Evaluation of Polyvinyl Alcohol as Binder during Continuous Twin Screw Wet Granulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods for Binder Characterization
2.2.1. Particle Size Analysis
2.2.2. Dissolution Kinetics
2.2.3. Wettability
2.2.4. Surface Tension
2.2.5. Viscosity
2.2.6. Inverse Gas Chromatography
2.3. Principal Component Analysis
2.4. Preparation of Granules
2.5. Preparation and Fluidized Bed Drying of Acetaminophen-Containing Granules
2.6. Preparation of Tablets
2.7. Evaluation of Granules
2.7.1. Loss-On-Drying
2.7.2. Friability
2.7.3. Particle Size Analysis
2.8. Evaluation of Tablets
Tensile Strength Analysis
3. Results
3.1. Binder Characterization
3.1.1. Clustering of Binders According to Their Attributes
3.1.2. Surface Energy
3.2. Evaluation of the Robustness of Granulation Process
3.3. Binder Effectiveness during Granulation of Water-Soluble and Water-Insoluble Formulations
3.3.1. PVA Grades
3.3.2. Corresponding Cluster Binders
3.4. Binder Effectiveness during Fluidized Bed Drying
3.4.1. Drying Behavior
3.4.2. Granule Breakage
3.5. Impact of Binder Properties on Tableting Behavior
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A. Binder Characterization
| Binder | Dv_10 (m) | Dv_50 (m) | Dv_90 (m) | D_span | DissRate_30 (%) | DissRate_60 (%) | DissRate_90 (%) | |
|---|---|---|---|---|---|---|---|---|
| PVA 4-88 | 9.6 (±0.2) | 27.1 (±1.1) | 93.8 (±1.1) | 3.1 (±0.1) | 3.7 (±0.0) | 6.1 (±0.3) | 7.3 (±0.0) | |
| PVA 18-88 | 18.5 (±0.3) | 68.3 (±0.3) | 121.5 (±2.6) | 1.5 (±0.0) | 2.2 (±0.0) | 3.0 (±0.2) | 4.6 (±0.3) | |
| PVA 40-88 | 19.5 (±0.1) | 66.4 (±0.9) | 168.8 (±4.2) | 2.3 (±0.0) | 1.5 (±0.3) | 2.3 (±0.4) | 4.3 (±0.3) | |
| SOS CO 01 * | 14.6 | 95.5 | 212.3 | 2.1 | 3.8 | 6.1 | 6.1 | |
| Maltodextrin 6 * | 27.6 (±3.5) | 123.8 (±2.4) | 270.0 (±8.7) | 2.0 (±0.1) | 9.7 (±0.0) | 10.0 (±0.0) | 10.0 (±0.0) | |
| HP pea starch * | 105.8 (±1.2) | 216.9 (±2.9) | 374.9 (±4.8) | 1.2 (±0.0) | 8.7 (±0.0) | 10.0 (±0.0) | 10.0 (±0.0) | |
| PVP K12 * | 13.5 (±0.4) | 49.3 (±1.0) | 100.4 (±1.0) | 1.8 (±0.0) | 9.6 (±0.0) | 9.9 (±0.0) | 9.9 (±0.0) | |
| PVP K30 * | 29.2 (±0.6) | 83.0 (±0.2) | 144.1 (±1.5) | 1.4 (±0.0) | 8.0 (±0.0) | 9.5 (±0.0) | 10.0 (±0.0) | |
| PVP K90 * | 83.0 (±2.1) | 173.0 (±4.7) | 315.1 (±7.1) | 1.3 (±0.0) | 3.0 (±0.0) | 4.0 (±0.0) | 6.6 (±0.0) | |
| HPMC E15 * | 38.6 (±0.3) | 89.5 (±0.5) | 177.7 (±2.0) | 1.6 (±0.0) | 0.5 (±0.0) | 2.5 (±0.0) | 2.5 (±0.0) | |
| Binder | CAbinder_t0 (°) | CAbinder_t30 (°) | CAmannitol_t0 (°) | CAmannitol_t30 (°) | CADCP_t0 (°) | ST (mN/m) | Dynamic Viscosity (mPa.s) | Viscosity Slope |
| PVA 4-88 | 62.9 (±3.1) | 33.4 (±2.4) | 45.9 (±2.1) | 33.2 (±2.9) | 34.6 (±1.4) | 31.2 (±0.0) | 8.3 (±0.2) | 0.2 |
| PVA 18-88 | 71.4 (±4.4) | 63.6 (±3.1) | 65.9 (±1.2) | 40.4 (±2.5) | 68.8 (±1.1) | 33.8 (±0.1) | 166.2 (±2.2) | 0.2 |
