Integrated miRNA Profiling of Extracellular Vesicles from Uterine Aspirates, Malignant Ascites and Primary-Cultured Ascites Cells for Ovarian Cancer Screening
Abstract
:1. Introduction
2. Materials and Methods
2.1. Clinical Samples
2.2. Sample Processing
2.3. Primary Culture of Ascites Cells and Collection of Conditioned Medium
2.4. Cell Staining
2.5. Extracellular Vesicles Isolation
2.6. Nanoparticle Tracking Analysis (NTA)
2.7. EV Visualization
2.8. Immunoblotting and Antibodies
2.9. RNA Extraction and Small RNA Deep Sequencing
2.10. Small RNA-Seq Data Analysis
2.11. Gene Ontology Enrichment Analysis
2.12. Statistics
3. Results
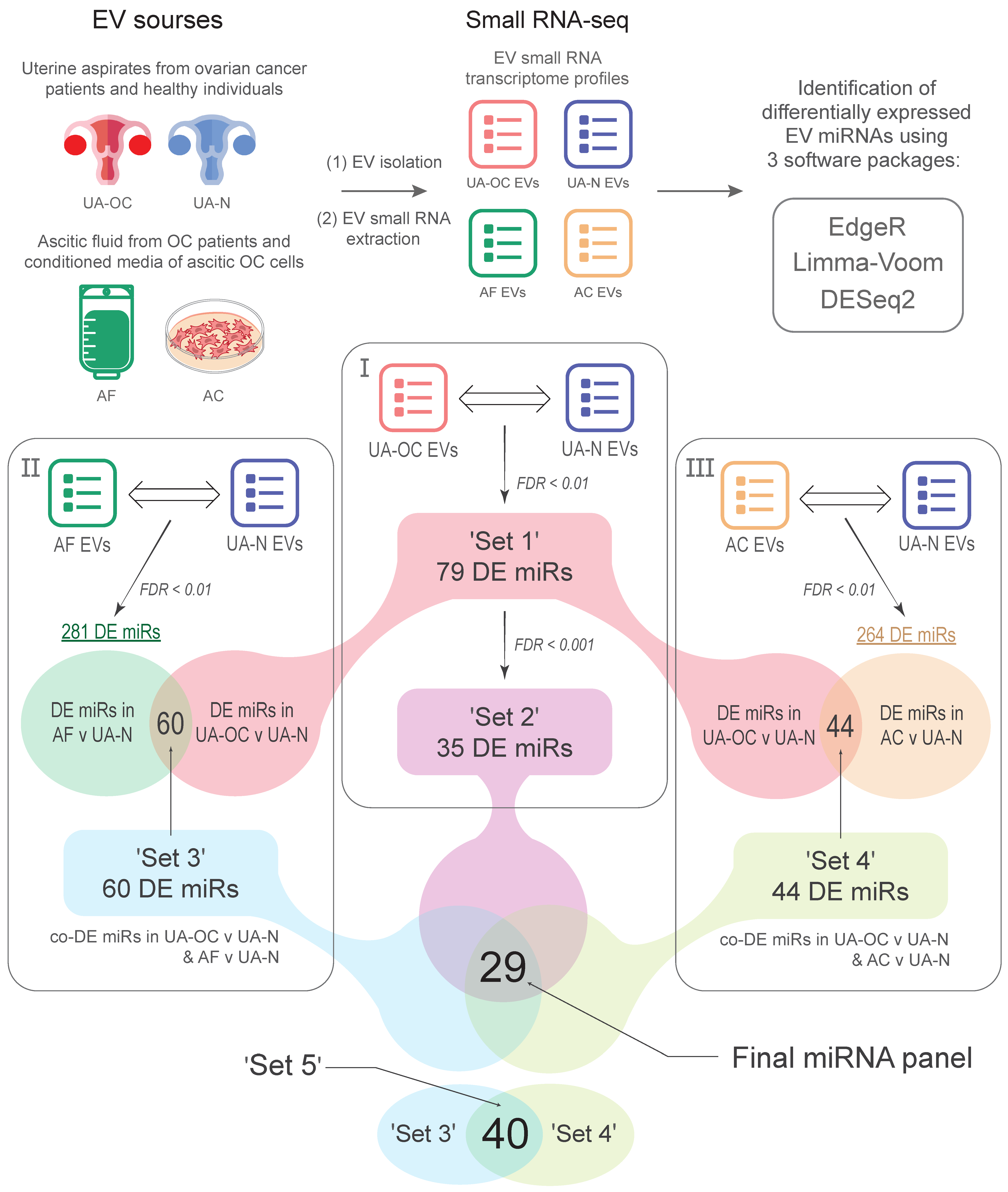
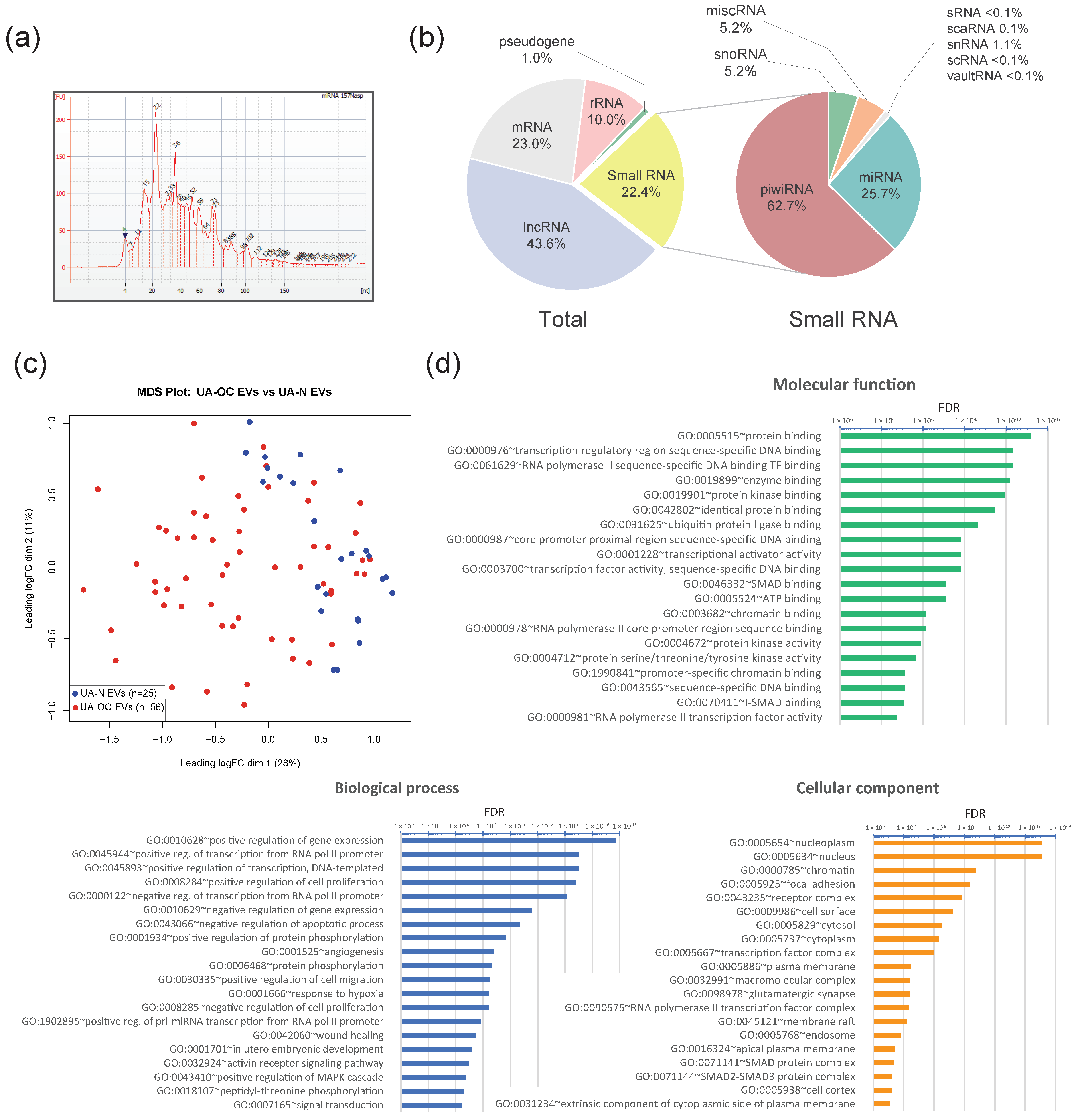
3.1. Differential Expression of miRNAs in EVs Isolated from the Uterine Aspirates of Ovarian Cancer and Non-Cancer Patients
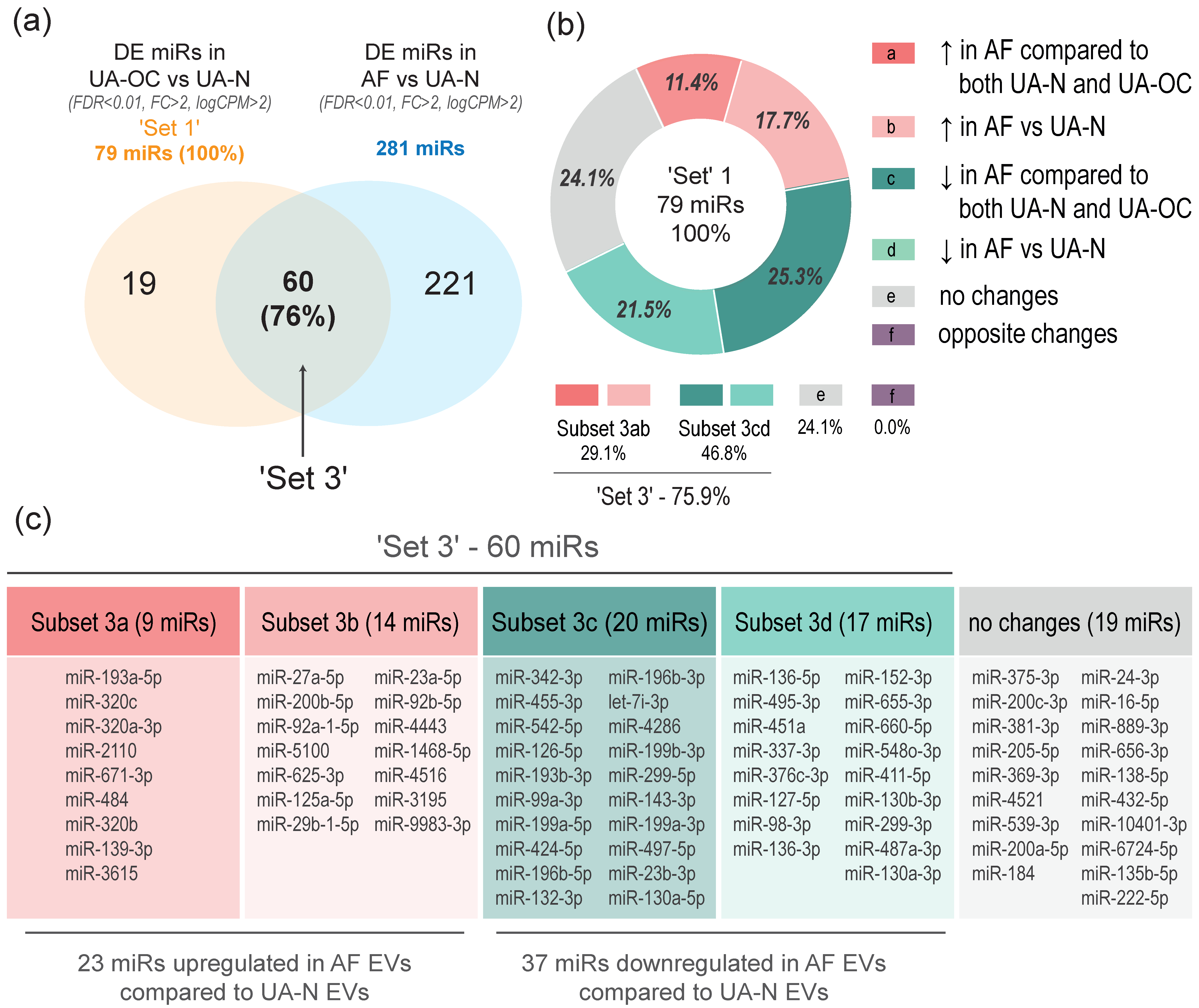
3.2. EVs from Malignant Ascites and the Uterine Aspirates of OC Patients Show a Common Spectrum of miRNA Alterations Compared to Non-Cancer Individuals (Differential Co-Expression)
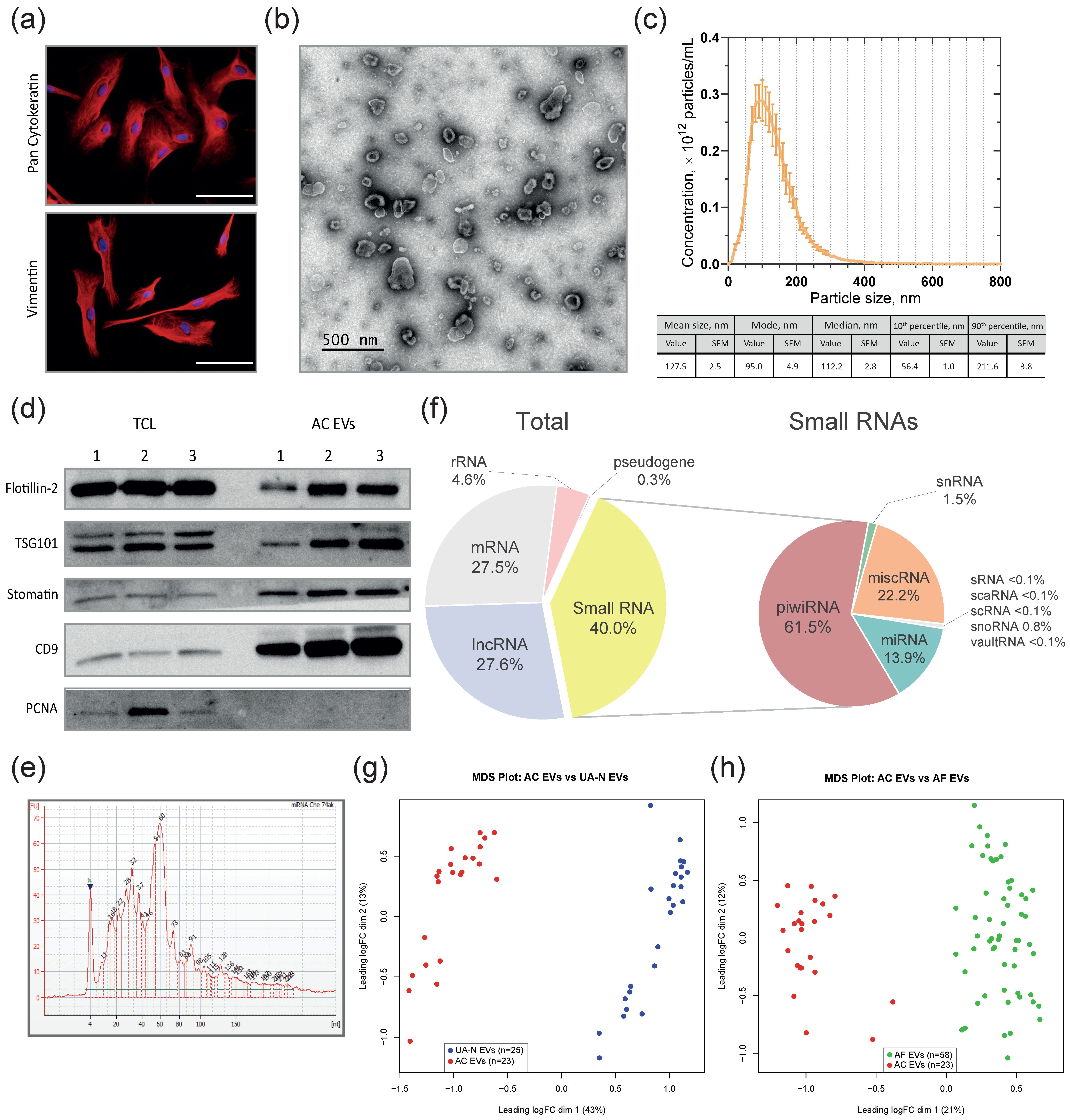
3.3. MiRNAs Differentially Co-Expressed in EVs from Primary-Cultured OC Cells, Malignant Ascites, and the Uterine Aspirates from OC Patients Compared to Non-Cancer Subjects
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Konstantinopoulos, P.A.; Matulonis, U.A. Clinical and translational advances in ovarian cancer therapy. Nat. Cancer 2023, 4, 1239–1257. [Google Scholar] [CrossRef] [PubMed]
- Raposo, G.; Stahl, P.D. Extracellular vesicles—On the cusp of a new language in the biological sciences. Extracell. Vesicles Circ. Nucleic Acids 2023, 4, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.-J.; Wang, C. A review of the regulatory mechanisms of extracellular vesicles-mediated intercellular communication. Cell Commun. Signal. 2023, 21, 77. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Siljander, P.R.-M.; Andreu, Z.; Bedina Zavec, A.; Borràs, F.E.; Buzas, E.I.; Buzas, K.; Casal, E.; Cappello, F.; Carvalho, J.; et al. Biological properties of extracellular vesicles and their physiological functions. J. Extracell. Vesicles 2015, 4, 27066. [Google Scholar] [CrossRef] [PubMed]
- Dixson, A.C.; Dawson, T.R.; Di Vizio, D.; Weaver, A.M. Context-specific regulation of extracellular vesicle biogenesis and cargo selection. Nat. Rev. Mol. Cell Biol. 2023, 24, 454–476. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.J.; James, V. The Role of Extracellular Vesicles in the Development of a Cancer Stem Cell Microenvironment Niche and Potential Therapeutic Targets: A Systematic Review. Cancers 2021, 13, 2435. [Google Scholar] [CrossRef] [PubMed]
- Croft, P.K.; Sharma, S.; Godbole, N.; Rice, G.E.; Salomon, C. Ovarian-Cancer-Associated Extracellular Vesicles: Microenvironmental Regulation and Potential Clinical Applications. Cells 2021, 10, 2272. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Sawada, K.; Kobayashi, M.; Miyamoto, M.; Shimizu, A.; Yamamoto, M.; Kinose, Y.; Kimura, T. Role of the Exosome in Ovarian Cancer Progression and Its Potential as a Therapeutic Target. Cancers 2019, 11, 1147. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Rai, A.; Chen, M.; Suwakulsiri, W.; Greening, D.W.; Simpson, R.J. Extracellular vesicles in cancer—Implications for future improvements in cancer care. Nat. Rev. Clin. Oncol. 2018, 15, 617–638. [Google Scholar] [CrossRef] [PubMed]
- Zhou, E.; Li, Y.; Wu, F.; Guo, M.; Xu, J.; Wang, S.; Tan, Q.; Ma, P.; Song, S.; Jin, Y. Circulating extracellular vesicles are effective biomarkers for predicting response to cancer therapy. EBioMedicine 2021, 67, 103365. [Google Scholar] [CrossRef]
- Logozzi, M.; Mizzoni, D.; Di Raimo, R.; Fais, S. Exosomes: A Source for New and Old Biomarkers in Cancer. Cancers 2020, 12, 2566. [Google Scholar] [CrossRef]
- Cheng, L.; Sharples, R.A.; Scicluna, B.J.; Hill, A.F. Exosomes provide a protective and enriched source of miRNA for biomarker profiling compared to intracellular and cell-free blood. J. Extracell. Vesicles 2014, 3, 23743. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445.e18. [Google Scholar] [CrossRef]
