Inhibition of Enzyme and Bacteria Activities in Diabetic Ulcer-like Scenarios via WAAPV-Loaded Electrospun Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. AAPV and WAAPV Synthesis and Characterization
2.3. Inhibition of Elastase and Bacteria Activities
2.4. Electrospun Fiber Production and Modification
2.5. Physical, Chemical, and Thermal Characterization
2.6. Peptide Release Kinetics
2.7. HNE Inhibition
2.8. Antibacterial Activity
3. Results and Discussion
3.1. Peptide Synthesis and Characterization
3.2. HNE Inhibition by AAPV and WAAPV
3.3. Minimum Inhibitory and Bactericidal Concentrations
3.4. Physical, Chemical, and Thermal Characterization of Electrospun Mats
3.4.1. Morphology
3.4.2. Chemical Composition
3.4.3. Thermal Response
3.4.4. Wettability
3.4.5. Air Permeability and Porosity
3.4.6. Degree of Swelling
3.4.7. Degradation Profile
3.5. Peptide Release Kinetics from Electrospun Fibers
3.6. HNE Inhibition by Peptide-Loaded Electrospun Fibers
3.7. Time-Kill Kinetics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Felgueiras, H.P.; Amorim, M.T.P. Functionalization of electrospun polymeric wound dressings with antimicrobial peptides. Colloids Surf. B Biointerfaces 2017, 156, 133–148. [Google Scholar] [CrossRef] [PubMed]
- Felgueiras, H.P. Emerging antimicrobial and immunomodulatory fiber-based scaffolding systems for treating diabetic foot ulcers. Pharmaceutics 2023, 15, 258. [Google Scholar] [CrossRef]
- Lindsay, S.; Oates, A.; Bourdillon, K. The detrimental impact of extracellular bacterial proteases on wound healing. Int. Wound J. 2017, 14, 1237–1247. [Google Scholar] [CrossRef] [PubMed]
- Westby, M.J.; Norman, G.; Watson, R.E.; Cullum, N.A.; Dumville, J.C. Protease activity as a prognostic factor for wound healing in complex wounds. Wound Repair Regen. 2020, 28, 631–644. [Google Scholar] [CrossRef] [PubMed]
- Vatansever, F.; de Melo, W.C.; Avci, P.; Vecchio, D.; Sadasivam, M.; Gupta, A.; Chandran, R.; Karimi, M.; Parizotto, N.A.; Yin, R. Antimicrobial strategies centered around reactive oxygen species–bactericidal antibiotics, photodynamic therapy, and beyond. FEMS Microbiol. Rev. 2013, 37, 955–989. [Google Scholar] [CrossRef] [PubMed]
- Stadelmann, W.K.; Digenis, A.G.; Tobin, G.R. Physiology and healing dynamics of chronic cutaneous wounds. Am. J. Surg. 1998, 176, 26S–38S. [Google Scholar] [CrossRef]
- Wilgus, T.A.; Roy, S.; McDaniel, J.C. Neutrophils and wound repair: Positive actions and negative reactions. Adv. Wound Care 2013, 2, 379–388. [Google Scholar] [CrossRef]
- Miranda, C.S.; Silva, A.F.G.; Seabra, C.L.; Reis, S.; Silva, M.M.P.; Pereira-Lima, S.M.; Costa, S.P.; Homem, N.C.; Felgueiras, H.P. Sodium alginate/polycaprolactone co-axial wet-spun microfibers modified with N-carboxymethyl chitosan and the peptide AAPV for Staphylococcus aureus and human neutrophil elastase inhibition in potential chronic wound scenarios. Biomater. Adv. 2023, 151, 213488. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, A.; Azoia, N.G.; Carvalho, A.C.; Gomes, A.C.; Güebitz, G.; Cavaco-Paulo, A. Tailoring elastase inhibition with synthetic peptides. Eur. J. Pharmacol. 2011, 666, 53–60. [Google Scholar] [CrossRef]
- Powers, J.C.; Plaskon, R.R.; Kam, C.-M. Low-molecular-weight inhibitors of neutrophil elastase. In Alpha 1-Antitrypsin Deficiency: Biology-Pathogenesis-Clinical Manifestations-Therapy; CRC Press: Boca Raton, FL, USA, 2014; Volume 341. [Google Scholar]
- Namjoshi, S.; Toth, I.; Blanchfield, J.T.; Trotter, N.; Mancera, R.L.; Benson, H.A.E. Enhanced Transdermal Peptide Delivery and Stability by Lipid Conjugation: Epidermal Permeation, Stereoselectivity and Mechanistic Insights. Pharm. Res. 2014, 31, 3304–3312. [Google Scholar] [CrossRef]
