Influence of the Acceptor Fluid on the Bupivacaine Release from the Prospective Intra-Articular Methylcellulose Hydrogel
Abstract
1. Introduction
2. Materials and Methods
2.1. Hydrogel Preparation
2.2. Acceptor Fluid Preparation
2.3. Viscosity Study
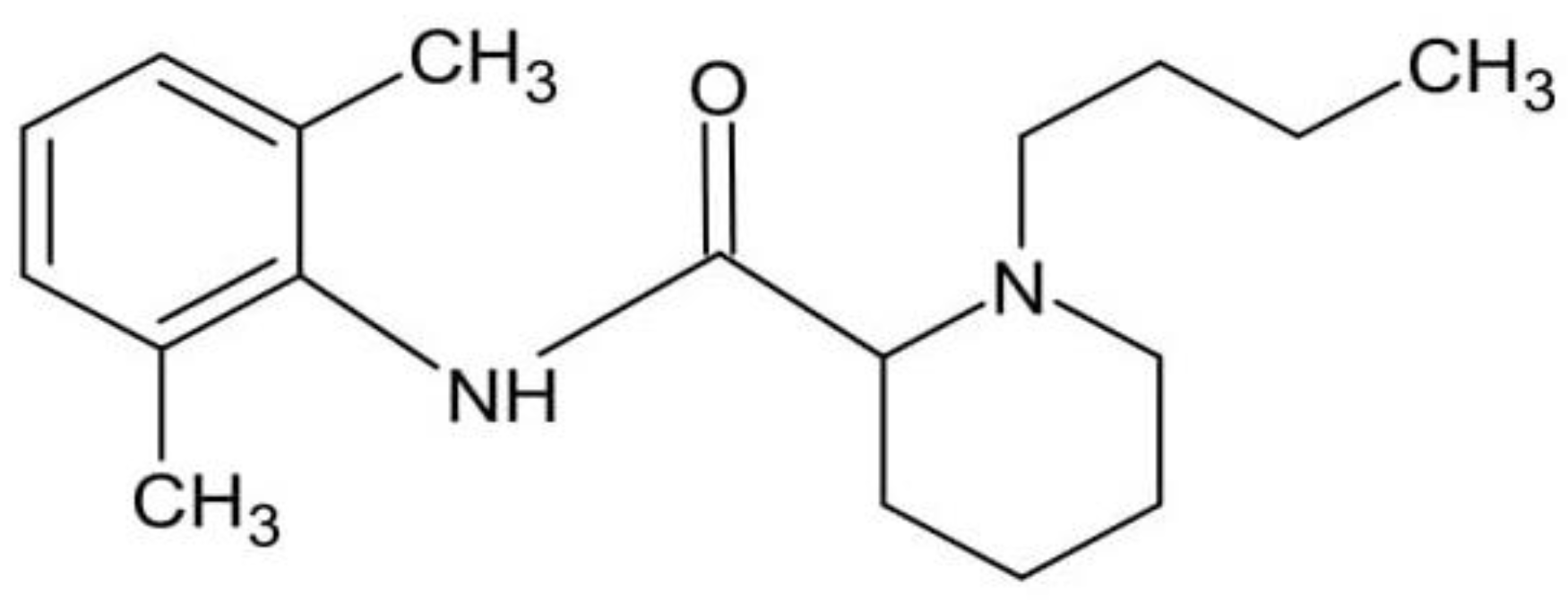
2.4. UV–Vis Spectra of Bupivacaine and Calibration Curves
2.5. Release Study
2.6. Difference Factor and Similarity Factor
2.7. Kinetic Study
2.8. Statistical Analysis
2.9. FTIR Measurements
2.10. DSC Measurements
3. Results and Discussion
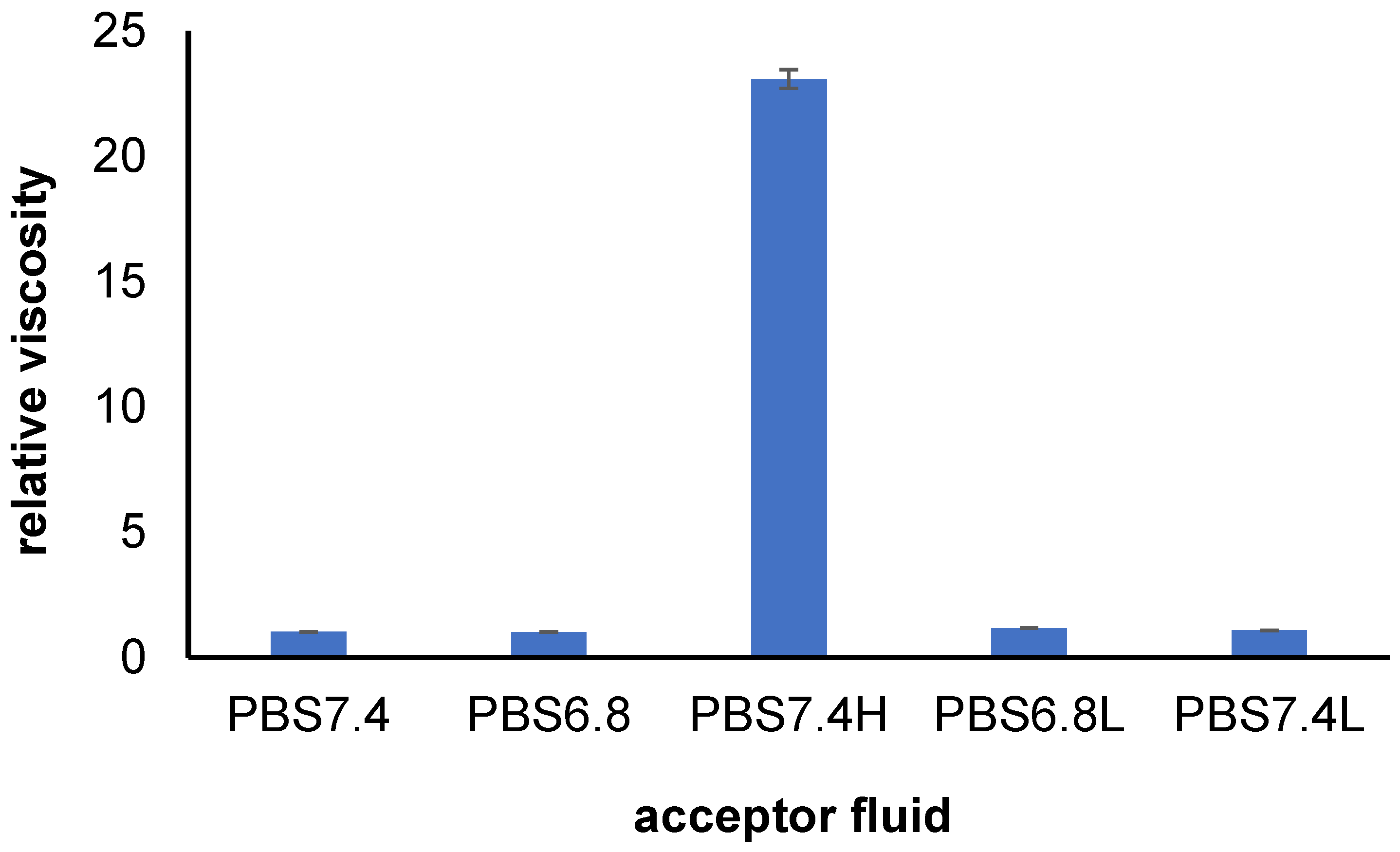
3.1. Viscosity Study
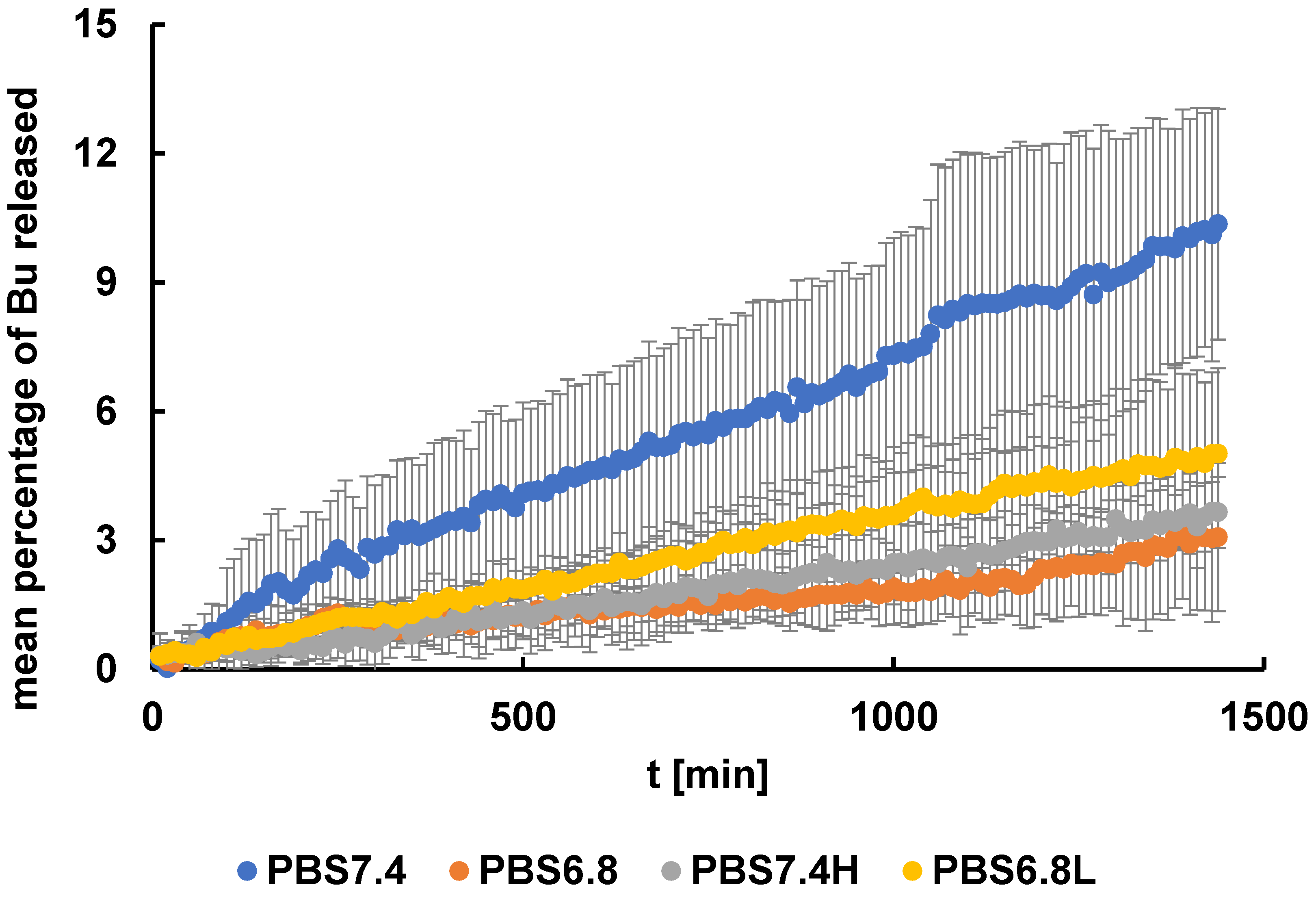
3.2. Release Study
3.3. Release Curves Comparison
3.4. Kinetic Analysis
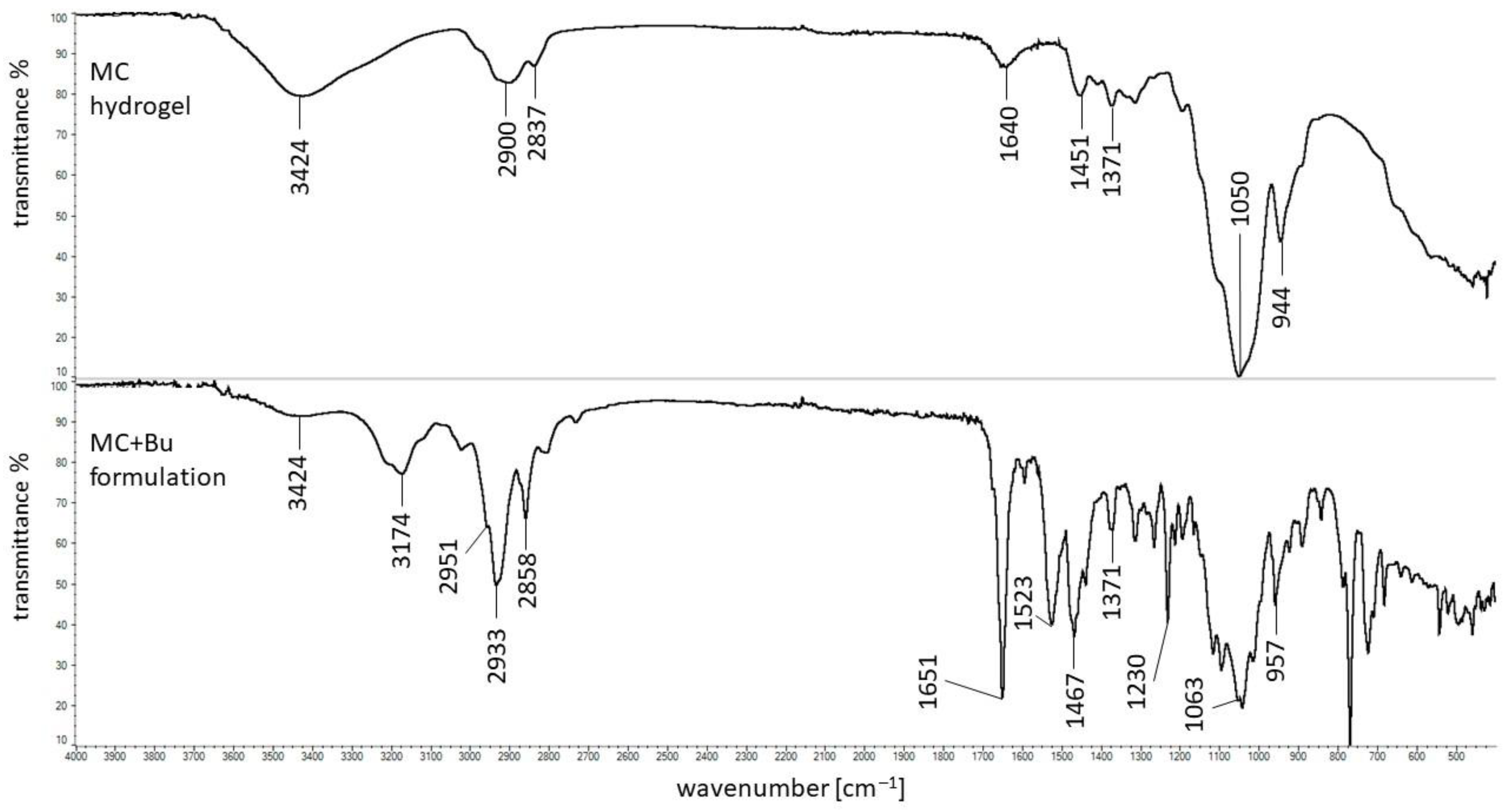
3.5. FTIR Study
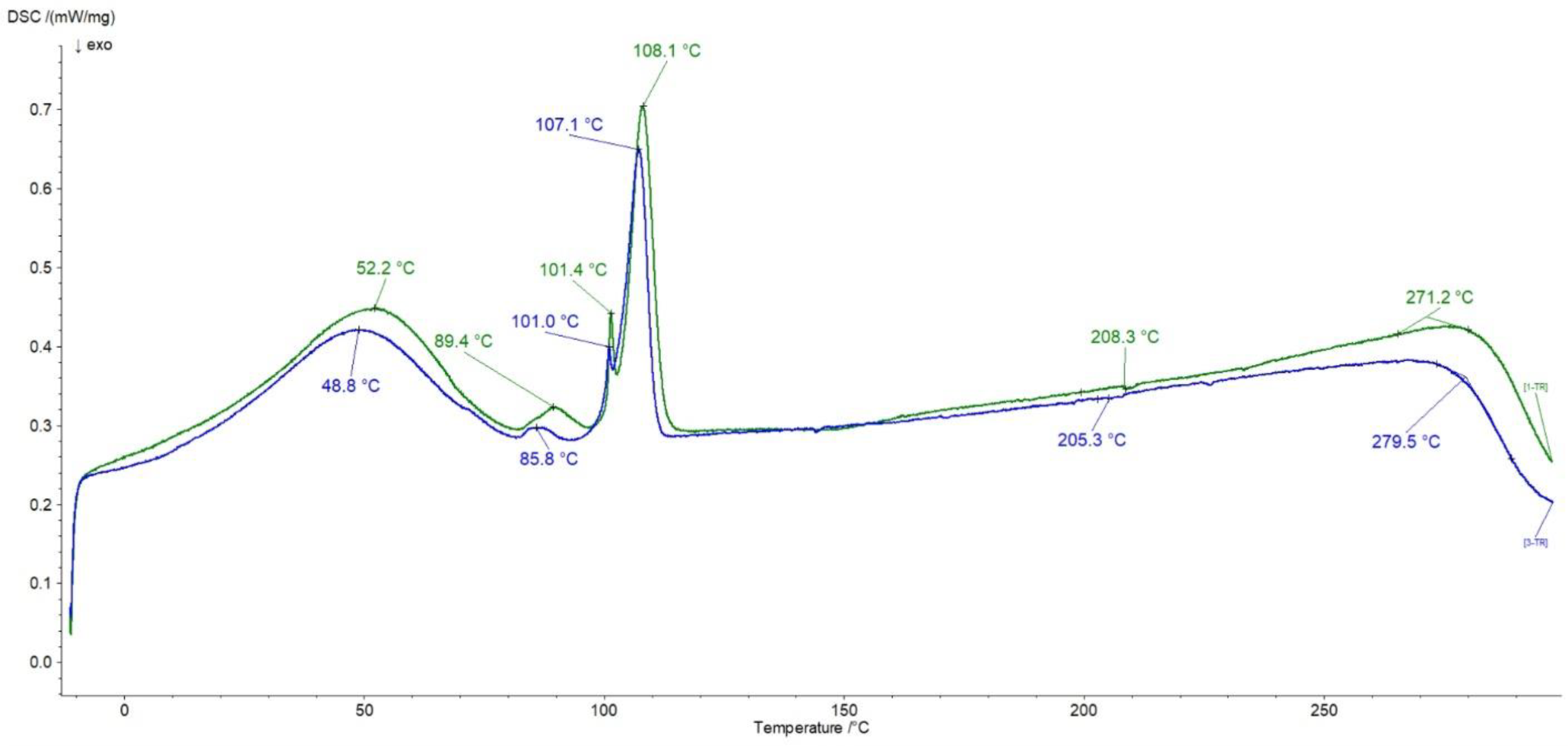
3.6. DSC Investigations
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lavelle, W.; Lavelle, E.D.; Lavelle, L. Intra-Articular Injections. Anesthesiol. Clin. 2007, 25, 853–862. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.H.; Kraus, V.B.; Setton, L.A. Progress in intra-articular therapy. Nat. Rev. Rheumatol. 