The Assessment of Physicochemical and Antimicrobial Properties of Hydrophilic Gels Containing Tetracycline Hydrochloride and Various Concentrations of Ethanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation of Hydrophilic Gels
2.3. Measurement of the pH of Preparations
2.4. HPLC Analysis of TC Stability
2.4.1. Samples Preparation
2.4.2. Chromatographic Analysis
2.5. Assessment of the Rheological Properties of Hydrogels
2.6. Evaluation of Microbiological Activity of the Preparations
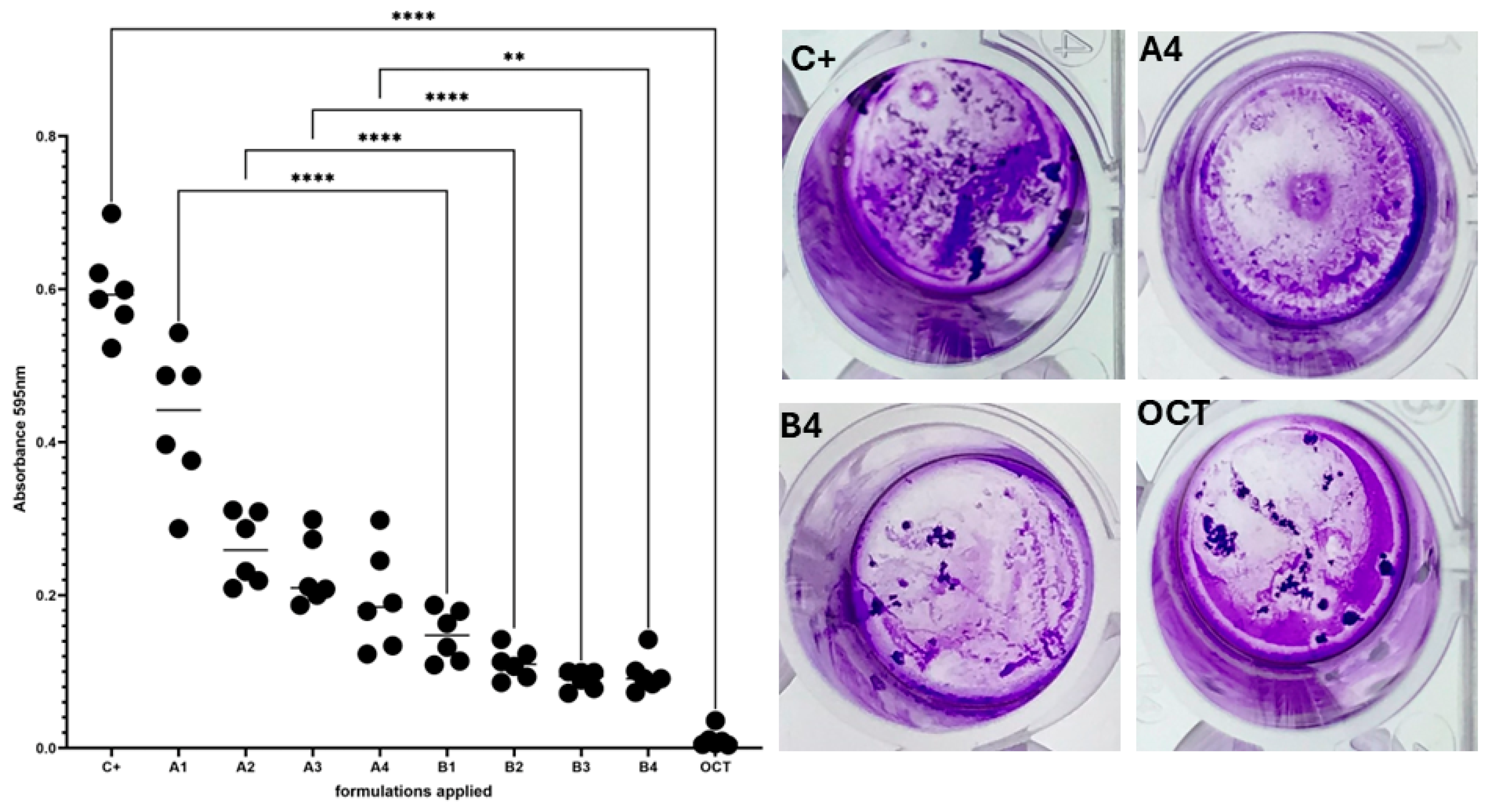
2.6.1. C. acnes Biofilm Formation in a Six-Well Plate Model and Assessment of Provided Formulations’ Activity Using Cristal Violet Assay
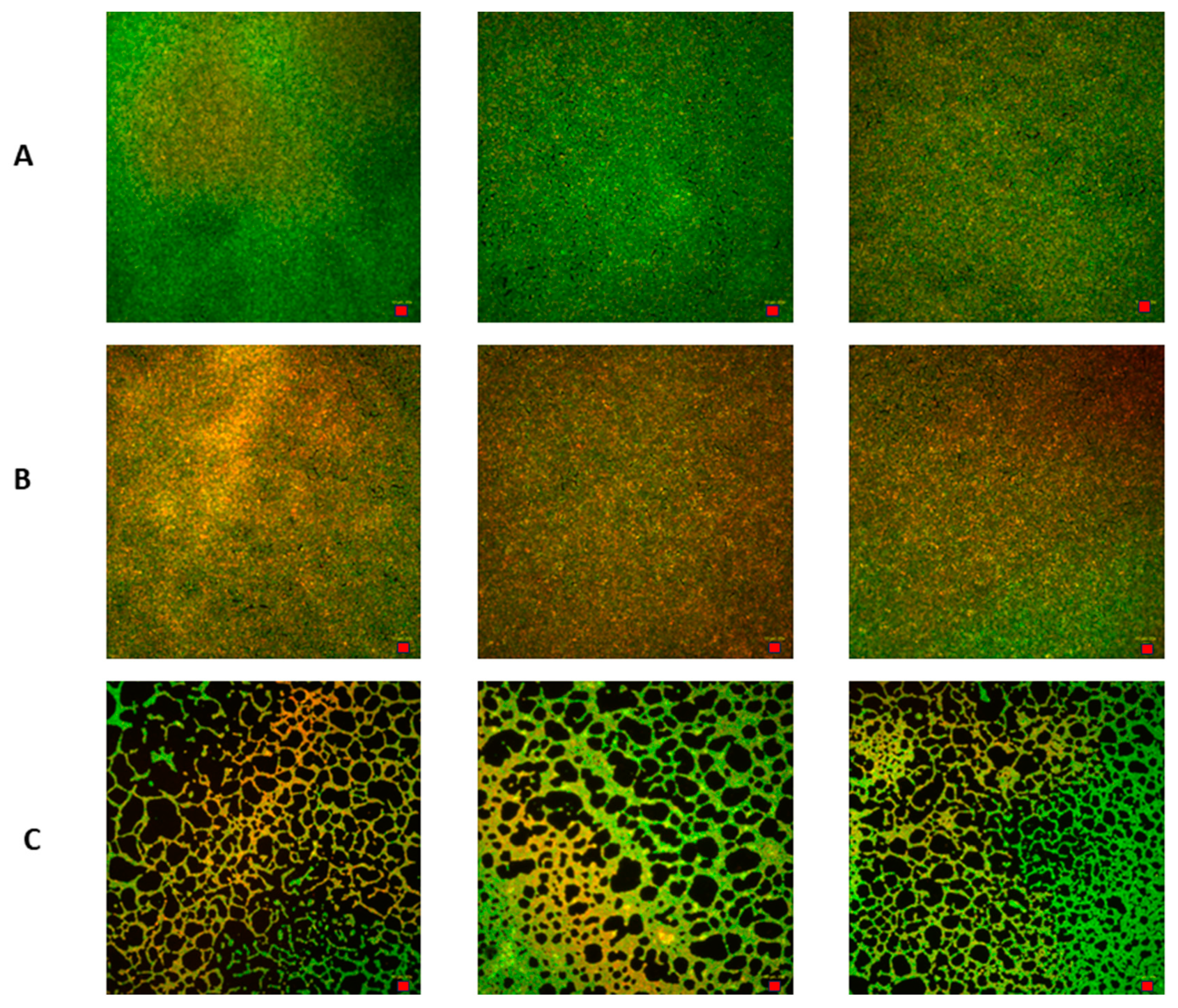
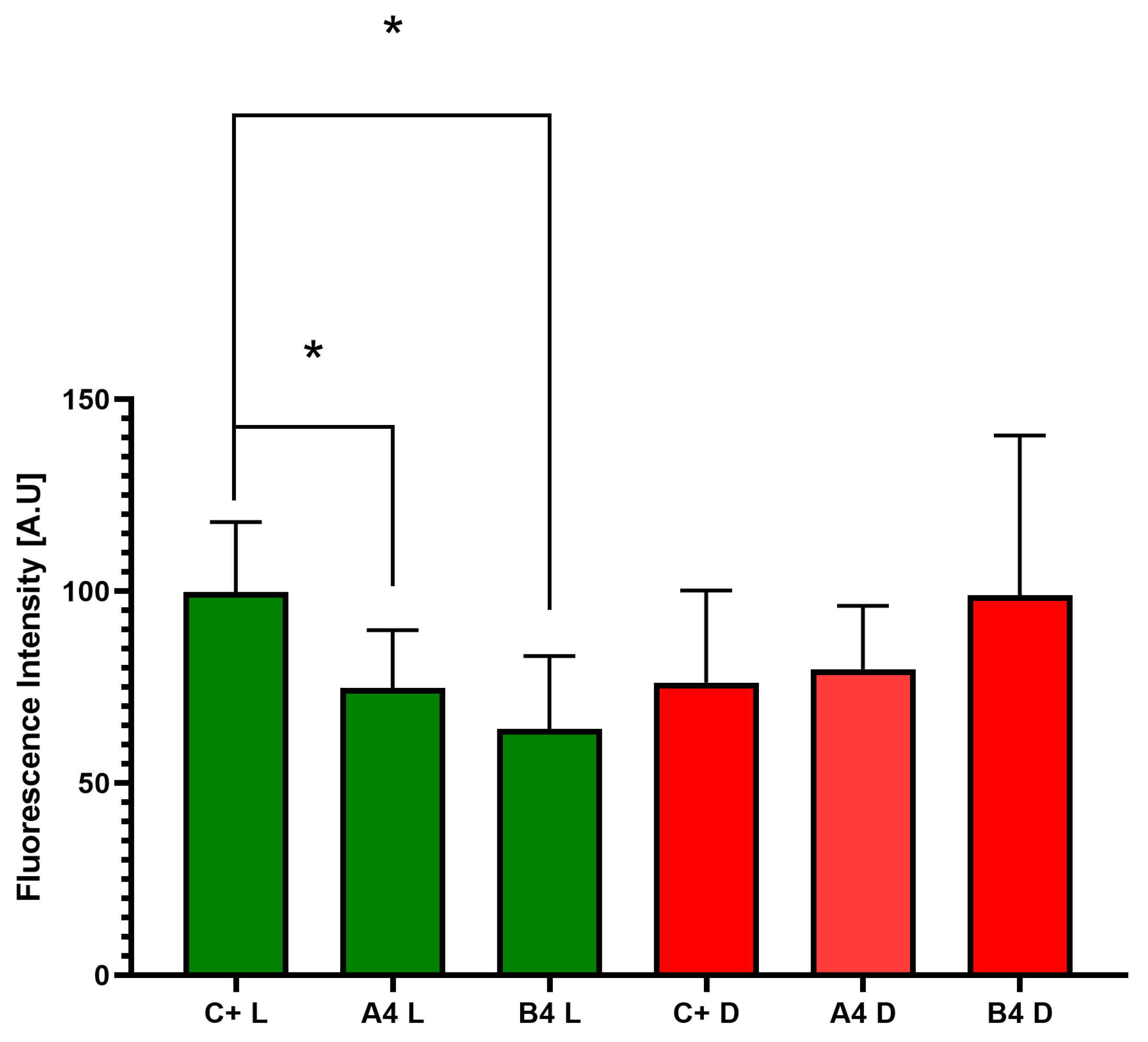
2.6.2. Measurement of Viability of C. acnes Biofilm Cells Using a Live/Dead Biofilm Viability Kit
2.6.3. Image Processing of C. acnes Biofilms Using ImageJ Software
2.7. The Statistical Analysis
3. Results
3.1. pH Value of Formulations
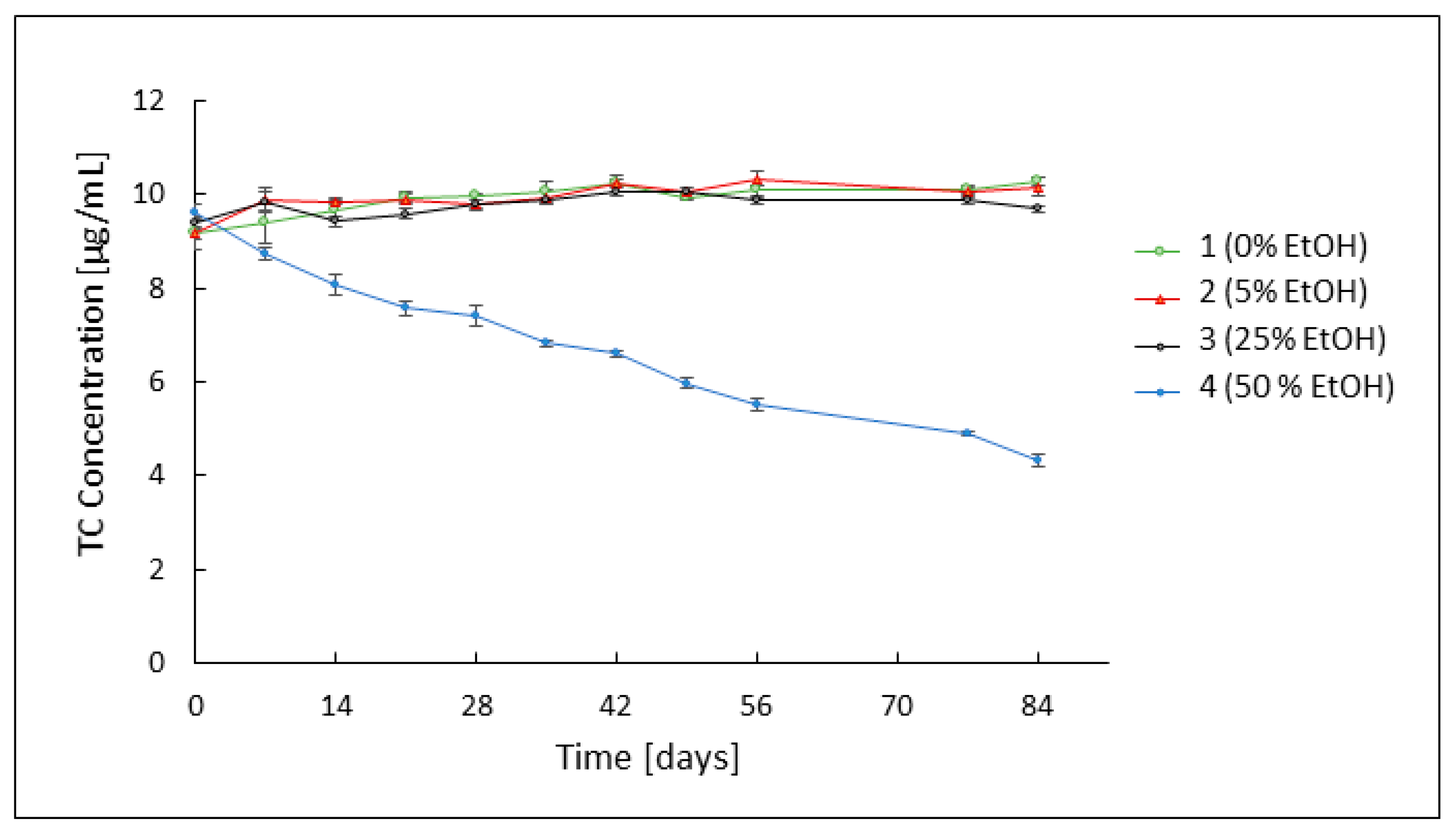
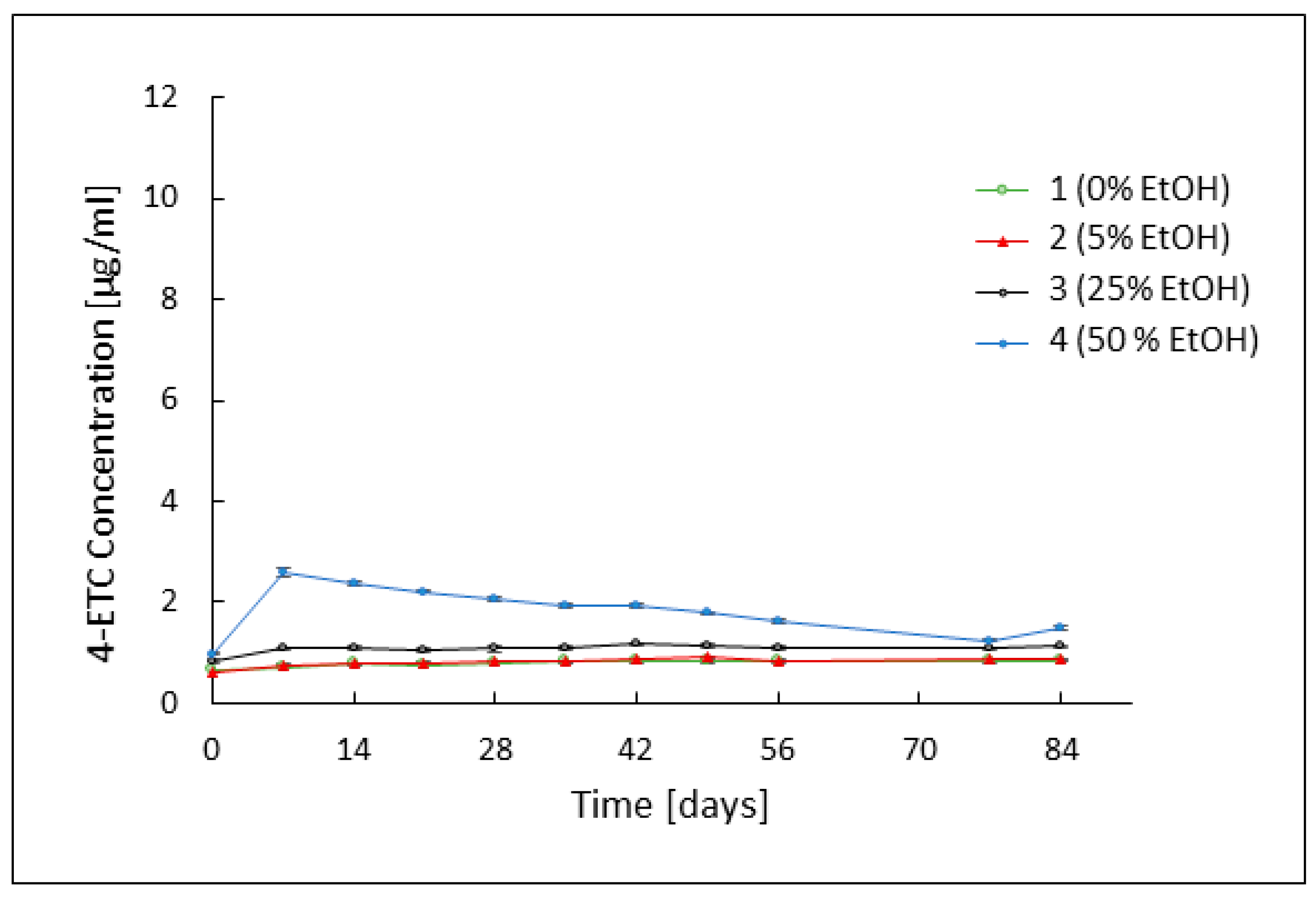
3.2. Assessment of TC Stability by HPLC Analysis
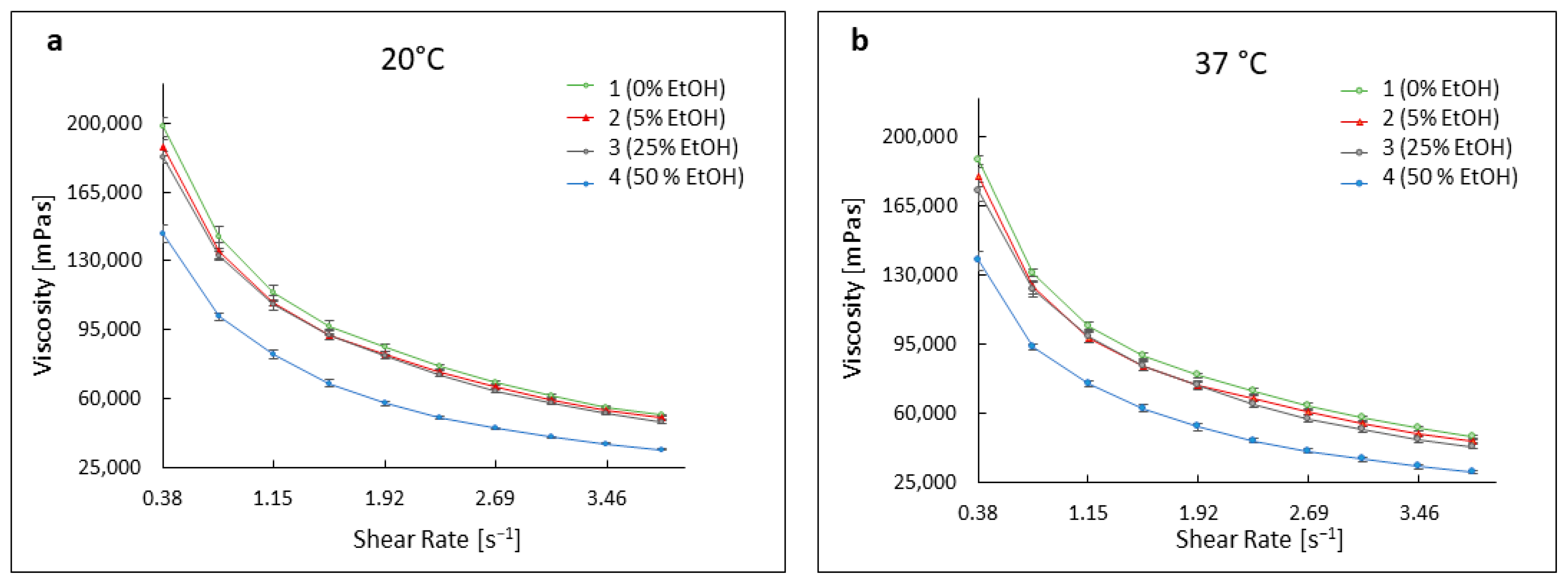
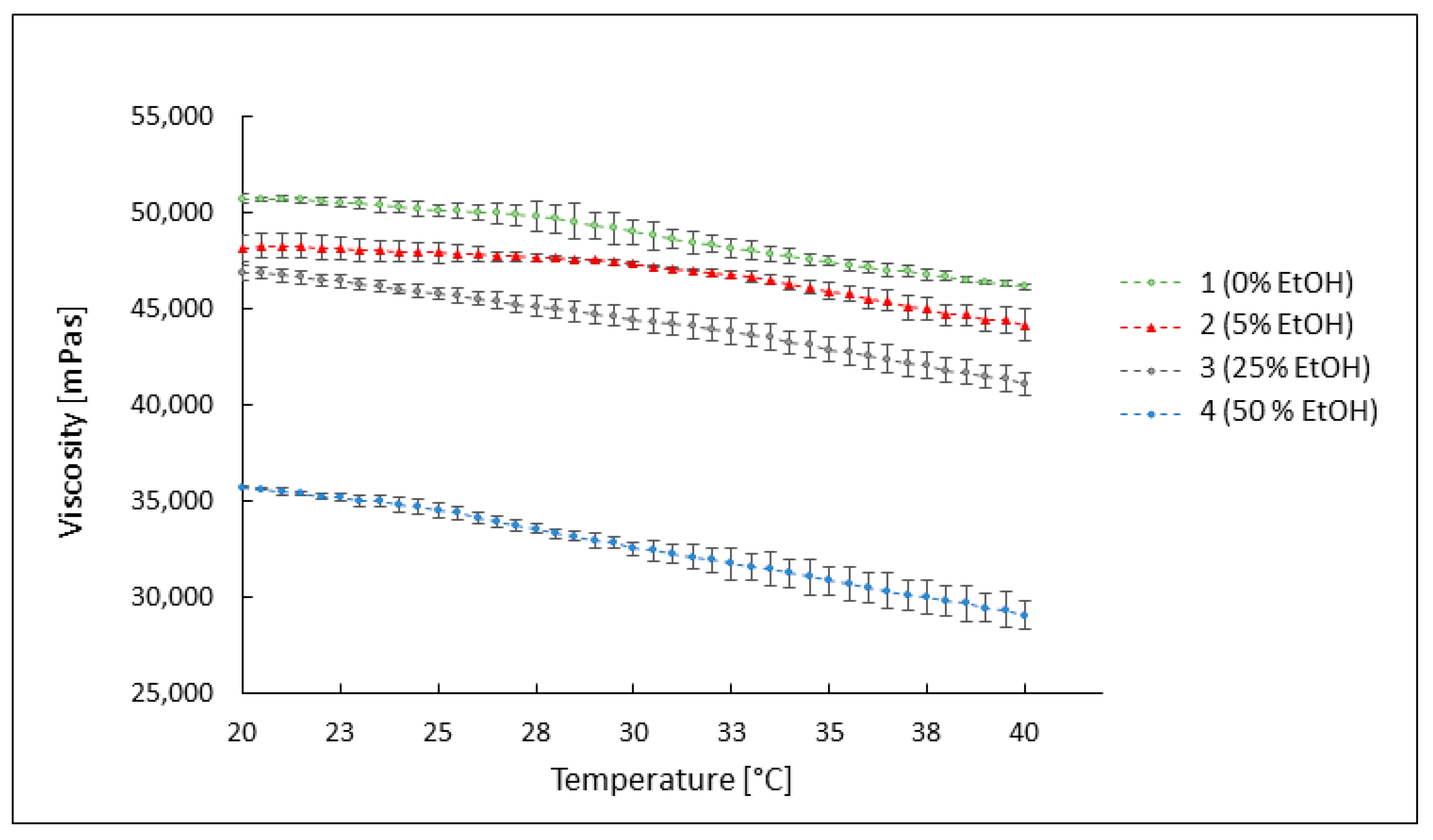
3.3. Evaluation of the Rheological Properties of the Developed Formulations
3.3.1. Viscosity Curves of Hydrogels
3.3.2. The Influence of Temperature on the Viscosity of the Preparations
3.4. Evaluation of Microbiological Activity of the Developed Preparations
3.5. Optical Evaluation of Color Changes in Preparations
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 4-EATC | 4-epianhydrotetracycline |
| 4-ETC | 4-epitetracycline hydrochloride |
| AMPD | 2-amino-2-methyl-1,3-propanediol |
| ATC | anhydrotetracycline |
| BHI | Brain Heart Infusion |
| C+ | positive control |
| HPLC | High-Performance Liquid Chromatography |
| OCT | octenidine dihydrochloride |
