Different Effects of Strong-Bonded Water with Different Degrees of Substitution of Sodium Sulfobutylether-β-cyclodextrin on Encapsulation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Materials
2.2. KFT
2.3. Computational Details
3. Results
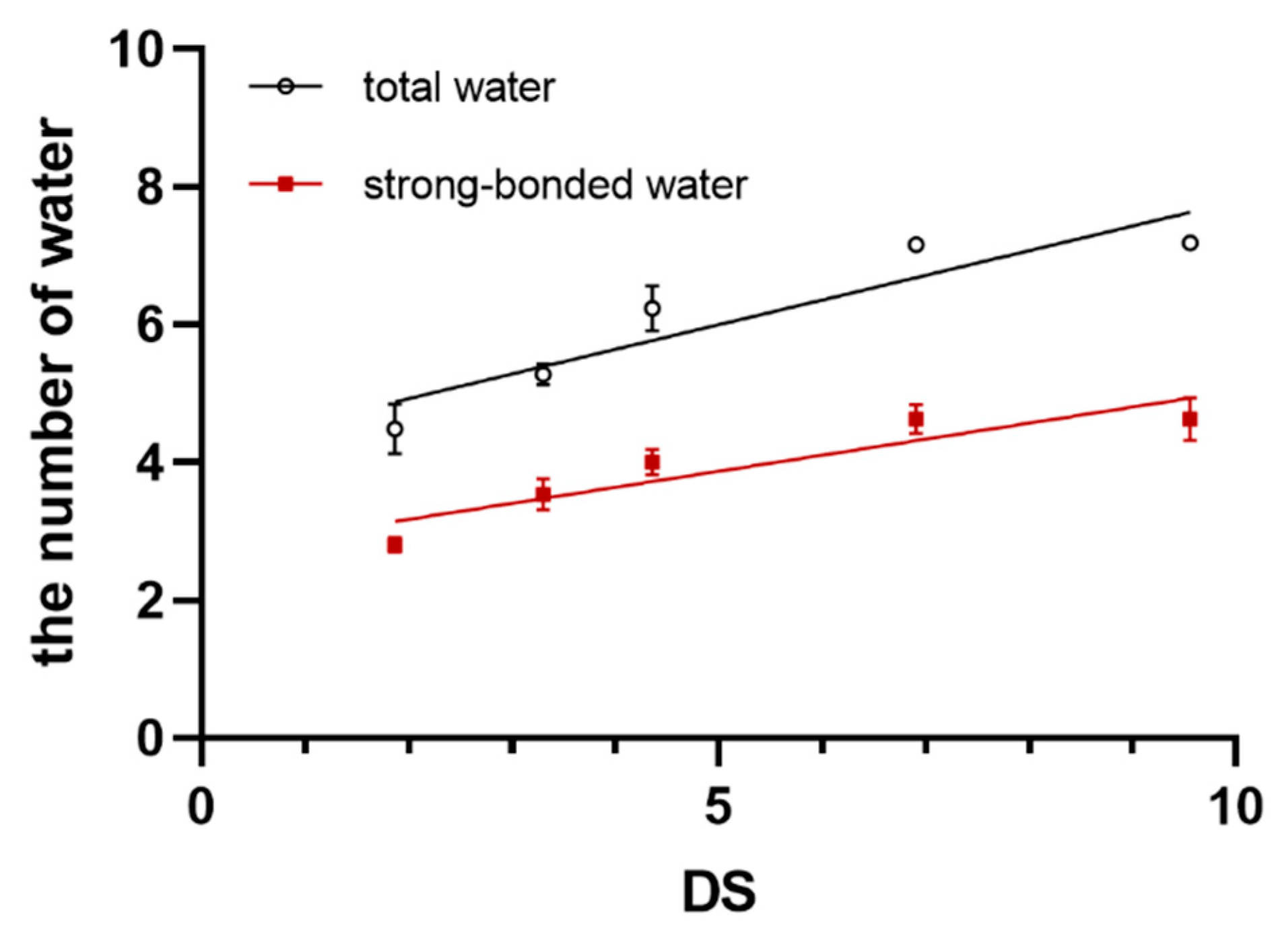
3.1. The Positive Correlation between the Content of SBW and DS
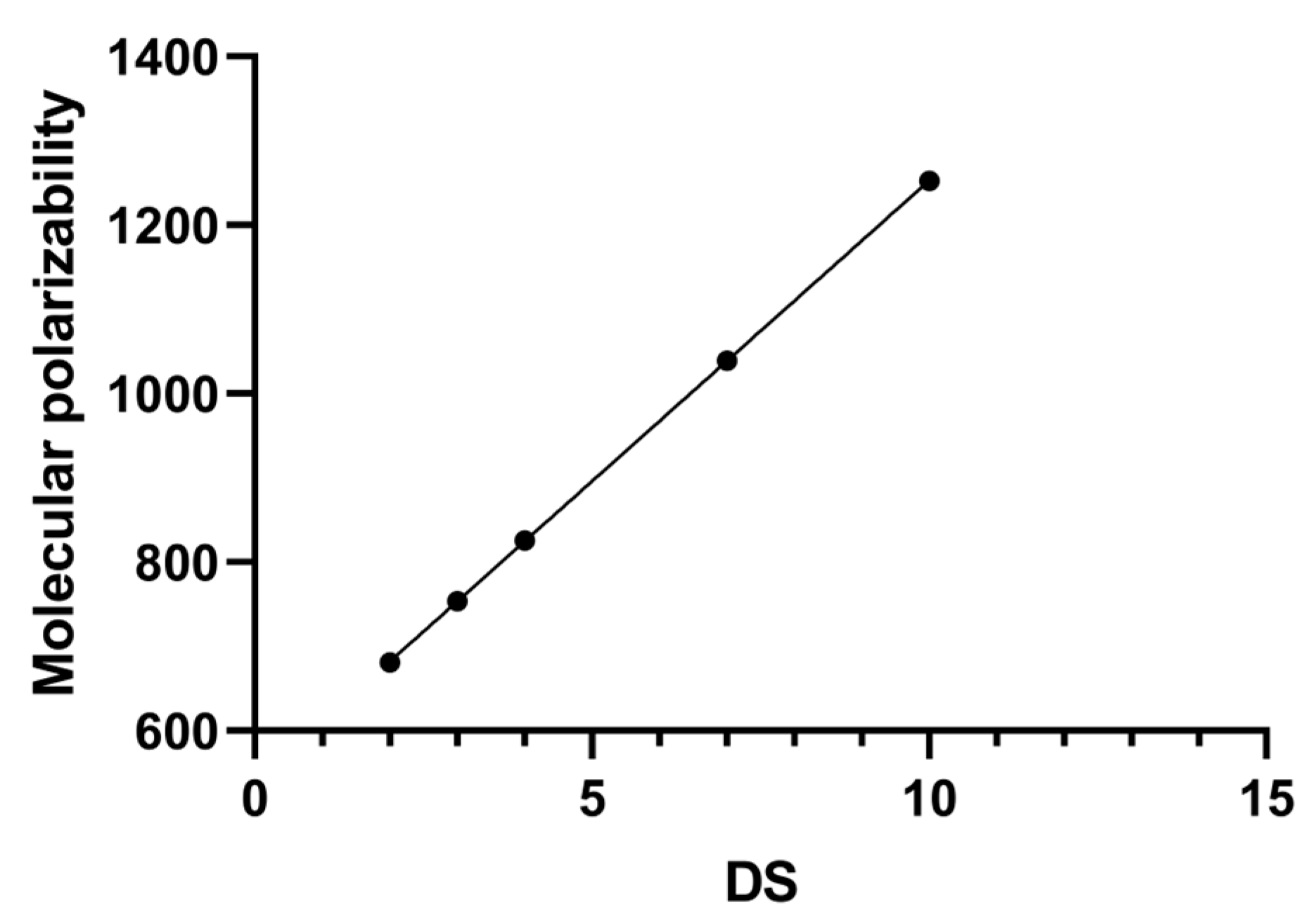
3.2. The Mechanism of DS Affecting the Content of SBW
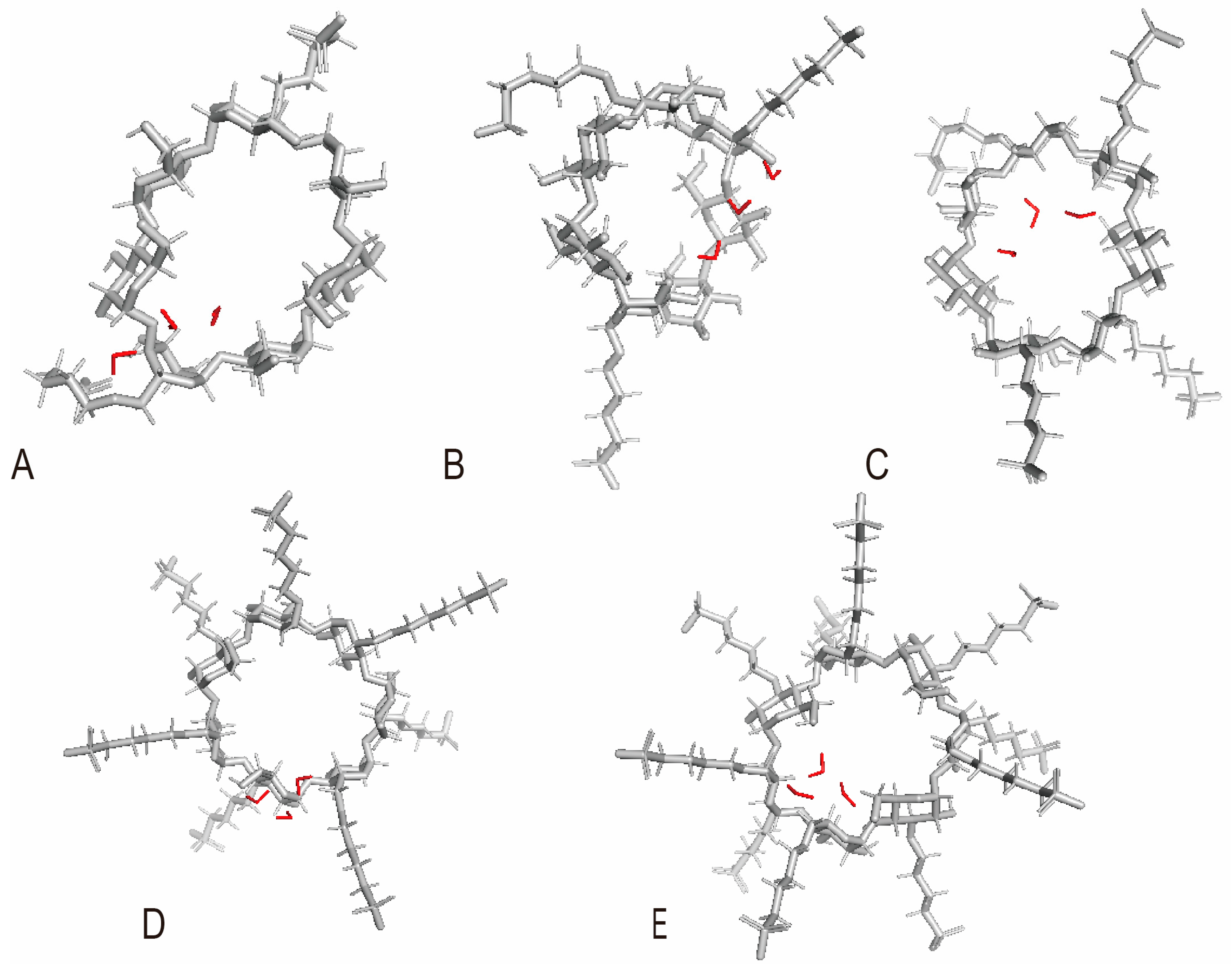
3.3. The Influence of SBW on Encapsulation
3.3.1. The Impact of the Quantity of SBW on the Encapsulation of SBE7-β-CD
3.3.2. The Effect of SBW in SBE-β-CD with Various DS on Encapsulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stella, V.J.; Rajewski, R.A. Sulfobutylether-Β-Cyclodextrin. Int. J. Pharm. 2020, 583, 119396. [Google Scholar] [CrossRef] [PubMed]
- Pardeshi, C.V.; Kothawade, R.V.; Markad, A.R.; Pardeshi, S.R.; Kulkarni, A.D.; Chaudhari, P.J.; Longhi, M.R.; Dhas, N.; Naik, J.B.; Surana, S.J.; et al. Sulfobutylether-Fi-Cyclodextrin: A Functional Biopolymer for Drug Delivery Applications. Carbohydr. Polym. 2023, 301, 120347. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Wang, X.; Huang, T.; Yang, Y.; Tu, J.; Zou, J.; Yang, H.; Yang, R. Application of Sodium Sulfobutylether-β-cyclodextrin Based on Encapsulation. Carbohydr. Polym. 2024, 333, 121985. [Google Scholar] [CrossRef] [PubMed]
- Jindrich, J.; Pitha, J.; Lindberg, B. Separation of Cyclodextrins and Their Derivatives by Thin-Layer and Preparative Column Chromatography. Carbohydr. Res. 1995, 275, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Ray, A.K.; Singh, P.K.; Pal, H. A Styryl Based Fluorogenic Probe with High Affinity for a Cyclodextrin Derivative. Org. Biomol. Chem. 2019, 17, 6895–6904. [Google Scholar] [CrossRef] [PubMed]
- Bayat, F.; Homami, S.S.; Monzavi, A.; Olyai, M.R.T.B. A Combined Molecular Docking and Molecular Dynamics Simulation Approach to Probing the Host-Guest Interactions of Ataluren with Natural and Modified Cyclodextrins. Mol. Simul. 2022, 48, 108–119. [Google Scholar] [CrossRef]
- Khomutov, S.M.; Sidorov, I.A.; Dovbnya, D.V.; Donova, M.V. Estimation of Cyclodextrin Affinity to Steroids. J. Pharm. Pharmacol. 