2014, 10, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Temple-Wong, M.M.; Ren, S.; Quach, P.; Hansen, B.C.; Chen, A.C.; Hasegawa, A.; D’Lima, D.D.; Koziol, J.; Masuda, K.; Lotz, M.K.; et al. Hyaluronan concentration and size distribution in human knee synovial fluid: Variations with age and cartilage degeneration. Arthritis Res. Ther. 2016, 18, 18. [Google Scholar] [CrossRef]
- Rajamäki, K.; Nordström, T.; Nurmi, K.; Åkerman, K.E.O.; Kovanen, P.T.; Öörni, K.; Eklund, K.K. Extracellular acidosis is a novel danger signal alerting innate immunity via the NLRP3 inflammasome. J. Biol. Chem. 2013, 288, 13410–13419. [Google Scholar] [CrossRef]
- MacWilliams, P.S.; Friedrichs, K.R. Laboratory evaluation and interpretation of synovial fluid. Vet. Clin. Small Anim. 2003, 33, 153–178. [Google Scholar]
- Cowman, M.K.; Schmidt, T.A.; Raghavan, P.; Stecco, A. Viscoelastic Properties of Hyaluronan in Physiological Conditions. F1000Research 2015, 4, 1–13. [Google Scholar] [CrossRef]
- Tamer, T.M. Hyaluronan and synovial joint: Function, distribution and healing. Interdiscip. Toxicol. 2013, 6, 111–125. [Google Scholar] [CrossRef]