| RCA | Reinforced Clostridial Agar |
| rpm | Revolutions per minute |
References
- Lynn, D.; Umari, T.; Dellavalle, R.; Dunnick, C. The Epidemiology of Acne Vulgaris in Late Adolescence. Adolesc. Health Med. Ther. 2016, 7, 13–25. [Google Scholar] [CrossRef]
- Bhate, K.; Williams, H.C. Epidemiology of Acne Vulgaris. Br. J. Dermatol. 2013, 168, 474–485. [Google Scholar] [CrossRef]
- Lee, Y.B.; Byun, E.J.; Kim, H.S. Potential Role of the Microbiome in Acne: A Comprehensive Review. J. Clin. Med. 2019, 8, 987. [Google Scholar] [CrossRef]
- Gollnick, H.; Cunliffe, W.; Berson, D.; Dreno, B.; Finlay, A.; Leyden, J.J.; Shalita, A.R.; Thiboutot, D. Management of Acne: A Report from a Global Alliance to Improve Outcomes in Acne. J. Am. Acad. Dermatol. 2003, 49, S1–S37. [Google Scholar] [CrossRef]
- Shaw, L.; Kennedy, C. The Treatment of Acne. Curr. Paediatr. 2003, 13, 423–428. [Google Scholar] [CrossRef]
- Leyden, J.J. The Evolving Role of Propionibacterium Acnes in Acne. Semin. Cutan. Med. Surg. 2001, 20, 139–143. [Google Scholar] [CrossRef]
- Dessinioti, C.; Katsambas, A.D. The Role of Propionibacterium Acnes in Acne Pathogenesis: Facts and Controversies. Clin. Dermatol. 2010, 28, 2–7. [Google Scholar] [CrossRef]
- Rozas, M.; de Ruijter, A.H.; Fabrega, M.J.; Zorgani, A.; Guell, M.; Paetzold, B.; Brillet, F. From Dysbiosis to Healthy Skin: Major Contributions of Cutibacterium Acnes to Skin Homeostasis. Microorganisms 2021, 9, 628. [Google Scholar] [CrossRef]
- Nørreslet, L.B.; Agner, T.; Clausen, M.L. The Skin Microbiome in Inflammatory Skin Diseases. Curr. Dermatol. Rep. 2020, 9, 141–151. [Google Scholar] [CrossRef]
- Akaza, N.; Akamatsu, H.; Numata, S.; Yamada, S.; Yagami, A.; Nakata, S.; Matsunaga, K. Microorganisms Inhabiting Follicular Contents of Facial Acne Are Not Only Propionibacterium but also Malassezia Spp. J. Dermatol. 2016, 43, 906–911. [Google Scholar] [CrossRef]
