Natural Killer-Based Therapy: A Prospective Thought for Cancer Treatment Related to Diversified Drug Delivery Pathways
Abstract
:1. Introduction
2. Receptors Expressed on NK Cells
2.1. Activating Receptors on NK Cells
2.2. Inhibitory Receptors on NK Cells
3. Drug Delivery System-Based NK Activation for Tumor Immunotherapy
3.1. Ligand Expression Stimulation of Activating Receptors
3.2. Cytokine-Related Application
3.3. Expression or Binding Prevention of Inhibitory Receptors’ Ligands
3.4. ADCC Induction of NK Cells
3.5. Other Strategies for NK Cell Stimulation
4. NK Cells and NK-Derived Exosomes as Drug Delivery Vehicles for Tumor Immunotherapy
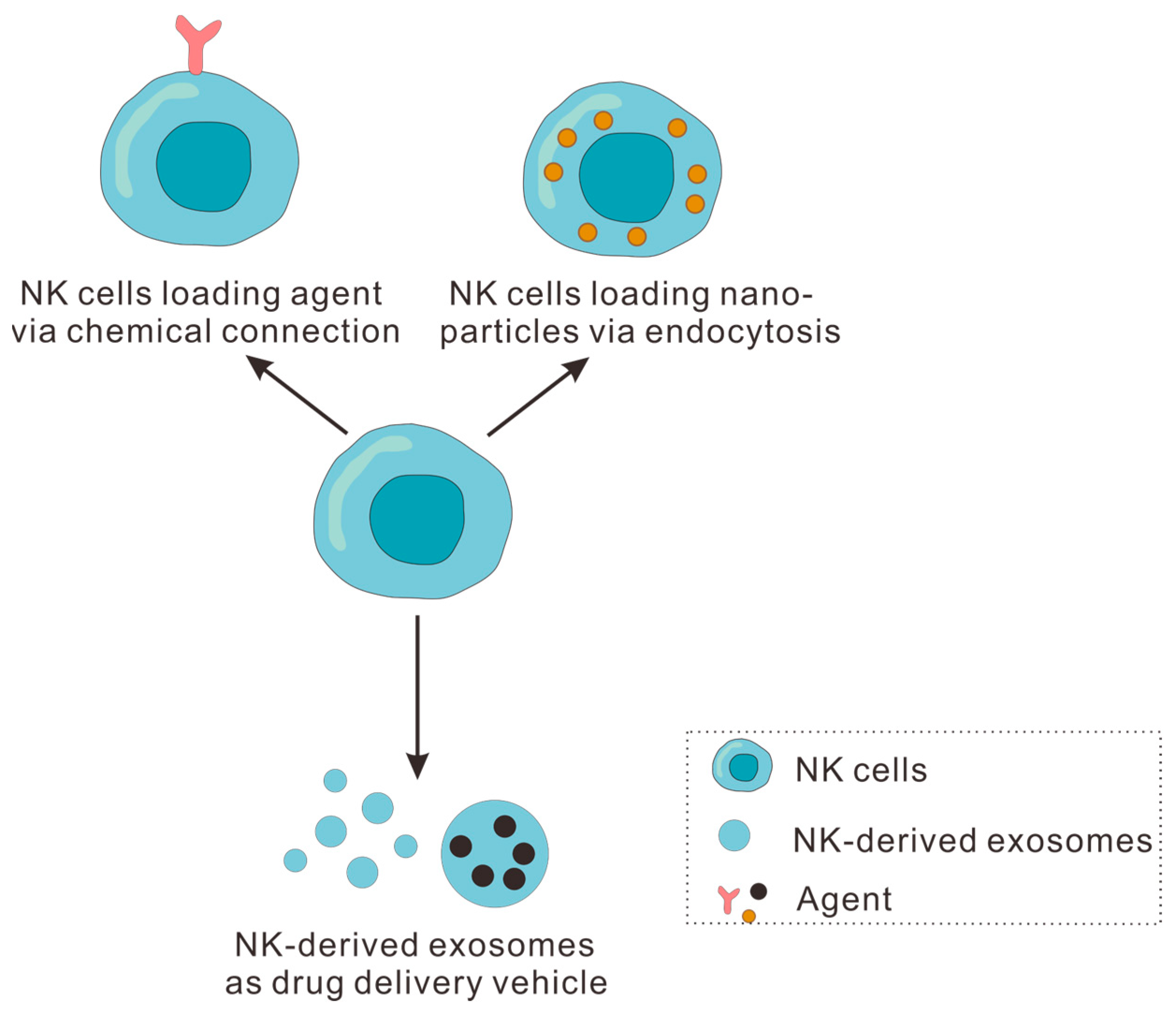
4.1. NK Cells as Drug Delivery Vehicle for Tumor Immunotherapy
4.1.1. NK Cell Loading Agent via Chemical Connection
4.1.2. NK Cells Loading Nanoparticles via Endocytosis
4.2. NK-Derived Exosomes as Drug Delivery Vehicle for Tumor Immunotherapy
5. Summary and Outlook
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| ADCC | Antibody-dependent cell-mediated cytotoxicity |
| APCs | Antigen-presenting cells |
| CRS | Cytokine release syndrome |
| DDS | Drug delivery system |
| HLA-E | Human leukocyte antigen-E |
| IFN-γ | Interferon-γ |
| IL-12R | Interleukin 12 receptor |
| IL-15 | Interleukin 15 |
| IL-15R | Interleukin 15 receptor |
| IL-18R | Interleukin 18 receptor |
| IL-21R | Interleukin 21 receptor |
| IL-2R | Interleukin 2 receptor |
| ISs | Immune synapses |
| KIRs | Killer cell immunoglobulin-like receptors |
| MHC | Major histocompatibility complex |
| MHT | Mild magnetothermal therapy |
| NCAM | Neural cell adhesion molecule |
| NCR1 | Natural cytotoxic triggering receptor 1 |
| NEO | NK-derived exosomes |
| NK | Natural killer |
| NKG2A | Natural Killer Group 2A |
| PBMCs | Peripheral blood monocytes |
| PD-1 | Programmed death 1 |
| PDA | Polydopamine |
| PS | Phosphatidylserine |
| ROS | Reactive oxygen species |
| siRNAs | Small interfering nucleic acids |
| Tim-3 | T-cell immunoglobulin and mucin domain Containing protein-3 |
| TNFR | Tumor necrosis factor receptor |
| TNF-α | Tumor necrosis factor-α |
| Tregs | Inhibiting regulatory T cells |
| ULBPs | UL16-binding proteins |
References
- Siegel, R.L.; Miller, K.D.; Wagle, N.S.; Jemal, A. Cancer statistics, 2023. CA-Cancer J. Clin. 2023, 73, 17–48. [Google Scholar] [CrossRef] [PubMed]
- Tohme, S.; Simmons, R.L.; Tsung, A. Surgery for cancer: A trigger for metastases. Cancer Res. 2017, 77, 1548–1552. [Google Scholar] [CrossRef]
- Pardoll, D.M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 2012, 12, 252–264. [Google Scholar] [CrossRef] [PubMed]
- Barker, H.E.; Paget, J.T.E.; Khan, A.A.; Harrington, K.J. The tumour microenvironment after radiotherapy: Mechanisms of resistance and recurrence. Nat. Rev. Cancer 2015, 15, 409–425. [Google Scholar] [CrossRef]
- DeVita, V.T., Jr.; Chu, E. A history of cancer chemotherapy. Cancer Res. 2008, 68, 8643–8653. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, R.; Alatise, O.I.; Anderson, B.O.; Audisio, R.; Autier, P.; Aggarwal, A.; Balch, C.; Brennan, M.F.; Dare, A.; D’Cruz, A.; et al. Global cancer surgery: Delivering safe, aff ordable, and timely cancer surgery. Lancet Oncol. 2015, 16, 1193–1224. [Google Scholar] [CrossRef] [PubMed]
- Strobel, O.; Neoptolemos, J.; Jaeger, D.; Buechler, M.W. Optimizing the outcomes of pancreatic cancer surgery. Nat. Rev. Clin. Oncol. 2019, 16, 11–26. [Google Scholar] [CrossRef] [PubMed]
- Bukowski, K.; Kciuk, M.; Kontek, R. Mechanisms of multidrug resistance in cancer chemotherapy. Int. J. Mol. Sci. 2020, 21, 3233. [Google Scholar] [CrossRef]
- Schaue, D.; McBride, W.H. Opportunities and challenges of radiotherapy for treating cancer. Nat. Rev. Clin. Oncol. 2015, 12, 527–540. [Google Scholar] [CrossRef]
- Li, B.; Shao, H.; Gao, L.; Li, H.; Sheng, H.; Zhu, L. Nano-drug co-delivery system of natural active ingredients and chemotherapy drugs for cancer treatment: A review. Drug Deliv. 2022, 29, 2130–2161. [Google Scholar] [CrossRef]
- Liu, Y.-Q.; Wang, X.-L.; He, D.-H.; Cheng, Y.-X. Protection against chemotherapy- and radiotherapy-induced side effects: A review based on the mechanisms and therapeutic opportunities of phytochemicals. Phytomedicine 2021, 80, 153402. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, M.S. Improving cancer immunotherapy through nanotechnology. Nat. Rev. Cancer 2019, 19, 587–602. [Google Scholar] [CrossRef] [PubMed]
- Gowd, V.; Ahmad, A.; Tarique, M.; Suhail, M.; Zughaibi, T.A.; Tabrez, S.; Khan, R. Advancement of cancer immunotherapy using nanoparticles-based nanomedicine. Semin. Cancer Biol. 2022, 86, 624–644. [Google Scholar] [CrossRef] [PubMed]
