Bioprospecting, Synergistic Antifungal and Toxicological Aspects of the Hydroxychalcones and Their Association with Azole Derivates against Candida spp. for Treating Vulvovaginal Candidiasis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Yeasts
2.3. In Vitro Susceptibility of Candida spp.
2.4. In Vitro Cytotoxicity
2.5. Combinatorial Antifungal Activity
2.6. In Vitro Cytotoxicity of the Combinations
2.7. Development of Lipid Carrier (LC)
2.7.1. Mechanical Characterization of LC
Texture Profile Analysis
In Vitro Determination of Mucoadhesive Strength
2.7.2. Analytical Method
2.7.3. Encapsulation Efficiency
2.8. Toxicity and Efficacy in Galleria mellonella Model
3. Results
3.1. In Vitro Susceptibility of Candida spp
3.2. In Vitro Cytotoxicity
3.3. In Vitro Combinatorial Antifungal Activity
3.4. In Vitro Cytotoxicity of the Combinations
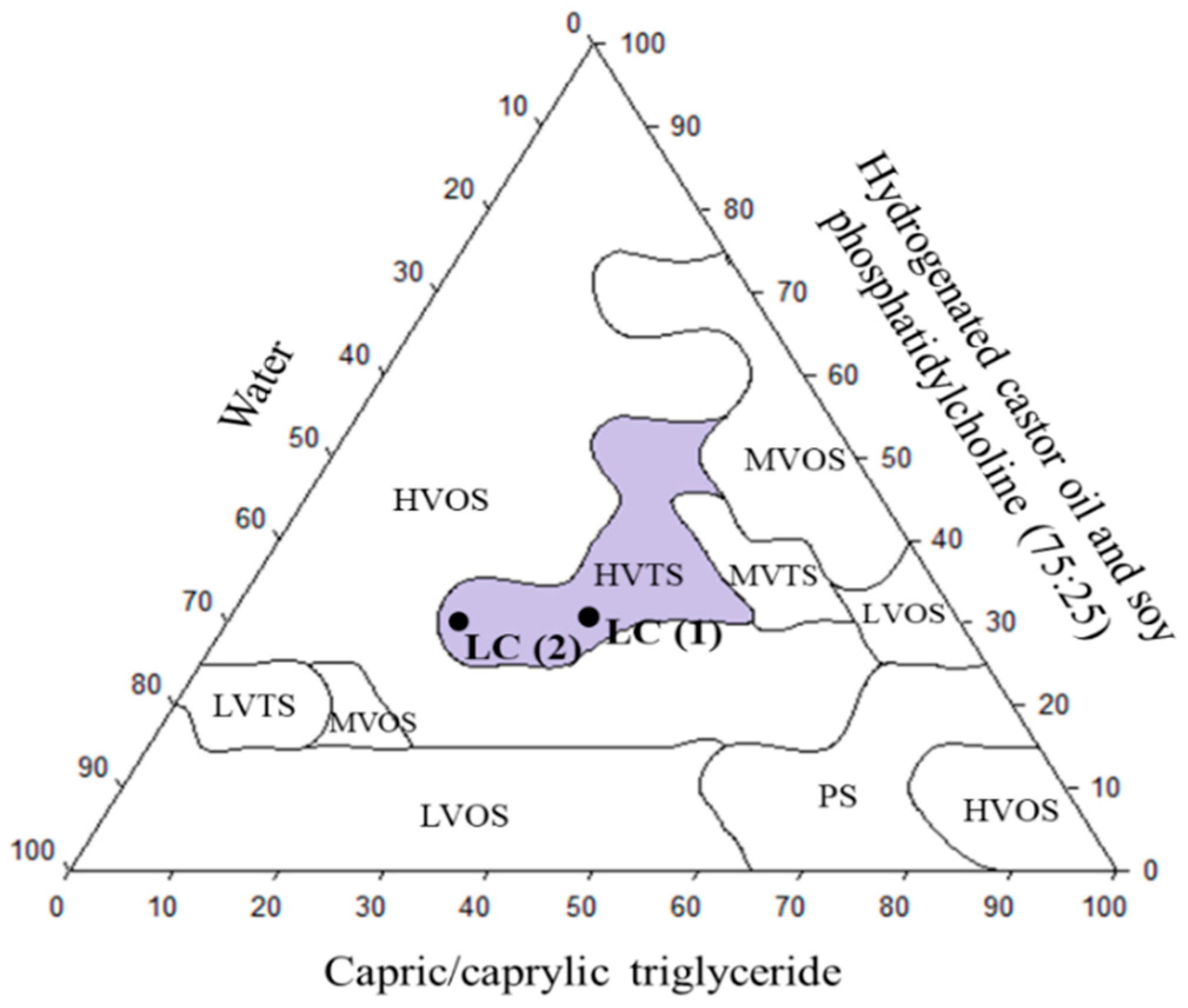
3.5. Development of Lipid Carrier (LC)
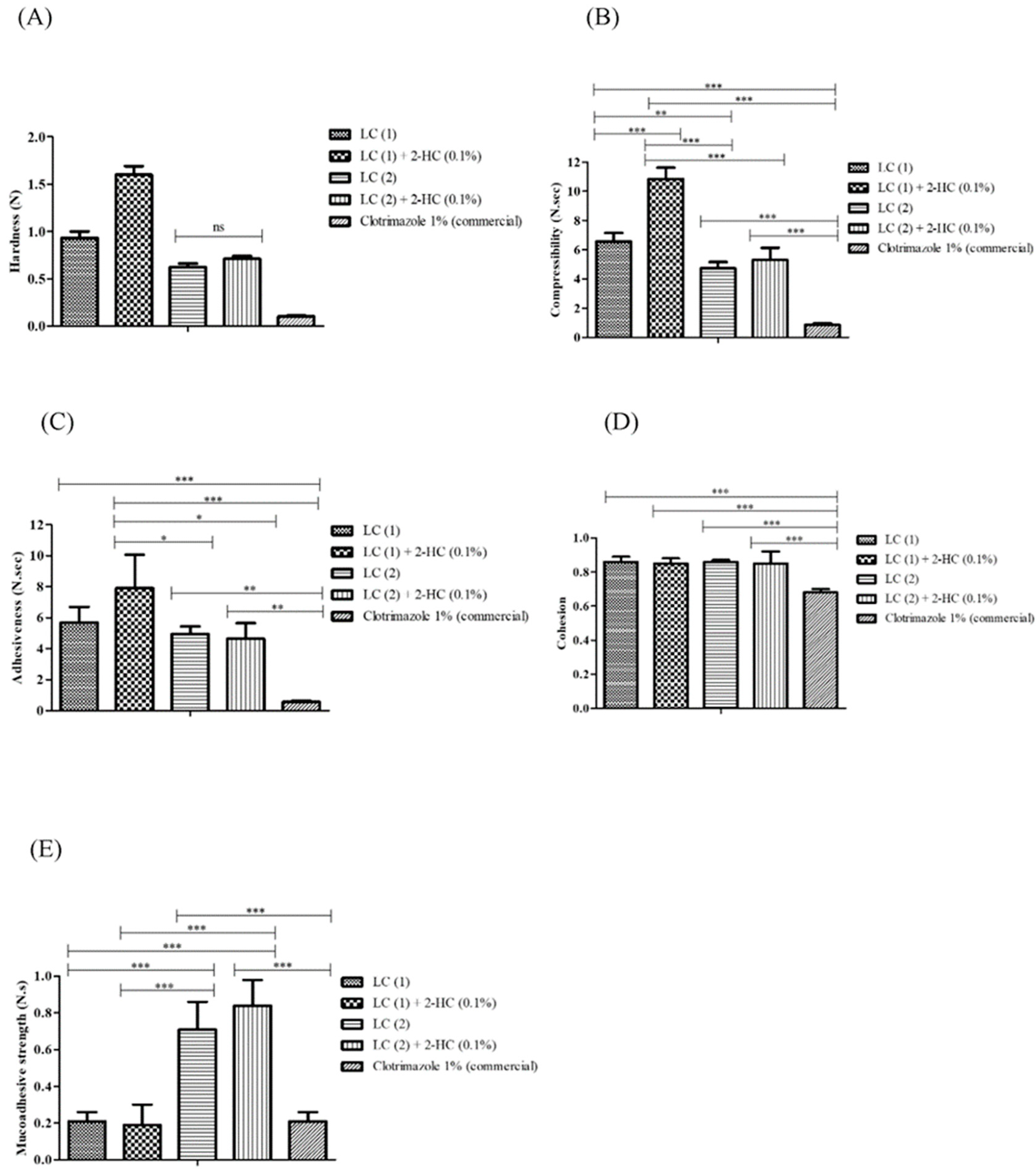
3.6. Mechanical Characterization of LCs and Encapsulation Efficiency
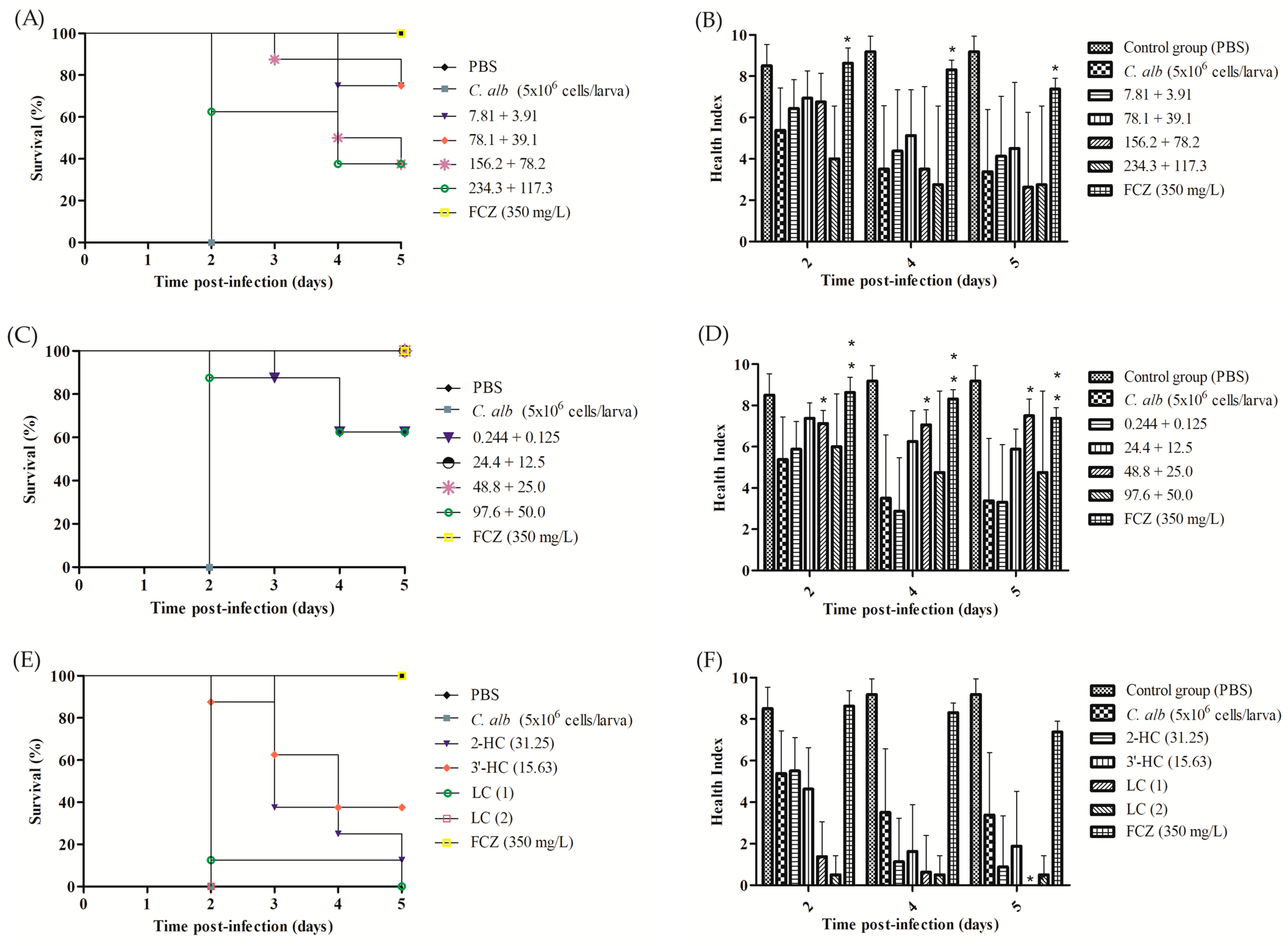
3.7. Evaluation of Toxicological and Therapeutic Efficacy In Vivo Model Galleria mellonella
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Willems, H.M.E.; Ahmed, S.S.; Liu, J.; Xu, Z.; Peters, B.M. Vulvovaginal Candidiasis: A Current Understanding and Burning Questions. J. Fungi 2020, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- Hurley, R.; De Louvois, J. Candida Vaginitis. Postgrad. Med. J. 1979, 55, 645–647. [Google Scholar] [CrossRef] [PubMed]
- Foxman, B.; Marsh, J.V.; Gillespie, B.; Sobel, J.D. Frequency and Response to Vaginal Symptoms among White and African American Women: Results of a Random Digit Dialing Survey. J. Womens Health 1998, 7, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vulvovaginal Candidosis. Lancet 2007, 369, 1961–1971. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D. Vaginitis. N. Engl. J. Med. 1997, 337, 1896–1903. [Google Scholar] [CrossRef] [PubMed]
- Irving, G.; Miller, D.; Robinson, A.; Reynolds, S.; Copas, A.J. Psychological Factors Associated with Recurrent Vaginal Candidiasis: A Preliminary Study. Sex. Transm. Infect. 1998, 74, 334–338. [Google Scholar] [CrossRef] [PubMed]
- Dan, M.; Poch, F.; Levin, D. High Rate of Vaginal Infections Caused by Non- C. albicans Candida Species among Asymptomatic Women. Med. Mycol. 2002, 40, 383–386. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Sobel, J.D. Vulvovaginal Candidiasis Caused by Non-Albicans candida Species: New Insights. Curr. Infect. Dis. Rep. 2010, 12, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Choukri, F.; Benderdouche, M.; Sednaoui, P. In Vitro Susceptibility Profile of 200 Recent Clinical Isolates of Candida Spp. to Topical Antifungal Treatments of Vulvovaginal Candidiasis, the Imidazoles and Nystatin Agents. J. Mycol. Médicale 2014, 24, 303–307. [Google Scholar] [CrossRef]