| PVA 40-88 | 75.5 (±1.8) | 73.0 (±0.2) | 76.4 (±3.4) | 45.6 (±3.1) | 73.4 (±2.6) | 34.3 (±0.1) | 574.9 (±6.4) | 0.3 |
| SOS CO 01 * | 68.7 | 47.2 | 47.3 | 33.9 | 32.8 | 30.2 | 4.1 | 0.1 |
| Maltodextrin 6 * | 60.0 (±3.9) | 47.8 (±2.5) | 40.9 (±2.5) | 35.4 (±7.8) | 103.0 (±5.3) | 66.8 (±0.2) | 2.1 (±0.0) | 0.1 |
| HP pea starch * | 67.0 (±3.8) | 57.4 (±4.6) | 43.7 (±0.5) | 33.0 (±0.5) | 95.3 (±0.5) | 57.7 (±0.5) | 15.7 (±0.5) | 0.1 |
| PVP K12 * | NA | NA | 39.8 (±2.0) | 26.9 (±1.5) | 109.6 (±3.1) | 50.7 (±0.5) | 1.6 (±0.0) | 0.0 |
| PVP K30 * | 66.9 (±3.2) | 37.3 (±3.3) | 40.3 (±2.0) | 27.2 (±1.8) | 81.3 (±2.9) | 43.3 (±0.2) | 4.3 (±0.1) | 0.1 |
| PVP K90 * | 76.7 (±2.9) | 56.0 (±1.0) | 55.4 (±1.9) | 38.0 (±0.9) | 84.7 (±1.3) | 44.7 (±0.4) | 99.5 (±1.6) | 0.2 |
| HPMC E15 * | 79.1 (±1.7) | 70.2 (±1.8) | 69.4 (±2.6) | 51.3 (±0.9) | 69.6 (±4.4) | 49.0 (±0.2) | 817.1 (±2.8) | 0.3 |
References
- Ryckaert, A.; Ghijs, M.; Portier, C.; Djuric, D.; Funke, A.; Vervaet, C.; De Beer, T. The influence of equipment design and process parameters on granule breakage in a semi-continuous fluid bed dryer after continuous twin-screw wet granulation. Pharmaceutics 2021, 13, 293. [Google Scholar] [CrossRef] [PubMed]
- Van Snick, B.; Holman, J.; Vanhoorne, V.; Kumar, A.; De Beer, T.; Remon, J.P.; Vervaet, C. Development of a continuous direct compression platform for low-dose drug products. Int. J. Pharm. 2017, 529, 329–346. [Google Scholar] [CrossRef] [PubMed]
- Portier, C.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: A review of recent progress and opportunities in formulation and equipment design. Pharmaceutics 2021, 13, 668. [Google Scholar] [CrossRef] [PubMed]
- Vervaet, C.; Remon, J.P. Continuous granulation in the pharmaceutical industry. Chem. Eng. Sci. 2005, 60, 3949–3957. [Google Scholar] [CrossRef]
- Fonteyne, M.; Vercruysse, J.; De Leersnyder, F.; Van Snick, B.; Vervaet, C.; Remon, J.P.; De Beer, T. Process Analytical Technology for continuous manufacturing of solid-dosage forms. TrAC Trends Anal. Chem. 2015, 67, 159–166. [Google Scholar] [CrossRef]
- Plath, T.; Korte, C.; Sivanesapillai, R.; Weinhart, T. Parametric study of residence time distributions and granulation kinetics as a basis for process modeling of twin-screwwet granulation. Pharmaceutics 2021, 13, 645. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Alakarjula, M.; Vanhoorne, V.; Toiviainen, M.; De Leersnyder, F.; Vercruysse, J.; Juuti, M.; Ketolainen, J.; Vervaet, C.; Remon, J.P.; et al. Linking granulation performance with residence time and granulation liquid distributions in twin-screw granulation: An experimental investigation. Eur. J. Pharm. Sci. 2016, 90, 25–37. [Google Scholar] [CrossRef] [PubMed]
