Liquid- and Semisolid-Filled Hard Gelatin Capsules Containing Alpha-Lipoic Acid as a Suitable Dosage Form for Compounding Medicines and Dietary Supplements
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Capsules Formulation
2.3. Flowability
2.4. Uniformity of Mass
2.5. Assay
2.6. Fourier Transform Infrared Spectroscopy
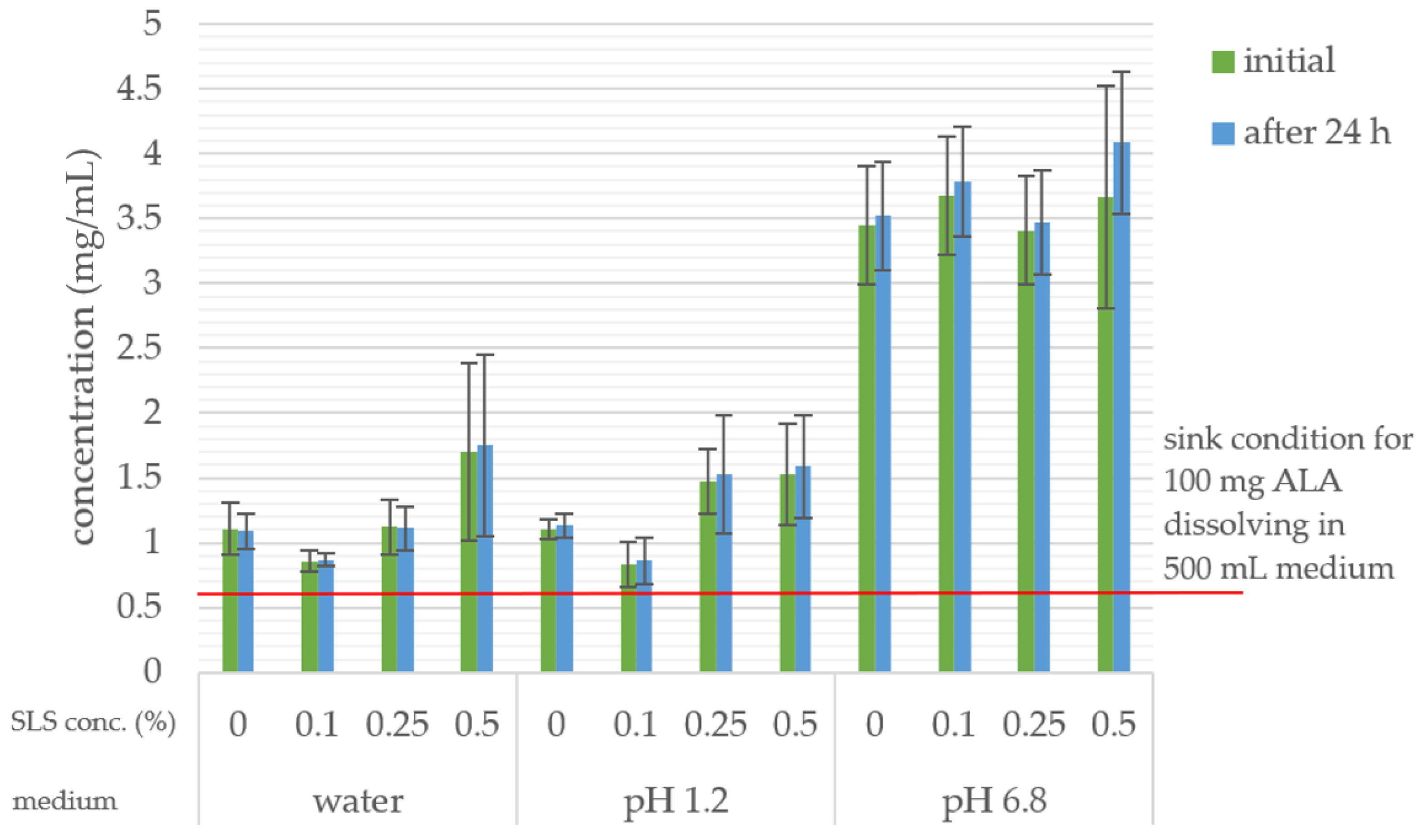
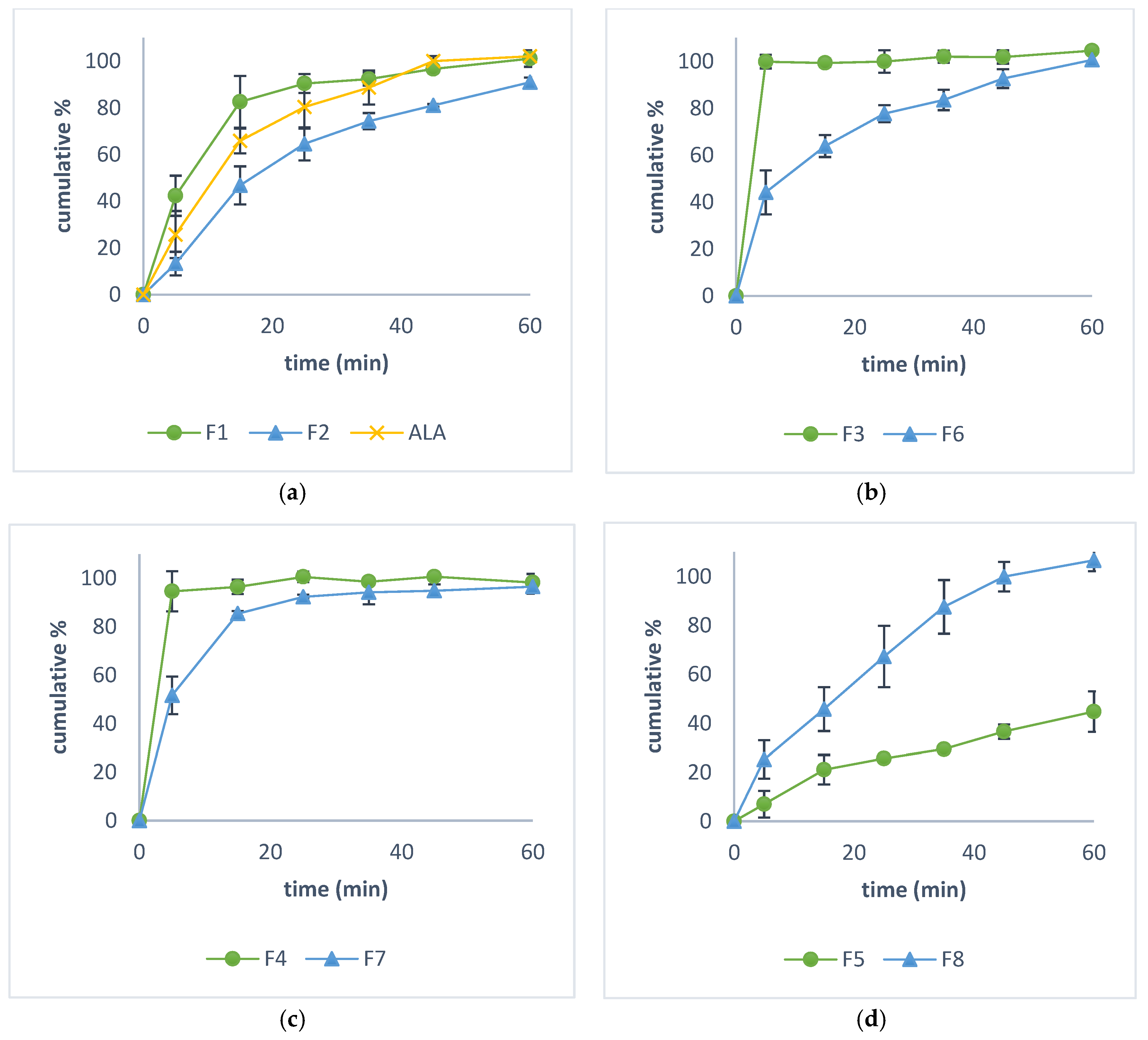
2.7. In Vitro Dissolution Study
2.8. Rheological Properties
3. Results
3.1. Flowability
3.2. Uniformity of Mass and Assay
3.3. Fourier Transform Infrared Spectroscopy
3.4. In Vitro Dissolution Study
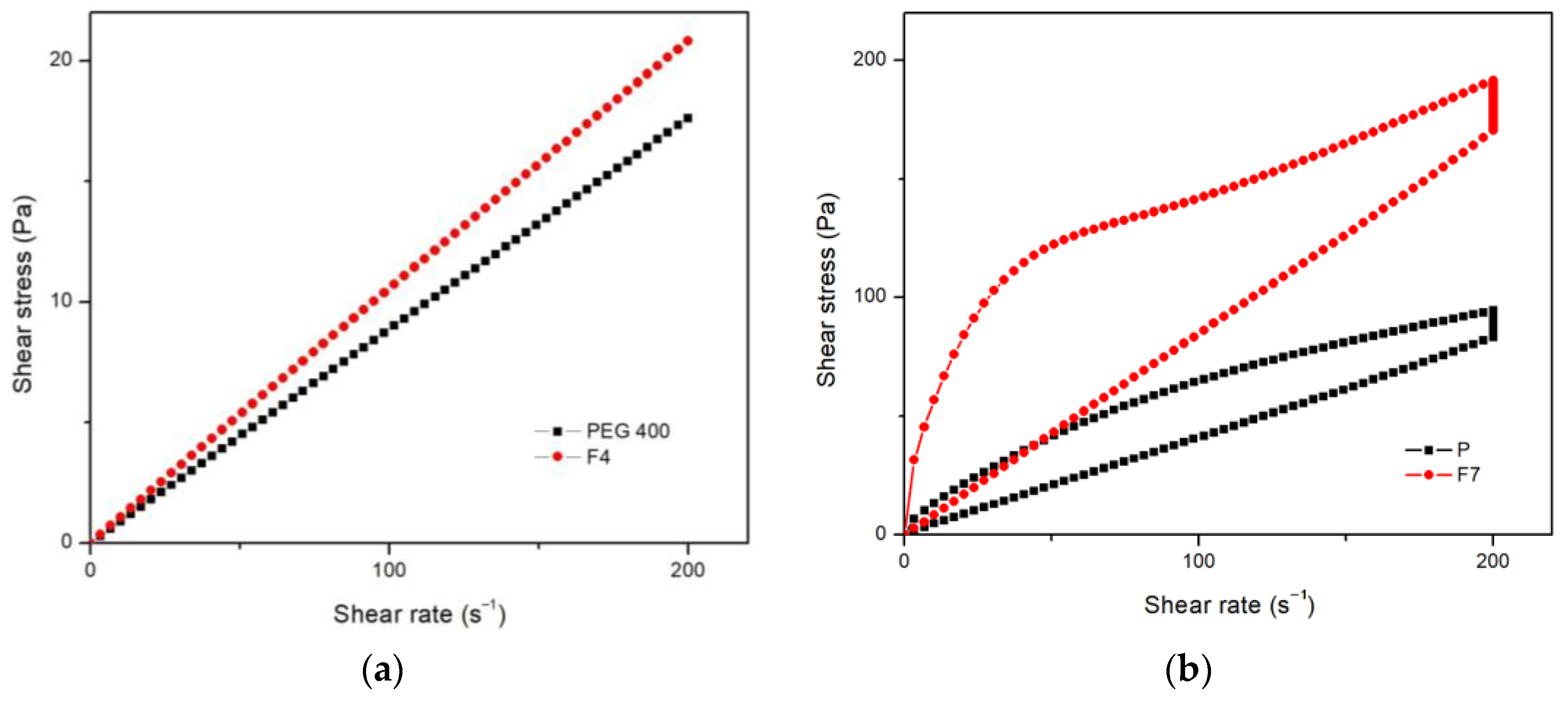
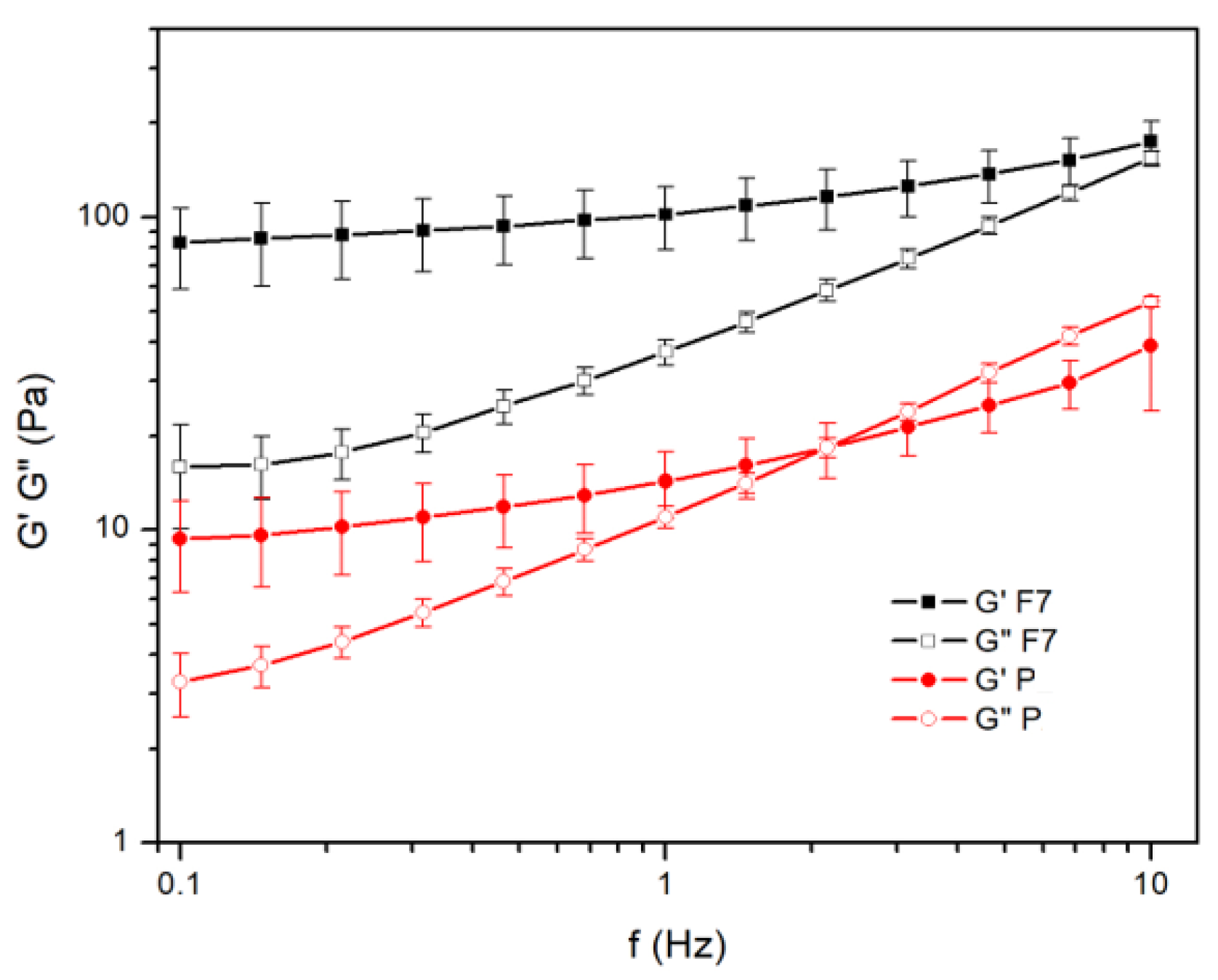
3.5. Rheological Properties
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rochette, L.; Ghibu, S.; Muresan, A.; Vergely, C.; Alpha, C.V. Alpha-lipoic acid: Molecular mechanisms and therapeutic potential in diabetes. Can. J. Physiol. Pharmacol. 2015, 93, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Shay, K.P.; Moreau, R.F.; Smith, E.J.; Smith, A.R.; Hagen, T.M. Alpha-lipoic acid as a dietary supplement: Molecular mechanisms and therapeutic potential. Biochim. Biophys. Acta (BBA) Gen. Subj. 2009, 1790, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Goraca, A.; Huk-Kolega, H.; Piechota, A.; Kleniewska, P.; Ciejka, E.; Skibska, B. Lipoic acid-biological activity and therapeutic potential. Pharmacol. Rep. 2011, 63, 849–858. [Google Scholar] [CrossRef] [PubMed]
- Padmalayam, I.; Hasham, S.; Saxena, U.; Pillarisetti, S. Lipoic acid: Synthase (LASY): A novel role in inflammation, mitochondrial function, and insulin resistance. Diabetes 2009, 58, 600–608. [Google Scholar] [CrossRef] [PubMed]
