From Tyrosine Kinases to Tyrosine Phosphatases: New Therapeutic Targets in Cancers and Beyond
Abstract
1. Introduction
2. Status of TK Inhibitors
2.1. Advantages of TKIs
2.2. Challenges for TKIs
2.2.1. Drug Resistance
2.2.2. Toxicity and Adverse Reactions
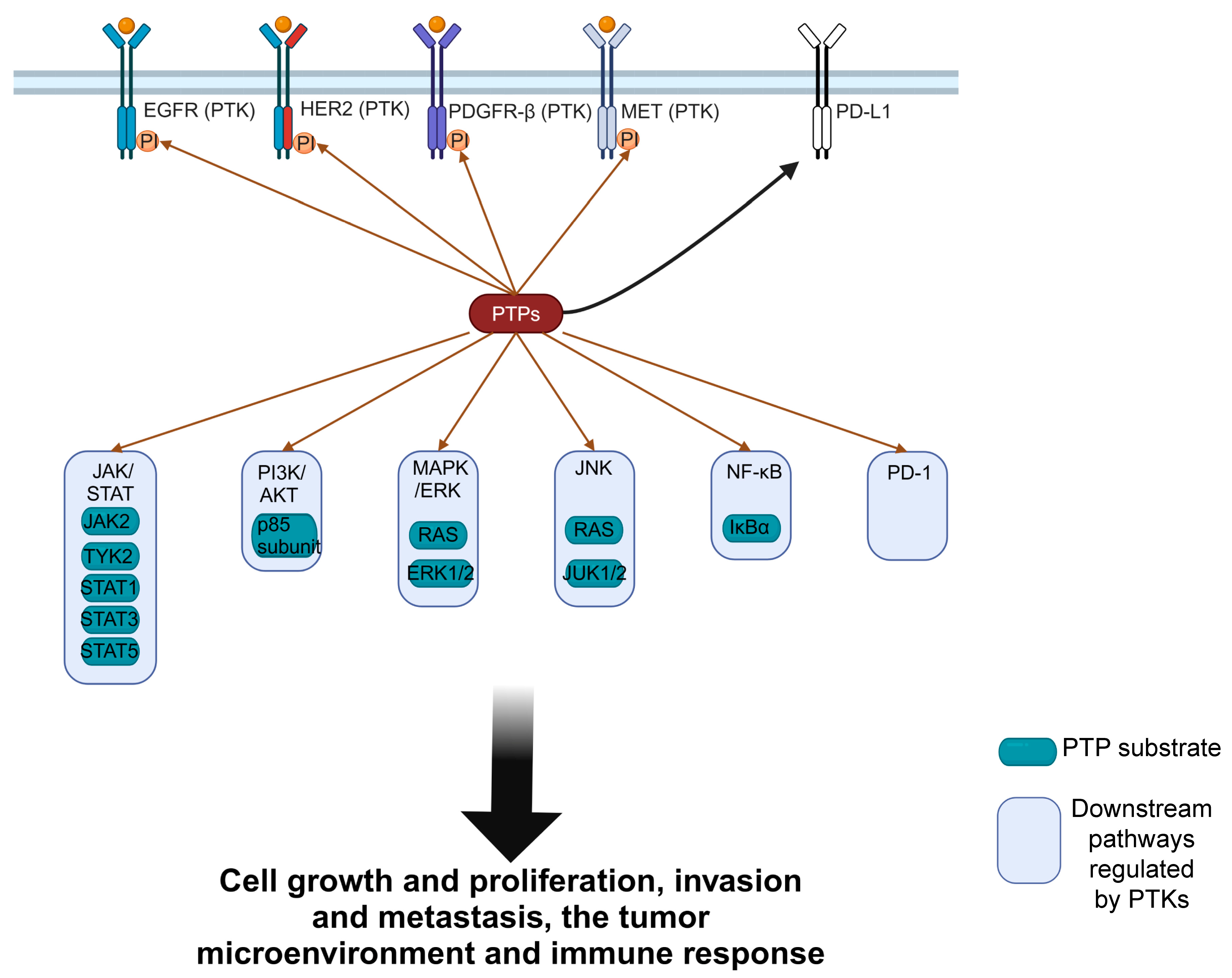
3. The Importance of PTPs in Various Diseases
3.1. PTPs in Cancers
3.1.1. Tumor-Suppressive PTPs
3.1.2. Oncogenic PTPs
3.2. PTPs in the Nervous System
3.3. PTPs in Cardiovascular and Metabolic Diseases
4. The Potential of PTPs as Drug Targets
4.1. The Challenges and Emerging Opportunities for PTPs in Cancer Therapy Development
4.2. PTP-Targeted Therapy to Overcome TKI Resistance
4.3. Potential Advantages of PTP Inhibitors in Reducing Toxicity
5. Exploration of PTPs as Drug Targets
6. Navigating the Challenges in PTP-Targeted Drug Development
6.1. Challenges for Small-Molecule Drugs
6.2. Delivery Challenges for Antibody and Nucleic Acid Drugs
6.3. Other Challenges
6.4. Integration and Innovation in Drug Development
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
Abbreviations
References
- Stanford, S.M.; Mustelin, T.M.; Bottini, N. Lymphoid tyrosine phosphatase and autoimmunity: Human genetics rediscovers tyrosine phosphatases. Semin. Immunopathol. 2010, 32, 127–136. [Google Scholar] [CrossRef] [PubMed]
- Al-Aidaroos, A.Q.; Yuen, H.F.; Guo, K.; Zhang, S.D.; Chung, T.H.; Chng, W.J.; Zeng, Q. Metastasis-associated PRL-3 induces EGFR activation and addiction in cancer cells. J. Clin. Investig. 2013, 123, 3459–3471. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.; Aceto, N.; Meerbrey, K.L.; Kessler, J.D.; Zhou, C.; Migliaccio, I.; Nguyen, D.X.; Pavlova, N.N.; Botero, M.; Huang, J.; et al. Activation of multiple proto-oncogenic tyrosine kinases in breast cancer via loss of the PTPN12 phosphatase. Cell 2011, 144, 703–718. [Google Scholar] [CrossRef] [PubMed]
- Roskoski, R., Jr. Properties of FDA-approved small molecule protein kinase inhibitors: A 2024 update. Pharmacol. Res. 2024, 200, 107059. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, S.; Wang, Y.; Zhao, Y.; Li, Q. Protein tyrosine kinase inhibitor resistance in malignant tumors: Molecular mechanisms and future perspective. Signal Transduct. Target. Ther. 2022, 7, 329. [Google Scholar] [CrossRef] [PubMed]
- Tibau, A.; Molto, C.; Ocana, A.; Templeton, A.J.; Del Carpio, L.P.; Del Paggio, J.C.; Barnadas, A.; Booth, C.M.; Amir, E. Magnitude of Clinical Benefit of Cancer Drugs Approved by the US Food and Drug Administration. J. Natl. Cancer Inst. 2018, 110, 486–492. [Google Scholar] [CrossRef] [PubMed]
- Bollu, L.R.; Mazumdar, A.; Savage, M.I.; Brown, P.H. Molecular Pathways: Targeting Protein Tyrosine Phosphatases in Cancer. Clin. Cancer Res. 2017, 23, 2136–2142. [Google Scholar] [CrossRef] [PubMed]
- Westermarck, J. Targeted therapies don’t work for a reason; the neglected tumor suppressor phosphatase PP2A strikes back. FEBS J. 2018, 285, 4139–4145. [Google Scholar] [CrossRef] [PubMed]
- Gillies, R.J.; Verduzco, D.; Gatenby, R.A. Evolutionary dynamics of carcinogenesis and why targeted therapy does not work. Nat. Rev. Cancer 2012, 12, 487–493. [Google Scholar] [CrossRef]
- Kauko, O.; Imanishi, S.Y.; Kulesskiy, E.; Yetukuri, L.; Laajala, T.D.; Sharma, M.; Pavic, K.; Aakula, A.; Rupp, C.; Jumppanen, M.; et al. Phosphoproteome and drug-response effects mediated by the three protein phosphatase 2A inhibitor proteins CIP2A, SET, and PME-1. J. Biol. Chem. 2020, 295, 4194–4211. [Google Scholar] [CrossRef]
- Meyer, D.S.; Aceto, N.; Sausgruber, N.; Brinkhaus, H.; Muller, U.; Pallen, C.J.; Bentires-Alj, M. Tyrosine phosphatase PTPalpha contributes to HER2-evoked breast tumor initiation and maintenance. Oncogene 2014, 33, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Kirouac, D.C.; Du, J.; Lahdenranta, J.; Onsum, M.D.; Nielsen, U.B.; Schoeberl, B.; McDonagh, C.F. HER2+ Cancer Cell Dependence on PI3K vs. MAPK Signaling Axes Is Determined by Expression of EGFR, ERBB3 and CDKN1B. PLoS Comput. Biol. 2016, 12, e1004827. [Google Scholar] [CrossRef] [PubMed]
- Gan, J.; Ke, X.; Jiang, J.; Dong, H.; Yao, Z.; Lin, Y.; Lin, W.; Wu, X.; Yan, S.; Zhuang, Y.; et al. Growth hormone-releasing hormone receptor antagonists inhibit human gastric cancer through downregulation of PAK1-STAT3/NF-kappaB signaling. Proc. Natl. Acad. Sci. USA 2016, 113, 14745–14750. [Google Scholar] [CrossRef] [PubMed]
- Ooms, L.M.; Binge, L.C.; Davies, E.M.; Rahman, P.; Conway, J.R.; Gurung, R.; Ferguson, D.T.; Papa, A.; Fedele, C.G.; Vieusseux, J.L.; et al. The Inositol Polyphosphate 5-Phosphatase PIPP Regulates AKT1-Dependent Breast Cancer Growth and Metastasis. Cancer Cell 2015, 28, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Chen, Q.; Hu, X.G.; Zhang, X.C.; Fu, T.W.; Liu, Q.; Liang, Y.; Zhao, X.L.; Zhang, X.; Ping, Y.F.; et al. PTP1B promotes aggressiveness of breast cancer cells by regulating PTEN but not EMT. Tumour Biol. 2016, 37, 13479–13487. [Google Scholar] [CrossRef] [PubMed]
- Xu, Q.; Wu, N.; Li, X.; Guo, C.; Li, C.; Jiang, B.; Wang, H.; Shi, D. Inhibition of PTP1B blocks pancreatic cancer progression by targeting the PKM2/AMPK/mTOC1 pathway. Cell Death Dis. 2019, 10, 874. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Qu, L.; Lian, S.; Meng, L.; Min, L.; Liu, J.; Song, Q.; Shen, L.; Shou, C. PRL-3 Promotes Ubiquitination and Degradation of AURKA and Colorectal Cancer Progression via Dephosphorylation of FZR1. Cancer Res. 2019, 79, 928–940. [Google Scholar] [CrossRef] [PubMed]
- Yao, Z.; Dong, H.; Zhu, J.; Du, L.; Luo, Y.; Liu, Q.; Liu, S.; Lin, Y.; Wang, L.; Wang, S.; et al. Age-related decline in hippocampal tyrosine phosphatase PTPRO is a mechanistic factor in chemotherapy-related cognitive impairment. JCI Insight 2023, 8, 166306. [Google Scholar] [CrossRef]
- Huang, T.; Li, X.; Wang, F.; Lu, L.; Hou, W.; Zhu, M.; Miao, C. The CREB/KMT5A complex regulates PTP1B to modulate high glucose-induced endothelial inflammatory factor levels in diabetic nephropathy. Cell Death Dis. 2021, 12, 333. [Google Scholar] [CrossRef]
- Marycz, K.; Bourebaba, N.; Serwotka-Suszczak, A.; Mularczyk, M.; Galuppo, L.; Bourebaba, L. In Vitro Generated Equine Hepatic-Like Progenitor Cells as a Novel Potent Cell Pool for Equine Metabolic Syndrome (EMS) Treatment. Stem Cell Rev. Rep. 2023, 19, 1124–1134. [Google Scholar] [CrossRef]
- Bourebaba, L.; Serwotka-Suszczak, A.; Pielok, A.; Sikora, M.; Mularczyk, M.; Marycz, K. The PTP1B inhibitor MSI-1436 ameliorates liver insulin sensitivity by modulating autophagy, ER stress and systemic inflammation in Equine metabolic syndrome affected horses. Front. Endocrinol. 2023, 14, 1149610. [Google Scholar] [CrossRef]
