Stimuli-Responsive Liposomes of 5-Fluorouracil: Progressive Steps for Safe and Effective Treatment of Colorectal Cancer
Abstract
1. Introduction
2. 5-Fluorouracil: Mechanism of Action and Resistance Pathways in Colorectal Cancer
3. Liposomes for Cancer Drug Delivery
4. Stimuli-Responsive Drug Delivery Platforms as a Potential Strategy to Improve 5-FU-Based Chemotherapy
5. Internal-Stimuli-Responsive Liposomes for 5-FU Delivery
5.1. pH-Responsive Liposomes
5.2. Enzyme-Responsive Liposomes
6. External-Stimuli-Responsive Drug Delivery System for 5-FU
6.1. Thermosensitive Liposomes
| Material | Formulation Composition | Drug | Phase Transition Temperature (Tm) | Refs. |
|---|---|---|---|---|
| DPPC | DPPC/DSPC, 9:1 + 3 mol% PEG | Doxorubicin | 42 °C | [60] |
| MPPC | DPPC/MPPC/DSPE-PEG-2000 (90:10:4) | Doxorubicin | 39–40 °C | [64] |
| DPPGOG | DPPC/DSPC/DPPGOG 50:20:30 (m/m) | Doxorubicin | 42 °C | [65] |
| Poloxamer 188 | DPPC, MSPC, poloxamer 188, and DSPE-PEG2000 (85:9.5:0.5:5, molar %) | Oxaliplatin | 42 °C | [68] |
| PNIPAM | DPPC/HSPC/CHOL/DSPE-PEG-2000 (100:50:30:6) with NIPAAm-AAM (83:17) 10 mg/mL | Doxorubicin | 40 °C | [70] |
| EOEOVE | EYPC/Chol/PEG-PE (50:45:4) + EOEOVE 2 mol% | Doxorubicin | 45 °C | [71] |
| Brij78 | DPPC/Brij78 (96:4 mol/mol) | Doxorubicin | 41.0 °C | [73] |
6.2. Magnetic Liposomes
6.3. Ultrasound-Responsive Liposomes
6.4. Photo-Triggerable Liposomes
7. Conclusions and Future Perspectives
Funding
Conflicts of Interest
Abbreviations
| AAm | hydrophilic acrylamide |
| CH | cholesterol |
| CHEMS | cholesterylhemisuccinate |
| CRC | colorectal cancer |
| CS | cationic chitosan |
| DC8,9PC | 1,2-bis(tricosa-10,12-diynoyl)-sn-glycero-3-phosphocholine |
| DDSs | drug delivery systems |
| DHSG | 1,5-O-dihexadecyl-N-succinyl-l-glutamate1,5-O-dihexadecyl-N-succinyll-glutamate |
| DMPC | dimyristoyl phosphatidyl choline |
| DOG | 1,2-dioleylglycerol |
| DOPC | 1,2-dioleoyl-snglycero-3-phosphocholine |
| DOPE | 1,2-dioleoyl-sn-glycerol-3 phosphoethanolamine |
| DOTAP | 1,2-dioleoyl-3-trimethylammonium-propane |
| DOX | doxorubicin |
| DPD | dihydropyrimidine dehydrogenase |
| DPPC | dipalmitoylphosphatidylcholine |
| DPPG | 1,2-dipalmitoyl-sn-glycerol-3-phosphoglycerol |
| DPPGOG | phosphatidyloligoglycerol |
| DSPC | 1,2-distearoyl-sn-glycero-3-phosphocholine |
| DSPE-PEG2000 | 1,2-distearoyl-sn-glycero-3-phosphoethanolamine-N-[amino(polyethyleneglycol)-2000] (DSPE-PEG2000) |
| EOEOVE | poly[2-(2-ethoxy) ethoxyethyl vinyl ether] |
| EYPC | egg yolk phosphatidylcholine |
| 5-FU | 5-fluorouracil |
| HA | sodium hyaluronate |
| HSPC | hydrogenated soybean phosphatidylcholine |
| HT29 | human colorectal adenocarcinoma cells |
| MMPs | matrix metalloproteinases |
| MPPC | 1-palmitoyl-2-hydroxy-sn-glycero-3 phosphocholine |
| MSPC | 1-myristoyl-2-stearoyl-sn-glycero-3-phosphocholine |
| NHE1 | Na+/H+ exchanger isoform 1 |
| NIR | near-infrared |
| OA | oleic acid |
| OFZG | 1-O-octadecyl-2-(5-fluorouracil)-N-acetyl-3-zidovudine-phosphorylglycerol |
| PAA | poly(acrylic acid) |
| PC | phosphatidylcholine |
| PCDA | 10,12-pentacosadiynoic acid |
| PDPA | poly(2-(diisopropylamino) ethylmethacrylate) |
| PE | phosphatidylethanolamine |
| PFH | perfluorohexane |
| PIVE | phenyl-substituted vinyl ether |
| sPLA2 | secretory phospholipase A2 |
| PNIPAM | poly(N-isopropyl acrylamide) |
| PTX | paclitaxel |
| ROS | reactive oxygen species |
| SMA | poly(styrene-co-maleic acid) |
| S100PC | soybean phosphatidylcholine |
| Tc | transition temperature |
| TME | tumor microenvironment |
| TS | thymidylate synthase |
References
- Xi, Y.; Xu, P. Global Colorectal Cancer Burden in 2020 and Projections to 2040. Transl. Oncol. 2021, 14, 101174. [Google Scholar] [CrossRef]