- Rocco, D.; Ross, J.; Murray, P.E.; Caccetta, R. Acyl lipidation of a peptide: Effects on activity and epidermal permeability in vitro. Drug Des. Dev. Ther. 2016, 10, 2203–2209. [Google Scholar]
- Strøm, M.B.; Rekdal, Ø.; Svendsen, J.S. Antimicrobial activity of short arginine-and tryptophan-rich peptides. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2002, 8, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Malikmammadov, E.; Tanir, T.E.; Kiziltay, A.; Hasirci, V.; Hasirci, N. PCL and PCL-based materials in biomedical applications. J. Biomater. Sci. Polym. Ed. 2018, 29, 863–893. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Xu, Y.; Lv, C.; Han, C.; Li, Y. Enhanced wound healing activity of PEG/PCL copolymer combined with bioactive nanoparticles in wound care after anorectal surgery: Via bio-inspired methodology. J. Photochem. Photobiol. B Biol. 2018, 187, 54–60. [Google Scholar] [CrossRef]
- Archer, E.; Torretti, M.; Madbouly, S. Biodegradable polycaprolactone (PCL) based polymer and composites. Phys. Sci. Rev. 2023, 8, 4391–4414. [Google Scholar] [CrossRef]
- D’souza, A.A.; Shegokar, R. Polyethylene glycol (PEG): A versatile polymer for pharmaceutical applications. Expert Opin. Drug Deliv. 2016, 13, 1257–1275. [Google Scholar] [CrossRef]
- Yu, H.; Jia, Y.; Yao, C.; Lu, Y. PCL/PEG core/sheath fibers with controlled drug release rate fabricated on the basis of a novel combined technique. Int. J. Pharm. 2014, 469, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Sowmya, B.; Panda, P. Electrospinning of poly (ε-caprolactone)(PCL) and poly ethylene glycol (PEG) composite nanofiber membranes using methyl ethyl ketone (MEK) and N N’-dimethyl acetamide (DMAc) solvent mixture for anti-adhesion applications. Mater. Today Commun. 2022, 33, 104718. [Google Scholar] [CrossRef]
- Alasbahi, R.; MELZIG, M. The in vitro inhibition of human neutrophil elastase activity by some Yemeni medicinal plants. Sci. Pharm. 2008, 76, 471–484. [Google Scholar] [CrossRef]
- Edwards, J.V.; Howley, P.; Cohen, I.K. In vitro inhibition of human neutrophil elastase by oleic acid albumin formulations from derivatized cotton wound dressings. Int. J. Pharm. 2004, 284, 1–12. [Google Scholar] [CrossRef]
- Wiegand, I.; Hilpert, K.; Hancock, R.E.W. Agar and broth dilution methods to determine the minimal inhibitory concentration (MIC) of antimicrobial substances. Nat. Protoc. 2008, 3, 163–175. [Google Scholar] [CrossRef] [PubMed]
- ASTM-D7334-08; Standard Practice for Surface Wettability of Coatings, Substrates and Pigments by Advancing Contact Angle Measurement. ASTM: West Conshohocken, PA, USA, 2022.
- ISO 9237; Determination of the Permeability of Fabrics to Air. ISO: Geneva, Switzerland, 1999.
- Gonçalves, M.M.; Carneiro, J.; Justus, B.; Espinoza, J.T.; Budel, J.M.; Farago, P.V.; Paula, J.P.d. Preparation and characterization of a novel antimicrobial film dressing for wound healing application. Braz. J. Pharm. Sci. 2021, 56, e18784. [Google Scholar] [CrossRef]
- Huang, A.; Peng, X.; Geng, L.; Zhang, L.; Huang, K.; Chen, B.; Gu, Z.; Kuang, T. Electrospun poly (butylene succinate)/cellulose nanocrystals bio-nanocomposite scaffolds for tissue engineering: Preparation, characterization and in vitro evaluation. Polym. Test. 2018, 71, 101–109. [Google Scholar] [CrossRef]
- ASTM E2149-20; Standard Test Method for Determining the Antimicrobial Activity of Antimicrobial Agents Under Dynamic Contact Conditions. ASTM: West Conshohocken, PA, USA, 2001.
