Personalized SO2 Prodrug for pH-Triggered Gas Enhancement in Anti-Tumor Radio-Immunotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
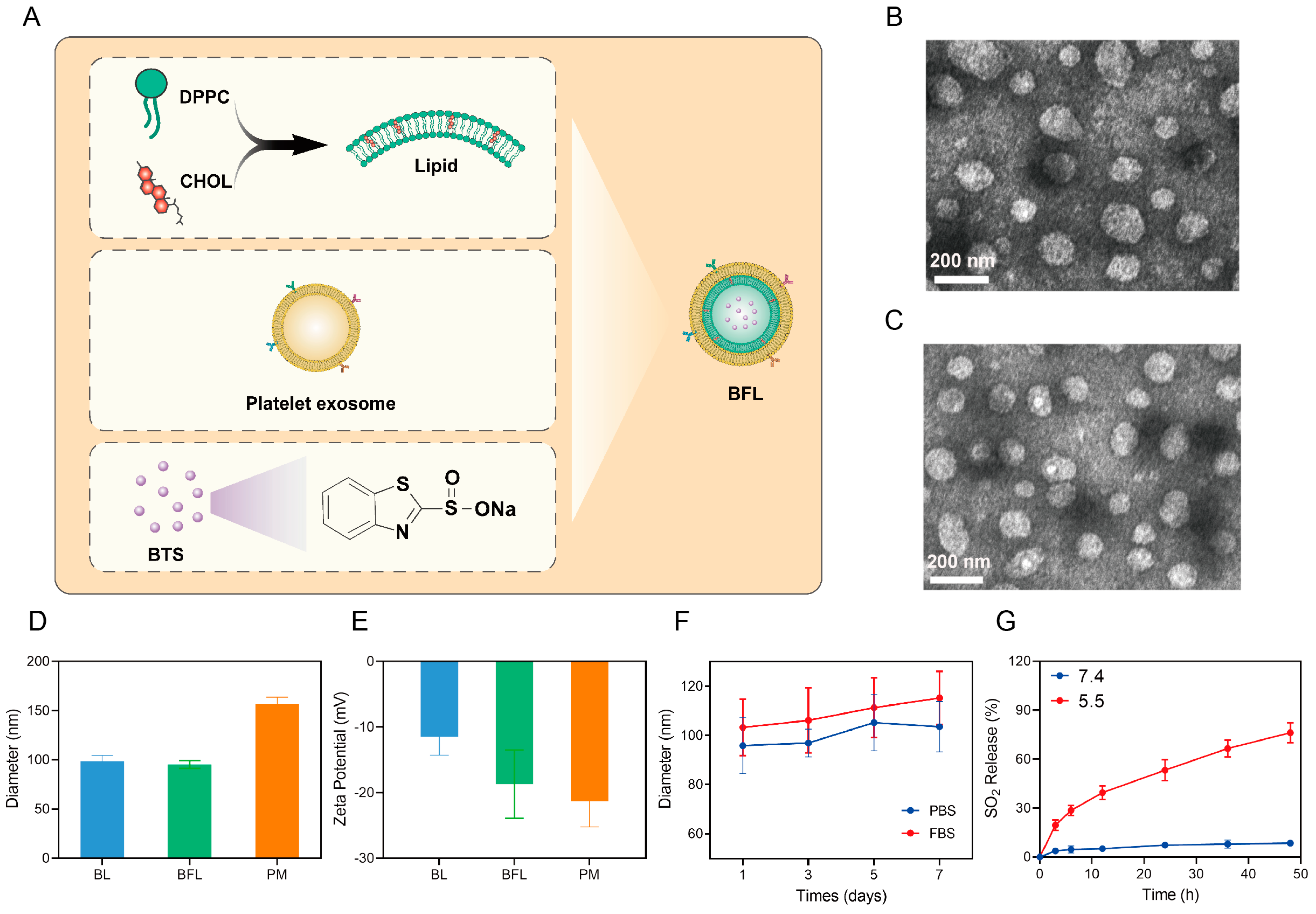
2.2. Preparation and Characterization of BTS Loaded Fusion Liposomes (BFL)
2.3. SO2 Release
2.4. Tumor Cell Targeting Assessment
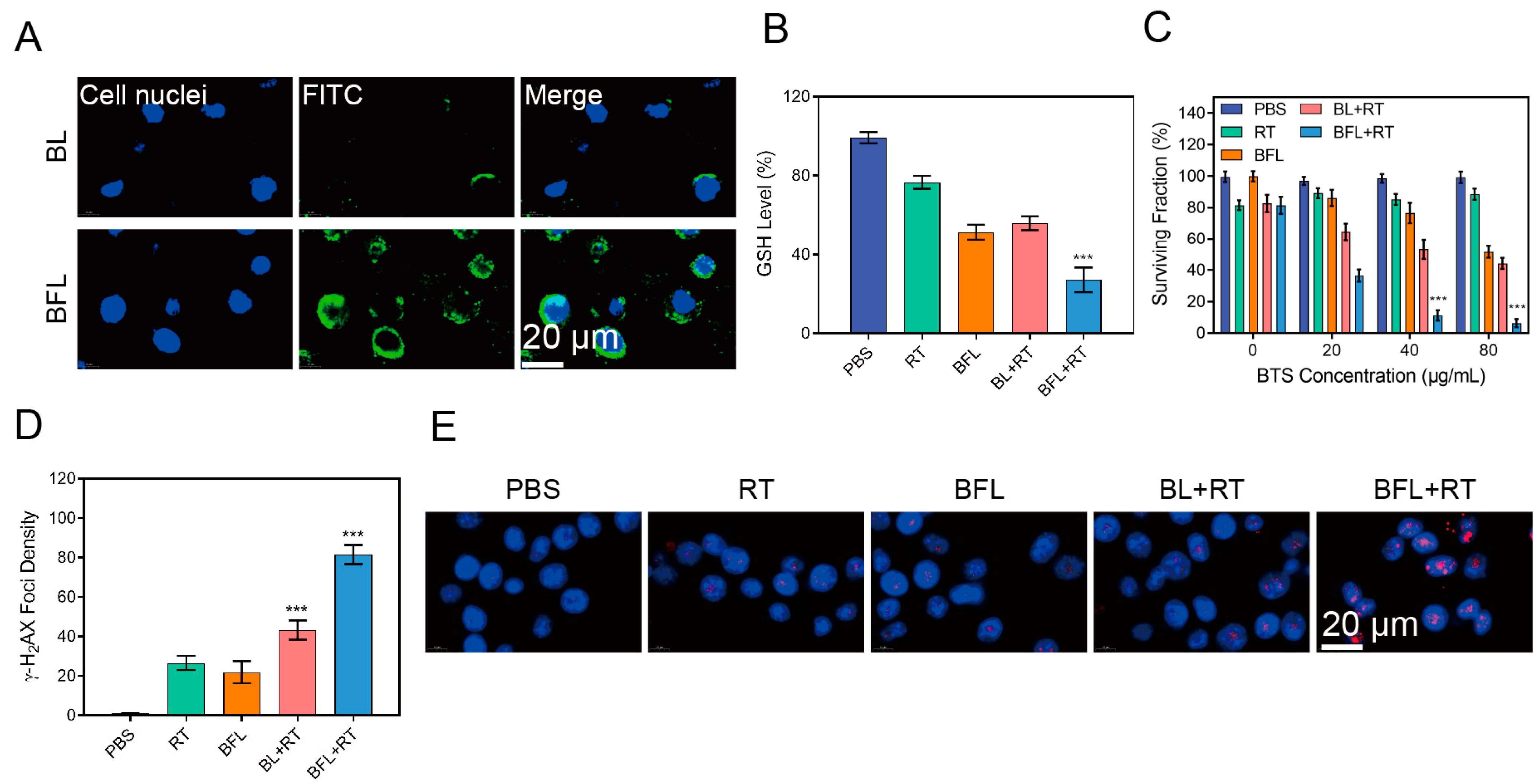
2.5. ROS, DNA Damage, and ICD Detection
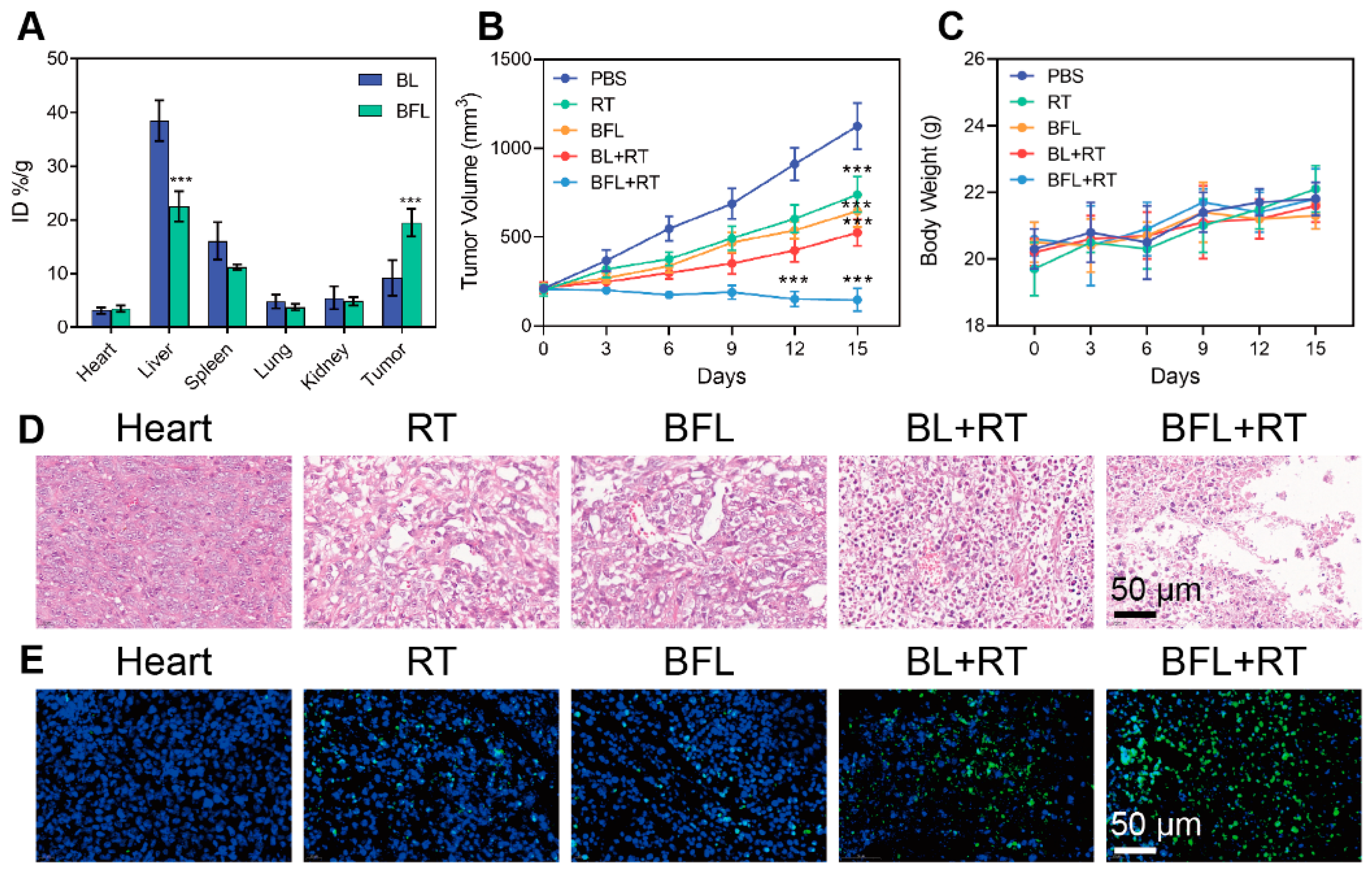
2.6. Bilateral Antitumor Study
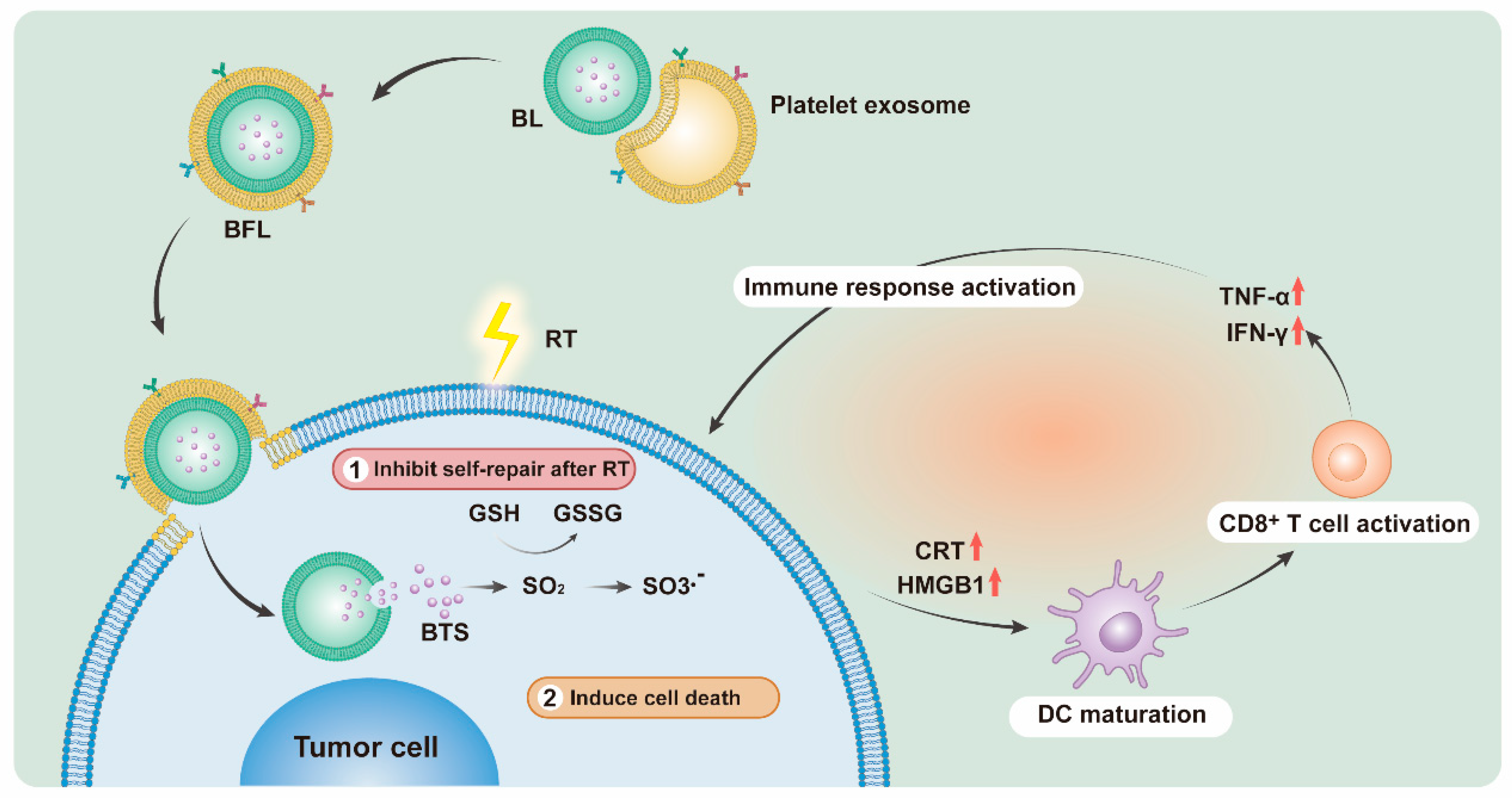
3. Results and Discussion
3.1. Characterization of BL and BFL
3.2. BFL Enhances RT In Vitro
3.3. BFL Enhances RT to Provoke an Immune Response In Vitro
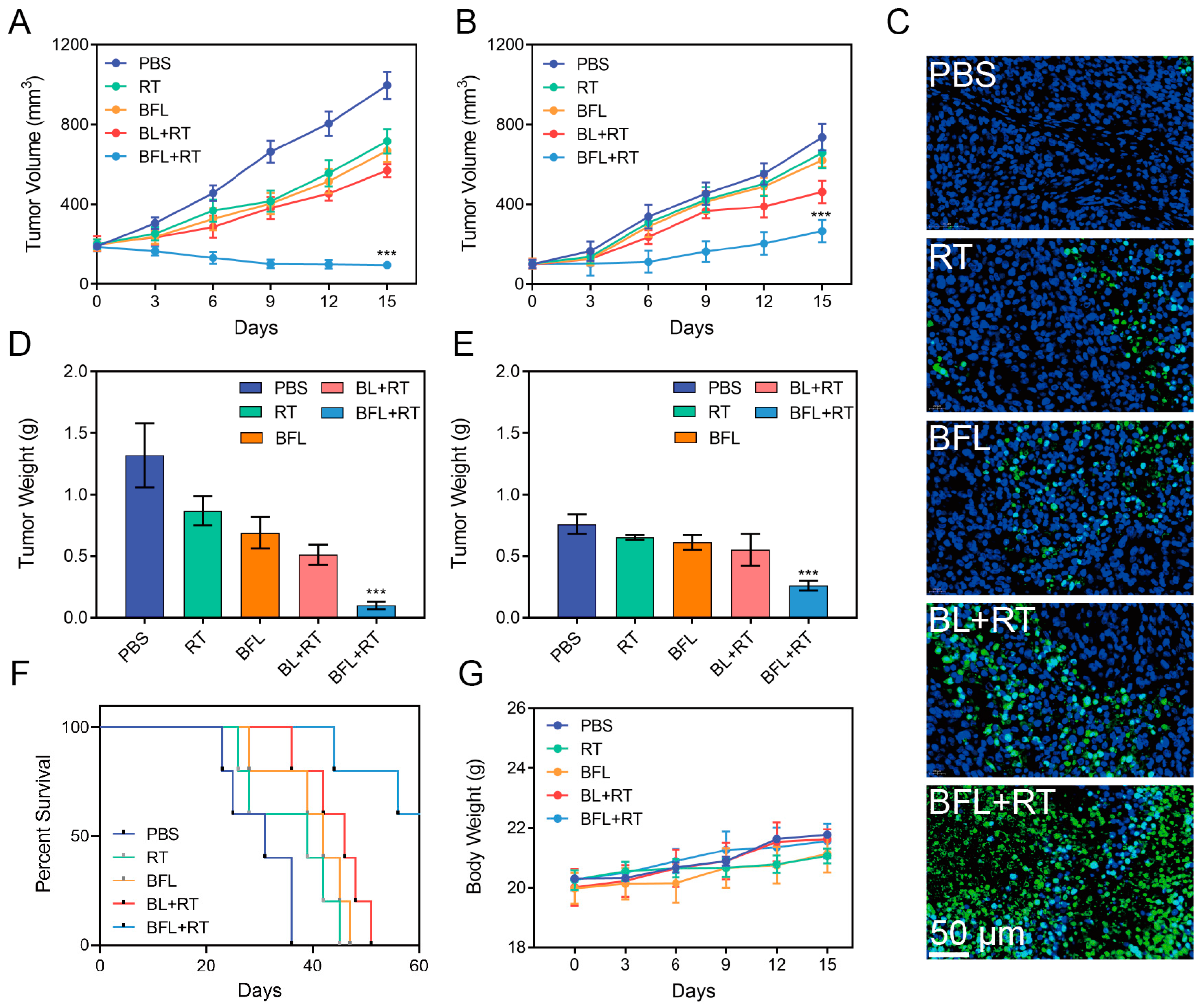
3.4. Radiosensitization by BFL In Vivo
3.5. In Vivo Antitumor Efficacy Evaluation
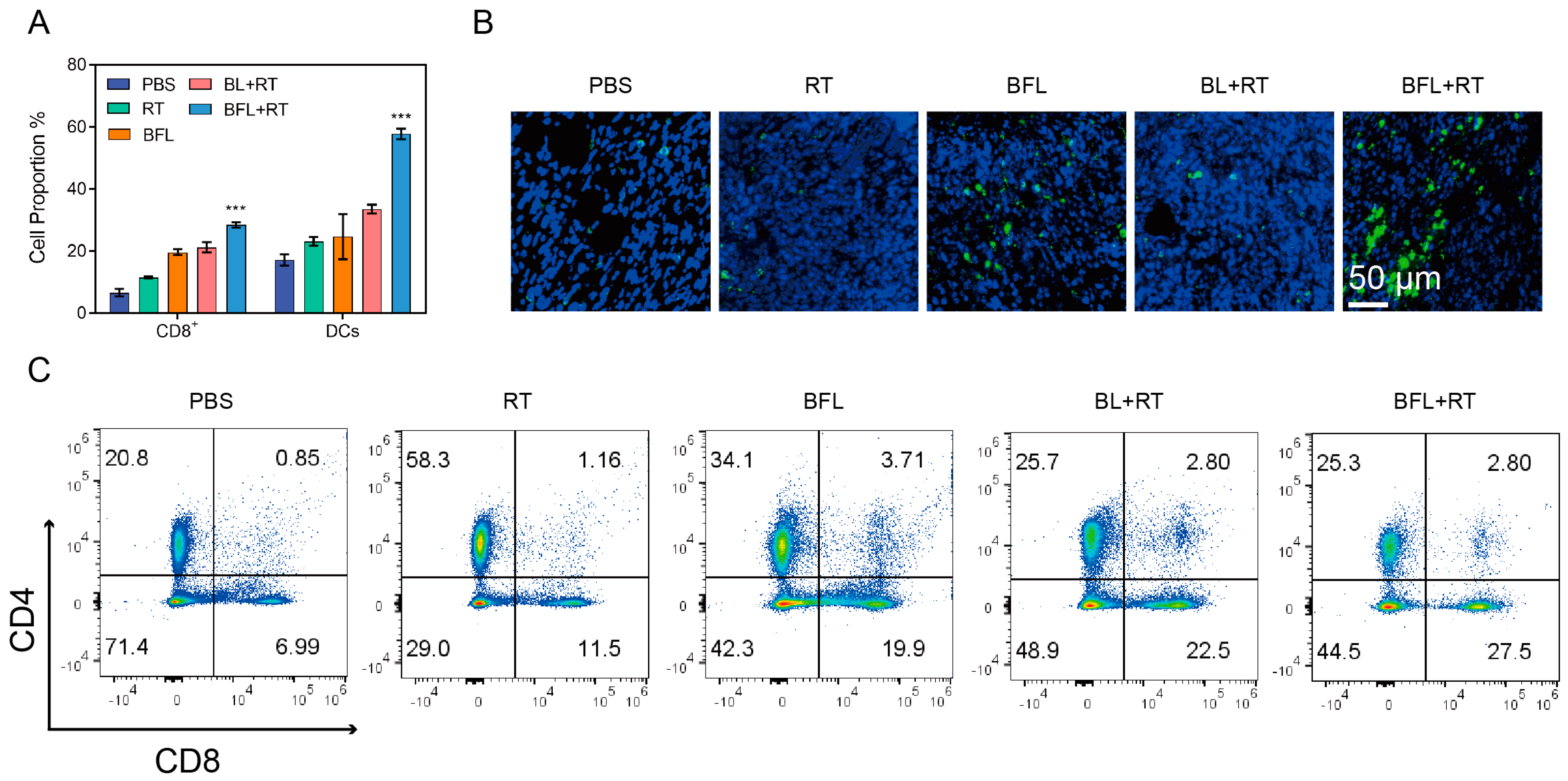
3.6. Immune Response Activated by BFL + RT Contributes to the Inhibition of Tumor Metastasis
3.7. Biosafety Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yang, N.; Gong, F.; Liu, B.; Hao, Y.; Chao, Y.; Lei, H.; Yang, X.; Gong, Y.; Wang, X.; Liu, Z.; et al. Magnesium galvanic cells produce hydrogen and modulate the tumor microenvironment to inhibit cancer growth. Nat. Commun. 2022, 13, 2336. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.-Z.; Li, S.-J.; Sun, Z.-J. Gas and gas-generating nanoplatforms in cancer therapy. J. Mater. Chem. B 2021, 9, 8541–8557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Pan, Y.; Suo, M.; Lyu, M.; Lam, J.W.Y.; Jin, Z.; Ning, S.; Tang, B.Z. Photothermal-Triggered Sulfur Oxide Gas Therapy Augments Type I Photodynamic Therapy for Potentiating Cancer Stem Cell Ablation and Inhibiting Radioresistant Tumor Recurrence. Adv. Sci. 2023, 10, 2304042. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, H.; Wang, Y.; Ji, Y.; Wang, F.; He, P. A Novel Cu(II) Loaded Polypeptide SO2 Prodrug Nanoformulation Combining Chemodynamic and Gas Anticancer Therapies. Macromol. Biosci. 2023, 24, 2300429. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Liang, S.; Wang, Z.; Sun, Q.; He, F.; Gai, S.; Yang, P.; Cheng, Z.; Lin, J. A Tumor-Microenvironment-Responsive Nanocomposite for Hydrogen Sulfide Gas and Trimodal-Enhanced Enzyme Dynamic Therapy. Adv. Mater. 2021, 33, 2101223. [Google Scholar] [CrossRef] [PubMed]
- Ding, H.; Chang, J.; He, F.; Gai, S.; Yang, P. Hydrogen Sulfide: An Emerging Precision Strategy for Gas Therapy. Adv. Healthc. Mater. 2022, 11, 2101984. [Google Scholar] [CrossRef] [PubMed]
- Fang, X.; Cai, S.; Wang, M.; Chen, Z.; Lu, C.; Yang, H. Photogenerated Holes Mediated Nitric Oxide Production for Hypoxic Tumor Treatment. Angew. Chem. Int. Ed. 2021, 60, 7046–7050. [Google Scholar] [CrossRef] [PubMed]
- An, J.; Hu, Y.-G.; Li, C.; Hou, X.-L.; Cheng, K.; Zhang, B.; Zhang, R.-Y.; Li, D.-Y.; Liu, S.-J.; Liu, B.; et al. A pH/Ultrasound dual-response biomimetic nanoplatform for nitric oxide gas-sonodynamic combined therapy and repeated ultrasound for relieving hypoxia. Biomaterials 2020, 230, 119636. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, S.; Chen, Z.; Hu, R.; Zhao, Y.; Wang, K.; Qu, J.; Liu, L. Nitric oxide release activated near-Infrared photothermal agent for synergistic tumor treatment. Biomaterials 2021, 276, 121017. [Google Scholar] [CrossRef]