- Sawicki, T.; Ruszkowska, M.; Danielewicz, A.; Niedźwiedzka, E.; Arłukowicz, T.; Przybyłowicz, K.E. A Review of Colorectal Cancer in Terms of Epidemiology, Risk Factors, Development, Symptoms and Diagnosis. Cancers 2021, 13, 2025. [Google Scholar] [CrossRef] [PubMed]
- Long, F.; Lin, Z.; Li, L.; Ma, M.; Lu, Z.; Jing, L.; Li, X.; Lin, C. Comprehensive Landscape and Future Perspectives of Circular RNAs in Colorectal Cancer. Mol. Cancer 2021, 20, 26. [Google Scholar] [CrossRef]
- Carethers, J.M. Review: Systemic Treatment of Advanced Colorectal Cancer: Tailoring Therapy to the Tumor. Ther. Adv. Gastroenterol. 2008, 1, 33–42. [Google Scholar] [CrossRef]
- Folprecht, G.; Cunningham, D.; Ross, P.; Glimelius, B.; Di Costanzo, F.; Wils, J.; Scheithauer, W.; Rougier, P.; Aranda, E.; Hecker, H.; et al. Efficacy of 5-Fluorouracil-Based Chemotherapy in Elderly Patients with Metastatic Colorectal Cancer: A Pooled Analysis of Clinical Trials. Ann. Oncol. 2004, 15, 1330–1338. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Yin, Y.; Xu, S.J.; Chen, W.S. 5-Fluorouracil: Mechanisms of Resistance and Reversal Strategies. Molecules 2008, 13, 1551–1569. [Google Scholar] [CrossRef]
- Kadoyama, K.; Miki, I.; Tamura, T.; Brown, J.B.; Sakaeda, T.; Okuno, Y. Adverse Event Profiles of 5-Fluorouracil and Capecitabine: Data Mining of the Public Version of the FDA Adverse Event Reporting System, AERS, and Reproducibility of Clinical Observations. Int. J. Med. Sci. 2012, 9, 33–39. [Google Scholar] [CrossRef]
- Yang, C.; Merlin, D. Lipid-Based Drug Delivery Nanoplatforms for Colorectal Cancer Therapy. Nanomaterials 2020, 10, 1424. [Google Scholar] [CrossRef]
- Bhattacharyya, S.; Kudgus, R.A.; Bhattacharya, R.; Mukherjee, P. Inorganic Nanoparticles in Cancer Therapy. Pharm. Res. 2011, 28, 237–259. [Google Scholar] [CrossRef] [PubMed]
- Mura, S.; Nicolas, J.; Couvreur, P. Stimuli-Responsive Nanocarriers for Drug Delivery. Nat. Mater. 2013, 12, 991–1003. [Google Scholar] [CrossRef]
- Parker, W.B.; Cheng, Y.C. Metabolism and Mechanism of Action of 5-Fluorouracil. Pharmacol. Ther. 1990, 48, 381–395. [Google Scholar] [CrossRef]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef] [PubMed]
- Chernyshev, A.; Fleischmann, T.; Kohen, A. Thymidyl Biosynthesis Enzymes as Antibiotic Targets. Appl. Microbiol. Biotechnol. 2007, 74, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Mazzuca, F.; Borro, M.; Botticelli, A.; Mazzotti, E.; Marchetti, L.; Gentile, G.; La Torre, M.; Lionetto, L.; Simmaco, M.; Marchetti, P. Pre-Treatment Evaluation of 5-Fluorouracil Degradation Rate: Association of Poor and Ultra-Rapid Metabolism with Severe Toxicity in a Colorectal Cancer Patients Cohort. Oncotarget 2016, 7, 20612–20620. [Google Scholar] [CrossRef] [PubMed]
- Blondy, S.; David, V.; Verdier, M.; Mathonnet, M.; Perraud, A.; Christou, N. 5-Fluorouracil Resistance Mechanisms in Colorectal Cancer: From Classical Pathways to Promising Processes. Cancer Sci. 2020, 111, 3142–3154. [Google Scholar] [CrossRef] [PubMed]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, Composition, Types, and Clinical Applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Akbarzadeh, A.; Rezaei-Sadabady, R.; Davaran, S.; Joo, S.W.; Zarghami, N.; Hanifehpour, Y.; Samiei, M.; Kouhi, M.; Nejati-Koshki, K. Liposome: Classification, Preparation, and Applications. Nanoscale Res. Lett. 2013, 8, 102. [Google Scholar] [CrossRef] [PubMed]
- Pasarin, D.; Ghizdareanu, A.I.; Enascuta, C.E.; Matei, C.B.; Bilbie, C.; Paraschiv-Palada, L.; Veres, P.A. Coating Materials to Increase the Stability of Liposomes. Polymers 2023, 15, 782. [Google Scholar] [CrossRef] [PubMed]