- Alharbi, M.; Sharma, S.; Guanzon, D.; Lai, A.; Zuñiga, F.; Shiddiky, M.J.A.; Yamauchi, Y.; Salas-Burgos, A.; He, Y.; Pejovic, T.; et al. miRNa signature in small extracellular vesicles and their association with platinum resistance and cancer recurrence in ovarian cancer. Nanomedicine 2020, 28, 102207. [Google Scholar] [CrossRef]
- Jo, A.; Green, A.; Medina, J.E.; Iyer, S.; Ohman, A.W.; McCarthy, E.T.; Reinhardt, F.; Gerton, T.; Demehin, D.; Mishra, R.; et al. Inaugurating High-Throughput Profiling of Extracellular Vesicles for Earlier Ovarian Cancer Detection. Adv. Sci. 2023, 10, 2301930. [Google Scholar] [CrossRef]
- Lopacinska-Jørgensen, J.; Oliveira, D.V.N.P.; Wayne Novotny, G.; Høgdall, C.K.; Høgdall, E.V. Integrated microRNA and mRNA signatures associated with overall survival in epithelial ovarian cancer. PLoS ONE 2021, 16, e0255142. [Google Scholar] [CrossRef]
- Meng, X.; Müller, V.; Milde-Langosch, K.; Trillsch, F.; Pantel, K.; Schwarzenbach, H. Diagnostic and prognostic relevance of circulating exosomal miR-373, miR-200a, miR-200b and miR-200c in patients with epithelial ovarian cancer. Oncotarget 2016, 7, 16923–16935. [Google Scholar] [CrossRef]
- Pan, C.; Stevic, I.; Müller, V.; Ni, Q.; Oliveira-Ferrer, L.; Pantel, K.; Schwarzenbach, H. Exosomal microRNAs as tumor markers in epithelial ovarian cancer. Mol. Oncol. 2018, 12, 1935–1948. [Google Scholar] [CrossRef]
- Shiao, M.-S.; Chang, J.-M.; Lertkhachonsuk, A.-A.; Rermluk, N.; Jinawath, N. Circulating Exosomal miRNAs as Biomarkers in Epithelial Ovarian Cancer. Biomedicines 2021, 9, 1433. [Google Scholar] [CrossRef]
- Salmond, N.; Williams, K.C. Isolation and characterization of extracellular vesicles for clinical applications in cancer—Time for standardization? Nanoscale Adv. 2021, 3, 1830–1852. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef]
- Alberro, A.; Iparraguirre, L.; Fernandes, A.; Otaegui, D. Extracellular Vesicles in Blood: Sources, Effects, and Applications. Int. J. Mol. Sci. 2021, 22, 8163. [Google Scholar] [CrossRef]
- Durin, L.; Pradines, A.; Basset, C.; Ulrich, B.; Keller, L.; Dongay, V.; Favre, G.; Mazieres, J.; Guibert, N. Liquid Biopsy of Non-Plasma Body Fluids in Non-Small Cell Lung Cancer: Look Closer to the Tumor! Cells 2020, 9, 2486. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Komelkov, A.V.; Zhordania, K.I.; Bagrov, D.V.; Vinokurova, S.V.; Galetsky, S.A.; Elkina, N.V.; Denisova, D.A.; Enikeev, A.D.; Tchevkina, E.M. Extracellular Vesicles from Uterine Aspirates Represent a Promising Source for Screening Markers of Gynecologic Cancers. Cells 2022, 11, 1064. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Vinokurova, S.V.; Galetsky, S.A.; Elkin, D.S.; Senkovenko, A.M.; Denisova, D.A.; Komelkov, A.V.; Stilidi, I.S.; Peregorodiev, I.N.; Malikhova, O.A.; et al. Isolation and Characterization of Extracellular Vesicles from Gastric Juice. Cancers 2022, 14, 3314. [Google Scholar] [CrossRef]
- Gutiérrez-Castañeda, L.D.; Tovar-Parra, D.; Quintero, G.; Amezquita, L.; Guerrero, C.; Sanabria, D. Isolation and phenotypic characterization of tumor cells of patients with a diagnosis of ovarian cancer. J. Cell. Physiol. 2020, 235, 3320–3328. [Google Scholar] [CrossRef]
- Théry, C.; Amigorena, S.; Raposo, G.; Clayton, A. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar] [CrossRef]
- Skryabin, G.O.; Komelkov, A.V.; Galetsky, S.A.; Bagrov, D.V.; Evtushenko, E.G.; Nikishin, I.I.; Zhordaniia, K.I.; Savelyeva, E.E.; Akselrod, M.E.; Paianidi, I.G.; et al. Stomatin is highly expressed in exosomes of different origin and is a promising candidate as an exosomal marker. J. Cell. Biochem. 2021, 122, 100–115. [Google Scholar] [CrossRef]
- Nikishin, I.; Dulimov, R.; Skryabin, G.; Galetsky, S.; Tchevkina, E.; Bagrov, D. ScanEV—A neural network-based tool for the automated detection of extracellular vesicles in TEM images. Micron 2021, 145, 103044. [Google Scholar] [CrossRef]