- Hamad, M.; Kazandji, N.; Awadallah, S.; Allam, H. Prevalence and Epidemiological Characteristics of Vaginal Candidiasis in the UAE. Mycoses 2014, 57, 184–190. [Google Scholar] [CrossRef]
- Kalaiarasan, K. Fungal Profile of Vulvovaginal Candidiasis in a Tertiary Care Hospital. J. Clin. Diagn. Res. 2017, 11, DC06–DC09. [Google Scholar] [CrossRef] [PubMed]
- Bitew, A.; Abebaw, Y. Vulvovaginal Candidiasis: Species Distribution of Candida and Their Antifungal Susceptibility Pattern. BMC Womens Health 2018, 18, 94. [Google Scholar] [CrossRef] [PubMed]
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action. Available online: https://www.who.int/publications-detail-redirect/9789240060241 (accessed on 5 March 2024).
- Pappas, P.G.; Kauffman, C.A.; Andes, D.R.; Clancy, C.J.; Marr, K.A.; Ostrosky-Zeichner, L.; Reboli, A.C.; Schuster, M.G.; Vazquez, J.A.; Walsh, T.J.; et al. Clinical Practice Guideline for the Management of Candidiasis: 2016 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2016, 62, e1–e50. [Google Scholar] [CrossRef] [PubMed]
- Sobel, J.D.; Faro, S.; Force, R.W.; Foxman, B.; Ledger, W.J.; Nyirjesy, P.R.; Reed, B.D.; Summers, P.R. Vulvovaginal Candidiasis: Epidemiologic, Diagnostic, and Therapeutic Considerations. Am. J. Obstet. Gynecol. 1998, 178, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Spinillo, A.; Nicola, S.; Colonna, L.; Marangoni, E.; Cavanna, C.; Michelone, G. Frequency and Significance of Drug Resistance in Vulvovaginal Candidiasis. Gynecol. Obstet. Investig. 1994, 38, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Richter, S.S.; Galask, R.P.; Messer, S.A.; Hollis, R.J.; Diekema, D.J.; Pfaller, M.A. Antifungal Susceptibilities of Candida Species Causing Vulvovaginitis and Epidemiology of Recurrent Cases. J. Clin. Microbiol. 2005, 43, 2155–2162. [Google Scholar] [CrossRef]
- Sobel, J.D. Use of Antifungal Drugs in Pregnancy: A Focus on Safety. Drug Saf. 2000, 23, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Grover, N. Echinocandins: A Ray of Hope in Antifungal Drug Therapy. Indian J. Pharmacol. 2010, 42, 9–11. [Google Scholar] [CrossRef] [PubMed]
- Conte, J.; Parize, A.L.; Caon, T. Advanced Solid Formulations for Vulvovaginal Candidiasis. Pharm. Res. 2023, 40, 593–610. [Google Scholar] [CrossRef]
- Felix, T.C.; De Brito Röder, D.V.D.; Dos Santos Pedroso, R. Alternative and Complementary Therapies for Vulvovaginal Candidiasis. Folia Microbiol. 2019, 64, 133–141. [Google Scholar] [CrossRef]
- Nasim, N.; Sandeep, I.S.; Mohanty, S. Plant-Derived Natural Products for Drug Discovery: Current Approaches and Prospects. Nucleus 2022, 65, 399–411. [Google Scholar] [CrossRef]