- Bourebaba, L.; Serwotka-Suszczak, A.; Bourebaba, N.; Zyzak, M.; Marycz, K. The PTP1B Inhibitor Trodusquemine (MSI-1436) Improves Glucose Uptake in Equine Metabolic Syndrome Affected Liver through Anti-Inflammatory and Antifibrotic Activity. Int. J. Inflam. 2023, 2023, 3803056. [Google Scholar] [CrossRef]
- Karaca Atabay, E.; Mecca, C.; Wang, Q.; Ambrogio, C.; Mota, I.; Prokoph, N.; Mura, G.; Martinengo, C.; Patrucco, E.; Leonardi, G.; et al. Tyrosine phosphatases regulate resistance to ALK inhibitors in ALK+ anaplastic large cell lymphoma. Blood 2022, 139, 717–731. [Google Scholar] [CrossRef] [PubMed]
- Balko, J.M.; Schwarz, L.J.; Bhola, N.E.; Kurupi, R.; Owens, P.; Miller, T.W.; Gomez, H.; Cook, R.S.; Arteaga, C.L. Activation of MAPK pathways due to DUSP4 loss promotes cancer stem cell-like phenotypes in basal-like breast cancer. Cancer Res. 2013, 73, 6346–6358. [Google Scholar] [CrossRef]
- Dong, H.; Xie, C.; Yao, Z.; Zhao, R.; Lin, Y.; Luo, Y.; Chen, S.; Qin, Y.; Chen, Y.; Zhang, H. PTPRO-related CD8(+) T-cell signatures predict prognosis and immunotherapy response in patients with breast cancer. Front. Immunol. 2022, 13, 947841. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Du, L.; Cai, S.; Lin, W.; Chen, C.; Still, M.; Yao, Z.; Coppes, R.P.; Pan, Y.; Zhang, D.; et al. Tyrosine Phosphatase PTPRO Deficiency in ERBB2-Positive Breast Cancer Contributes to Poor Prognosis and Lapatinib Resistance. Front. Pharmacol. 2022, 13, 838171. [Google Scholar] [CrossRef]
- Stanford, S.M.; Bottini, N. Targeting Tyrosine Phosphatases: Time to End the Stigma. Trends Pharmacol. Sci. 2017, 38, 524–540. [Google Scholar] [CrossRef]
- He, R.J.; Yu, Z.H.; Zhang, R.Y.; Zhang, Z.Y. Protein tyrosine phosphatases as potential therapeutic targets. Acta Pharmacol. Sin. 2014, 35, 1227–1246. [Google Scholar] [CrossRef]
- Fan, L.C.; Teng, H.W.; Shiau, C.W.; Lin, H.; Hung, M.H.; Chen, Y.L.; Huang, J.W.; Tai, W.T.; Yu, H.C.; Chen, K.F. SHP-1 is a target of regorafenib in colorectal cancer. Oncotarget 2014, 5, 6243–6251. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.N.; LaMarche, M.J.; Chan, H.M.; Fekkes, P.; Garcia-Fortanet, J.; Acker, M.G.; Antonakos, B.; Chen, C.H.; Chen, Z.; Cooke, V.G.; et al. Allosteric inhibition of SHP2 phosphatase inhibits cancers driven by receptor tyrosine kinases. Nature 2016, 535, 148–152. [Google Scholar] [CrossRef]
- Liu, W.; Yu, B.; Xu, G.; Xu, W.R.; Loh, M.L.; Tang, L.D.; Qu, C.K. Identification of cryptotanshinone as an inhibitor of oncogenic protein tyrosine phosphatase SHP2 (PTPN11). J. Med. Chem. 2013, 56, 7212–7221. [Google Scholar] [CrossRef] [PubMed]
- Krishnan, N.; Koveal, D.; Miller, D.H.; Xue, B.; Akshinthala, S.D.; Kragelj, J.; Jensen, M.R.; Gauss, C.M.; Page, R.; Blackledge, M.; et al. Targeting the disordered C terminus of PTP1B with an allosteric inhibitor. Nat. Chem. Biol. 2014, 10, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Sarver, P.; Acker, M.; Bagdanoff, J.T.; Chen, Z.; Chen, Y.N.; Chan, H.; Firestone, B.; Fodor, M.; Fortanet, J.; Hao, H.; et al. 6-Amino-3-methylpyrimidinones as Potent, Selective, and Orally Efficacious SHP2 Inhibitors. J. Med. Chem. 2019, 62, 1793–1802. [Google Scholar] [CrossRef] [PubMed]
- Elhassan, R.M.; Hou, X.; Fang, H. Recent advances in the development of allosteric protein tyrosine phosphatase inhibitors for drug discovery. Med. Res. Rev. 2022, 42, 1064–1110. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Guo, W.; Wu, Y.; Yang, C.; Zhong, L.; Deng, G.; Zhu, Y.; Liu, W.; Gu, Y.; Lu, Y.; et al. SHP2 inhibition triggers anti-tumor immunity and synergizes with PD-1 blockade. Acta Pharm. Sin. B 2019, 9, 304–315. [Google Scholar] [CrossRef] [PubMed]
- Baghbani, E.; Baradaran, B.; Pak, F.; Mohammadnejad, L.; Shanehbandi, D.; Mansoori, B.; Khaze, V.; Montazami, N.; Mohammadi, A.; Kokhaei, P. Suppression of protein tyrosine phosphatase PTPN22 gene induces apoptosis in T-cell leukemia cell line (Jurkat) through the AKT and ERK pathways. Biomed. Pharmacother. 2017, 86, 41–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wu, H.; Yi, B.; Zhou, J.; Wei, L.; Chen, Y.; Zhang, L. RING finger protein 38 induces gastric cancer cell growth by decreasing the stability of the protein tyrosine phosphatase SHP-1. FEBS Lett. 2018, 592, 3092–3100. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Ji, Q.; Ji, J.; Ju, S.; Xu, C.; Yong, X.; Xu, X.; Muddassir, M.; Chen, X.; Xie, J.; et al. Co-delivery of siPTPN13 and siNOX4 via (myo)fibroblast-targeting polymeric micelles for idiopathic pulmonary fibrosis therapy. Theranostics 2021, 11, 3244–3261. [Google Scholar] [CrossRef]
- Miao, J.; Dong, J.; Miao, Y.; Bai, Y.; Qu, Z.; Jassim, B.A.; Huang, B.; Nguyen, Q.; Ma, Y.; Murray, A.A.; et al. Discovery of a selective TC-PTP degrader for cancer immunotherapy. Chem. Sci. 2023, 14, 12606–12614. [Google Scholar] [CrossRef]
- Zheng, L.; Wang, L.; Gan, J.; Zhang, H. RNA activation: Promise as a new weapon against cancer. Cancer Lett. 2014, 355, 18–24. [Google Scholar] [CrossRef]
- Cheung, L.W.; Walkiewicz, K.W.; Besong, T.M.; Guo, H.; Hawke, D.H.; Arold, S.T.; Mills, G.B. Regulation of the PI3K pathway through a p85alpha monomer-homodimer equilibrium. eLife 2015, 4, e06866. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, S.R.; Till, J.H. Protein tyrosine kinase structure and function. Annu. Rev. Biochem. 2000, 69, 373–398. [Google Scholar] [CrossRef] [PubMed]
- Siveen, K.S.; Prabhu, K.S.; Achkar, I.W.; Kuttikrishnan, S.; Shyam, S.; Khan, A.Q.; Merhi, M.; Dermime, S.; Uddin, S. Role of Non Receptor Tyrosine Kinases in Hematological Malignances and its Targeting by Natural Products. Mol. Cancer 2018, 17, 31. [Google Scholar] [CrossRef] [PubMed]
- Du, Z.; Lovly, C.M. Mechanisms of receptor tyrosine kinase activation in cancer. Mol. Cancer 2018, 17, 58. [Google Scholar] [CrossRef] [PubMed]
- Castelo-Soccio, L.; Kim, H.; Gadina, M.; Schwartzberg, P.L.; Laurence, A.; O’Shea, J.J. Protein kinases: Drug targets for immunological disorders. Nat. Rev. Immunol. 2023, 23, 787–806. [Google Scholar] [CrossRef] [PubMed]
- Novoplansky, O.; Shnerb, A.B.; Marripati, D.; Jagadeeshan, S.; Abu Shareb, R.; Conde-Lopez, C.; Zorea, J.; Prasad, M.; Ben Lulu, T.; Yegodayev, K.M.; et al. Activation of the EGFR/PI3K/AKT pathway limits the efficacy of trametinib treatment in head and neck cancer. Mol. Oncol. 2023, 17, 2618–2636. [Google Scholar] [CrossRef]
- Zhou, J.; Du, T.; Li, B.; Rong, Y.; Verkhratsky, A.; Peng, L. Crosstalk Between MAPK/ERK and PI3K/AKT Signal Pathways During Brain Ischemia/Reperfusion. ASN Neuro 2015, 7, 1759091415602463. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.C.; Wu, Y.L.; Schuler, M.; Sebastian, M.; Popat, S.; Yamamoto, N.; Zhou, C.; Hu, C.P.; O’Byrne, K.; Feng, J.; et al. Afatinib versus cisplatin-based chemotherapy for EGFR mutation-positive lung adenocarcinoma (LUX-Lung 3 and LUX-Lung 6): Analysis of overall survival data from two randomised, phase 3 trials. Lancet Oncol. 2015, 16, 141–151. [Google Scholar] [CrossRef]
