, S.J.; Hutchinson, D.K.; Barbachyn, M.R.; Manninen, P.R.; Ulanowicz, D.A.; Garmon, S.A.; Grega, K.C.; Hendges, S.K.; Toops, D.S.; Ford, C.W.; et al. Synthesis and Antibacterial Activity of U-100592 and U-100766, Two Oxazolidinone Antibacterial Agents for the Potential Treatment of Multidrug-Resistant Gram-Positive Bacterial Infections. J. Med. Chem. 1996, 39, 673–679. [Google Scholar] [CrossRef] [PubMed]
- Lin, A.H.; Murray, R.W.; Vidmar, T.J.; Marotti, K.R. The oxazolidinone eperezolid binds to the 50S ribosomal subunit and competes with binding of chloramphenicol and lincomycin. Antimicrob. Agents Chemother. 1997, 41, 2127–2131. [Google Scholar] [CrossRef] [PubMed]
- Zurenko, G.E.; Yagi, B.H.; Schaadt, R.D.; Allison, J.W.; Kilburn, J.O.; Glickman, S.E.; Hutchinson, D.K.; Barbachyn, M.R.; Brickner, S.J. In vitro activities of U-100592 and U-100766, novel oxazolidinone antibacterial agents. Antimicrob. Agents Chemother. 1996, 40, 839–845. [Google Scholar] [CrossRef] [PubMed]
- Pawsey, S.D.; Harry, J.D.; Stalker, D.J. 1st Administration of a New Oxazolidinone Antibiotic (U-100592) to Man. Abstract F225. In Abstracts of the 35th Interscience Conference on Antimicrobial Agents and Chemotherapy: 17-20 September 1995, Moscone Center, San Francisco, California; American Society for Microbiology: Washington, DC, USA, 1995. [Google Scholar]
- Zurenko, G.E.; Ford, C.W.; Hutchinson, D.K.; Brickner, S.J.; Barbachyn, M.R. Oxazolidinone antibacterial agents: Development of the clinical candidates eperezolid and linezolid. Expert Opin. Investig. Drugs 1997, 6, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.W.; Zurenko, G.E.; Barbachyn, M.R. The discovery of linezolid, the first oxazolidinone antibacterial agent. Curr. Drug Targets Infect. Disord. 2001, 1, 181–199. [Google Scholar] [CrossRef] [PubMed]
- Hoy, S.M. Contezolid: First Approval. Drugs 2021, 81, 1587–1591. [Google Scholar] [CrossRef]
- Wang, W.; Voss, K.M.; Liu, J.; Gordeev, M.F. Nonclinical Evaluation of Antibacterial Oxazolidinones Contezolid and Contezolid Acefosamil with Low Serotonergic Neurotoxicity. Chem. Res. Toxicol. 2021, 34, 1348–1354. [Google Scholar] [CrossRef]
- Li, B.; Liu, Y.; Luo, J.; Cai, Y.; Chen, M.; Wang, T. Contezolid, a novel oxazolidinone antibiotic, may improve drug-related thrombocytopenia in clinical antibacterial treatment. Front. Pharmacol. 2023, 14, 1157437. [Google Scholar] [CrossRef] [PubMed]
- Shoen, C.; DeStefano, M.; Hafkin, B.; Cynamon, M. In Vitro and In Vivo Activities of Contezolid (MRX-I) against Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2018, 62, e00493-18. [Google Scholar] [CrossRef] [PubMed]
- Almeida, D.; Li, S.-Y.; Lee, J.; Hafkin, B.; Mdluli, K.; Fotouhi, N.; Nuermberger, E.L. Contezolid can replace linezolid in a novel combination with bedaquiline and pretomanid in a murine model of tuberculosis. Antimicrob. Agents Chemother. 2023, 67, e0078923. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Cao, G.; Wu, H.; Chen, Y.; Guo, B.; Wu, X.; Yu, J.; Ni, K.; Qian, J.; Wang, L.; et al. Evaluation of the Effect of Contezolid (MRX-I) on the Corrected QT Interval in a Randomized, Double-Blind, Placebo- and Positive-Controlled Crossover Study in Healthy Chinese Volunteers. Antimicrob. Agents Chemother. 2020, 64, e02158-19. [Google Scholar] [CrossRef]