- Dürig, T.; Karan, K. Chapter 9—Binders in Wet Granulation. In Handbook of Pharmaceutical Wet Granulation: Theory and Practice in a Quality by Design Paradigm; Academic Press: New York, NY, USA, 2018; pp. 317–349. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw wet granulation: Effects of properties of granulation liquid. Powder Technol. 2012, 229, 126–136. [Google Scholar] [CrossRef]
- Dhenge, R.M.; Washino, K.; Cartwright, J.J.; Hounslow, M.J.; Salman, A.D. Twin screw granulation using conveying screws: Effects of viscosity of granulation liquids and flow of powders. Powder Technol. 2013, 238, 77–90. [Google Scholar] [CrossRef]
- Ito, A.; Kleinebudde, P. Influence of granulation temperature on particle size distribution of granules in twin-screw granulation (TSG). Pharm. Dev. Technol. 2019, 24, 874–882. [Google Scholar] [CrossRef]
- Willecke, N.; Szepes, A.; Wunderlich, M.; Remon, J.P.; Vervaet, C.; De Beer, T. A novel approach to support formulation design on twin screw wet granulation technology: Understanding the impact of overarching excipient properties on drug product quality attributes. Int. J. Pharm. 2018, 545, 128–143. [Google Scholar] [CrossRef]
- Köster, C.; Pohl, S.; Kleinebudde, P. Evaluation of binders in twin-screw wet granulation. Pharmaceutics 2021, 13, 241. [Google Scholar] [CrossRef] [PubMed]
- Vandevivere, L.; Denduyver, P.; Portier, C.; Häusler, O.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Influence of binder attributes on binder effectiveness in a continuous twin screw wet granulation process via wet and dry binder addition. Int. J. Pharm. 2020, 585, 119466. [Google Scholar] [CrossRef] [PubMed]
- Vandevivere, L.; Vangampelaere, M.; Portier, C.; de Backere, C.; Häusler, O.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Identifying critical binder attributes to facilitate binder selection for efficient formulation development in a continuous twin screw wet granulation process. Pharmaceutics 2021, 13, 210. [Google Scholar] [CrossRef] [PubMed]
- Vandevivere, L.; Denduyver, P.; Portier, C.; Häusler, O.; De Beer, T.; Vervaet, C.; Vanhoorne, V. The Effect of Binder Types on the Breakage and Drying Behavior of Granules in a Semi-Continuous Fluid Bed Dryer after Twin Screw Wet Granulation. Int. J. Pharm. 2022, 614, 121449. [Google Scholar] [CrossRef] [PubMed]
- Portier, C.; Vigh, T.; Di Pretoro, G.; De Beer, T.; Vervaet, C.; Vanhoorne, V. Continuous twin screw granulation: Impact of binder addition method and surfactants on granulation of a high-dosed, poorly soluble API. Int. J. Pharm. 2020, 577, 119068. [Google Scholar] [CrossRef] [PubMed]
- Kuraray. Basic Physical Properties of PVOH Resin. 2022. Available online: https://www.kuraray-poval.com/fileadmin/technical_information/brochures/poval/kuraray_poval_basic_physical_properties_web.pdf (accessed on 11 December 2023).