2002, 54, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Oo, A.; Kerdpol, K.; Mahalapbutr, P.; Rungrotmongkol, T. Molecular Encapsulation of Emodin with Various β-Cyclodextrin Derivatives: A Computational Study. J. Mol. Liq. 2022, 347, 118002. [Google Scholar] [CrossRef]
- Varnai, B.; Malanga, M.; Sohajda, T.; Beni, S. Molecular Interactions in Remdesivir-Cyclodextrin Systems. J. Pharm. Biomed. Anal. 2022, 209, 114482. [Google Scholar] [CrossRef]
- Jafari, G.; Raissi, H.; Shahabi, M. Assessment of Sulfobutylether-β-cyclodextrin as a Promising Fluorometholone Molecule Container: DFT, Docking, Molecular Dynamics and Mm-PBSA Free Energy Calculations. Mol. Simul. 2022, 48, 168–175. [Google Scholar] [CrossRef]
- Maeda, H.; Shiobara, R.; Tanaka, M.; Kajinami, A.; Nakayama, H. Effect of Mechanochemical Inclusion of Triamterene into Sulfobutylether-β-cyclodextrin and Its Improved Dissolution Behavior. Drug Dev. Ind. Pharm. 2021, 47, 535–541. [Google Scholar] [CrossRef]
- Szente, L.; Puskas, I.; Sohajda, T.; Varga, E.; Vass, P.; Nagy, Z.K.; Farkas, A.; Varnai, B.; Beni, S.; Hazai, E. Sulfobutylether-β-cyclodextrin-Enabled Antiviral Remdesivir: Characterization of Electrospun- and Lyophilized Formulations. Carbohydr. Polym. 