Functional Mechanisms of Dietary Crocin Protection in Cardiovascular Models under Oxidative Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Crocin and Cell-Free Antioxidant Assay
2.2.1. DPPH Radical Scavenging Test
2.2.2. Peroxyl Radical Scavenging Activity (ORAC)
2.3. Cell Viability and Induction of Oxidative Stress Conditions
2.4. Geno-Protective Effect of Crocin
2.4.1. DAPI Staining and DNA Ladder Assay
2.4.2. Analysis of Cellular DNA Contents by Flow Cytometry: Cell Cycle Arrest Test
2.5. Effects of Crocin on Mitochondrial Membrane Potential and Cell Signaling
2.5.1. The Mitochondrial Membrane Changes under Oxidative Stress and Crocin Enhancement
2.5.2. Signaling Regulation by Crocin in HUVEC under Oxidative Stress Conditions
2.6. Animal Studies
2.6.1. Animals and Grouping
2.6.2. Determination of Serum Biochemical Parameters, Total Antioxidant Status, and Lipid Peroxidation
2.6.3. Histopathological Analysis of the Heart Tissue
2.6.4. Western Blot Analysis
2.7. Statistical Analysis
3. Results
3.1. Antioxidant Activity of Crocin
3.2. Crocin Protects Endothelial Cells against Exogenous Stress Induced by AAPH and H2O2 In Vitro
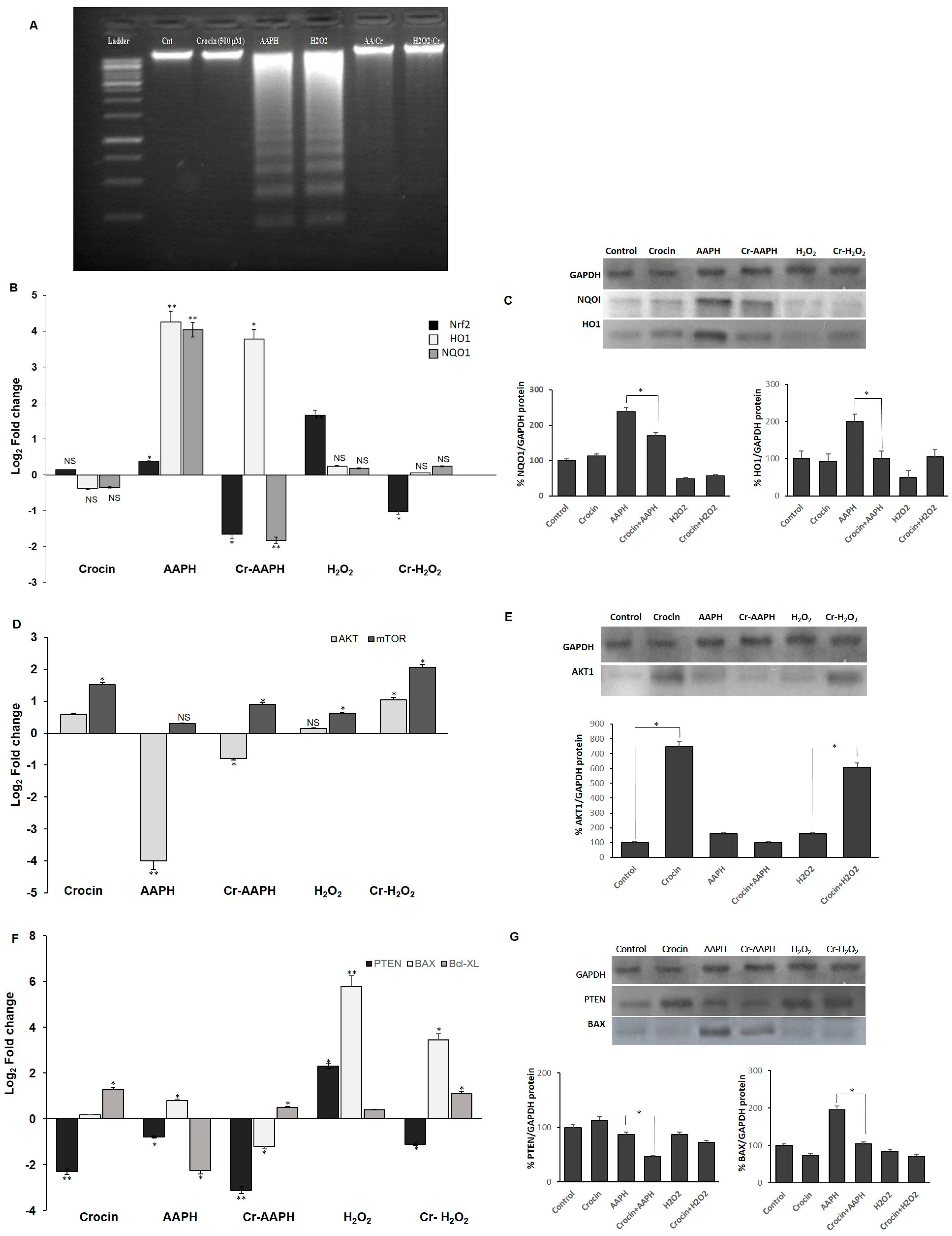
3.3. Crocin Protection against HUVEC DNA Damage
3.4. Influence of Crocin on the Signaling Pathway of HUVEC under Oxidative Stress
3.4.1. Crocin Carries out Its Antioxidant Activity through the Nrf2/HO1/NQO1 Pathway
3.4.2. Crocin Exerts Its Cytoprotective and Survival Potential via the AKT/mTOR Signaling Pathway on HUVEC Cells
3.4.3. Crocin Inhibits HUVEC Apoptotic Genes Induced by AAPH and H2O2
3.5. AAPH and H2O2 Induce G0/G1 Phase Arrest and Apoptosis Phase
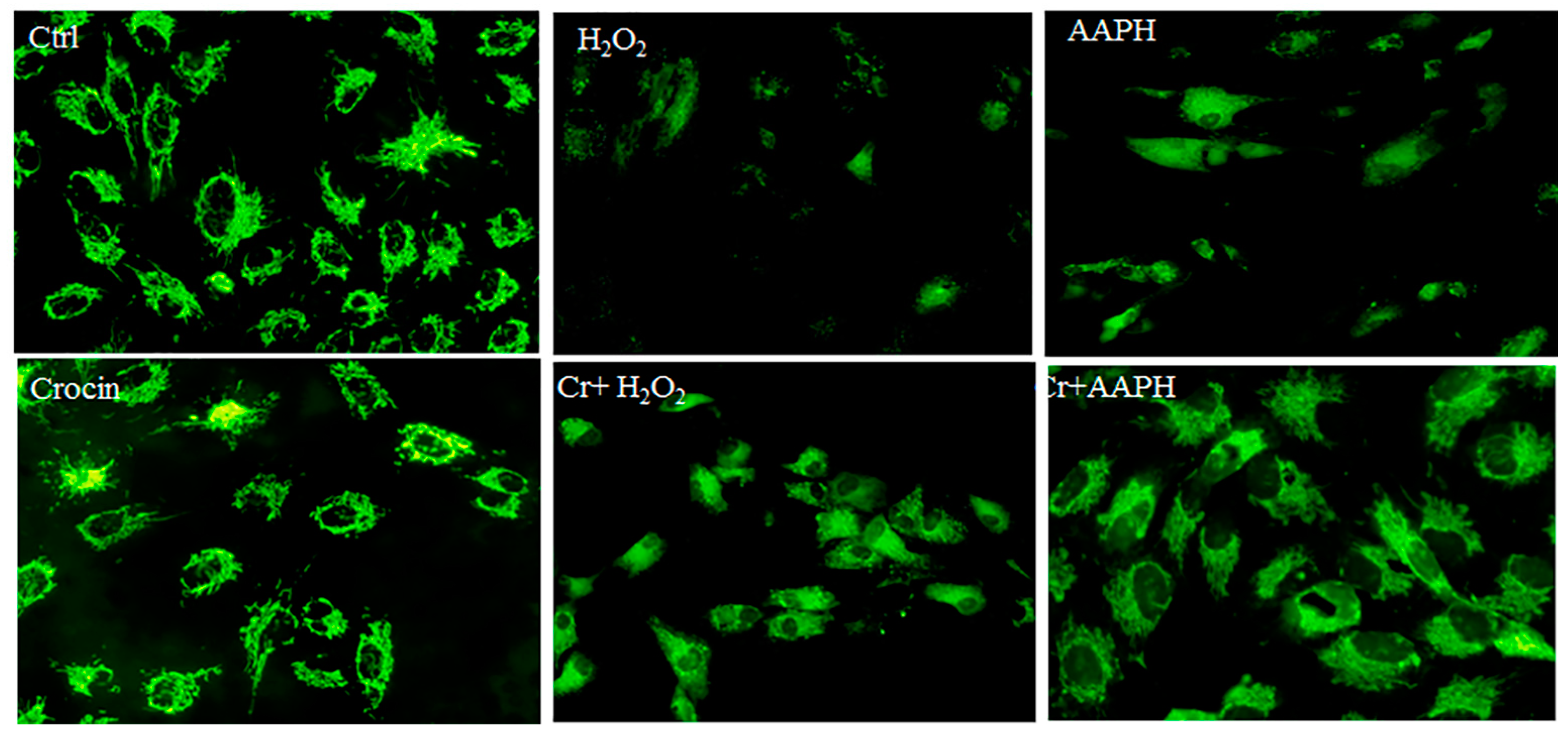
3.6. Mitochondrial Protection by Crocin
3.7. Effects of Crocin on Stress-Induced Heart Injury in Mice
3.7.1. Protective Effects of Crocin on the Histology of Mice Heart
3.7.2. Serum Biochemical Parameters
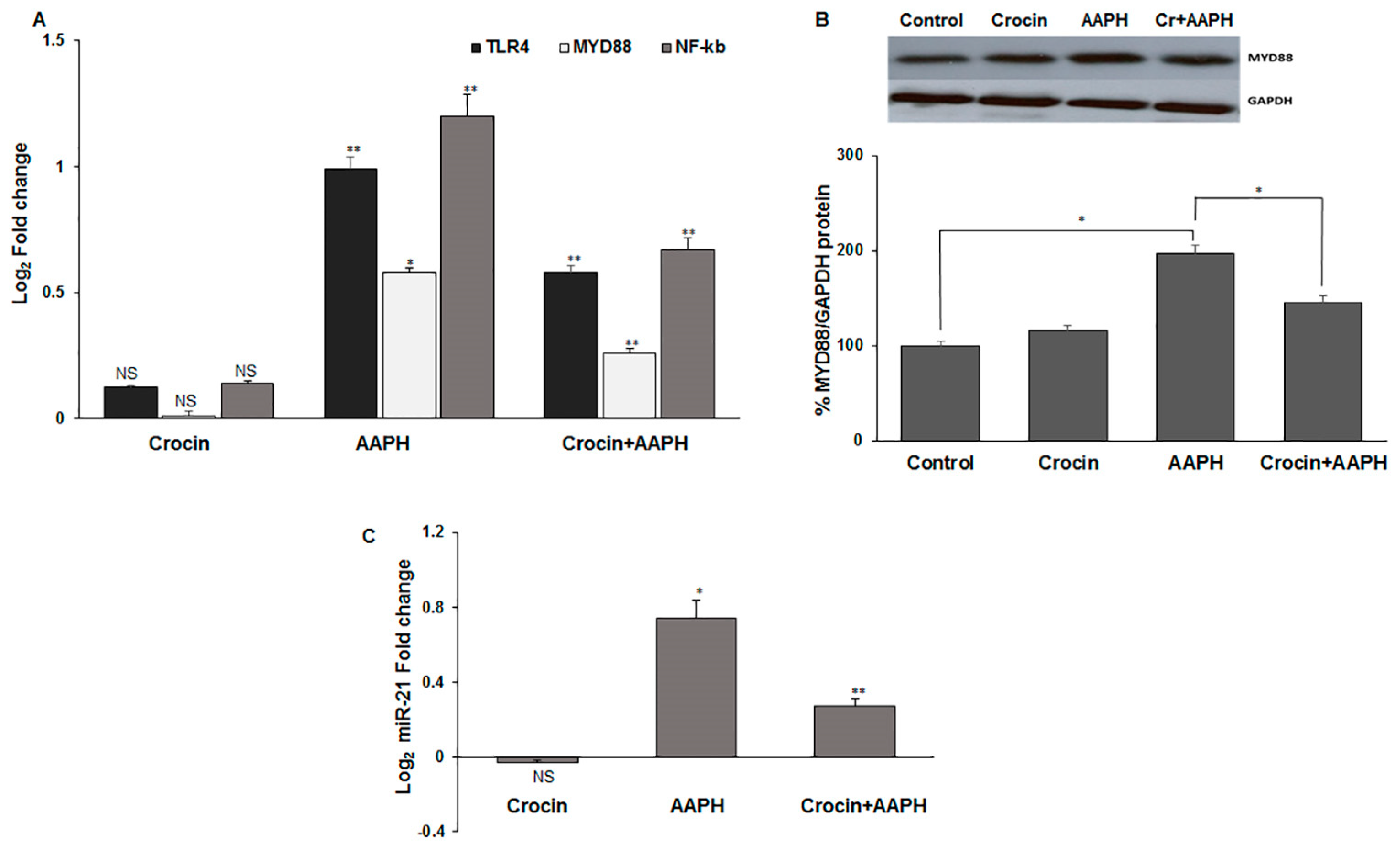
3.7.3. Crocin Exerts Its Antioxidant Activity via TLR4/MYD88 Receptors in Mice
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brieger, K.; Schiavone, S.; Miller, F.J., Jr.; Krause, K.