- Berry, J.D.; Neeson, M.J.; Dagastine, R.R.; Chan, D.Y.; Tabor, R.F. Measurement of surface and interfacial tension using pendant drop tensiometry. J. Colloid Interface Sci. 2015, 454, 226–237. [Google Scholar] [CrossRef]
- Shi, B.; Wang, Y.; Jia, L. Comparison of Dorris-Gray and Schultz methods for the calculation of surface dispersive free energy by inverse gas chromatography. J. Chromatogr. 2011, 1218, 860–862. [Google Scholar] [CrossRef] [PubMed]
- Della Volpe, C.; Siboni, S. Some reflections on acid-base solid surface free energy theories. J. Colloid Interface Sci. 1997, 195, 121–136. [Google Scholar] [CrossRef]
- Murtagh, F.; Legendre, P. Ward’s Hierarchical Agglomerative Clustering Method: Which Algorithms Implement Ward’s Criterion? J. Classif. 2014, 31, 274–295. [Google Scholar] [CrossRef]
- Lookingbill, B.D. The tensile strength of lactose tablets. In A Companion to Custer and the Little Bighorn Campaign; Wiley: Hoboken, NJ, USA, 2016; pp. 387–403. [Google Scholar] [CrossRef]
- Ylä-Mäihäniemi, P.P.; Heng, J.Y.; Thielmann, F.; Williams, D.R. Inverse gas chromatographic method for measuring the dispersive surface energy distribution for particulates. Langmuir 2008, 24, 9551–9557. [Google Scholar] [CrossRef]
- Jefferson, A.E.; Williams, D.R.; Heng, J.Y. Computing the surface energy distributions of heterogeneous crystalline powders. J. Adhes. Sci. Technol. 2011, 25, 339–355. [Google Scholar] [CrossRef]
- Lapčík, L.; Otyepka, M.; Otyepková, E.; Lapčíková, B.; Gabriel, R.; Gavenda, A.; Prudilová, B. Surface heterogeneity: Information from inverse gas chromatography and application to model pharmaceutical substances. Curr. Opin. Colloid Interface Sci. 2016, 24, 64–71. [Google Scholar] [CrossRef]
- Ali, J.; Saigal, N.; Baboota, S.; Ahuja, A. Microcrystalline cellulose as a versatile excipient in drug research. J. Young Pharm. 2009, 1, 6. [Google Scholar] [CrossRef]
- Hong, P.D.; Chou, C.M.; He, C.H. Solvent effects on aggregation behavior of polyvinyl alcohol solutions. Polymer 2001, 42, 6105–6112. [Google Scholar] [CrossRef]
- Rumyantsev, M. Influences of co-solvent on hydrogen bond reorganization in ternary poly(vinyl alcohol) solutions. Eur. Polym. J. 2013, 49, 2257–2266. [Google Scholar] [CrossRef]