- Llovet, J.M.; Ricci, S.; Mazzaferro, V.; Hilgard, P.; Gane, E.; Blanc, J.F.; de Oliveira, A.C.; Santoro, A.; Raoul, J.L.; Forner, A.; et al. Sorafenib in advanced hepatocellular carcinoma. N. Engl. J. Med. 2008, 359, 378–390. [Google Scholar] [CrossRef]
- Sarah, A.; Dondi, E.; De Francia, S. Tyrosine kinase inhibitors: The role of pharmacokinetics and pharmacogenetics. Expert. Opin. Drug Metab. Toxicol. 2023, 19, 733–739. [Google Scholar] [CrossRef]
- Wanika, L.; Evans, N.D.; Johnson, M.; Tomkinson, H.; Chappell, M.J. In vitro PK/PD modeling of tyrosine kinase inhibitors in non-small cell lung cancer cell lines. Clin. Transl. Sci. 2024, 17, e13714. [Google Scholar] [CrossRef] [PubMed]
- Meng, A.; Humeniuk, R.; Jurgensmeier, J.M.; Hsueh, C.H.; Matzkies, F.; Grant, E.; Truong, H.; Billin, A.N.; Yu, H.; Feng, J.; et al. Semi-Mechanistic PK/PD Modeling and Simulation of Irreversible BTK Inhibition to Support Dose Selection of Tirabrutinib in Subjects with RA. Clin. Pharmacol. Ther. 2022, 111, 416–424. [Google Scholar] [CrossRef]
- Kelderman, S.; Schumacher, T.N.; Haanen, J.B. Acquired and intrinsic resistance in cancer immunotherapy. Mol. Oncol. 2014, 8, 1132–1139. [Google Scholar] [CrossRef]
- Rosenzweig, S.A. Acquired Resistance to Drugs Targeting Tyrosine Kinases. Adv. Cancer Res. 2018, 138, 71–98. [Google Scholar] [CrossRef]
- Mansoori, B.; Mohammadi, A.; Davudian, S.; Shirjang, S.; Baradaran, B. The Different Mechanisms of Cancer Drug Resistance: A Brief Review. Adv. Pharm. Bull. 2017, 7, 339–348. [Google Scholar] [CrossRef]
- Alexander, P.B.; Wang, X.F. Resistance to receptor tyrosine kinase inhibition in cancer: Molecular mechanisms and therapeutic strategies. Front. Med. 2015, 9, 134–138. [Google Scholar] [CrossRef]
- Maione, P.; Sacco, P.C.; Sgambato, A.; Casaluce, F.; Rossi, A.; Gridelli, C. Overcoming resistance to targeted therapies in NSCLC: Current approaches and clinical application. Ther. Adv. Med. Oncol. 2015, 7, 263–273. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.J.; Bell, D.W.; Sordella, R.; Gurubhagavatula, S.; Okimoto, R.A.; Brannigan, B.W.; Harris, P.L.; Haserlat, S.M.; Supko, J.G.; Haluska, F.G.; et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N. Engl. J. Med. 2004, 350, 2129–2139. [Google Scholar] [CrossRef]
- Salemme, V.; Centonze, G.; Avalle, L.; Natalini, D.; Piccolantonio, A.; Arina, P.; Morellato, A.; Ala, U.; Taverna, D.; Turco, E.; et al. The role of tumor microenvironment in drug resistance: Emerging technologies to unravel breast cancer heterogeneity. Front. Oncol. 2023, 13, 1170264. [Google Scholar] [CrossRef] [PubMed]
- Dai, Z.; Gu, X.Y.; Xiang, S.Y.; Gong, D.D.; Man, C.F.; Fan, Y. Research and application of single-cell sequencing in tumor heterogeneity and drug resistance of circulating tumor cells. Biomark. Res. 2020, 8, 60. [Google Scholar] [CrossRef]
- Bean, J.; Brennan, C.; Shih, J.Y.; Riely, G.; Viale, A.; Wang, L.; Chitale, D.; Motoi, N.; Szoke, J.; Broderick, S.; et al. MET amplification occurs with or without T790M mutations in EGFR mutant lung tumors with acquired resistance to gefitinib or erlotinib. Proc. Natl. Acad. Sci. USA 2007, 104, 20932–20937. [Google Scholar] [CrossRef] [PubMed]
- Engelman, J.A.; Zejnullahu, K.; Mitsudomi, T.; Song, Y.; Hyland, C.; Park, J.O.; Lindeman, N.; Gale, C.M.; Zhao, X.; Christensen, J.; et al. MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 2007, 316, 1039–1043. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.P.; Wellbrock, C. Molecular Pathways: Maintaining MAPK Inhibitor Sensitivity by Targeting Nonmutational Tolerance. Clin. Cancer Res. 2016, 22, 5966–5970. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Mason, S.; Wilson, R.C.; Hazard, S.E.; Wang, Y.; Fang, R.; Wang, Q.; Yeh, E.S.; Yang, M.; Roberts, T.M.; et al. Inhibition of the transcriptional kinase CDK7 overcomes therapeutic resistance in HER2-positive breast cancers. Oncogene 2020, 39, 50–63. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J.; Wang, L.; Zhou, F.M.; Liu, S.W.; Wang, W.; Zhao, E.J.; Yao, Q.J.; Li, W.; Zhao, Y.Q.; Shi, Z.; et al. Elevated NOX4 promotes tumorigenesis and acquired EGFR-TKIs resistance via enhancing IL-8/PD-L1 signaling in NSCLC. Drug Resist. Updat. 2023, 70, 100987. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Liu, D.; Wang, L.; Liu, K.; Chen, C.; Wang, L.; Ren, Y.; Ju, B.; Zhong, F.; Jiang, X.; et al. Methyltransferase like 7B is a potential therapeutic target for reversing EGFR-TKIs resistance in lung adenocarcinoma. Mol. Cancer 2022, 21, 43. [Google Scholar] [CrossRef] [PubMed]
- Lu, X.; Smaill, J.B.; Ding, K. New Promise and Opportunities for Allosteric Kinase Inhibitors. Angew. Chem. Int. Ed. Engl. 2020, 59, 13764–13776. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Kurzrock, R. Toxicity of targeted therapy: Implications for response and impact of genetic polymorphisms. Cancer Treat. Rev. 2014, 40, 883–891. [Google Scholar] [CrossRef] [PubMed]
- Shah, D.R.; Shah, R.R.; Morganroth, J. Tyrosine kinase inhibitors: Their on-target toxicities as potential indicators of efficacy. Drug Saf. 2013, 36, 413–426. [Google Scholar] [CrossRef]
- Chen, Z.I.; Ai, D.I. Cardiotoxicity associated with targeted cancer therapies. Mol. Clin. Oncol. 2016, 4, 675–681. [Google Scholar] [CrossRef]
- Wang, H.; Sheehan, R.P.; Palmer, A.C.; Everley, R.A.; Boswell, S.A.; Ron-Harel, N.; Ringel, A.E.; Holton, K.M.; Jacobson, C.A.; Erickson, A.R.; et al. Adaptation of Human iPSC-Derived Cardiomyocytes to Tyrosine Kinase Inhibitors Reduces Acute Cardiotoxicity via Metabolic Reprogramming. Cell Syst. 2019, 8, 412–426.e7. [Google Scholar] [CrossRef] [PubMed]
- Celutkiene, J.; Pudil, R.; Lopez-Fernandez, T.; Grapsa, J.; Nihoyannopoulos, P.; Bergler-Klein, J.; Cohen-Solal, A.; Farmakis, D.; Tocchetti, C.G.; von Haehling, S.; et al. Role of cardiovascular imaging in cancer patients receiving cardiotoxic therapies: A position statement on behalf of the Heart Failure Association (HFA), the European Association of Cardiovascular Imaging (EACVI) and the Cardio-Oncology Council of the European Society of Cardiology (ESC). Eur. J. Heart Fail. 2020, 22, 1504–1524. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Lenihan, D.; Fradley, M.; Ganatra, S.; Barac, A.; Blaes, A.; Herrmann, J.; Porter, C.; Lyon, A.R.; Lancellotti, P.; et al. Management of cardiac disease in cancer patients throughout oncological treatment: ESMO consensus recommendations. Ann. Oncol. 2020, 31, 171–190. [Google Scholar] [CrossRef] [PubMed]