-H. Reactive Oxygen Species: From Health to Disease. Swiss Med. Wkly. 2012, 142, w13659. [Google Scholar] [CrossRef] [PubMed]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Krylatov, A.V.; Maslov, L.N.; Voronkov, N.S.; Boshchenko, A.A.; Popov, S.V.; Gomez, L.; Wang, H.; Jaggi, A.S.; Downey, J.M. Reactive Oxygen Species as Intracellular Signaling Molecules in the Cardiovascular System. Curr. Cardiol. Rev. 2018, 14, 290–300. [Google Scholar] [CrossRef] [PubMed]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F. Oxidative Stress and Reactive Oxygen Species in Endothelial Dysfunction Associated with Cardiovascular and Metabolic Diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Zhou, G.B.; Hou, Y.P.; Jin, F.; Yang, Q.E.; Yang, Z.Q.; Quan, G.B.; Tan, H.M.; Zhu, S.E. Vitrification of Mouse Embryos at Various Stages by Open-Pulled Straw (OPS) Method. Anim. Biotechnol. 2005, 16, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Magno, S.; Ceccarini, G.; Pelosini, C.; Jaccheri, R.; Vitti, J.; Fierabracci, P.; Salvetti, G.; Airoldi, G.; Minale, M.; Saponati, G.; et al. LDL-Cholesterol Lowering Effect of a New Dietary Supplement: An Open Label, Controlled, Randomized, Cross-over Clinical Trial in Patients with Mild-to-Moderate Hypercholesterolemia. Lipids Health Dis. 2018, 17, 124. [Google Scholar] [CrossRef] [PubMed]
- Zurbau, A.; Au-Yeung, F.; Blanco Mejia, S.; Khan, T.A.; Vuksan, V.; Jovanovski, E.; Leiter, L.A.; Kendall, C.W.C.; Jenkins, D.J.A.; Sievenpiper, J.L. Relation of Different Fruit and Vegetable Sources With Incident Cardiovascular Outcomes: A Systematic Review and Meta-Analysis of Prospective Cohort Studies. J. Am. Heart Assoc. 2020, 9, e017728. [Google Scholar] [CrossRef] [PubMed]
- Palacio, C.; Mooradian, A.D. Clinical Trials and Antioxidant Outcomes. In Oxidative Stress and Antioxidant Protection; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2016; pp. 493–506. ISBN 978-1-118-83243-1. [Google Scholar]
- José Bagur, M.; Alonso Salinas, L.G.; Jiménez-Monreal, M.A.; Chaouqi, S.; Llorens, S.; Martínez-Tomé, M.; Alonso, L.G. Saffron: An Old Medicinal Plant and a Potential Novel Functional Food. Molecules 2018, 23, 30. [Google Scholar] [CrossRef] [PubMed]
- Cerdá-Bernad, D.; Valero-Cases, E.; Pastor, J.-J.; Frutos, M.J. Saffron Bioactives Crocin, Crocetin and Safranal: Effect on Oxidative Stress and Mechanisms of Action. Crit. Rev. Food Sci. Nutr. 2022, 62, 3232–3249. [Google Scholar] [CrossRef]
- Licón, C.C.; Carmona, M.; Rubio, R.; Molina, A.; Berruga, M.I. Preliminary Study of Saffron (Crocus sativus L. Stigmas) Color Extraction in a Dairy Matrix. Dye. Pigment. 2012, 92, 1355–1360. [Google Scholar] [CrossRef]