- Thielmann, F.; Naderi, M.; Ansari, M.A.; Stepanek, F. The effect of primary particle surface energy on agglomeration rate in fluidised bed wet granulation. Powder Technol. 2008, 181, 160–168. [Google Scholar] [CrossRef]
- Iveson, S.M.; Litster, J.D. Fundamental studies of granule consolidation. Part 2: Quantifying the effects of particle and binder properties. Powder Technol. 1998, 99, 243–250. [Google Scholar] [CrossRef]
- Pharmaceutical Networking. 2.9.7 Friability of uncoated tablets. In European Pharmacopoeia 11.0; Council of Europe: London, UK, 2022; p. 359. [Google Scholar]


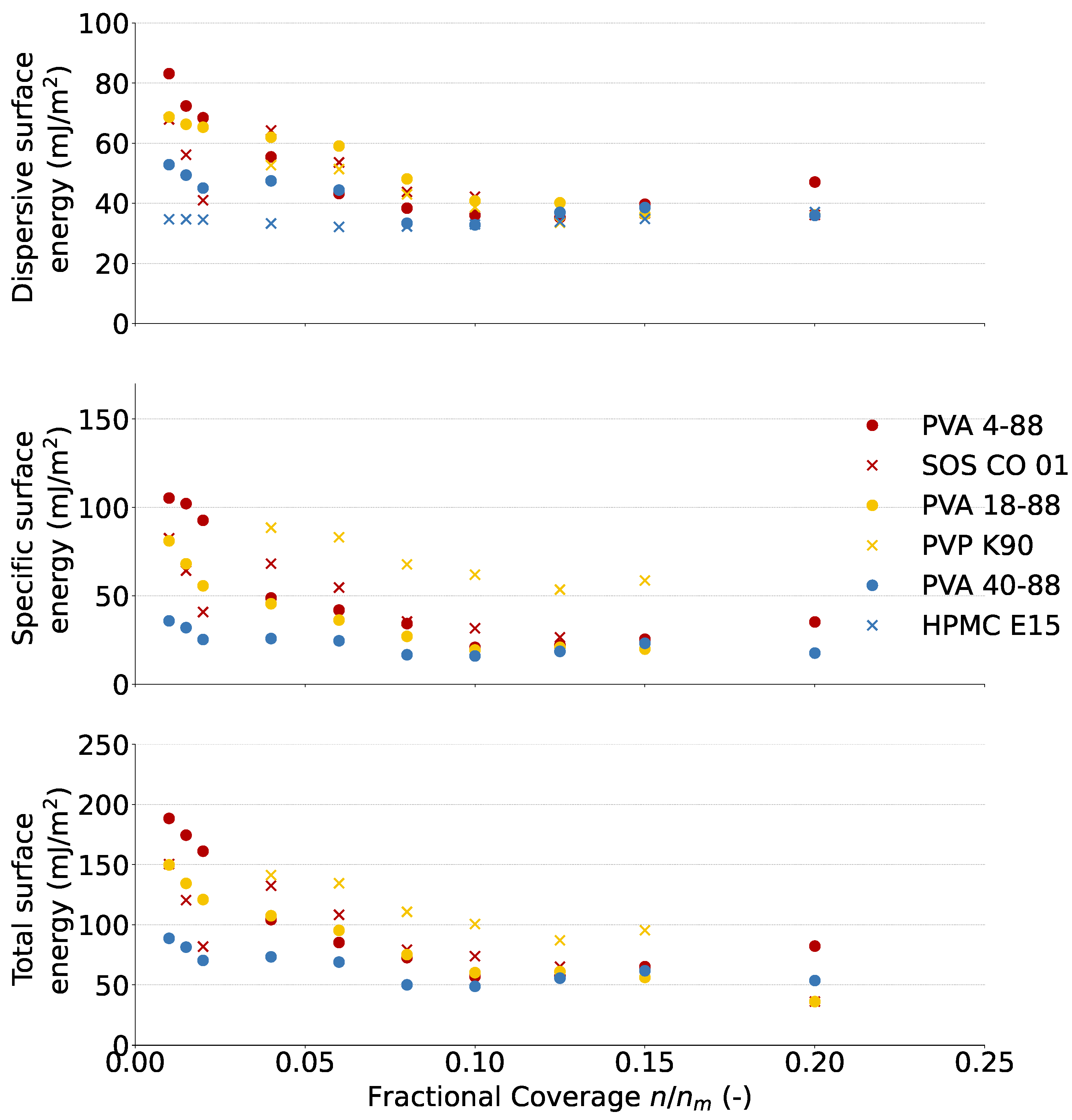
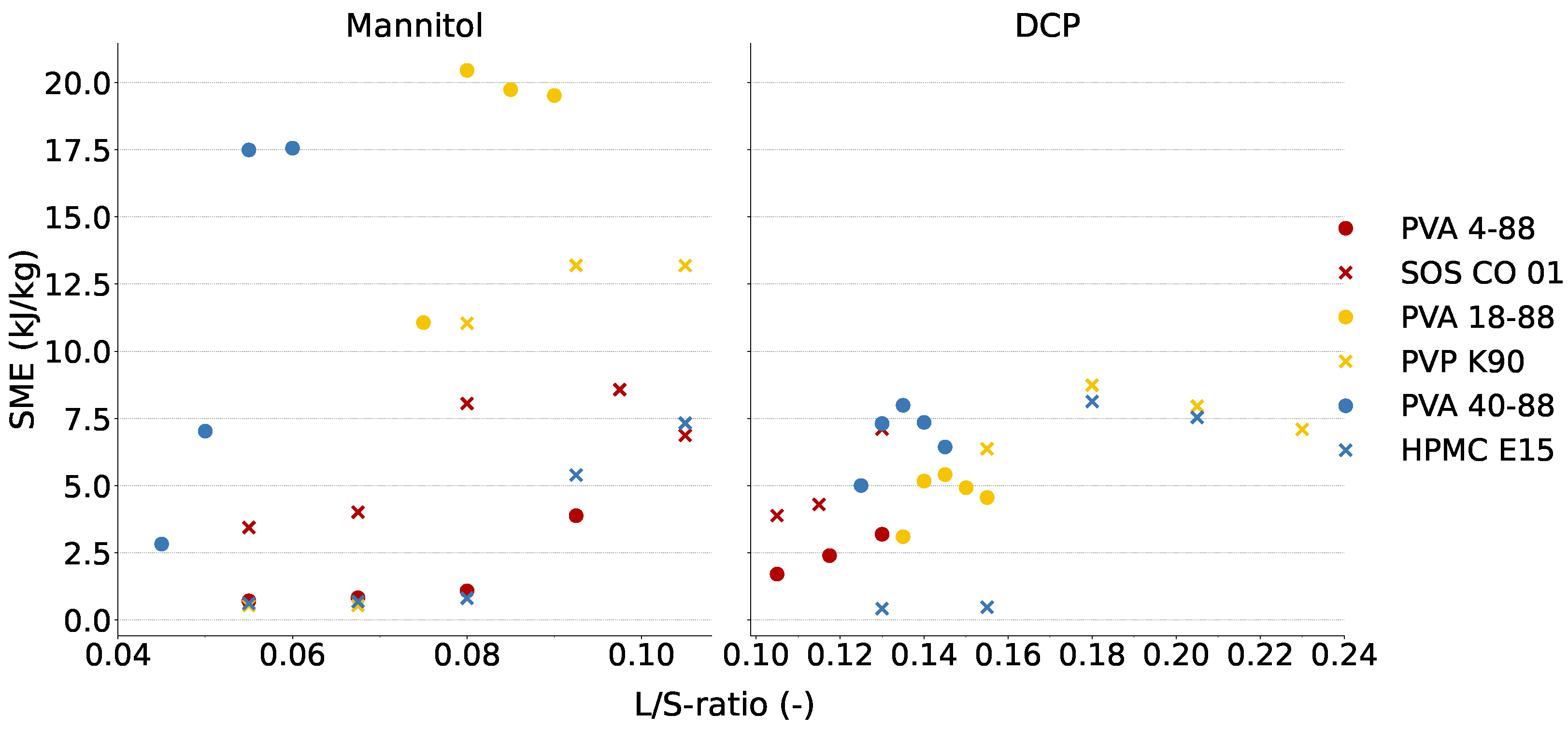
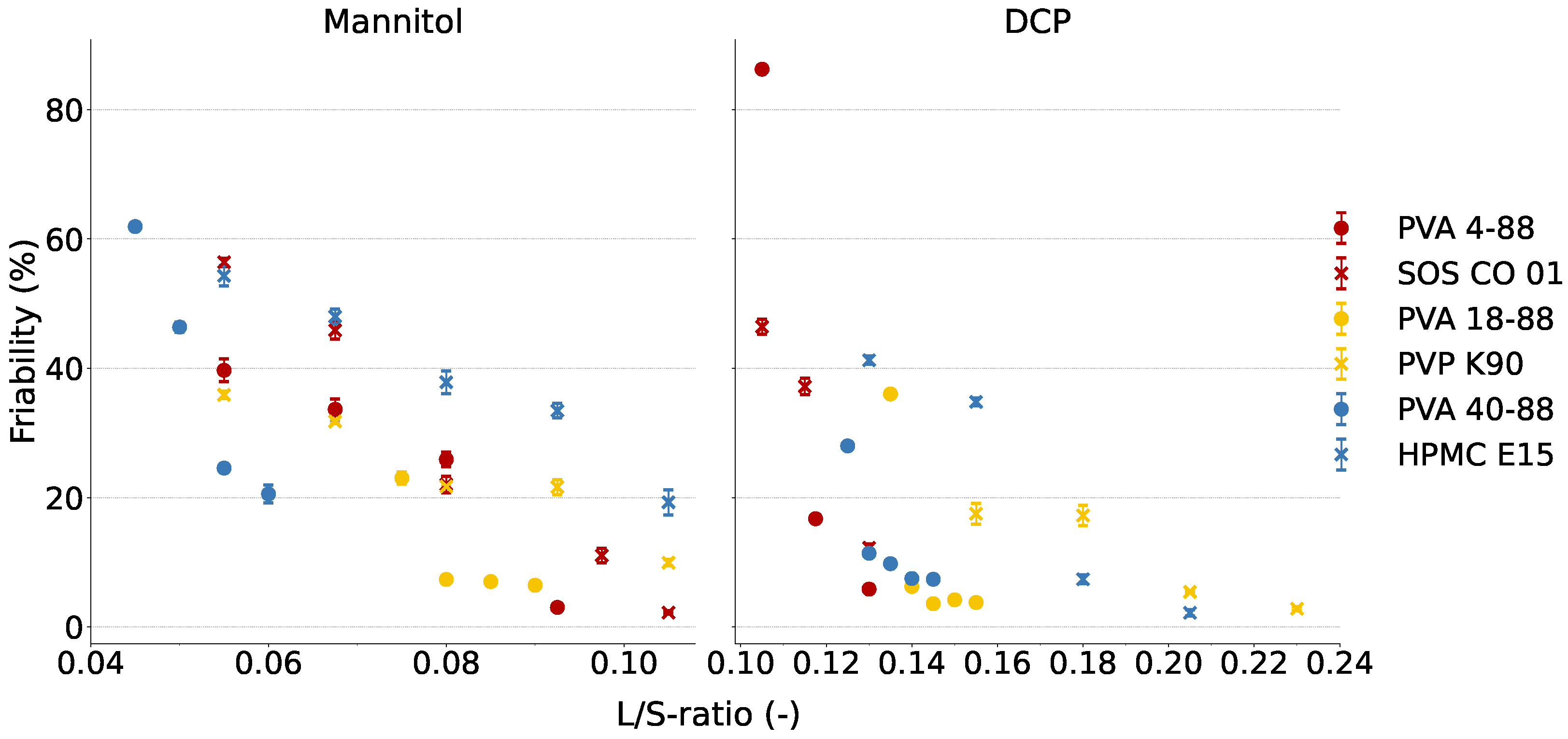



| Binder | C25 | C1 | ||
|---|---|---|---|---|
| Mannitol Formulation | DCP Formulation | Model Drug Formulation | ||
| L/S-Ratio Range | L/S-Ratio Range | L/S-Ratio | Drying Time (s) | |
| PVA 4-88 | 0.0550–0.0925 * | 0.1050–0.1300 * | 0.1000 | 325; 425 |
| SOS CO 01 | 0.0550–0.1050 * | 0.1050–0.1300 * | 0.1050 * | 450 * |