- Gang, H.; Yu-ping, W.; Jie, F. Malassezia Infection: Is There Any Chance or Necessity in Refractory Acne? Chin. Med. J. 2010, 123, 628–632. [Google Scholar]
- Nishijima, S.; Kurokawa, I.; Katoh, N.; Watanabe, K. The Bacteriology of Acne Vulgaris and Antimicrobial Susceptibility of Propionibacterium Aches and Staphylococcus Epidermidis Isolated from Acne Lesions. J. Dermatol. 2000, 27, 318–323. [Google Scholar] [CrossRef]
- Katsambas, A.; Papakonstantinou, A. Acne: Systemic Treatment. Clin. Dermatol. 2004, 22, 412–418. [Google Scholar] [CrossRef]
- Salvaggio, H.L.; Zaenglein, A.L. Examining the Use of Oral Contraceptives in the Management of Acne. Int. J. Women’s Health 2010, 2, 69–76. [Google Scholar]
- Zouboulis, C.C.; Piquero-Martin, J. Update and Future of Systemic Acne Treatment. Dermatology 2003, 206, 37–53. [Google Scholar] [CrossRef]
- Fried, R.G.; Wechsler, A. Psychological Problems in the Acne Patient. Dermatol. Ther. 2006, 19, 237–240. [Google Scholar] [CrossRef]
- Ramos-E-Silva, M.; Ramos-E-Silva, S.; Carneiro, S. Acne in Women. Br. J. Dermatol. 2015, 172, 20–26. [Google Scholar] [CrossRef]
- Adler, B.L.; Kornmehl, H.; Armstrong, A.W. Antibiotic Resistance in Acne Treatment. JAMA Dermatol. 2017, 153, 810–811. [Google Scholar] [CrossRef]
- Dessinioti, C.; Katsambas, A. Propionibacterium Acnes and Antimicrobial Resistance in Acne. Clin. Dermatol. 2017, 35, 163–167. [Google Scholar] [CrossRef]
- Nord, C.E.; Oprica, C. Antibiotic Resistance in Propionibacterium Acnes. Microbiological and Clinical Aspects. Anaerobe 2006, 12, 207–210. [Google Scholar] [CrossRef]
- Humbert, P.; Treffel, P.; Chapuis, J.F.; Buchet, S.; Derancourt, C.; Agache, P. The Tetracyclines in Dermatology. J. Am. Acad. Dermatol. 1991, 25, 691–697. [Google Scholar] [CrossRef]
- Simonart, T.; Dramaix, M.; De Maertelaer, V. Efficacy of Tetracyclines in the Treatment of Acne Vulgaris: A Review. Br. J. Dermatol. 2008, 158, 208–216. [Google Scholar] [CrossRef]
