Uncovering the Therapeutic Potential of Lithium Chloride in Type 2 Diabetic Cardiomyopathy: Targeting Tau Hyperphosphorylation and TGF-β Signaling via GSK-3β Inhibition
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Models
2.2. Echocardiography
2.3. ELISA Measurement in Serum Samples
2.4. LV Histology
2.5. Immunohistochemistry Analysis
2.6. Western Blot Analysis
2.7. PCR Analysis
2.8. Statistical Analysis
3. Results
3.1. Metabolic Profile Alterations in T2DM Rats
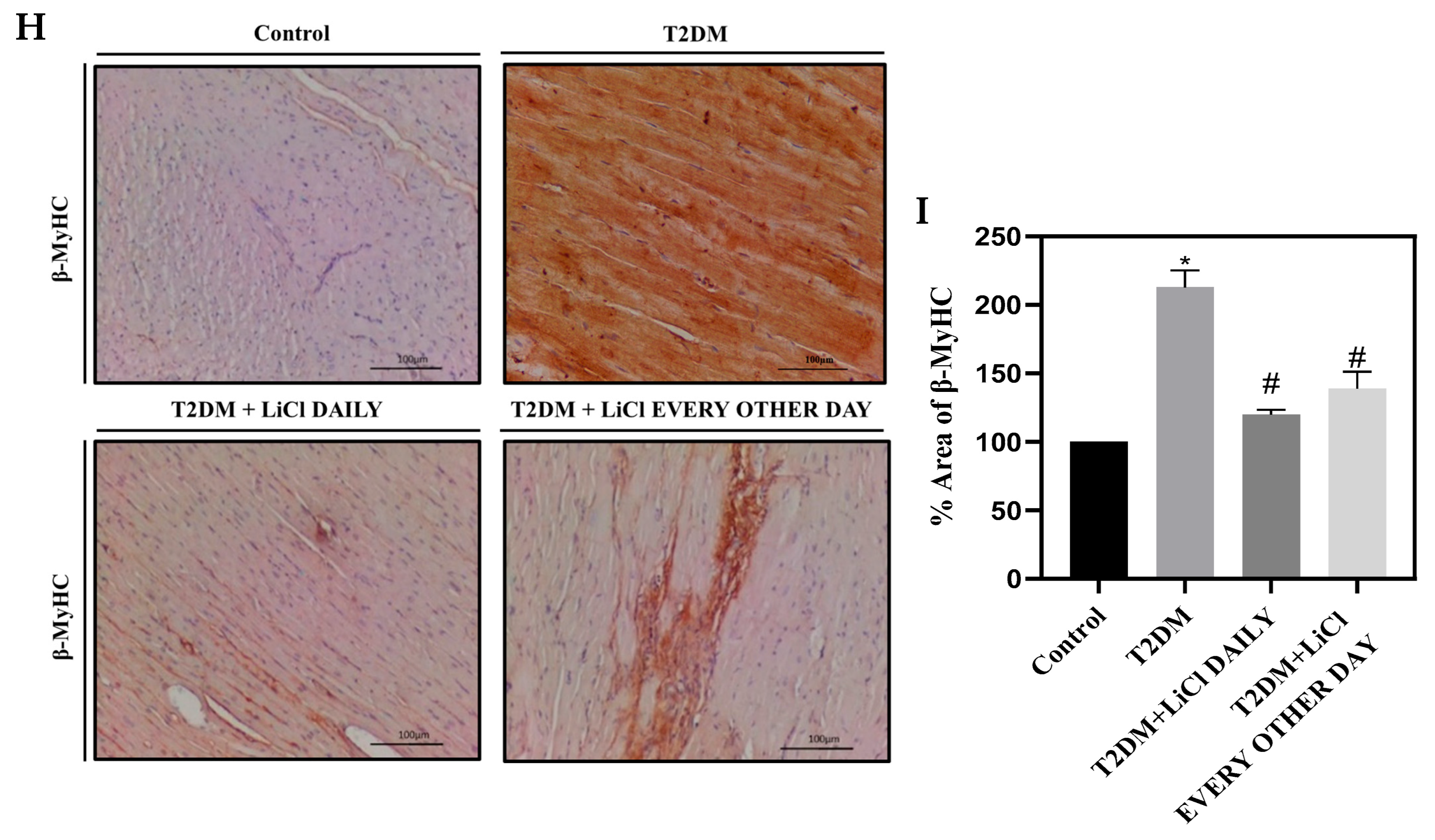
3.2. T2DM Induces Hyperphosphorylation of Tau Protein in the Rats’ LV
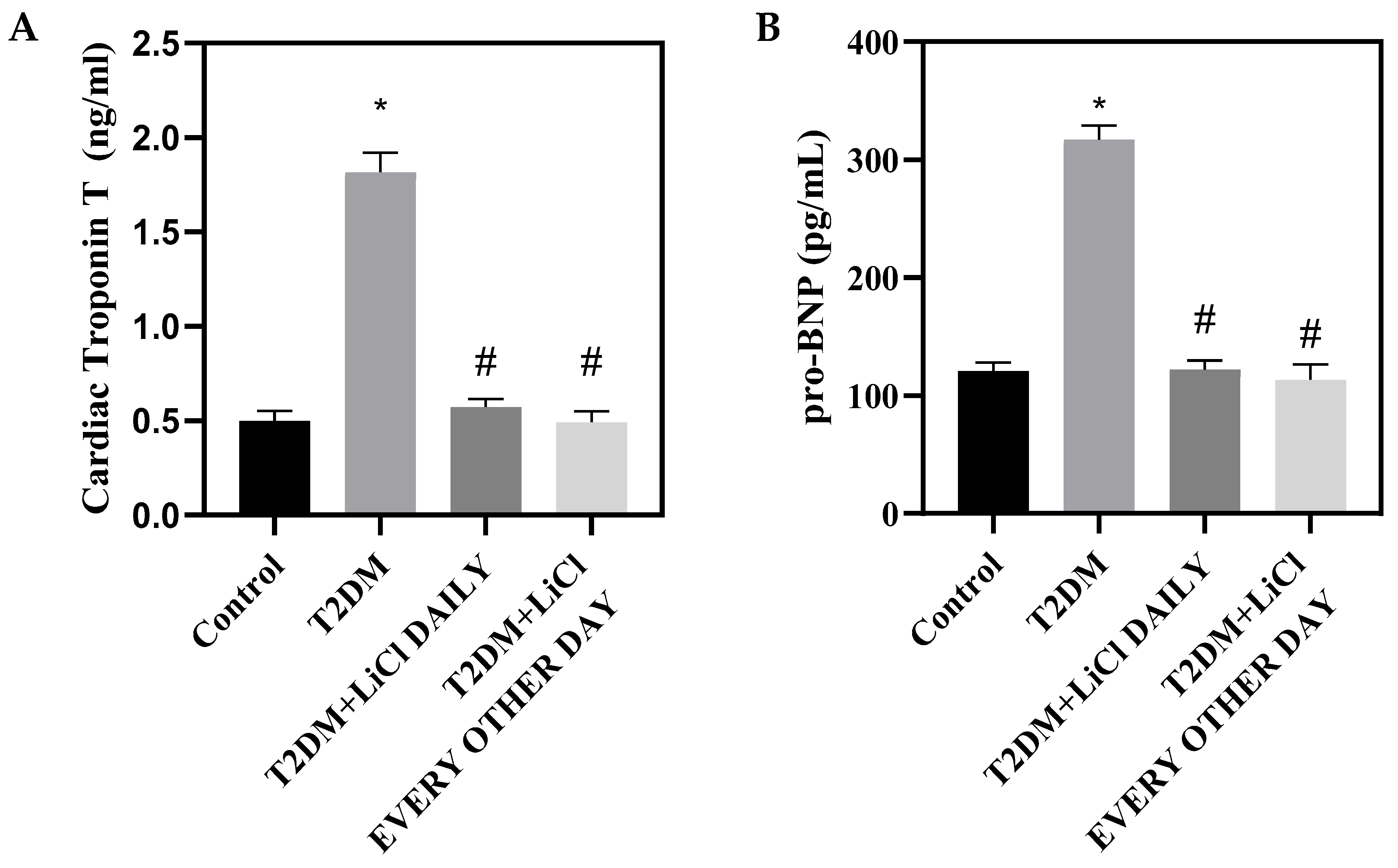
3.3. Tau Hyperphosphorylation Inhibition by LiCl Is Associated with Attenuation of Cardiac Dysfunction and Structural Changes
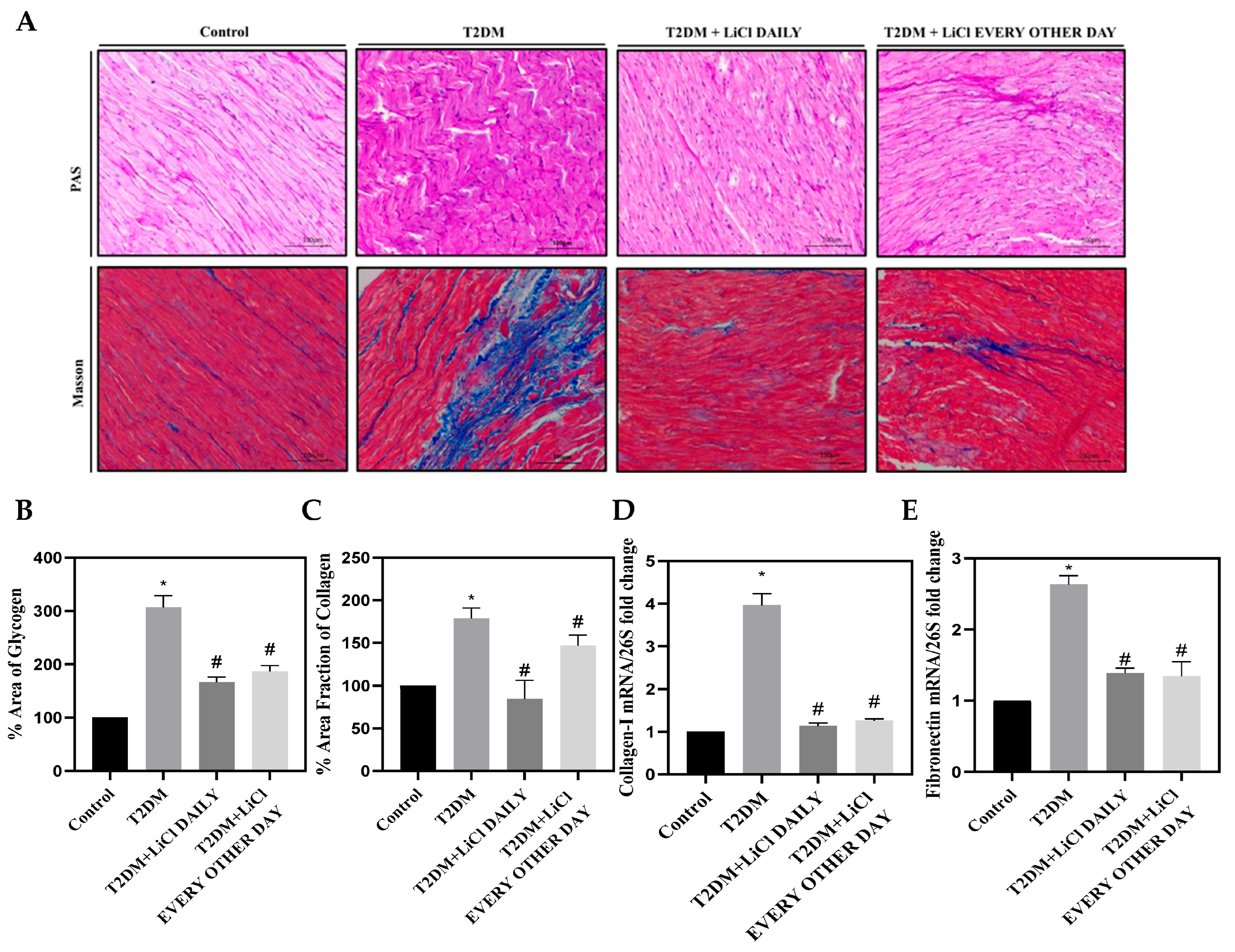
3.4. Cardiac Fibrosis Is Correlated to Tau Hyperphosphorylation in T2DM and Is Attenuated by LiCl Treatment
3.5. Tau Hyperphosphorylation Is Coupled with Cardiac Inflammation in T2DM and Is Attenuated by LiCl Treatment
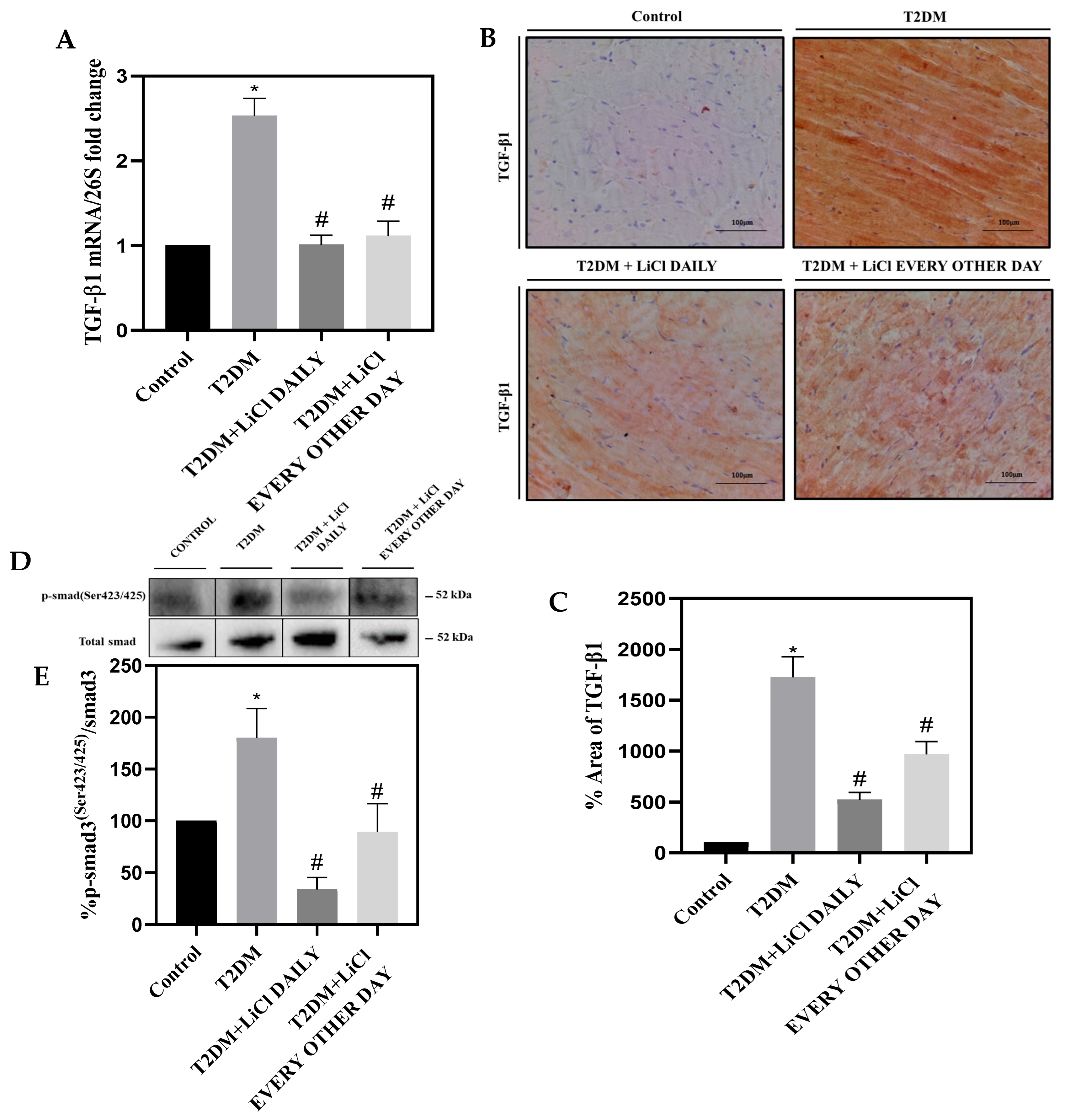
3.6. LiCl Treatment Attenuates TGF-β1 Overexpression in the Heart of Type 2 Diabetic Rats
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef]
- Jia, G.; Hill, M.A.; Sowers, J.R. Diabetic Cardiomyopathy: An Update of Mechanisms Contributing to This Clinical Entity. Circ. Res. 2018, 122, 624–638. [Google Scholar] [CrossRef] [PubMed]
- Young, M.E.; McNulty, P.; Taegtmeyer, H. Adaptation and maladaptation of the heart in diabetes: Part II: Potential mechanisms. Circulation 2002, 105, 1861–1870. [Google Scholar] [CrossRef] [PubMed]
- Alaeddine, L.M.; Harb, F.; Hamza, M.; Dia, B.; Mogharbil, N.; Azar, N.S.; Noureldein, M.H.; El Khoury, M.; Sabra, R.; Eid, A.A. Pharmacological regulation of cytochrome P450 metabolites of arachidonic acid attenuates cardiac injury in diabetic rats. Transl. Res. 2021, 235, 85–101. [Google Scholar] [CrossRef] [PubMed]
- Piera-Velazquez, S.; Jimenez, S.A. Endothelial to Mesenchymal Transition: Role in Physiology and in the Pathogenesis of Human Diseases. Physiol. Rev. 2019, 99, 1281–1324. [Google Scholar] [CrossRef] [PubMed]
- Khalil, H.; Kanisicak, O.; Prasad, V.; Correll, R.N.; Fu, X.; Schips, T.; Vagnozzi, R.J.; Liu, R.; Huynh, T.; Lee, S.J.; et al. Fibroblast-specific TGF-β-Smad2/3 signaling underlies cardiac fibrosis. J. Clin. Investig. 2017, 127, 3770–3783. [Google Scholar] [CrossRef] [PubMed]