- dos Ferreira, N.R.; Sanz, C.K.; Raybolt, A.; Pereira, C.M.; dos Santos, M.F. Action of Hyaluronic Acid as a Damage-Associated Molecular Pattern Molecule and Its Function on the Treatment of Temporomandibular Disorders. Front. Pain Res. 2022, 3, 852249. [Google Scholar] [CrossRef]
- Snetkov, P.; Zakharova, K.; Morozkina, S.; Olekhnovich, R. Hyaluronic Acid: The Influence of Molecular Weight and Degradable Properties of Biopolymer. Polymers 2020, 12, 1800. [Google Scholar] [CrossRef] [PubMed]
- Iaconisi, G.N.; Lunetti, P.; Gallo, N.; Cappello, A.R.; Fiermonte, G.; Dolce, V.; Capobianco, L. Hyaluronic Acid: A Powerful Biomolecule with Wide-Ranging Applications—A Comprehensive Review. Int. J. Mol. Sci. 2023, 24, 10296. [Google Scholar] [CrossRef]
- Shah, V.R.; Mackwana, R.; Vora, K. Spinal Anaesthesia in Ambulatory Surgery: Dose-Response Characteristics of Constant Volume Bupivacaine. Indian J. Anaesth. 2008, 52, 191–195. [Google Scholar]
- Leone, S.; Di Cianni, S.; Casati, A.; Fanelli, G. Pharmacology, toxicology, and clinical use of new long acting local anesthetics, ropivacaine and levobupivacaine. Acta Biomed. 2008, 79, 92–105. [Google Scholar] [PubMed]