- Burton, J. A Placebo-Controlled Study to Evaluate the Efficacy of Topical Tetracycline and Oral Tetracycline in the Treatment of Mild to Moderate Acne. J. Int. Med. Res. 1990, 18, 94–103. [Google Scholar] [CrossRef]
- Sapadin, A.N.; Fleischmajer, R. Tetracyclines: Nonantibiotic Properties and Their Clinical Implications. J. Am. Acad. Dermatol. 2006, 54, 258–265. [Google Scholar] [CrossRef]
- Morimoto, Y.; Wada, Y.; Seki, T.; Sugibayashi, K. In Vitro Skin Permeation of Morphine Hydrochloride during the Finite Application of Penetration-Enhancing System Containing Water, Ethanol and l-Menthol. Biol. Pharm. Bull. 2002, 25, 134–136. [Google Scholar] [CrossRef]
- Wosicka, H.; Cal, K. Targeting to the Hair Follicles: Current Status and Potential. J. Dermatol. Sci. 2010, 57, 83–89. [Google Scholar] [CrossRef]
- Gupta, R.; Badhe, Y.; Rai, B.; Mitragotri, S. Molecular Mechanism of the Skin Permeation Enhancing Effect of Ethanol: A Molecular Dynamics Study. RSC Adv. 2020, 10, 12234–12248. [Google Scholar] [CrossRef]
- Pershing, L.K.; Lambert, L.D.; Knutson, K. Mechanism of Ethanol-Enhanced Estradiol Permeation Across Human Skin in Vivo. Pharm. Res. 1990, 7, 170–175. [Google Scholar] [CrossRef]
- Grice, J.E.; Ciotti, S.; Weiner, N.; Lockwood, P.; Cross, S.E.; Roberts, M.S. Relative Uptake of Minoxidil into Appendages and Stratum Corneum and Permeation through Human Skin In Vitro. J. Pharm. Sci. 2010, 99, 712–718. [Google Scholar] [CrossRef]
- Grams, Y.Y.; Bouwstra, J.A. Penetration and Distribution of Three Lipophilic Probes in vitro in Human Skin Focusing on the Hair Follicle. J. Control. Release 2002, 83, 253–262. [Google Scholar] [CrossRef]
- Jain, S.K.; Verma, A.; Jain, A.; Hurkat, P. Transfollicular Drug Delivery: Current Perspectives. Res. Rep. Transdermal Drug Deliv. 2016, 1, 1–17. [Google Scholar] [CrossRef]