2021, 264, 118011. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, M.; Ilyich, T.; Zavodnik, I.; Palecz, B.; Stepniak, A. Studies of the Formation and Stability of Ezetimibe-Cyclodextrin Inclusion Complexes. Int. J. Mol. Sci. 2022, 23, 455. [Google Scholar] [CrossRef] [PubMed]
- Kadam, V.; Kakatkar, A.S.; Barooah, N.; Chatterjee, S.; Bhasikuttan, A.C.; Mohanty, J. Supramolecular Interaction of Sanguinarine Dye with Sulfobutylether-β-cyclodextrin: Modulation of the Photophysical Properties and Antibacterial Activity. RSC Adv. 2020, 10, 25370–25378. [Google Scholar] [CrossRef] [PubMed]
- Dsouza, R.N.; Pischel, U.; Nau, W.M. Fluorescent Dyes and Their Supramolecular Host/Guest Complexes with Macrocycles in Aqueous Solution. Chem. Rev. 2011, 111, 7941–7980. [Google Scholar] [CrossRef] [PubMed]
- Zhao, D.; Song, L.; Hou, L. Efficient Persistent Luminescence Tuning Using a Cyclodextrin Inclusion Complex as Efficient Light Conversion Materials. Acs Omega 2021, 6, 25585–25593. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Wang, Z.; Yuan, X.; Zhang, H.; Liu, Z.; Liu, Y. Configurationally Confined Multilevel Supramolecular Assemblies for Modulating Multicolor Luminescence. Adv. Funct. Mater. 2023, 33, 2300779. [Google Scholar] [CrossRef]