- Mirnasrollahi Parsa, R.S.; Aryaeian, N.; Mokhtare, M.; Kavianipour, F.; Janani, L.; Agah, S.; Moradi, N. The Effects of Saffron (Crocus sativus L) Supplementation on Cardiovascular Risk Factors, Liver Steatosis, and Fibrosis in Patients With Non-Alcoholic Fatty Liver Disease: A Randomised, Double-Blind, Placebo-Controlled Study. J. Herb. Med. 2024, 45, 100877. [Google Scholar] [CrossRef]
- Yang, X.; Huo, F.; Liu, B.; Liu, J.; Chen, T.; Li, J.; Zhu, Z.; Lv, B. Crocin Inhibits Oxidative Stress and Pro-Inflammatory Response of Microglial Cells Associated with Diabetic Retinopathy Through the Activation of PI3K/Akt Signaling Pathway. J. Mol. Neurosci. 2017, 61, 581–589. [Google Scholar] [CrossRef] [PubMed]
- Rocchi, R.; Mascini, M.; Sergi, M.; Compagnone, D.; Mastrocola, D.; Pittia, P. Crocins Pattern in Saffron Detected by UHPLC-MS/MS as Marker of Quality, Process and Traceability. Food Chem. 2018, 264, 241–249. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Díaz, J.; Sánchez, A.M.; Maggi, L.; Martínez-Tomé, M.; García-Diz, L.; Murcia, M.A.; Alonso, G.L. Increasing the Applications of Crocus sativus Flowers as Natural Antioxidants. J. Food Sci. 2012, 77, C1162–C1168. [Google Scholar] [CrossRef] [PubMed]
- Dogan, T.; Yildirim, B.A.; Kapakin, K.A.T. Investigation of the Effects of Crocin on Inflammation, Oxidative Stress, Apoptosis, NF-κB, TLR-4 and Nrf-2/HO-1 Pathways in Gentamicin-Induced Nephrotoxicity in Rats. Environ. Toxicol. Pharmacol. 2024, 106, 104374. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Yuan, B.; Cheng, B.; Liu, Y.; Zhang, B.; Wang, X.; Lin, X.; Yang, B.; Gong, G. Crocin Alleviates Myocardial Ischemia/Reperfusion-Induced Endoplasmic Reticulum Stress via Regulation of miR-34a/Sirt1/Nrf2 Pathway. Shock 2019, 51, 123–130. [Google Scholar] [CrossRef]
- Han, S.; Song, R.; Cao, Y.; Yan, X.; Gao, H.; Lian, F. Crocin Mitigates Atherosclerotic Progression in LDLR Knockout Mice by Hepatic Oxidative Stress and Inflammatory Reaction Reduction, and Intestinal Barrier Improvement and Gut Microbiota Modulation. J. Funct. Foods 2022, 96, 105221. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Maleki, M.; Rashid-Farrokhi, F.; Abdullahi, P.R.; Hemmati, M.A.; Jamialahmadi, T.; Sahebkar, A. Modulating Effects of Crocin on Lipids and Lipoproteins: Mechanisms and Potential Benefits. Heliyon 2024, 10, e28837. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, V.; Salatti-Dorado, Á.J.; Barzegari, A.; Nicolas-Boluda, A.; Houaoui, A.; Caballo, C.; Caballero-Casero, N.; Sicilia, D.; Bastias Venegas, J.; Pauthe, E.; et al. Astaxanthin-Loaded Nanostructured Lipid Carriers for Preservation of Antioxidant Activity. Molecules 2018, 23, 2601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, O.P.; Bhat, T.K. DPPH Antioxidant Assay Revisited. Food Chem. 2009, 113, 1202–1205. [Google Scholar] [CrossRef]
- Ou, B.; Huang, D.; Hampsch-Woodill, M.; Flanagan, J.A.; Deemer, E.K. Analysis of Antioxidant Activities of Common Vegetables Employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) Assays: A Comparative Study. J. Agric. Food Chem. 2002, 50, 3122–3128. [Google Scholar] [CrossRef] [PubMed]
- Regnier, P.; Bastias, J.; Rodriguez-Ruiz, V.; Caballero-Casero, N.; Caballo, C.; Sicilia, D.; Fuentes, A.; Maire, M.; Crepin, M.; Letourneur, D.; et al. Astaxanthin from Haematococcus Pluvialis Prevents Oxidative Stress on Human Endothelial Cells without Toxicity. Mar. Drugs 2015, 13, 2857–2874. [Google Scholar] [CrossRef] [PubMed]
- RahbarSaadat, Y.; Saeidi, N.; Vahed, S.Z.; Barzegari, A.; Barar, J. An Update to DNA Ladder Assay for Apoptosis Detection. Biolmpacts 2015, 5, 25. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Ruiz, V.; Barzegari, A.; Zuluaga, M.; Zunooni-Vahed, S.; Rahbar-Saadat, Y.; Letourneur, D.; Gueguen, V.; Pavon-Djavid, G. Potential of Aqueous Extract of Saffron (Crocus sativus L.) in Blocking the Oxidative Stress by Modulation of Signal Transduction in Human Vascular Endothelial Cells. J. Funct. Foods 2016, 26, 123–134. [Google Scholar] [CrossRef]
- Zuluaga Tamayo, M.; Choudat, L.; Aid-Launais, R.; Thibaudeau, O.; Louedec, L.; Letourneur, D.; Gueguen, V.; Meddahi-Pellé, A.; Couvelard, A.; Pavon-Djavid, G. Astaxanthin Complexes to Attenuate Muscle Damage after In Vivo Femoral Ischemia-Reperfusion. Mar. Drugs 2019, 17, 354. [Google Scholar] [CrossRef]
- Gokila Vani, M.; Kumar, K.J.S.; Liao, J.-W.; Chien, S.-C.; Mau, J.-L.; Chiang, S.-S.; Lin, C.-C.; Kuo, Y.-H.; Wang, S.-Y. Antcin C from Antrodia Cinnamomea Protects Liver Cells Against Free Radical-Induced Oxidative Stress and Apoptosis In Vitro and In Vivo through Nrf2-Dependent Mechanism. Evid. -Based Complement. Altern. Med. 2013, 2013, 17. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.J.; Kim, D.H.; Lee, E.K.; Song, C.W.; Yu, B.P.; Chung, H.Y. Oxidative Stress Induces Inactivation of Protein Phosphatase 2A, Promoting Proinflammatory NF-κB in Aged Rat Kidney. Free Radic. Biol. Med. 2013, 61, 206–217. [Google Scholar] [CrossRef]