- Tata, S.; Flynn, G.L.; Weiner, N.D. Penetration of Minoxidil from Ethanol/Propylene Glycol Solutions: Effect of Application Volume and Occlusion. J. Pharm. Sci. 1995, 84, 688–691. [Google Scholar] [CrossRef]
- Jimenez, M.M.; Fresno, M.J.; Ramirez, A. The Influence of Cosolvent Polarity on the Flow Properties of Hydroalcoholic Gels. Empirical Models. Chem. Pharm. Bull. 2005, 53, 1097–1102. [Google Scholar] [CrossRef]
- Kim, J.Y.; Song, J.Y.; Lee, E.J.; Park, S.K. Rheological Properties and Microstructures of Carbopol Gel Network System. Colloid Polym. Sci. 2003, 281, 614–623. [Google Scholar] [CrossRef]
- Islam, M.T.; Rodríguez-Hornedo, N.; Ciotti, S.; Ackermann, C. Rheological Characterization of Topical Carbomer Gels Neutralized to Different PH. Pharm. Res. 2004, 21, 1192–1199. [Google Scholar] [CrossRef]
- Kolman, M.; Smith, C.; Chakrabarty, D.; Amin, S. Rheological Stability of Carbomer in Hydroalcoholic Gels: Influence of Alcohol Type. Int. J. Cosmet. Sci. 2021, 43, 748–763. [Google Scholar] [CrossRef]
- Fresno, M.J.C.; Ramírez, A.D.; Jiménez, M.M. Systematic Study of the Flow Behaviour and Mechanical Properties of Carbopol w Ultreze 10 Hydroalcoholic Gels. Eurepean J. Pharm. Biopharm. 2002, 54, 329–335. [Google Scholar] [CrossRef]
- Pavlačková, J.; Pecháčková, H.; Egner, P.; Mokrejš, P.; Gál, R.; Janalíková, M. The Effect of Cosmetic Treatment and Gel Laser Therapy on the Improvement of Comedogenic Skin Type. Gels 2023, 9, 370. [Google Scholar] [CrossRef]
- Goodman, G. Cleansing and Moisturizing in Acne Patients. Am. J. Clin. Dermatol. 2009, 10, 1–6. [Google Scholar] [CrossRef]
- Drexel, R.E.; Olack, G.; Jones, C.; Santim, R.; Morrison, H.; Chmurny, G. Lumitetracycline: A Novel New Tetracycline Photoproduct. J. Org. Chem. 1990, 55, 2471–2478. [Google Scholar] [CrossRef]