- Zhao, X.; Huang, H.; Yuan, H.; Yuan, Z.; Zhang, Y. A Phase III multicentre, randomized, double-blind trial to evaluate the efficacy and safety of oral contezolid versus linezolid in adults with complicated skin and soft tissue infections. J. Antimicrob. Chemother. 2022, 77, 1762–1769. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Nie, W.; Ma, L.; Li, Q.; Geng, R.; Shi, W.; Chu, N. Clinical Utility of Contezolid-Containing Regimens in 25 Cases of Linezolid-Intolerable Tuberculosis Patients. Infect. Drug Resist. 2023, 16, 6237–6245. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.; Hu, M.; Xu, N.; Shangguan, Y.; Xia, J.; Hu, W.; Li, X.; Zhao, Q.; Xu, K. Concentration of contezolid in cerebrospinal fluid and serum in a patient with tuberculous meningoencephalitis: A case report. Int. J. Antimicrob. Agents 2023, 62, 106875. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, V.; Solapure, S.; Iyer, H.; Ghosh, A.; Sharma, S.; Kaur, P.; Deepthi, R.; Subbulakshmi, V.; Ramya, V.; Ramachandran, V.; et al. Bactericidal Activity and Mechanism of Action of AZD5847, a Novel Oxazolidinone for Treatment of Tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Gravestock, M.B.; Acton, D.G.; Betts, M.J.; Dennis, M.; Hatter, G.; McGregor, A.; Swain, M.L.; Wilson, R.G.; Woods, L.; Wookey, A. New classes of antibacterial oxazolidinones with C-5, methylene O-Linked heterocyclic side chains. Bioorganic Med. Chem. Lett. 2003, 13, 4179–4186. [Google Scholar] [CrossRef]
- Werngren, J.; Wijkander, M.; Perskvist, N.; Balasubramanian, V.; Sambandamurthy, V.K.; Rodrigues, C.; Hoffner, S. In Vitro Activity of AZD5847 against Geographically Diverse Clinical Isolates of Mycobacterium tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 4222–4223. [Google Scholar] [CrossRef]
- Balasubramanian, V.; Solapure, S.; Shandil, R.; Gaonkar, S.; Mahesh, K.N.; Reddy, J.; Deshpande, A.; Bharath, S.; Kumar, N.; Wright, L.; et al. Pharmacokinetic and Pharmacodynamic Evaluation of AZD5847 in a Mouse Model of Tuberculosis. Antimicrob. Agents Chemother. 2014, 58, 4185–4190. [Google Scholar] [CrossRef] [PubMed]
- Alsultan, A.; Furin, J.J.; Du Bois, J.; Van Brakel, E.; Chheng, P.; Venter, A.; Thiel, B.; Debanne, S.A.; Boom, W.H.; Diacon, A.H.; et al. Population Pharmacokinetics of AZD-5847 in Adults with Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 2017, 61, e01066-17. [Google Scholar] [CrossRef] [PubMed]
- Furin, J.J.; Du Bois, J.; Van Brakel, E.; Chheng, P.; Venter, A.; Peloquin, C.A.; Alsultan, A.; Thiel, B.A.; Debanne, S.M.; Boom, W.H.; et al. Early Bactericidal Activity of AZD5847 in Patients with Pulmonary Tuberculosis. Antimicrob. Agents Chemother. 2016, 60, 6591–6599. [Google Scholar] [CrossRef] [PubMed]
- Working Group on New Drugs. AZD5847. Available online: https://www.newtbdrugs.org/pipeline/compound/azd5847 (accessed on 6 April 2024).
- Yuan, S.; Shen, D.-D.; Bai, Y.-R.; Zhang, M.; Zhou, T.; Sun, C.; Zhou, L.; Wang, S.-Q.; Liu, H.-M. Oxazolidinone: A promising scaffold for the development of antibacterial drugs. Eur. J. Med. Chem. 2023, 250, 115239. [Google Scholar] [CrossRef] [PubMed]
- TB Alliance. TBI-223. Available online: https://www.tballiance.org/portfolio/compound/tbi-223-oxazolidinone (accessed on 6 April 2024).
- Working Group on New Drugs. TBI-223. Available online: https://www.newtbdrugs.org/pipeline/compound/tbi-223 (accessed on 6 April 2024).
