A Second Wind for Inorganic APIs: Leishmanicidal and Antileukemic Activity of Hydrated Bismuth Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Hydrated Bismuth Oxide Nanoparticles (BiNP)
2.2. Bismuth Assay
2.3. Nanoparticle Characterization
2.3.1. Dynamic Light Scattering (DLS)
2.3.2. Transmission Electron Microscopy (TEM)
2.4. Cell Culture
2.4.1. Biological Assays in Promastigote Forms
2.4.2. Biological Assays in Amastigote Forms
2.4.3. Cytotoxicity Assays
Resazurin Assay
MTT Assay
2.4.4. Colony Formation Assay
2.5. Data Analysis
3. Results
3.1. Obtaining and Characterization of Nanoparticles
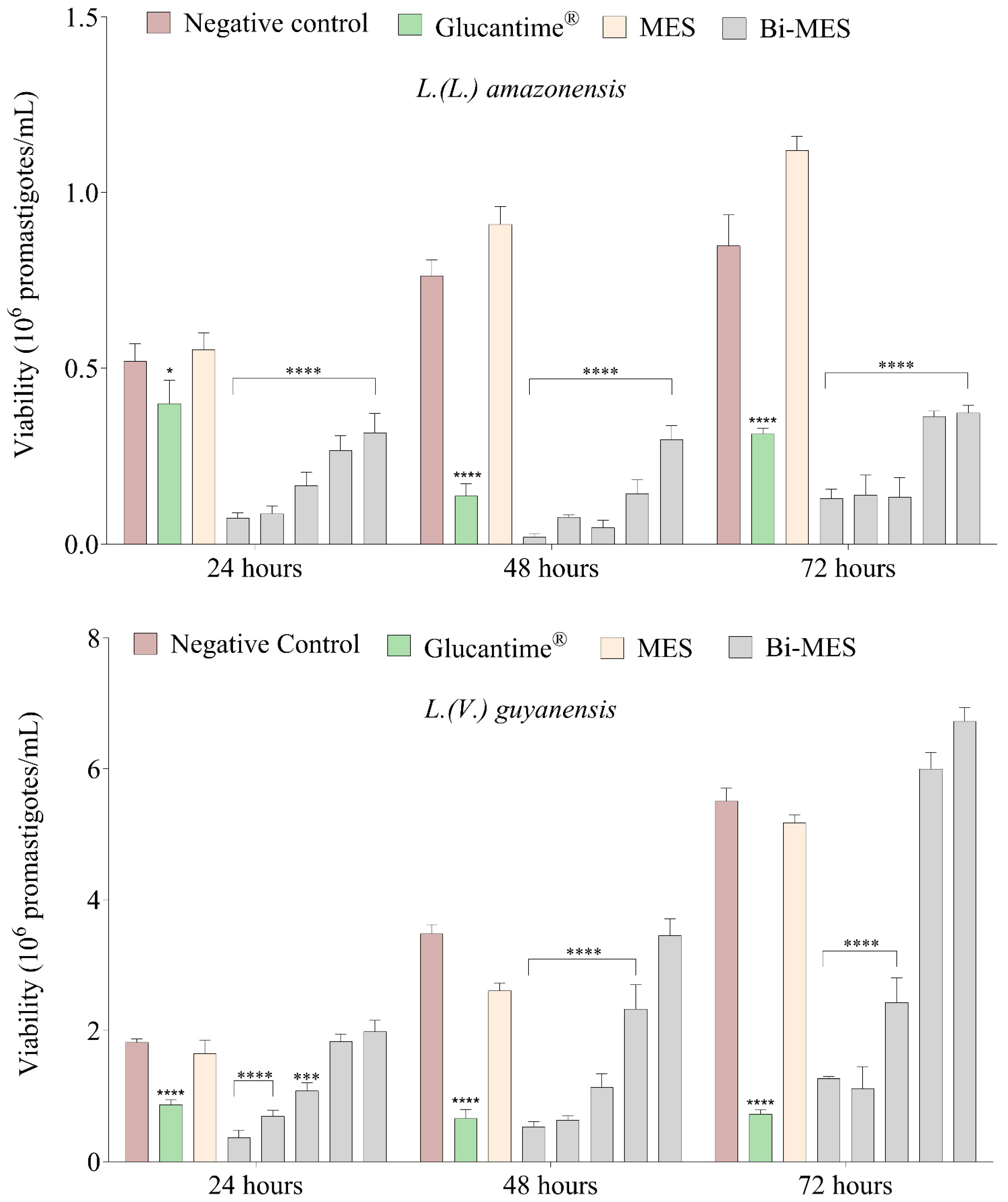
3.2. Leishmanicidal Activity of Bismuth Nanoparticles
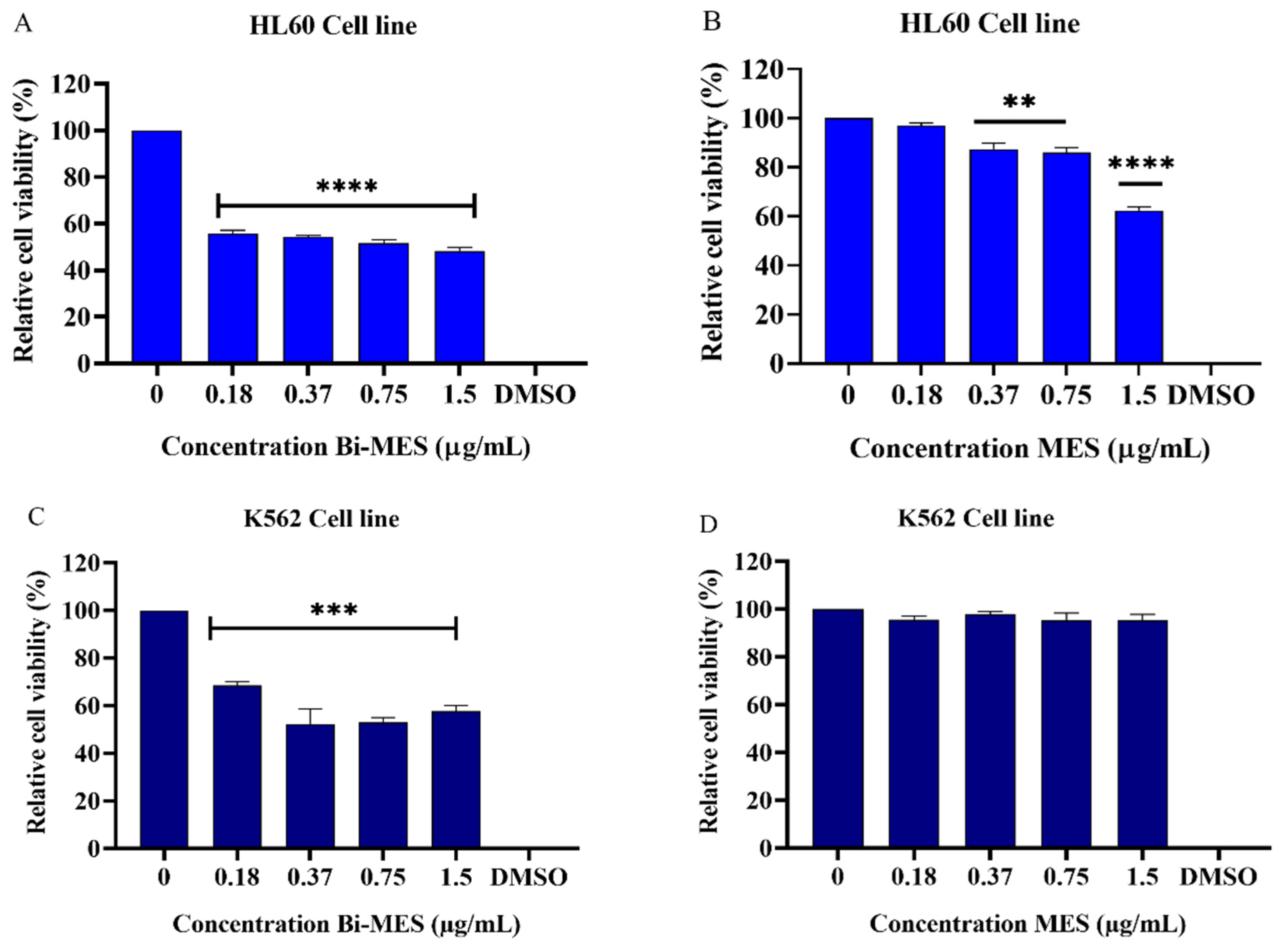
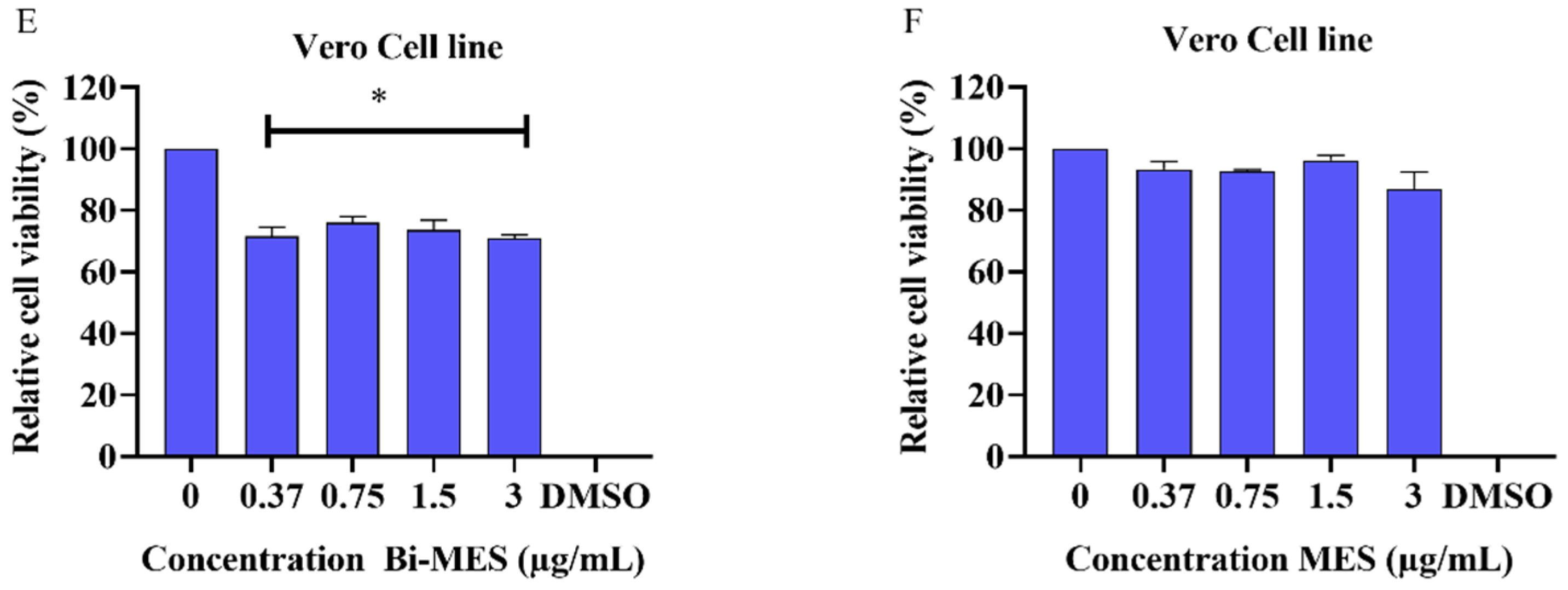
3.3. Cytotoxic Activity of Bismuth Nanoparticles
3.4. Colony Formation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- de Marcillac, P.; Coron, N.; Dambier, G.; Leblanc, J.; Moalic, J.-P. Experimental Detection of α-Particles from the Radioactive Decay of Natural Bismuth. Nature 2003, 422, 876–878. [Google Scholar] [CrossRef] [PubMed]
- Odier, L. Observations on the Effects of Magistery of Bismuth, Given Internally, as an Antispasmodic. Lond. Med. J. 1786, 7, 321–325. [Google Scholar] [PubMed]