- Mei, Y.; Tang, L.; Zhang, L.; Hu, J.; Zhang, Z.; He, S.; Zang, J.; Wang, W. A minimally designed PD-L1-targeted nanocomposite for positive feedback-based multimodal cancer therapy. Mater. Today 2022, 60, 52–68. [Google Scholar] [CrossRef]
- Peng, S.; Xiao, F.; Chen, M.; Gao, H. Tumor-microenvironment-responsive nanomedicine for enhanced cancer immunotherapy. Adv. Sci. 2022, 9, 2103836. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Hu-Lieskovan, S.; Wargo, J.A.; Ribas, A. Primary, adaptive, and acquired resistance to cancer immunotherapy. Cell 2017, 168, 707–723. [Google Scholar] [CrossRef]
- Sivori, S.; Pende, D.; Quatrini, L.; Pietra, G.; Della Chiesa, M.; Vacca, P.; Tumino, N.; Moretta, F.; Mingari, M.C.; Locatelli, F.; et al. NK cells and ILCs in tumor immunotherapy. Mol. Asp. Med. 2021, 80, 100870. [Google Scholar] [CrossRef]
- Seidel, U.J.E.; Schlegel, P.; Lang, P. Natural killer cell mediated antibody-dependent cellular cytotoxicity in tumor immunotherapy with therapeutic antibodies. Front. Immunol. 2013, 4, 76. [Google Scholar] [CrossRef]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” killer cells in the mouse. I. cytotoxic cells with specificity for mouse moloney leukemia cells. specificity and distribution according to genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef]
- Herberman, R.B.; Nunn, M.E.; Lavrin, D.H. Natural cytotoxic reactivity of mouse lymphoid cells against syngeneic acid allogeneic tumors. I. Distribution of reactivity and specificity. Int. J. Cancer 1975, 16, 216–229. [Google Scholar] [CrossRef]
- Liu, S.; Galat, V.; Galat, Y.; Lee, Y.K.A.; Wainwright, D.; Wu, J. NK cell-based cancer immunotherapy: From basic biology to clinical development. J. Hematol. Oncol. 2021, 14, 7. [Google Scholar] [CrossRef] [PubMed]
- Mestre-Duran, C.; Martin-Cortazar, C.; Garcia-Solis, B.; Pernas, A.; Pertinez, L.; Galan, V.; Sisinni, L.; Clares-Villa, L.; Navarro-Zapata, A.; Al-Akioui, K.; et al. Ruxolitinib does not completely abrogate the functional capabilities of TLR4/9 ligand-activated NK cells. Front. Immunol. 2023, 13, 1045316. [Google Scholar] [CrossRef] [PubMed]
- Lanier, L.L.; Testi, R.; Bindl, J.; Phillips, J.H. Identity of Leu-19 (CD56) leukocyte differentiation antigen and neural cell adhesion molecule. J. Exp. Med. 1989, 169, 2233–2238. [Google Scholar] [CrossRef] [PubMed]
- Shimasaki, N.; Jain, A.; Campana, D. NK cells for cancer immunotherapy. Nat. Rev. Drug Discov. 2020, 19, 200–218. [Google Scholar] [CrossRef] [PubMed]
- Freud, A.G.; Zhao, S.; Wei, S.; Gitana, G.M.; Molina-Kirsch, H.F.; Atwater, S.K.; Natkunam, Y. Expression of the activating receptor, NKp46 (CD335), in human natural killer and T-cell neoplasia. Am. J. Clin. Pathol. 2013, 140, 853–866. [Google Scholar] [CrossRef] [PubMed]
- Pende, D.; Falco, M.; Vitale, M.; Cantoni, C.; Vitale, C.; Munari, E.; Bertaina, A.; Moretta, F.; Del Zotto, G.; Pietra, G.; et al. Killer Ig-like receptors (KIRs): Their role in NK cell modulation and developments leading to their clinical exploitation. Front. Immunol. 2019, 10, 1179. [Google Scholar] [CrossRef]
- Cong, J.; Wei, H. Natural killer cells in the lungs. Front. Immunol. 2019, 10, 1416. [Google Scholar] [CrossRef] [PubMed]
- Wagner, J.A.; Rosario, M.; Romee, R.; Berrien-Elliott, M.M.; Schneider, S.E.; Leong, J.W.; Sullivan, R.P.; Jewell, B.A.; Becker-Hapak, M.; Schappe, T.; et al. CD56bright NK cells exhibit potent antitumor responses following IL-15 priming. J. Clin. Investig. 2017, 127, 4042–4058. [Google Scholar] [CrossRef] [PubMed]
- Prager, I.; Liesche, C.; van Ooijen, H.; Urlaub, D.; Verron, Q.; Sandstrom, N.; Fasbender, F.; Claus, M.; Eils, R.; Beaudouin, J.; et al. NK cells switch from granzyme B to death receptor-mediated cytotoxicity during serial killing. J. Exp. Med. 2019, 216, 2113–2127. [Google Scholar] [CrossRef]
- Yu, Y. The function of NK cells in tumor metastasis and NK cell-based immunotherapy. Cancers 2023, 15, 2323. [Google Scholar] [CrossRef]
- Morvan, M.G.; Lanier, L.L. NK cells and cancer: You can teach innate cells new tricks. Nat. Rev. Cancer 2016, 16, 7–19. [Google Scholar] [CrossRef]
- Zamai, L.; Ponti, C.; Mirandola, P.; Gobbi, G.; Papa, S.; Galeotti, L.; Cocco, L.; Vitale, M. NK cells and cancer. J. Immunol. 2007, 178, 4011–4016. [Google Scholar] [CrossRef] [PubMed]
- Lyu, M.A.; Rosenblum, M.G. The immunocytokine scFv23/TNF sensitizes HER-2/neu-overexpressing SKBR-3 cells to tumor necrosis factor (TNF) via up-regulation of TNF receptor-1. Mol. Cancer Ther. 2005, 4, 1205–1213. [Google Scholar] [CrossRef] [PubMed]
- Ebert, L.M.; Meuter, S.; Moser, B. Homing and function of human skin γδ T cells and NK cells:: Relevance for tumor surveillance. J. Immunol. 2006, 176, 4331–4336. [Google Scholar] [CrossRef] [PubMed]