- Orr, M.E.; Sullivan, A.C.; Frost, B. A Brief Overview of Tauopathy: Causes, Consequences, and Therapeutic Strategies. Trends Pharmacol. Sci. 2017, 38, 637–648. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Oyama, F.; Ihara, Y. Tau is widely expressed in rat tissues. J. Neurochem. 1996, 67, 1235–1244. [Google Scholar] [CrossRef] [PubMed]
- Hanger, D.P.; Noble, W. Functional implications of glycogen synthase kinase-3-mediated tau phosphorylation. Int. J. Alzheimers Dis. 2011, 2011, 352805. [Google Scholar] [CrossRef]
- Pei, J.J.; Braak, E.; Braak, H.; Grundke-Iqbal, I.; Iqbal, K.; Winblad, B.; Cowburn, R.F. Distribution of active glycogen synthase kinase 3beta (GSK-3beta) in brains staged for Alzheimer disease neurofibrillary changes. J. Neuropathol. Exp. Neurol. 1999, 58, 1010–1019. [Google Scholar] [CrossRef]
- Evans, D.B.; Rank, K.B.; Bhattacharya, K.; Thomsen, D.R.; Gurney, M.E.; Sharma, S.K. Tau phosphorylation at serine 396 and serine 404 by human recombinant tau protein kinase II inhibits tau’s ability to promote microtubule assembly. J. Biol. Chem. 2000, 275, 24977–24983. [Google Scholar] [CrossRef] [PubMed]
- Ho, K.H.; Yang, X.; Osipovich, A.B.; Cabrera, O.; Hayashi, M.L.; Magnuson, M.A.; Gu, G.; Kaverina, I. Glucose Regulates Microtubule Disassembly and the Dose of Insulin Secretion via Tau Phosphorylation. Diabetes 2020, 69, 1936–1947. [Google Scholar] [CrossRef] [PubMed]
- Heydemann, A. An Overview of Murine High Fat Diet as a Model for Type 2 Diabetes Mellitus. J. Diabetes Res. 2016, 2016, 2902351. [Google Scholar] [CrossRef] [PubMed]
- Gheibi, S.; Kashfi, K.; Ghasemi, A. A practical guide for induction of type-2 diabetes in rat: Incorporating a high-fat diet and streptozotocin. Biomed. Pharmacother. 2017, 95, 605–613. [Google Scholar] [CrossRef] [PubMed]
- Reed, M.J.; Meszaros, K.; Entes, L.J.; Claypool, M.D.; Pinkett, J.G.; Gadbois, T.M.; Reaven, G.M. A new rat model of type 2 diabetes: The fat-fed, streptozotocin-treated rat. Metabolism 2000, 49, 1390–1394. [Google Scholar] [CrossRef] [PubMed]
- Farrag, E.A.E.; Hammad, M.O.; Safwat, S.M.; Hamed, S.; Hellal, D. Artemisinin attenuates type 2 diabetic cardiomyopathy in rats through modulation of AGE-RAGE/HMGB-1 signaling pathway. Sci. Rep. 2023, 13, 11043. [Google Scholar] [CrossRef]