- Hasan, T.; Allen, M.; Cooperman, B.S. Anhydrotetracycline Is a Major Product of Tetracycline Photolysis. J. Org. Chem. 1985, 50, 1755–1757. [Google Scholar] [CrossRef]
- Wu, Y.; Fassihi, R. Stability of Metronidazole, Tetracycline HCl and Famotidine Alone and in Combination. Int. J. Pharm. 2005, 290, 1–13. [Google Scholar] [CrossRef]
- Pena, A.; Carmona, A.; Barbosa, A.; Lino, C.M.; Silveira, M.I.; Castillo, B. Determination of Tetracycline and Its Major Degradation Products by Liquid Chromatography with Fluorescence Detection. J. Pharm. Biomed. Anal. 1998, 18, 839–845. [Google Scholar] [CrossRef]
- Schlecht, K.D.; Frank, C.W. Dehydration of Tetracycline. J. Pharm. Sci. 1975, 64, 352–354. [Google Scholar] [CrossRef]
- Kelly, R.G. Determination of Anhydrotetracycline and 4-Epianhydrotetracycline in a Tetracycline Mixture. J. Pharm. Sci. 1964, 53, 1551–1552. [Google Scholar] [CrossRef]
- Hoener, B.-A.; Sokoloski, T.D.; Mitscher, L.A.; Malspeis, L. Kinetics of Dehydration of Epitetracycline in Solution. J. Pharm. Sci. 1974, 63, 1901–1904. [Google Scholar] [CrossRef]
- Morrison, H.; Olack, G.; Xiao, C. Photochemical and Photophysical Studies of Tetracycline. J. Am. Chem. Soc. 1991, 113, 8110–8118. [Google Scholar] [CrossRef]
- Grobben-Verpoorten, A.; Dihuidi, K.; Roets, E.; Hoogmartens, J.; Vanderhaeghe, H. Determination of the Stability of Tetracycline Suspensions by High Performance Liquid Chromatography. Pharm. Weekbl. 1985, 7, 104–108. [Google Scholar] [CrossRef]
- Khan, N.H.; Wera, P.; Roets, E.; Hoogmartens, J. Quantitative Analysis of Tetracycline by High Performance Liquid Chromatography on Polystyrene-Divinylbenzene Packing Materials. J. Liq. Chromatogr. 1990, 13, 1351–1374. [Google Scholar] [CrossRef]
- Kostrzębska, A.; Złocińska, A.; Musiał, W. Evaluation of the Influence of a Hydrogel Containing AMPD on the Stability of Tetracycline Hydrochloride. Pharmaceutics 2021, 13, 1381. [Google Scholar] [CrossRef]