- Mastronarde, D.N. SerialEM: A Program for Automated Tilt Series Acquisition on Tecnai Microscopes Using Prediction of Specimen Position. Microsc. Microanal. 2003, 9, 1182–1183. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Robinson, M.D.; McCarthy, D.J.; Smyth, G.K. edgeR: A Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 2010, 26, 139–140. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, M.E.; Phipson, B.; Wu, D.; Hu, Y.; Law, C.W.; Shi, W.; Smyth, G.K. limma powers differential expression analyses for RNA-sequencing and microarray studies. Nucleic Acids Res. 2015, 43, e47. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Welsh, J.A.; Goberdhan, D.C.I.; O’Driscoll, L.; Buzas, E.I.; Blenkiron, C.; Bussolati, B.; Cai, H.; Di Vizio, D.; Driedonks, T.A.P.; Erdbrügger, U.; et al. Minimal information for studies of extracellular vesicles (MISEV2023): From basic to advanced approaches. J. Extracell. Vesicle 2024, 13, e12404. [Google Scholar] [CrossRef] [PubMed]
- Emelyanov, A.; Shtam, T.; Kamyshinsky, R.; Garaeva, L.; Verlov, N.; Miliukhina, I.; Kudrevatykh, A.; Gavrilov, G.; Zabrodskaya, Y.; Pchelina, S.; et al. Cryo-electron microscopy of extracellular vesicles from cerebrospinal fluid. PLoS ONE 2020, 15, e0227949. [Google Scholar] [CrossRef] [PubMed]
- Höög, J.L.; Lötvall, J. Diversity of extracellular vesicles in human ejaculates revealed by cryo-electron microscopy. J. Extracell. Vesicles 2015, 4, 28680. [Google Scholar] [CrossRef] [PubMed]
- Neyroud, A.-S.; Chiechio, R.M.; Moulin, G.; Ducarre, S.; Heichette, C.; Dupont, A.; Budzynski, M.; Even-Hernandez, P.; Faro, M.J.L.; Yefimova, M.; et al. Diversity of Extracellular Vesicles in Human Follicular Fluid: Morphological Analysis and Quantification. Int. J. Mol. Sci. 2022, 23, 11676. [Google Scholar] [CrossRef] [PubMed]
- Skryabin, G.O.; Vinokurova, S.V.; Elkina, N.V.; Denisova, D.A.; Beliaeva, A.A.; Zhordania, K.I.; Bagrov, D.V.; Enikeev, A.D.; Galetsky, S.A.; Komelkov, A.V.; et al. Comparison of Methods for MicroRNA Isolation from Extracellular Vesicles Obtained from Ascitic Fluids. Biochemistry 2022, 87, 1354–1366. [Google Scholar] [CrossRef]
- Li, Y.; Andrade, J. DEApp: An interactive web interface for differential expression analysis of next generation sequence data. Source Code Biol. Med. 2017, 12, 2. [Google Scholar] [CrossRef]
- Kossaï, M.; Leary, A.; Scoazec, J.-Y.; Genestie, C. Ovarian Cancer: A Heterogeneous Disease. Pathobiology 2017, 85, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Webb, P.M.; Jordan, S.J. Epidemiology of epithelial ovarian cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Eisenhauer, E.A. Real-world evidence in the treatment of ovarian cancer. Ann. Oncol. 2017, 28, viii61–viii65. [Google Scholar] [CrossRef] [PubMed]
- Grunewald, T.; Ledermann, J.A. Targeted Therapies for Ovarian Cancer. Best Pract. Res. Clin. Obstet. Gynaecol. 2017, 41, 139–152. [Google Scholar] [CrossRef] [PubMed]
- Nik Mohamed Kamal, N.N.S.B.; Shahidan, W.N.S. Non-Exosomal and Exosomal Circulatory MicroRNAs: Which Are More Valid as Biomarkers? Front. Pharmacol. 2020, 10, 1500. [Google Scholar] [CrossRef]
- Kinde, I.; Bettegowda, C.; Wang, Y.; Wu, J.; Agrawal, N.; Shih, I.-M.; Kurman, R.; Dao, F.; Levine, D.A.; Giuntoli, R.; et al. Evaluation of DNA from the Papanicolaou Test to Detect Ovarian and Endometrial Cancers. Sci. Transl. Med. 2013, 5, 167ra4. [Google Scholar] [CrossRef] [PubMed]
- Otsuka, I.; Kameda, S.; Hoshi, K. Early detection of ovarian and fallopian tube cancer by examination of cytological samples from the endometrial cavity. Br. J. Cancer 2013, 109, 603–609. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, L.; Douville, C.; Cohen, J.D.; Yen, T.-T.; Kinde, I.; Sundfelt, K.; Kjær, S.K.; Hruban, R.H.; Shih, I.-M.; et al. Evaluation of liquid from the Papanicolaou test and other liquid biopsies for the detection of endometrial and ovarian cancers. Sci. Transl. Med. 2018, 10, eaap8793. [Google Scholar] [CrossRef]
- Jafarzadeh, A.; Noori, M.; Sarrafzadeh, S.; Tamehri Zadeh, S.S.; Nemati, M.; Chatrabnous, N.; Jafarzadeh, S.; Hamblin, M.R.; Abadi, M.H.J.N.; Mirzaei, H. MicroRNA-383: A tumor suppressor miRNA in human cancer. Front. Cell Dev. Biol. 2022, 10, 955486. [Google Scholar] [CrossRef]
- Vilming Elgaaen, B.; Olstad, O.K.; Haug, K.B.F.; Brusletto, B.; Sandvik, L.; Staff, A.C.; Gautvik, K.M.; Davidson, B. Global miRNA expression analysis of serous and clear cell ovarian carcinomas identifies differentially expressed miRNAs including miR-200c-3p as a prognostic marker. BMC Cancer 2014, 14, 80. [Google Scholar] [CrossRef]