- Owen, C.A.; Campbell, M.A.; Boukedes, S.S.; Campbell, E.J. Inducible binding of bioactive cathepsin-G to the cell-surface of neutrophils—A novel mechanism for mediating extracellular catalytic activity of cathepsin-G. J. Immunol. 1995, 155, 5803–5810. [Google Scholar] [CrossRef] [PubMed]
- Aqbi, H.F.; Wallace, M.; Sappal, S.; Payne, K.K.; Manjili, M.H. IFN-γ orchestrates tumor elimination, tumor dormancy, tumor escape, and progression. J. Leukoc. Biol. 2018, 103, 1219–1223. [Google Scholar] [CrossRef] [PubMed]
- Klingemann, H.; Boissel, L.; Toneguzzo, F. Natural killer cells for immunotherapy-advantages of the NK-92 cell line over blood NK cells. Front. Immunol. 2016, 7, 91. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Erbe, A.K.; Hank, J.A.; Morris, Z.S.; Sondel, P.M. NK cell-mediated antibody-dependent cellular cytotoxicity in cancer immunotherapy. Front. Immunol. 2015, 6, 368. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Xiong, T.; He, S.; Sun, H.; Huang, C.; Ren, X.; Wu, L.; Patterson, L.H.; Zhang, J. Pulmonary targeting crosslinked cyclodextrin metal-organic frameworks for lung cancer therapy. Adv. Funct. Mater. 2021, 31, 2004550. [Google Scholar] [CrossRef]
- Zhu, T.; Chen, Z.; Jiang, G.; Huang, X. Sequential targeting hybrid nanovesicles composed of chimeric antigen receptor T-cell-derived exosomes and liposomes for enhanced cancer immunochemotherapy. Acs Nano 2023, 17, 16770–16786. [Google Scholar] [CrossRef]
- Liau, L.M.; Ashkan, K.; Tran, D.D.; Campian, J.L.; Trusheim, J.E.; Cobbs, C.S.; Heth, J.A.; Salacz, M.; Taylor, S.; D’Andre, S.D.; et al. First results on survival from a large Phase 3 clinical trial of an autologous dendritic cell vaccine in newly diagnosed glioblastoma. J. Transl. Med. 2018, 16, 142. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Wang, Y.; Yang, F.; Shen, H.; Weng, Z.; Wu, D. Charge-reversible and pH-responsive biodegradable micelles and vesicles from linear-dendritic supramolecular amphiphiles for anticancer drug delivery. Polym. Chem. 2017, 8, 6675–6687. [Google Scholar] [CrossRef]
- Pan, J.; Xu, Y.; Wu, Q.; Hu, P.; Shi, J. Mild magnetic hyperthermia-activated innate immunity for liver cancer therapy. J. Am. Chem. Soc. 2021, 143, 8116–8128. [Google Scholar] [CrossRef] [PubMed]
- Gauthier, L.; Morel, A.; Anceriz, N.; Rossi, B.; Blanchard-Alvarez, A.; Grondin, G.; Trichard, S.; Cesari, C.; Sapet, M.; Bosco, F.; et al. Multifunctional natural killer cell engagers targeting nkp46 trigger protective tumor immunity. Cell 2019, 177, 1701–1713. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Pan, S.; Gao, S.; Xiang, W.; Sun, C.; Cao, W.; Xu, H. Diselenide-pemetrexed assemblies for combined cancer immuno-, radio-, and chemotherapies. Angew. Chem. Int. Ed. 2020, 59, 2700–2704. [Google Scholar] [CrossRef]
- Luo, H.; Zhou, Y.; Zhang, J.; Zhang, Y.; Long, S.; Lin, X.; Yang, A.; Duan, J.; Yang, N.; Yang, Z.; et al. NK cell-derived exosomes enhance the anti-tumor effects against ovarian cancer by delivering cisplatin and reactivating NK cell functions. Front. Immunol. 2023, 13, 1087689. [Google Scholar] [CrossRef]
- Zmievskaya, E.A.; Mukhametshin, S.A.; Ganeeva, I.A.; Gilyazova, E.M.; Siraeva, E.T.; Kutyreva, M.P.; Khannanov, A.A.; Yuan, Y.; Bulatov, E.R. Artificial Extracellular Vesicles Generated from T Cells Using Different Induction Techniques. Biomedicines 2024, 12, 919. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.; Kyewski, B.; Allen, P.M.; Hogquist, K.A. Positive and negative selection of the T cell repertoire: What thymocytes see (and don’t see). Nat. Rev. Immunol. 2014, 14, 377–391. [Google Scholar] [CrossRef]
- Roche, P.A.; Furuta, K. The ins and outs of MHC class II-mediated antigen processing and presentation. Nat. Rev. Immunol. 2015, 15, 203–216. [Google Scholar] [CrossRef] [PubMed]
- Merino, A.; Maakaron, J.; Bachanova, V. Advances in NK cell therapy for hematologic malignancies: NK source, persistence and tumor targeting. Blood Rev. 2023, 60, 101073. [Google Scholar] [CrossRef]
- Han, B.; Song, Y.; Park, J.; Doh, J. Nanomaterials to improve cancer immunotherapy based on ex vivo engineered T cells and NK cells. J. Control. Release 2022, 343, 379–391. [Google Scholar] [CrossRef] [PubMed]
- Carotta, S. Targeting NK cells for anticancer immunotherapy: Clinical and preclinical approaches. Front. Immunol. 2016, 7, 152. [Google Scholar] [CrossRef]
- Yang, L.; Yang, Y.; Chen, Y.; Xu, Y.; Peng, J. Cell-based drug delivery systems and their in vivo fate. Adv. Drug Deliver. Rev. 2022, 187, 114394. [Google Scholar] [CrossRef] [PubMed]