- Muller, L.; Theile, K.; Bohm, V. In Vitro Antioxidant Activity of Tocopherols and Tocotrienols and Comparison of Vitamin E Concentration and Lipophilic Antioxidant Capacity in Human Plasma. Mol. Nutr. Food Res. 2010, 54, 731–742. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The Chemistry behind Antioxidant Capacity Assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Vali, F.; Changizi, V.; Safa, M. Synergistic Apoptotic Effect of Crocin and Paclitaxel or Crocin and Radiation on MCF-7 Cells, a Type of Breast Cancer Cell Line. Int. J. Breast Cancer 2015, 2015, 139349. [Google Scholar] [CrossRef]
- Mann, G.E. Nrf2-Mediated Redox Signalling in Vascular Health and Disease. Free Radic. Biol. Med. 2014, 75, S1. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-S.; Chau, L.-Y. Heme Oxygenase-1 Mediates the Anti-Inflammatory Effect of Interleukin-10 in Mice. Nat. Med. 2002, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-L.; Ho, Y.-C.; Yet, S.-F. A Central Role of Heme Oxygenase-1 in Cardiovascular Protection. Antioxid. Redox Signal. 2011, 15, 1835–1846. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Volpe, M.; Sadoshima, J. Mammalian Target of Rapamycin Signaling in Cardiac Physiology and Disease. Circ. Res. 2014, 114, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Liu, A.; Zhu, Y.; Chen, W.; Merlino, G.; Yu, Y. PTEN Dual Lipid- and Protein-Phosphatase Function in Tumor Progression. Cancers 2022, 14, 3666. [Google Scholar] [CrossRef] [PubMed]
- Bu, L.; Wang, H.; Pan, J.; Chen, L.; Xing, F.; Wu, J.; Li, S.; Guo, D. PTEN Suppresses Tumorigenesis by Directly Dephosphorylating Akt. Signal Transduct. Target. Ther. 2021, 6, 262. [Google Scholar] [CrossRef] [PubMed]
- Zorova, L.D.; Popkov, V.A.; Plotnikov, E.Y.; Silachev, D.N.; Pevzner, I.B.; Jankauskas, S.S.; Babenko, V.A.; Zorov, S.D.; Balakireva, A.V.; Juhaszova, M.; et al. Mitochondrial Membrane Potential. Anal. Biochem. 2018, 552, 50–59. [Google Scholar] [CrossRef] [PubMed]
