Clinical Ocular Exposure Extrapolation for a Complex Ophthalmic Suspension Using Physiologically Based Pharmacokinetic Modeling and Simulation
Abstract
:1. Introduction
2. Methods
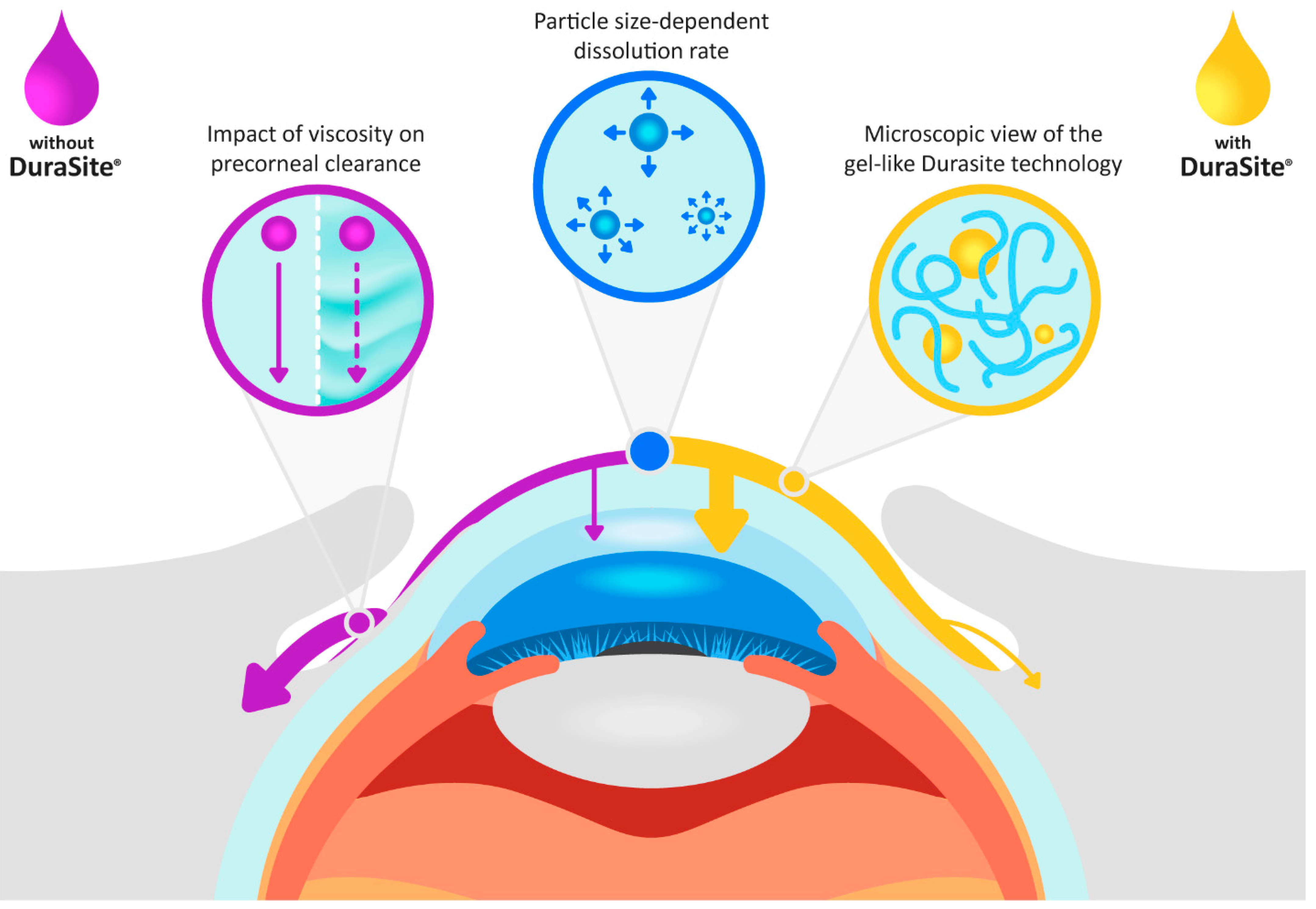
2.1. Besifloxacin Drug Product
2.2. Software and Model Structure
2.3. Parameterization of Besifloxacin Suspension Parameters
2.4. Ophthalmic PK Clinical Extrapolation Strategy
3. Results
3.1. Rabbit OCAT Models
3.2. Ophthalmic PK Clinical Extrapolation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Le Merdy, M.; Fan, J.; Bolger, M.B.; Lukacova, V.; Spires, J.; Tsakalozou, E.; Patel, V.; Xu, L.; Stewart, S.; Chockalingam, A.; et al. Application of Mechanistic Ocular Absorption Modeling and Simulation to Understand the Impact of Formulation Properties on Ophthalmic Bioavailability in Rabbits: A Case Study Using Dexamethasone Suspension. AAPS J. 2019, 21, 65. [Google Scholar] [CrossRef]
- U.S. FDA. Bioavailability and Bioequivalence Studies for Orally Administered Drug Products—General Considerations. 2002. Available online: https://www.fda.gov/files/drugs/published/Guidance-for-Industry-Bioavailability-and-Bioequivalence-Studies-for-Orally-Administered-Drug-Products---General-Considerations.PDF (accessed on 1 June 2024).
- U.S. FDA. Science & Research; 2024. Available online: https://www.fda.gov/drugs/generic-drugs/science-research (accessed on 1 June 2024).
- Bellantone, R.A.; Shah, K.B.; Patel, P.G.; Kaplan, M.; Xu, X.; Li, V.; Newman, B.; Abul Kaisar, M. Cyclosporine release and distribution in ophthalmic emulsions determined by pulsatile microdialysis. Int. J. Pharm. 2022, 615, 121521. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.H.; Lionberger, R.A. Clinical, Pharmacokinetic, and In Vitro Studies to Support Bioequivalence of Ophthalmic Drug Products. AAPS J. 2016, 18, 1032–1038. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Seo, P.; Lionberger, R. Current Scientific Considerations to Verify Physiologically-Based Pharmacokinetic Models and Their Implications for Locally Acting Products. CPT Pharmacomet. Syst. Pharmacol. 2019, 8, 347–351. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA. Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations. Available online: https://www.accessdata.fda.gov/scripts/cder/ob/ (accessed on 28 February 2022).
- Sager, J.E.; Yu, J.; Ragueneau-Majlessi, I.; Isoherranen, N. Physiologically Based Pharmacokinetic (PBPK) Modeling and Simulation Approaches: A Systematic Review of Published Models, Applications, and Model Verification. Drug Metab. Dispos. Biol. Fate Chem. 2015, 43, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, K.B.; Dedrick, R.L.; Zaharko, D.S.; Longstreth, J.A. Methotrexate pharmacokinetics. J. Pharm. Sci. 1971, 60, 1128–1133. [Google Scholar] [CrossRef] [PubMed]
- Worakul, N.; Robinson, J.R. Ocular pharmacokinetics/pharmacodynamics. Eur. J. Pharm. Biopharm. 1997, 44, 71–83. [Google Scholar] [CrossRef]
- Le Merdy, M.; AlQaraghuli, F.; Tan, M.-L.; Walenga, R.; Babiskin, A.; Zhao, L.; Lukacova, V. Clinical Ocular Exposure Extrapolation for Ophthalmic Solutions Using PBPK Modeling and Simulation. Pharm. Res. 2022, 40, 431–447. [Google Scholar] [CrossRef] [PubMed]
- U.S. FDA. Besivance® FDA Label. 2009. Available online: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/022308s013lbl.pdf (accessed on 1 June 2024).