- Gordon, O.; Dikeman, D.A.; Ortines, R.V.; Wang, Y.; Youn, C.; Mumtaz, M.; Orlando, N.; Zhang, J.; Patel, A.M.; Gough, E.; et al. The Novel Oxazolidinone TBI-223 Is Effective in Three Preclinical Mouse Models of Methicillin-Resistant Staphylococcus aureus Infection. Microbiol. Spectr. 2022, 10, e0245121. [Google Scholar] [CrossRef]
- Krutikov, M.; Bruchfeld, J.; Migliori, G.B.; Borisov, S.; Tiberi, S. New and repurposed drugs. In Tuberculosis; Migliori, G.B., Bothamley, G., Duarte, R., Rendon, A., Eds.; European Respiratory Society: Sheffield, UK, 2018; pp. 179–204. [Google Scholar]
- Mdluli, K.; Cooper, C.; Yang, T.; Lotlikar, M.; Betoudji, F.; Pinn, M.; Converse, P.; Nuermberger, E.; Cho, S.; Oh, T. TBI-223: A safer oxazolidinone in pre-clinical development for tuberculosis. In Proceedings of the ASM Microbe 2017, New Orleans, LA, USA, 1–5 June 2017; p. Session 335-AAID-Sunday-50. [Google Scholar]
- Li, S.-Y.; Converse, P.J.; Betoudji, F.; Lee, J.; Mdluli, K.; Upton, A.; Fotouhi, N.; Nuermberger, E.L. Next-Generation Diarylquinolines Improve Sterilizing Activity of Regimens with Pretomanid and the Novel Oxazolidinone TBI-223 in a Mouse Tuberculosis Model. Antimicrob. Agents Chemother. 2023, 67, e0003523. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Kim, A.; Lee, S.; Choi, S.-H.; Lee, D.Y.; Song, J.-S.; Lee, H.; Jang, I.-J.; Yu, K.-S. Pharmacokinetics, Safety, and Tolerability of Tedizolid Phosphate After Single-dose Administration in Healthy Korean Male Subjects. Clin. Ther. 2017, 39, 1849–1857. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Shen, K.; Chang, X.; Tanaka, T.; Li, L.; Hu, P. Pharmacokinetics and Safety of Tedizolid after Single and Multiple Intravenous/Oral Sequential Administrations in Healthy Chinese Subjects. Clin. Ther. 2016, 38, 1869–1879. [Google Scholar] [CrossRef]
- Lanoix, J.-P.; Lenaerts, A.J.; Nuermberger, E.L. Heterogeneous disease progression and treatment response in a C3HeB/FeJ mouse model of tuberculosis. Dis. Models Mech. 2015, 8, 603–610. [Google Scholar] [CrossRef]
- Williams, K.; Minkowski, A.; Amoabeng, O.; Peloquin, C.A.; Taylor, D.; Andries, K.; Wallis, R.S.; Mdluli, K.E.; Nuermberger, E.L. Sterilizing Activities of Novel Combinations Lacking First- and Second-Line Drugs in a Murine Model of Tuberculosis. Antimicrob. Agents Chemother. 2012, 56, 3114–3120. [Google Scholar] [CrossRef]
- Shoen, C.M. Short-course treatment regimen to identify potential antituberculous agents in a murine model of tuberculosis. J. Antimicrob. Chemother. 2004, 53, 641–645. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Jakubiec, W.; Kumar, V.; Bedarida, G.; Silvia, A.; Paige, D.; Zhu, T.; Mitton-Fry, M.; Ladutko, L.; Campbell, S.; et al. Biomarker-Assisted Dose Selection for Safety and Efficacy in Early Development of PNU-100480 for Tuberculosis. Antimicrob. Agents Chemother. 2011, 55, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Wallis, R.S.; Jakubiec, W.M.; Kumar, V.; Silvia, A.M.; Paige, D.; Dimitrova, D.; Li, X.; Ladutko, L.; Campbell, S.; Friedland, G.; et al. Pharmacokinetics and Whole-Blood Bactericidal Activity against Mycobacterium tuberculosis of Single Doses of PNU-100480 in Healthy Volunteers. J. Infect. Dis. 2010, 202, 745–751. [Google Scholar] [CrossRef] [PubMed]
