Recent Advances in Photodynamic Therapy: Metal-Based Nanoparticles as Tools to Improve Cancer Therapy
Abstract
:1. Introduction
2. Metal Nanoparticles and Their Role in Cancer Therapy
3. PDT: Mechanism of Action and Applications
4. MBNPs as PSs in PDT
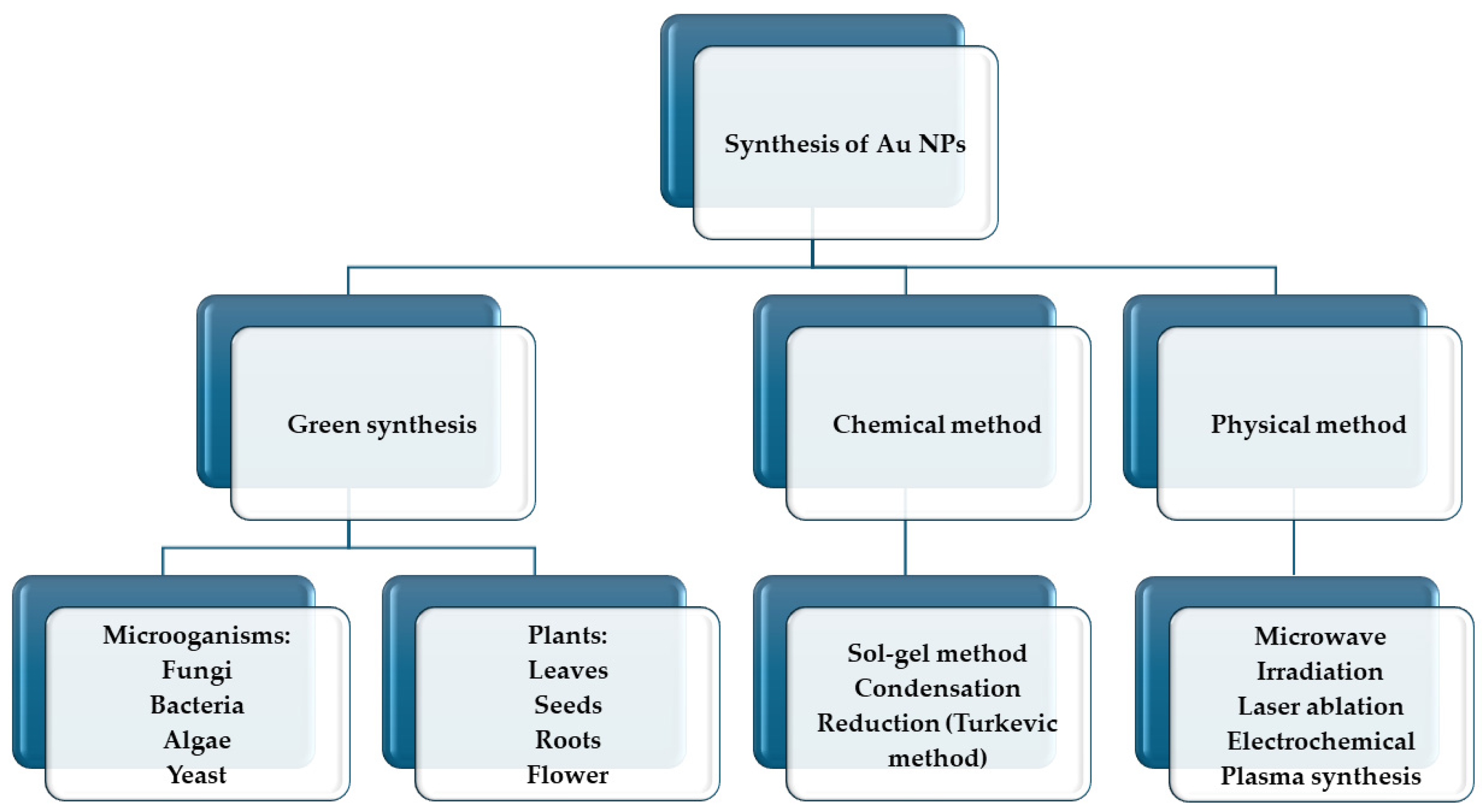
4.1. Gold Nanoparticles (AuNPs)
4.2. Silver Nanoparticles (AgNPs)
4.3. Titanium Dioxide Nanoparticles (TiO2NPs)
4.4. Magnetic Nanoparticles (MNPs)
5. Challenges and Limitations in MBNP-Mediated PDT
6. Conclusions: Future Directions and Opportunities in Research for Cancer Therapy
Author Contributions
Funding
Conflicts of Interest
References
- Westwood, L.; Nixon, I.J.; Emmerson, E.; Callanan, A. The Road after Cancer: Biomaterials and Tissue Engineering Approaches to Mediate the Tumor Microenvironment Post-Cancer Treatment. Front. Biomater. Sci. 2024, 3, 1347324. [Google Scholar] [CrossRef]
- Ingole, S.; Vasdev, N.; Tekade, M.; Gupta, T.; Pawar, B.; Mhatre, M.; Prasad, A.G.; Tekade, R.K. Toxic Effects of Cancer Therapies. In Public Health and Toxicology Issues Drug Research; Elsevier: Amsterdam, The Netherlands, 2024; Volume 2, pp. 353–379. [Google Scholar] [CrossRef]
- Anand, U.; Dey, A.; Chandel, A.K.S.; Sanyal, R.; Mishra, A.; Pandey, D.K.; De Falco, V.; Upadhyay, A.; Kandimalla, R.; Chaudhary, A. Cancer Chemotherapy and beyond: Current Status, Drug Candidates, Associated Risks and Progress in Targeted Therapeutics. Genes Dis. 2023, 10, 1367–1401. [Google Scholar] [CrossRef] [PubMed]
- Bogart, J.A.; Waqar, S.N.; Mix, M.D. Radiation and Systemic Therapy for Limited-Stage Small-Cell Lung Cancer. J. Clin. Oncol. 2022, 40, 661. [Google Scholar] [CrossRef]
- Mekuye, B.; Abera, B. Nanomaterials: An Overview of Synthesis, Classification, Characterization, and Applications. Nano Select 2023, 4, 486–501. [Google Scholar] [CrossRef]
- Xu, M.; Han, X.; Xiong, H.; Gao, Y.; Xu, B.; Zhu, G.; Li, J. Cancer Nanomedicine: Emerging Strategies and Therapeutic Potentials. Molecules 2023, 28, 5145. [Google Scholar] [CrossRef] [PubMed]
- Tuli, H.S.; Joshi, R.; Kaur, G.; Garg, V.K.; Sak, K.; Varol, M.; Kaur, J.; Alharbi, S.A.; Alahmadi, T.A.; Aggarwal, D. Metal Nanoparticles in Cancer: From Synthesis and Metabolism to Cellular Interactions. J. Nanostruct. Chem. 2023, 13, 321–348. [Google Scholar] [CrossRef]
- Vergallo, C.; Panzarini, E.; Carata, E.; Ahmadi, M.; Mariano, S.; Tenuzzo, B.A.; Dini, L. Cytotoxicity of β-D-glucose/sucrose-coated silver nanoparticles depends on cell type, nanoparticles concentration and time of incubation. AIP Conf. Proc. 2016, 1749, 020012. [Google Scholar] [CrossRef]
- Panzarini, E.; Mariano, S.; Dini, L. Glycans coated silver nanoparticles induces autophagy and necrosis in HeLa cells. AIP Conf. Proc. 2015, 1667, 020017. [Google Scholar]
- Talei, M.R. Metal Nanoparticles as Novel Drug Delivery Systems: A Review of Current Challenges and Opportunities. Int. J. Nat. Sci. Nanotechnol. 2023, 4, 113–140. [Google Scholar] [CrossRef]
- Mariano, S.; Panzarini, E.; Inverno, M.D.; Voulvoulis, N.; Dini, L. Toxicity, bioaccumulation and biotransformation of glucose-capped silver nanoparticles in green microalgae Chlorella vulgaris. Nanomaterials 2020, 10, 1377. [Google Scholar] [CrossRef]
- Narayan, N.; Meiyazhagan, A.; Vajtai, R. Metal Nanoparticles as Green Catalysts. Materials 2019, 12, 3602. [Google Scholar] [CrossRef] [PubMed]
- Vogt, C.; Meirer, F.; Monai, M.; Groeneveld, E.; Ferri, D.; van Santen, R.A.; Nachtegaal, M.; Unocic, R.R.; Frenkel, A.I.; Weckhuysen, B.M. Dynamic Restructuring of Supported Metal Nanoparticles and Its Implications for Structure Insensitive Catalysis. Nat. Commun. 2021, 12, 7096. [Google Scholar] [CrossRef] [PubMed]
- Ai, K.; Huang, J.; Xiao, Z.; Yang, Y.; Bai, Y.; Peng, J. Localized Surface Plasmon Resonance Properties and Biomedical Applications of Copper Selenide Nanomaterials. Mater. Today Chem. 2021, 20, 100402. [Google Scholar] [CrossRef]
- Wang, L.; Hasanzadeh Kafshgari, M.; Meunier, M. Optical Properties and Applications of Plasmonic-metal Nanoparticles. Adv. Funct. Mater. 2020, 30, 2005400. [Google Scholar] [CrossRef]
- Guo, Z.; Yu, G.; Zhang, Z.; Han, Y.; Guan, G.; Yang, W.; Han, M.-Y. Intrinsic Optical Properties and Emerging Applications of Gold Nanostructures. Adv. Mater. 2023, 35, 2206700. [Google Scholar] [CrossRef] [PubMed]