- Ogoshi, T.; Harada, A. Chemical Sensors Based on Cyclodextrin Derivatives. Sensors 2008, 8, 4961–4982. [Google Scholar] [CrossRef]
- Cong, L.; Ding, Z.; Lan, T.; Guo, M.; Yan, F.; Zhao, J. Simultaneous Determination of Nitrophenol Isomers Based on Reduced Graphene Oxide Modified with Sulfobutylether-β-cyclodextrin. Carbohydr. Polym. 2021, 271, 118446. [Google Scholar] [CrossRef]
- Brown, R.S.; Szolar, O.H.J.; Luong, J.H.T. Cyclodextrin-Aided Capillary Electrophoretic Separation and Laser-Induced Fluorescence Detection of Polynuclear Aromatic Hydrocarbons (PAHs). J. Mol. Recognit. 1996, 9, 515–523. [Google Scholar] [CrossRef]
- Ma, X.; Cao, J.; Yu, J.; Cai, L. Evaluation of an Ionic Liquid Chiral Selector Based on Sulfobutylether-β-cyclodextrin in Capillary Electrophoresis. J. Mol. Liq. 2022, 362, 119782. [Google Scholar] [CrossRef]
- The United States Pharmacopeia. In Monograph: Betadex Sulfobutyl Ether Sodium; The United States Pharmacopeial Convention: Rockville, MD, USA, 2023.