- Yuan, Z.; Lin, C.; Dai, L.; He, Y.; Hu, J.; Xu, K.; Tao, B.; Liu, P.; Cai, K. Near-Infrared Light-Activatable Dual-Action Nanoparticle Combats the Established Biofilms of Methicillin-Resistant Staphylococcus aureus and Its Accompanying Inflammation. Small 2021, 17, 2007522. [Google Scholar] [CrossRef]
- Qi, M.; Ren, X.; Li, W.; Sun, Y.; Sun, X.; Li, C.; Yu, S.; Xu, L.; Zhou, Y.; Song, S.; et al. NIR responsive nitric oxide nanogenerator for enhanced biofilm eradication and inflammation immunotherapy against periodontal diseases. Nano Today 2022, 43, 101447. [Google Scholar] [CrossRef]
- Li, N.; Huang, C.; Zhang, J.; Zhang, J.; Huang, J.; Li, S.; Xia, X.; Wu, Z.; Chen, C.; Tang, S.; et al. Chemotactic NO/H2S Nanomotors Realizing Cardiac Targeting of G-CSF against Myocardial Ischemia-Reperfusion Injury. ACS Nano 2023, 17, 12573–12593. [Google Scholar] [CrossRef] [PubMed]
- Lu, Q.; Lu, T.; Xu, M.; Yang, L.; Song, Y.; Li, N. SO2 prodrug doped nanorattles with extra-high drug payload for “collusion inside and outside” photothermal/pH triggered—Gas therapy. Biomaterials 2020, 257, 120236. [Google Scholar] [CrossRef] [PubMed]
- Chu, Q.; Liao, L.; Liu, B.; Han, G.; Li, X. Sulfite-Inserted MgAl Layered Double Hydroxides Loaded with Glucose Oxidase to Enable SO2-Mediated Synergistic Tumor Therapy. Adv. Funct. Mater. 2021, 31, 2103262. [Google Scholar] [CrossRef]
- Zhu, M.; Yang, X.; You, J.; Zheng, L.; Yi, C.; Huang, Y. Nanobiotechnology-mediated radioimmunotherapy treatment for triple-negative breast cancer. MedComm Biomater. Appl. 2023, 2, e32. [Google Scholar] [CrossRef]
- Xin, J.; Deng, C.; Zheng, M.; An, F. Amphiphilic photosensitizer polymer as a nanocarrier of cytotoxic molecule for carrier-free combination therapy. MedComm Biomater. Appl. 2023, 2, e28. [Google Scholar] [CrossRef]