- Baracca, A.; Sgarbi, G.; Solaini, G.; Lenaz, G. Rhodamine 123 as a Probe of Mitochondrial Membrane Potential: Evaluation of Proton Flux through F(0) during ATP Synthesis. Biochim. Biophys. Acta 2003, 1606, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Sun, L.; Chen, X.; Zhang, D. Oxidative Stress, Mitochondrial Damage and Neurodegenerative Diseases. Neural Regen. Res. 2013, 8, 2003–2014. [Google Scholar] [CrossRef]
- Kanno, T.; Utsumi, T.; Ide, A.; Takehara, Y.; Saibara, T.; Akiyama, J.; Yoshioka, T.; Utsumi, K. Dysfunction of Mouse Liver Mitochondria Induced by 2,2′-Azobis-(2-Amidinopropane) Dihydrochloride, a Radical Initiator, in Vitro and in Vivo. Free Radic. Res. 1994, 21, 223–234. [Google Scholar] [CrossRef]
- Li, L.; Pan, H.; Wang, H.; Li, X.; Bu, X.; Wang, Q.; Gao, Y.; Wen, G.; Zhou, Y.; Cong, Z.; et al. Interplay between VEGF and Nrf2 Regulates Angiogenesis Due to Intracranial Venous Hypertension. Sci. Rep. 2016, 6, 37338. [Google Scholar] [CrossRef] [PubMed]
- Chao, W. Toll-like Receptor Signaling: A Critical Modulator of Cell Survival and Ischemic Injury in the Heart. Am. J. Physiol. Heart Circ. Physiol. 2009, 296, H1–H12. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Zhang, C. MicroRNA-21 in Cardiovascular Disease. J. Cardiovasc. Transl. Res. 2010, 3, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Roy, S. MicroRNA 21 in Tissue Injury and Inflammation: AUTHORS’ RETROSPECTIVE. Cardiovasc. Res. 2012, 96, 230–233. [Google Scholar] [CrossRef]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.-M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-Coding RNAs in Cardiovascular Diseases: Diagnostic and Therapeutic Perspectives. Eur. Heart J. 2018, 39, 2704–2716. [Google Scholar] [CrossRef] [PubMed]
- Dinh, Q.N.; Chrissobolis, S.; Diep, H.; Chan, C.T.; Ferens, D.; Drummond, G.R.; Sobey, C.G. Advanced Atherosclerosis Is Associated with Inflammation, Vascular Dysfunction and Oxidative Stress, but Not Hypertension. Pharmacol. Res. 2017, 116, 70–76. [Google Scholar] [CrossRef] [PubMed]
- Forstermann, U.; Xia, N.; Li, H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017, 120, 713–735. [Google Scholar] [CrossRef] [PubMed]
- Cai, H.; Harrison, D.G. Endothelial Dysfunction in Cardiovascular Diseases: The Role of Oxidant Stress. Circ. Res. 2000, 87, 840–844. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Jacobi, A.; Vater, C.; Zou, X.; Stiehler, M. Salvianolic Acid B Protects Human Endothelial Progenitor Cells against Oxidative Stress-Mediated Dysfunction by Modulating Akt/mTOR/4EBP1, P38 MAPK/ATF2, and ERK1/2 Signaling Pathways. Biochem. Pharmacol. 2014, 90, 34–49. [Google Scholar] [CrossRef]