- Deng, W.; Chen, W.; Clement, S.; Guller, A.; Zhao, Z.; Engel, A.; Goldys, E.M. Controlled Gene and Drug Release from a Liposomal Delivery Platform Triggered by X-Ray Radiation. Nat. Commun. 2018, 9, 2713. [Google Scholar] [CrossRef]
- Federman, N.; Denny, C.T. Targeting Liposomes toward Novel Pediatric Anticancer Therapeutics. Pediatr. Res. 2010, 67, 514–519. [Google Scholar] [CrossRef]
- Alavi, M.; Hamidi, M. Passive and Active Targeting in Cancer Therapy by Liposomes and Lipid Nanoparticles. Drug Metab. Pers. Ther. 2019, 34, 20180032. [Google Scholar] [CrossRef] [PubMed]
- Yuba, E. Development of Functional Liposomes by Modification of Stimuli-Responsive Materials and Their Biomedical Applications. J. Mater. Chem. B 2020, 8, 1093–1107. [Google Scholar] [CrossRef] [PubMed]
- Noble, G.T.; Stefanick, J.F.; Ashley, J.D.; Kiziltepe, T.; Bilgicer, B. Ligand-Targeted Liposome Design: Challenges and Fundamental Considerations. Trends Biotechnol. 2014, 32, 32–45. [Google Scholar] [CrossRef] [PubMed]
- Dewhirst, M.W.; Secomb, T.W. Transport of Drugs from Blood Vessels to Tumour Tissue. Nat. Rev. Cancer 2017, 17, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Hu, W.; Cai, C.; Wu, Y.; Li, J.; Dong, S. Advanced Application of Stimuli-Responsive Drug Delivery System for Inflammatory Arthritis Treatment. Mater. Today Bio 2022, 14, 100223. [Google Scholar] [CrossRef] [PubMed]
- Raza, A.; Rasheed, T.; Nabeel, F.; Hayat, U.; Bilal, M.; Iqbal, H.M.N. Endogenous and Exogenous Stimuli-Responsive Drug Delivery Systems for Programmed Site-Specific Release. Molecules 2019, 24, 1117. [Google Scholar] [CrossRef] [PubMed]
- Rahim, M.A.; Jan, N.; Khan, S.; Shah, H.; Madni, A.; Khan, A.; Jabar, A.; Khan, S.; Elhissi, A.; Hussain, Z.; et al. Recent Advancements in Stimuli Responsive Drug Delivery Platforms for Active and Passive Cancer Targeting. Cancers 2021, 13, 670. [Google Scholar] [CrossRef] [PubMed]
- Gallo, G.; Vescio, G.; De Paola, G.; Sammarco, G. Therapeutic Targets and Tumor Microenvironment in Colorectal Cancer. J. Clin. Med. 2021, 10, 2295. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Teng, L.; Gao, L.; Su, T.; Fu, L.; Qiu, Z.; Bi, Y. Advances in Multiple Stimuli-Responsive Drug-Delivery Systems for Cancer Therapy. Int. J. Nanomed. 2021, 16, 1525–1551. [Google Scholar] [CrossRef]
- Raza, A.; Hayat, U.; Rasheed, T.; Bilal, M.; Iqbal, H.M.N. “smart” Materials-Based near-Infrared Light-Responsive Drug Delivery Systems for Cancer Treatment: A Review. J. Mater. Res. Technol. 2019, 8, 1497–1509. [Google Scholar] [CrossRef]
- Warburg, O.; Wind, F.; Negelein, E. The Metabolism of Tumors in the Body. J. General. Physiol. 1927, 8, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Huntington, K.E.; Louie, A.; Zhou, L.; Seyhan, A.A.; Maxwell, A.W.; El-Deiry, W.S. Colorectal Cancer Extracellular Acidosis Decreases Immune Cell Killing and Is Partially Ameliorated by PH-Modulating Agents That Modify Tumor Cell Cytokine Profiles. Am. J. Cancer Res. 2022, 12, 138–151. [Google Scholar] [PubMed]
- Udofot, O.; Affram, K.; Israel, B.; Agyare, E. Cytotoxicity of 5-Fluorouracil-Loaded PH-Sensitive Liposomal Nanoparticles in Colorectal Cancer Cell Lines. Integr. Cancer Sci. Ther. 2015, 2, 245–252. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Sen, K.; Pal, T.K.; Guha, S.K. Poly(Styrene-Co-Maleic Acid)-Based PH-Sensitive Liposomes Mediate Cytosolic Delivery of Drugs for Enhanced Cancer Chemotherapy. Int. J. Pharm. 2012, 436, 786–797. [Google Scholar] [CrossRef] [PubMed]
- Mansoori, B.; Mohammadi, A.; Abedi-Gaballu, F.; Abbaspour, S.; Ghasabi, M.; Yekta, R.; Shirjang, S.; Dehghan, G.; Hamblin, M.R.; Baradaran, B. Hyaluronic Acid-Decorated Liposomal Nanoparticles for Targeted Delivery of 5-Fluorouracil into HT-29 Colorectal Cancer Cells. J. Cell Physiol. 2020, 235, 6817–6830. [Google Scholar] [CrossRef]