- Du, G.; Qiao, Y.; Zhuo, Z.; Zhou, J.; Li, X.; Liu, Z.; Li, Y.; Chen, H. Lipoic acid rejuvenates aged intestinal stem cells by preventing age-associated endosome reduction. EMBO Rep. 2020, 21, e49583. [Google Scholar] [CrossRef] [PubMed]
- Salehi, B.; Berkay Yılmaz, Y.; Antika, G.; Boyunegmez Tumer, T.; Fawzi Mahomoodally, M.; Lobine, D.; Akram, M.; Riaz, M.; Capanoglu, E.; Sharopov, F.; et al. Insights on the use of α-lipoic acid for therapeutic purposes. Biomolecules 2019, 9, 356. [Google Scholar] [CrossRef]
- Wang, J.Q.; Ling, X.; Wang, H.J.; Chen, F.E. α-Lipoic acid chemistry: The past 70 years. RSC Adv. 2023, 13, 36346–36363. [Google Scholar] [CrossRef] [PubMed]
- Bustamante, J.; Lodge, J.K.; Marcocci, L.; Tritschler, H.J.; Packer, L.; Rihn, B.H. α-Lipoic acid in liver metabolism and disease. Free Radic. Biol. Med. 1998, 24, 1023–1039. [Google Scholar] [CrossRef] [PubMed]
- Cronan, J.E. Progress in the enzymology of the mitochondrial diseases of lipoic acid requiring enzymes. Front. Genet. 2020, 11, 510. [Google Scholar] [CrossRef]
- Turkowicz, M.; Jastrzebska, I.; Hryniewicka, M.; Kotowska, U.; Gudalewska, D.; Karpińska, J. Investigation of lipoic acid—4-Methoxybenzyl alcohol reaction and evaluation of its analytical usefulness. Food Chem. 2020, 309, 125750. [Google Scholar] [CrossRef]
- Dos Santos, S.M.; Romeiro, C.F.R.; Rodrigues, C.A.; Cerqueira, A.R.L.; Monteiro, M.C. Mitochondrial dysfunction and alpha-lipoic acid: Beneficial or harmful in Alzheimer’s disease? Oxid. Med. Cell. Longev. 2019, 2019, 8409329. [Google Scholar] [CrossRef]
- Viana, M.D.M.; Lauria, P.S.S.; Lima, A.A.; de Opretzka, L.C.F.; Marcelino, H.R.; Villarreal, C.F. Alpha-Lipoic Acid as an Antioxidant Strategy for Managing Neuropathic Pain. Antioxidants 2022, 11, 2420. [Google Scholar] [CrossRef]