- Hong, M.; Chen, D.C.; Klein, P.S.; Lee, V.M. Lithium reduces tau phosphorylation by inhibition of glycogen synthase kinase-3. J. Biol. Chem. 1997, 272, 25326–25332. [Google Scholar] [CrossRef]
- Tajes, M.; Gutierrez-Cuesta, J.; Folch, J.; Ferrer, I.; Caballero, B.; Smith, M.A.; Casadesus, G.; Camins, A.; Pallás, M. Lithium treatment decreases activities of tau kinases in a murine model of senescence. J. Neuropathol. Exp. Neurol. 2008, 67, 612–623. [Google Scholar] [CrossRef]
- Li, Q.; Li, H.; Roughton, K.; Wang, X.; Kroemer, G.; Blomgren, K.; Zhu, C. Lithium reduces apoptosis and autophagy after neonatal hypoxia-ischemia. Cell Death Dis. 2010, 1, e56. [Google Scholar] [CrossRef]
- Maalouf, R.M.; Eid, A.A.; Gorin, Y.C.; Block, K.; Escobar, G.P.; Bailey, S.; Abboud, H.E. Nox4-derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am. J. Physiol. Cell Physiol. 2012, 302, C597–C604. [Google Scholar] [CrossRef]
- Anton, P.E.; Rutt, L.N.; Capper, C.; Orlicky, D.J.; McCullough, R.L. Profiling the oxylipidome in aged mice after chronic ethanol feeding: Identifying lipid metabolites as drivers of hepatocyte stress. Alcohol 2023, 107, 119–135. [Google Scholar] [CrossRef] [PubMed]
- Corbett, C.B.; St Paul, A.; Leigh, T.; Kelemen, S.E.; Peluzzo, A.M.; Okune, R.N.; Eguchi, S.; Haines, D.S.; Autieri, M.V. Genetic Deletion of FXR1 Reduces Intimal Hyperplasia and Induces Senescence in Vascular Smooth Muscle Cells. Am. J. Pathol. 2023, 193, 638–653. [Google Scholar] [CrossRef] [PubMed]
- Youssef, N.; Noureldein, M.; Njeim, R.; Ghadieh, H.E.; Harb, F.; Azar, S.T.; Fares, N.; Eid, A.A. Reno-Protective Effect of GLP-1 Receptor Agonists in Type1 Diabetes: Dual Action on TRPC6 and NADPH Oxidases. Biomedicines 2021, 9, 1360. [Google Scholar] [CrossRef]
- Noureldein, M.; Nawfal, R.; Bitar, S.; Maxwell, S.S.; Khurana, I.; Kassouf, H.K.; Khuri, F.R.; El-Osta, A.; Eid, A.A. Intestinal microbiota regulates diabetes and cancer progression by IL-1β and NOX4 dependent signaling cascades. Cell Mol. Life Sci. 2022, 79, 502. [Google Scholar] [CrossRef] [PubMed]
- Elia, E.; Ministrini, S.; Carbone, F.; Montecucco, F. Diabetic cardiomyopathy and inflammation: Development of hostile microenvironment resulting in cardiac damage. Minerva Cardiol. Angiol. 2022, 70, 357–369. [Google Scholar] [CrossRef] [PubMed]