- Paliwal, S.R.; Paliwal, R.; Vyas, S.P. A Review of Mechanistic Insight and Application of PH-Sensitive Liposomes in Drug Delivery. Drug Deliv. 2015, 22, 231–242. [Google Scholar] [CrossRef]
- Shahidi, M.; Abazari, O.; Dayati, P.; Haghiralsadat, B.F.; Oroojalian, F.; Tofighi, D. Targeted Delivery of 5-Fluorouracil, MiR-532-3p, and Si-KRAS to the Colorectal Tumor Using Layer-by-Layer Liposomes. Front. Bioeng. Biotechnol. 2022, 10, 1013541. [Google Scholar] [CrossRef]
- Farshbaf, M.; Davaran, S.; Zarebkohan, A.; Annabi, N.; Akbarzadeh, A.; Salehi, R. Significant Role of Cationic Polymers in Drug Delivery Systems. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1872–1891. [Google Scholar] [CrossRef]
- Ye, P.; Zhang, W.; Yang, T.; Lu, Y.; Lu, M.; Gai, Y.; Ma, X.; Xiang, G. Folate Receptor-Targeted Liposomes Enhanced the Antitumor Potency of Imatinib through the Combination of Active Targeting and Molecular Targeting. Int. J. Nanomed. 2014, 9, 2167–2178. [Google Scholar] [CrossRef]
- Zhang, H.; Li, R.Y.; Lu, X.; Mou, Z.Z.; Lin, G.M. Docetaxel-Loaded Liposomes: Preparation, PH Sensitivity, Pharmacokinetics, and Tissue Distribution. J. Zhejiang Univ. Sci. B 2012, 13, 981–989. [Google Scholar] [CrossRef]
- Chen, D.; Liu, W.; Shen, Y.; Mu, H.; Zhang, Y.; Liang, R.; Wang, A.; Sun, K.; Fu, F. Effects of a Novel PH-Sensitive Liposome with Cleavable Esterase-Catalyzed and PH-Responsive Double Smart MPEG Lipid Derivative on ABC Phenomenon. Int. J. Nanomed. 2011, 6, 2053–2061. [Google Scholar] [CrossRef] [PubMed]
- Hao, W.; Han, X.; Shang, Y.; Xu, S.; Liu, H. Insertion of PH-Sensitive Bola-Type Copolymer into Liposome as a “Stability Anchor” for Control of Drug Release. Colloids Surf. B Biointerfaces 2015, 136, 809–816. [Google Scholar] [CrossRef]
- Lee, S.M.; Chen, H.; O’Halloran, T.V.; Nguyen, S.B.T. “Clickable” Polymer-Caged Nanobins as a Modular Drug Delivery Platform. J. Am. Chem. Soc. 2009, 131, 9311–9320. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Shum, P.; Grey, J.; Fujiwara, S.I.; Malhotra, G.S.; González-Bonet, A.; Hyun, S.H.; Moase, E.; Allen, T.M.; Thompson, D.H. Acid-Labile MPEG-Vinyl Ether-1,2-Dioleylglycerol Lipids with Tunable Ph Sensitivity: Synthesis and Structural Effects on Hydrolysis Rates, DOPE Liposome Release Performance, and Pharmacokinetics. Mol. Pharm. 2012, 9, 3266–3276. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.K.; Van Den Bossche, J.; Hyun, S.H.; Thompson, D.H. Acid-Triggered Release via DePEGylation of Fusogenic Liposomes Mediated by Heterobifunctional Phenyl-Substituted Vinyl Ethers with Tunable PH-Sensitivity. Bioconjug. Chem. 2012, 23, 2071–2077. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Geng, D.; Su, H. Safe and Efficient PH Sensitive Tumor Targeting Modified Liposomes with Minimal Cytotoxicity. Colloids Surf. B Biointerfaces 2014, 123, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Bersani, S.; Vila-Caballer, M.; Brazzale, C.; Barattin, M.; Salmaso, S. PH-Sensitive Stearoyl-PEG-Poly(Methacryloyl Sulfadimethoxine) Decorated Liposomes for the Delivery of Gemcitabine to Cancer Cells. Eur. J. Pharm. Biopharm. 2014, 88, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Yoshizaki, Y.; Yuba, E.; Sakaguchi, N.; Koiwai, K.; Harada, A.; Kono, K. Potentiation of PH-Sensitive Polymer-Modified Liposomes with Cationic Lipid Inclusion as Antigen Delivery Carriers for Cancer Immunotherapy. Biomaterials 2014, 35, 8186–8196. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Ma, H.; Wei, T.; Liang, X.J. CO2 Gas Induced Drug Release from PH-Sensitive Liposome to Circumvent Doxorubicin Resistant Cells. Chem. Commun. 2012, 48, 4869–4871. [Google Scholar] [CrossRef]