- Skibska, B.; Kochan, E.; Stanczak, A.; Lipert, A.; Skibska, A. Antioxidant and anti-inflammatory effects of α-lipoic acid on lipopolysaccharide-induced oxidative stress in rat kidney. Arch. Immunol. Ther. Exp. 2023, 71, 16. [Google Scholar] [CrossRef]
- Karalis, D.T.; Karalis, T.; Karalis, S.; Kleisiari, A.S.; Malakoudi, F.; Maimari, K.E.V. The effect of alpha-lipoic acid on diabetic peripheral neuropathy and the upcoming depressive disorders of type II diabetics. Cureus 2021, 13, e12773. [Google Scholar] [CrossRef]
- Teichert, J.; Hermann, R.; Ruus, P.; Preiss, R. Plasma kinetics, metabolism, and urinary excretion of alpha-lipoic acid following oral administration in healthy volunteers. J. Clin. Pharmacol. 2003, 43, 1257–1267. [Google Scholar] [CrossRef]
- Gomes, M.B.; Negrato, C.A. Alpha-lipoic acid as a pleiotropic compound with potential therapeutic use in diabetes and other chronic diseases. Diabetol. Metab. Syndr. 2014, 6, 80. [Google Scholar] [CrossRef]
- Capece, U.; Moffa, S.; Improta, I.; Di Giuseppe, G.; Nista, E.C.; Cefalo, C.M.A.; Cinti, F.; Pontecorvi, A.; Gasbarrini, A.; Giaccari, A.; et al. Alpha-Lipoic Acid and Glucose Metabolism: A Comprehensive Update on Biochemical and Therapeutic Features. Nutrients 2023, 15, 18. [Google Scholar] [CrossRef]
- Seifar, F.; Khalili, M.; Khaledyan, H.; Amiri Moghadam, S.; Izadi, A.; Azimi, A.; Shakouri, S.K. α-Lipoic acid, functional fatty acid, as a novel therapeutic alternative for central nervous system diseases: A review. Nutr. Neurosci. 2019, 22, 306–316. [Google Scholar] [CrossRef]