- Kostrzębska, A.; Pączek, K.; Weselak, A.; Musiał, W. Effect of Hydrogel Substrate Components on the Stability of Tetracycline Hydrochloride and Swelling Activity against Model Skin Sebum. Int. J. Mol. Sci. 2023, 24, 2678. [Google Scholar] [CrossRef]
- Younis, U.S.; Fazel, M.; Myrdal, P.B. Characterization of Tetracycline Hydrochloride Compounded in a Miracle Mouthwash Formulation. AAPS PharmSciTech 2019, 20, 178. [Google Scholar] [CrossRef]
- Gajda, A.; Posyniak, A. Tetracyclines and Their Epimers in Animal Tissues by High-Performance Liquid Chromatography. Bull. Vet. Inst. Pulawy 2009, 53, 263–267. [Google Scholar]
- Cherlet, M.; Schelkens, M.; Croubels, S.; De Backer, P. Quantitative Multi-Residue Analysis of Tetracyclines and Their 4-Epimers in Pig Tissues by High-Performance Liquid Chromatography Combined with Positive-Ion Electrospray Ionization Mass Spectrometry. Anal. Chim. Acta 2003, 492, 199–213. [Google Scholar] [CrossRef]
- Davies, A.K.; McKellar, J.F.; Phillips, G.O.; Reid, A.G. Photochemical Oxidation of Tetracycline in Aqueous Solution. J. Chem. Soc. Perkin Trans. 2 1979, 3, 369–375. [Google Scholar] [CrossRef]
- Oka, H.; Ikai, Y.; Kawamura, N.; Yamada, M.; Harada, K.; Ito, S.; Suzuki, M. Photodecomposition Products of Tetracycline in Aqueous Solution. J. Agric. Food Chem. 1989, 37, 226–231. [Google Scholar] [CrossRef]
- Contreras, M.D.; Sánchez, R. Application of a Factorial Design to the Study of Specific Parameters of a Carbopol ETD 2020 Gel. Part I. Viscoelastic Parameters. Int. J. Pharm. 2002, 234, 139–147. [Google Scholar] [CrossRef]
- Contreras, M.D.; Sanchez, R. Application of a Factorial Design to the Study of the Flow Behavior, Spreadability and Transparency of a Carbopol ETD 2020 Gel. Part II. Int. J. Pharm. 2002, 234, 149–157. [Google Scholar] [CrossRef]