- Melo, W.C.M.A.; Santos, M.B.D.; Marques, B.D.C.; Regasini, L.O.; Giannini, M.J.S.M.; Almeida, A.M.F. Selective Photoinactivation of Histoplasma Capsulatum by Water-Soluble Derivatives Chalcones. Photodiagn. Photodyn. Ther. 2017, 18, 232–235. [Google Scholar] [CrossRef]
- Palanco, A.C.; Lacorte Singulani, J.D.; Costa-Orlandi, C.B.; Gullo, F.P.; Strohmayer Lourencetti, N.M.; Gomes, P.C.; Ayusso, G.M.; Dutra, L.A.; Silva Bolzani, V.D.; Regasini, L.O.; et al. Activity of 3′-Hydroxychalcone against Cryptococcus gattii and Toxicity, and Efficacy in Alternative Animal Models. Future Microbiol. 2017, 12, 1123–1134. [Google Scholar] [CrossRef]
- Bila, N.M.; Costa-Orlandi, C.B.; Vaso, C.O.; Bonatti, J.L.C.; De Assis, L.R.; Regasini, L.O.; Fontana, C.R.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. 2-Hydroxychalcone as a Potent Compound and Photosensitizer Against Dermatophyte Biofilms. Front. Cell. Infect. Microbiol. 2021, 11, 679470. [Google Scholar] [CrossRef]
- Medina-Alarcón, K.P.; Tobias Da Silva, I.P.; Ferin, G.G.; Pereira-da-Silva, M.A.; Marcos, C.M.; Dos Santos, M.B.; Regasini, L.O.; Chorilli, M.; Mendes-Giannini, M.J.S.; Pavan, F.R.; et al. Mycobacterium tuberculosis and Paracoccidioides brasiliensis Formation and Treatment of Mixed Biofilm In Vitro. Front. Cell. Infect. Microbiol. 2021, 11, 681131. [Google Scholar] [CrossRef]
- Sato, M.R.; Oshiro-Junior, J.A.; Rodero, C.F.; Boni, F.I.; Araújo, V.H.S.; Bauab, T.M.; Nicholas, D.; Callan, J.F.; Chorilli, M. Photodynamic Therapy-Mediated Hypericin-Loaded Nanostructured Lipid Carriers against Vulvovaginal Candidiasis. J. Med. Mycol. 2022, 32, 101296. [Google Scholar] [CrossRef]
- Shakibaei, M.; Kraehe, P.; Popper, B.; Shayan, P.; Goel, A.; Buhrmann, C. Curcumin Potentiates Antitumor Activity of 5-Fluorouracil in a 3D Alginate Tumor Microenvironment of Colorectal Cancer. BMC Cancer 2015, 15, 250. [Google Scholar] [CrossRef]
- Ayaz, M.; Ullah, F.; Sadiq, A.; Ullah, F.; Ovais, M.; Ahmed, J.; Devkota, H.P. Synergistic Interactions of Phytochemicals with Antimicrobial Agents: Potential Strategy to Counteract Drug Resistance. Chem. Biol. Interact. 2019, 308, 294–303. [Google Scholar] [CrossRef]
- Buhrmann, C.; Yazdi, M.; Popper, B.; Shayan, P.; Goel, A.; Aggarwal, B.B.; Shakibaei, M. Evidence That TNF-β Induces Proliferation in Colorectal Cancer Cells and Resveratrol Can down-Modulate It. Exp. Biol. Med. 2019, 244, 1–12. [Google Scholar] [CrossRef]
- Cheng, Y.-S.; Williamson, P.R.; Zheng, W. Improving Therapy of Severe Infections through Drug Repurposing of Synergistic Combinations. Curr. Opin. Pharmacol. 2019, 48, 92–98. [Google Scholar] [CrossRef]
- Shaban, S.; Patel, M.; Ahmad, A. Improved Efficacy of Antifungal Drugs in Combination with Monoterpene Phenols against Candida auris. Sci. Rep. 2020, 10, 1162. [Google Scholar] [CrossRef]