- Tan, D.S.; Agarwal, R.; Kaye, S.B. Mechanisms of transcoelomic metastasis in ovarian cancer. Lancet Oncol. 2006, 7, 925–934. [Google Scholar] [CrossRef] [PubMed]
- Ford, C.E.; Werner, B.; Hacker, N.F.; Warton, K. The untapped potential of ascites in ovarian cancer research and treatment. Br. J. Cancer 2020, 123, 9–16. [Google Scholar] [CrossRef] [PubMed]
- Eldh, M.; Lötvall, J.; Malmhäll, C.; Ekström, K. Importance of RNA isolation methods for analysis of exosomal RNA: Evaluation of different methods. Mol. Immunol. 2012, 50, 278–286. [Google Scholar] [CrossRef] [PubMed]
- Patel, G.K.; Khan, M.A.; Zubair, H.; Srivastava, S.K.; Khushman, M.; Singh, S.; Singh, A.P. Comparative analysis of exosome isolation methods using culture supernatant for optimum yield, purity and downstream applications. Sci. Rep. 2019, 9, 5335. [Google Scholar] [CrossRef] [PubMed]
- Godoy, P.M.; Bhakta, N.R.; Barczak, A.J.; Cakmak, H.; Fisher, S.; MacKenzie, T.C.; Patel, T.; Price, R.W.; Smith, J.F.; Woodruff, P.G.; et al. Large Differences in Small RNA Composition Between Human Biofluids. Cell Rep. 2018, 25, 1346–1358. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Jo, H.; Park, S.; Kim, H.; Kim, S.I.; Han, Y.; Patel, T.; Price, R.W.; Smith, J.F.; Woodruff, P.G.; et al. Integrated analysis of ascites and plasma extracellular vesicles identifies a miRNA-based diagnostic signature in ovarian cancer. Cancer Lett. 2022, 542, 215735. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, C.M.; Oakes, M.L.; Murakami, T.; Muto, M.G.; Berkowitz, R.S.; Ng, S.-W. Comparison of benign peritoneal fluid- and ovarian cancer ascites-derived extracellular vesicle RNA biomarkers. J. Ovarian Res. 2018, 11, 20. [Google Scholar] [CrossRef] [PubMed]
- Iorio, M.V.; Visone, R.; Di Leva, G.; Donati, V.; Petrocca, F.; Casalini, P.; Taccioli, C.; Volinia, S.; Liu, C.-G.; Alder, H.; et al. MicroRNA Signatures in Human Ovarian Cancer. Cancer Res. 2007, 67, 8699–8707. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.N.P.; Carlsen, A.L.; Heegaard, N.H.H.; Prahm, K.P.; Christensen, I.J.; Høgdall, C.K.; Høgdall, E.V. Diagnostic plasma miRNA-profiles for ovarian cancer in patients with pelvic mass. PLoS ONE 2019, 14, e0225249. [Google Scholar] [CrossRef]
- Bagnoli, M.; Canevari, S.; Califano, D.; Losito, S.; Maio, M.D.; Raspagliesi, F.; Carcangiu, M.L.; Toffoli, G.; Cecchin, E.; Sorio, R.; et al. Development and validation of a microRNA-based signature (MiROvaR) to predict early relapse or progression of epithelial ovarian cancer: A cohort study. Lancet Oncol. 2016, 17, 1137–1146. [Google Scholar] [CrossRef]
- De Cecco, L.; Bagnoli, M.; Chiodini, P.; Pignata, S.; Mezzanzanica, D. Prognostic Evidence of the miRNA-Based Ovarian Cancer Signature MiROvaR in Independent Datasets. Cancers 2021, 13, 1544. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, V.H.L.; Yue, C.; Du, K.Y.; Salem, M.; O’Brien, J.; Peng, C. The Role of microRNAs in Epithelial Ovarian Cancer Metastasis. Int. J. Mol. Sci. 2020, 21, 7093. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Hong, S.; Zhu, X. The Accuracy of Single MicroRNAs in Peripheral Blood to Diagnose Ovarian Cancer: An Updated Meta-Analysis. Dis. Markers 2020, 2020, 1075942. [Google Scholar] [CrossRef]
- Yoshida, K.; Yokoi, A.; Kato, T.; Ochiya, T.; Yamamoto, Y. The clinical impact of intra- and extracellular miRNAs in ovarian cancer. Cancer Sci. 2020, 111, 3435–3444. [Google Scholar] [CrossRef] [PubMed]
- Chu, P.; Liang, A.; Jiang, A.; Zong, L. miR-205 regulates the proliferation and invasion of ovarian cancer cells via suppressing PTEN/SMAD4 expression. Oncol. Lett. 2018, 15, 7571–7578. [Google Scholar] [CrossRef]
- He, L.; Zhu, W.; Chen, Q.; Yuan, Y.; Wang, Y.; Wang, J.; Wu, X. Ovarian cancer cell-secreted exosomal miR-205 promotes metastasis by inducing angiogenesis. Theranostics 2019, 9, 8206–8220. [Google Scholar] [CrossRef]
- Niu, K.; Shen, W.; Zhang, Y.; Zhao, Y.; Lu, Y. MiR-205 promotes motility of ovarian cancer cells via targeting ZEB1. Gene 2015, 574, 330–336. [Google Scholar] [CrossRef]