- Yang, N.; Sun, H. Bismuth: Environmental Pollution and Health Effects. In Encyclopedia of Environmental Health; Elsevier: Amsterdam, The Netherlands, 2011; pp. 414–420. [Google Scholar]
- Yang, Y.; Ouyang, R.; Xu, L.; Guo, N.; Li, W.; Feng, K.; Ouyang, L.; Yang, Z.; Zhou, S.; Miao, Y. Review: Bismuth Complexes: Synthesis and Applications in Biomedicine. J. Coord. Chem. 2015, 68, 379–397. [Google Scholar] [CrossRef]
- Andrews, P.C.; Deacon, G.B.; Junk, P.C.; Kumar, I.; Silberstein, M. Synthetic and Structural Comparisons of Bismuth(Iii) Carboxylates Synthesised under Solvent-Free and Reflux Conditions. Dalt. Trans. 2006, 4852–4858. [Google Scholar] [CrossRef] [PubMed]
- Rosário, J.d.S.; Moreira, F.H.; Rosa, L.H.F.; Guerra, W.; Silva-Caldeira, P.P. Biological Activities of Bismuth Compounds: An Overview of the New Findings and the Old Challenges Not Yet Overcome. Molecules 2023, 28, 5921. [Google Scholar] [CrossRef] [PubMed]
- Cheng, T.; Lai, Y.-T.; Wang, C.; Wang, Y.; Jiang, N.; Li, H.; Sun, H.; Jin, L. Bismuth Drugs Tackle Porphyromonas Gingivalis and Attune Cytokine Response in Human Cells. Metallomics 2019, 11, 1207–1218. [Google Scholar] [CrossRef] [PubMed]