- Le Merdy, M.; Tan, M.-L.; Babiskin, A.; Zhao, L. Physiologically Based Pharmacokinetic Model to Support Ophthalmic Suspension Product Development. AAPS J. 2020, 22, 26. [Google Scholar] [CrossRef]
- Malhotra, R.; Gira, J.; Berdy, G.J.; Brusatti, R. Safety of besifloxacin ophthalmic suspension 0.6% as a prophylactic antibiotic following routine cataract surgery: Results of a prospective, parallel-group, investigator-masked study. Clin. Ophthalmol. Auckl. NZ 2012, 6, 855–863. [Google Scholar] [CrossRef]
- Ward, K.W.; Lepage, J.-F.; Driot, J.-Y. Nonclinical pharmacodynamics, pharmacokinetics, and safety of BOL-303224-A, a novel fluoroquinolone antimicrobial agent for topical ophthalmic use. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2007, 23, 243–256. [Google Scholar] [CrossRef]
- Santa Cruz Biotechnology. Besifloxacin Hydrochloride (CAS 405165-61-9). 2024. Available online: https://www.scbt.com/p/besifloxacin-hydrochloride-405165-61-9 (accessed on 1 June 2024).
- Therapeutic Goods Administration. Australian Public Assessment Report for Besifloxacin Hydrochloride. 2014. Available online: https://www.tga.gov.au/sites/default/files/auspar-besifloxacin-hydrochloride-140203.pdf (accessed on 1 June 2024).
- Lu, A.T.K.; Frisella, M.E.; Johnson, K.C. Dissolution Modeling: Factors Affecting the Dissolution Rates of Polydisperse Powders. Pharm. Res. 1993, 10, 1308–1314. [Google Scholar] [CrossRef]
- Baush & Lomb BesivanceTM (Besifloxacin Ophthalmic Suspension) 0.6%—MATERIAL SAFETY DATA SHEET. Available online: https://s3-us-west-2.amazonaws.com/drugbank/msds/DB06771.pdf?1365982762 (accessed on 1 June 2024).
- Gu, X.-F.; Mao, B.-Y.; Xia, M.; Yang, Y.; Zhang, J.-L.; Yang, D.-S.; Wu, W.-X.; Du, Y.-X.; Di, B.; Su, M.-X. Rapid, sensitive and selective HPLC-MS/MS method for the quantification of topically applied besifloxacin in rabbit plasma and ocular tissues: Application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2016, 117, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Chung, J.L.; Lim, E.H.; Song, S.W.; Kim, B.Y.; Lee, J.H.; Mah, F.S.; Seo, K.Y. Comparative intraocular penetration of 4 fluoroquinolones after topical instillation. Cornea 2013, 32, 1046–1051. [Google Scholar] [CrossRef]
- Proksch, J.W.; Granvil, C.P.; Siou-Mermet, R.; Comstock, T.L.; Paterno, M.R.; Ward, K.W. Ocular pharmacokinetics of besifloxacin following topical administration to rabbits, monkeys, and humans. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2009, 25, 335–344. [Google Scholar] [CrossRef]
- Proksch, J.W.; Ward, K.W. Ocular pharmacokinetics/pharmacodynamics of besifloxacin, moxifloxacin, and gatifloxacin following topical administration to pigmented rabbits. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2010, 26, 449–458. [Google Scholar] [CrossRef]
- Torkildsen, G.; Proksch, J.W.; Shapiro, A.; Lynch, S.K.; Comstock, T.L. Concentrations of besifloxacin, gatifloxacin, and moxifloxacin in human conjunctiva after topical ocular administration. Clin. Ophthalmol. Auckl. NZ 2010, 4, 331–341. [Google Scholar]