- Freitas, S.C.; Sanderson, D.; Caspani, S.; Magalhães, R.; Cortés-Llanos, B.; Granja, A.; Reis, S.; Belo, J.H.; Azevedo, J.; Gómez-Gaviro, M.V. New Frontiers in Colorectal Cancer Treatment Combining Nanotechnology with Photo-and Radiotherapy. Cancers 2023, 15, 383. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Wu, Y.; Zuo, H.; Chen, W.; Wang, K. Metal Nanoparticles as Novel Agents for Lung Cancer Diagnosis and Therapy. Small 2023, 19, 2206624. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Chen, L.; Wang, Y.; Zhang, L.; Zheng, X.; Yang, Y.; Zhu, Y. Tumor Microenvironment Responsive Metal Nanoparticles in Cancer Immunotherapy. Front. Immunol. 2023, 14, 1237361. [Google Scholar] [CrossRef] [PubMed]
- De la Encarnación Bermúdez, C. Magnetic-Plasmonic Nanoparticles for Multimodal Bioimaging and Hyperthermia; Universidad del País Vasco, Euskal Herriko Unibertsitatea: Biscay, Spain, 2023. [Google Scholar]
- Desai, N.; Momin, M.; Khan, T.; Gharat, S.; Ningthoujam, R.S.; Omri, A. Metallic Nanoparticles as Drug Delivery System for the Treatment of Cancer. Expert Opin. Drug Deliv. 2021, 18, 1261–1290. [Google Scholar] [CrossRef]
- Yang, W.; Xia, B.; Wang, L.; Ma, S.; Liang, H.; Wang, D.; Huang, J. Shape Effects of Gold Nanoparticles in Photothermal Cancer Therapy. Mater. Today Sustain. 2021, 13, 100078. [Google Scholar] [CrossRef]
- Wang, R.; Yang, H.; Fu, R.; Su, Y.; Lin, X.; Jin, X.; Du, W.; Shan, X.; Huang, G. Biomimetic Upconversion Nanoparticles and Gold Nanoparticles for Novel Simultaneous Dual-Modal Imaging-Guided Photothermal Therapy of Cancer. Cancers 2020, 12, 3136. [Google Scholar] [CrossRef] [PubMed]
- Liao, S.; Yue, W.; Cai, S.; Tang, Q.; Lu, W.; Huang, L.; Qi, T.; Liao, J. Improvement of Gold Nanorods in Photothermal Therapy: Recent Progress and Perspective. Front. Pharmacol. 2021, 12, 664123. [Google Scholar] [CrossRef] [PubMed]
- Alhussan, A.; Bozdoğan, E.P.D.; Chithrani, D.B. Combining Gold Nanoparticles with Other Radiosensitizing Agents for Unlocking the Full Potential of Cancer Radiotherapy. Pharmaceutics 2021, 13, 442. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Han, G.; Chen, Q.; Yu, L.; Wang, P.; Zhang, Q.; Dong, J.; Zhang, W.; Huang, J. Au-Pt Nanoparticle Formulation as a Radiosensitizer for Radiotherapy with Dual Effects. Int. J. Nanomed. 2021, 16, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Thiruppathi, J.; Vijayan, V.; Park, I.-K.; Lee, S.E.; Rhee, J.H. Enhancing Cancer Immunotherapy with Photodynamic Therapy and Nanoparticle: Making Tumor Microenvironment Hotter to Make Immunotherapeutic Work Better. Front. Immunol. 2024, 15, 1375767. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Cong, X. Surface-Engineered Nanoparticles in Cancer Immune Response and Immunotherapy: Current Status and Future Prospects. Biomed. Pharmacother. 2023, 157, 113998. [Google Scholar] [CrossRef] [PubMed]
- Prasad, R.; Selvaraj, K. Choice of Nanoparticles for Theranostics Engineering: Surface Coating to Nanovalves Approach. Nanotheranostics 2024, 8, 12. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Liang, M.; Lei, Q.; Li, G.; Wu, S. The Current Status of Photodynamic Therapy in Cancer Treatment. Cancers 2023, 15, 585. [Google Scholar] [CrossRef] [PubMed]
- Subhan, M.A.; Parveen, F.; Filipczak, N.; Yalamarty, S.S.K.; Torchilin, V.P. Approaches to Improve EPR-Based Drug Delivery for Cancer Therapy and Diagnosis. J. Pers. Med. 2023, 13, 389. [Google Scholar] [CrossRef]
- Maharjan, P.S.; Bhattarai, H.K. Singlet Oxygen, Photodynamic Therapy, and Mechanisms of Cancer Cell Death. J. Oncol. 2022, 2022, 7211485. [Google Scholar] [CrossRef]
- Lu, B.; Wang, L.; Tang, H.; Cao, D. Recent advances in type I organic photosensitizers for efficient photodynamic therapy for overcoming tumor hypoxia. J. Mater. Chem. B 2023, 11, 4600–4618. [Google Scholar] [CrossRef]
- Sharma, D.; Singh, S.; Kumar, P.; Jain, G.K.; Aggarwal, G.; Almalki, W.H.; Kesharwani, P. Mechanisms of Photodynamic Therapy. In Nanomaterials for Photodynamic Therapy; Elsevier: Amsterdam, The Netherlands, 2023; pp. 41–54. [Google Scholar] [CrossRef]
- Valerio, T.I.; Furrer, C.L.; Sadeghipour, N.; Patrock, S.-J.X.; Tillery, S.A.; Hoover, A.R.; Liu, K.; Chen, W.R. Immune Modulations of the Tumor Microenvironment in Response to Phototherapy. J. Innov. Opt. Health Sci. 2023, 16, 2330007. [Google Scholar] [CrossRef]
- Calvillo-Rodríguez, K.M.; Lorenzo-Anota, H.Y.; Rodríguez-Padilla, C.; Martínez-Torres, A.C.; Scott-Algara, D. Immunotherapies Inducing Immunogenic Cell Death in Cancer: Insight of the Innate Immune System. Front. Immunol. 2023, 14, 1294434. [Google Scholar] [CrossRef]
- Huis in‘t Veld, R.V.; Heuts, J.; Ma, S.; Cruz, L.J.; Ossendorp, F.A.; Jager, M.J. Current Challenges and Opportunities of Photodynamic Therapy against Cancer. Pharmaceutics 2023, 15, 330. [Google Scholar] [CrossRef]