- Thompson, D.O. Cyclodextrins—Enabling Excipients: Their Present and Future Use in Pharmaceuticals. Crit. Rev. Ther. Drug Carr. Syst. 1997, 14, 1–104. [Google Scholar] [CrossRef]
- Jain, A.C.; Adeyeye, M.C. Hygroscopicity, Phase Solubility and Dissolution of Various Substituted Sulfobutylether-β-cyclodextrins (SBE) and Danazol–SBE Inclusion Complexes. Int. J. Pharm. 2001, 212, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, G.; Chattaraj, S.; Pal, H. Ph Assisted Modulation in the Binding Affinity for Bodipy-Benzimidazole Conjugate with Anionic Cyclodextrin. J. Photochem. Photobiol. A Chem. 2023, 434, 114266. [Google Scholar] [CrossRef]
- Biedermann, F.; Nau, W.M.; Schneider, H. The Hydrophobic Effect Revisited—Studies with Supramolecular Complexes Imply High-Energy Water as a Noncovalent Driving Force. Angew. Chem. Int. Ed. 2014, 53, 11158–11171. [Google Scholar] [CrossRef] [PubMed]
- Loftsson, T.; Sigurdsson, H.H.; Jansook, P. Anomalous Properties of Cyclodextrins and Their Complexes in Aqueous Solutions. Materials 2023, 16, 2223. [Google Scholar] [CrossRef]
- Rekharsky, M.V.; Inoue, Y. Complexation Thermodynamics of Cyclodextrins. Chem. Rev. 1998, 98, 1875–1917. [Google Scholar] [CrossRef]
- Jafari, G.; Raissi, H.; Saberinasab, A.; Pasban, S. Phosphatidylcholine in the Tear Film of the Eye: Enhanced Topical Delivery of Fluorometholone to the Eye. Inorg. Chem. Commun. 2023, 150, 110506. [Google Scholar] [CrossRef]
- Pereva, S.; Nikolova, V.; Angelova, S.; Spassov, T.; Dudev, T. Water inside β-Cyclodextrin Cavity: Amount, Stability and Mechanism of Binding. Beilstein J. Org. Chem. 2019, 15, 1592–1600. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Isengard, H.-D. Water Content of Natural Cyclodextrins and Their Essential Oil Complexes: A Comparative Study between Karl Fischer Titration and Thermal Methods. Food Chem. 2012, 132, 1741–1748. [Google Scholar] [CrossRef]
- Hădărugă, N.G.; Hădărugă, D.I.; Isengard, H.-D. “Surface Water” and “Strong-Bonded Water” in Cyclodextrins: A Karl Fischer Titration Approach. J. Incl. Phenom. Macrocycl. Chem. 2012, 75, 297–302. [Google Scholar] [CrossRef]
- Gaussian 16 Rev. B.01; Gaussian: Wallingford, CT, USA, 2016.
- Zhao, Y.; Truhlar, D.G. The M06 Suite of Density Functionals for Main Group Thermochemistry, Thermochemical Kinetics, Noncovalent Interactions, Excited States, and Transition Elements: Two New Functionals and Systematic Testing of Four M06-Class Functionals and 12 Other Functionals. Theor. Chem. Acc. 2008, 120, 215–241. [Google Scholar]
- Hehre, W.J.; Ditchfield, R.; People, J.A. Self-Consistent Molecular Orbital Methods. XII. Further Extensions of Gaussian-Type Basis Sets for Use in Molecular Orbital Studies of Organic Molecules. J. Chem. Phys. 1972, 56, 2257–2261. [Google Scholar] [CrossRef]
- Dill, J.D.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XV. Extended Gaussian-Type Basis Sets for Lithium, Beryllium, and Boron. J. Chem. Phys. 1975, 62, 2921–2923. [Google Scholar] [CrossRef]
- Francl, M.M.; Pietro, W.J.; Hehre, W.J.; Binkley, J.S.; Gordon, M.S.; Defrees, D.J.; People, J.A. Self-Consistent Molecular Orbital Methods. XXIII. A Polarization-Type Basis Set for Second-Row Elements. J. Chem. Phys. 1982, 77, 3654–3665. [Google Scholar] [CrossRef]