- Zeraik, M.L.; Ximenes, V.F.; Regasini, L.O.; Dutra, L.A.; Silva, D.H.S.; Fonseca, L.M.; Coelho, D.; Machado, S.A.S.; Bolzani, V.S. 4′-Aminochalcones as Novel Inhibitors of the Chlorinating Activity of Myeloperoxidase. Curr. Med. Chem. 2012, 19, 5405–5413. [Google Scholar] [CrossRef]
- Passalacqua, T.G.; Dutra, L.A.; De Almeida, L.; Velásquez, A.M.A.; Torres, F.A.E.; Yamasaki, P.R.; Dos Santos, M.B.; Regasini, L.O.; Michels, P.A.M.; Bolzani, V.D.S.; et al. Synthesis and Evaluation of Novel Prenylated Chalcone Derivatives as Anti-Leishmanial and Anti-Trypanosomal Compounds. Bioorg. Med. Chem. Lett. 2015, 25, 3342–3345. [Google Scholar] [CrossRef]
- M27-A3; Reference Method for Broth Dilution Antifungal Susceptibility Testing of Yeasts. 3rd ed. Approved Standard. Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2008.
- Fernandes, L.D.S.; Amorim, Y.M.; Da Silva, E.L.; Silva, S.C.; Santos, A.J.A.; Peixoto, F.N.; Neves Pires, L.M.; Sakamoto, R.Y.; Horta Pinto, F.D.C.; Scarpa, M.V.C.; et al. Formulation, Stability Study and Preclinical Evaluation of a Vaginal Cream Containing Curcumin in a Rat Model of Vulvovaginal Candidiasis. Mycoses 2018, 61, 723–730. [Google Scholar] [CrossRef]
- De Freitas Araújo, M.G.; Pacífico, M.; Vilegas, W.; Dos Santos, L.C.; Icely, P.A.; Miró, M.S.; Scarpa, M.V.C.; Bauab, T.M.; Sotomayor, C.E. Evaluation of Syngonanthus nitens (Bong.) Ruhl. Extract as Antifungal and in Treatment of Vulvovaginal Candidiasis. Med. Mycol. 2013, 51, 673–682. [Google Scholar] [CrossRef]
- OECD. Test No. 491: Short Time Exposure In Vitro Test Method for Identifying i) Chemicals Inducing Serious Eye Damage and ii) Chemicals Not Requiring Classification for Eye Irritation or Serious Eye Damage; OECD Guidelines for the Testing of Chemicals, Section 4; OECD: Paris, France, 2023; ISBN 978-92-64-24243-2. [Google Scholar]
- De Abreu Costa, L.; Henrique Fernandes Ottoni, M.; Dos Santos, M.G.; Meireles, A.B.; Gomes de Almeida, V.; De Fátima Pereira, W.; Alves de Avelar-Freitas, B.; Eustáquio Alvim Brito-Melo, G. Dimethyl Sulfoxide (DMSO) Decreases Cell Proliferation and TNF-α, IFN-γ, and IL-2 Cytokines Production in Cultures of Peripheral Blood Lymphocytes. Molecules 2017, 22, 1789. [Google Scholar] [CrossRef]
- Gullo, F.P.; Sardi, J.C.O.; Santos, V.A.F.F.M.; Sangalli-Leite, F.; Pitangui, N.S.; Rossi, S.A.; de Paula e Silva, A.C.A.; Soares, L.A.; Silva, J.F.; Oliveira, H.C.; et al. Antifungal Activity of Maytenin and Pristimerin. Evid. Based Complement. Alternat. Med. 2012, 2012, e340787. [Google Scholar] [CrossRef]
- Bellio, P.; Fagnani, L.; Nazzicone, L.; Celenza, G. New and Simplified Method for Drug Combination Studies by Checkerboard Assay. MethodsX 2021, 8, 101543. [Google Scholar] [CrossRef]
- Feng, W.; Yang, J. Interpretation of Fractional Inhibitory Concentration Index (FICI). Available online: https://bio-protocol.org/exchange/preprintdetail?id=2404&type=3 (accessed on 6 March 2024).