- Marzano, I.M.; Tomco, D.; Staples, R.J.; Lizarazo-Jaimes, E.H.; Gomes, D.A.; Bucciarelli-Rodriguez, M.; Guerra, W.; de Souza, Í.P.; Verani, C.N.; Pereira Maia, E.C. Dual Anticancer and Antibacterial Activities of Bismuth Compounds Based on Asymmetric [NN’O] Ligands. J. Inorg. Biochem. 2021, 222, 111522. [Google Scholar] [CrossRef]
- Tiekink, E.R.T. Antimony and Bismuth Compounds in Oncology. Crit. Rev. Oncol. Hematol. 2002, 42, 217–224. [Google Scholar] [CrossRef]
- Li, Y.-K.; Yang, M.; Li, M.-X.; Yu, H.; Wu, H.-C.; Xie, S.-Q. Synthesis, Crystal Structure and Biological Evaluation of a Main Group Seven-Coordinated Bismuth(III) Complex with 2-Acetylpyridine N4-Phenylthiosemicarbazone. Bioorg. Med. Chem. Lett. 2013, 23, 2288–2292. [Google Scholar] [CrossRef]
- Lin, C.Y.; Shen, Y.H.; Wu, S.H.; Lin, C.H.; Hwang, S.M.; Tsai, Y.C. Effect of Bismuth Subgallate on Nitric Oxide and Prostaglandin E2 Production by Macrophages. Biochem. Biophys. Res. Commun. 2004, 315, 830–835. [Google Scholar] [CrossRef]
- Keogan, D.; Griffith, D. Current and Potential Applications of Bismuth-Based Drugs. Molecules 2014, 19, 15258–15297. [Google Scholar] [CrossRef] [PubMed]