- Frati, G.; Schirone, L.; Chimenti, I.; Yee, D.; Biondi-Zoccai, G.; Volpe, M.; Sciarretta, S. An overview of the inflammatory signalling mechanisms in the myocardium underlying the development of diabetic cardiomyopathy. Cardiovasc. Res. 2017, 113, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Otero Losada, M.E.; Rubio, M.C. Acute effects of lithium chloride on noradrenergic neurons from rat cerebral cortex. Gen. Pharmacol. 1984, 15, 31–35. [Google Scholar] [CrossRef]
- Klemfuss, H. Dietary potassium effects on lithium concentration and toxicity in hamsters. Biol. Psychiatry 1995, 37, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Linakis, J.G.; Eisenberg, M.S.; Lacouture, P.G.; Maher, T.J.; Lewander, W.J.; Driscoll, J.L.; Woolf, A. Multiple-dose sodium polystyrene sulfonate in lithium intoxication: An animal model. Pharmacol. Toxicol. 1992, 70, 38–40. [Google Scholar] [CrossRef] [PubMed]
- Nikoulina, S.E.; Ciaraldi, T.P.; Mudaliar, S.; Carter, L.; Johnson, K.; Henry, R.R. Inhibition of glycogen synthase kinase 3 improves insulin action and glucose metabolism in human skeletal muscle. Diabetes 2002, 51, 2190–2198. [Google Scholar] [CrossRef]
- Chen, X.; McMahon, E.G.; Gulve, E.A. Stimulatory effect of lithium on glucose transport in rat adipocytes is not mediated by elevation of IP1. Am. J. Physiol. 1998, 275, E272–E277. [Google Scholar] [CrossRef]
- Klein, P.S.; Melton, D.A. A molecular mechanism for the effect of lithium on development. Proc. Natl. Acad. Sci. USA 1996, 93, 8455–8459. [Google Scholar] [CrossRef]
- Rossetti, L. Normalization of insulin sensitivity with lithium in diabetic rats. Diabetes 1989, 38, 648–652. [Google Scholar] [CrossRef]
- Dangana, E.O.; Michael, O.S.; Omolekulo, T.E.; Areola, E.D.; Olatunji, L.A. Enhanced hepatic glycogen synthesis and suppressed adenosine deaminase activity by lithium attenuates hepatic triglyceride accumulation in nicotine-exposed rats. Biomed. Pharmacother. 2019, 109, 1417–1427. [Google Scholar] [CrossRef]
- Samad, N.; Bilal, K.; Yasmin, F.; Khaliq, S.; Zaman, A.; Ayaz, M.M. Effect of lithium chloride on d-galactose induced organs injury: Possible antioxidative role. Pak. J. Pharm. Sci. 2020, 33, 1795–1803. [Google Scholar]