- Lalić-Popović, M.; Vuković, M.; Jovičić-Bata, J.; Čanji-Panić, J.; Todorović, N. Comparison of formulation characteristics of drugs and dietary supplements containing alpha-lipoic acid relevant to therapeutic efficacy. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 3159–3170. [Google Scholar] [CrossRef]
- Pop, A.L.; Crișan, S.; Bârcă, M.; Ciobanu, A.M.; Varlas, V.N.; Pop, C.; Pali, M.-A.; Cauni, D.; Ozon, E.A.; Udeanu, D.; et al. Evaluation of dissolution profiles of a newly developed solid oral immediate-release formula containing alpha-lipoic acid. Processes 2021, 9, 176. [Google Scholar] [CrossRef]
- Fogacci, F.; Rizzo, M.; Krogager, C.; Kennedy, C.; Georges, C.M.G.; Knežević, T.; Liberopoulos, E.; Vallée, A.; Pérez-Martínez, P.; Wenstedt, E.F.; et al. Safety Evaluation of α-Lipoic Acid Supplementation: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Clinical Studies. Antioxidants 2020, 9, 1011. [Google Scholar] [CrossRef] [PubMed]
- Teichert, J.; Tuemmers, T.; Achenbach, H.; Preiss, C.; Hermann, R.; Ruus, P.; Preiss, R. Pharmacokinetics of alpha-lipoic acid in subjects with severe kidney damage and end-stage renal disease. J. Clin. Pharmacol. 2005, 45, 313–328. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, Y.; Miyamoto, T.; Kakizawa, T.; Shigematsu, S.; Hashizume, K. Insulin autoimmune syndrome Possibly Caused by Alpha Lipoic Acid. Intern. Med. 2007, 46, 237–239. [Google Scholar] [CrossRef] [PubMed]
- Gullo, D.; Evans, J.L.; Sortino, G.; Goldfine, I.D.; Vigneri, R. Insulin autoimmune syndrome (Hirata Disease) in European Caucasians taking α-lipoic acid. Clin. Endocrinol. 2014, 81, 204–209. [Google Scholar] [CrossRef] [PubMed]