- Marinaccio, L.; Stefanucci, A.; Scioli, G.; Della Valle, A.; Zengin, G.; Cichelli, A.; Mollica, A. Peptide Human Neutrophil Elastase Inhibitors from Natural Sources: An Overview. Int. J. Mol. Sci. 2022, 23, 2924. [Google Scholar] [CrossRef] [PubMed]
- Musa, N.H.; Chang, Z.H.; Teow, Y.H.; Rosli, N.A.; Mohammad, A.W. A review on polymer based antimicrobial coating. J. Biochem. Microbiol. Biotechnol. 2022, 10, 1–8. [Google Scholar] [CrossRef]
- Valuckaite, V.; Zaborina, O.; Long, J.; Hauer-Jensen, M.; Wang, J.; Holbrook, C.; Zaborin, A.; Drabik, K.; Katdare, M.; Mauceri, H. Oral PEG 15–20 protects the intestine against radiation: Role of lipid rafts. Am. J. Physiol.-Gastrointest. Liver Physiol. 2009, 297, G1041–G1052. [Google Scholar] [CrossRef]
- Tavares, T.D.; Antunes, J.C.; Padrão, J.; Ribeiro, A.I.; Zille, A.; Amorim, M.T.P.; Ferreira, F.; Felgueiras, H.P. Activity of specialized biomolecules against gram-positive and gram-negative bacteria. Antibiotics 2020, 9, 314. [Google Scholar] [CrossRef]
- Ruhal, R.; Kataria, R. Biofilm patterns in gram-positive and gram-negative bacteria. Microbiol. Res. 2021, 251, 126829. [Google Scholar] [CrossRef]
- Domingues, J.M.; Teixeira, M.O.; Teixeira, M.A.; Freitas, D.; Silva, S.F.d.; Tohidi, S.D.; Fernandes, R.D.; Padrão, J.; Zille, A.; Silva, C. Inhibition of Escherichia virus MS2, surrogate of SARS-CoV-2, via essential oils-loaded electrospun fibrous mats: Increasing the multifunctionality of antivirus protection masks. Pharmaceutics 2022, 14, 303. [Google Scholar] [CrossRef] [PubMed]