- Bulitta, J.B.; Fang, E.; Stryjewski, M.E.; Wang, W.; Atiee, G.J.; Stark, J.G.; Hafkin, B. Population pharmacokinetic rationale for intravenous contezolid acefosamil followed by oral contezolid dosage regimens. Antimicrob. Agents Chemother. 2024, 68, e0140023. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Jin, Y.; Wang, H.; Yuan, H.; Wang, J.; Li, S.; Hu, Y.; Yang, H.; Li, X.; Liang, H.; et al. A phase I study of the safety, tolerability, and pharmacokinetics of contezolid acefosamil after intravenous and oral administration in healthy Chinese subjects. Antimicrob. Agents Chemother. 2023, 67, e0079623. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wu, H.; Chen, Y.; Yuan, H.; Wu, J.; Wu, X.; Zhang, Y.; Cao, G.; Guo, B.; Wu, J.; et al. Population Pharmacokinetics Study of Contezolid (MRX-I), a Novel Oxazolidinone Antibacterial Agent, in Chinese Patients. Clin. Ther. 2020, 42, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Eckburg, P.B.; Ge, Y.; Hafkin, B. Single- and Multiple-Dose Study To Determine the Safety, Tolerability, Pharmacokinetics, and Food Effect of Oral MRX-I versus Linezolid in Healthy Adult Subjects. Antimicrob. Agents Chemother. 2017, 61, e02181-16. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, H.; Wang, Y.; Chen, Y.; Guo, B.; Cao, G.; Wu, X.; Yu, J.; Wu, J.; Zhu, D.; et al. Tolerability and Pharmacokinetics of Contezolid at Therapeutic and Supratherapeutic Doses in Healthy Chinese Subjects, and Assessment of Contezolid Dosing Regimens Based on Pharmacokinetic/Pharmacodynamic Analysis. Clin. Ther. 2019, 41, 1164–1174.e4. [Google Scholar] [CrossRef] [PubMed]
- Chang, V.; Phillips, P.P.J.; Imperial, M.Z.; Nahid, P.; Savic, R.M. A comparison of clinical development pathways to advance tuberculosis regimen development. BMC Infect. Dis. 2022, 22, 920. [Google Scholar] [CrossRef]
- du Cros, P.; Greig, J.; Alffenaar, J.W.C.; Cross, G.B.; Cousins, C.; Berry, C.; Khan, U.; Phillips, P.P.J.; Velásquez, G.E.; Furin, J.; et al. Standards for clinical trials for treating TB. Int. J. Tuberc. Lung Dis. 2023, 27, 885–898. [Google Scholar] [CrossRef]
- Global Tuberculosis Report 2023. Available online: https://iris.who.int/bitstream/handle/10665/373828/9789240083851-eng.pdf?sequence=1 (accessed on 9 May 2024).
- Frontières, M.S. Transparency CORE: Clinical Trial Cost Reporting Toolkit. Available online: https://msfaccess.org/transparency-core-clinical-trial-cost-reporting-toolkit (accessed on 9 May 2024).