- Weinberg, G.L.; Ripper, R.; Murphy, P.; Edelman, L.B.; Hoffman, W.; Stricharz, G.; Feinstein, D.L. Lipid Infusion Accelerates Removal of Bupivacaine and Recovery From Bupivacaine Toxicity in the Isolated Rat Heart. Reg. Anesth. Pain Med. 2006, 31, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Rogobete, A.F.; Bedreag, O.H.; Sărăndan, M.; Păpurică, M.; Preda, G.; Dumbuleu, M.C.; Vernic, C.; Stoicescu, E.R.; Săndesc, D. Liposomal bupivacaine—New trends in Anesthesia and Intensive Care Units. Egypt. J. Anaesth. 2015, 31, 89–95. [Google Scholar] [CrossRef][Green Version]
- Chapman, P.J. Review: Bupivacaine—A long-acting local anaesthetic. Aust. Dent. J. 1987, 32, 288–291. [Google Scholar] [CrossRef] [PubMed]
- Dumlu, E.G.; Tokaç, M.; Öcal, H.; Durak, D.; Kara, H.; Kiliç, M.; Yalçin, A. Local bupivacaine for postoperative pain management in thyroidectomized patients: A prospective and controlled clinical study. Turk. J. Surg. 2016, 32, 173–177. [Google Scholar] [CrossRef]
- Hellyer, P.; Rodan, I.; Brunt, J.; Downing, R.; Hagedorn, J.; Robertson, S. AAHA/AAFP pain management guidelines for dogs and cats. J. Am. Anim. Hosp. Assoc. 2007, 9, 466–480. [Google Scholar] [CrossRef] [PubMed]
- Devi, M.M.; Gupta, S.; Amaravathi, R.; Udupa, S.; Hegde, A.; Ghosh, S. Comparison of efficacy of intra-articular plain bupivacaine and bupivacaine with adjuvants (dexmedetomidine and magnesium sulfate) for postoperative analgesia in arthroscopic knee surgeries: A prospective, randomized controlled trial. Anesth. Essays Res. 2018, 12, 848–854. [Google Scholar] [CrossRef] [PubMed]
- Fox, M.G.; Patrie, J.T. Does reducing the concentration of bupivacaine when performing therapeutic shoulder joint injections impact the clinical outcome? Am. J. Roentgenol. 2016, 206, 805–809. [Google Scholar] [CrossRef] [PubMed]
- Almoshari, Y.H. Novel Hydrogels for Topical Applications: An Updated Comprehensive Review Based on Source. Gels 2022, 8, 174–198. [Google Scholar] [CrossRef]
- Pluta, J.; Karolewicz, B. Hydrożele: Właściwości i zastosowanie w technologii postaci leku. I. Charakterystyka hydrożeli. Polim. Med. 2004, 34, 63–81. Available online: https://www.dbc.wroc.pl/Content/2293/201_Plut.pdf (accessed on 19 September 2023).