- Warszyńska, M.; Repetowski, P.; Dąbrowski, J.M. Photodynamic therapy combined with immunotherapy: Recent advances and future research directions. Coord. Chem. Rev. 2023, 495, 215350. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, Z.; Wang, Y.; Wang, J.; Zhou, L.; Cheng, H.-B.; Yoon, J. Activatable Nano-Photosensitizers for Precise Photodynamic Cancer Therapy. Coord. Chem. Rev. 2023, 493, 215324. [Google Scholar] [CrossRef]
- Carobeli, L.R.; Santos, A.B.C.; Martins, L.B.M.; Damke, E.; Consolaro, M.E.L. Recent Advances in Photodynamic Therapy Combined with Chemotherapy for Cervical Cancer: A Systematic Review. Expert Rev. Anticancer Ther. 2024, 24, 263–282. [Google Scholar] [CrossRef]
- Aebisher, D.; Rogóż, K.; Myśliwiec, A.; Dynarowicz, K.; Wiench, R.; Cieślar, G.; Kawczyk-Krupka, A.; Bartusik-Aebisher, D. The Use of Photodynamic Therapy in Medical Practice. Front. Oncol. 2024, 14, 1373263. [Google Scholar] [CrossRef]
- Yao, Y.; Pan, L.; Song, W.; Yuan, Y.; Yan, S.; Yu, S.; Chen, S. Elsinochrome A Induces Cell Apoptosis and Autophagy in Photodynamic Therapy. J. Cell. Biochem. 2023, 124, 1346–1365. [Google Scholar] [CrossRef]
- Moloudi, K.; Abrahamse, H.; George, B.P. Photodynamic Therapy Induced Cell Cycle Arrest and Cancer Cell Synchronization. Front. Oncol. 2023, 13, 1225694. [Google Scholar] [CrossRef]
- Przygoda, M.; Bartusik-Aebisher, D.; Dynarowicz, K.; Cieślar, G.; Kawczyk-Krupka, A.; Aebisher, D. Cellular Mechanisms of Singlet Oxygen in Photodynamic Therapy. Int. J. Mol. Sci. 2023, 24, 16890. [Google Scholar] [CrossRef]
- Jiang, P.; Mizushima, N. Autophagy and Human Diseases. Cell Res. 2014, 24, 69–79. [Google Scholar] [CrossRef]
- García-Prat, L.; Martínez-Vicente, M.; Perdiguero, E.; Ortet, L.; Rodríguez-Ubreva, J.; Rebollo, E.; Ruiz-Bonilla, V.; Gutarra, S.; Ballestar, E.; Serrano, A.; et al. Autophagy maintains stemness by preventing senescence. Nature 2016, 529, 37–42. [Google Scholar] [CrossRef]
- Oliveira, C.S.; Turchiello, R.; Kowaltowski, A.J.; Indig, G.L.; Baptista, M.S. Major Determinants of Photoinduced Cell Death: Subcellular Localization versus Photosensitization Efficiency. Free Radic. Biol. Med. 2011, 51, 824–833. [Google Scholar] [CrossRef]
- Gunaydin, G.; Gedik, M.E.; Ayan, S. Photodynamic therapy for the treatment and diagnosis of cancer—A review of the current clinical status. Front. Chem. 2021, 9, 686303. [Google Scholar] [CrossRef]
- Kim, T.E.; Chang, J.-E. Recent Studies in Photodynamic Therapy for Cancer Treatment: From Basic Research to Clinical Trials. Pharmaceutics 2023, 15, 2257. [Google Scholar] [CrossRef]
- Mosaddad, S.A.; Mahootchi, P.; Rastegar, Z.; Abbasi, B.; Alam, M.; Abbasi, K.; Fani-Hanifeh, S.; Amookhteh, S.; Sadeghi, S.; Soufdoost, R.S. Photodynamic Therapy in Oral Cancer: A Narrative Review. Photobiomodul. Photomed. Laser Surg. 2023, 41, 248–264. [Google Scholar] [CrossRef]
- Hu, T.; Wang, Z.; Shen, W.; Liang, R.; Yan, D.; Wei, M. Recent Advances in Innovative Strategies for Enhanced Cancer Photodynamic Therapy. Theranostics 2021, 11, 3278. [Google Scholar] [CrossRef]
- Dantas, K.C.F.; Rosário, J.d.S.; Silva-Caldeira, P.P. Polymeric Nanosystems Applied for Metal-Based Drugs and Photosensitizers Delivery: The State of the Art and Recent Advancements. Pharmaceutics 2022, 14, 1506. [Google Scholar] [CrossRef]
- Chandrakala, V.; Aruna, V.; Angajala, G. Review on Metal Nanoparticles as Nanocarriers: Current Challenges and Perspectives in Drug Delivery Systems. Emergent Mater. 2022, 5, 1593–1615. [Google Scholar] [CrossRef]
- Son, J.; Yi, G.; Yoo, J.; Park, C.; Koo, H.; Choi, H.S. Light-Responsive Nanomedicine for Biophotonic Imaging and Targeted Therapy. Adv. Drug Deliv. Res. 2019, 138, 133–147. [Google Scholar] [CrossRef] [PubMed]
- Azharuddin, M.; Zhu, G.H.; Das, D.; Ozgur, E.; Uzun, L.; Turner, A.P.; Patra, H.K. A Repertoire of Biomedical Applications of Noble Metal Nanoparticles. Chem. Commun. 2019, 55, 6964–6996. [Google Scholar] [CrossRef] [PubMed]
- Fothergill, S.M.; Joyce, C.; Xie, F. Metal Enhanced Fluorescence Biosensing: From Ultra-Violet towards Second near-Infrared Window. Nanoscale 2018, 10, 20914–20929. [Google Scholar] [CrossRef] [PubMed]
- George, B.P.; Chota, A.; Sarbadhikary, P.; Abrahamse, H. Fundamentals and Applications of Metal Nanoparticle-Enhanced Singlet Oxygen Generation for Improved Cancer Photodynamic Therapy. Front. Chem. 2022, 10, 964674. [Google Scholar] [CrossRef] [PubMed]
- Mahboob, S.; Nivetha, R.; Gopinath, K.; Balalakshmi, C.; Al-Ghanim, K.A.; Al-Misned, F.; Ahmed, Z.; Govindarajan, M. Facile Synthesis of Gold and Platinum Doped Titanium Oxide Nanoparticles for Antibacterial and Photocatalytic Activity: A Photodynamic Approach. Photodiagn. Photodyn. Ther. 2021, 33, 102148. [Google Scholar] [CrossRef] [PubMed]