- He, H.; Guo, C.; Liu, W.; Chen, S.; Wang, X.Y.; Yang, H. Engineering nanostructured pure cancer cell membrane-derived vesicles as a novel therapeutic cancer vaccine. MedComm Biomater. Appl. 2022, 1, e22. [Google Scholar] [CrossRef]
- Wang, H.; Zhang, Y.; Liang, X.; Zhang, Y. Smart Fibers and Textiles for Personal Health Management. ACS Nano 2021, 15, 12497–12508. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Luo, M.; Li, J.; Akakuru, O.U.; Fan, X.; Cao, Z.; Fan, K.; Jiang, W. Personalized Carbon Monoxide-Loaded Biomimetic Single-Atom Nanozyme for Ferroptosis-Enhanced FLASH Radioimmunotherapy. Adv. Funct. Mater. 2023, 33, 2306930. [Google Scholar] [CrossRef]
- Wu, L.; Xie, W.; Zan, H.-M.; Liu, Z.; Wang, G.; Wang, Y.; Liu, W.; Dong, W. Platelet membrane-coated nanoparticles for targeted drug delivery and local chemo-photothermal therapy of orthotopic hepatocellular carcinoma. J. Mater. Chem. B 2020, 8, 4648–4659. [Google Scholar] [CrossRef]
- Bu, L.-L.; Rao, L.; Yu, G.-T.; Chen, L.; Deng, W.-W.; Liu, J.-F.; Wu, H.; Meng, Q.-F.; Guo, S.-S.; Zhao, X.-Z.; et al. Cancer Stem Cell-Platelet Hybrid Membrane-Coated Magnetic Nanoparticles for Enhanced Photothermal Therapy of Head and Neck Squamous Cell Carcinoma. Adv. Funct. Mater. 2019, 29, 1807733. [Google Scholar] [CrossRef]
- Zhang, Q.; Dehaini, D.; Zhang, Y.; Zhou, J.; Chen, X.; Zhang, L.; Fang, R.H.; Gao, W.; Zhang, L. Neutrophil membrane-coated nanoparticles inhibit synovial inflammation and alleviate joint damage in inflammatory arthritis. Nat. Nanotechnol. 2018, 13, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Gao, M.; Liang, C.; Song, X.; Chen, Q.; Jin, Q.; Wang, C.; Liu, Z. Erythrocyte-Membrane-Enveloped Perfluorocarbon as Nanoscale Artificial Red Blood Cells to Relieve Tumor Hypoxia and Enhance Cancer Radiotherapy. Adv Mater 2017, 29, 1701429. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Lin, Z.; Wu, Z.; Lin, X.; He, Q. Stem-Cell-Membrane Camouflaging on Near-Infrared Photoactivated Upconversion Nanoarchitectures for In Vivo Remote-Controlled Photodynamic Therapy. ACS Appl. Mater. Interfaces 2016, 8, 34252–34260. [Google Scholar] [CrossRef]
- Liu, B.; Wang, W.; Fan, J.; Long, Y.; Xiao, F.; Daniyal, M.; Tong, C.; Xie, Q.; Jian, Y.; Li, B.; et al. RBC membrane camouflaged prussian blue nanoparticles for gamabutolin loading and combined chemo/photothermal therapy of breast cancer. Biomaterials 2019, 217, 119301. [Google Scholar] [CrossRef]
- Nguyen, P.H.D.; Jayasinghe, M.K.; Le, A.H.; Peng, B.; Le, M.T.N. Advances in Drug Delivery Systems Based on Red Blood Cells and Their Membrane-Derived Nanoparticles. ACS Nano 2023, 17, 5187–5210. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Lang, T.; Zheng, C.; Yan, W.; Li, Y.; Zhu, R.; Huang, X.; Xu, H.; Li, Y.; Yin, Q. Promote Intratumoral Drug Release and Penetration to Counteract Docetaxel-Induced Metastasis by Photosensitizer-Modified Red Blood Cell Membrane-Coated Nanoparticle. Adv. Funct. Mater. 2023, 33, 2212109. [Google Scholar] [CrossRef]
- Chen, Y.; Zhu, M.; Huang, B.; Jiang, Y.; Su, J. Advances in cell membrane-coated nanoparticles and their applications for bone therapy. Biomater. Adv. 2023, 144, 213232. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.; Zhang, T.; Li, Y.; Xiang, J.; Zhu, D.; Xia, L.; Guo, B.; Xu, Y.; Yu, H.; Tang, B. AIEgen-based nanotherapeutic strategy for enhanced FLASH irradiation to prevent tumour recurrence and avoid severe side effects. Chem. Eng. J. 2023, 473, 145179. [Google Scholar] [CrossRef]