- De Leo, A.; Di Simone, E.; Spano, A.; Puliani, G.; Petrone, F. Nursing Management and Adverse Events in Thyroid Cancer Treatments with Tyrosine Kinase Inhibitors. A Narrative Review. Cancers 2021, 13, 5961. [Google Scholar] [CrossRef] [PubMed]
- Jabbour, E.; Deininger, M.; Hochhaus, A. Management of adverse events associated with tyrosine kinase inhibitors in the treatment of chronic myeloid leukemia. Leukemia 2011, 25, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.P.; Li, J.; Kros, J.M. Breakthroughs in modern cancer therapy and elusive cardiotoxicity: Critical research-practice gaps, challenges, and insights. Med. Res. Rev. 2018, 38, 325–376. [Google Scholar] [CrossRef]
- Wei, Q.; Li, P.; Yang, T.; Zhu, J.; Sun, L.; Zhang, Z.; Wang, L.; Tian, X.; Chen, J.; Hu, C.; et al. The promise and challenges of combination therapies with antibody-drug conjugates in solid tumors. J. Hematol. Oncol. 2024, 17, 1. [Google Scholar] [CrossRef] [PubMed]
- Martin, M.; Fumoleau, P.; Dewar, J.A.; Albanell, J.; Limentani, S.A.; Campone, M.; Chang, J.C.; Patre, M.; Strasak, A.; de Haas, S.L.; et al. Trastuzumab emtansine (T-DM1) plus docetaxel with or without pertuzumab in patients with HER2-positive locally advanced or metastatic breast cancer: Results from a phase Ib/IIa study. Ann. Oncol. 2016, 27, 1249–1256. [Google Scholar] [CrossRef] [PubMed]
- Luongo, F.; Colonna, F.; Calapa, F.; Vitale, S.; Fiori, M.E.; De Maria, R. PTEN Tumor-Suppressor: The Dam of Stemness in Cancer. Cancers 2019, 11, 1076. [Google Scholar] [CrossRef]
- Haddadi, N.; Lin, Y.; Travis, G.; Simpson, A.M.; Nassif, N.T.; McGowan, E.M. PTEN/PTENP1: ‘Regulating the regulator of RTK-dependent PI3K/Akt signalling’, new targets for cancer therapy. Mol. Cancer 2018, 17, 37. [Google Scholar] [CrossRef]
- Lee, C.; Rhee, I. Important roles of protein tyrosine phosphatase PTPN12 in tumor progression. Pharmacol. Res. 2019, 144, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Morales, J.E.; Guerrero, P.A.; Sun, H.; McCarty, J.H. PTPN12/PTP-PEST Regulates Phosphorylation-Dependent Ubiquitination and Stability of Focal Adhesion Substrates in Invasive Glioblastoma Cells. Cancer Res. 2018, 78, 3809–3822. [Google Scholar] [CrossRef] [PubMed]
- Behjati, S.; Tarpey, P.S.; Sheldon, H.; Martincorena, I.; Van Loo, P.; Gundem, G.; Wedge, D.C.; Ramakrishna, M.; Cooke, S.L.; Pillay, N.; et al. Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat. Genet. 2014, 46, 376–379. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.; Majumdar, A.; Li, X.; Adler, J.; Sun, Z.; Vertuani, S.; Hellberg, C.; Mellberg, S.; Koch, S.; Dimberg, A.; et al. VE-PTP regulates VEGFR2 activity in stalk cells to establish endothelial cell polarity and lumen formation. Nat. Commun. 2013, 4, 1672. [Google Scholar] [CrossRef]
- Dong, H.; Lin, W.; Du, L.; Yao, Z.; Li, F.; Chen, S.; Huang, Y.; Ren, H.; Luo, Y.; Cai, S.; et al. PTPRO suppresses lymph node metastasis of esophageal carcinoma by dephosphorylating MET. Cancer Lett. 2023, 567, 216283. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Ma, L.; Gan, J.; Lin, W.; Chen, C.; Yao, Z.; Du, L.; Zheng, L.; Ke, C.; Huang, X.; et al. PTPRO represses ERBB2-driven breast oncogenesis by dephosphorylation and endosomal internalization of ERBB2. Oncogene 2017, 36, 410–422. [Google Scholar] [CrossRef] [PubMed]
- You, Y.J.; Chen, Y.P.; Zheng, X.X.; Meltzer, S.J.; Zhang, H. Aberrant methylation of the PTPRO gene in peripheral blood as a potential biomarker in esophageal squamous cell carcinoma patients. Cancer Lett. 2012, 315, 138–144. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Asbagh, L.A.; Vazquez, I.; Vecchione, L.; Budinska, E.; De Vriendt, V.; Baietti, M.F.; Steklov, M.; Jacobs, B.; Hoe, N.; Singh, S.; et al. The tyrosine phosphatase PTPRO sensitizes colon cancer cells to anti-EGFR therapy through activation of SRC-mediated EGFR signaling. Oncotarget 2014, 5, 10070–10083. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Li, R.; Chen, Y.L.; Xiong, L.K.; Wang, H.L.; Rong, L.; Luo, R.C. Aberrant PTPRO methylation in tumor tissues as a potential biomarker that predicts clinical outcomes in breast cancer patients. BMC Genet. 2014, 15, 67. [Google Scholar] [CrossRef]
- Huang, Y.T.; Li, F.F.; Ke, C.; Li, Z.; Li, Z.T.; Zou, X.F.; Zheng, X.X.; Chen, Y.P.; Zhang, H. PTPRO promoter methylation is predictive of poorer outcome for HER2-positive breast cancer: Indication for personalized therapy. J. Transl. Med. 2013, 11, 245. [Google Scholar] [CrossRef]
- Ramaswamy, B.; Majumder, S.; Roy, S.; Ghoshal, K.; Kutay, H.; Datta, J.; Younes, M.; Shapiro, C.L.; Motiwala, T.; Jacob, S.T. Estrogen-mediated suppression of the gene encoding protein tyrosine phosphatase PTPRO in human breast cancer: Mechanism and role in tamoxifen sensitivity. Mol. Endocrinol. 2009, 23, 176–187. [Google Scholar] [CrossRef]
- Motiwala, T.; Majumder, S.; Kutay, H.; Smith, D.S.; Neuberg, D.S.; Lucas, D.M.; Byrd, J.C.; Grever, M.; Jacob, S.T. Methylation and silencing of protein tyrosine phosphatase receptor type O in chronic lymphocytic leukemia. Clin. Cancer Res. 2007, 13, 3174–3181. [Google Scholar] [CrossRef][Green Version]
- Xie, F.; Dong, H.; Zhang, H. Regulatory Functions of Protein Tyrosine Phosphatase Receptor Type O in Immune Cells. Front. Immunol. 2021, 12, 783370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Liu, Y.; Yan, Z.; Yang, H.; Sun, W.; Yao, Y.; Chen, Y.; Jiang, R. IL-6 promotes PD-L1 expression in monocytes and macrophages by decreasing protein tyrosine phosphatase receptor type O expression in human hepatocellular carcinoma. J. Immunother. Cancer 2020, 8, e000285. [Google Scholar] [CrossRef]
- Hou, X.; Du, J.; Fang, H. PTPRO is a therapeutic target and correlated with immune infiltrates in pancreatic cancer. J. Cancer 2021, 12, 7445–7453. [Google Scholar] [CrossRef]
- Chen, L.; Juszczynski, P.; Takeyama, K.; Aguiar, R.C.; Shipp, M.A. Protein tyrosine phosphatase receptor-type O truncated (PTPROt) regulates SYK phosphorylation, proximal B-cell-receptor signaling, and cellular proliferation. Blood 2006, 108, 3428–3433. [Google Scholar] [CrossRef] [PubMed]
- Juszczynski, P.; Chen, L.; O’Donnell, E.; Polo, J.M.; Ranuncolo, S.M.; Dalla-Favera, R.; Melnick, A.; Shipp, M.A. BCL6 modulates tonic BCR signaling in diffuse large B-cell lymphomas by repressing the SYK phosphatase, PTPROt. Blood 2009, 114, 5315–5321. [Google Scholar] [CrossRef]
- Dai, W.; Xiang, W.; Han, L.; Yuan, Z.; Wang, R.; Ma, Y.; Yang, Y.; Cai, S.; Xu, Y.; Mo, S.; et al. PTPRO represses colorectal cancer tumorigenesis and progression by reprogramming fatty acid metabolism. Cancer Commun. 2022, 42, 848–867. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Wang, X.; Yan, S.; Yin, Y.; Hou, J.; Wang, X.; Sun, B. Interaction of PTPRO and TLR4 signaling in hepatocellular carcinoma. Tumour Biol. 2014, 35, 10267–10273. [Google Scholar] [CrossRef]