- Lasoń, E.; Sikora, E.; Miastkowska, M.; Socha, P.; Ogonowski, J. NLC delivery systems for alpha lipoic acid: Physicochemical characteristics and release study. Colloids Surf. A Physicochem. Eng. Asp 2017, 532, 57–62. [Google Scholar] [CrossRef]
- Bernkop-Schnürch, A.; Schuhbauer, H.; Clausen, A.E.; Hanel, R. Development of a sustained release dosage form for α-lipoic acid. I. Design and in vitro evaluation. Drug Dev. Ind. Pharm. 2004, 30, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Gleiter, C.H.; Schug, B.S.; Hermann, R.; Elze, M.; Blume, H.H.; Gundert-Remy, U. Influence of food intake on the bioavailability of thioctic acid enantiomers. Eur. J. Clin. Pharmacol. 1996, 50, 513–514. [Google Scholar] [CrossRef]
- Amidon, G.; Lennernäs, H.; Shah, V.; Crison, J. A theoretical basis for a biopharmaceutic drug classification: The correlation of in vitro drug product dissolution and in vivo bioavailability. Pharm. Res. 1995, 12, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Brufani, M. Acido α-lipoico farmaco o integratore. Una panoramica sulla farmacocinetica, le formulazioni disponibili e le evidenze cliniche nelle complicanze del diabete. Prog. Nutr. 2014, 16, 62–74. [Google Scholar]
- Maglione, E.; Marrese, C.; Migliaro, E.; Marcuccio, F.; Panico, C.; Salvati, C.; Citro, G.; Quercio, M.; Roncagliolo, F.; Torello, C.; et al. Increasing bioavailability of (R)-alpha-lipoic acid to boost antioxidant activity in the treatment of neuropathic pain. Acta Biomed. 2015, 86, 226–233. [Google Scholar] [PubMed]
- DTU Food National Institute. Safety of Alpha-Lipoic Acid Use in Food Supplements. 2017. Available online: https://food.ec.europa.eu/system/files/2020-06/labelling-nutrition_vitamins-minerals_add_alpha-lipoic-acid_nfi-da.pdf (accessed on 29 June 2024).