- Herszényi, L.; Barabás, L.; Hritz, I.; István, G.; Tulassay, Z. Impact of Proteolytic Enzymes in Colorectal Cancer Development and Progression. World J. Gastroenterol. 2014, 20, 13246–13257. [Google Scholar] [CrossRef]
- Lou, J.; Best, M.D. A General Approach to Enzyme-Responsive Liposomes. Chem.-A Eur. J. 2020, 26, 8597–8607. [Google Scholar] [CrossRef]
- Jin, Y.; Yang, F.; Du, L. Nanoassemblies Containing a Fluorouracil/Zidovudine Glyceryl Prodrug with Phospholipase A2-Triggered Drug Release for Cancer Treatment. Colloids Surf. B Biointerfaces 2013, 112, 421–428. [Google Scholar] [CrossRef] [PubMed]
- Roomi, M.W.; Monterrey, J.C.; Kalinovsky, T.; Rath, M.; Niedzwiecki, A. Patterns of MMP-2 and MMP-9 Expression in Human Cancer Cell Lines. Oncol. Rep. 2009, 21, 1323–1333. [Google Scholar] [CrossRef] [PubMed]
- Xu, P.; Meng, Q.; Sun, H.; Yin, Q.; Yu, H.; Zhang, Z.; Cao, M.; Zhang, Y.; Li, Y. Shrapnel Nanoparticles Loading Docetaxel Inhibit Metastasis and Growth of Breast Cancer. Biomaterials 2015, 64, 10–20. [Google Scholar] [CrossRef] [PubMed]
- Pak, C.C.; Ali, S.; Janoff, A.S.; Meers, P. Triggerable Liposomal Fusion by Enzyme Cleavage of a Novel Peptide-Lipid Conjugate. Biochim. Biophys. Acta Biomembr. 1998, 1372, 13–27. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Song, S.J.; Lee, J.; Ha, T.H.; Choi, J.S. Cathepsin B-Responsive Liposomes for Controlled Anticancer Drug Delivery in Hep G2 Cells. Pharmaceutics 2020, 12, 876. [Google Scholar] [CrossRef] [PubMed]
- Nieva, J.L.; Goñi, F.M.; Alonso, A. Liposome Fusion Catalytically Induced by Phospholipase C. Biochemistry 1989, 28, 7364–7367. [Google Scholar] [CrossRef]
- Jo, S.M.; Lee, H.Y.; Kim, J.C. Glucose-Sensitivity of Liposomes Incorporating Conjugates of Glucose Oxidase and Poly(N-Isopropylacrylamide-Co-Methacrylic Acid-Co-Octadecylacrylate). Int. J. Biol. Macromol. 2009, 45, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Ta, T.; Porter, T.M. Thermosensitive Liposomes for Localized Delivery and Triggered Release of Chemotherapy. J. Control. Release 2013, 169, 112–125. [Google Scholar] [CrossRef] [PubMed]
- Unezaki, S.; Maruyama, K.; Takahashi, N.; Koyama, M.; Yuda, T.; Suginaka, A.; Iwatsuru, M. Enhanced Delivery and Antitumor Activity of Doxorubicin Using Long-Circulating Thermosensitive Liposomes Containing Amphipathic Polyethylene Glycol in Combination with Local Hyperthermia. Pharm. Res. 1994, 11, 11805. [Google Scholar] [CrossRef]
- Al Sabbagh, C.; Tsapis, N.; Novell, A.; Calleja-Gonzalez, P.; Escoffre, J.M.; Bouakaz, A.; Chacun, H.; Denis, S.; Vergnaud, J.; Gueutin, C.; et al. Formulation and Pharmacokinetics of Thermosensitive Stealth® Liposomes Encapsulating 5-Fluorouracil. Pharm. Res. 2015, 32, 1585–1603. [Google Scholar] [CrossRef] [PubMed]
- Clares, B.; Biedma-Ortiz, R.A.; Sáez-Fernández, E.; Prados, J.C.; Melguizo, C.; Cabeza, L.; Ortiz, R.; Arias, J.L. Nano-Engineering of 5-Fluorouracil-Loaded Magnetoliposomes for Combined Hyperthermia and Chemotherapy against Colon Cancer. Eur. J. Pharm. Biopharm. 2013, 85, 329–338. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Pan, H.; Raza, F.; Zafar, H.; Ge, Y.; Wang, N.; Zheng, R.; Zhang, D.; Yang, Y. Thermosensitive Drug-Loaded Liposomes for Photothermal and Chemotherapeutic Treatment of Colon Cancer. Mater. Adv. 2024, 5, 2456–2469. [Google Scholar] [CrossRef]
- Needham, D.; Anyarambhatla, G.; Kong, G.; Dewhirst, M.W. A New Temperature-Sensitive Liposome for Use with Mild Hyperthermia: Characterization and Testing in a Human Tumor Xenograft Model. Cancer Res. 2000, 60, 1197–1201. [Google Scholar] [PubMed]