- Chae, D.; Ban, E.; Yoo, Y.S.; Kim, E.E.; Baik, J.; Song, E.J. MIR-27a regulates the TGF-β signaling pathway by targeting SMAD2 and SMAD4 in lung cancer. Mol. Carcinog. 2017, 56, 1992–1998. [Google Scholar] [CrossRef]
- Che, X.; Jian, F.; Chen, C.; Liu, C.; Liu, G.; Feng, W. PCOS serum-derived exosomal miR-27a-5p stimulates endometrial cancer cells migration and invasion. J. Mol. Endocrinol. 2020, 64, 1–12. [Google Scholar] [CrossRef]
- Min, S.; Li, L.; Zhang, M.; Zhang, Y.; Liang, X.; Xie, Y.; He, Q.; Li, Y.; Sun, J.; Liu, Q.; et al. TGF-β-associated miR-27a inhibits dendritic cell-mediated differentiation of Th1 and Th17 cells by TAB3, p38 MAPK, MAP2K4 and MAP2K7. Genes. Immun. 2012, 13, 621–631. [Google Scholar] [CrossRef]
- Shi, J.; Yang, C.; An, J.; Hao, D.; Liu, C.; Liu, J.; Sun, J.; Jiang, J. KLF5-induced BBOX1-AS1 contributes to cell malignant phenotypes in non-small cell lung cancer via sponging miR-27a-5p to up-regulate MELK and activate FAK signaling pathway. J. Exp. Clin. Cancer Res. 2021, 40, 148. [Google Scholar] [CrossRef] [PubMed]
- Towers, C.G.; Guarnieri, A.L.; Micalizzi, D.S.; Harrell, J.C.; Gillen, A.E.; Kim, J.; Wang, C.-A.; Oliphant, M.U.; Drasin, D.J.; Guney, M.A.; et al. The Six1 oncoprotein downregulates p53 via concomitant regulation of RPL26 and microRNA-27a-3p. Nat. Commun. 2015, 6, 10077. [Google Scholar] [CrossRef] [PubMed]
- Prahm, K.P.; Høgdall, C.; Karlsen, M.A.; Christensen, I.J.; Novotny, G.W.; Høgdall, E. Identification and validation of potential prognostic and predictive miRNAs of epithelial ovarian cancer. PLoS ONE 2018, 13, e0207319. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.-Y.; Chen, Y.; Jia, J.; Zhu, X.; He, Y.; Wu, L.-M. MiR-27a promotes EMT in ovarian cancer through active Wnt/β-catenin signalling by targeting FOXO1. CBM 2019, 24, 31–42. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Hu, S.; Wang, J.; Cai, J.; Xiao, L.; Yu, L.; Wang, Z. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol. Oncol. 2010, 119, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Wambecke, A.; Ahmad, M.; Morice, P.-M.; Lambert, B.; Weiswald, L.-B.; Vernon, M.; Vigneron, N.; Abeilard, E.; Brotin, E.; Figeac, M.; et al. The lncRNA ‘UCA1’ modulates the response to chemotherapy of ovarian cancer through direct binding to miR-27a-5p and control of UBE2N levels. Mol. Oncol. 2021, 15, 3659–3678. [Google Scholar] [CrossRef] [PubMed]
- Chhabra, R.; Dubey, R.; Saini, N. Cooperative and individualistic functions of the microRNAs in the miR-23a~27a~24-2 cluster and its implication in human diseases. Mol. Cancer 2010, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Haier, J.; Ströse, A.; Matuszcak, C.; Hummel, R. miR clusters target cellular functional complexes by defining their degree of regulatory freedom. Cancer Metastasis Rev. 2016, 35, 289–322. [Google Scholar] [CrossRef] [PubMed]
- Quan, J.; Liu, S.; Dai, K.; Jin, L.; He, T.; Pan, X.; Lai, Y. MicroRNA-23a/24-2/27a as a potential diagnostic biomarker for cancer: A systematic review and meta-analysis. Mol. Clin. Oncol. 2018, 8, 159–169. [Google Scholar] [CrossRef]
- Zhang, S.; Lu, Z.; Unruh, A.K.; Ivan, C.; Baggerly, K.A.; Calin, G.A.; Li, Z.; Bast, R.C.; Le, X.-F. Clinically Relevant microRNAs in Ovarian Cancer. Mol. Cancer Res. 2015, 13, 393–401. [Google Scholar] [CrossRef]
- Bai, H.; Wu, S. miR-451: A Novel Biomarker and Potential Therapeutic Target for Cancer. Onco Targets Ther. 2019, 12, 11069–11082. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Alvero, A.B.; Silasi, D.A.; Kelly, M.G.; Fest, S.; Visintin, I.; Leiser, A.L.; Schwartz, P.E.; Rutherford, T.J.; Mor, G. Regulation of IKKbeta by miR-199a affects NF-kappaB activity in ovarian cancer cells. Oncogene 2008, 27, 4712–4723. [Google Scholar] [CrossRef]
- Yin, G.; Chen, R.; Alvero, A.B.; Fu, H.-H.; Holmberg, J.; Glackin, C.; Rutherford, T.; Mor, G. TWISTing stemness, inflammation and proliferation of epithelial ovarian cancer cells through MIR199A2/214. Oncogene 2010, 29, 3545–3553. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.X.; Siu, M.K.Y.; Liu, S.S.; Yam, J.W.P.; Ngan, H.Y.S.; Chan, D.W. Epigenetic silencing of microRNA-199b-5p is associated with acquired chemoresistance via activation of JAG1-Notch1 signaling in ovarian cancer. Oncotarget 2014, 5, 944–958. [Google Scholar] [CrossRef]