- Vivier, E.; Tomasello, E.; Paul, P. Lymphocyte activation via NKG2D: Towards a new paradigm in immune recognition? Curr. Opin. Immunol. 2002, 14, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Gong, Y.; Klein Wolterink, R.G.J.; Wang, J.; Bos, G.M.J.; Germeraad, W.T.V. Chimeric antigen receptor natural killer (CAR-NK) cell design and engineering for cancer therapy. J. Hematol. Oncol. 2021, 14, 73. [Google Scholar] [CrossRef] [PubMed]
- Koene, H.R.; Kleijer, M.; Algra, J.; Roos, D.; vondemBorne, A.; deHaas, M. Fc gamma RIIIa-158V/F polymorphism influences the binding of IgG by natural killer cell Fc gamma RIIIa, independently of the Fc gamma IIIa-48L/R/H phenotype. Blood 1997, 90, 1109–1114. [Google Scholar] [CrossRef]
- Brandt, C.S.; Baratin, M.; Yi, E.C.; Kennedy, J.; Gao, Z.; Fox, B.; Haldeman, B.; Ostrander, C.D.; Kaifu, T.; Chabannon, C.; et al. The B7 family member B7-H6 is a tumor cell ligand for the activating natural killer cell receptor NKp30 in humans. J. Exp. Med. 2009, 206, 1495–1503. [Google Scholar] [CrossRef]
- Barrow, A.D.; Martin, C.J.; Colonna, M. The natural cytotoxicity receptors in health and disease. Front. Immunol. 2019, 10, 909. [Google Scholar] [CrossRef]
- Satoh-Takayama, N.; Dumoutier, L.; Lesjean-Pottier, S.; Ribeiro, V.S.G.; Mandelboim, O.; Renauld, J.-C.; Vosshenrich, C.A.J.; Di Santo, J.P. The natural cytotoxicity receptor NKp46 is dispensable for IL-22-mediated innate intestinal immune defense against citrobacter rodentium. J. Immunol. 2009, 183, 6579–6587. [Google Scholar] [CrossRef]
- Fuchs, A.; Cella, M.; Kondo, T.; Colonna, M. Paradoxic inhibition of human natural interferon-producing cells by the activating receptor NKp44. Blood 2005, 106, 2076–2082. [Google Scholar] [CrossRef]
- Cho, D.; Campana, D. Expansion and activation of natural killer cells for cancer immunotherapy. Korean J. Lab. Med. 2009, 29, 89–96. [Google Scholar] [CrossRef]
- Cheng, M.; Chen, Y.; Xiao, W.; Sun, R.; Tian, Z. NK cell-based immunotherapy for malignant diseases. Cell. Mol. Immunol. 2013, 10, 230–252. [Google Scholar] [CrossRef]
- Tomala, J.; Chmelova, H.; Mrkvan, T.; Rihova, B.; Kovar, M. In vivo expansion of activated naive CD8(+) T cells and NK cells driven by complexes of IL-2 and anti-IL-2 monoclonal antibody as novel approach of cancer immunotherapy. J. Immunol. 2009, 183, 4904–4912. [Google Scholar] [CrossRef]
- Hydes, T.; Noll, A.; Salinas-Riester, G.; Abuhilal, M.; Armstrong, T.; Hamady, Z.; Primrose, J.; Takhar, A.; Walter, L.; Khakoo, S.I. IL-12 and IL-15 induce the expression of CXCR6 and CD49a on peripheral natural killer cells. Immun. Inflamm. Dis. 2018, 6, 34–46. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, K.B.; Salazar-Mather, T.P.; Dalod, M.Y.; Van Deusen, J.B.; Wei, X.Q.; Liew, F.Y.; Caligiuri, M.A.; Durbin, J.E.; Biron, C.A. Coordinated and distinct roles for IFN-alpha beta, IL-12, and IL-15 regulation of NK cell responses to viral infection. J. Immunol. 2002, 169, 4279–4287. [Google Scholar] [CrossRef]
- Mamessier, E.; Sylvain, A.; Thibult, M.-L.; Houvenaeghel, G.; Jacquemier, J.; Castellano, R.; Goncalves, A.; Andre, P.; Romagne, F.; Thibault, G.; et al. Human breast cancer cells enhance self tolerance by promoting evasion from NK cell antitumor immunity. J. Clin. Investig. 2011, 121, 3609–3622. [Google Scholar] [CrossRef]
- Hiam-Galvez, K.J.; Allen, B.M.; Spitzer, M.H. Systemic immunity in cancer. Nat. Rev. Cancer 2021, 21, 345–359. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, N.; Lu, Y.; Davidson, D.; Colonna, M.; Veillette, A. DNAM-1 controls NK cell activation via an ITT-like motif. J. Exp. Med. 2015, 212, 2165–2182. [Google Scholar] [CrossRef] [PubMed]
- Long, E.O. Negative signaling by inhibitory receptors: The NK cell paradigm. Immunol. Rev. 2008, 224, 70–84. [Google Scholar] [CrossRef]
- Cozar, B.; Greppi, M.; Carpentier, S.; Narni-Mancinelli, E.; Chiossone, L.; Vivier, E. Tumor-infiltrating natural killer cells. Cancer Discov. 2021, 11, 34–44. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Angelo, L.S.; Banerjee, P.P.; Monaco-Shawver, L.; Rosen, J.B.; Makedonas, G.; Forbes, L.R.; Mace, E.M.; Orange, J.S. Practical NK cell phenotyping and variability in healthy adults. Immunol. Res. 2015, 62, 341–356. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.A.; Stanczak, M.A.; Mantuano, N.R.; Xiao, H.; Pijnenborg, J.F.A.; Malaker, S.A.; Miller, C.L.; Weidenbacher, P.A.; Tanzo, J.T.; Ahn, G.; et al. Targeted glycan degradation potentiates the anticancer immune response in vivo. Nat. Chem. Biol. 2020, 16, 1376–1384. [Google Scholar] [CrossRef] [PubMed]
- Pesce, S.; Greppi, M.; Tabellini, G.; Rampinelli, F.; Parolini, S.; Olive, D.; Moretta, L.; Moretta, A.; Marcenaro, E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J. Allergy Clin. Immun. 2017, 139, 335–346. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Li, M.; Qin, X.; Tan, S.; Du, L.; Ma, C.; Li, M. Photophosphatidylserine guides natural killer cell photoimmunotherapy via Tim-3. J. Am. Chem. Soc. 2022, 144, 3863–3874. [Google Scholar] [CrossRef] [PubMed]