- Rassolov, V.A.; Pople, J.A.; Ratner, M.A.; Windus, T.L. 6-31g* Basis Set for Atoms K through Zn. J. Chem. Phys. 1998, 109, 1223–1229. [Google Scholar] [CrossRef]
- Krishnan, R.; Binkley, J.S.; Seeger, R.; Pople, J.A. Self-Consistent Molecular Orbital Methods. XX. A Basis Set for Correlated Wave Functions. J. Chem. Phys. 1980, 72, 650–654. [Google Scholar] [CrossRef]
- Bannwarth, C.; Ehlert, S.; Grimme, S. Gfn2-Xtb-an Accurate and Broadly Parametrized Self-Consistent Tight-Binding Quantum Chemical Method with Multipole Electrostatics and Density-Dependent Dispersion Contributions. J. Chem. Theory Comput. 2019, 15, 1652–1671. [Google Scholar] [CrossRef]


| DS | Limit Range (%) |
|---|---|
| 1 | 0–0.3 |
| 2 | 0–0.9 |
| 3 | 0.5–5.0 |
| 4 | 2.0–10.0 |
| 5 | 10.0–20.0 |
| 6 | 15.0–25.0 |
| 7 | 20.0–30.0 |
| 8 | 10.0–25.0 |
| 9 | 2.0–12.0 |
| 10 | 0–4.0 |
| DS (Average ± Standard Deviation) (n = 3) | F | p | Pearson Correlation Coefficient | |||||
|---|---|---|---|---|---|---|---|---|
| 1.87 | 3.30 | 4.36 | 6.91 | 9.56 | ||||
| Total water | 4.49 ± 0.36 | 5.28 ± 0.15 | 6.24 ± 0.33 | 7.16 ± 0.04 | 7.19 ± 0.04 | 78.886 | 0.000 ** | 0.911 ** |
| SBW | 2.80 ± 0.11 | 3.54 ± 0.23 | 4.00 ± 0.19 | 4.63 ± 0.21 | 4.63 ± 0.31 | 37.030 | 0.000 ** | 0.885 ** |
| The Number of SBW | 3 | 4 | 5 |
|---|---|---|---|
| ΔH (cal/mol) | 27,945.3922 | 38,470.6708 | 47,125.2312 |
| ΔS (cal/mol) | 98.7140 | 137.1910 | 161.9910 |
| ΔG (cal/mol) | −1486 | −2433 | −1172 |
| DS | 2 | 3 | 4 | 7 | 10 |
|---|---|---|---|---|---|
| ΔH (cal/mol) | 58,464.1512 | 49,554.6063 | 31,432.1753 | 27,945.3922 | 31,805.9224 |
| ΔS (cal/mol) | 122.3380 | 115.0210 | 106.6270 | 98.7140 | 106.6240 |
| ΔG (cal/mol) | 21,989 | 15,261 | −359 | −1486 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Huang, J.; Yang, D.; Huang, T.; Yang, Y.; Tu, J.; Zou, J.; Sun, H.; Zhao, X.; Yang, R. Different Effects of Strong-Bonded Water with Different Degrees of Substitution of Sodium Sulfobutylether-β-cyclodextrin on Encapsulation. Pharmaceutics 2024, 16, 919. https://doi.org/10.3390/pharmaceutics16070919
Wang X, Huang J, Yang D, Huang T, Yang Y, Tu J, Zou J, Sun H, Zhao X, Yang R. Different Effects of Strong-Bonded Water with Different Degrees of Substitution of Sodium Sulfobutylether-β-cyclodextrin on Encapsulation. Pharmaceutics. 2024; 16(7):919. https://doi.org/10.3390/pharmaceutics16070919
Chicago/Turabian StyleWang, Xiaofeng, Jiaqi Huang, Dengchen Yang, Ting Huang, Yang Yang, Jiasheng Tu, Jian Zou, Huimin Sun, Xia Zhao, and Rui Yang. 2024. "Different Effects of Strong-Bonded Water with Different Degrees of Substitution of Sodium Sulfobutylether-β-cyclodextrin on Encapsulation" Pharmaceutics 16, no. 7: 919. https://doi.org/10.3390/pharmaceutics16070919
APA StyleWang, X., Huang, J., Yang, D., Huang, T., Yang, Y., Tu, J., Zou, J., Sun, H., Zhao, X., & Yang, R. (2024). Different Effects of Strong-Bonded Water with Different Degrees of Substitution of Sodium Sulfobutylether-β-cyclodextrin on Encapsulation. Pharmaceutics, 16(7), 919. https://doi.org/10.3390/pharmaceutics16070919