- Tripathi, P.; Chandra, M.; Misra, M.K. Oral Administration of L-Arginine in Patients with Angina or Following Myocardial Infarction May Be Protective by Increasing Plasma Superoxide Dismutase and Total Thiols with Reduction in Serum Cholesterol and Xanthine Oxidase. Oxid. Med. Cell Longev. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Shihabi, A.; Li, W.-G.; Miller, F.J.J.; Weintraub, N.L. Antioxidant Therapy for Atherosclerotic Vascular Disease: The Promise and the Pitfalls. Am. J. Physiol. Heart Circ. Physiol. 2002, 282, H797–H802. [Google Scholar] [CrossRef]
- Toledo-Ibelles, P.; Mas-Oliva, J. Antioxidants in the Fight Against Atherosclerosis: Is This a Dead End? Curr. Atheroscler. Rep. 2018, 20, 36. [Google Scholar] [CrossRef] [PubMed]
- Hosseinzadeh, H.; Sadeghnia, H.R.; Ziaee, T.; Danaee, A. Protective Effect of Aqueous Saffron Extract (Crocus sativus L.) and Crocin, Its Active Constituent, on Renal Ischemia-Reperfusion-Induced Oxidative Damage in Rats. J. Pharm. Pharm. Sci. 2005, 8, 387–393. [Google Scholar] [PubMed]
- Hosseinzadeh, H.; Modaghegh, M.H.; Saffari, Z. Crocus sativus L. (Saffron) Extract and Its Active Constituents (Crocin and Safranal) on Ischemia-Reperfusion in Rat Skeletal Muscle. Evid. Based Complement. Altern. Med. 2009, 6, 343–350. [Google Scholar] [CrossRef]
- Hosseinzadeh, H.; Abootorabi, A.; Sadeghnia, H.R. Protective Effect of Crocus sativus Stigma Extract and Crocin (Trans-Crocin 4) on Methyl Methanesulfonate–Induced DNA Damage in Mice Organs. DNA Cell Biol. 2008, 27, 657–664. [Google Scholar] [CrossRef]
- Premkumar, K.; Abraham, S.K.; Santhiya, S.T.; Ramesh, A. Protective Effects of Saffron (Crocus sativus Linn.) on Genotoxins-Induced Oxidative Stress in Swiss Albino Mice. Phytother. Res. 2003, 17, 614–617. [Google Scholar] [CrossRef] [PubMed]
- Andreucci, M.; Fuiano, G.; Presta, P.; Lucisano, G.; Leone, F.; Fuiano, L.; Bisesti, V.; Esposito, P.; Russo, D.; Memoli, B.; et al. Downregulation of Cell Survival Signalling Pathways and Increased Cell Damage in Hydrogen Peroxide-Treated Human Renal Proximal Tubular Cells by Alpha-Erythropoietin. Cell Prolif. 