- Schwing, A.; Pomares, C.; Majoor, A.; Boyer, L.; Marty, P.; Michel, G. Leishmania Infection: Misdiagnosis as Cancer and Tumor-Promoting Potential. Acta Trop. 2019, 197, 104855. [Google Scholar] [CrossRef] [PubMed]
- Kevric, I.; Cappel, M.A.; Keeling, J.H. New World and Old World Leishmania Infections: A Practical Review. Dermatol. Clin. 2015, 33, 579–593. [Google Scholar] [CrossRef] [PubMed]
- Perez-Franco, J.E.; Cruz-Barrera, M.L.; Robayo, M.L.; Lopez, M.C.; Daza, C.D.; Bedoya, A.; Mariño, M.L.; Saavedra, C.H.; Echeverry, M.C. Clinical and Parasitological Features of Patients with American Cutaneous Leishmaniasis That Did Not Respond to Treatment with Meglumine Antimoniate. PLoS Negl. Trop. Dis. 2016, 10, e0004739. [Google Scholar] [CrossRef] [PubMed]
- Lockard, R.D.; Wilson, M.E.; Rodríguez, N.E. Sex-Related Differences in Immune Response and Symptomatic Manifestations to Infection with Leishmania Species. J. Immunol. Res. 2019, 2019, 4103819. [Google Scholar] [CrossRef] [PubMed]
- Global Leishmaniasis Surveillance, 2022: Assessing Trends over the Past 10 Years [EN/FR]—World|ReliefWeb. Available online: https://reliefweb.int/report/world/global-leishmaniasis-surveillance-2022-assessing-trends-over-past-10-years-enfr (accessed on 3 November 2023).