- Fang, C.X.; Dong, F.; Thomas, D.P.; Ma, H.; He, L.; Ren, J. Hypertrophic cardiomyopathy in high-fat diet-induced obesity: Role of suppression of forkhead transcription factor and atrophy gene transcription. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H1206–H1215. [Google Scholar] [CrossRef]
- Pulinilkunnil, T.; Kienesberger, P.C.; Nagendran, J.; Sharma, N.; Young, M.E.; Dyck, J.R. Cardiac-specific adipose triglyceride lipase overexpression protects from cardiac steatosis and dilated cardiomyopathy following diet-induced obesity. Int. J. Obes. 2014, 38, 205–215. [Google Scholar] [CrossRef]
- Malachias, M.V.B.; Wijkman, M.O.; Bertoluci, M.C. NT-proBNP as a predictor of death and cardiovascular events in patients with type 2 diabetes. Diabetol. Metab. Syndr. 2022, 14, 64. [Google Scholar] [CrossRef]
- Rørth, R.; Jhund, P.S.; Kristensen, S.L.; Desai, A.S.; Køber, L.; Rouleau, J.L.; Solomon, S.D.; Swedberg, K.; Zile, M.R.; Packer, M.; et al. The prognostic value of troponin T and N-terminal pro B-type natriuretic peptide, alone and in combination, in heart failure patients with and without diabetes. Eur. J. Heart Fail. 2019, 21, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Wu, X. The first line of defense against cardiac hypertrophy. Curr. Mol. Med. 2013, 13, 670–680. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Feng, H.Z.; Wei, H.; Cala, S.; Jin, J.P. Up-regulation of alpha-smooth muscle actin in cardiomyocytes from non-hypertrophic and non-failing transgenic mouse hearts expressing N-terminal truncated cardiac troponin I. FEBS Open Bio 2013, 4, 11–17. [Google Scholar] [CrossRef]
- Camelliti, P.; Borg, T.K.; Kohl, P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc. Res. 2005, 65, 40–51. [Google Scholar] [CrossRef]
- Ignotz, R.A.; Massagué, J. Transforming growth factor-beta stimulates the expression of fibronectin and collagen and their incorporation into the extracellular matrix. J. Biol. Chem. 1986, 261, 4337–4345. [Google Scholar] [CrossRef]
- Jin, D.; Takai, S.; Sugiyama, T.; Hayashi, T.; Fukumoto, M.; Oku, H.; Kitaura, Y.; Ikeda, T.; Miyazaki, M. Long-term angiotensin II blockade may improve not only hyperglycemia but also age-associated cardiac fibrosis. J. Pharmacol. Sci. 2009, 109, 275–284. [Google Scholar] [CrossRef]