- de Pra, M.A.A.; Coelho, D.S.; Ribeiro-do-Valle, R.M.; Lopes-da-Silva, J.A.; Maraschin, M.; Veleirinho, B. The Effect of Polyethylene Glycol on Polycaprolactone Electrospun Scaffolds: Morphology, Mechanical Properties, and Nerve Growth Factor Delivery Profile. Nano Prog. 2020, 2, 24–31. [Google Scholar]
- Pan, L.; Yang, J.; Xu, L. Preparation and characterization of simvastatin-loaded PCL/PEG nanofiber membranes for drug sustained release. Molecules 2022, 27, 7158. [Google Scholar] [CrossRef]
- Abdelrazek, E.; Hezma, A.; El-Khodary, A.; Elzayat, A. Spectroscopic studies and thermal properties of PCL/PMMA biopolymer blend. Egypt. J. Basic Appl. Sci. 2016, 3, 10–15. [Google Scholar] [CrossRef]
- Gorodzha, S.; Surmeneva, M.; Surmenev, R. Fabrication and characterization of polycaprolactone cross-linked and highly-aligned 3-D artificial scaffolds for bone tissue regeneration via electrospinning technology. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2015. [Google Scholar]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR study of polycaprolactone chain organization at interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Padín-González, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the role and impact of poly (ethylene glycol)(PEG) on nanoparticle formulation: Implications for COVID-19 vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef] [PubMed]
- Kheilnezhad, B.; Hadjizadeh, A. Ibuprofen-loaded electrospun PCL/PEG nanofibrous membranes for preventing postoperative abdominal adhesion. ACS Appl. Bio Mater. 2022, 5, 1766–1778. [Google Scholar] [CrossRef] [PubMed]