- Motiwala, T.; Kutay, H.; Zanesi, N.; Frissora, F.W.; Mo, X.; Muthusamy, N.; Jacob, S.T. PTPROt-mediated regulation of p53/Foxm1 suppresses leukemic phenotype in a CLL mouse model. Leukemia 2015, 29, 1350–1359. [Google Scholar] [CrossRef][Green Version]
- Sivaganesh, V.; Sivaganesh, V.; Scanlon, C.; Iskander, A.; Maher, S.; Le, T.; Peethambaran, B. Protein Tyrosine Phosphatases: Mechanisms in Cancer. Int. J. Mol. Sci. 2021, 22, 12865. [Google Scholar] [CrossRef] [PubMed]
- Salmond, R.J. Targeting Protein Tyrosine Phosphatases to Improve Cancer Immunotherapies. Cells 2024, 13, 231. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhang, L.; Bourne, P.A.; Reeder, J.E.; di Sant’Agnese, P.A.; Yao, J.L.; Na, Y.; Huang, J. Protein tyrosine phosphatase PTP1B is involved in neuroendocrine differentiation of prostate cancer. Prostate 2006, 66, 1125–1135. [Google Scholar] [CrossRef] [PubMed]
- LaFleur, M.W.; Nguyen, T.H.; Coxe, M.A.; Miller, B.C.; Yates, K.B.; Gillis, J.E.; Sen, D.R.; Gaudiano, E.F.; Al Abosy, R.; Freeman, G.J.; et al. PTPN2 regulates the generation of exhausted CD8(+) T cell subpopulations and restrains tumor immunity. Nat. Immunol. 2019, 20, 1335–1347. [Google Scholar] [CrossRef] [PubMed]
- Baumgartner, C.K.; Ebrahimi-Nik, H.; Iracheta-Vellve, A.; Hamel, K.M.; Olander, K.E.; Davis, T.G.R.; McGuire, K.A.; Halvorsen, G.T.; Avila, O.I.; Patel, C.H.; et al. The PTPN2/PTPN1 inhibitor ABBV-CLS-484 unleashes potent anti-tumour immunity. Nature 2023, 622, 850–862. [Google Scholar] [CrossRef] [PubMed]
- Wiede, F.; Lu, K.H.; Du, X.; Liang, S.; Hochheiser, K.; Dodd, G.T.; Goh, P.K.; Kearney, C.; Meyran, D.; Beavis, P.A.; et al. PTPN2 phosphatase deletion in T cells promotes anti-tumour immunity and CAR T-cell efficacy in solid tumours. EMBO J. 2020, 39, e103637. [Google Scholar] [CrossRef]
- Wiede, F.; Lu, K.H.; Du, X.; Zeissig, M.N.; Xu, R.; Goh, P.K.; Xirouchaki, C.E.; Hogarth, S.J.; Greatorex, S.; Sek, K.; et al. PTP1B Is an Intracellular Checkpoint that Limits T-cell and CAR T-cell Antitumor Immunity. Cancer Discov. 2022, 12, 752–773. [Google Scholar] [CrossRef]
- Richine, B.M.; Virts, E.L.; Bowling, J.D.; Ramdas, B.; Mali, R.; Naoye, R.; Liu, Z.; Zhang, Z.Y.; Boswell, H.S.; Kapur, R.; et al. Syk kinase and Shp2 phosphatase inhibition cooperate to reduce FLT3-ITD-induced STAT5 activation and proliferation of acute myeloid leukemia. Leukemia 2016, 30, 2094–2097. [Google Scholar] [CrossRef]
- Nabinger, S.C.; Li, X.J.; Ramdas, B.; He, Y.; Zhang, X.; Zeng, L.; Richine, B.; Bowling, J.D.; Fukuda, S.; Goenka, S.; et al. The protein tyrosine phosphatase, Shp2, positively contributes to FLT3-ITD-induced hematopoietic progenitor hyperproliferation and malignant disease in vivo. Leukemia 2013, 27, 398–408. [Google Scholar] [CrossRef][Green Version]
- Matalkah, F.; Martin, E.; Zhao, H.; Agazie, Y.M. SHP2 acts both upstream and downstream of multiple receptor tyrosine kinases to promote basal-like and triple-negative breast cancer. Breast Cancer Res. 2016, 18, 2. [Google Scholar] [CrossRef]
- Li, J.; Reed, S.A.; Johnson, S.E. Hepatocyte growth factor (HGF) signals through SHP2 to regulate primary mouse myoblast proliferation. Exp. Cell Res. 2009, 315, 2284–2292. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Libring, S.; Ruddraraju, K.V.; Miao, J.; Solorio, L.; Zhang, Z.Y.; Wendt, M.K. SHP2 is a multifunctional therapeutic target in drug resistant metastatic breast cancer. Oncogene 2020, 39, 7166–7180. [Google Scholar] [CrossRef] [PubMed]
- Araki, T.; Nawa, H.; Neel, B.G. Tyrosyl phosphorylation of Shp2 is required for normal ERK activation in response to some, but not all, growth factors. J. Biol. Chem. 2003, 278, 41677–41684. [Google Scholar] [CrossRef] [PubMed]
- Agazie, Y.M.; Hayman, M.J. Molecular mechanism for a role of SHP2 in epidermal growth factor receptor signaling. Mol. Cell Biol. 2003, 23, 7875–7886. [Google Scholar] [CrossRef] [PubMed]
- Aceto, N.; Sausgruber, N.; Brinkhaus, H.; Gaidatzis, D.; Martiny-Baron, G.; Mazzarol, G.; Confalonieri, S.; Quarto, M.; Hu, G.; Balwierz, P.J.; et al. Tyrosine phosphatase SHP2 promotes breast cancer progression and maintains tumor-initiating cells via activation of key transcription factors and a positive feedback signaling loop. Nat. Med. 2012, 18, 529–537. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Fan, Y.; Gao, Z.; Sun, X.; Zhang, H.; Wang, Z.; Cui, Y.; Song, W.; Wang, Z.; Zhang, F.; et al. SHP2 promotes proliferation of breast cancer cells through regulating Cyclin D1 stability via the PI3K/AKT/GSK3beta signaling pathway. Cancer Biol. Med. 2020, 17, 707–725. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Li, Y.; Huang, X.; Cheng, H.; Ke, Y.; Wang, L. The prognostic significance of SHP2 and its binding protein Hook1 in non-small cell lung cancer. Onco Targets Ther. 2019, 12, 5897–5906. [Google Scholar] [CrossRef] [PubMed]
- Nagamura, Y.; Miyazaki, M.; Nagano, Y.; Tomiyama, A.; Ohki, R.; Yanagihara, K.; Sakai, R.; Yamaguchi, H. SHP2 as a Potential Therapeutic Target in Diffuse-Type Gastric Carcinoma Addicted to Receptor Tyrosine Kinase Signaling. Cancers 2021, 13, 4309. [Google Scholar] [CrossRef] [PubMed]
- Martin, E.; Agazie, Y.M. SHP2 Potentiates the Oncogenic Activity of beta-Catenin to Promote Triple-Negative Breast Cancer. Mol. Cancer Res. 2021, 19, 1946–1956. [Google Scholar] [CrossRef]
- Sodir, N.M.; Pathria, G.; Adamkewicz, J.I.; Kelley, E.H.; Sudhamsu, J.; Merchant, M.; Chiarle, R.; Maddalo, D. SHP2: A Pleiotropic Target at the Interface of Cancer and Its Microenvironment. Cancer Discov. 2023, 13, 2339–2355. [Google Scholar] [CrossRef]
- Marasco, M.; Berteotti, A.; Weyershaeuser, J.; Thorausch, N.; Sikorska, J.; Krausze, J.; Brandt, H.J.; Kirkpatrick, J.; Rios, P.; Schamel, W.W.; et al. Molecular mechanism of SHP2 activation by PD-1 stimulation. Sci. Adv. 2020, 6, eaay4458. [Google Scholar] [CrossRef] [PubMed]
- Stanford, S.M.; Bottini, N. Targeting protein phosphatases in cancer immunotherapy and autoimmune disorders. Nat. Rev. Drug Discov. 2023, 22, 273–294. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.G.; Van Vactor, D. Receptor protein tyrosine phosphatases in nervous system development. Physiol. Rev. 2003, 83, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Stoker, A.W. RPTPs in axons, synapses and neurology. Semin. Cell Dev. Biol. 2015, 37, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, D.; Huang, H.Z.; Wang, Z.H.; Hou, T.Y.; Yang, X.; Pang, P.; Wei, N.; Zhou, Y.F.; Dupras, M.J.; et al. A Novel MicroRNA-124/PTPN1 Signal Pathway Mediates Synaptic and Memory Deficits in Alzheimer’s Disease. Biol. Psychiatry 2018, 83, 395–405. [Google Scholar] [CrossRef] [PubMed]