| PVA 18-88 | 0.0750–0.0900 | 0.1350–0.1550 | 0.1000 | 325; 475 |
| PVP K90 | 0.0550–0.1050 * | 0.1550–0.2300 * | 0.1200 | 550 |
| PVA 40-88 | 0.0450–0.0600 | 0.1250–0.1450 | 0.1100 | 400; 475; 550 |
| HPMC E15 | 0.0550–0.1050 * | 0.1300–0.2050 * | 0.1800 * | 600 * |
| Binder | Mannitol Formulation | DCP Formulation | |||||
|---|---|---|---|---|---|---|---|
| L/S-Ratio | Friability (%) | SME (kJ/kg) | L/S-Ratio | Friability (%) | SME (kJ/kg) | ||
| Cluster 1 | PVA 4-88 | 0.0800 | 3.03 (±0.26) | 1.09 | 0.1175 | 5.85 (±0.35) | 2.40 |
| SOS CO 01 | 0.0800 | 2.23 (±0.26) | 8.05 | 0.1300 | 12.26 (±0.58) | 7.11 | |
| Cluster 2 | PVA 18-88 | 0.0750 | 6.45 (±0.12) | 11.07 | 0.1400 | 3.79 (±0.17) | 5.17 |
| PVP K90 | 0.0800 | 9.94 (±0.48) | 11.04 | 0.1550 | 2.82 (±0.31) | 6.37 | |
| Cluster 3 | PVA 40-88 | 0.0550 | 20.57 (±1.38) | 17.49 | 0.1250 | 7.38 (±0.08) | 5.00 |
| HPMC E15 | 0.1050 | 19.29 (±1.94) | 7.32 | 0.1800 | 2.19 (±0.51) | 8.13 | |
| Binder | Model Drug Formulation | |||
|---|---|---|---|---|
| L/S-Ratio | Friability (%) | d50 (m) | ||