- Cremer, D.R.; Rabeler, R.; Roberts, A.; Lynch, B. Safety evaluation of α-lipoic acid (ALA). Regul. Toxicol. Pharmacol. 2006, 46, 29–41. [Google Scholar] [CrossRef]
- Cremer, D.R.; Rabeler, R.; Roberts, A.; Lynch, B. Long-term safety of α-lipoic acid (ALA) consumption: A 2-year study. Regul. Toxicol. Pharmacol. 2006, 46, 193–201. [Google Scholar] [CrossRef] [PubMed]
- EFSA Panel on Nutrition, Novel Foods and Food Allergens (NDA); Turck, D.; Castenmiller, J.; de Henauw, S.; Hirsch-Ernst, K.I.; Kearney, J.; Knutsen, H.K.; Mangelsdorf, I.; McArdle, H.J.; Naska, A.; et al. Scientific opinion on the relationship between intake of alpha-lipoic acid (thioctic acid) and the risk of insulin autoimmune syndrome. EFSA J. 2021, 19, e06577. [Google Scholar] [CrossRef] [PubMed]
- Wagner, A.F.; Walton, E.; Boxer, G.E.; Pruss, M.P.; Holly, F.W.; Folkers, K. Properties and derivatives of α-lipoic acid. J. Am. Chem. Soc. 1956, 78, 5079–5081. [Google Scholar] [CrossRef]
- FDA.gov. FDA Continues Taking Key Actions on Bulk Drug Substances Used for Compounding to Advance the Regulatory Framework Governing Compounded Drugs and to Protect Patients. Available online: https://www.fda.gov/news-events/press-announcements/fda-continues-taking-key-actions-bulk-drug-substances-used-compounding-advance-regulatory-framework (accessed on 23 May 2024).
- Council of Europe. European Pharmacopoeia, 11th ed.; European Directorate for the Quality of Medicines & Healthcare; Council of Europe: Strasbourg, France, 2022. [Google Scholar]
- GotI, P.P.; Savsani, J.J.; Patel, P.B. Spectrophotometric method development and validation for estimation of α-lipoic acid in tablet dosage form. Int. J. Pharm. Pharm. Sci. 2012, 4, 519–522. [Google Scholar]
- Silverstein, R.M.; Bassler, G.C.; Morrill, T.C. Spectrometric Identification of Organic Compounds, 3rd ed.; John Willey and Sons, Inc.: New York, NY, USA, 1974. [Google Scholar]
- Nikolic, R.S.; Krstic, N.S.; Nikolic, G.M.; Kocic, G.M.; Cakic, M.D.; Andelkovic, D.H. Molecular mechanisms of beneficial effects of lipoic acid in copper intoxicated rats assessment by FTIR and ESI-MS. Polyhedron 2014, 80, 223–227. [Google Scholar] [CrossRef]
- Leane, M.; Pitt, K.; Reynolds, G.K.; Dawson, N.; Ziegler, I.; Szepes, A.; Crean, A.M.; Agnol, R.D.; Sy, T.M.C. Manufacturing classification system in the real world: Factors influencing manufacturing process choices for filed commercial oral solid dosage formulations, case studies from industry and considerations for continuous processing. Pharm. Dev. Technol. 2018, 23, 964–977. [Google Scholar] [CrossRef] [PubMed]
- Todorović, N.; Čanji Panić, J.; Zavišić, M.; Krtolica, J.; Ratajac, R.; Petrović, J.; Bosiljčić, D.; Kladar, N.; Milošević, N.; Lalić-Popović, M. Compounding of Liquid and Solid Dose Adjustable Formulations with Pantoprazole: Comparison of Stability, Applicability and Suitability. Pharmaceutics 2023, 15, 717. [Google Scholar] [CrossRef] [PubMed]