- Brahmachari, U. Pentavalent Antimonial. Therapeutic Efficacy in Kala-Azar. Indian J. Med. Res. 1922, 10, 492. [Google Scholar]
- Ong, Y.C.; Kedzierski, L.; Andrews, P.C. Do Bismuth Complexes Hold Promise as Antileishmanial Drugs? Future Med. Chem. 2018, 10, 1721–1733. [Google Scholar] [CrossRef] [PubMed]
- Ministério da Saude. Manual de Vigilância Da Lieshmaniose Tegumentar; Ministério da Saude: Brasília, Brazil, 2017; ISBN 978-85-334-2474-6. [Google Scholar]
- Franco, A.M.R.; Grafova, I.; Soares, F.V.; Gentile, G.; Wyrepkowski, C.D.C.; Bolson, M.A.; Sargentini, E., Jr.; Carfagna, C.; Leskelä, M.; Grafov, A. Nanoscaled Hydrated Antimony (V) Oxide as a New Approach to First-Line Antileishmanial Drugs. Int. J. Nanomed. 2016, 11, 6771–6780. [Google Scholar] [CrossRef] [PubMed]
- Macedo Bastos, M.; Villas Bôas Hoelz, L.; Boechat, N.; de Oliveira, A.P. Antileishmanial Chemotherapy: A Literature Review. Rev. Virtual Química 2016, 8, 2072–2104. [Google Scholar] [CrossRef]
- Andrews, P.C.; Frank, R.; Junk, P.C.; Kedzierski, L.; Kumar, I.; MacLellan, J.G. Anti-Leishmanial Activity of Homo- and Heteroleptic Bismuth(III) Carboxylates. J. Inorg. Biochem. 2011, 105, 454–461. [Google Scholar] [CrossRef]
- Sampaio, M.M.; Santos, M.L.C.; Marques, H.S.; de Souza Gonçalves, V.L.; Araújo, G.R.L.; Lopes, L.W.; Apolonio, J.S.; Silva, C.S.; de Sá Santos, L.K.; Cuzzuol, B.R.; et al. Chronic Myeloid Leukemia-from the Philadelphia Chromosome to Specific Target Drugs: A Literature Review. World J. Clin. Oncol. 2021, 12, 69–94. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Estimativa 2023: Incidência de Câncer No Brasil. Available online: https://www.inca.gov.br/publicacoes/livros/estimativa-2023-incidencia-de-cancer-no-brasil (accessed on 21 September 2023).
- Schmiegelow, K.; Müller, K.; Mogensen, S.S.; Mogensen, P.R.; Wolthers, B.O.; Stoltze, U.K.; Tuckuviene, R.; Frandsen, T. Non-Infectious Chemotherapy-Associated Acute Toxicities during Childhood Acute Lymphoblastic Leukemia Therapy. F1000Research 2017, 6, 444. [Google Scholar] [CrossRef] [PubMed]
- Lejman, M.; Kuśmierczuk, K.; Bednarz, K.; Ostapińska, K.; Zawitkowska, J. Targeted Therapy in the Treatment of Pediatric Acute Lymphoblastic Leukemia—Therapy and Toxicity Mechanisms. Int. J. Mol. Sci. 2021, 22, 9827. [Google Scholar] [CrossRef] [PubMed]
- Bin Emran, T.; Shahriar, A.; Mahmud, A.R.; Rahman, T.; Abir, M.H.; Siddiquee, M.F.-R.; Ahmed, H.; Rahman, N.; Nainu, F.; Wahyudin, E.; et al. Multidrug Resistance in Cancer: Understanding Molecular Mechanisms, Immunoprevention and Therapeutic Approaches. Front. Oncol. 2022, 12, 891652. [Google Scholar] [CrossRef] [PubMed]