- Lößlein, S.M.; Merz, R.; Müller, D.W.; Kopnarski, M.; Mücklich, F. An in-depth evaluation of sample and measurement induced influences on static contact angle measurements. Sci. Rep. 2022, 12, 19389. [Google Scholar] [CrossRef] [PubMed]
- Eğri, Ö.; Erdemir, N. Production of Hypericum perforatum oil-loaded membranes for wound dressing material and in vitro tests. Artif. Cells Nanomed. Biotechnol. 2019, 47, 1404–1415. [Google Scholar] [CrossRef] [PubMed]
- Wiegand, C.; Tittelbach, J.; Hipler, U.-C.; Elsner, P. Clinical efficacy of dressings for treatment of heavily exuding chronic wounds. Chronic Wound Care Manag. Res. 2015, 2, 101–111. [Google Scholar] [CrossRef]
- Homem, N.C.; Tavares, T.D.; Miranda, C.S.; Antunes, J.C.; Amorim, M.T.P.; Felgueiras, H.P. Functionalization of crosslinked sodium alginate/gelatin wet-spun porous fibers with nisin Z for the inhibition of Staphylococcus aureus-induced infections. Int. J. Mol. Sci. 2021, 22, 1930. [Google Scholar] [CrossRef]
- Dardeer, H.M.; Toghan, A.; Zaki, M.E.; Elamary, R.B. Design, synthesis and evaluation of novel antimicrobial polymers based on the inclusion of polyethylene glycol/TiO2 nanocomposites in cyclodextrin as drug carriers for sulfaguanidine. Polymers 2022, 14, 227. [Google Scholar] [CrossRef]
- Klode, J.; Schöttler, L.; Stoffels, I.; Körber, A.; Schadendorf, D.; Dissemond, J. Investigation of adhesion of modern wound dressings: A comparative analysis of 56 different wound dressings. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 933–939. [Google Scholar] [CrossRef]
- Acharyya, A.; Zhang, W.; Gai, F. Tryptophan as a template for development of visible fluorescent amino acids. J. Phys. Chem. B 2021, 125, 5458–5465. [Google Scholar] [CrossRef]