- Ono, H. Molecular Mechanisms of Hypothalamic Insulin Resistance. Int. J. Mol. Sci. 2019, 20, 1317. [Google Scholar] [CrossRef] [PubMed]
- Aluise, C.D.; Sultana, R.; Tangpong, J.; Vore, M.; St Clair, D.; Moscow, J.A.; Butterfield, D.A. Chemo brain (chemo fog) as a potential side effect of doxorubicin administration: Role of cytokine-induced, oxidative/nitrosative stress in cognitive dysfunction. Adv. Exp. Med. Biol. 2010, 678, 147–156. [Google Scholar] [CrossRef] [PubMed]
- Vannorsdall, T.D. Cognitive Changes Related to Cancer Therapy. Med. Clin. N. Am. 2017, 101, 1115–1134. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, D.A. The 2013 SFRBM discovery award: Selected discoveries from the butterfield laboratory of oxidative stress and its sequela in brain in cognitive disorders exemplified by Alzheimer disease and chemotherapy induced cognitive impairment. Free Radic. Biol. Med. 2014, 74, 157–174. [Google Scholar] [CrossRef]
- Ahles, T.A.; Saykin, A.J. Candidate mechanisms for chemotherapy-induced cognitive changes. Nat. Rev. Cancer 2007, 7, 192–201. [Google Scholar] [CrossRef]
- Bodden, K.; Bixby, J.L. CRYP-2: A receptor-type tyrosine phosphatase selectively expressed by developing vertebrate neurons. J. Neurobiol. 1996, 31, 309–324. [Google Scholar] [CrossRef]
- Wiggins, R.C.; Wiggins, J.E.; Goyal, M.; Wharram, B.L.; Thomas, P.E. Molecular cloning of cDNAs encoding human GLEPP1, a membrane protein tyrosine phosphatase: Characterization of the GLEPP1 protein distribution in human kidney and assignment of the GLEPP1 gene to human chromosome 12p12-p13. Genomics 1995, 27, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, S.S.; Korashy, H.M.; Zeidan, A.; Agouni, A. The Role of Protein Tyrosine Phosphatase (PTP)-1B in Cardiovascular Disease and Its Interplay with Insulin Resistance. Biomolecules 2019, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.J.; Hou, B.; Wang, X.; Zhu, X.X.; Li, K.X.; Qiu, L.Y. Endothelial dysfunction and cardiometabolic diseases: Role of long non-coding RNAs. Life Sci. 2016, 167, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Zhong, S.; Li, L.; Shen, X.; Li, Q.; Xu, W.; Wang, X.; Tao, Y.; Yin, H. An update on lipid oxidation and inflammation in cardiovascular diseases. Free Radic. Biol. Med. 2019, 144, 266–278. [Google Scholar] [CrossRef] [PubMed]
- Legeay, S.; Fautrat, P.; Norman, J.B.; Antonova, G.; Kennard, S.; Bruder-Nascimento, T.; Patel, V.S.; Faure, S.; Belin de Chantemele, E.J. Selective deficiency in endothelial PTP1B protects from diabetes and endoplasmic reticulum stress-associated endothelial dysfunction via preventing endothelial cell apoptosis. Biomed. Pharmacother. 2020, 127, 110200. [Google Scholar] [CrossRef]
- Zhang, Y.; Guan, Q.; Wang, Z. PTP1B inhibition ameliorates inflammatory injury and dysfunction in ox-LDL-induced HUVECs by activating the AMPK/SIRT1 signaling pathway via negative regulation of KLF2. Exp. Ther. Med. 2022, 24, 467. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Tao, W.; Bu, D.; Zhao, Y.; Zhang, T.; Chong, D.; Xue, B.; Xing, Z.; Li, C. Egr-1 transcriptionally activates protein phosphatase PTP1B to facilitate hyperinsulinemia-induced insulin resistance in the liver in type 2 diabetes. FEBS Lett. 2019, 593, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- da Cruz Rodrigues, K.C.; Martins Pereira, R.; Peruca, G.F.; Torres Barbosa, L.W.; Ramos Sant’Ana, M.; Rosetto Munoz, V.; Morelli, A.P.; Moreira Simabuco, F.; Sanchez Ramos da Silva, A.; Esper Cintra, D.; et al. Short-Term Strength Exercise Reduces Hepatic Insulin Resistance in Obese Mice by Reducing PTP1B Content, Regardless of Changes in Body Weight. Int. J. Mol. Sci. 2021, 22, 6402. [Google Scholar] [CrossRef]
- Loh, K.; Fukushima, A.; Zhang, X.; Galic, S.; Briggs, D.; Enriori, P.J.; Simonds, S.; Wiede, F.; Reichenbach, A.; Hauser, C.; et al. Elevated Hypothalamic TCPTP in Obesity Contributes to Cellular Leptin Resistance. Cell Metab. 2022, 34, 1892. [Google Scholar] [CrossRef]
- Stanford, S.M.; Aleshin, A.E.; Zhang, V.; Ardecky, R.J.; Hedrick, M.P.; Zou, J.; Ganji, S.R.; Bliss, M.R.; Yamamoto, F.; Bobkov, A.A.; et al. Diabetes reversal by inhibition of the low-molecular-weight tyrosine phosphatase. Nat. Chem. Biol. 2017, 13, 624–632. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Liu, Y.; Deng, J.; Zhu, C.; Ma, X.; Jiang, M.; Fan, D. Ginsenoside F4 Alleviates Skeletal Muscle Insulin Resistance by Regulating PTP1B in Type II Diabetes Mellitus. J. Agric. Food Chem. 2023, 71, 14263–14275. [Google Scholar] [CrossRef] [PubMed]
- Yoon, S.Y.; Kang, H.J.; Ahn, D.; Hwang, J.Y.; Kwon, S.J.; Chung, S.J. Identification of chebulinic acid as a dual targeting inhibitor of protein tyrosine phosphatases relevant to insulin resistance. Bioorg Chem. 2019, 90, 103087. [Google Scholar] [CrossRef] [PubMed]
- Thiebaut, P.A.; Besnier, M.; Gomez, E.; Richard, V. Role of protein tyrosine phosphatase 1B in cardiovascular diseases. J. Mol. Cell Cardiol. 2016, 101, 50–57. [Google Scholar] [CrossRef] [PubMed]
- Delibegovic, M.; Dall’Angelo, S.; Dekeryte, R. Protein tyrosine phosphatase 1B in metabolic diseases and drug development. Nat. Rev. Endocrinol. 2024, 20, 366–378. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Sun, R.; Yagimuma, H.; Taki, K.; Mizoguchi, A.; Kobayashi, T.; Sugiyama, M.; Onoue, T.; Tsunekawa, T.; Takagi, H.; et al. Protein Tyrosine Phosphatase 1B Deficiency Improves Glucose Homeostasis in Type 1 Diabetes Treated with Leptin. Diabetes 2022, 71, 1902–1914. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Xie, C.; Jiang, Y.; Li, K.; Lin, Y.; Pang, X.; Xiong, X.; Zheng, J.; Ke, X.; Chen, Y.; et al. Tumor-Derived Exosomal Protein Tyrosine Phosphatase Receptor Type O Polarizes Macrophage to Suppress Breast Tumor Cell Invasion and Migration. Front. Cell Dev. Biol. 2021, 9, 703537. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Yu, T.; Li, X.; Zhang, N.; Foster, L.J.; Peng, C.; Huang, W.; He, G. Recent advances in targeting the “undruggable” proteins: From drug discovery to clinical trials. Signal Transduct. Target. Ther. 2023, 8, 335. [Google Scholar] [CrossRef]
- Senis, Y.A.; Barr, A.J. Targeting Receptor-Type Protein Tyrosine Phosphatases with Biotherapeutics: Is Outside-in Better than Inside-Out? Molecules 2018, 23, 569. [Google Scholar] [CrossRef]
- Liang, S.; Tran, E.; Du, X.; Dong, J.; Sudholz, H.; Chen, H.; Qu, Z.; Huntington, N.D.; Babon, J.J.; Kershaw, N.J.; et al. A small molecule inhibitor of PTP1B and PTPN2 enhances T cell anti-tumor immunity. Nat. Commun. 2023, 14, 4524. [Google Scholar] [CrossRef]