- Lindner, L.H.; Eichhorn, M.E.; Eibl, H.; Teichert, N.; Schmitt-Sody, M.; Issels, R.D.; Dellian, M. Novel Temperature-Sensitive Liposomes with Prolonged Circulation Time. Clin. Cancer Res. 2004, 10, 2168–2178. [Google Scholar] [CrossRef] [PubMed]
- Abuwatfa, W.H.; Awad, N.S.; Pitt, W.G.; Husseini, G.A. Thermosensitive Polymers and Thermo-Responsive Liposomal Drug Delivery Systems. Polymers 2022, 14, 925. [Google Scholar] [CrossRef] [PubMed]
- Hassler, J.F.; Lawson, M.; Arroyo, E.C.; Bates, F.S.; Hackel, B.J.; Lodge, T.P. Discovery of Kinetic Trapping of Poloxamers inside Liposomes via Thermal Treatment. Langmuir 2023, 39, 14263–14274. [Google Scholar] [CrossRef] [PubMed]
- Zeng, C.; Yu, F.; Yang, Y.; Cheng, X.; Liu, Y.; Zhang, H.; Zhao, S.; Yang, Z.; Li, M.; Li, Z.; et al. Preparation and Evaluation of Oxaliplatin Thermosensitive Liposomes with Rapid Release and High Stability. PLoS ONE 2016, 11, e0158517. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, H.; Kono, K.; Takfagishi, T. Temperature Sensitization of Liposomes Using Copolymers of N-Isopropylacrylamide. Bioconjug. Chem. 1999, 10, 4128. [Google Scholar] [CrossRef]
- Han, H.D.; Shin, B.C.; Choi, H.S. Doxorubicin-Encapsulated Thermosensitive Liposomes Modified with Poly(N-Isopropylacrylamide-Co-Acrylamide): Drug Release Behavior and Stability in the Presence of Serum. Eur. J. Pharm. Biopharm. 2006, 62, 1106. [Google Scholar] [CrossRef]
- Kono, K.; Ozawa, T.; Yoshida, T.; Ozaki, F.; Ishizaka, Y.; Maruyama, K.; Kojima, C.; Harada, A.; Aoshima, S. Highly Temperature-Sensitive Liposomes Based on a Thermosensitive Block Copolymer for Tumor-Specific Chemotherapy. Biomaterials 2010, 31, 7096–7105. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; May, J.P.; Ernsting, M.J.; Li, S.D. A Thermosensitive Liposome Prepared with a Cu2+ Gradient Demonstrates Improved Pharmacokinetics, Drug Delivery and Antitumor Efficacy. J. Control. Release 2012, 161, 1429. [Google Scholar] [CrossRef] [PubMed]
- Tagami, T.; Ernsting, M.J.; Li, S.D. Efficient Tumor Regression by a Single and Low Dose Treatment with a Novel and Enhanced Formulation of Thermosensitive Liposomal Doxorubicin. J. Control. Release 2011, 152, 3039. [Google Scholar] [CrossRef] [PubMed]
- Amstad, E.; Kohlbrecher, J.; Müller, E.; Schweizer, T.; Textor, M.; Reimhult, E. Triggered Release from Liposomes through Magnetic Actuation of Iron Oxide Nanoparticle Containing Membranes. Nano Lett. 2011, 11, 1664–1670. [Google Scholar] [CrossRef]
- Kulshrestha, P.; Gogoi, M.; Bahadur, D.; Banerjee, R. In Vitro Application of Paclitaxel Loaded Magnetoliposomes for Combined Chemotherapy and Hyperthermia. Colloids Surf. B Biointerfaces 2012, 96, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Azevedo, A.; Coelho, M.P.; Pinho, J.O.; Soares, P.I.P.; Reis, C.P.; Borges, J.P.; Gaspar, M.M. An Alternative Hybrid Lipid Nanosystem Combining Cytotoxic and Magnetic Properties as a Tool to Potentiate Antitumor Effect of 5-Fluorouracil. Life Sci. 2024, 344, 122558. [Google Scholar] [CrossRef] [PubMed]
- Riva, E.R.; Sinibaldi, E.; Grillone, A.F.; Del Turco, S.; Mondini, A.; Li, T.; Takeoka, S.; Mattoli, V. Enhanced in Vitro Magnetic Cell Targeting of Doxorubicin-Loaded Magnetic Liposomes for Localized Cancer Therapy. Nanomaterials 2020, 10, 2104. [Google Scholar] [CrossRef] [PubMed]
- Jose, G.; Lu, Y.J.; Chen, H.A.; Hsu, H.L.; Hung, J.T.; Anilkumar, T.S.; Chen, J.P. Hyaluronic Acid Modified Bubble-Generating Magnetic Liposomes for Targeted Delivery of Doxorubicin. J. Magn. Magn. Mater. 2019, 474, 355–364. [Google Scholar] [CrossRef]