- Chiwele, I.; Jones, B.E.; Podczeck, F. The shell dissolution of various empty hard capsules. Chem. Pharm. Bull. 2000, 48, 951–956. [Google Scholar] [CrossRef] [PubMed]
- Glube, N.; von Moos, L.; Duchateau, G. Capsule shell material impacts the in vitro disintegration and dissolution behaviour of a green tea extract. Results Pharma Sci. 2013, 3, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Brufani, M.; Figliola, R. (R)-α-lipoic acid oral liquid formulation: Pharmacokinetic parameters and therapeutic efficacy. Acta Biomed. 2014, 85, 108–115. [Google Scholar] [PubMed]
- Jambhekar, S.S.; Breen, P.J. Drug dissolution: Significance of physicochemical properties and physiological conditions. Drug Discov. Today 2013, 18, 1173–1184. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.J.; Parrott, E.L. Influence of viscosity and solubilization on dissolution rate. J. Pharm. Sci. 1972, 61, 175–178. [Google Scholar] [CrossRef] [PubMed]
- EMA. Guideline on the Investigation of Bioequivalence; (CPMP/EWP/QWP/1401/98 Rev. 1/Corr**); European Medicines Agency, Committee for Medicinal Products for Human Use (EMEA): Amsterdam, The Netherlands, 2010. [Google Scholar]
- Sultana, M.; Butt, M.A.; Saeed, T.; Mahmood, R.; ul Hassan, S.; Hussain, K.; Raza, S.A.; Ahsan, M.; Bukhari, N.I. Effect of Rheology and Poloxamers Properties on Release of Drugs from Silicon Dioxide Gel-Filled Hard Gelatin Capsules—A Further Enhancement of Viability of Liquid Semisolid Matrix Technology. AAPS PharmSciTech 2017, 18, 1998–2010. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.; Lampard, J.F.; Carruthers, K.M.; Regan, P. The filling of molten ibuprofen into hard gelatin capsules. Int. J. Pharm. 1990, 59, 115–119. [Google Scholar] [CrossRef]
- McTaggart, C.; Wood, R.; Bedford, K.; Walker, S.E. The evaluation of an automatic system for filling liquids into hard gelatin capsules. J. Pharm. Pharmacol. 1984, 36, 119–121. [Google Scholar] [CrossRef] [PubMed]
- Hawley, A.R. Investigation of Thermosoftened Solid Dispersion Formulations of Ibuprofen for Hard Gelatin Capsules. Ph.D. Thesis, University of Sunderland, Sunderland, UK, 1993. [Google Scholar]
- Fahr, A. Voigt’s Pharmaceutical Technology; John Wiley & Sons: Hoboken, NY, USA, 2018; pp. 468–477. [Google Scholar]
- Biglarian, N.; Rafe, A.; Shahidi, S.A.; Lorenzo, J.M. Rheological, textural and structural properties of dairy cream as affected by some natural stabilizers. Chem. Biol. Technol. Agric. 2022, 9, 96. [Google Scholar] [CrossRef]
- Krstonošić, V.; Dokić, L.; Nikolić, I.; Milanović, M. Influence of xanthan gum on oil-in-water emulsion characteristics stabilized by OSA starch. Food Hydrocoll. 2015, 45, 9–17. [Google Scholar] [CrossRef]
- Mandala, I.G.; Savvas, T.P.; Kostaropoulos, A.E. Xanthan and locust bean gum influence on the rheology and structure of a white model-sauce. J. Food Eng. 2004, 64, 335–342. [Google Scholar] [CrossRef]
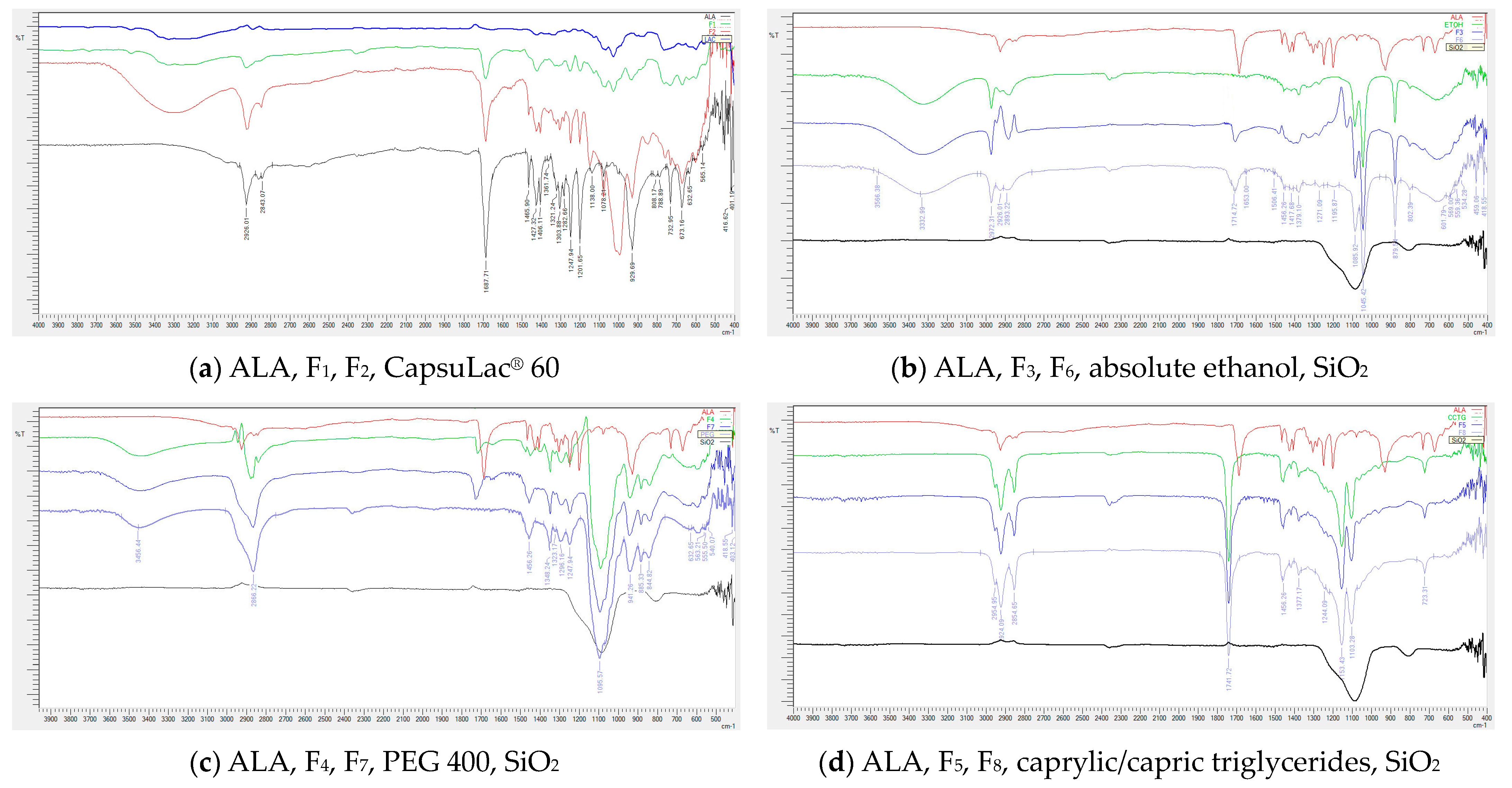
| Formulation | Ingredient | ||
|---|---|---|---|
| ALA | CapsuLac® 60 | Excipient II | |
| F0 | 100 mg | / | / |