- Joshi, H.P.; Subramanian, I.V.; Schnettler, E.K.; Ghosh, G.; Rupaimoole, R.; Evans, C.; Saluja, M.; Jing, Y.; Cristina, I.; Roy, S.; et al. Dynamin 2 along with microRNA-199a reciprocally regulate hypoxia-inducible factors and ovarian cancer metastasis. Proc. Natl. Acad. Sci. USA 2014, 111, 5331–5336. [Google Scholar] [CrossRef]
- Cheng, W.; Liu, T.; Wan, X.; Gao, Y.; Wang, H. MicroRNA-199a targets CD44 to suppress the tumorigenicity and multidrug resistance of ovarian cancer-initiating cells. FEBS J. 2012, 279, 2047–2059. [Google Scholar] [CrossRef]
- Wei, L.; He, Y.; Bi, S.; Li, X.; Zhang, J.; Zhang, S. miRNA-199b-3p suppresses growth and progression of ovarian cancer via the CHK1/E-cadherin/EMT signaling pathway by targeting ZEB1. Oncol. Rep. 2021, 45, 569–581. [Google Scholar] [CrossRef] [PubMed]
- Alshamrani, A.A. Roles of microRNAs in Ovarian Cancer Tumorigenesis: Two Decades Later, What Have We Learned? Front. Oncol. 2020, 10, 1084. [Google Scholar] [CrossRef]
- He, J.; Jing, Y.; Li, W.; Qian, X.; Xu, Q.; Li, F.-S.; Liu, L.-Z.; Jiang, B.-H.; Jiang, Y. Roles and mechanism of miR-199a and miR-125b in tumor angiogenesis. PLoS ONE 2013, 8, e56647. [Google Scholar] [CrossRef]
- Guo, T.; Pan, G. MicroRNA-136 functions as a tumor suppressor in osteosarcoma via regulating metadherin. CBM 2018, 22, 79–87. [Google Scholar] [CrossRef]
- Zhang, M.; Xu, T.; Tong, D.; Yu, X.; Liu, B.; Jiang, L.; Liu, K. MiR-136-5p in cancer: Roles, mechanisms, and chemotherapy resistance. Gene 2024, 909, 148265. [Google Scholar] [CrossRef] [PubMed]
- Niu, J.; Li, Z.; Li, F. Overexpressed microRNA-136 works as a cancer suppressor in gallbladder cancer through suppression of JNK signaling pathway via inhibition of MAP2K4. Am. J. Physiol.-Gastrointest. Liver Physiol. 2019, 317, G670–G681. [Google Scholar] [CrossRef] [PubMed]
- Hsu, C.-Y.; Allela, O.Q.B.; Mahdi, S.A.-H.; Doshi, O.P.; Adil, M.; Ali, M.S.; Saadh, M.J. miR-136–5p: A key player in human cancers with diagnostic, prognostic and therapeutic implications. Pathol. Res. Pract. 2023, 250, 154794. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Li, J.; Qin, F.; Dai, S. miR-152 as a tumor suppressor microRNA: Target recognition and regulation in cancer. Oncol. Lett. 2016, 11, 3911–3916. [Google Scholar] [CrossRef]
- Wang, Y.; Bao, W.; Liu, Y.; Wang, S.; Xu, S.; Li, X.; Li, Y.; Wu, S. miR-98-5p contributes to cisplatin resistance in epithelial ovarian cancer by suppressing miR-152 biogenesis via targeting Dicer1. Cell Death Dis. 2018, 9, 447. [Google Scholar] [CrossRef]
- Xiang, Y.; Ma, N.; Wang, D.; Zhang, Y.; Zhou, J.; Wu, G.; Zhao, R.; Huang, H.; Wang, X.; Qiao, Y.; et al. MiR-152 and miR-185 co-contribute to ovarian cancer cells cisplatin sensitivity by targeting DNMT1 directly: A novel epigenetic therapy independent of decitabine. Oncogene 2014, 33, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Huldani, H.; Alshahrani, S.H.; Almajidi, Y.Q.; Romero-Parra, R.M.; Hjazi, A.; Alsaab, H.O.; Oudaha, K.H.; Hussien, B.M.; Ahmed, M.; Fard, S.R.H. miR-495-3p as a promising tumor suppressor in human cancers. Pathol. Res. Pract. 2023, 248, 154610. [Google Scholar] [CrossRef]
- Taghehchian, N.; Samsami, Y.; Maharati, A.; Zangouei, A.S.; Boroumand-Noughabi, S.; Moghbeli, M. Molecular biology of microRNA-342 during tumor progression and invasion. Pathol. Res. Pract. 2023, 248, 154672. [Google Scholar] [CrossRef]
- Li, C.; Hong, Z.; Ou, M.; Zhu, X.; Zhang, L.; Yang, X. Integrated miRNA-mRNA Expression Profiles Revealing Key Molecules in Ovarian Cancer Based on Bioinformatics Analysis. Biomed. Res. Int. 2021, 2021, 6673655. [Google Scholar] [CrossRef]
- Xuan, J.; Liu, Y.; Zeng, X.; Wang, H. Sequence Requirements for miR-424-5p Regulating and Function in Cancers. Int. J. Mol. Sci. 2022, 23, 4037. [Google Scholar] [CrossRef]
- Khordadmehr, M.; Shahbazi, R.; Sadreddini, S.; Baradaran, B. miR-193: A new weapon against cancer. J. Cell. Physiol. 2019, 234, 16861–16872. [Google Scholar] [CrossRef] [PubMed]