- Anilkumar, T.S.; Lu, Y.J.; Chen, H.A.; Hsu, H.L.; Jose, G.; Chen, J.P. Dual Targeted Magnetic Photosensitive Liposomes for Photothermal/Photodynamic Tumor Therapy. J. Magn. Magn. Mater. 2019, 473, 241–252. [Google Scholar] [CrossRef]
- Pradhan, P.; Giri, J.; Rieken, F.; Koch, C.; Mykhaylyk, O.; Döblinger, M.; Banerjee, R.; Bahadur, D.; Plank, C. Targeted Temperature Sensitive Magnetic Liposomes for Thermo-Chemotherapy. J. Control. Release 2010, 142, 10821. [Google Scholar] [CrossRef]
- Deshpande, P.P.; Biswas, S.; Torchilin, V.P. Current Trends in the Use of Liposomes for Tumor Targeting. Nanomedicine 2013, 8, 1509–1528. [Google Scholar] [CrossRef]
- Awad, N.S.; Paul, V.; AlSawaftah, N.M.; Husseini, G.A. Effect of Phospholipid Head Group on Ultrasound-Triggered Drug Release and Cellular Uptake of Immunoliposomes. Sci. Rep. 2023, 13, 16644. [Google Scholar] [CrossRef]
- Tharkar, P.; Varanasi, R.; Wong, W.S.F.; Jin, C.T.; Chrzanowski, W. Nano-Enhanced Drug Delivery and Therapeutic Ultrasound for Cancer Treatment and Beyond. Front. Bioeng. Biotechnol. 2019, 7, 324. [Google Scholar] [CrossRef]
- Kim, Y.S.; Ko, M.J.; Moon, H.; Sim, W.; Cho, A.S.; Gil, G.; Kim, H.R. Ultrasound-Responsive Liposomes for Targeted Drug Delivery Combined with Focused Ultrasound. Pharmaceutics 2022, 14, 1314. [Google Scholar] [CrossRef]
- Ezekiel, C.I.; Bapolisi, A.M.; Walker, R.B.; Krause, R.W.M. Ultrasound-Triggered Release of 5-Fluorouracil from Soy Lecithin Echogenic Liposomes. Pharmaceutics 2021, 13, 821. [Google Scholar] [CrossRef]
- Mitragotri, S. Healing Sound: The Use of Ultrasound in Drug Delivery and Other Therapeutic Applications. Nat. Rev. Drug Discov. 2005, 4, 255–260. [Google Scholar] [CrossRef]
- Hynynen, K.; McDannold, N.; Sheikov, N.A.; Jolesz, F.A.; Vykhodtseva, N. Local and Reversible Blood-Brain Barrier Disruption by Noninvasive Focused Ultrasound at Frequencies Suitable for Trans-Skull Sonications. Neuroimage 2005, 24, 12–20. [Google Scholar] [CrossRef]
- Massiot, J.; Abuillan, W.; Konovalov, O.; Makky, A. Photo-Triggerable Liposomes Based on Lipid-Porphyrin Conjugate and Cholesterol Combination: Formulation and Mechanistic Study on Monolayers and Bilayers. Biochim. Biophys. Acta Biomembr. 2022, 1864, 183812. [Google Scholar] [CrossRef]
- Carter, K.A.; Shao, S.; Hoopes, M.I.; Luo, D.; Ahsan, B.; Grigoryants, V.M.; Song, W.; Huang, H.; Zhang, G.; Pandey, R.K.; et al. Porphyrin-Phospholipid Liposomes Permeabilized by near-Infrared Light. Nat. Commun. 2014, 5, 3546. [Google Scholar] [CrossRef]
- Luo, D.; Carter, K.A.; Razi, A.; Geng, J.; Shao, S.; Giraldo, D.; Sunar, U.; Ortega, J.; Lovell, J.F. Doxorubicin Encapsulated in Stealth Liposomes Conferred with Light-Triggered Drug Release. Biomaterials 2016, 75, 193–202. [Google Scholar] [CrossRef]
- Mertins, O.; Bacellar, I.O.L.; Thalmann, F.; Marques, C.M.; Baptista, M.S.; Itri, R. Physical Damage on Giant Vesicles Membrane as a Result of Methylene Blue Photoirradiation. Biophys. J. 2014, 106, 162–171. [Google Scholar] [CrossRef]
- Bacellar, I.O.L.; Oliveira, M.C.; Dantas, L.S.; Costa, E.B.; Junqueira, H.C.; Martins, W.K.; Durantini, A.M.; Cosa, G.; Di Mascio, P.; Wainwright, M.; et al. Photosensitized Membrane Permeabilization Requires Contact-Dependent Reactions between Photosensitizer and Lipids. J. Am. Chem. Soc. 2018, 140, 9606–9615. [Google Scholar] [CrossRef]
- Kim, M.A.; Lee, C.M. NIR-Mediated Drug Release and Tumor Theranostics Using Melanin-Loaded Liposomes. Biomater. Res. 2022, 26, 22. [Google Scholar] [CrossRef]
- Yavlovich, A.; Singh, A.; Blumenthal, R.; Puri, A. A Novel Class of Photo-Triggerable Liposomes Containing DPPC:DC 8,9PC as Vehicles for Delivery of Doxorubcin to Cells. Biochim. Biophys. Acta Biomembr. 2011, 1808, 117–126. [Google Scholar] [CrossRef]