- Bahram, M.; Mohseni, N.; Moghtader, M. An Introduction to Hydrogels and Some Recent Applications. Emerg. Concepts Anal. Appl. Hydrogels 2018, 2, 9–38. [Google Scholar] [CrossRef]
- Hynninen, V.; Patrakka, J.; Nonappa. Methylcellulose–cellulose nanocrystal composites for optomechanically tunable hydrogels and fibers. Materials 2021, 14, 5137. [Google Scholar] [CrossRef] [PubMed]
- Nasatto, P.L.; Pignon, F.; Silveira, J.L.M.; Duarte, M.E.R.; Noseda, M.D.; Rinaudo, M. Methylcellulose, a cellulose derivative with original physical properties and extended applications. Polymers 2015, 7, 777–803. [Google Scholar] [CrossRef]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Thirumala, S.; Gimble, J.M.; Devireddy, R.V. Methylcellulose based thermally reversible hydrogel system for tissue engineering applications. Cells 2013, 2, 460–475. [Google Scholar] [CrossRef] [PubMed]
- Budai, L.; Budai, M.; Fülöpné Pápay, Z.E.; Vilimi, Z.; Antal, I. Rheological Considerations of Pharmaceutical Formulations: Focus on Viscoelasticity. Gels 2023, 9, 469. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Wang, B.; Hu, C.; Zhao, J. An overview of hydrogel-based intra-articular drug delivery for the treatment of osteoarthritis. Colloids Surfaces B Biointerfaces 2017, 154, 33–39. [Google Scholar] [CrossRef]
- Malik, D.S.; Mital, N.; Kaur, G. Topical drug delivery systems: A patent review. Expert Opin. Ther. Pat. 2016, 26, 213–228. [Google Scholar] [CrossRef]
- Council of Europe; European Directorate for the Quality of Medicines & Health Care (EDQM). European Pharmacopeia 10.8; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- Wójcik-Pastuszka, D.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osiński, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials 2019, 12, 1202. [Google Scholar] [CrossRef]
- Wójcik-Pastuszka, D.; Krzak, J.; Prządka, P.; Twarda, M.; Osiński, B.; Musiał, W. Release of bupivacaine from artificial ligament implants modified with the silica coating. Ceram. Int. 2023, 49, 2852–2859. [Google Scholar] [CrossRef]
- US Department of Health and Human Services; Food and Drug Administration; Center for Drug Evaluation and Research. Guidance for Industry, Dissolution Testing of Immediate Release Solid Oral Dosage Forms; Part 4; Food and Drug Administration: Rockville, MD, USA, 1997; pp. 15–22. [Google Scholar]
- Wójcik-Pastuszka, D.; Stawicka, K.; Dryś, A.; Musiał, W. Influence of HA on Release Process of Anionic and Cationic API Incorporated into Hydrophilic Gel. Int. J. Mol. Sci. 2023, 24, 5606. [Google Scholar] [CrossRef] [PubMed]
- Paarakh, M.P.; Jose, P.A.; Setty, C.M.; Peterchristoperr, G.V. Release Kinetics—Concepts and Applications. Int. J. Pharm. Res. Technol. 2018, 8, 12–20. [Google Scholar] [CrossRef]
- Saitarly, S.; Pushkarev, Y.; Nesterkina, M.; Öztürk, S.; Salih, B.; Kravchenko, I. Rheological properties of hyaluronic acid diluted solutions as components of cosmetics. Biointerface Res. Appl. Chem. 1907. [Google Scholar] [CrossRef]
- Serajuddin, A.T.M.; Jarowski, C.I. Effect of Diffusion Layer pH and Solubility on The Dissolution Rate of Pharmaceutical Acids and Their Sodium Salts II: Salicylic Acid, Theophylline, and Benzoic Acid. J. Pharm. Sci. 1985, 74, 148–154. [Google Scholar] [CrossRef] [PubMed]