- Ramesh, P.; Yeo, J.L.; Brady, E.M.; McCann, G.P. Role of inflammation in diabetic cardiomyopathy. Ther. Adv. Endocrinol. Metab. 2022, 13, 20420188221083530. [Google Scholar] [CrossRef]
- Hanna, A.; Frangogiannis, N.G. Inflammatory Cytokines and Chemokines as Therapeutic Targets in Heart Failure. Cardiovasc. Drugs Ther. 2020, 34, 849–863. [Google Scholar] [CrossRef]
- Yokoyama, T.; Nakano, M.; Bednarczyk, J.L.; McIntyre, B.W.; Entman, M.; Mann, D.L. Tumor necrosis factor-alpha provokes a hypertrophic growth response in adult cardiac myocytes. Circulation 1997, 95, 1247–1252. [Google Scholar] [CrossRef]
- Siwik, D.A.; Chang, D.L.; Colucci, W.S. Interleukin-1beta and tumor necrosis factor-alpha decrease collagen synthesis and increase matrix metalloproteinase activity in cardiac fibroblasts in vitro. Circ. Res. 2000, 86, 1259–1265. [Google Scholar] [CrossRef]
- Palmer, J.N.; Hartogensis, W.E.; Patten, M.; Fortuin, F.D.; Long, C.S. Interleukin-1 beta induces cardiac myocyte growth but inhibits cardiac fibroblast proliferation in culture. J. Clin. Investig. 1995, 95, 2555–2564. [Google Scholar] [CrossRef] [PubMed]
- Krown, K.A.; Page, M.T.; Nguyen, C.; Zechner, D.; Gutierrez, V.; Comstock, K.L.; Glembotski, C.C.; Quintana, P.J.; Sabbadini, R.A. Tumor necrosis factor alpha-induced apoptosis in cardiac myocytes. Involvement of the sphingolipid signaling cascade in cardiac cell death. J. Clin. Investig. 1996, 98, 2854–2865. [Google Scholar] [CrossRef] [PubMed]
- Biernacka, A.; Dobaczewski, M.; Frangogiannis, N.G. TGF-β signaling in fibrosis. Growth Factors 2011, 29, 196–202. [Google Scholar] [CrossRef] [PubMed]
- Heger, J.; Schulz, R.; Euler, G. Molecular switches under TGFβ signalling during progression from cardiac hypertrophy to heart failure. Br. J. Pharmacol. 2016, 173, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Ma, C.; Yang, H.; Zhang, P.Y. Transforming growth factor β and its role in heart disease. Exp. Ther. Med. 2017, 13, 2123–2128. [Google Scholar] [CrossRef]
- Rosenkranz, S.; Flesch, M.; Amann, K.; Haeuseler, C.; Kilter, H.; Seeland, U.; Schlüter, K.D.; Böhm, M. Alterations of beta-adrenergic signaling and cardiac hypertrophy in transgenic mice overexpressing TGF-beta(1). Am. J. Physiol. Heart Circ. Physiol. 2002, 283, H1253–H1262. [Google Scholar] [CrossRef] [PubMed]