- Sultana, R.; Yadav, D.; Puranik, N.; Chavda, V.; Kim, J.; Song, M. A Review on the Use of Gold Nanoparticles in Cancer Treatment. Curr. Med. Chem. Anticancer Agents 2023, 23, 2171–2182. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, A.E.F.; Pereira, A.C.; Resende, M.A.; Ferreira, L.F. Gold Nanoparticles: A didactic step-by-step of the synthesis using the turkevich method, mechanisms, and characterizations. Analytica 2023, 4, 250–263. [Google Scholar] [CrossRef]
- Chota, A.; George, B.P.; Abrahamse, H. Recent advances in green metallic nanoparticles for enhanced drug delivery in photodynamic therapy: A therapeutic approach. Int. J. Mol. Sci. 2023, 24, 4808. [Google Scholar] [CrossRef] [PubMed]
- Lv, J.; Yi, Y.; Wu, G.; Liu, W. Gold nanotriangles: Green synthesis and PDT & PTT effect. Mater. Lett. 2017, 187, 148–150. [Google Scholar]
- Bhatia, P.; Verma, S.S. Enhancement of LSPR properties of temperature-dependent gold nanoparticles. Mater. Today Proc. 2023, 78, 871–876. [Google Scholar] [CrossRef]
- Truong, D.H.; Tran, P.T.T.; Tran, T.H. Nanoparticles as Carriers of Photosensitizers to Improve Photodynamic Therapy in Cancer. Pharm. Dev. Technol. 2024, 29, 221–235. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Ali, M.R.; Chen, K.; Fang, N.; El-Sayed, M.A. Gold Nanoparticles in Biological Optical Imaging. Nano Today 2019, 24, 120–140. [Google Scholar] [CrossRef]
- Polte, J.; Emmerling, F.; Radtke, M.; Reinholz, U.; Riesemeier, H.; Thünemann, A.F. Real-Time Monitoring of Copolymer Stabilized Growing Gold Nanoparticles. Langmuir 2010, 26, 5889–5894. [Google Scholar] [CrossRef] [PubMed]
- Koc, S.N.T.; Benam, S.R.; Aral, I.P.; Shahbazi, R.; Ulubayram, K. Gold Nanoparticles-Mediated Photothermal and Photodynamic Therapies for Cancer. Int. J. Pharm. 2024, 655, 124057. [Google Scholar] [CrossRef]
- Campu, A.; Focsan, M.; Lerouge, F.; Borlan, R.; Tie, L.; Rugina, D.; Astilean, S. ICG-Loaded Gold Nano-Bipyramids with NIR Activatable Dual PTT-PDT Therapeutic Potential in Melanoma Cells. Colloids Surf. B Biointerfaces 2020, 194, 111213. [Google Scholar] [CrossRef] [PubMed]
- Meyers, J.D.; Cheng, Y.; Broome, A.-M.; Agnes, R.S.; Schluchter, M.D.; Margevicius, S.; Wang, X.; Kenney, M.E.; Burda, C.; Basilion, J.P. Peptide-targeted Gold Nanoparticles for Photodynamic Therapy of Brain Cancer. Part. Part. Syst. Charact. 2015, 32, 448–457. [Google Scholar] [CrossRef] [PubMed]
- Debele, T.A.; Peng, S.; Tsai, H.-C. Drug Carrier for Photodynamic Cancer Therapy. Int. J. Mol. Sci. 2015, 16, 22094–22136. [Google Scholar] [CrossRef] [PubMed]
- Shang, L.-L.; Song, X.; Niu, C.-B.; Lv, Q.-Y.; Li, C.-L.; Cui, H.-F.; Zhang, S. Red Fluorescent Nanoprobe Based on Ag@ Au Nanoparticles and Graphene Quantum Dots for H2O2 Determination and Living Cell Imaging. Microchim. Acta 2021, 188, 291. [Google Scholar] [CrossRef] [PubMed]
- Wang, C. Study on the stability and targeting of AuNPs based on PEG and pepducin surface modification. TNS 2024, 33, 136–143. [Google Scholar] [CrossRef]
- Ovais, M.; Khalil, A.T.; Raza, A.; Khan, M.A.; Ahmad, I.; Islam, N.U.; Saravanan, M.; Ubaid, M.F.; Ali, M.; Shinwari, Z.K. Green Synthesis of Silver Nanoparticles via Plant Extracts: Beginning a New Era in Cancer Theranostics. Nanomedicine 2016, 12, 3157–3177. [Google Scholar] [CrossRef]
- Yesilot, S.; Aydin, C. Silver Nanoparticles; A New Hope in Cancer Therapy? East. J. Med. 2019, 24, 111–116. [Google Scholar] [CrossRef]
- Kah, G.; Chandran, R.; Abrahamse, H. Biogenic Silver Nanoparticles for Targeted Cancer Therapy and Enhancing Photodynamic Therapy. Cells 2023, 12, 2012. [Google Scholar] [CrossRef] [PubMed]
- Wei, L.; Lu, J.; Xu, H.; Patel, A.; Chen, Z.-S.; Chen, G. Silver Nanoparticles: Synthesis, Properties, and Therapeutic Applications. Drug Discov. Today 2015, 20, 595–601. [Google Scholar] [CrossRef] [PubMed]
- Mfouo-Tynga, I.; El-Hussein, A.; Abdel-Harith, M.; Abrahamse, H. Photodynamic Ability of Silver Nanoparticles in Inducing Cytotoxic Effects in Breast and Lung Cancer Cell Lines. Int. J. Nanomed. 2014, 9, 3771–3780. [Google Scholar]
- Khoza, P.; Ndhundhuma, I.; Karsten, A.; Nyokong, T. Photodynamic Therapy Activity of Phthalocyanine-Silver Nanoparticles on Melanoma Cancer Cells. J. Nanosci. Nanotechnol. 2020, 20, 3097–3104. [Google Scholar] [CrossRef]