- Feng, W.; Yang, J.; Ma, Y.; Xi, Z.; Ji, Y.; Ren, Q.; Ning, H.; Wang, S. Cotreatment with Aspirin and Azole Drugs Increases Sensitivity of Candida albicans in Vitro. Infect. Drug Resist. 2021, 14, 2027–2038. [Google Scholar] [CrossRef]
- Sader, H.S.; Huynh, H.K.; Jones, R.N. Contemporary in Vitro Synergy Rates for Aztreonam Combined with Newer Fluoroquinolones and β-Lactams Tested against Gram-Negative Bacilli. Diagn. Microbiol. Infect. Dis. 2003, 47, 547–550. [Google Scholar] [CrossRef]
- D’Cruz, O.J.; Uckun, F.M. Gel-Microemulsions as Vaginal Spermicides and Intravaginal Drug Delivery Vehicles. Contraception 2001, 64, 113–123. [Google Scholar] [CrossRef]
- Owen, D.H.; Katz, D.F. A Vaginal Fluid Simulant. Contraception 1999, 59, 91–95. [Google Scholar] [CrossRef]
- International Conference on Harmonization (ICH). Q2b: Validation of Analytical Procedures: Methodology; US FDA Federal Register; U.S. Food and Drug: Rockville, MD, USA, 1997; Volume 62, p. 27463. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/q2b-validation-analytical-procedures-methodology (accessed on 19 May 2024).
- Scorzoni, L.; De Paula E Silva, A.C.A.; Singulani, J.D.L.; Leite, F.S.; De Oliveira, H.C.; Moraes Da Silva, R.A.; Fusco-Almeida, A.M.; Mendes-Giannini, M.J.S. Comparison of Virulence between Paracoccidioides brasiliensis and Paracoccidioides lutzii Using Galleria mellonella as a Host Model. Virulence 2015, 6, 766–776. [Google Scholar] [CrossRef]
- Scorzoni, L.; de Lucas, M.P.; Mesa-Arango, A.C.; Fusco-Almeida, A.M.; Lozano, E.; Cuenca-Estrella, M.; Mendes-Giannini, M.J.; Zaragoza, O. Antifungal Efficacy during Candida krusei Infection in Non-Conventional Models Correlates with the Yeast in Vitro Susceptibility Profile. PLoS ONE 2013, 8, e60047. [Google Scholar] [CrossRef]
- Astvad, K.M.T.; Meletiadis, J.; Whalley, S.; Arendrup, M.C. Fluconazole Pharmacokinetics in Galleria mellonella Larvae and Performance Evaluation of a Bioassay Compared to Liquid Chromatography-Tandem Mass Spectrometry for Hemolymph Specimens. Antimicrob. Agents Chemother. 2017, 61, e00895-17. [Google Scholar] [CrossRef]
- Loh, J.M.; Adenwalla, N.; Wiles, S.; Proft, T. Galleria mellonella Larvae as an Infection Model for Group A Streptococcus. Virulence 2013, 4, 419–428. [Google Scholar] [CrossRef]
- ISO 10993-5:2009; Biological Evaluation of Medical Devices—Part 5: Tests for In Vitro Cytotoxicity. International Organization for Standardization: Geneva, Switzerland, 2009.
- Kay, S.; Edwards, J.; Brown, J.; Dixon, R. Galleria mellonella Infection Model Identifies Both High and Low Lethality of Clostridium perfringens Toxigenic Strains and Their Response to Antimicrobials. Front. Microbiol. 2019, 10, 1281. [Google Scholar] [CrossRef]
- Torres, M.; Pinzón, E.N.; Rey, F.M.; Martinez, H.; Parra Giraldo, C.M.; Celis Ramírez, A.M. Galleria mellonella as a Novelty in Vivo Model of Host-Pathogen Interaction for Malassezia furfur CBS 1878 and Malassezia pachydermatis CBS 1879. Front. Cell. Infect. Microbiol. 2020, 10, 199. [Google Scholar] [CrossRef]
- Anifowose, S.O.; Alqahtani, W.S.N.; Al-Dahmash, B.A.; Sasse, F.; Jalouli, M.; Aboul-Soud, M.A.M.; Badjah-Hadj-Ahmed, A.Y.; Elnakady, Y.A. Efforts in Bioprospecting Research: A Survey of Novel Anticancer Phytochemicals Reported in the Last Decade. Molecules 2022, 27, 8307. [Google Scholar] [CrossRef]
- Valli, M.; Russo, H.M.; Bolzani, V.S. The Potential Contribution of the Natural Products from Brazilian Biodiversity to Bioeconomy. An. Acad. Bras. Ciênc. 2018, 90, 763–778. [Google Scholar] [CrossRef]
- Srivastava, V.; Negi, A.S.; Kumar, J.K.; Gupta, M.M.; Khanuja, S.P.S. Plant-Based Anticancer Molecules: A Chemical and Biological Profile of Some Important Leads. Bioorg. Med. Chem. 2005, 13, 5892–5908. [Google Scholar] [CrossRef]
- Oberlies, N.H.; Kroll, D.J. Camptothecin and Taxol: Historic Achievements in Natural Products Research. J. Nat. Prod. 2004, 67, 129–135. [Google Scholar] [CrossRef]
- Singh, P.; Anand, A.; Kumar, V. Recent Developments in Biological Activities of Chalcones: A Mini Review. Eur. J. Med. Chem. 2014, 85, 758–777. [Google Scholar] [CrossRef]
- Rosa, G.P.; Seca, A.M.L.; Barreto, M.D.C.; Silva, A.M.S.; Pinto, D.C.G.A. Chalcones and Flavanones Bearing Hydroxyl and/or Methoxyl Groups: Synthesis and Biological Assessments. Appl. Sci. 2019, 9, 2846. [Google Scholar] [CrossRef]