| Cluster 1 | PVA 4-88 | 0.100 | 2.57 (±0.12) | 2026.3 (±23.8) |
| SOS CO 01 | 0.105 | 7.03 (±0.95) | 1699.6 (±55.5) | |
| Cluster 2 | PVA 18-88 | 0.100 | 7.25 (±0.50) | 2032.2 (±71.7) |
| Cluster 3 | PVA 40-88 | 0.110 | 5.07 (±0.22) | 2324.9 (±19.6) |
| HPMC E15 | 0.180 | 0.77 (±0.05) | 1529.0 (±13.8) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Denduyver, P.; Birk, G.; Ambruosi, A.; Vervaet, C.; Vanhoorne, V. Evaluation of Polyvinyl Alcohol as Binder during Continuous Twin Screw Wet Granulation. Pharmaceutics 2024, 16, 854. https://doi.org/10.3390/pharmaceutics16070854
Denduyver P, Birk G, Ambruosi A, Vervaet C, Vanhoorne V. Evaluation of Polyvinyl Alcohol as Binder during Continuous Twin Screw Wet Granulation. Pharmaceutics. 2024; 16(7):854. https://doi.org/10.3390/pharmaceutics16070854
Chicago/Turabian StyleDenduyver, Phaedra, Gudrun Birk, Alessandra Ambruosi, Chris Vervaet, and Valérie Vanhoorne. 2024. "Evaluation of Polyvinyl Alcohol as Binder during Continuous Twin Screw Wet Granulation" Pharmaceutics 16, no. 7: 854. https://doi.org/10.3390/pharmaceutics16070854