- Aimetti, A.A.; Tibbitt, M.W.; Anseth, K.S. Human Neutrophil Elastase Responsive Delivery from Poly(ethylene glycol) Hydrogels. Biomacromolecules 2009, 10, 1484–1489. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-J.; Cheng, Y.-A.; Chen, Y.-T.; Li, C.-C.; Huang, B.-C.; Hong, S.-T.; Chen, I.-J.; Ho, K.-W.; Chen, C.-Y.; Chen, F.-M. Targeting and internalizing PEGylated nanodrugs to enhance the therapeutic efficacy of hematologic malignancies by anti-PEG bispecific antibody (mPEG × CD20). Cancer Nanotechnol. 2023, 14, 78. [Google Scholar] [CrossRef]
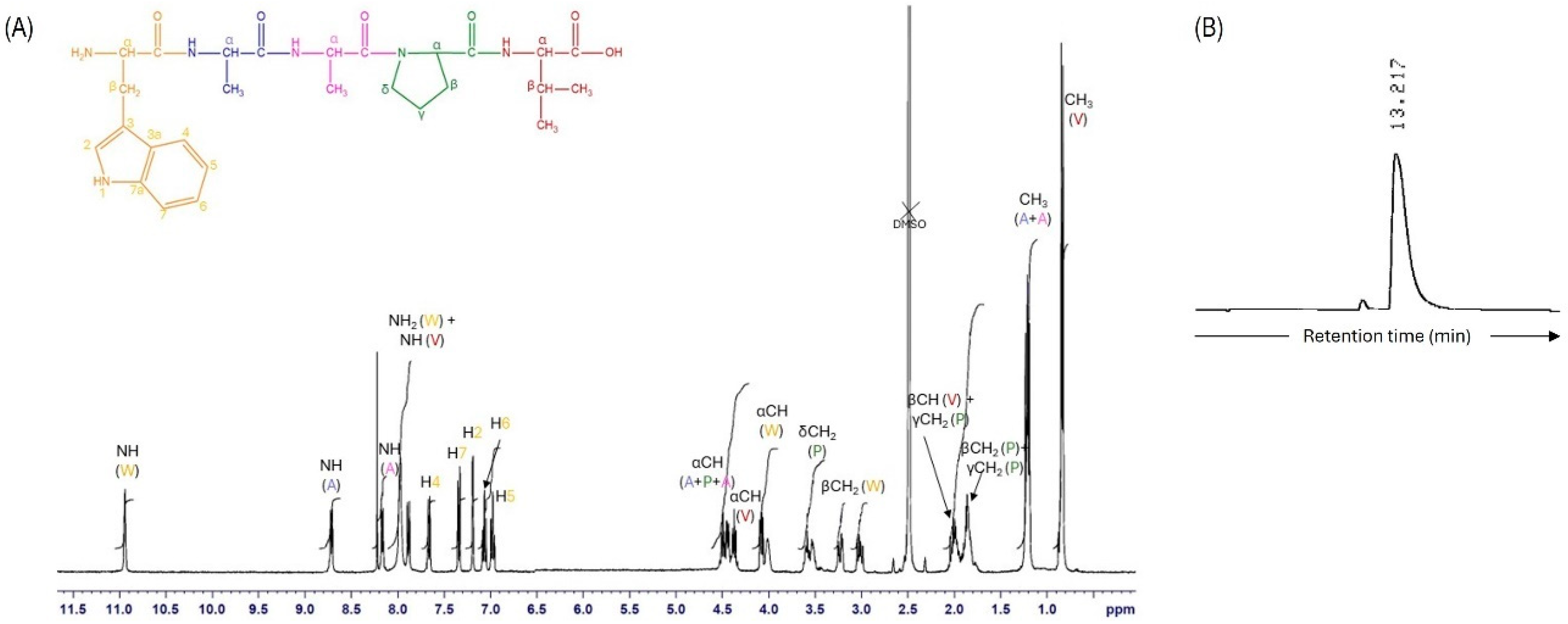
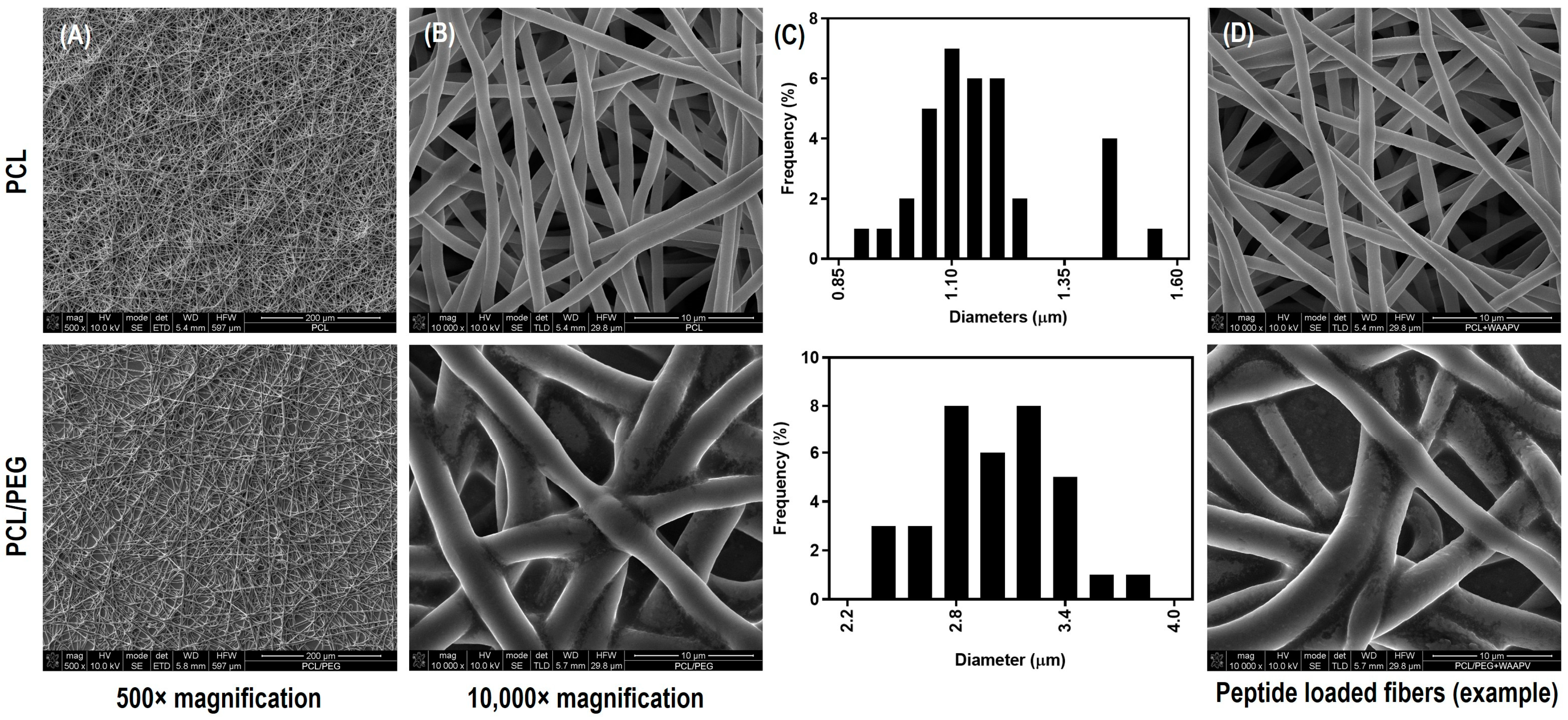
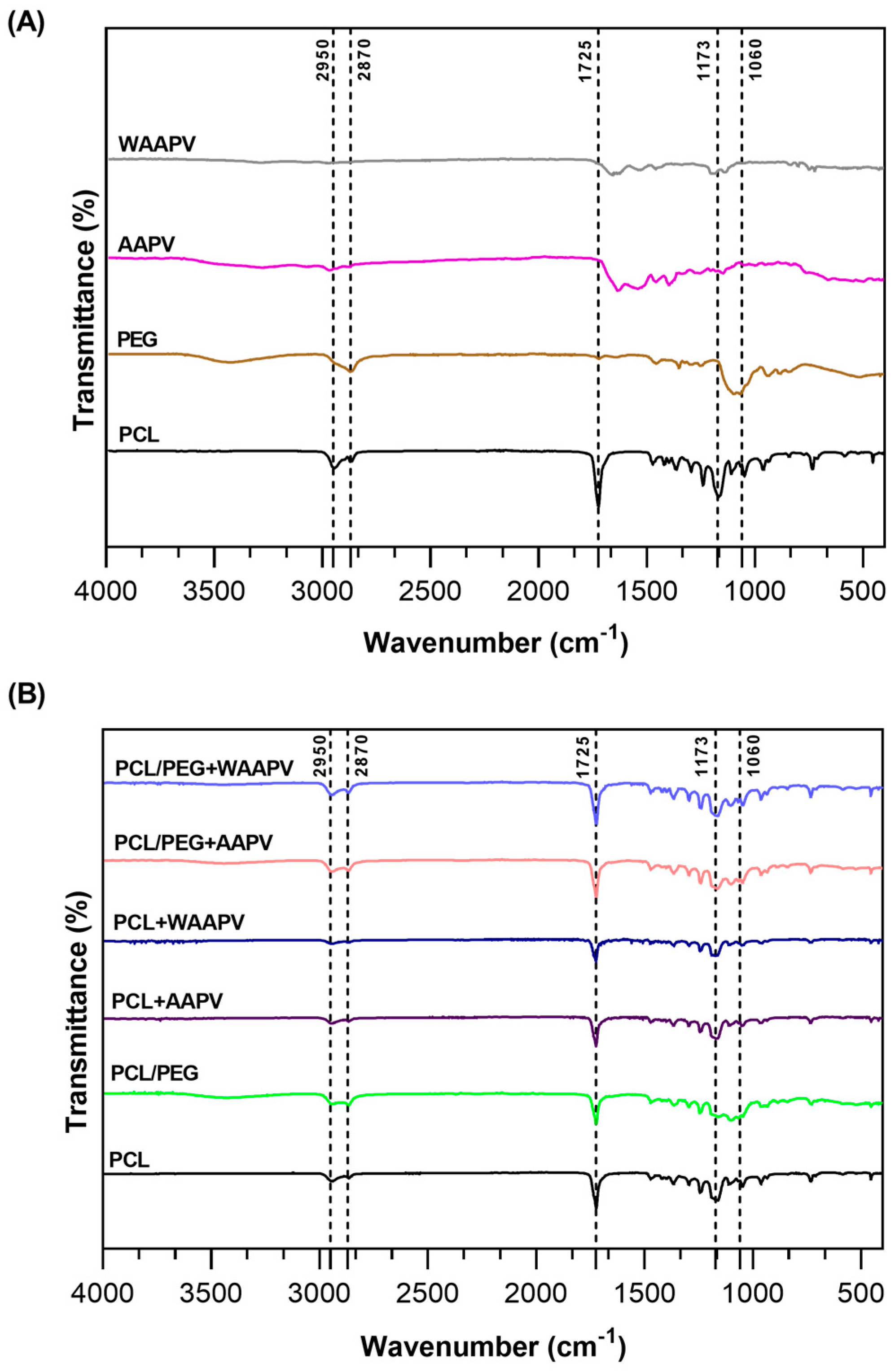
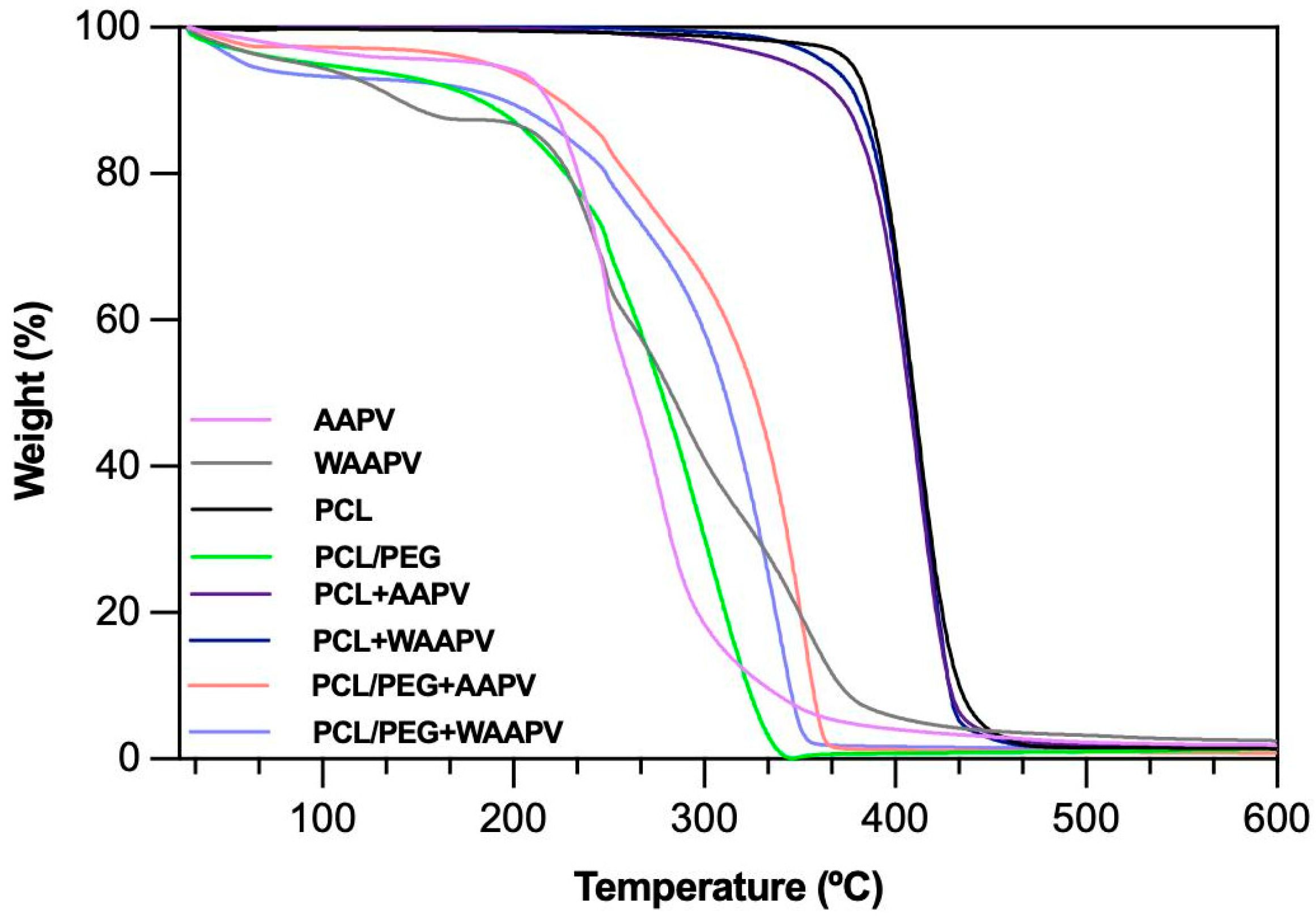



| Concentration (μg/mL) | HNE Inhibition (%) | |
|---|---|---|
| AAPV | WAAPV | |
| 200.00 | 5.74 ± 0.010 | 11.94 ± 6.530 |
| 100.00 | 9.53 ± 0.010 | 12.22 ± 9.426 |
| 50.00 | 16.89 ± 0.010 | 17.38 ± 5.441 |
| 25.00 | 12.69 ± 0.002 | 9.66 ± 9.400 |
| 12.50 | 9.56 ± 0.011 | 11.68 ± 1.811 |
| 6.25 | 8.84 ± 0.002 | 14.25 ± 3.009 |
| 3.13 | 8.07 ± 0.007 | 15.36 ± 8.564 |
| 1.56 | 6.43 ± 0.005 | 21.91 ± 3.562 |
| 0.78 | 4.07 ± 0.004 | 13.36 ± 6.001 |
| 0.39 | 3.43 ± 0.005 | 9.09 ± 10.200 |
| 0.20 | 2.37 ± 0.007 | 10.20 ± 3.002 |
| 0.10 | 0.79 ± 0.008 | 7.92 ± 1.781 |
| Samples | Water Contact Angle (°) | Image |
|---|---|---|
| PCL | 131.43 ± 9.04 |  |
| PCL/PEG | 17.16 ± 3.19 | 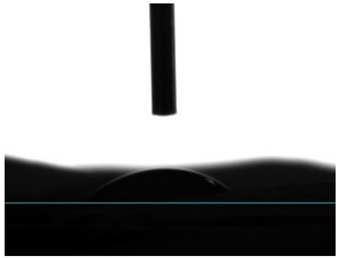 |
| PCL + AAPV | 109.55 ± 16.56 |  |
| PCL + WAAPV | 127.86 ± 6.08 |  |
| PCL/PEG + AAPV | 0 | - |
| PCL/PEG + WAAPV | 0 | - |
| Samples | Porosity (%) | Air Permeability (L/min) |
|---|---|---|