- Choi, S.-E.; Khoo, H.; Hur, S.C. Recent Advances in Microscale Electroporation. Chem. Rev. 2022, 122, 11247–11286. [Google Scholar] [CrossRef]
- Lopes, D.; Lopes, J.; Pereira-Silva, M.; Peixoto, D.; Rabiee, N.; Veiga, F.; Moradi, O.; Guo, Z.-H.; Wang, X.-D.; Conde, J.; et al. Bioengineered exosomal-membrane-camouflaged abiotic nanocarriers: Neurodegenerative diseases, tissue engineering and regenerative medicine. Mil. Med. Res. 2023, 10, 19. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Chiang, C.-l.; Wang, X.; Bertani, P.; Ma, Y.; Cheng, J.; Talesara, V.; Lee, L.J.; Lu, W. Cell membrane damage and cargo delivery in nano-electroporation. Nanoscale 2023, 15, 4080–4089. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Deng, J.; Wang, Y.; Wu, C.-Q.; Li, X.; Dai, H.-W. Hybrid cell membrane-coated nanoparticles: A multifunctional biomimetic platform for cancer diagnosis and therapy. Acta Biomater. 2020, 112, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Dash, P.; Piras, A.M.; Dash, M. Cell membrane coated nanocarriers—An efficient biomimetic platform for targeted therapy. J. Control. Release 2020, 327, 546–570. [Google Scholar] [CrossRef] [PubMed]
- Chugh, V.; Vijaya Krishna, K.; Pandit, A. Cell Membrane-Coated Mimics: A Methodological Approach for Fabrication, Characterization for Therapeutic Applications, and Challenges for Clinical Translation. ACS Nano 2021, 15, 17080–17123. [Google Scholar] [CrossRef] [PubMed]