- Caraci, F.; Battaglia, G.; Bruno, V.; Bosco, P.; Carbonaro, V.; Giuffrida, M.L.; Drago, F.; Sortino, M.A.; Nicoletti, F.; Copani, A. TGF-β1 pathway as a new target for neuroprotection in Alzheimer’s disease. CNS Neurosci. Ther. 2011, 17, 237–249. [Google Scholar] [CrossRef]
- Chalmers, K.A.; Love, S. Neurofibrillary tangles may interfere with Smad 2/3 signaling in neurons. J. Neuropathol. Exp. Neurol. 2007, 66, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Kashima, R.; Hata, A. The role of TGF-β superfamily signaling in neurological disorders. Acta Biochim. Biophys. Sin. 2018, 50, 106–120. [Google Scholar] [CrossRef] [PubMed]
- Betrie, A.H.; Ayton, S.; Bush, A.I.; Angus, J.A.; Lei, P.; Wright, C.E. Evidence of a Cardiovascular Function for Microtubule-Associated Protein Tau. J. Alzheimers Dis. 2017, 56, 849–860. [Google Scholar] [CrossRef] [PubMed]
- Daniele, G.; DiLucia, S.; Masci, P.G.; Del Monte, F. Heart and Brain: Complex Relationships for Left Ventricular Dysfunction. Curr. Cardiol. Rep. 2020, 22, 72. [Google Scholar] [CrossRef]
- Ke, Y.D.; Delerue, F.; Gladbach, A.; Götz, J.; Ittner, L.M. Experimental diabetes mellitus exacerbates tau pathology in a transgenic mouse model of Alzheimer’s disease. PLoS ONE 2009, 4, e7917. [Google Scholar] [CrossRef]



| Parameter | Control | T2DM | T2DM Treated with LiCl Daily | T2DM Treated with LiCl Every Other Day |
|---|---|---|---|---|
| Body weight (g) | 350.10 ± 62.07 | 456.40 ± 20.59 * | 347.20 ± 24.82 # | 491.3 ± 4.93 *^ |
| Blood glucose (mg/dL) | 114.58 ± 5.81 | 355.45 ± 22.69 * | 302.04 ± 40.03 * | 347.93 ± 34.20 * |
| Insulin (pmol/L) | 120 ± 16 | 207 ± 21 * | 167 ± 18 *# | 158 ± 16 *# |
| FFA (mmol/L) | 1.7 ± 0.05 | 5.2 ± 0.12 * | 3.4 ± 0.09 *# | 3.7 ± 0.07 *# |
| TG (mmol/L) | 1.2 ± 0.02 | 9.9 ± 0.36 * | 5.3 ± 0.44 *# | 4.9 ± 0.33 *# |
| Tibia length (cm) | 4.60 ± 0.05 | 3.89 ± 0.08 * | 4.62 ± 0.04 # | 4.48 ± 0.06 # |
| HW/TL (g/cm) | 0.38 ± 0.04 | 0.53 ± 0.03 * | 0.37 ± 0.02 # | 0.40 ± 0.02 # |
| Parameter | Control | T2DM | T2DM Treated with LiCl Daily | T2DM Treated with LiCl Every Other Day |
|---|---|---|---|---|
| LV Structure | ||||
| LVM (g) | 1.73 ± 0.32 | 1.81 ± 0.10 | 1.51 ± 0.07 | 1.66 ± 0.10 |
| LVM/TL (g/cm) | 0.32 ± 0.04 | 0.46 ± 0.02 * | 0.33 ± 0.02 # | 0.38 ± 0.01 # |