- Serajuddin, A.T.M.; Jarowski, C.I. Effect of Diffusion Layer pH and Solubility on the Dissolution Rate of Pharmaceutical Bases and Their Hydrochloride Salts I: Phenazopyridine. J. Pharm. Sci. 1985, 74, 142–147. [Google Scholar] [CrossRef] [PubMed]
- Shah, J.C.; Maniar, M. pH-Dependent solubility and dissolution of bupivacaine and its relevance to the formulation of a controlled release system. J. Control. Release 1993, 23, 261–270. [Google Scholar] [CrossRef]
- Zheng, X.; Wang, B.; Tang, X.; Mao, B.; Zhang, Q.; Zhang, T.; Zhao, J.; Cui, S.; Chen, W. Absorption, metabolism, and functions of hyaluronic acid and its therapeutic prospects in combination with microorganisms: A review. Carbohydr. Polym. 2023, 299, 120153–120171. [Google Scholar] [CrossRef] [PubMed]
- Hassan, D.S.; Hasary, H.J. The impact of viscosity on the dissolution of naproxen immediate-release tablets. J. Taibah. Univ. Med. Sci. 2023, 18, 687–695. [Google Scholar] [CrossRef] [PubMed]
- Babaie, S.; Taghvimi, A.; Charkhpour, M.; Zarebkohan, A.; Keyhanvar, P.; Hamishehkar, H. Optimization of influential variables in the development of buprenorphine and bupivacaine loaded invasome for dermal delivery. Adv. Pharm. Bull. 2021, 11, 522–529. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Domingues, J.S.; dos Santos, S.M.D.; das Neves Rodrigues Ferreira, J.; Monti, B.M.; Baggio, D.F.; Hummig, W.; Araya, E.I.; de Paula, E.; Chichorro, J.G.; Ferreira, L.E.N. Antinociceptive effects of bupivacaine and its sulfobutylether-β-cyclodextrin inclusion complex in orofacial pain. Naunyn. Schmiedebergs. Arch. Pharmacol. 2022, 395, 1405–1417. [Google Scholar] [CrossRef]
- Shivakumar, B.S.; Gopalakrishnan-Prema, V.; Raju, G.; Mathew, S.E.; Katiyar, N.; Menon, D.; Shankarappa, S.A. Anisotropic microparticles for differential drug release in nerve block anesthesia. RSC Adv. 2021, 11, 4623–4630. [Google Scholar] [CrossRef]
- Martins, M.L.; Eckert, J.; Jacobsen, H.; dos Santos, E.C.; Ignazzi, R.; de Araujo, D.R.; Bellissent-Funel, M.-C.; Natali, F.; Koza, M.M.; Matic, A.; et al. Raman and Infrared spectroscopies and X-ray diffraction data on bupivacaine and ropivacaine complexed with 2-hydroxypropyl–β–cyclodextrin. Data Brief 2017, 15, 25–29. [Google Scholar] [CrossRef] [PubMed]
- Nadour, M.; Boukraa, F.; Ouradi, A.; Benaboura, A. Effects of methylcellulose on the properties and morphology of polysulfone membranes prepared by phase inversion. Mater. Res. 2017, 20, 339–348. [Google Scholar] [CrossRef]
- Mojet, B.L.; Ebbesen, S.D.; Lefferts, L. Light at the interface: The potential of attenuated total reflection infrared spectroscopy for understanding heterogeneous catalysis in water. Chem. Soc. Rev. 2010, 39, 4643–4655. [Google Scholar] [CrossRef] [PubMed]
- Abdeltawab, H.; Jaiswal, J.K.; Young, S.W.; Svirskis, D.; Hill, A.; Sharma, M. Stability and compatibility of admixtures containing bupivacaine hydrochloride and ketorolac tromethamine for parenteral use. Eur. J. Hosp. Pharm. 2023, 30, E48–E54. [Google Scholar] [CrossRef] [PubMed]