- Ji, T.; Lang, J.; Ning, B.; Qi, F.; Wang, H.; Zhang, Y.; Zhao, R.; Yang, X.; Zhang, L.; Li, W.; et al. Enhanced natural killer cell immunotherapy by rationally assembling Fc fragments of antibodies onto tumor membranes. Adv. Mater. 2019, 31, 1804395. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Wang, Q.; Wang, Y.; Zhao, X.; Gao, K.; Liu, Q.; Zhao, Y.; Zhang, Z.; Zheng, Y.; Cao, J.; et al. In situ modification of the tumor cell surface with immunomodulating nanoparticles for effective suppression of tumor growth in mice. Adv. Mater. 2019, 31, 1902542. [Google Scholar] [CrossRef] [PubMed]
- Song, Q.; Yin, Y.; Shang, L.; Wu, T.; Zhang, D.; Kong, M.; Zhao, Y.; He, Y.; Tan, S.; Guo, Y.; et al. Tumor microenvironment responsive nanogel for the combinatorial antitumor effect of chemotherapy and immunotherapy. Nano Lett. 2017, 17, 6366–6375. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Liu, X.; Ran, M.; Yang, T.; Li, T.; Wu, Y.; Lin, Y.; Qian, Z.; Gao, X. Simultaneous delivery of immune stimulatory gene and checkpoint blocker via targeted nanoparticles to strengthen antitumor immunity. Mater. Today Nano 2022, 17, 100151. [Google Scholar] [CrossRef]
- Jiang, D.; Gao, T.; Liang, S.; Mu, W.; Fu, S.; Liu, Y.; Yang, R.; Zhang, Z.; Liu, Y.; Zhang, N. Lymph lymph node delivery strategy enables the activation of cytotoxic T lymphocytes and natural killer cells to augment cancer immunotherapy. Acs Appl. Mater. Inter. 2021, 13, 22213–22224. [Google Scholar] [CrossRef]
- Medina-Echeverz, J.; Hinterberger, M.; Testori, M.; Geiger, M.; Giessel, R.; Bathke, B.; Kassub, R.; Graebnitz, F.; Fiore, G.; Wennier, S.T.; et al. Synergistic cancer immunotherapy combines MVA-CD40L induced innate and adaptive immunity with tumor targeting antibodies. Nat. Commun. 2019, 10, 5041. [Google Scholar] [CrossRef] [PubMed]
- Perry, J.L.; Tian, S.; Sengottuvel, N.; Harrison, E.B.; Gorentla, B.K.; Kapadia, C.H.; Cheng, N.; Luft, J.C.; Ting, J.P.-Y.; DeSimone, J.M.; et al. Pulmonary delivery of nanoparticle-bound toll-like receptor 9 agonist for the treatment of metastatic lung cancer. Acs Nano 2020, 14, 7200–7215. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhao, J.; Hao, C.; Hu, S.; Chen, C.; Cao, Y.; Xu, Z.; Guo, J.; Xu, L.; Sun, M.; et al. The development of chiral nanoparticles to target NK cells and CD8(+) T cells for cancer immunotherapy. Adv. Mater. 2022, 34, 2109354. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.-Q.; Zhang, C.; Zhang, X.; Yan, J.; Zeng, C.; Talebian, F.; Lynch, K.; Zhao, W.; Hou, X.; Du, S.; et al. Intratumoral delivery of IL-12 and IL-27 mRNA using lipid nanoparticles for cancer immunotherapy. J. Control. Release 2022, 345, 306–313. [Google Scholar] [CrossRef] [PubMed]
- Vallera, D.A.; Oh, F.; Kodal, B.; Hinderlie, P.; Geller, M.A.; Miller, J.S.; Felices, M. A HER2 Tri-specific NK cell engager mediates efficient targeting of human ovarian cancer. Cancers 2021, 13, 3994. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Lee, J.Y.; Han, J.; Hwang, H.S.; Lee, J.; Na, K. Local immune-triggered surface-modified stem cells for solid tumor immunotherapy. Adv. Funct. Mater. 2019, 29, 1900773. [Google Scholar] [CrossRef]
- Wei, Z.; Yi, Y.; Luo, Z.; Gong, X.; Jiang, Y.; Hou, D.; Zhang, L.; Liu, Z.; Wang, M.; Wang, J.; et al. Selenopeptide nanomedicine activates natural killer cells for enhanced tumor chemoimmunotherapy. Adv. Mater. 2022, 34, 2108167. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Li, T.; Guo, Y.; Sun, C.; Xianyu, B.; Xu, H. Selenium-containing nanoparticles combine the NK cells mediated immunotherapy with radiotherapy and chemotherapy. Adv. Mater. 2020, 32, 1907568. [Google Scholar] [CrossRef]
- Chen, Q.; He, L.; Li, X.; Xu, L.; Chen, T. Ruthenium complexes boost NK cell immunotherapy via sensitizing triple-negative breast cancer and shaping immuno-microenvironment. Biomaterials 2022, 281, 121371. [Google Scholar] [CrossRef]
- Qian, M.; Chen, L.; Du, Y.; Jiang, H.; Huo, T.; Yang, Y.; Guo, W.; Wang, Y.; Huang, R. Biodegradable mesoporous silica achieved via carbon nanodots-incorporated framework swelling for debris-mediated photothermal synergistic immunotherapy. Nano Lett. 2019, 19, 8409–8417. [Google Scholar] [CrossRef]