- Chan, P.F.; Ang, K.P.; Hamid, R.A. A Bismuth Diethyldithiocarbamate Compound Induced Apoptosis via Mitochondria-Dependent Pathway and Suppressed Invasion in MCF-7 Breast Cancer Cells. BioMetals 2021, 34, 365–391. [Google Scholar] [CrossRef]
- McNeil, S.E. Nanoparticle Therapeutics: A Personal Perspective. WIREs Nanomed. Nanobiotechnol. 2009, 1, 264–271. [Google Scholar] [CrossRef] [PubMed]
- Soni, G.; Yadav, K.S. Applications of Nanoparticles in Treatment and Diagnosis of Leukemia. Mater. Sci. Eng. C 2015, 47, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Herrero, E.; Fernández-Medarde, A. Advanced Targeted Therapies in Cancer: Drug Nanocarriers, the Future of Chemotherapy. Eur. J. Pharm. Biopharm. 2015, 93, 52–79. [Google Scholar] [CrossRef]
- Cabral-Romero, C.; Solís-Soto, J.M.; Sánchez-Pérez, Y.; Pineda-Aguilar, N.; Meester, I.; Pérez-Carrillo, E.; Nakagoshi-Cepeda, S.E.; Sánchez-Nájera, R.I.; Nakagoshi-Cepeda, M.A.A.; Hernandez-Delgadillo, R.; et al. Antitumor Activity of a Hydrogel Loaded with Lipophilic Bismuth Nanoparticles on Cervical, Prostate, and Colon Human Cancer Cells. Anticancer Drugs 2020, 31, 251–259. [Google Scholar] [CrossRef]
- Grafov, A.; Grafova, I.; Pereira, A.M.R.F.; Leskelä, M. Process of Preparation of Nanohybrid Material, Pharmaceutical Composition and Use of the Same. Patent BR 10 2013 029618, 18 November 2013. [Google Scholar]
- Domingos, P.R.C.; Pereira, A.M.R.F.; Grafova, I.; Grafov, A. Bioactive Inorganic Nanomatrices Based on Hydrated Polyvalent Transition Metal Oxides: Preparation Process and Applications. Patent BR 10 2019 005007 1, 14 March 2019. [Google Scholar]
- Petiti, J.; Revel, L.; Divieto, C. Standard Operating Procedure to Optimize Resazurin-Based Viability Assays. Biosensors 2024, 14, 156. [Google Scholar] [CrossRef]
- do Nascimento, N.R.F.; de Aguiar, F.L.N.; Santos, C.F.; Costa, A.M.L.; de Jesus Hardoim, D.; da Silva Calabrese, K.; Almeida-Souza, F.; de Sousa, E.H.S.; de França Lopes, L.G.; Teixeira, M.J.; et al. In Vitro and in Vivo Leishmanicidal Activity of a Ruthenium Nitrosyl Complex against Leishmania (Viannia) Braziliensis. Acta Trop. 2019, 192, 61–65. [Google Scholar] [CrossRef] [PubMed]
- Guzmán, C.; Bagga, M.; Kaur, A.; Westermarck, J.; Abankwa, D. ColonyArea: An ImageJ Plugin to Automatically Quantify Colony Formation in Clonogenic Assays. PLoS ONE 2014, 9, e92444. [Google Scholar] [CrossRef]
- Biltz, W. Ueber Colloidale Hydroxyde. Berichte der Dtsch. Chem. Ges. 1902, 35, 4431–4438. [Google Scholar] [CrossRef]
- Paal, C.; di Pol, L. Über Kolloides Wismuthydroxyd. Berichte der Dtsch. Chem. Ges. A B Ser. 1926, 59, 874–877. [Google Scholar] [CrossRef]