- Sykuła-Zając, A.; Łodyga-Chruścińska, E.; Pałecz, B.; Dinnebier, R.E.; Griesser, U.J.; Niederwanger, V. Thermal and X-ray analysis of racemic bupivacaine hydrochloride. J. Therm. Anal. Calorim. 2011, 105, 1031–1036. [Google Scholar] [CrossRef][Green Version]
- Viera, R.G.P.; Filho, G.R.; de Assunção, R.M.N.; da Carla, C.; Vieira, J.G.; de Oliveira, G.S. Synthesis and characterization of methylcellulose from sugar cane bagasse cellulose. Carbohydr. Polym. 2007, 67, 182–189. [Google Scholar] [CrossRef]




| Fluid | PBS7.4 | PBS6.8 | PBS7.4H | PBS6.8L | PBS7.4L |
|---|---|---|---|---|---|
| pH of PBS | 7.4 | 6.8 | 7.4 | 6.8 | 7.4 |
| HA | — | — | High molecular | Low molecular | Low molecular |
| Fluid | PBS6.8 | PBS7.4H | ||
|---|---|---|---|---|
| f1 | f2 | f1 | f2 | |
| PBS7.4 | 71.4 | 67.0 | 66.7 | 68.9 |
| PBS6.8L | 71.7 | 88.8 | 46.0 | 93.0 |
| Kinetic Model | Kinetic Parameters | PBS7.4 | PBS6.8 | PBS7.4H | PBS6.8L |
|---|---|---|---|---|---|
| Z-O | k0 × 103 [mg × min−1] | 1.91 ± 0.02 | 0.51 ± 0.01 | 0.63 ± 0.01 | 0.90 ± 0.01 |
| t0.5 [min] | 7532 ± 64 | 28,666 ± 828 | 21,650 ± 340 | 15,443 ± 172 | |
| R2 | 0.94 ± 0.03 | 0.53 ± 0.27 | 0.86 ± 0.06 | 0.96 ± 0.02 | |
| F-O | k1 × 105 [min−1] | 7.20 ± 0.01 | 1.60 ± 0.07 | 2.36 ± 0.06 | 3.5 ± 0.05 |
| t0.5 [min] | 10,561 ± 156 | 59,002 ± 4380 | 30,411 ± 860 | 26,653 ± 546 | |
| R2 | 0.96 ± 0.01 | 0.64 ± 0.23 | 0.88 ± 0.07 | 0.95 ± 0.05 | |
| S-O | k2 × 106 [mg−1 × min−1] | 3.00 ± 0.04 | 0.67 ± 0.003 | 0.96 ± 0.03 | 1.45 ± 0.02 |
| t0.5 [min] | 14,468 ± 217 | 84,016 ± 6258 | 43,072 ± 1220 | 37,577 ± 771 | |
| R2 | 0.96 ± 0.01 | 0.63 ± 0.23 | 0.88 ± 0.07 | 0.95 ± 0.05 | |
| H | kH × 102 [mg × min−1/2] | 5.85 ± 0.08 | 1.57 ± 0.03 | 1.93 ± 0.03 | 2.74 ± 0.04 |
| t0.5 [min] | 66,471 ± 2109 | 969,656 ± 51,061 | 548,209 ± 26,166 | 272,192 ± 6665 | |
| R2 | 0.84 ± 0.07 | 0.60 ± 0.16 | 0.75 ± 0.15 | 0.83 ± 0.04 | |
| K-P | kK-P × 103 [min−n] | 3.6 ± 0.2 | 25.9 ± 2.5 | 5.2 ± 0.6 | 16.0 ± 0.6 |
| t0.5 [min] | 712 ± 73 | 810 ± 190 | 848 ± 169 | 615 ± 57 | |
| R2 | 0.87 ± 0.06 | 0.47 ± 0.21 | 0.62 ± 0.16 | 0.88 ± 0.03 | |
| n | 0.82 ± 0.02 | 0.54 ± 0.04 | 0.70 ± 0.03 | 0.75 ± 0.02 | |
| best fit | F-O, S-O | F-O, S-O | F-O, S-O | Z-O, F-O, S-O | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wójcik-Pastuszka, D.; Frąk, A.; Musiał, W. Influence of the Acceptor Fluid on the Bupivacaine Release from the Prospective Intra-Articular Methylcellulose Hydrogel. Pharmaceutics 2024, 16, 867. https://doi.org/10.3390/pharmaceutics16070867
Wójcik-Pastuszka D, Frąk A, Musiał W. Influence of the Acceptor Fluid on the Bupivacaine Release from the Prospective Intra-Articular Methylcellulose Hydrogel. Pharmaceutics. 2024; 16(7):867. https://doi.org/10.3390/pharmaceutics16070867
Chicago/Turabian StyleWójcik-Pastuszka, Dorota, Anna Frąk, and Witold Musiał. 2024. "Influence of the Acceptor Fluid on the Bupivacaine Release from the Prospective Intra-Articular Methylcellulose Hydrogel" Pharmaceutics 16, no. 7: 867. https://doi.org/10.3390/pharmaceutics16070867
APA StyleWójcik-Pastuszka, D., Frąk, A., & Musiał, W. (2024). Influence of the Acceptor Fluid on the Bupivacaine Release from the Prospective Intra-Articular Methylcellulose Hydrogel. Pharmaceutics, 16(7), 867. https://doi.org/10.3390/pharmaceutics16070867