| PCL | 16.455 ± 0.066 | 0.523 ± 0.057 |
| PCL/PEG | 28.118 ± 1.001 | 1.878 ± 0.172 |
| PCL + AAPV | 15.012 ± 0.106 | 0.510 ± 0.018 |
| PCL + WAAPV | 17.588 ± 1.090 | 0.540 ± 0.105 |
| PCL/PEG + AAPV | 30.180 ± 1.255 | 1.997 ± 0.101 |
| PCL/PEG + WAAPV | 27.992 ± 1.880 | 1.769 ± 0.033 |
| Samples | Degree of Swelling (%) | |||
|---|---|---|---|---|
| 4 h | 6 h | 8 h | 24 h | |
| PCL | 33.65 ± 0.22 | 22.08 ± 0.20 | 14.75 ± 0.48 | 12.11 ± 0.05 |
| PCL/PEG | 91.18 ± 1.62 | 85.83 ± 2.31 | 79.60 ± 1.50 | 78.08 ± 1.59 |
| PCL + AAPV | 41.05 ± 1.00 | 33.36 ± 0.02 | 25.06 ± 0.81 | 27.23 ± 1.22 |
| PCL + WAAPV | 28.05 ± 1.09 | 12.84 ± 1.06 | 22.75 ± 0.54 | 20.41 ± 0.65 |
| PCL/PEG + AAPV | 98.01 ± 0.24 | 95.00 ± 0.68 | 88.60 ± 0.45 | 80.11 ± 0.79 |
| PCL/PEG + WAAPV | 87.15 ± 1.09 | 80.45 ± 1.06 | 62.00 ± 1.26 | 59.17 ± 0.44 |
| Samples | Mass Loss (%) | ||||||
|---|---|---|---|---|---|---|---|
| Day 1 | Day 2 | Day 3 | Day 4 | Day 5 | Day 6 | Day 7 | |
| PCL | −5.03 ± 0.11 | −26.17 ± 0.35 | −47.10 ± 1.78 | −62.21 ± 0.88 | −56.07 ± 0.95 | −67.12 ± 0.35 | −55.50 ± 1.17 |
| PCL/PEG | 15.02 ± 0.35 | 9.98 ± 1.23 | 17.66 ± 1.64 | 18.02 ± 1.04 | 17.82 ± 1.20 | 17.95 ± 0.99 | 20.43 ± 0.28 |
| PCL + AAPV | 1.88 ± 0.02 | −4.41 ± 0.23 | −8.22 ± 1.00 | −22.59 ± 1.02 | −36.08 ± 1.60 | −41.11 ± 0.55 | −40.06 ± 0.77 |
| PCL + WAAPV | −3.87 ± 0.88 | −16.25 ± 0.87 | −29.58 ± 1.07 | −42.00 ± 1.58 | −40.12 ± 0.33 | −50.24 ± 0.69 | −53.02 ± 0.94 |
| PCL/PEG + AAPV | 22.88 ± 0.16 | 19.00 ± 1.20 | 27.47 ± 0.48 | 28.11 ± 0.94 | 30.01 ± 1.55 | 29.05 ± 1.92 | 30.11 ± 0.97 |