- Damrongrungruang, T.; Puasiri, S.; Vongtavatchai, V.; Saeng-On, C.; Petcharapiruch, T.; Teerakapong, A.; Sangpanya, A. Anticandidal Efficacy of Erythrosine with Nano-TiO2 and Blue LED-Mediated Photodynamic Therapy against Candida Albicans Biofilms on Acrylic Resin: A Preliminary Study. Eur. J. Dent. 2024, 18, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Balas, M.; Nistorescu, S.; Badea, M.A.; Dinischiotu, A.; Boni, M.; Dinache, A.; Smarandache, A.; Udrea, A.-M.; Prepelita, P.; Staicu, A. Photodynamic Activity of TMPyP4/TiO2 Complex under Blue Light in Human Melanoma Cells: Potential for Cancer-Selective Therapy. Pharmaceutics 2023, 15, 1194. [Google Scholar] [CrossRef]
- Zhang, H.; Shan, Y.; Dong, L. A Comparison of TiO2 and ZnO Nanoparticles as Photosensitizers in Photodynamic Therapy for Cancer. J. Biomed. Nanotechnol. 2014, 10, 1450–1457. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Mahmoud, Z.H.; Abdullaev, S.; Ali, F.K.; Naeem, Y.A.; Mizher, R.M.; Karim, M.M.; Abdulwahid, A.S.; Ahmadi, Z.; Habibzadeh, S.; et al. Nano titanium oxide (nano-TiO2): A review of synthesis methods, properties, and applications. Case Stud. Chem. Environ. Eng. 2024, 9, 100626. [Google Scholar] [CrossRef]
- Sargazi, S.; Simge, E.R.; Gelen, S.S.; Rahdar, A.; Bilal, M.; Arshad, R.; Ajalli, N.; Khan, M.F.A.; Pandey, S. Application of Titanium Dioxide Nanoparticles in Photothermal and Photodynamic Therapy of Cancer: An Updated and Comprehensive Review. J. Drug Deliv. Sci. Technol. 2022, 75, 103605. [Google Scholar] [CrossRef]
- Akakuru, O.U.; Iqbal, Z.M.; Wu, A. TiO2 Nanoparticles: Properties and Applications. In TiO2 Nanoparticles: Applications in Nanobiotechnology and Nanomedicine; Wiley: Hoboken, NJ, USA, 2020; pp. 1–66. [Google Scholar] [CrossRef]
- Ziental, D.; Czarczynska-Goslinska, B.; Mlynarczyk, D.T.; Glowacka-Sobotta, A.; Stanisz, B.; Goslinski, T.; Sobotta, L. Titanium Dioxide Nanoparticles: Prospects and Applications in Medicine. Nanomaterials 2020, 10, 387. [Google Scholar] [CrossRef] [PubMed]
- Haghighi, F.H.; Mercurio, M.; Cerra, S.; Salamone, T.A.; Bianymotlagh, R.; Palocci, C.; Spica, V.R.; Fratoddi, I. Surface modification of TiO2 nanoparticles with organic molecules and their biological applications. J. Mater. Chem. B 2023, 11, 2334–2366. [Google Scholar] [CrossRef] [PubMed]
- Youssef, Z.; Jouan-Hureaux, V.; Colombeau, L.; Arnoux, P.; Moussaron, A.; Baros, F.; Toufaily, J.; Hamieh, T.; Roques-Carmes, T.; Frochot, C. Titania and silica nanoparticles coupled to Chlorin e6 for anti-cancer photodynamic therapy. Photodiagn. Photodyn. Ther. 2018, 22, 115–126. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Xie, Y.; Wu, J.; Wang, J.; Petković, M.; Stepić, M.; Zhao, J.; Ma, J.; Mi, L. Functional titanium dioxide nanoparticle conjugated with phthalocyanine and folic acid as a promising photosensitizer for targeted photodynamic therapy in vitro and in vivo. J. Photochem. Photobiol. B 2021, 215, 112122. [Google Scholar] [CrossRef] [PubMed]
- Pan, Q.; Li, M.; Xiao, M.; He, Y.; Sun, G.; Xue, T.; Luo, Y.; Chen, L.; Ai, B.; Xiong, J. Semiconductor Quantum Dots (CdX, X = S, Te, Se) Modify Titanium Dioxide Nanoparticles for Photodynamic Inactivation of Leukemia HL60 Cancer Cells. J. Nanomater. 2021, 2021, 4125350. [Google Scholar] [CrossRef]
- Salama, B.; Chang, C.J.; Kanehira, K.; El-Sherbini, E.S.; El-Sayed, G.; El-Adl, M.; Taniguchi, A. EGF Conjugation Improves Safety and Uptake Efficacy of Titanium Dioxide Nanoparticles. Molecules 2020, 25, 4467. [Google Scholar] [CrossRef]
- Nasir, A.; Khan, A.; Li, J.; Naeem, M.; Khalil, A.A.; Khan, K.; Qasim, M. Nanotechnology, a Tool for Diagnostics and Treatment of Cancer. Cur. Top. Med. Chem. 2021, 21, 1360–1376. [Google Scholar] [CrossRef]
- Fu, Y.; Jang, M.-S.; Liu, C.; Li, Y.; Lee, J.H.; Yang, H.Y. Oxygen-Generating Organic/Inorganic Self-Assembled Nanocolloids for Tumor-Activated Dual-Model Imaging-Guided Photodynamic Therapy. ACS Appl. Mater. Interfaces 2023, 15, 36013–36024. [Google Scholar] [CrossRef]
- Chen, Q.; Ma, X.; Xie, L.; Chen, W.; Xu, Z.; Song, E.; Zhu, X.; Song, Y. Iron-Based Nanoparticles for MR Imaging-Guided Ferroptosis in Combination with Photodynamic Therapy to Enhance Cancer Treatment. Nanoscale 2021, 13, 4855–4870. [Google Scholar] [CrossRef]
- Sun, Z.; Luo, M.; Li, J.; Wang, A.; Sun, X.; Wu, Q.; Li, K.; Ma, Y.; Yang, C.; Li, X. Folic Acid Functionalized Chlorin E6-Superparamagnetic Iron Oxide Nanocarriers as a Theranostic Agent for MRI-Guided Photodynamic Therapy. J. Biomed. Nanotechnol. 2021, 17, 205–215. [Google Scholar] [CrossRef]