- Jing, L.; Qu, H.; Wu, D.; Zhu, C.; Yang, Y.; Jin, X.; Zheng, J.; Shi, X.; Yan, X.; Wang, Y. Platelet-camouflaged nanococktail: Simultaneous inhibition of drug-resistant tumor growth and metastasis via a cancer cells and tumor vasculature dual-targeting strategy. Theranostics 2018, 8, 2683–2695. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Liu, Y.; Li, X.; Wang, H.; Mao, D.; Wei, L.; Ye, X.; Qu, D.; Huo, J.; Chen, Y. Magnetic Metal–Organic Framework-Based Nanoplatform with Platelet Membrane Coating as a Synergistic Programmed Cell Death Protein 1 Inhibitor against Hepatocellular Carcinoma. ACS Nano 2023, 17, 23829–23849. [Google Scholar] [CrossRef] [PubMed]
- Weiss, F.; Lauffenburger, D.; Friedl, P. Towards targeting of shared mechanisms of cancer metastasis and therapy resistance. Nat. Rev. Cancer 2022, 22, 157–173. [Google Scholar] [CrossRef]
- Lyu, M.; Chen, M.; Liu, L.; Zhu, D.; Wu, X.; Li, Y.; Rao, L.; Bao, Z. A platelet-mimicking theranostic platform for cancer interstitial brachytherapy. Theranostics 2021, 11, 7589–7599. [Google Scholar] [CrossRef]
- Wang, J.; Chen, D.; Ho, E.A. Challenges in the development and establishment of exosome-based drug delivery systems. J. Control. Release 2021, 329, 894–906. [Google Scholar] [CrossRef]
- Xu, M.; Lu, Q.; Song, Y.; Yang, L.; Li, J.; Li, N. Enhanced Bax upregulating in mitochondria for deep tumor therapy based on SO2 prodrug loaded Au–Ag hollow nanotriangles. Biomaterials 2020, 250, 120076. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.-C.; Tang, X.-F.; Xu, Y.-C.; Jiang, W.; Xin, Y.-J.; Zhao, W.; He, X.; Lu, L.-G.; Zhan, M.-X. Nano-enabled coordination platform of bismuth nitrate and cisplatin prodrug potentiates cancer chemoradiotherapy via DNA damage enhancement. Biomater. Sci. 2021, 9, 3401–3409. [Google Scholar] [CrossRef] [PubMed]