- Siddiqui, I.R.; Mahdihassan, S. Preparation of Colloidal Bismuth Hydroxide. Pak. J. Sci. Ind. Res. 1964, 7, 75–76. [Google Scholar]
- Pathak, A.; Blair, V.L.; Ferrero, R.L.; Kedzierski, L.; Andrews, P.C. Structural Influences on the Activity of Bismuth(III) Indole-Carboxylato Complexes towards Helicobacter pylori and Leishmania. J. Inorg. Biochem. 2017, 177, 266–275. [Google Scholar] [CrossRef] [PubMed]
- Andleeb, S.; Imtiaz-ud-Din; Rauf, M.K.; Azam, S.S.; Haq, I.; Tahir, M.N.; Ahmad, S. Bioactive Heteroleptic Bismuth(V) Complexes: Synthesis, Structural Analysis and Binding Pattern Validation. Appl. Organomet. Chem. 2019, 33, e5061. [Google Scholar] [CrossRef]
- da Luz, J.Z.; Machado, T.N.; Bezerra, A.G.; de Oliveira Ribeiro, C.A.; Neto, F.F. Cytotoxicity of Bismuth Nanoparticles in the Murine Macrophage Cell Line RAW 264.7. J. Mater. Sci. Mater. Med. 2020, 31, 95. [Google Scholar] [CrossRef]
- Islam, A.; Da Silva, J.; Berbet, F.; da Silva, S.; Rodrigues, B.; Beraldo, H.; Melo, M.; Frézard, F.; Demicheli, C. Novel Triphenylantimony(V) and Triphenylbismuth(V) Complexes with Benzoic Acid Derivatives: Structural Characterization, in Vitro Antileishmanial and Antibacterial Activities and Cytotoxicity against Macrophages. Molecules 2014, 19, 6009–6030. [Google Scholar] [CrossRef]
- Lizarazo-Jaimes, E.; Monte-Neto, R.; Reis, P.; Fernandes, N.; Speziali, N.; Melo, M.; Frézard, F.; Demicheli, C. Improved Antileishmanial Activity of Dppz through Complexation with Antimony(III) and Bismuth(III): Investigation of the Role of the Metal. Molecules 2012, 17, 12622–12635. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhang, L.; Chen, X.; Li, S.; Han, Q.; Li, L.; Wang, C. Biomolecules-Assisted Synthesis of Degradable Bismuth Nanoparticles for Dual-Modal Imaging-Guided Chemo-Photothermal Therapy. Chem. Eng. J. 2020, 382, 122720. [Google Scholar] [CrossRef]
- Hernandez-Delgadillo, R.; García-Cuellar, C.M.; Sánchez-Pérez, Y.; Pineda-Aguilar, N.; Martínez-Martínez, M.A.; Rangel-Padilla, E.E.; Nakagoshi-Cepeda, S.E.; Solís-Soto, J.M.; Sánchez-Nájera, R.I.; Nakagoshi-Cepeda, M.A.A.; et al. In Vitro Evaluation of the Antitumor Effect of Bismuth Lipophilic Nanoparticles (BisBAL NPs) on Breast Cancer Cells. Int. J. Nanomed. 2018, 13, 6089–6097. [Google Scholar] [CrossRef] [PubMed]
- Ahamed, M.; Akhtar, M.J.; Khan, M.A.M.; Alaizeri, Z.M.; Alhadlaq, H. Facile Synthesis of Zn-Doped Bi2O3 Nanoparticles and Their Selective Cytotoxicity toward Cancer Cells. ACS Omega 2021, 6, 17353–17361. [Google Scholar] [CrossRef] [PubMed]