- Martínez-Matamoros, D.; Castro-García, S.; Balado, M.; Matamoros-Veloza, A.; Camargo-Valero, M.A.; Cespedes, O.; Rodriguez, J.; Lemos, L.M.; Jiménez, C. Preparation of functionalized magnetic nanoparticles conjugated with feroxamine and their evaluation for pathogen detection. RSC Adv. 2019, 9, 13533–13542. [Google Scholar] [CrossRef] [PubMed]
- Kumar, Y.; Sinha, A.S.K.; Nigam, K.D.P.; Dwivedi, D.; Sangwai, J.S. Functionalized nanoparticles: Tailoring properties through surface energetics and coordination chemistry for advanced biomedical applications. Nanoscale 2023, 15, 6075–6104. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Peng, H.; Tian, Y.; Shen, S.; Yang, W. Mitochondria-targeting Magnetic Composite Nanoparticles for Enhanced Phototherapy of Cancer. Small 2016, 12, 4541–4552. [Google Scholar] [CrossRef]
- Bellouard, M.; Gasser, M.; Lenglet, S.; Gilardi, F.; Bararpour, N.; Augsburger, M.; Thomas, A.; Alvarez, J.-C. Toxicity and Metabolomic Impact of Cobalt, Chromium, and Nickel Exposure on HepaRG Hepatocytes. Chem. Res. Toxicol. 2022, 35, 807–816. [Google Scholar] [CrossRef] [PubMed]
- Janik-Olchawa, N.; Drozdz, A.; Wajda, A.; Sitarz, M.; Planeta, K.; Setkowicz, Z.; Ryszawy, D.; Kmita, A.; Chwiej, J. Biochemical Changes of Macrophages and U87MG Cells Occurring as a Result of the Exposure to Iron Oxide Nanoparticles Detected with the Raman Microspectroscopy. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 278, 121337. [Google Scholar] [CrossRef]
- Alavi, M.; Kamarasu, P.; McClements, D.J.; Moore, M.D. Metal and Metal Oxide-Based Antiviral Nanoparticles: Properties, Mechanisms of Action, and Applications. Adv. Colloid Interface Sci. 2022, 306, 102726. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, L.; Dekkers, S.; Zhang, L.G.; Cassee, F.R.; Zuo, Y.Y. Aggregation State of Metal-Based Nanomaterials at the Pulmonary Surfactant Film Determines Biophysical Inhibition. Environ. Sci. Technol. 2018, 52, 8920–8929. [Google Scholar] [CrossRef] [PubMed]
- Pedroso-Santana, S.; Fleitas-Salazar, N. The Use of Capping Agents in the Stabilization and Functionalization of Metallic Nanoparticles for Biomedical Applications. Part Syst. Charact. 2023, 40, 2200146. [Google Scholar] [CrossRef]
- Madkour, M.; Bumajdad, A.; Al-Sagheer, F. To What Extent Do Polymeric Stabilizers Affect Nanoparticles Characteristics? Adv. Colloid Interface Sci. 2019, 270, 38–53. [Google Scholar] [CrossRef]
- Deb, M.; Hunter, R.; Taha, M.; Abdelbary, H.; Anis, H. Rapid Detection of Bacteria Using Gold Nanoparticles in SERS with Three Different Capping Agents: Thioglucose, Polyvinylpyrrolidone, and Citrate. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2022, 280, 121533. [Google Scholar] [CrossRef]
- Iqbal, M.; Usanase, G.; Oulmi, K.; Aberkane, F.; Bendaikha, T.; Fessi, H.; Zine, N.; Agusti, G.; Errachid, E.-S.; Elaissari, A. Preparation of Gold Nanoparticles and Determination of Their Particles Size via Different Methods. Mater. Res. Bull. 2016, 79, 97–104. [Google Scholar] [CrossRef]
- Kamalesh, T. Advances in Stabilization of Metallic Nanoparticle with Biosurfactants—A Review on Current Trends. Heliyon 2024, 10, E29773. [Google Scholar] [CrossRef]
- Park, J.W.; Shumaker-Parry, J.S. Structural Study of Citrate Layers on Gold Nanoparticles: Role of Intermolecular Interactions in Stabilizing Nanoparticles. J. Am. Chem. Soc. 2014, 136, 1907–1921. [Google Scholar] [CrossRef]
- Badawy, A.M.E.; Luxton, T.P.; Silva, R.G.; Scheckel, K.G.; Suidan, M.T.; Tolaymat, T.M. Impact of Environmental Conditions (pH, Ionic Strength, and Electrolyte Type) on the Surface Charge and Aggregation of Silver Nanoparticles Suspensions. Environ. Sci. Technol. 2010, 44, 1260–1266. [Google Scholar] [CrossRef] [PubMed]
- Beach, M.A.; Nayanathara, U.; Gao, Y.; Zhang, C.; Xiong, Y.; Wang, Y.; Such, G.K. Polymeric Nanoparticles for Drug Delivery. Chem. Rev. 2024, 124, 5505–5616. [Google Scholar] [CrossRef] [PubMed]
- Hariharan, K.; Patel, P.; Mehta, T. Surface Modifications of Gold Nanoparticles: Stabilization and Recent Applications in Cancer Therapy. Pharm. Dev. Technol. 2022, 27, 665–683. [Google Scholar] [CrossRef]
- Ahangaran, F.; Navarchian, A.H. Recent Advances in Chemical Surface Modification of Metal Oxide Nanoparticles with Silane Coupling Agents: A Review. Adv. Colloid Interface Sci. 2020, 286, 102298. [Google Scholar] [CrossRef]
- Kumawat, M.; Madhyastha, H.; Umapathi, A.; Singh, M.; Revaprasadu, N.; Daima, H.K. Surface Engineered Peroxidase-Mimicking Gold Nanoparticles to Subside Cell Inflammation. Langmuir 2022, 38, 1877–1887. [Google Scholar] [CrossRef]
- Song, L.; Falzone, N.; Vallis, K.A. EGF-Coated Gold Nanoparticles Provide an Efficient Nano-Scale Delivery System for the Molecular Radiotherapy of EGFR-Positive Cancer. Int. J. Radiat. Biol. 2016, 92, 716–723. [Google Scholar] [CrossRef]