- Leite, F.F.; De Sousa, N.F.; De Oliveira, B.H.M.; Duarte, G.D.; Ferreira, M.D.L.; Scotti, M.T.; Filho, J.M.B.; Rodrigues, L.C.; De Moura, R.O.; Mendonça-Junior, F.J.B.; et al. Anticancer Activity of Chalcones and Its Derivatives: Review and In Silico Studies. Molecules 2023, 28, 4009. [Google Scholar] [CrossRef]
- Mascarello, A.; Chiaradia, L.D.; Vernal, J.; Villarino, A.; Guido, R.V.C.; Perizzolo, P.; Poirier, V.; Wong, D.; Martins, P.G.A.; Nunes, R.J.; et al. Inhibition of Mycobacterium Tuberculosis Tyrosine Phosphatase PtpA by Synthetic Chalcones: Kinetics, Molecular Modeling, Toxicity and Effect on Growth. Bioorg. Med. Chem. 2010, 18, 3783–3789. [Google Scholar] [CrossRef]
- Abdullah, M.I.; Mahmood, A.; Madni, M.; Masood, S.; Kashif, M. Synthesis, Characterization, Theoretical, Anti-Bacterial and Molecular Docking Studies of Quinoline Based Chalcones as a DNA Gyrase Inhibitor. Bioorganic Chem. 2014, 54, 31–37. [Google Scholar] [CrossRef]
- Wang, L.; Chen, G.; Lu, X.; Wang, S.; Han, S.; Li, Y.; Ping, G.; Jiang, X.; Li, H.; Yang, J.; et al. Novel Chalcone Derivatives as Hypoxia-Inducible Factor (HIF)-1 Inhibitor: Synthesis, Anti-Invasive and Anti-Angiogenic Properties. Eur. J. Med. Chem. 2015, 89, 88–97. [Google Scholar] [CrossRef]
- Whaley, S.G.; Berkow, E.L.; Rybak, J.M.; Nishimoto, A.T.; Barker, K.S.; Rogers, P.D. Azole Antifungal Resistance in Candida albicans and Emerging Non-albicans Candida Species. Front. Microbiol. 2017, 7, 2173. [Google Scholar] [CrossRef]
- Wong, S.S.W.; Kao, R.Y.T.; Yuen, K.Y.; Wang, Y.; Yang, D.; Samaranayake, L.P.; Seneviratne, C.J. In Vitro and In Vivo Activity of a Novel Antifungal Small Molecule against Candida Infections. PLoS ONE 2014, 9, e85836. [Google Scholar] [CrossRef]
- Kant, R.; Kumar, D.; Agarwal, D.; Gupta, R.D.; Tilak, R.; Awasthi, S.K.; Agarwal, A. Synthesis of Newer 1,2,3-Triazole Linked Chalcone and Flavone Hybrid Compounds and Evaluation of Their Antimicrobial and Cytotoxic Activities. Eur. J. Med. Chem. 2016, 113, 34–49. [Google Scholar] [CrossRef]
- Ahmad, A.; Wani, M.Y.; Patel, M.; Sobral, A.J.F.N.; Duse, A.G.; Aqlan, F.M.; Al-Bogami, A.S. Synergistic Antifungal Effect of Cyclized Chalcone Derivatives and Fluconazole against Candida albicans. MedChemComm 2017, 8, 2195–2207. [Google Scholar] [CrossRef]
- Soares, L.A.; Gullo, F.P.; de Sardi, J.C.O.; de Pitangui, N.S.; Costa-Orlandi, C.B.; Sangalli-Leite, F.; Scorzoni, L.; Regasini, L.O.; Petrônio, M.S.; Souza, P.F.; et al. Anti-Trichophyton Activity of Protocatechuates and Their Synergism with Fluconazole. Evid. Based Complement. Alternat. Med. 2014, 2014, e957860. [Google Scholar] [CrossRef]
- Chai, N.; Sun, A.; Zhu, X.; Li, Y.; Wang, R.; Zhang, Y.; Mao, Z. Antifungal Evaluation of Quinoline-Chalcone Derivatives Combined with FLC against Drug-Resistant Candida albicans. Bioorg. Med. Chem. Lett. 2023, 86, 129242. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Dong, H.-H.; Zhao, F.; Wang, J.; Yan, F.; Jiang, Y.-Y.; Jin, Y.-S. The Synthesis and Synergistic Antifungal Effects of Chalcones against Drug Resistant Candida albicans. Bioorg. Med. Chem. Lett. 2016, 26, 3098–3102. [Google Scholar] [CrossRef]
- da Silva, C.R.; de Andrade Neto, J.B.; de Sousa Campos, R.; Figueiredo, N.S.; Sampaio, L.S.; Magalhães, H.I.F.; Cavalcanti, B.C.; Gaspar, D.M.; de Andrade, G.M.; Lima, I.S.P.; et al. Synergistic Effect of the Flavonoid Catechin, Quercetin, or Epigallocatechin Gallate with Fluconazole Induces Apoptosis in Candida tropicalis Resistant to Fluconazole. Antimicrob. Agents Chemother. 2014, 58, 1468–1478. [Google Scholar] [CrossRef]
- Medina-Alarcón, K.P.; Singulani, J.L.; Voltan, A.R.; Sardi, J.C.O.; Petrônio, M.S.; Santos, M.B.; Polaquini, C.R.; Regasini, L.O.; Bolzani, V.S.; da Silva, D.H.S.; et al. Alkyl Protocatechuate-Loaded Nanostructured Lipid Systems as a Treatment Strategy for Paracoccidioides brasiliensis and Paracoccidioides lutzii In Vitro. Front. Microbiol. 2017, 8, 1048. [Google Scholar] [CrossRef]
- Teng, Z.; Yu, M.; Ding, Y.; Zhang, H.; Shen, Y.; Jiang, M.; Liu, P.; Opoku-Damoah, Y.; Webster, T.J.; Zhou, J. Preparation and Characterization of Nimodipine-Loaded Nanostructured Lipid Systems for Enhanced Solubility and Bioavailability. Int. J. Nanomed. 2018, 14, 119–133. [Google Scholar] [CrossRef]
- Kumbhar, D.D.; Pokharkar, V.B. Engineering of a Nanostructured Lipid Carrier for the Poorly Water-Soluble Drug, Bicalutamide: Physicochemical Investigations. Colloids Surf. Physicochem. Eng. Asp. 2013, 416, 32–42. [Google Scholar] [CrossRef]
- Davis, S.S. Viscoelastic Properties of Pharmaceutical Semisolids III: Nondestructive Oscillatory Testing. J. Pharm. Sci. 1971, 60, 1351–1356. [Google Scholar] [CrossRef]