- Donnenfeld, E.D.; Comstock, T.L.; Proksch, J.W. Human aqueous humor concentrations of besifloxacin, moxifloxacin, and gatifloxacin after topical ocular application. J. Cataract Refract. Surg. 2011, 37, 1082–1089. [Google Scholar] [CrossRef]
- Yoshida, J.; Kim, A.; Pratzer, K.A.; Stark, W.J. Aqueous penetration of moxifloxacin 0.5% ophthalmic solution and besifloxacin 0.6% ophthalmic suspension in cataract surgery patients. J. Cataract Refract. Surg. 2010, 36, 1499–1502. [Google Scholar] [CrossRef]
- Schoenwald, R.D.; Stewart, P. Effect of particle size on ophthalmic bioavailability of dexamethasone suspensions in rabbits. J. Pharm. Sci. 1980, 69, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Sieg, J.W.; Robinson, J.R. Mechanistic studies on transcorneal permeation of fluorometholone. J. Pharm. Sci. 1981, 70, 1026–1029. [Google Scholar] [CrossRef] [PubMed]
- Sieg, J.W.; Robinson, J.R. Vehicle Effects on Ocular Drug Bioavailability I: Evaluation of Fluorometholone. J. Pharm. Sci. 1975, 64, 931–936. [Google Scholar] [CrossRef]
- Toropainen, E.; Fraser-Miller, S.J.; Novakovic, D.; Del Amo, E.M.; Vellonen, K.-S.; Ruponen, M.; Viitala, T.; Korhonen, O.; Auriola, S.; Hellinen, L.; et al. Biopharmaceutics of Topical Ophthalmic Suspensions: Importance of Viscosity and Particle Size in Ocular Absorption of Indomethacin. Pharmaceutics 2021, 13, 452. [Google Scholar] [CrossRef] [PubMed]
- Le Merdy, M.; Spires, J.; Lukacova, V.; Tan, M.-L.; Babiskin, A.; Xu, X.; Zhao, L.; Bolger, M.B. Ocular Physiologically Based Pharmacokinetic Modeling for Ointment Formulations. Pharm. Res. 2020, 37, 245. [Google Scholar] [CrossRef] [PubMed]
- Patton, T.F.; Robinson, J.R. Ocular evaluation of polyvinyl alcohol vehicle in rabbits. J. Pharm. Sci. 1975, 64, 1312–1316. [Google Scholar] [CrossRef] [PubMed]
- Bowman, L.M.; Si, E.; Pang, J.; Archibald, R.; Friedlaender, M. Development of a topical polymeric mucoadhesive ocular delivery system for azithromycin. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther. 2009, 25, 133–139. [Google Scholar] [CrossRef]
- Sousa, J.; Alves, G.; Abrantes, J.; Fortuna, A.; Falcão, A. Analytical methods for determination of new fluoroquinolones in biological matrices and pharmaceutical formulations by liquid chromatography: A review. Anal. Bioanal. Chem. 2012, 403, 93–129. [Google Scholar] [CrossRef]



| Chemical | Role |
|---|---|
| Besifloxacin | Active pharmaceutical ingredient |
| Polycarbophil | Bioadhesive support matrix |
| Mannitol | Diluent, Tonicity agent |
| Poloxamer 407 | Wetting agent, Viscosity enhancer |
| Sodium chloride | Tonicity agent |
| Edetate disodium dihydrate | Preservatives, Chelating agent |
| Sodium hydroxide | Buffering agent |
| Water | Volume for injection |
| Benzalkonium chloride | Preservative |
| Parameter | Definition | Units | Besifloxacin | |
|---|---|---|---|---|
| Physicochemical Properties | Value | Source | ||
| MWt | Molecular weight | g/mol | 393.85 | AP10.4 * |
| logP(neutral) | Log octanol/water partition coefficient | - | 0.26 | AP10.4 |
| Fu | Plasma unbound percent | % | 41.5 | [15] |
| Fu melanin | Percent unbound to melanin | % | 100 | GP ** |
| Rbp | Blood-to-plasma-concentration ratio | 1.33 | AP10.4 | |