- Zhan, M.; Qiu, J.; Fan, Y.; Chen, L.; Guo, Y.; Wang, Z.; Li, J.; Majoral, J.-P.; Shi, X. Phosphorous dendron micelles as a nanomedicine platform for cooperative tumor chemoimmunotherapy via synergistic modulation of immune cells. Adv. Mater. 2023, 35, 2208277. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Gong, X.; Li, J.; Wang, H.; Xu, X.; Li, Y.; Sha, X.; Zhang, Z. Oxygen-delivering polyfluorocarbon nanovehicles improve tumor oxygenation and potentiate photodynamic-mediated antitumor immunity. Acs Nano 2021, 15, 5405–5419. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, Y.; Wang, J.; Xu, X.; Zhang, A.; Li, Y.; Zhang, Z. Macrophage membrane-coated nano- gemcitabine promotes lymphocyte infiltration and synergizes AntiPD-L1 to restore the tumoricidal function. Acs Nano 2023, 17, 322–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhao, J.; Hu, X.; Wang, C.; Jia, Y.; Zhu, C.; Xie, S.; Lee, J.; Li, F.; Ling, D. A peritumorally injected immunomodulating adjuvant elicits robust and safe metalloimmunotherapy against solid tumors. Adv. Mater. 2022, 34, 2206915. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-S.; Han, J.-H.; Choi, S.H.; Jung, H.-Y.; Park, J.D.; An, H.-J.; Kim, S.-E.; Kim, D.-H.; Doh, J.; Han, D.K.; et al. Cationic nanoparticle-mediated activation of natural killer cells for effective cancer immunotherapy. Acs Appl. Mater. Inter. 2020, 12, 56731–56740. [Google Scholar] [CrossRef] [PubMed]
- Hellstrand, K.; Hermodsson, S.; Naredi, P.; Mellqvist, U.H.; Brune, M. Histamine and cytokine therapy. Acta Oncol. 1998, 37, 347–353. [Google Scholar] [CrossRef] [PubMed]
- Liao, N.; Su, L.; Zheng, Y.; Zhao, B.; Wu, M.; Zhang, D.; Yang, H.; Liu, X.; Song, J. In vivo tracking of cell viability for adoptive natural killer cell-based immunotherapy by ratiometric NIR-II fluorescence imaging. Angew. Chem. Int. Edit. 2021, 60, 20888–20896. [Google Scholar] [CrossRef] [PubMed]
- Khushalani, N.I.; Diab, A.; Ascierto, P.A.; Larkin, J.; Sandhu, S.; Sznol, M.; Koon, H.B.; Jarkowski, A.; Zhou, M.; Statkevich, P.; et al. Bempegaldesleukin plus nivolumab in untreated, unresectable or metastatic melanoma: Phase III PIVOT IO 001 study design. Future Oncol. 2020, 16, 2165–2175. [Google Scholar] [CrossRef] [PubMed]
- Dubois, S.P.; Miljkovic, M.D.; Fleisher, T.A.; Pittaluga, S.; Hsu-Albert, J.; Bryant, B.R.; Petrus, M.N.; Perera, L.P.; Muller, J.R.; Shih, J.H.; et al. Short-course IL-15 given as a continuous infusion led to a massive expansion of effective NK cells: Implications for combination therapy with antitumor antibodies. J. Immunother. Cancer 2021, 9, e002193. [Google Scholar] [CrossRef]
- Lin, C.-W.; Tsai, M.-H.; Li, S.-T.; Tsai, T.-I.; Chu, K.-C.; Liu, Y.-C.; Lai, M.-Y.; Wu, C.-Y.; Tseng, Y.-C.; Shivatare, S.S.; et al. A common glycan structure on immunoglobulin G for enhancement of effector functions. Proc. Natl. Acad. Sci. USA 2015, 112, 10611–10616. [Google Scholar] [CrossRef]
- Poupot, M.; Turrin, C.-O.; Caminade, A.-M.; Fournie, J.-J.; Attal, M.; Poupot, R.; Fruchon, S. Poly(phosphorhydrazone) dendrimers: Yin and yang of monocyte activation for human NK cell amplification applied to immunotherapy against multiple myeloma. Nanomed.-Nano Technol. 2016, 12, 2321–2330. [Google Scholar] [CrossRef] [PubMed]
- Portevin, D.; Poupot, M.; Rolland, O.; Turrin, C.-O.; Fournie, J.-J.; Majoral, J.-P.; Caminade, A.-M.; Poupot, R. Regulatory activity of azabisphosphonate-capped dendrimers on human CD4(+) T cell proliferation enhances ex-vivo expansion of NK cells from PBMCs for immunotherapy. J. Transl. Med. 2009, 7, 82. [Google Scholar] [CrossRef] [PubMed]
- Yim, H.; Park, W.; Kim, D.; Fahmy, T.M.; Na, K. A self-assembled polymeric micellar immunomodulator for cancer treatment based on cationic amphiphilic polymers. Biomaterials 2014, 35, 9912–9919. [Google Scholar] [CrossRef] [PubMed]
- Mulens-Arias, V.; Rojas, J.M.; Perez-Yaguee, S.; Morales, M.P.; Barber, D.F. Polyethylenimine-coated SPIONs trigger macrophage activation through TLR-4 signaling and ROS production and modulate podosome dynamics. Biomaterials 2015, 52, 494–506. [Google Scholar] [CrossRef]
- Grote, S.; Urena-Bailen, G.; Chan, K.C.-H.; Baden, C.; Mezger, M.; Handgretinger, R.; Schleicher, S. In vitro evaluation of CD276-CAR NK-92 functionality, migration and invasion potential in the presence of immune inhibitory factors of the tumor microenvironment. Cells 2021, 10, 1020. [Google Scholar] [CrossRef]
- Surace, L.; Doisne, J.-M.; Escoll, P.; Marie, S.; Dardalhon, V.; Croft, C.; Thaller, A.; Topazio, D.; Sparaneo, A.; Cama, A.; et al. Polarized mitochondria as guardians of NK cell fitness. Blood Adv. 2021, 5, 26–38. [Google Scholar] [CrossRef]
- Mitwasi, N.; Feldmann, A.; Arndt, C.; Koristka, S.; Berndt, N.; Jureczek, J.; Loureiro, L.R.; Bergmann, R.; Mathe, D.; Hegedues, N.; et al. “UniCAR”-modified off-the-shelf NK-92 cells for targeting of GD2-expressing tumour cells. Sci. Rep. 2020, 10, 2141. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Han, W. Biomarkers of cytokine release syndrome and neurotoxicity related to CAR-T cell therapy. Biomark. Res. 2018, 6, 4. [Google Scholar] [CrossRef]