- Shahbazi, M.-A.; Faghfouri, L.; Ferreira, M.P.A.; Figueiredo, P.; Maleki, H.; Sefat, F.; Hirvonen, J.; Santos, H.A. The Versatile Biomedical Applications of Bismuth-Based Nanoparticles and Composites: Therapeutic, Diagnostic, Biosensing, and Regenerative Properties. Chem. Soc. Rev. 2020, 49, 1253–1321. [Google Scholar] [CrossRef] [PubMed]
- Passemard, S.; Staedler, D.; Sonego, G.; Magouroux, T.; Schneiter, G.S.; Juillerat-Jeanneret, L.; Bonacina, L.; Gerber-Lemaire, S. Functionalized Bismuth Ferrite Harmonic Nanoparticles for Cancer Cells Labeling and Imaging. J. Nanopart. Res. 2015, 17, 414. [Google Scholar] [CrossRef]
- Huang, R.; Wallqvist, A.; Covell, D.G. Anticancer Metal Compounds in NCI’s Tumor-Screening Database: Putative Mode of Action. Biochem. Pharmacol. 2005, 69, 1009–1039. [Google Scholar] [CrossRef]
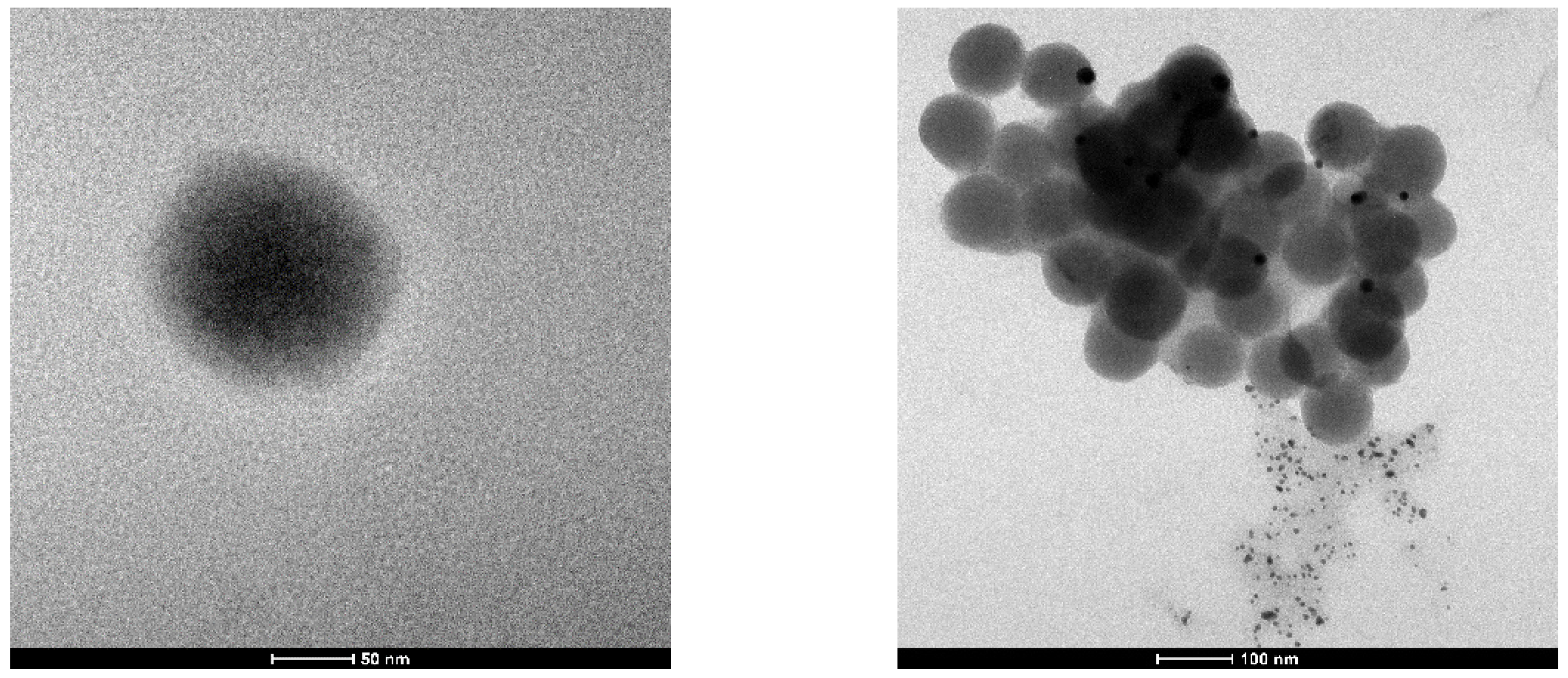


| Stabilizing Ligand | [Bi3+] Concentration, mM | Particle Size, nm | ζ-Potential, mV |
|---|---|---|---|
| MES | 25.93 ± 0.22 | 70 ± 23 | −38.1 ± 5.53 |
| MPS | 20.31 ± 0.41 | 54 ± 11 | −35.1 ± 4.28 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grafov, A.; da Silva Chagas, A.F.; de Freitas Gomes, A.; Ouedrhiri, W.; Cerruti, P.; Del Barone, M.C.; de Souza Mota, B.; de Castro Alves, C.E.; Brasil, A.M.V.; Pereira, A.M.R.F.; et al. A Second Wind for Inorganic APIs: Leishmanicidal and Antileukemic Activity of Hydrated Bismuth Oxide Nanoparticles. Pharmaceutics 2024, 16, 874. https://doi.org/10.3390/pharmaceutics16070874
Grafov A, da Silva Chagas AF, de Freitas Gomes A, Ouedrhiri W, Cerruti P, Del Barone MC, de Souza Mota B, de Castro Alves CE, Brasil AMV, Pereira AMRF, et al. A Second Wind for Inorganic APIs: Leishmanicidal and Antileukemic Activity of Hydrated Bismuth Oxide Nanoparticles. Pharmaceutics. 2024; 16(7):874. https://doi.org/10.3390/pharmaceutics16070874
Chicago/Turabian StyleGrafov, Andriy, Ana Flávia da Silva Chagas, Alice de Freitas Gomes, Wessal Ouedrhiri, Pierfrancesco Cerruti, Maria Cristina Del Barone, Breno de Souza Mota, Carlos Eduardo de Castro Alves, Anny Maíza Vargas Brasil, Antonia Maria Ramos Franco Pereira, and et al. 2024. "A Second Wind for Inorganic APIs: Leishmanicidal and Antileukemic Activity of Hydrated Bismuth Oxide Nanoparticles" Pharmaceutics 16, no. 7: 874. https://doi.org/10.3390/pharmaceutics16070874
APA StyleGrafov, A., da Silva Chagas, A. F., de Freitas Gomes, A., Ouedrhiri, W., Cerruti, P., Del Barone, M. C., de Souza Mota, B., de Castro Alves, C. E., Brasil, A. M. V., Pereira, A. M. R. F., & Soares Pontes, G. (2024). A Second Wind for Inorganic APIs: Leishmanicidal and Antileukemic Activity of Hydrated Bismuth Oxide Nanoparticles. Pharmaceutics, 16(7), 874. https://doi.org/10.3390/pharmaceutics16070874