- Schwartz, N.O. Adaptation of the Sensory Texture Profile Method to Skin Care Products. J. Texture Stud. 1975, 6, 33–42. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Djokic, J. Texture Profile Analysis of Bioadhesive Polymeric Semisolids: Mechanical Characterization and Investigation of Interactions between Formulation Components. J. Appl. Polym. Sci. 1996, 61, 2229–2234. [Google Scholar] [CrossRef]
- Jones, D.S.; Woolfson, A.D.; Brown, A.F. Textural, Viscoelastic and Mucoadhesive Properties of Pharmaceutical Gels Composed of Cellulose Polymers. Int. J. Pharm. 1997, 151, 223–233. [Google Scholar] [CrossRef]
- Passos, J.S.; Martino, L.C.D.; Dartora, V.F.C.; Araujo, G.L.B.D.; Ishida, K.; Lopes, L.B. Development, Skin Targeting and Antifungal Efficacy of Topical Lipid Nanoparticles Containing Itraconazole. Eur. J. Pharm. Sci. 2020, 149, 105296. [Google Scholar] [CrossRef]
- Salmazi, R.; Calixto, G.; Bernegossi, J.; dos Ramos, M.A.S.; Bauab, T.M.; Chorilli, M. A Curcumin-Loaded Liquid Crystal Precursor Mucoadhesive System for the Treatment of Vaginal Candidiasis. Int. J. Nanomed. 2015, 10, 4815–4824. [Google Scholar] [CrossRef]
- Su, S.; Shi, X.; Xu, W.; Li, Y.; Chen, X.; Jia, S.; Sun, S. Antifungal Activity and Potential Mechanism of Panobinostat in Combination with Fluconazole against Candida albicans. Front. Microbiol. 2020, 11, 1584. [Google Scholar] [CrossRef]
- MacCallum, D.M.; Desbois, A.P.; Coote, P.J. Enhanced Efficacy of Synergistic Combinations of Antimicrobial Peptides with Caspofungin versus Candida albicans in Insect and Murine Models of Systemic Infection. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 1055–1062. [Google Scholar] [CrossRef]
- Baldim, I.; Paziani, M.H.; Grizante Barião, P.H.; Kress, M.R.V.Z.; Oliveira, W.P. Nanostructured Lipid Carriers Loaded with Lippia sidoides Essential Oil as a Strategy to Combat the Multidrug-Resistant Candida auris. Pharmaceutics 2022, 14, 180. [Google Scholar] [CrossRef]



| Yeasts | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| C. albicans (ATCC 90028) | C. tropicalis (ATCC 750) | C. krusei (ATCC 6258) | C. glabrata (ATCC 90030) | C. parapsilosis (ATCC 22019) | ||||||
| MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | MIC | MFC | |
| 2-HC | 31.3 | >125.0 | 31.3 | >125.0 | 7.8 | 125.0 | 15.6 | >125.0 | 7.8 | 15.6 |
| 3′-HC | 31.3 | >125.0 | 31.3 | 125.0 | 7.8 | 31.3 | 7.8 | 31.3 | 15.6 | 31.3 |
| CTZ | 0.01 | >4.0 | 0.01 | >4.0 | 0.06 | >4.0 | 0.004 | >2.0 | 0.03 | 0.1 |
| FCZ | 0.25 | >64.0 | 0.50 | >64.0 | 32.0 | 128.0 | 0.50 | >64.0 | 1.00 | 2.0 |
| AMB | 0.20 | 1.0 | 0.05 | 1.0 | 0.10 | 1.0 | 0.40 | 0.5 | 0.10 | 0.3 |
| 2-HC | 3′-HC | |||||
|---|---|---|---|---|---|---|
| MIC50 (mg/L) | IC50 (mg/L) | SI | MIC50 (mg/L) | IC50 (mg/L) | SI | |
| C. albicans ATCC 90028 | 15.63 | 12.97 | 0.83 | 15.63 | 11.86 | 0.76 |
| C. tropicalis ATCC 750 | 7.81 | 1.66 | 7.81 | 1.52 | ||
| C. krusei ATCC 6258 | 15.63 | 0.83 | 7.81 | 1.52 | ||
| MIC80 2-HC (individual) | MIC80 2-HC (combination) | FIC 2-HC | MIC80 FCZ (individual) | MIC80 FCZ (combination) | FIC FCZ | FICI | ||
| 2-HC + FCZ | C. albicans | 31.25 | 0.24 | 0.01 | 0.50 | 0.13 | 0.25 | 0.3 |
| C. tropicalis | 31.25 | 0.06 | 0.002 | 0.50 | 0.50 | 1.00 | 1.0 | |
| C. krusei | 31.25 | 15.63 | 0.50 | 32.00 | 0.50 | 0.02 | 0.5 | |
| MIC80 2-HC (individual) | MIC80 2-HC (combination) | FIC 2-HC | MIC80 CTZ (individual) | MIC80 CTZ (combination) | FIC CTZ | FICI | ||
| 2-HC + CTZ | C. albicans | 31.25 | 0.06 | 0.002 | 0.03 | 0.02 | 0.50 | 0.5 |
| C. tropicalis | 31.25 | 15.63 | 0.50 | 0.03 | 0.002 | 0.06 | 0.6 | |
| C. krusei | 31.25 | 0.06 | 0.002 | 0.13 | 0.13 | 1.00 | 1.0 | |
| MIC80 3′-HC (individual) | MIC80 3′-HC (combination) | FIC 3′-HC | MIC80 FCZ (individual) | MIC80 FCZ (combination) | FIC FCZ | FICI | ||
| 3′-HC + FCZ | C. albicans | 31.25 | 0.06 | 0.002 | 0.50 | 0.25 | 0.50 | 0.5 |
| C. tropicalis | 62.50 | 0.06 | 0.001 | 0.50 | 0.25 | 0.50 | 0.5 | |
| C. krusei | 15.63 | 0.06 | 0.00 | 32.00 | 32.00 | 1.00 | 1.0 | |
| MIC80 3′-HC (individual) | MIC80 3′-HC (combination) | FIC 3′-HC | MIC80 CTZ (individual) | MIC80 CTZ (combination) | FIC CTZ | FICI | ||
| 3′-HC + CTZ | C. albicans | 31.25 | 15.63 | 0.50 | 0.03 | 0.002 | 0.06 | 0.6 |
| C. tropicalis | 62.50 | 62.50 | 1.00 | 0.03 | 0.002 | 0.06 | 1.1 | |
| C. krusei | 15.63 | 7.81 | 0.50 | 0.13 | 0.01 | 0.06 | 0.6 | |