2009, 42, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xu, B.; Liu, L.; Luo, Y.; Yin, J.; Zhou, H.; Chen, W.; Shen, T.; Han, X.; Huang, S. Hydrogen Peroxide Inhibits mTOR Signaling by Activation of AMPKα Leading to Apoptosis of Neuronal Cells. Lab. Investig. 2010, 90, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Avlas, O.; Fallach, R.; Shainberg, A.; Porat, E.; Hochhauser, E. Toll-Like Receptor 4 Stimulation Initiates an Inflammatory Response That Decreases Cardiomyocyte Contractility. Antioxid. Redox Signal. 2010, 15, 1895–1909. [Google Scholar] [CrossRef]
- Liu, L.; Wang, Y.; Cao, Z.-Y.; Wang, M.-M.; Liu, X.-M.; Gao, T.; Hu, Q.-K.; Yuan, W.-J.; Lin, L. Up-Regulated TLR4 in Cardiomyocytes Exacerbates Heart Failure after Long-Term Myocardial Infarction. J. Cell Mol. Med. 2015, 19, 2728–2740. [Google Scholar] [CrossRef]
- Feng, Y.; Chao, W. Toll-like Receptors and Myocardial Inflammation. Int. J. Inflam. 2011, 2011, 170352. [Google Scholar] [CrossRef] [PubMed]
- Morote, L.; Lobato-Gómez, M.; Ahrazem, O.; Argandoña, J.; Olmedilla-Alonso, B.; López-Jiménez, A.J.; Diretto, G.; Cuciniello, R.; Bergamo, P.; Frusciante, S.; et al. Crocins-Rich Tomato Extracts Showed Enhanced Protective Effects in Vitro. J. Funct. Foods 2023, 101, 105432. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zununi Vahed, S.; Zuluaga Tamayo, M.; Rodriguez-Ruiz, V.; Thibaudeau, O.; Aboulhassanzadeh, S.; Abdolalizadeh, J.; Meddahi-Pellé, A.; Gueguen, V.; Barzegari, A.; Pavon-Djavid, G. Functional Mechanisms of Dietary Crocin Protection in Cardiovascular Models under Oxidative Stress. Pharmaceutics 2024, 16, 840. https://doi.org/10.3390/pharmaceutics16070840
Zununi Vahed S, Zuluaga Tamayo M, Rodriguez-Ruiz V, Thibaudeau O, Aboulhassanzadeh S, Abdolalizadeh J, Meddahi-Pellé A, Gueguen V, Barzegari A, Pavon-Djavid G. Functional Mechanisms of Dietary Crocin Protection in Cardiovascular Models under Oxidative Stress. Pharmaceutics. 2024; 16(7):840. https://doi.org/10.3390/pharmaceutics16070840
Chicago/Turabian StyleZununi Vahed, Sepideh, Marisol Zuluaga Tamayo, Violeta Rodriguez-Ruiz, Olivier Thibaudeau, Sobhan Aboulhassanzadeh, Jalal Abdolalizadeh, Anne Meddahi-Pellé, Virginie Gueguen, Abolfazl Barzegari, and Graciela Pavon-Djavid. 2024. "Functional Mechanisms of Dietary Crocin Protection in Cardiovascular Models under Oxidative Stress" Pharmaceutics 16, no. 7: 840. https://doi.org/10.3390/pharmaceutics16070840