| PCL/PEG + WAAPV | 18.12 ± 0.99 | 18.77 ± 0.03 | 19.51 ± 1.00 | 18.55 ± 0.58 | 22.12 ± 1.88 | 20.84 ± 0.67 | 20.00 ± 0.90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, A.R.M.; Miranda, C.S.; Silva, A.F.G.; Mendes, F.D.P.; Silva, B.M.; Oliveira, B.A.S.; Paiva, E.D.; Gonçalves, S.P.; Pereira-Lima, S.M.M.A.; Costa, S.P.G.; et al. Inhibition of Enzyme and Bacteria Activities in Diabetic Ulcer-like Scenarios via WAAPV-Loaded Electrospun Fibers. Pharmaceutics 2024, 16, 911. https://doi.org/10.3390/pharmaceutics16070911
Ribeiro ARM, Miranda CS, Silva AFG, Mendes FDP, Silva BM, Oliveira BAS, Paiva ED, Gonçalves SP, Pereira-Lima SMMA, Costa SPG, et al. Inhibition of Enzyme and Bacteria Activities in Diabetic Ulcer-like Scenarios via WAAPV-Loaded Electrospun Fibers. Pharmaceutics. 2024; 16(7):911. https://doi.org/10.3390/pharmaceutics16070911
Chicago/Turabian StyleRibeiro, Ana R. M., Catarina S. Miranda, Ana Francisca G. Silva, Filipa D. P. Mendes, Beatriz M. Silva, Bruna A. S. Oliveira, Eduardo D. Paiva, Sónia P. Gonçalves, Sílvia M. M. A. Pereira-Lima, Susana P. G. Costa, and et al. 2024. "Inhibition of Enzyme and Bacteria Activities in Diabetic Ulcer-like Scenarios via WAAPV-Loaded Electrospun Fibers" Pharmaceutics 16, no. 7: 911. https://doi.org/10.3390/pharmaceutics16070911
APA StyleRibeiro, A. R. M., Miranda, C. S., Silva, A. F. G., Mendes, F. D. P., Silva, B. M., Oliveira, B. A. S., Paiva, E. D., Gonçalves, S. P., Pereira-Lima, S. M. M. A., Costa, S. P. G., & Felgueiras, H. P. (2024). Inhibition of Enzyme and Bacteria Activities in Diabetic Ulcer-like Scenarios via WAAPV-Loaded Electrospun Fibers. Pharmaceutics, 16(7), 911. https://doi.org/10.3390/pharmaceutics16070911