- Porter, D.; Frey, N.; Wood, P.A.; Weng, Y.; Grupp, S.A. Grading of cytokine release syndrome associated with the CAR T cell therapy tisagenlecleucel. J. Hematol. Oncol. 2018, 11, 35. [Google Scholar] [CrossRef]
- Martinet, L.; Smyth, M.J. Balancing natural killer cell activation through paired receptors. Nat. Rev. Immunol. 2015, 15, 243–254. [Google Scholar] [CrossRef]
- Xie, G.; Dong, H.; Liang, Y.; Ham, J.D.; Rizwan, R.; Chen, J. CAR-NK cells: A promising cellular immunotherapy for cancer. Ebiomedicine 2020, 59, 102975. [Google Scholar] [CrossRef]
- Liu, E.; Marin, D.; Banerjee, P.; Macapinlac, H.A.; Thompson, P.; Basar, R.; Kerbauy, L.N.; Overman, B.; Thall, P.; Kaplan, M.; et al. Use of CAR-transduced natural killer cells in CD19-positive lymphoid tumors. N. Engl. J. Med. 2020, 382, 545–553. [Google Scholar] [CrossRef] [PubMed]
- Papayannakos, C.; Daniel, R. Understanding lentiviral vector chromatin targeting: Working to reduce insertional mutagenic potential for gene therapy. Gene Ther. 2013, 20, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Chang, Y.-H.; Connolly, J.; Shimasaki, N.; Mimura, K.; Kono, K.; Campana, D. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 2013, 73, 1777–1786. [Google Scholar] [CrossRef]
- Shimasaki, N.; Fujisaki, H.; Cho, D.; Masselli, M.; Lockey, T.; Eldridge, P.; Leung, W.; Campana, D. A clinically adaptable method to enhance the cytotoxicity of natural killer cells against B-cell malignancies. Cytotherapy 2012, 14, 830–840. [Google Scholar] [CrossRef] [PubMed]
- Tong, L.; Jimenez-Cortegana, C.; Tay, A.H.M.; Wickstrom, S.; Galluzzi, L.; Lundqvist, A. NK cells and solid tumors: Therapeutic potential and persisting obstacles. Molecular Cancer 2022, 21, 206. [Google Scholar] [CrossRef]
- Teng, K.-Y.; Mansour, A.G.; Zhu, Z.; Li, Z.; Tian, L.; Ma, S.; Xu, B.; Lu, T.; Chen, H.; Hou, D.; et al. Off-the-shelf prostate stem cell antigen-directed chimeric antigen receptor natural killer cell therapy to treat pancreatic cancer. Gastroenterology 2022, 162, 1319–1333. [Google Scholar] [CrossRef]
- Yang, S.; Wen, J.; Li, H.; Xu, L.; Liu, Y.; Zhao, N.; Zeng, Z.; Qi, J.; Jiang, W.; Han, W.; et al. Aptamer-engineered natural killer cells for cell-specific adaptive immunotherapy. Small 2019, 15, 1900903. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Liu, Z.; Zhang, L.; Felding, B.H.; Moremen, K.W.; Lauvau, G.; Abadier, M.; Ley, K.; Wu, P. A single-step chemoenzymatic reaction for the construction of antibody-cell conjugates. Acs Cent. Sci. 2018, 4, 1633–1641. [Google Scholar] [CrossRef]
- Yilmaz, A.; Cui, H.; Caligiuri, M.A.; Yu, J. Chimeric antigen receptor-engineered natural killer cells for cancer immunotherapy. J. Hematol. Oncol. 2020, 13, 168. [Google Scholar] [CrossRef]
- Simonetta, F.; Alvarez, M.; Negrin, R.S. Natural killer cells in graft-versus-host-disease after allogeneic hematopoietic cell transplantation. Front. Immunol. 2017, 8, 465. [Google Scholar] [CrossRef]
- Zhang, L.; Meng, Y.; Feng, X.; Han, Z. CAR-NK cells for cancer immunotherapy: From bench to bedside. Biomark. Res. 2022, 10, 12. [Google Scholar] [CrossRef]
- Quintarelli, C.; Sivori, S.; Caruso, S.; Carlomagno, S.; Falco, M.; Boffa, I.; Orlando, D.; Guercio, M.; Abbaszadeh, Z.; Sinibaldi, M.; et al. Efficacy of third-party chimeric antigen receptor modified peripheral blood natural killer cells for adoptive cell therapy of B-cell precursor acute lymphoblastic leukemia. Leukemia 2020, 34, 1102–1115. [Google Scholar] [CrossRef]
- Meng, D.; Pan, H.; He, W.; Jiang, X.; Liang, Z.; Zhang, X.; Xu, X.; Wang, Z.; Zheng, J.; Gong, P.; et al. In situ activated NK cell as bio-orthogonal targeted live-cell nanocarrier augmented solid tumor immunotherapy. Adv. Funct. Mater. 2022, 32, 2202603. [Google Scholar] [CrossRef]
- Im, S.; Jang, D.; Saravanakumar, G.; Lee, J.; Kang, Y.; Lee, Y.M.; Lee, J.; Doh, J.; Yang, Z.Y.; Jang, M.H.; et al. Harnessing the formation of natural killer-tumor cell immunological synapses for enhanced therapeutic effect in solid tumors. Adv. Mater. 2020, 32, 2000020. [Google Scholar] [CrossRef]
- Chandrasekaran, S.; Chan, M.F.; Li, J.; King, M.R. Super natural killer cells that target metastases in the tumor draining lymph nodes. Biomaterials 2016, 77, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Zhao, Z.; Zhang, L.; Xue, L.; Shen, S.; Wen, Y.; Wei, Z.; Wang, L.; Kong, L.; Sun, H.; et al. Neutrophil-mediated anticancer drug delivery for suppression of postoperative malignant glioma recurrence. Nat. Nanotechnol. 2017, 12, 692–700. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, F.; Wei, Z.; Li, X.; Zhao, H.; Lv, H.; Ge, R.; Ma, H.; Zhang, H.; Yang, B.; et al. Magnetic delivery of Fe3O4@polydopamine nanoparticle-loaded natural killer cells suggest a promising anticancer treatment. Biomater. Sci. 2018, 6, 2714–2725. [Google Scholar] [CrossRef] [PubMed]