- Duciel, L.; Monraz Gomez, L.C.; Kondratova, M.; Kuperstein, I.; Saule, S. The Phosphatase PRL-3 Is Involved in Key Steps of Cancer Metastasis. J. Mol. Biol. 2019, 431, 3056–3067. [Google Scholar] [CrossRef]
- Thura, M.; Al-Aidaroos, A.Q.O.; Yong, W.P.; Kono, K.; Gupta, A.; Lin, Y.B.; Mimura, K.; Thiery, J.P.; Goh, B.C.; Tan, P.; et al. PRL3-zumab, a first-in-class humanized antibody for cancer therapy. JCI Insight 2016, 1, e87607. [Google Scholar] [CrossRef]
- Liu, C.; Lu, H.; Wang, H.; Loo, A.; Zhang, X.; Yang, G.; Kowal, C.; Delach, S.; Wang, Y.; Goldoni, S.; et al. Combinations with Allosteric SHP2 Inhibitor TNO155 to Block Receptor Tyrosine Kinase Signaling. Clin. Cancer Res. 2021, 27, 342–354. [Google Scholar] [CrossRef] [PubMed]
- LaMarche, M.J.; Acker, M.; Argintaru, A.; Bauer, D.; Boisclair, J.; Chan, H.; Chen, C.H.; Chen, Y.N.; Chen, Z.; Deng, Z.; et al. Identification of TNO155, an Allosteric SHP2 Inhibitor for the Treatment of Cancer. J. Med. Chem. 2020, 63, 13578–13594. [Google Scholar] [CrossRef]
- Nichols, R.J.; Haderk, F.; Stahlhut, C.; Schulze, C.J.; Hemmati, G.; Wildes, D.; Tzitzilonis, C.; Mordec, K.; Marquez, A.; Romero, J.; et al. RAS nucleotide cycling underlies the SHP2 phosphatase dependence of mutant BRAF-, NF1- and RAS-driven cancers. Nat. Cell Biol. 2018, 20, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Meyers, B.A.; Czako, B.; Leonard, P.; Mseeh, F.; Harris, A.L.; Wu, Q.; Johnson, S.; Parker, C.A.; Cross, J.B.; et al. Allosteric SHP2 Inhibitor, IACS-13909, Overcomes EGFR-Dependent and EGFR-Independent Resistance Mechanisms toward Osimertinib. Cancer Res. 2020, 80, 4840–4853. [Google Scholar] [CrossRef]
- Cross, D.A.; Ashton, S.E.; Ghiorghiu, S.; Eberlein, C.; Nebhan, C.A.; Spitzler, P.J.; Orme, J.P.; Finlay, M.R.; Ward, R.A.; Mellor, M.J.; et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov. 2014, 4, 1046–1061. [Google Scholar] [CrossRef]
- Eno, M.S.; Brubaker, J.D.; Campbell, J.E.; De Savi, C.; Guzi, T.J.; Williams, B.D.; Wilson, D.; Wilson, K.; Brooijmans, N.; Kim, J.; et al. Discovery of BLU-945, a Reversible, Potent, and Wild-Type-Sparing Next-Generation EGFR Mutant Inhibitor for Treatment-Resistant Non-Small-Cell Lung Cancer. J. Med. Chem. 2022, 65, 9662–9677. [Google Scholar] [CrossRef]
- Hashimoto, Y.; Koyama, K.; Kamai, Y.; Hirotani, K.; Ogitani, Y.; Zembutsu, A.; Abe, M.; Kaneda, Y.; Maeda, N.; Shiose, Y.; et al. A Novel HER3-Targeting Antibody-Drug Conjugate, U3-1402, Exhibits Potent Therapeutic Efficacy through the Delivery of Cytotoxic Payload by Efficient Internalization. Clin. Cancer Res. 2019, 25, 7151–7161. [Google Scholar] [CrossRef]
- Lim, S.M.; Fujino, T.; Kim, C.; Lee, G.; Lee, Y.H.; Kim, D.W.; Ahn, J.S.; Mitsudomi, T.; Jin, T.; Lee, S.Y. BBT-176, a Novel Fourth-Generation Tyrosine Kinase Inhibitor for Osimertinib-Resistant EGFR Mutations in Non-Small Cell Lung Cancer. Clin. Cancer Res. 2023, 29, 3004–3016. [Google Scholar] [CrossRef]
- Tan, Y.; Tian, D.; Li, C.; Chen, Y.; Shen, Y.; Li, J.; Tang, J. Naphthoquinones and triterpenoids from Arnebia euchroma (Royle) Johnst and their hypoglycemic and lipid-lowering effects. Fitoterapia 2022, 162, 105288. [Google Scholar] [CrossRef] [PubMed]
- He, Q.F.; Wu, Z.L.; Huang, X.J.; Xia, T.Q.; Tang, G.; Tang, W.; Shi, L.; Ye, W.C.; Wang, Y. Cajanusoids A-D, Unusual Atropisomeric Stilbene Dimers with PTP1B Inhibitory Activities from the Leaves of Cajanus cajan. J. Org. Chem. 2021, 86, 5870–5882. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wu, Z.; Oyawaluja, B.O.; Coker, H.A.B.; Odukoya, O.A.; Yao, G.; Che, C.T. Protein Tyrosine Phosphatase 1B Inhibitory Iridoids from Psydrax subcordata. J. Nat. Prod. 2019, 82, 2916–2924. [Google Scholar] [CrossRef]
- Huang, J.H.; Lv, J.M.; Wang, Q.Z.; Zou, J.; Lu, Y.J.; Wang, Q.L.; Chen, D.N.; Yao, X.S.; Gao, H.; Hu, D. Biosynthesis of an anti-tuberculosis sesterterpenoid asperterpenoid A. Org. Biomol. Chem. 2019, 17, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Sharma, C.; Kim, Y.; Ahn, D.; Chung, S.J. Protein tyrosine phosphatases (PTPs) in diabetes: Causes and therapeutic opportunities. Arch. Pharm. Res. 2021, 44, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Jiang, Q.; Chang, N.; Wang, X.; Liu, C.; Xiong, J.; Cao, H.; Liang, Z. Small activating RNA binds to the genomic target site in a seed-region-dependent manner. Nucleic Acids Res. 2016, 44, 2274–2282. [Google Scholar] [CrossRef] [PubMed]
- Chu, Y.; Yue, X.; Younger, S.T.; Janowski, B.A.; Corey, D.R. Involvement of argonaute proteins in gene silencing and activation by RNAs complementary to a non-coding transcript at the progesterone receptor promoter. Nucleic Acids Res. 2010, 38, 7736–7748. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Lee, J.D.; Zhang, J.; Lin, S.Y.; Fu, T.M.; Chen, H.; Ishikawa, T.; Chiang, S.Y.; Katon, J.; et al. Reactivation of PTEN tumor suppressor for cancer treatment through inhibition of a MYC-WWP1 inhibitory pathway. Science 2019, 364, eaau0159. [Google Scholar] [CrossRef]
- Fuentes-Antras, J.; Genta, S.; Vijenthira, A.; Siu, L.L. Antibody-drug conjugates: In search of partners of choice. Trends Cancer 2023, 9, 339–354. [Google Scholar] [CrossRef]
- Moon, E.K.; Wang, L.C.; Dolfi, D.V.; Wilson, C.B.; Ranganathan, R.; Sun, J.; Kapoor, V.; Scholler, J.; Pure, E.; Milone, M.C.; et al. Multifactorial T-cell hypofunction that is reversible can limit the efficacy of chimeric antigen receptor-transduced human T cells in solid tumors. Clin. Cancer Res. 2014, 20, 4262–4273. [Google Scholar] [CrossRef]
- Manguso, R.T.; Pope, H.W.; Zimmer, M.D.; Brown, F.D.; Yates, K.B.; Miller, B.C.; Collins, N.B.; Bi, K.; LaFleur, M.W.; Juneja, V.R.; et al. In vivo CRISPR screening identifies Ptpn2 as a cancer immunotherapy target. Nature 2017, 547, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Huang, P.; Mao, K.; He, C.; Xu, Q.; Zhang, M.; Liu, H.; Zhou, Z.; Zhou, Q.; Zhou, Q.; et al. Anti-oncogene PTPN13 inactivation by hepatitis B virus X protein counteracts IGF2BP1 to promote hepatocellular carcinoma progression. Oncogene 2021, 40, 28–45. [Google Scholar] [CrossRef] [PubMed]
- Tzenaki, N.; Aivaliotis, M.; Papakonstanti, E.A. Focal adhesion kinase phosphorylates the phosphatase and tensin homolog deleted on chromosome 10 under the control of p110delta phosphoinositide-3 kinase. FASEB J. 2015, 29, 4840–4852. [Google Scholar] [CrossRef] [PubMed]