| FS % | 49.11 ± 0.68 | 31.84 ± 2.08 * | 44.24 ± 3.36 # | 41.67 ± 3.16 # |
| EDD (mm) | 7.12 ± 0.70 | 7.91 ± 0.67 | 7.27 ± 0.25 | 7.69 ± 0.19 |
| ESD (mm) | 3.64 ± 0.38 | 5.39 ± 0.26 * | 4.08 ± 0.22 # | 4.58 ± 0.3 * |
| LV Function | ||||
| EF % | 84.60 ± 0.51 | 64.68 ± 3.28 * | 79.56 ± 3.54 # | 76.20 ± 3.60 # |
| EDV (Teich) (mL) | 0.93 ± 0.23 | 1.19 ± 0.19 | 0.91 ± 0.08 | 1.02 ± 0.07 |
| ESV (Teich) (mL) | 0.15 ± 0.03 | 0.40 ± 0.05 * | 0.18 ± 0.03 # | 0.24 ± 0.04 # |
| Representative 2D Echo | 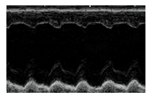 | 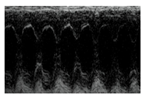 |  |  |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abou Assi, L.; Alkhansa, S.; Njeim, R.; Ismail, J.; Madi, M.; Ghadieh, H.E.; Al Moussawi, S.; Azar, T.S.; Ayoub, M.; Azar, W.S.; et al. Uncovering the Therapeutic Potential of Lithium Chloride in Type 2 Diabetic Cardiomyopathy: Targeting Tau Hyperphosphorylation and TGF-β Signaling via GSK-3β Inhibition. Pharmaceutics 2024, 16, 955. https://doi.org/10.3390/pharmaceutics16070955
Abou Assi L, Alkhansa S, Njeim R, Ismail J, Madi M, Ghadieh HE, Al Moussawi S, Azar TS, Ayoub M, Azar WS, et al. Uncovering the Therapeutic Potential of Lithium Chloride in Type 2 Diabetic Cardiomyopathy: Targeting Tau Hyperphosphorylation and TGF-β Signaling via GSK-3β Inhibition. Pharmaceutics. 2024; 16(7):955. https://doi.org/10.3390/pharmaceutics16070955
Chicago/Turabian StyleAbou Assi, Layal, Sahar Alkhansa, Rachel Njeim, Jaafar Ismail, Mikel Madi, Hilda E. Ghadieh, Sarah Al Moussawi, Tanya S. Azar, Maurice Ayoub, William S. Azar, and et al. 2024. "Uncovering the Therapeutic Potential of Lithium Chloride in Type 2 Diabetic Cardiomyopathy: Targeting Tau Hyperphosphorylation and TGF-β Signaling via GSK-3β Inhibition" Pharmaceutics 16, no. 7: 955. https://doi.org/10.3390/pharmaceutics16070955
APA StyleAbou Assi, L., Alkhansa, S., Njeim, R., Ismail, J., Madi, M., Ghadieh, H. E., Al Moussawi, S., Azar, T. S., Ayoub, M., Azar, W. S., Hamade, S., Nawfal, R., Haddad, N.-R., Harb, F., Faour, W., Khalil, M. I., & Eid, A. A. (2024). Uncovering the Therapeutic Potential of Lithium Chloride in Type 2 Diabetic Cardiomyopathy: Targeting Tau Hyperphosphorylation and TGF-β Signaling via GSK-3β Inhibition. Pharmaceutics, 16(7), 955. https://doi.org/10.3390/pharmaceutics16070955