- Jang, E.-S.; Shin, J.-H.; Ren, G.; Park, M.-J.; Cheng, K.; Chen, X.; Wu, J.C.; Sunwoo, J.B.; Cheng, Z. The manipulation of natural killer cells to target tumor sites using magnetic nanoparticles. Biomaterials 2012, 33, 5584–5592. [Google Scholar] [CrossRef]
- Burga, R.A.; Khan, D.H.; Agrawal, N.; Bollard, C.M.; Fernandes, R. Designing magnetically responsive biohybrids composed of cord blood-derived natural killer cells and iron oxide nanoparticles. Bioconjugate Chem. 2019, 30, 552–560. [Google Scholar] [CrossRef]
- Greening, D.W.; Gopal, S.K.; Xu, R.; Simpson, R.J.; Chen, W. Exosomes and their roles in immune regulation and cancer. Semin. Cell Dev. Biol. 2015, 40, 72–81. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Hu, W.; Chen, H.; Shou, X.; Ye, T.; Xu, Y. Cocktail strategy based on NK cell-derived exosomes and their biomimetic nanoparticles for dual tumor therapy. Cancers 2019, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Weng, J.; Xiang, X.; Ding, L.; Wong, A.L.-A.; Zeng, Q.; Sethi, G.; Wang, L.; Lee, S.C.; Goh, B.C. Extracellular vesicles, the cornerstone of next-generation cancer diagnosis? Semin. Cancer Biol. 2021, 74, 105–120. [Google Scholar] [CrossRef] [PubMed]
- Jong, A.Y.; Wu, C.-H.; Li, J.; Sun, J.; Fabbri, M.; Wayne, A.S.; Seeger, R.C. Large-scale isolation and cytotoxicity of extracellular vesicles derived from activated human natural killer cells. J. Extracell. Vesicles 2017, 6, 1294368. [Google Scholar] [CrossRef]
- Zhang, M.; Shao, W.; Yang, T.; Liu, H.; Guo, S.; Zhao, D.; Weng, Y.; Liang, X.-J.; Huang, Y. Conscription of immune cells by light-activatable silencing NK-derived exosome (LASNEO) for synergetic tumor eradication. Adv. Sci. 2022, 9, 2201135. [Google Scholar] [CrossRef]




| Target Receptors on NK Cells | Nanoparticles | Mechanisms of NK Activation | Ref. |
|---|---|---|---|
| CD16 | pHLIP-Fc or pHLIP-mAb | Inducing ADCC effect | [76] |
| IMN | Inducing ADCC effect | [77] | |
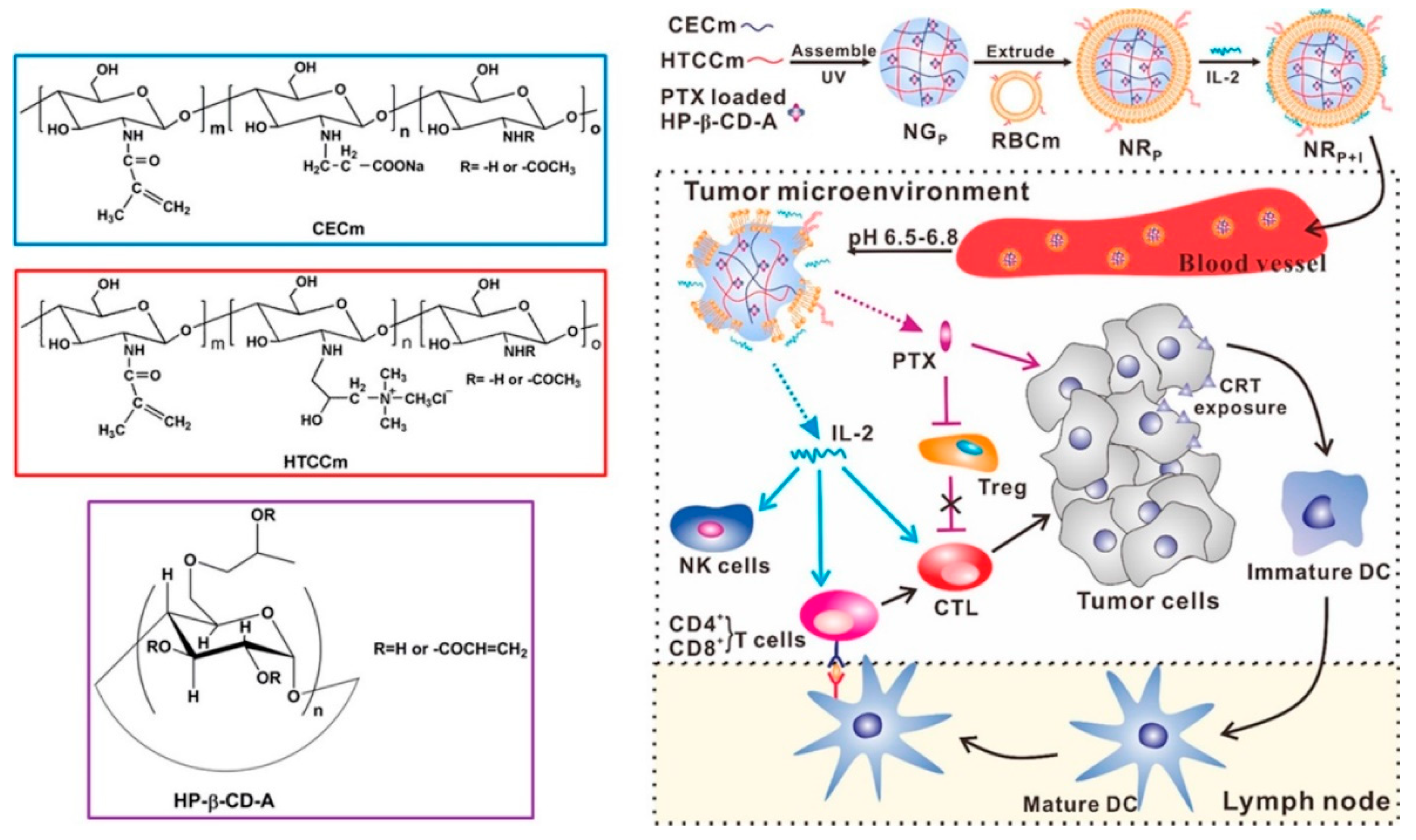
| IL-2R | NRP+I | Delivering IL-2 (immunotherapeutic agent) | [78] |
| IL-12R | RDMCM/pIL-12 + PD-1/PD-L1i | Delivering IL-12 encoding plasmid | [79] |
| IL-15R | IL-15-NPs | Delivering IL-15 (immunotherapeutic agent) | [80] |
| IL-18R | MVA-CD40L | Activating the secretion of IL-18, IFN-α, etc. | [81] |
| IL-6R and IL-12R | PRINT-CpG | Activating the secretion of IFN-γ, IL-6, IL-12, etc. | [82] |
| IL-12R and IL-18R | D- or L-type NPs | Activating the secretion of IL-18 and IL-12 | [83] |
| IL-12R and IL-27R | DAL4-LNP-IL-12 + IL-27 mRNA | Delivering IL-12 and IL-27 mRNA | [84] |
| IL-15R and CD16 | TriKEs | Delivering IL-15 and inducing ADCC effect | [85] |
| Multiple Cytokine receptor | hMSC-DPP | Activating the secretion of IFN-γ, IL-2, IL-4, IL-12, GM-CSF, etc. | [86] |
| NKG2A | SeP/DOX | Blocking the binding between HLA-E and NKG2A | [87] |
| Pem/Se | Blocking the binding between HLA-E and NKG2A | [45] | |
| PSeR/DOX NPs | Blocking the binding between HLA-E and NKG2A | [88] | |
| NKG2D | ZCMF | Upregulating the expression of NKG2D ligands | [43] |
| RuPOP | Upregulating the expression of NKG2D ligands | [89] | |
| CD@MSN | Upregulating the expression of NKG2D ligands | [90] | |
| Undefined | M-G1-TBPNa@DOX | Inhibiting the regulatory T cells and regulating the signaling between NK and monocytes | [91] |
| PF11DG | Activating the cytotoxicity of NK cells | [92] | |
| MNGs | Activating the cytotoxicity of NK cells | [93] | |
| Zn-LDH | Neutralizing tumor acidity to restore the function of NK cells | [94] | |
| cNPs | Increasing the expression of CCR4 and CXCR4 on NK | [95] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zang, J.; Mei, Y.; Zhu, S.; Yin, S.; Feng, N.; Ci, T.; Lyu, Y. Natural Killer-Based Therapy: A Prospective Thought for Cancer Treatment Related to Diversified Drug Delivery Pathways. Pharmaceutics 2024, 16, 939. https://doi.org/10.3390/pharmaceutics16070939
Zang J, Mei Y, Zhu S, Yin S, Feng N, Ci T, Lyu Y. Natural Killer-Based Therapy: A Prospective Thought for Cancer Treatment Related to Diversified Drug Delivery Pathways. Pharmaceutics. 2024; 16(7):939. https://doi.org/10.3390/pharmaceutics16070939
Chicago/Turabian StyleZang, Jing, Yijun Mei, Shiguo Zhu, Shaoping Yin, Nianping Feng, Tianyuan Ci, and Yaqi Lyu. 2024. "Natural Killer-Based Therapy: A Prospective Thought for Cancer Treatment Related to Diversified Drug Delivery Pathways" Pharmaceutics 16, no. 7: 939. https://doi.org/10.3390/pharmaceutics16070939