| F1 | 100 mg | q.s. * | / |
| F2 | 100 mg | / | q.s. |
| Formulation | Ingredient | ||||
|---|---|---|---|---|---|
| ALA | Absolute Ethanol | PEG 400 | Caprylic/Capric Triglycerides | Silicon Dioxide | |
| F3 | 100 mg | ad 476 µL | / | / | / |
| F4 | 100 mg | / | ad 476 µL | / | / |
| F5 | 100 mg | / | / | ad 476 µL | / |
| F6 | 100 mg | ad 476 µL | / | / | q.s.* |
| F7 | 100 mg | / | ad 476 µL | / | q.s. |
| F8 | 100 mg | / | / | ad 476 µL | q.s. |
| Substance/ Mixture | Hausner Ratio | Compressibility Index (%) | Flow Character According to Hausner Ratio and Compressibility Index | Angle of Repose | Flow Property According to Angle of Repose | |||
|---|---|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | Mean | SD | |||
| ALA | 1.51 | 0.01 | 33.63 | 0.30 | Very poor | 49.09 | 0.55 | Poor |
| CapsuLac® 60 | 1.31 | 0.02 | 23.80 | 1.21 | Passable | 32.87 | 1.43 | Good |
| Excipient II | 1.43 | 0.04 | 30.26 | 2.11 | Poor | 36.71 | 0.79 | Fair |
| F1 | 1.14 | 0.03 | 12.08 | 2.22 | Good | 39.74 | 0.29 | Fair |
| F2 | 1.29 | 0.01 | 22.43 | 0.37 | Passable | 41.22 | 0.36 | Passable |
| Formulation | Uniformity of Mass (%) | Assay (%) | ||
|---|---|---|---|---|
| Min | Max | Mean | SD | |
| F1 | 98.42 | 102.03 | 96.61 | 1.38 |
| F2 | 95.37 | 102.21 | 97.79 | 2.05 |
| F3 | 98.33 | 105.98 | 98.93 | 0.97 |
| F4 | 95.72 | 106.11 | 98.09 | 2.45 |
| F5 | 95.19 | 107.27 | 98.17 | 1.09 |
| F6 | 97.43 | 104.68 | 96.05 | 0.84 |
| F7 | 95.63 | 104.95 | 101.18 | 3.01 |
| F8 | 96.44 | 104.99 | 99.82 | 2.17 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jovičić-Bata, J.; Todorović, N.; Krstonošić, V.; Ristić, I.; Kovačević, Z.; Vuković, M.; Lalić-Popović, M. Liquid- and Semisolid-Filled Hard Gelatin Capsules Containing Alpha-Lipoic Acid as a Suitable Dosage Form for Compounding Medicines and Dietary Supplements. Pharmaceutics 2024, 16, 892. https://doi.org/10.3390/pharmaceutics16070892
Jovičić-Bata J, Todorović N, Krstonošić V, Ristić I, Kovačević Z, Vuković M, Lalić-Popović M. Liquid- and Semisolid-Filled Hard Gelatin Capsules Containing Alpha-Lipoic Acid as a Suitable Dosage Form for Compounding Medicines and Dietary Supplements. Pharmaceutics. 2024; 16(7):892. https://doi.org/10.3390/pharmaceutics16070892
Chicago/Turabian StyleJovičić-Bata, Jelena, Nemanja Todorović, Veljko Krstonošić, Ivan Ristić, Zorana Kovačević, Milana Vuković, and Mladena Lalić-Popović. 2024. "Liquid- and Semisolid-Filled Hard Gelatin Capsules Containing Alpha-Lipoic Acid as a Suitable Dosage Form for Compounding Medicines and Dietary Supplements" Pharmaceutics 16, no. 7: 892. https://doi.org/10.3390/pharmaceutics16070892
APA StyleJovičić-Bata, J., Todorović, N., Krstonošić, V., Ristić, I., Kovačević, Z., Vuković, M., & Lalić-Popović, M. (2024). Liquid- and Semisolid-Filled Hard Gelatin Capsules Containing Alpha-Lipoic Acid as a Suitable Dosage Form for Compounding Medicines and Dietary Supplements. Pharmaceutics, 16(7), 892. https://doi.org/10.3390/pharmaceutics16070892