- Eniafe, J.; Jiang, S. MicroRNA-99 family in cancer and immunity. Wiley Interdiscip. Rev. RNA 2021, 12, e1635. [Google Scholar] [CrossRef] [PubMed]
- Zhan, T.; Zhu, Q.; Han, Z.; Tan, J.; Liu, M.; Liu, W.; Chen, W.; Chen, X.; Chen, X.; Deng, J.; et al. miR-455-3p Functions as a Tumor Suppressor by Restraining Wnt/β-Catenin Signaling via TAZ in Pancreatic Cancer. Cancer Manag. Res. 2020, 12, 1483–1492. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.; Shi, Y.; Lyu, T.; Liu, H.; Shen, L.; Zhou, T.; Feng, W. Identification and Functional Validation of Differentially Expressed microRNAs in Ascites-Derived Ovarian Cancer Cells Compared with Primary Tumour Tissue. Cancer Manag. Res. 2021, 13, 6585–6597. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Han, Q.; Cai, J.; Xiao, M.; Huang, D.; Cao, J. The clinical validity of miR-126 as a prognostic marker in epithelial ovarian cancer. Medicine 2023, 102, e33085. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liang, X.; Wang, L.; Zhang, X. The Role of miRNA in Ovarian Cancer: An Overview. Reprod. Sci. 2022, 29, 2760–2767. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; He, K.; Xia, Z.; Liu, S.; Liu, H.; Xiang, G. Regulation of microRNA-497 expression in human cancer. Oncol. Lett. 2021, 21, 23. [Google Scholar] [CrossRef]
- Zeng, X.; Cao, Z.; Luo, W.; Zheng, L.; Zhang, T. MicroRNA-381-A Key Transcriptional Regulator: Its Biological Function and Clinical Application Prospects in Cancer. Front. Oncol. 2020, 10, 535665. [Google Scholar] [CrossRef]



| Histology | Serous Carcinoma | Endometrioid Carcinoma | Mucinous Carcinoma | Clear Cell Carcinoma |
|---|---|---|---|---|
| Cases N | 48 | 5 | 1 | 2 |
| Stage | ||||
| I | 2 (4%) | 2 (40%) | - | 1 (50%) |
| II | 2 (4%) | - | - | - |
| III | 34 (71%) | 3 (60%) | 1 (100%) | 1 (50%) |
| IV | 10 (21%) | - | - | - |
| Grade | ||||
| G1 | 8 (17%) | 1 (20%) | - | - |
| G2 | 3 (6%) | 3 (60%) | - | 1 (50%) |
| G3 | 36 (75%) | 1 (20%) | 1 (100%) | - |
| Gunknown | 1 (2%) | - | - | 1 (50%) |
| miRNAs Upregulated in UA-OC Compared to UA-N | miRNAs Downregulated in UA-OC Compared to UA-N | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| miRNA | logFC | logCPM | LR | p-Value | FDR | FC | miRNA | logFC | logCPM | LR | p-Value | FDR | FC | |
| miR-27a-5p | 4.237 | 8.517 | 60.574 | 7.09 × 10−15 | 9.56 × 10−12 | 18.857 | miR-495-3p | −1.904 | 7.556 | 40.765 | 1.72 × 10−10 | 4.64 × 10−8 | 0.267 | |
| miR-200b-5p | 2.840 | 8.773 | 43.317 | 4.65 × 10−11 | 3.14 × 10−8 | 7.161 | miR-381-3p | −1.824 | 6.177 | 37.324 | 1.00 × 10−9 | 1.88 × 10−7 | 0.282 | |
| miR-375-3p | 2.438 | 11.469 | 41.710 | 1.06 × 10−10 | 3.57 × 10−8 | 5.420 | miR-369-3p | −2.102 | 6.561 | 31.711 | 1.79 × 10−8 | 2.20 × 10−6 | 0.233 | |
| miR-200c-3p | 1.823 | 12.823 | 39.722 | 2.93 × 10−10 | 6.59 × 10−8 | 3.538 | miR-451a | −2.672 | 12.793 | 30.373 | 3.57 × 10−8 | 3.33 × 10−6 | 0.157 | |
| miR-193a-5p | 2.718 | 7.332 | 37.110 | 1.12 × 10−9 | 1.88 × 10−7 | 6.579 | miR-337-3p | −1.643 | 6.122 | 29.255 | 6.34 × 10−8 | 5.35 × 10−6 | 0.320 | |
| miR-205-5p | 3.358 | 10.035 | 35.724 | 2.27 × 10−9 | 3.41 × 10−7 | 10.257 | miR-376c-3p | −1.944 | 3.657 | 27.124 | 1.91 × 10−7 | 1.29 × 10−5 | 0.260 | |
| miR-92a-1-5p | 3.339 | 3.944 | 28.781 | 8.10 × 10−8 | 6.43 × 10−6 | 10.119 | miR-127-5p | −1.676 | 7.121 | 26.997 | 2.04 × 10−7 | 1.31 × 10−5 | 0.313 | |
| miR-2110 | 2.480 | 4.822 | 28.131 | 1.13 × 10−7 | 8.50 × 10−6 | 5.577 | miR-4521 | −1.672 | 4.424 | 22.549 | 2.05 × 10−6 | 8.92 × 10−5 | 0.314 | |
| miR-5100 | 5.146 | 5.276 | 27.980 | 1.23 × 10−7 | 8.71 × 10−6 | 35.400 | miR-98-3p | −1.555 | 5.206 | 22.025 | 2.69 × 10−6 | 1.04 × 10−4 | 0.340 | |
| miR-625-3p | 1.567 | 6.313 | 26.049 | 3.33 × 10−7 | 1.95 × 10−5 | 2.962 | miR-539-3p | −2.135 | 5.598 | 20.607 | 5.64 × 10−6 | 1.77 × 10−4 | 0.228 | |
| miR-125a-5p | 1.830 | 13.350 | 23.467 | 1.27 × 10−6 | 6.13 × 10−5 | 3.556 | miR-136-3p | −1.753 | 7.573 | 20.357 | 6.42 × 10−6 | 1.97 × 10−4 | 0.297 | |
| miR-671-3p | 1.764 | 5.307 | 23.134 | 1.51 × 10−6 | 7.04 × 10−5 | 3.396 | miR-152-3p | −1.188 | 10.297 | 20.020 | 7.66 × 10−6 | 2.30 × 10−4 | 0.439 | |