- Vazquez, F.; Grossman, S.R.; Takahashi, Y.; Rokas, M.V.; Nakamura, N.; Sellers, W.R. Phosphorylation of the PTEN tail acts as an inhibitory switch by preventing its recruitment into a protein complex. J. Biol. Chem. 2001, 276, 48627–48630. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Hepner, K.; Castelino-Prabhu, S.; Do, D.; Kaye, M.B.; Yuan, X.J.; Wood, J.; Ross, C.; Sawyers, C.L.; Whang, Y.E. Evidence for regulation of the PTEN tumor suppressor by a membrane-localized multi-PDZ domain containing scaffold protein MAGI-2. Proc. Natl. Acad. Sci. USA 2000, 97, 4233–4238. [Google Scholar] [CrossRef]
- Zhang, Z.Y. Drugging the Undruggable: Therapeutic Potential of Targeting Protein Tyrosine Phosphatases. Acc. Chem. Res. 2017, 50, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Schneider, P.; Walters, W.P.; Plowright, A.T.; Sieroka, N.; Listgarten, J.; Goodnow, R.A., Jr.; Fisher, J.; Jansen, J.M.; Duca, J.S.; Rush, T.S.; et al. Rethinking drug design in the artificial intelligence era. Nat. Rev. Drug Discov. 2020, 19, 353–364. [Google Scholar] [CrossRef] [PubMed]
- Leonard, D.; Huang, W.; Izadmehr, S.; O’Connor, C.M.; Wiredja, D.D.; Wang, Z.; Zaware, N.; Chen, Y.; Schlatzer, D.M.; Kiselar, J.; et al. Selective PP2A Enhancement through Biased Heterotrimer Stabilization. Cell 2020, 181, 688–701.e16. [Google Scholar] [CrossRef]
- Sangodkar, J.; Perl, A.; Tohme, R.; Kiselar, J.; Kastrinsky, D.B.; Zaware, N.; Izadmehr, S.; Mazhar, S.; Wiredja, D.D.; O’Connor, C.M.; et al. Activation of tumor suppressor protein PP2A inhibits KRAS-driven tumor growth. J. Clin. Investig. 2017, 127, 2081–2090. [Google Scholar] [CrossRef]

| Effectors | Functions | Tumor Types | References |
|---|---|---|---|
| HER2 (immediate) | PTPRO inhibited HER2-driven breast cancer through dephosphorylation, leading to dual effects of HER2. | Breast cancer | [86] |
| SYK (immediate) | Inhibited cell proliferation. Induced apoptosis. | Lymphoma Diffuse large B-cell lymphomas | [96,97] |
| MET (immediate) | PTPRO could inhibit MET-mediated metastasis. | Esophageal squamous cell carcinoma (ESCC) | [85] |
| AKT serine/threonine kinase (AKT)/mammalian target of rapamycin (mTOR) (immediate) | PTPRO silencing induced the activation of the AKT serine/threonine kinase (AKT)/mammalian target of rapamycin (mTOR) signaling axis. | Colorectal cancer | [98] |
| TLR4 | Inhibited cell proliferation. | Hepatocellular carcinoma | [99] |
| P53/FOXM1 (immediate) | Inhibited the leukemic cell population. Suppressed inflammatory cells in the spleen. | Chronic lymphocytic leukemia | [100] |
| NFkB | Inhibited cell proliferation. | Hepatocellular carcinoma | [99] |
| Oncogenic fusion protein BCR–ABL | Inhibited cell growth. Enhanced drug-induced apoptosis. Reduced tumorigenic potential. | Chronic myelogenous leukemia | [100] |
| Compound | Target | Stage of Development | Preclinical Study Results * | Refs. |
|---|---|---|---|---|
| TNO155 | SHP2 (PTP) | Phase I clinical trial (NCT03114319) | TNO155 showed benefit in four out of six mouse PDX models. | [153,154] |
| RMC-4630 | SHP2 (PTP) | Phase I clinical trial (NCT04916236) and Phase I/II clinical trial (NCT03989115) | RMC-4630 accelerated and increased the magnitude of the regression of ocitinib-sensitive EGFR-mutant tumors in mice. | [155] |
| PRL3-zumab | PRL3 (PTP) | Phase II clinical trials (NCT04118114, NCT04452955) | PRL3-zumab showed efficacy in the treatment of nude mouse PRL-3+ SNU-484 orthotopic tumors. | [156] |
| ABBV-CLS-484 | PTPN2/PTP1B (PTP) | Phase I clinical trial (NCT04777994) | AC484 treatment induced highly significant tumor regression and improved survival in all four mouse models. | [105] |
| JBJ-04-125-02 | Fourth-generation EGFR TKI (PTK) | Preclinical | JBJ-04-125-02 reduced the minimum residual tumor size by an average of nearly 60 mm3 in H1975 xenograft mice. | [157] |
| BLU-945 | Fourth-generation EGFR TKI (PTK) | Phase I/II clinical trial (NCT04862780) | BLU-945 strongly inhibited tumor growth in both NCI-H1975 mouse models. | [158] |
| U3-1402 | HER3 Fourth-generation EGFR TKI (PTK) | Phase III clinical trial (NCT05338970) | In the human MDA-MB-453 breast cancer cell line xenograft model, U3-1402 treatment led to significant tumor regression with a TGI of 87%. | [159] |
| BBT-176 | Fourth-generation EGFR TKI (PTK) | Phase I/II clinical trial (NCT04820023; terminated) | In all three EGFR-mutant PDX models, BBT-176 showed dose-dependent tumor suppression. | [160] |
| Drug Design Method | Function and Gene | Mechanism or Manner | Reference |
|---|---|---|---|
| Small-molecule targeting agent | Activates PTEN | Natural compounds inhibit WWP1 and reactivate PTEN | Indole-3-carbinol [168] |
| Activates SHP-1 | SHP-1 reactivation by multiple protein kinase inhibitors | Regorafenib [29] | |
| Suppresses SHP2 | Active-site inhibitors | Cryptotanshinone and II-B08 [30,31] | |
| Allosteric inhibitor | SHP099 [30,35] | ||
| Suppresses PTP1B | Reversible and non-competitive inhibitor | MSI-1436 [32] | |
| Suppresses PTP1B, PTPN2 | Active-site inhibitors | ABBV-CLS-484 (AC484); compound 182 [105,150] | |
| Antibody drug | PRL3 | Humanized antibody | PRL3-zumab [152] |
| HER2 | HER2-directed antibody–drug conjugates (ADCs) | Trastuzumab emtansine, trastuzumab deruxtecan [169] | |
| Genome editing | Knocks out PTPN6 | CRISPR-Cas9 | [170] |
| Knocks out PTPN2 | CRISPR-Cas9 | [171] | |
| Nucleic acid drugs | PTPN22 | siRNA; PTPN22 silencing | [36] |
| SHP-1 | siRNA; SHP-1 reactivation by RNF38 silencing | [37] | |
| PTPN13 | siRNA-mediated PTPN13 silencing | [38] | |
| / | small activating RNAs (saRNAs) | [40] | |
| Epigenetic modifications | / | DNA methylation; histone methylation and acetylation | [172] |
| Other | Suppresses TC-PTP | Proteolysis-targeting chimera (PROTAC) | [39] |
| PTEN | PIK3R1 positively regulates PTEN activity in combination with PTEN (protein–protein interaction (PPI)) | [41] | |
| PTEN | Phosphorylation of Tyr336 by FAK increases the phosphatase activity of PTEN | [173,174,175] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, Y.; Yao, Z.; Lin, Y.; Zhang, H. From Tyrosine Kinases to Tyrosine Phosphatases: New Therapeutic Targets in Cancers and Beyond. Pharmaceutics 2024, 16, 888. https://doi.org/10.3390/pharmaceutics16070888
Zhou Y, Yao Z, Lin Y, Zhang H. From Tyrosine Kinases to Tyrosine Phosphatases: New Therapeutic Targets in Cancers and Beyond. Pharmaceutics. 2024; 16(7):888. https://doi.org/10.3390/pharmaceutics16070888
Chicago/Turabian StyleZhou, Yu, Zhimeng Yao, Yusheng Lin, and Hao Zhang. 2024. "From Tyrosine Kinases to Tyrosine Phosphatases: New Therapeutic Targets in Cancers and Beyond" Pharmaceutics 16, no. 7: 888. https://doi.org/10.3390/pharmaceutics16070888
APA StyleZhou, Y., Yao, Z., Lin, Y., & Zhang, H. (2024). From Tyrosine Kinases to Tyrosine Phosphatases: New Therapeutic Targets in Cancers and Beyond. Pharmaceutics, 16(7), 888. https://doi.org/10.3390/pharmaceutics16070888