- Sur, I.; Cam, D.; Kahraman, M.; Baysal, A.; Culha, M. Interaction of Multi-Functional Silver Nanoparticles with Living Cells. Nanotechnology 2010, 21, 175104. [Google Scholar] [CrossRef]
- Thapa, R.K.; Soe, Z.C.; Ou, W.; Poudel, K.; Jeong, J.-H.; Jin, S.G.; Ku, S.K.; Choi, H.-G.; Lee, Y.M.; Yong, C.S. Palladium Nanoparticle-Decorated 2-D Graphene Oxide for Effective Photodynamic and Photothermal Therapy of Prostate Solid Tumors. Colloids Surf. B Biointerfaces 2018, 169, 429–437. [Google Scholar] [CrossRef]
- Nguyen, H.T.; Soe, Z.C.; Yang, K.Y.; Dai Phung, C.; Nguyen, L.T.-T.; Jeong, J.-H.; Jin, S.G.; Choi, H.-G.; Ku, S.K.; Yong, C.S. Transferrin-Conjugated pH-Sensitive Platform for Effective Delivery of Porous Palladium Nanoparticles and Paclitaxel in Cancer Treatment. Colloids Surf. B Biointerfaces 2019, 176, 265–275. [Google Scholar] [CrossRef]
- Zhang, Y.; Zheng, D.; Talaei, S.; Abasi, M. Albumin Stabilized Pt Nanoparticles as Radiosensitizer for Sensitization of Breast Cancer Cells under X-Ray Radiation Therapy. Inorg. Chem. Commun. 2022, 140, 109423. [Google Scholar] [CrossRef]
- Patel, P.; Umapathy, D.; Manivannan, S.; Nadar, V.M.; Venkatesan, R.; Arokiyam, V.A.J.; Pappu, S.; Ponnuchamy, K. A Doxorubicin–Platinum Conjugate System: Impacts on PI3K/AKT Actuation and Apoptosis in Breast Cancer Cells. RSC Adv. 2021, 11, 4818–4828. [Google Scholar] [CrossRef]
- Lickmichand, M.; Shaji, C.S.; Valarmathi, N.; Benjamin, A.S.; Kumar, R.A.; Nayak, S.; Saraswathy, R.; Sumathi, S.; Raj, N.A.N. In Vitro Biocompatibility and Hyperthermia Studies on Synthesized Cobalt Ferrite Nanoparticles Encapsulated with Polyethylene Glycol for Biomedical Applications. Mater. Today Proc. 2019, 15, 252–261. [Google Scholar] [CrossRef]
- Karges, J.; Jakubaszek, M.; Mari, C.; Zarschler, K.; Goud, B.; Stephan, H.; Gasser, G. Synthesis and Characterization of an Epidermal Growth Factor Receptor-Selective RuII Polypyridyl–Nanobody Conjugate as a Photosensitizer for Photodynamic Therapy. ChemBioChem 2020, 21, 531–542. [Google Scholar] [CrossRef]
- Lameijer, L.N.; Hopkins, S.L.; Brevé, T.G.; Askes, S.H.; Bonnet, S. d-Versus l-Glucose Conjugation: Mitochondrial Targeting of a Light-Activated Dual-Mode-of-Action Ruthenium-Based Anticancer Prodrug. Chem.-A Eur. J. 2016, 22, 18484–18491. [Google Scholar] [CrossRef]
- Huang, Y.; Huang, W.; Chan, L.; Zhou, B.; Chen, T. A multifunctional DNA origami as carrier of metal complexes to achieve enhanced tumoral delivery and nullified systemic toxicity. Biomaterials 2016, 103, 183–196. [Google Scholar] [CrossRef]
- Yücel, O.; Şengelen, A.; Emik, S.; Önay-Uçar, E.; Arda, N.; Gürdağ, G. Folic Acid-Modified Methotrexate-Conjugated Gold Nanoparticles as Nano-Sized Trojans for Drug Delivery to Folate Receptor-Positive Cancer Cells. Nanotechnology 2020, 31, 355101. [Google Scholar] [CrossRef]
- Karges, J.; Li, J.; Zeng, L.; Chao, H.; Gasser, G. Polymeric Encapsulation of a Ruthenium Polypyridine Complex for Tumor Targeted One-and Two-Photon Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 54433–54444. [Google Scholar] [CrossRef]
- Novohradsky, V.; Zamora, A.; Gandioso, A.; Brabec, V.; Ruiz, J.; Marchán, V. Somatostatin Receptor-Targeted Organometallic Iridium (III) Complexes as Novel Theranostic Agents. Chem. Commun. 2017, 53, 5523–5526. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Zhai, J.; Hu, D.; Yang, R.; Wang, G.; Li, Y.; Liang, G. “Targeting Design” of Nanoparticles in Tumor Therapy. Pharmaceutics 2022, 14, 1919. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Yu, G.; Yang, W.; Wang, Z.; Jacobson, O.; Tian, R.; Deng, H.; Lin, L.; Chen, X. Photodynamic-chemodynamic Cascade Reactions for Efficient Drug Delivery and Enhanced Combination Therapy. Adv. Sci. 2021, 8, 2002927. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhang, Z.; Liu, J.; Wang, B.; Pu, G.; Li, J.; Huang, Y.; Chu, M. Nanodrug-Loaded Bifidobacterium Bifidum Conjugated with Anti-Death Receptor Antibody for Tumor-Targeted Photodynamic and Sonodynamic Synergistic Therapy. Acta Biomater. 2022, 146, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Tang, L.; Xu, L.; Zhang, Y.; Li, G.; Peng, W.; Guo, X.; Zhou, L.; Liu, C.; Shen, X.-C. A NIR-II Light-Modulated Injectable Self-Healing Hydrogel for Synergistic Photothermal/Chemodynamic/Chemo-Therapy of Melanoma and Wound Healing Promotion. J. Mater. Chem. B 2022, 10, 7717–7731. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Shi, H.; Li, T.; Yu, J.; Guo, Z.; Cheng, J.; Liu, Y. A Dual Functional Nanoreactor for Synergistic Starvation and Photodynamic Therapy. ACS Appl. Mater. Interfaces 2020, 12, 18309–18318. [Google Scholar] [CrossRef] [PubMed]