- Cox, E.; Laessig, K. FDA Approval of Bedaquiline—The Benefit–Risk Balance for Drug-Resistant Tuberculosis. N. Engl. J. Med. 2014, 371, 689–691. [Google Scholar] [CrossRef] [PubMed]
- Alffenaar, J.-W.C.; Sintchenko, V.; Marais, B.J. Acquired Drug Resistance: Recognizing the Potential of Repurposed Drugs. Clin. Infect. Dis. 2019, 69, 2038–2039. [Google Scholar] [CrossRef] [PubMed]
- Renslo, A.R. Antibacterial oxazolidinones: Emerging structure–toxicity relationships. Expert Rev. Anti-Infect. Ther. 2010, 8, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Wang, B.; Fu, L.; Li, G.; Lu, H.; Liu, Y.; Sheng, L.; Li, Y.; Zhang, B.; Lu, Y.; et al. Discovery of a Conformationally Constrained Oxazolidinone with Improved Safety and Efficacy Profiles for the Treatment of Multidrug-Resistant Tuberculosis. J. Med. Chem. 2020, 63, 9316–9339. [Google Scholar] [CrossRef] [PubMed]
- Reck, F.; Zhou, F.; Girardot, M.; Kern, G.; Eyermann, C.J.; Hales, N.J.; Ramsay, R.R.; Gravestock, M.B. Identification of 4-Substituted 1,2,3-Triazoles as Novel Oxazolidinone Antibacterial Agents with Reduced Activity against Monoamine Oxidase A. J. Med. Chem. 2005, 48, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Bolhuis, M.S.; Van Altena, R.; Van Soolingen, D.; De Lange, W.C.M.; Uges, D.R.A.; Van Der Werf, T.S.; Kosterink, J.G.W.; Alffenaar, J.-W.C. Clarithromycin increases linezolid exposure in multidrug-resistant tuberculosis patients. Eur. Respir. J. 2013, 42, 1614–1621. [Google Scholar] [CrossRef]
- Okazaki, F.; Tsuji, Y.; Seto, Y.; Ogami, C.; Yamamoto, Y.; To, H. Effects of a rifampicin pre-treatment on linezolid pharmacokinetics. PLoS ONE 2019, 14, e0214037. [Google Scholar] [CrossRef]
- Douros, A.; Grabowski, K.; Stahlmann, R. Drug–drug interactions and safety of linezolid, tedizolid, and other oxazolidinones. Expert Opin. Drug Metab. Toxicol. 2015, 11, 1849–1859. [Google Scholar] [CrossRef] [PubMed]
- Hasan, T.; Medcalf, E.; Nyang’wa, B.-T.; Egizi, E.; Berry, C.; Dodd, M.; Foraida, S.; Gegia, M.; Li, M.; Mirzayev, F.; et al. The Safety and Tolerability of Linezolid in Novel Short-Course Regimens Containing Bedaquiline, Pretomanid, and Linezolid to Treat Rifampicin-Resistant Tuberculosis: An Individual Patient Data Meta-analysis. Clin. Infect. Dis. 2024, 78, 730–741. [Google Scholar] [CrossRef]
- Wang, S.; Forsman, L.D.; Xu, C.; Zhang, H.; Zhu, Y.; Shao, G.; Wang, S.; Cao, J.; Xiong, H.; Niward, K.; et al. Second-line antituberculosis drug exposure thresholds predictive of adverse events in multidrug-resistant tuberculosis treatment. Int. J. Infect. Dis. 2024, 140, 62–69. [Google Scholar] [CrossRef]
- Lau, C.; Marriott, D.; Bui, J.; Figtree, M.; Gould, M.; Chubaty, A.; Su, Y.; Adhikari, S.; Konecny, P.; Kozierowski, K.; et al. LInezolid Monitoring to MInimise Toxicity (LIMMIT1): A multicentre retrospective review of patients receiving linezolid therapy and the impact of therapeutic drug monitoring. Int. J. Antimicrob. Agents 2023, 61, 106783. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Ruiter, E.; Jongedijk, E.M.; Ak, H.K.; Marais, B.J.; Pk, B.; Sawleshwarkar, S.; Touw, D.J.; Alffenaar, J.-W. Saliva-based linezolid monitoring on a mobile UV spectrophotometer. J. Antimicrob. Chemother. 2021, 76, 1786–1792. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, R.H.; Burke, A.; Cho, J.-G.; Alffenaar, J.-W.; Davies Forsman, L. New Oxazolidinones for Tuberculosis: Are Novel Treatments on the Horizon? Pharmaceutics 2024, 16, 818. https://doi.org/10.3390/pharmaceutics16060818
Chen RH, Burke A, Cho J-G, Alffenaar J-W, Davies Forsman L. New Oxazolidinones for Tuberculosis: Are Novel Treatments on the Horizon? Pharmaceutics. 2024; 16(6):818. https://doi.org/10.3390/pharmaceutics16060818
Chicago/Turabian StyleChen, Ricky Hao, Andrew Burke, Jin-Gun Cho, Jan-Willem Alffenaar, and Lina Davies Forsman. 2024. "New Oxazolidinones for Tuberculosis: Are Novel Treatments on the Horizon?" Pharmaceutics 16, no. 6: 818. https://doi.org/10.3390/pharmaceutics16060818