- An, L.; Zhang, P.; Shen, W.; Yi, X.; Yin, W.; Jiang, R.; Xiao, C. A sulfur dioxide polymer prodrug showing combined effect with doxorubicin in combating subcutaneous and metastatic melanoma. Bioact. Mater. 2021, 6, 1365–1374. [Google Scholar] [CrossRef]
- Chen, J.L.-Y.; Yang, S.-J.; Pan, C.-K.; Lin, L.-C.; Tsai, C.-Y.; Wang, C.-H.; Huang, Y.-S.; Lin, Y.-L.; Kuo, S.-H.; Shieh, M.-J. Cisplatin and Albumin-Based Gold–Cisplatin Nanoparticles Enhance Ablative Radiation Therapy–Induced Antitumor Immunity in Local and Distant Tumor Microenvironment. Int. J. Radiat. Oncol. Biol. Phys. 2023, 116, 1135–1149. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Z.; Zhou, X.; Wu, B.; Tang, H.; Wei, W.; Zhu, D.; Ding, Y.; Chen, L. Personalized SO2 Prodrug for pH-Triggered Gas Enhancement in Anti-Tumor Radio-Immunotherapy. Pharmaceutics 2024, 16, 833. https://doi.org/10.3390/pharmaceutics16060833
Chen Z, Zhou X, Wu B, Tang H, Wei W, Zhu D, Ding Y, Chen L. Personalized SO2 Prodrug for pH-Triggered Gas Enhancement in Anti-Tumor Radio-Immunotherapy. Pharmaceutics. 2024; 16(6):833. https://doi.org/10.3390/pharmaceutics16060833
Chicago/Turabian StyleChen, Zhiran, Xiaoxiang Zhou, Bo Wu, Han Tang, Wei Wei, Daoming Zhu, Yi Ding, and Longyun Chen. 2024. "Personalized SO2 Prodrug for pH-Triggered Gas Enhancement in Anti-Tumor Radio-Immunotherapy" Pharmaceutics 16, no. 6: 833. https://doi.org/10.3390/pharmaceutics16060833
APA StyleChen, Z., Zhou, X., Wu, B., Tang, H., Wei, W., Zhu, D., Ding, Y., & Chen, L. (2024). Personalized SO2 Prodrug for pH-Triggered Gas Enhancement in Anti-Tumor Radio-Immunotherapy. Pharmaceutics, 16(6), 833. https://doi.org/10.3390/pharmaceutics16060833