| Solubility | Maximum amount dissolved in water *** | µg/mL | 1 (pH 7) | [16] |
| pKa | Acidity constant | - | 1.8/6/9.9 | [17] |
| Base/Acid/Base | ||||
| Peff | Intestinal permeability | ×10−4 cm/s | 0.39 | AP10.4 |
| Systemic parameters | ||||
| Vc | Rabbit volume of distribution | L/kg | 1.62 | AP10.4 |
| CL | Rabbit systemic clearance | L/h | 15.42 | AP10.4 |
| OCAT™ parameters | ||||
| PermCornea_epi | Cornea epithelium permeability | ×10−7 cm/s | 1 | fitted |
| PermCornea_str | Cornea stroma permeability | ×10−5 cm/s | 1.86 | GP |
| PermConjunctiva | Conjunctiva permeability | ×10−7 cm/s | 1.4 | fitted |
| PermAH. | Aqueous humor permeability | ×10−6 cm/s | 8.51 | GP |
| PermICB | Iris–ciliary body permeability | ×10−4 cm/s | 7.74 | GP |
| PermSclera | Sclera permeability | ×10−5 cm/s | 1.02 | GP |
| PermChoroid | Choroid permeability | ×10−4 cm/s | 1.84 | GP |
| PermRetina | Retina permeability | ×10−5 cm/s | 1.73 | GP |
| PermV.H. | Vitreous humor permeability | ×10−6 cm/s | 6.7 | GP |
| SARChoroid | Choroid systemic absorption rate | ×10−4 s−1 | 2.75 | GP |
| SARRetina | Retina systemic absorption rate | ×10−3 s−1 | 1.2 | GP |
| SARConjunctiva | Conjunctiva systemic absorption rate | ×10−4 s−1 | 3.81 | GP |
| SARICB | Iris–ciliary body systemic absorption rate | ×10−3 s−1 | 5 | fitted |
| Formulation parameters | ||||
| PS | Suspended particles’ mean diameter | µm | 3 | assumed |
| Max | Weibull total released parameter | % | 25.47 | fitted |
| A | Weibull time scale | hb | 11.93 | fitted |
| b | Weibull shape | - | 0.88 | fitted |
| Study Code | Species | Conc (%W/V) | Dose | Volume (µL) | Tissue of Interest | Source |
|---|---|---|---|---|---|---|
| Preclinical Studies | ||||||
| Bes.NZ.1 | NZ | 0.6 | single | 50 | Cornea, Conj, AH, Tears, Plasma | [20] |
| Bes.NZ.2 | NZ | 0.6 | single | 50 | Cornea, Conj, AH | [21] |
| Bes.DB.1 | DB | 0.6 | single | 50 | Cornea, Conj, AH, Tears | [22] |
| Bes.DB.2 | DB | 0.6 | single | 50 | Cornea, Conj, AH, Tears | [23] |
| Clinical Studies | ||||||
| Bes.Hum.1 | HS | 0.6 | single | 50 | Conj | [24] |
| Bes.Hum.2 | HS | 0.6 | single | 50 | Tears, Plasma | [22] |
| Bes.Hum.3 | CP | 0.6 | single | 50 | AH | [25] |
| Bes.Hum.4 | CP | 0.6 | multiple | 50 | AH | [26] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le Merdy, M.; Spires, J.; Tan, M.-L.; Zhao, L.; Lukacova, V. Clinical Ocular Exposure Extrapolation for a Complex Ophthalmic Suspension Using Physiologically Based Pharmacokinetic Modeling and Simulation. Pharmaceutics 2024, 16, 914. https://doi.org/10.3390/pharmaceutics16070914
Le Merdy M, Spires J, Tan M-L, Zhao L, Lukacova V. Clinical Ocular Exposure Extrapolation for a Complex Ophthalmic Suspension Using Physiologically Based Pharmacokinetic Modeling and Simulation. Pharmaceutics. 2024; 16(7):914. https://doi.org/10.3390/pharmaceutics16070914
Chicago/Turabian StyleLe Merdy, Maxime, Jessica Spires, Ming-Liang Tan, Liang Zhao, and Viera Lukacova. 2024. "Clinical Ocular Exposure Extrapolation for a Complex Ophthalmic Suspension Using Physiologically Based Pharmacokinetic Modeling and Simulation" Pharmaceutics 16, no. 7: 914. https://doi.org/10.3390/pharmaceutics16070914