- Fresno Contreras, M.J.; Ramírez Diéguez, A.; Jiménez Soriano, M.M. Rheological Characterization of Hydroalcoholic Gels—15% Ethanol—Of Carbopol® UltrezTM 10. Il Farm. 2001, 56, 437–441. [Google Scholar] [CrossRef]
- Weber, E.; Moyers-González, M.; Burghelea, T.I. Thermorheological Properties of a Carbopol Gel under Shear. J. Non-Newton. Fluid Mech. 2012, 183–184, 14–24. [Google Scholar] [CrossRef]
| Formulation | TC [g] | Ethanol [g] | AMPD [g] | Carbopol 980 NF [g] | Water [g] |
|---|---|---|---|---|---|
| A1 | 0.0 | 0.0 | 1.1 | 1.0 | 97.9 |
| A2 | 0.0 | 5.0 | 1.1 | 1.0 | 92.9 |
| A3 | 0.0 | 25.0 | 1.1 | 1.0 | 72.9 |
| A4 | 0.0 | 50.0 | 1.1 | 1.0 | 47.9 |
| 1, B1 | 0.2 | 0.0 | 1.1 | 1.0 | 97.7 |
| 2, B2 | 0.2 | 5.0 | 1.1 | 1.0 | 92.7 |
| 3, B3 | 0.2 | 25.0 | 1.1 | 1.0 | 72.7 |
| 4, B4 | 0.2 | 50.0 | 1.1 | 1.0 | 47.7 |
| Formulation | 1 | 2 | 3 | 4 |
|---|---|---|---|---|
| pH value | 6.66 ± 0.045 | 6.74 ± 0.017 | 6.82 ± 0.007 | 6.95 ± 0.034 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kostrzębska, A.; Junka, A.; Brożyna, M.; Musiał, W. The Assessment of Physicochemical and Antimicrobial Properties of Hydrophilic Gels Containing Tetracycline Hydrochloride and Various Concentrations of Ethanol. Pharmaceutics 2024, 16, 830. https://doi.org/10.3390/pharmaceutics16060830
Kostrzębska A, Junka A, Brożyna M, Musiał W. The Assessment of Physicochemical and Antimicrobial Properties of Hydrophilic Gels Containing Tetracycline Hydrochloride and Various Concentrations of Ethanol. Pharmaceutics. 2024; 16(6):830. https://doi.org/10.3390/pharmaceutics16060830
Chicago/Turabian StyleKostrzębska, Agnieszka, Adam Junka, Malwina Brożyna, and Witold Musiał. 2024. "The Assessment of Physicochemical and Antimicrobial Properties of Hydrophilic Gels Containing Tetracycline Hydrochloride and Various Concentrations of Ethanol" Pharmaceutics 16, no. 6: 830. https://doi.org/10.3390/pharmaceutics16060830