- Chong, L.M.; Tng, D.J.H.; Tan, L.L.Y.; Chua, M.L.K.; Zhang, Y. Recent Advances in Radiation Therapy and Photodynamic Therapy. Appl. Phys. Rev. 2021, 8, 041322. [Google Scholar] [CrossRef]
- Liu, Z.; Xie, Z.; Li, W.; Wu, X.; Jiang, X.; Li, G.; Cao, L.; Zhang, D.; Wang, Q.; Xue, P. Photodynamic Immunotherapy of Cancers Based on Nanotechnology: Recent Advances and Future Challenges. J. Nanobiotechnol. 2021, 19, 160. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Liu, X.; Mao, Y.; He, Y.; Xu, J.; Zheng, F.; Tan, W.; Rong, S.; Chen, Y.; Jia, X. Oxygen-Generating Hydrogels Overcome Tumor Hypoxia to Enhance Photodynamic/Gas Synergistic Therapy. ACS Appl. Mater. Interfaces 2022, 14, 27551–27563. [Google Scholar] [CrossRef]
- Wei, F.; Chen, Z.; Shen, X.-C.; Ji, L.; Chao, H. Recent Progress in Metal Complexes Functionalized Nanomaterials for Photodynamic Therapy. Chem. Commun. 2023, 59, 6956–6968. [Google Scholar] [CrossRef]
- Bhole, R.; Bonde, C.; Kadam, P.; Wavwale, R. A Comprehensive Review on Photodynamic Therapy (PDT) and Photothermal Therapy (PTT) for Cancer Treatment. Turk. J. Oncol. 2021, 36, 125–132. [Google Scholar] [CrossRef]
- Kim, H.S.; Lee, D.Y. Near-Infrared-Responsive Cancer Photothermal and Photodynamic Therapy Using Gold Nanoparticles. Polymers 2018, 10, 961. [Google Scholar] [CrossRef] [PubMed]




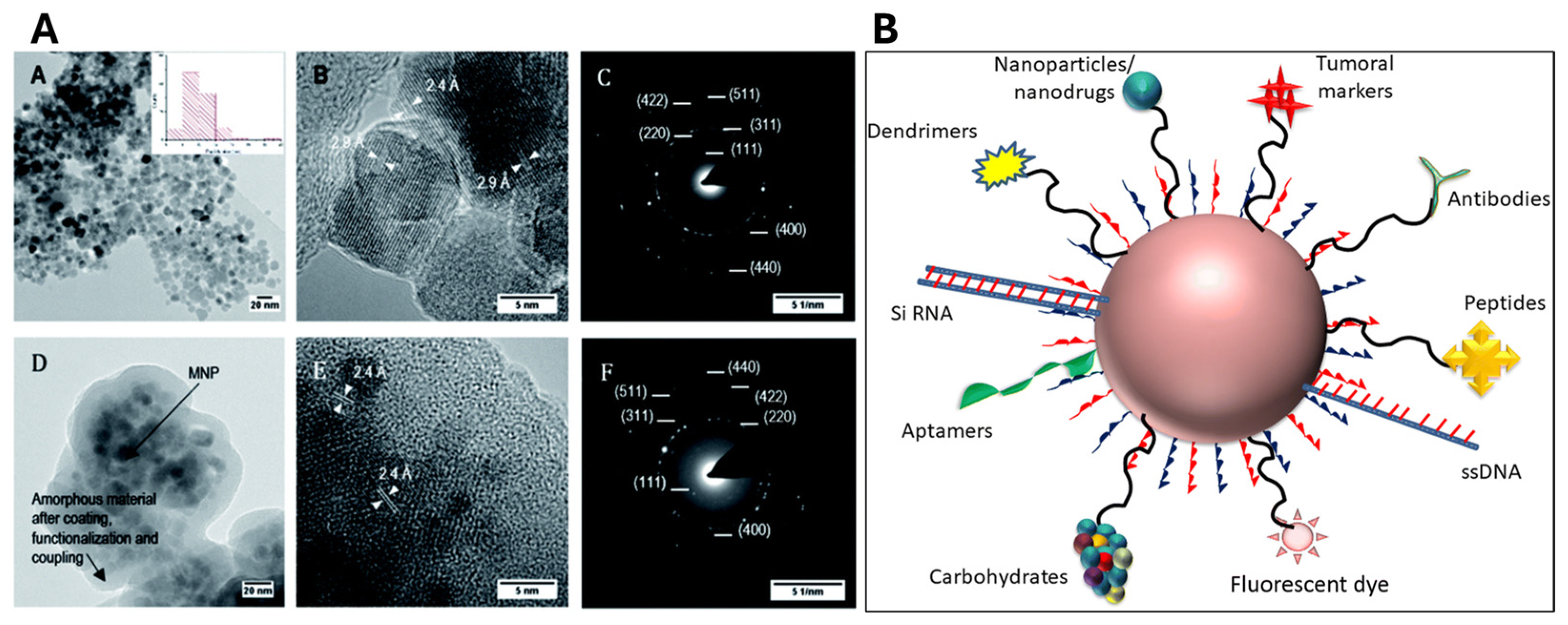

| MNPs | Surface Modification | In Vitro System | Highlights | Reference |
|---|---|---|---|---|
| Gold NPs | Curcumin, isoniazid, tyrosine, and quercitin | Raw 264.7 cells | The peroxidase-mimicking nanoparticle interactions with red blood cells and mouse macrophages confirmed their hemocompatible and biocompatible nature | [112] |
| Gold NPs | Epidermal growth factor (EGF) | MDA-MB-468 cells and MCF7cells | 111In-EGF-Au NPs were significantly more radiotoxic to MDA-MB-468 than MCF-7 cells with a surviving fraction of 17.1 ± 4.4% versus 89.8 ± 1.4% (p < 0.001) after exposure for 4 h | [113] |
| Silver NPs | Glucose, lactose, and oligonucleotides | L929 and A549 cells | The binding of oligonucleotides along with the carbohydrate on the AgNP surfaces influenced the differential uptake rate pattern into the cells. The cytotoxicity study with the modified AgNPs revealed that only naked AgNPs influence the viability of A549 cells | [114] |
| Palladium NPs | Graphene oxide | PC3 cells | Compared to GO or Pd NPs alone, GO-Pd NPs showed higher cytotoxic effects in prostate cancer 3 (PC3) cells. The irradiation of treated cells with a near-infrared (NIR) laser considerably enhanced apoptosis induced by the synergistic photothermal effect and reactive oxygen species (ROS) generation | [115] |
| Palladium NPs | Transferrin | MCF7 cells | The combination of phototherapy induced by PdNPs and a chemotherapeutic agent (PTX) could exhibit synergistic anticancer activities. | [116] |
| Platinum NPs | Bovine serum albumin | 4T1 cells | The results showed a greater cytotoxic effect compared to cells treated with only the BSA-PtNPs, suggesting that these nanomaterials may act as a potential radiosensitizer by improving the efficacy of radiotherapy | [117] |
| Silver NPs | Doxorubicin | MCF7 cells and MDA-MB-231 cells | The effect was mediated by activation of the tumor suppressor gene (PTEN), which restricts the PI3K/AKT signaling pathway, leading to mitochondrial dysfunction and the activation of caspases three and nine, ultimately resulting in cell apoptosis | [118] |
| Cobalt ferrite NPs | Polyethylene glycol (PEG) | Lymphocytes | The cytotoxicity of PEG-encapsulated MNPs was better than the bare particles and showed very low toxicity values. | [119] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mariano, S.; Carata, E.; Calcagnile, L.; Panzarini, E. Recent Advances in Photodynamic Therapy: Metal-Based Nanoparticles as Tools to Improve Cancer Therapy. Pharmaceutics 2024, 16, 932. https://doi.org/10.3390/pharmaceutics16070932
Mariano S, Carata E, Calcagnile L, Panzarini E. Recent Advances in Photodynamic Therapy: Metal-Based Nanoparticles as Tools to Improve Cancer Therapy. Pharmaceutics. 2024; 16(7):932. https://doi.org/10.3390/pharmaceutics16070932
Chicago/Turabian StyleMariano, Stefania, Elisabetta Carata, Lucio Calcagnile, and Elisa Panzarini. 2024. "Recent Advances in Photodynamic Therapy: Metal-Based Nanoparticles as Tools to Improve Cancer Therapy" Pharmaceutics 16, no. 7: 932. https://doi.org/10.3390/pharmaceutics16070932