| miR-29b-1-5p | 2.515 | 3.417 | 22.098 | 2.59 × 10−6 | 1.03 × 10−4 | 5.718 | miR-542-5p | −1.700 | 4.504 | 19.259 | 1.14 × 10−5 | 3.06 × 10−4 | 0.308 | |
| miR-23a-5p | 3.774 | 3.797 | 21.943 | 2.81 × 10−6 | 1.04 × 10−4 | 13.682 | miR-655-3p | −1.516 | 5.976 | 19.235 | 1.16 × 10−5 | 3.06 × 10−4 | 0.350 | |
| miR-484 | 1.278 | 9.722 | 16.749 | 4.27 × 10−5 | 8.73 × 10−4 | 2.425 | miR-193b-3p | −1.176 | 7.429 | 17.635 | 2.68 × 10−5 | 6.12 × 10−4 | 0.443 | |
| miR-99a-3p | −1.082 | 6.079 | 17.541 | 2.81 × 10−5 | 6.33 × 10−4 | 0.473 | ||||||||
| miR-199a-5p | −1.093 | 14.674 | 17.337 | 3.13 × 10−5 | 6.82 × 10−4 | 0.469 | ||||||||
| miR-130a-5p | −1.780 | 4.420 | 25.248 | 5.04 × 10−7 | 2.72 × 10−5 | 0.290 | ||||||||
| miR-136-5p | −2.468 | 5.759 | 42.428 | 7.33 × 10−11 | 3.30 × 10−8 | 0.181 | ||||||||
| miR-487a-3p | −1.535 | 3.802 | 15.046 | 1.05 × 10−5 | 9.63 × 10−4 | 0.345 | ||||||||
| DE miRs in UA-OC vs UA-N | DE miRs in AF vs UA-N | DE miRs in AC vs UA-N | DE miRs in UA-OC vs UA-N | DE miRs in AF vs UA-N | DE miRs in AC vs UA-N | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| (FDR < 0.001) | (FDR < 0.01) | (FDR < 0.01) | (FDR < 0.001) | (FDR < 0.01) | (FDR < 0.01) | |||||
| ‘Set 2’ | ‘Set 3’ | ‘Set 4’ | ‘Set 2’ | ‘Set 3’ | ‘Set 4’ | |||||
| UPREGULATED | UP or DOWN | FDR | UP or DOWN | FDR | DOWNREGULATED | UP or DOWN | FDR | UP or DOWN | FDR | |
| miR-27a-5p | ↑ | 4.27 × 10−8 | ↑ | 2.84 × 10−15 | miR-451a | ↓ | 7.16 × 10−9 | ↓ | 9.19 × 10−27 | |
| miR-193a-5p | ↑ | 1.85 × 10−4 | ↑ | 2.53 × 10−28 | miR-376c-3p | ↓ | 2.29 × 10−7 | ↓ | 5.65 × 10−3 | |
| miR-5100 | ↑ | 2.99 × 10−17 | ↑ | 1.57 × 10−16 | miR-127-5p | ↓ | 4.96 × 10−10 | ↓ | 6.61 × 10−3 | |
| miR-625-3p | ↑ | 2.55 × 10−8 | ↑ | 7.07 × 10−7 | miR-136-3p | ↓ | 3.43 × 10−3 | ↓ | 3.03 × 10−5 | |
| miR-125a-5p | ↑ | 9.78 × 10−5 | ↑ | 2.58 × 10−3 | miR-542-5p | ↓ | 1.44 × 10−20 | ↓ | 8.87 × 10−16 | |
| miR-671-3p | ↑ | 2.23 × 10−18 | ↑ | 2.90 × 10−12 | miR-193b-3p | ↓ | 1.37 × 10−9 | ↓ | 1.42 × 10−3 | |
| miR-29b-1-5p | ↑ | 5.92 × 10−6 | ↑ | 1.70 × 10−7 | miR-99a-3p | ↓ | 1.88 × 10−25 | ↓ | 4.61 × 10−35 | |
| miR-23a-5p | ↑ | 8.50 × 10−15 | ↑ | 2.52 × 10−6 | miR-199a-5p | ↓ | 6.82 × 10−26 | ↓ | 4.95 × 10−77 | |
| miR-200b-5p | ↑ | 1.15 × 10−7 | n/s | miR-130a-5p | ↓ | 6.60 × 10−15 | ↓ | 5.18 × 10−11 | ||
| miR-92a-1-5p | ↑ | 4.13 × 10−8 | n/s | miR-4521 | n/s | ↓ | 6.91 × 10−10 | |||
| miR-2110 | ↑ | 5.80 × 10−24 | n/s | miR-136-5p | ↓ | 5.59 × 10−5 | n/s | |||
| miR-484 | ↑ | 2.33 × 10−16 | n/s | miR-495-3p | ↓ | 1.01 × 10−17 | n/s | |||
| miR-375-3p | n/s | n/s | miR-337-3p | ↓ | 5.10 × 10−13 | n/s | ||||
| miR-200c-3p | n/s | n/s | miR-98-3p | ↓ | 5.26 × 10−9 | n/s | ||||
| miR-205-5p | n/s | n/s | miR-152-3p | ↓ | 8.95 × 10−3 | n/s | ||||
| miR-655-3p | ↓ | 4.36 × 10−4 | n/s | |||||||
| miR-487a-3p | ↓ | 2.07 × 10−4 | n/s | |||||||
| miR-381-3p | n/s | n/s | ||||||||
| miR-369-3p | n/s | n/s | ||||||||
| miR-6393p | n/s | n/s | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Skryabin, G.O.; Komelkov, A.V.; Zhordania, K.I.; Bagrov, D.V.; Enikeev, A.D.; Galetsky, S.A.; Beliaeva, A.A.; Kopnin, P.B.; Moiseenko, A.V.; Senkovenko, A.M.; et al. Integrated miRNA Profiling of Extracellular Vesicles from Uterine Aspirates, Malignant Ascites and Primary-Cultured Ascites Cells for Ovarian Cancer Screening. Pharmaceutics 2024, 16, 902. https://doi.org/10.3390/pharmaceutics16070902
Skryabin GO, Komelkov AV, Zhordania KI, Bagrov DV, Enikeev AD, Galetsky SA, Beliaeva AA, Kopnin PB, Moiseenko AV, Senkovenko AM, et al. Integrated miRNA Profiling of Extracellular Vesicles from Uterine Aspirates, Malignant Ascites and Primary-Cultured Ascites Cells for Ovarian Cancer Screening. Pharmaceutics. 2024; 16(7):902. https://doi.org/10.3390/pharmaceutics16070902
Chicago/Turabian StyleSkryabin, Gleb O., Andrei V. Komelkov, Kirill I. Zhordania, Dmitry V. Bagrov, Adel D. Enikeev, Sergey A. Galetsky, Anastasiia A. Beliaeva, Pavel B. Kopnin, Andey V. Moiseenko, Alexey M. Senkovenko, and et al. 2024. "Integrated miRNA Profiling of Extracellular Vesicles from Uterine Aspirates, Malignant Ascites and Primary-Cultured Ascites Cells for Ovarian Cancer Screening" Pharmaceutics 16, no. 7: 902. https://doi.org/10.3390/pharmaceutics16070902