| MIC80 3′-HC (individual) | MIC80 3′-HC (combination) | FIC 3′-HC | MIC80 2-HC (individual) | MIC80 2-HC (combination) | FIC 2-HC | FICI | ||
| 3′-HC + 2-HC | C. albicans | 31.25 | 7.81 | 0.25 | 31.25 | 3.91 | 0.13 | 0.4 |
| C. tropicalis | 31.25 | 7.81 | 0.25 | 62.50 | 15.63 | 0.25 | 0.5 | |
| C. krusei | 15.63 | 3.91 | 0.25 | 31.25 | 7.81 | 0.25 | 0.5 |
| Associations | Yeast | ||
|---|---|---|---|
| C. albicans | C. tropicalis | C. krusei | |
| 2-HC + FCZ | 0.3 | 1.0 | 0.5 |
| Synergistic | Additive | Synergistic | |
| 2-HC + CTZ | 0.5 | 0.6 | 1.00 |
| Synergistic | Synergistic (partial) | Additive | |
| 3′-HC + FCZ | 0.5 | 0.5 | 1.00 |
| Synergistic | Synergistic | Additive | |
| 3′-HC + CTZ | 0.6 | 1.1 | 0.6 |
| Synergistic (partial) | Indifferent | Synergistic (partial) | |
| 3′-HC + 2-HC | 0.4 | 0.5 | 0.5 |
| Synergistic | Synergistic | Synergistic | |
| 2-HC + FCZ | MIC80 2-HC (combination) | MIC80 FCZ (combination) | Interaction | % Cell viability | |
| C. albicans | 0.24 | 0.13 | SYN | 94.92 | |
| C. tropicalis | 0.06 | 0.50 | AD | 98.35 | |
| C. krusei | 15.63 | 0.50 | SYN | 38.85 | |
| 2-HC + CTZ | MIC80 2-HC (combination) | MIC80 CTZ (combination) | Interaction | % Cell viability | |
| C. albicans | 0.06 | 0.02 | SYN | 95.04 | |
| C. tropicalis | 15.63 | 0.002 | SYN (P) | 15.19 | |
| C. krusei | 0.06 | 0.13 | AD | 100.00 | |
| 3′-HC + FCZ | MIC80 3′-HC (combination) | MIC80 FCZ (combination) | Interaction | % Cell viability | |
| C. albicans | 0.06 | 0.25 | SYN | 100.00 | |
| C. tropicalis | 0.06 | 0.25 | SYN | 100.00 | |
| C. krusei | 0.06 | 32.00 | AD | ** | |
| 3′-HC + CTZ | MIC80 3′-HC (combination) | MIC80 CTZ (combination) | Interaction | % Cell viability | |
| C. albicans | 15.63 | 0.002 | SYN (P) | 10.07 | |
| C. tropicalis | 62.50 | 0.002 | IND | 0.00 | |
| C. krusei | 7.81 | 0.01 | SYN (P) | 90.60 | |
| 3′-HC + 2-HC | MIC80 2-HC (combination) | MIC80 3′-HC (combination) | Interaction | % Cell viability | |
| C. albicans | 3.91 | 7.81 | SYN | 86.91 | |
| C. tropicalis | 15.63 | 7.81 | SYN | 0.00 | |
| C. krusei | 7.81 | 3.91 | SYN | 75.63 |
| MIC80 2-HC (combination) | IC80 | SI | ||
| 2-HC + FCZ | C. albicans | 0.24 | 16.96 | 70.67 |
| C. tropicalis | 0.06 | 282.67 | ||
| C. krusei | 15.63 | 1.08 | ||
| MIC80 2-HC (combination) | ||||
| 2-HC + CTZ | C. albicans | 0.06 | 22.93 | 382.16 |
| C. tropicalis | 15.63 | 1.47 | ||
| C. krusei | 0.06 | 382.16 | ||
| MIC80 3′-HC (combination) | ||||
| 3′-HC + FCZ | C. albicans | 0.06 | 14.50 | 241.67 |
| C. tropicalis | 0.06 | 241.67 | ||
| C. krusei | 0.06 | 241.67 | ||
| MIC80 3′-HC (combination) | ||||
| 3′-HC + CTZ | C. albicans | 15.63 | 14.72 | 0.94 |
| C. tropicalis | 62.50 | 0.23 | ||
| C. krusei | 7.81 | 1.88 |
| 3′-HC + 2-HC (mg/L) | 2-HC + FCZ (mg/L) | ||
|---|---|---|---|
| 3′-HC + 2-HC (MIC80 combination) | 7.81 + 3.91 | 2-HC + FCZ (MIC80 combination) | 0.244 + 0.125 |
| x 10 | 78.10 + 39.10 | x 100 | 24.40 + 12.50 |
| x 20 | 156.20 + 78.20 | x 200 | 48.80 + 25.00 |
| x 30 | 234.30 + 117.30 | x 400 | 97.60 + 50.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fernandes, L.d.S.; Ogasawara, L.S.; Medina-Alarcón, K.P.; dos Santos, K.S.; de Matos Silva, S.; de Assis, L.R.; Regasini, L.O.; de Oliveira, A.G.; Mendes Giannini, M.J.S.; Scarpa, M.V.; et al. Bioprospecting, Synergistic Antifungal and Toxicological Aspects of the Hydroxychalcones and Their Association with Azole Derivates against Candida spp. for Treating Vulvovaginal Candidiasis. Pharmaceutics 2024, 16, 843. https://doi.org/10.3390/pharmaceutics16070843
Fernandes LdS, Ogasawara LS, Medina-Alarcón KP, dos Santos KS, de Matos Silva S, de Assis LR, Regasini LO, de Oliveira AG, Mendes Giannini MJS, Scarpa MV, et al. Bioprospecting, Synergistic Antifungal and Toxicological Aspects of the Hydroxychalcones and Their Association with Azole Derivates against Candida spp. for Treating Vulvovaginal Candidiasis. Pharmaceutics. 2024; 16(7):843. https://doi.org/10.3390/pharmaceutics16070843
Chicago/Turabian StyleFernandes, Lígia de Souza, Letícia Sayuri Ogasawara, Kaila Petronila Medina-Alarcón, Kelvin Sousa dos Santos, Samanta de Matos Silva, Letícia Ribeiro de Assis, Luís Octavio Regasini, Anselmo Gomes de Oliveira, Maria José Soares Mendes Giannini, Maria Virginia Scarpa, and et al. 2024. "Bioprospecting, Synergistic Antifungal and Toxicological Aspects of the Hydroxychalcones and Their Association with Azole Derivates against Candida spp. for Treating Vulvovaginal Candidiasis" Pharmaceutics 16, no. 7: 843. https://doi.org/10.3390/pharmaceutics16070843