- Guo, C.; Liu, S.; Jiang, C.; Li, W.; Dai, Z.; Fritz, H.; Wu, X. A Promising Drug Controlled-Release System Based on Diacetylene/ Phospholipid Polymerized Vesicles. Langmuir 2009, 25, 13114–13119. [Google Scholar] [CrossRef]
- Peng, P.C.; Hong, R.L.; Tsai, Y.J.; Li, P.T.; Tsai, T.; Chen, C.T. Dual-Effect Liposomes Encapsulated with Doxorubicin and Chlorin E6 Augment the Therapeutic Effect of Tumor Treatment. Lasers Surg. Med. 2015, 47, 77–87. [Google Scholar] [CrossRef]
- Li, M.; Teh, C.; Ang, C.Y.; Tan, S.Y.; Luo, Z.; Qu, Q.; Zhang, Y.; Korzh, V.; Zhao, Y. Near-Infrared Light-Absorptive Stealth Liposomes for Localized Photothermal Ablation of Tumors Combined with Chemotherapy. Adv. Funct. Mater. 2015, 25, 5602–5610. [Google Scholar] [CrossRef]
- Feng, Q.; Wang, J.; Song, H.; Zhuo, L.G.; Wang, G.; Liao, W.; Feng, Y.; Wei, H.; Chen, Y.; Yang, Y.; et al. Uptake and Light-Induced Cytotoxicity of Hyaluronic Acid-Grafted Liposomes Containing Porphyrin in Tumor Cells. J. Drug Deliv. Sci. Technol. 2018, 47, 137–143. [Google Scholar] [CrossRef]
- Sine, J.; Urban, C.; Thayer, D.; Charron, H.; Valim, N.; Tata, D.B.; Schiff, R.; Blumenthal, R.; Joshi, A.; Puri, A. Photo Activation of HPPH Encapsulated in “Pocket” Liposomes Triggers Multiple Drug Release and Tumor Cell Killing in Mouse Breast Cancer Xenografts. Int. J. Nanomed. 2014, 10, 125–145. [Google Scholar] [CrossRef]
- Seo, H.J.; Kim, J.C. 7-Acetoxycoumarin Dimer-Incorporated and Folate-Decorated Liposomes: Photoresponsive Release and in Vitro Targeting and Efficacy. Bioconjug. Chem. 2014, 25, 533–542. [Google Scholar] [CrossRef]
- Wang, H.; Liu, Z.; Wang, S.; Dong, C.; Gong, X.; Zhao, P.; Chang, J. MC540 and Upconverting Nanocrystal Coloaded Polymeric Liposome for Near-Infrared Light-Triggered Photodynamic Therapy and Cell Fluorescent Imaging. ACS Appl. Mater. Interfaces 2014, 6, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Ostrowski, A.D.; Lin, B.F.; Tirrell, M.V.; Ford, P.C. Liposome Encapsulation of a Photochemical NO Precursor for Controlled Nitric Oxide Release and Simultaneous Fluorescence Imaging. Mol. Pharm. 2012, 9, 2950–2955. [Google Scholar] [CrossRef] [PubMed]
- Hester, T.J.; Dennison, S.R.; Baker, M.J.; Snape, T.J. Functionalising the Azobenzene Motif Delivers a Light-Responsive Membrane-Interactive Compound with the Potential for Photodynamic Therapy Applications. Org. Biomol. Chem. 2015, 13, 8067–8070. [Google Scholar] [CrossRef] [PubMed]
- Reshetov, V.; Lassalle, H.P.; François, A.; Dumas, D.; Hupont, S.; Gräfe, S.; Filipe, V.; Jiskoot, W.; Guillemin, F.; Zorin, V.; et al. Photodynamic Therapy with Conventional and Pegylated Liposomal Formulations of Mthpc(Temoporfin): Comparison of Treatment Efficacy and Distribution Characteristics in vivo. Int. J. Nanomed. 2013, 8, 3817–3831. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Wang, Z.; Wang, T.; He, W.; Zhou, W.; Li, M.; Yao, C.; Li, X. Improved Antitumor Activity of Novel Redox-Responsive Paclitaxel-Encapsulated Liposomes Based on Disulfide Phosphatidylcholine. Mol. Pharm. 2020, 17, 262–273. [Google Scholar] [CrossRef]
- Fu, H.; Shi, K.; Hu, G.; Yang, Y.; Kuang, Q.; Lu, L.; Zhang, L.; Chen, W.; Dong, M.; Chen, Y.; et al. Tumor-Targeted Paclitaxel Delivery and Enhanced Penetration Using TAT-Decorated Liposomes Comprising Redox-Responsive Poly(Ethylene Glycol). J. Pharm. Sci. 2015, 104, 1160–1173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Lu, A.; Long, M.; Cui, L.; Chen, Z.; Zhu, L. Nitroimidazole Derivative Incorporated Liposomes for Hypoxia-Triggered Drug Delivery and Enhanced Therapeutic Efficacy in Patient-Derived Tumor Xenografts. Acta Biomater. 2019, 83, 334–348. [Google Scholar] [CrossRef]
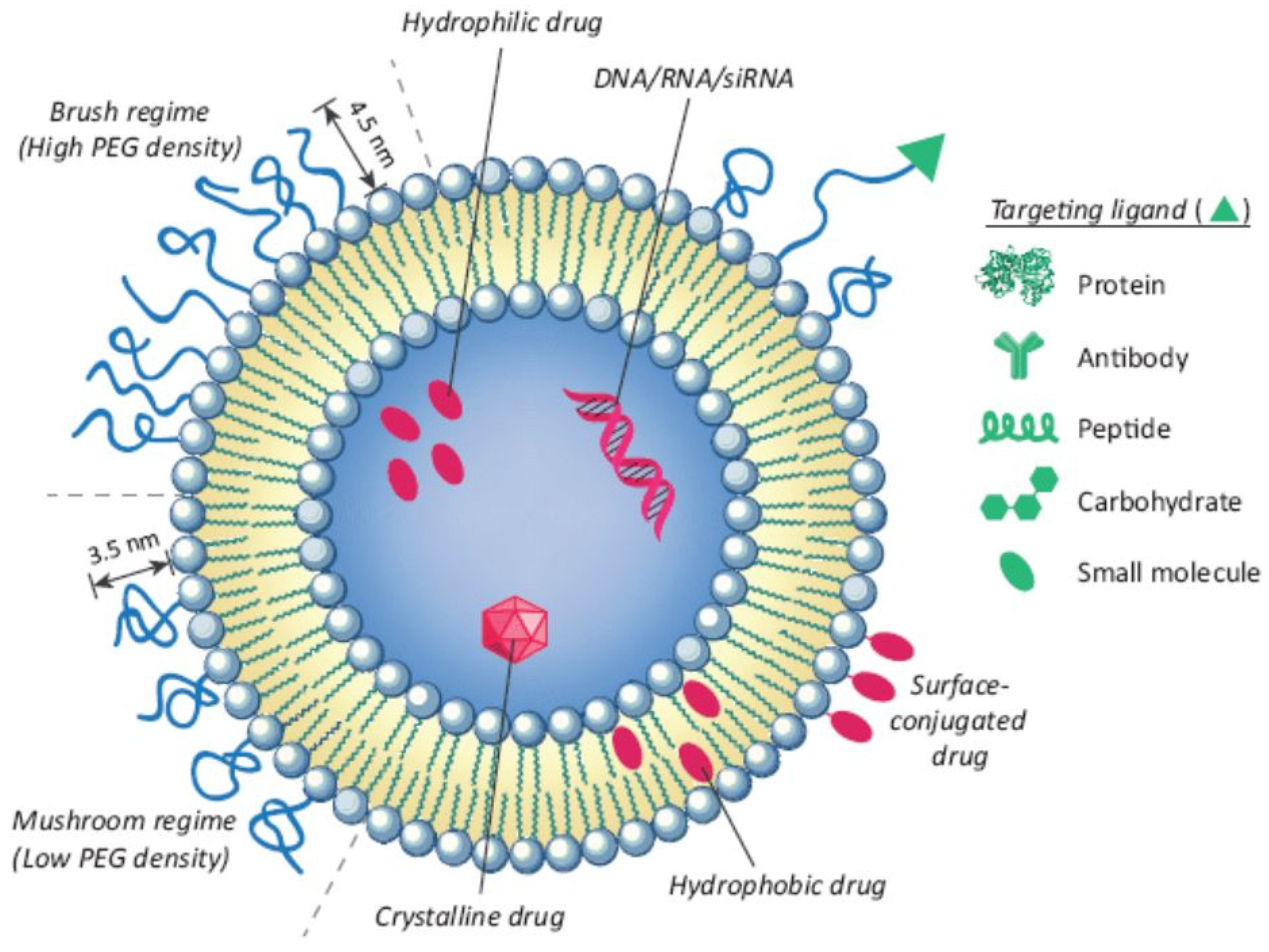

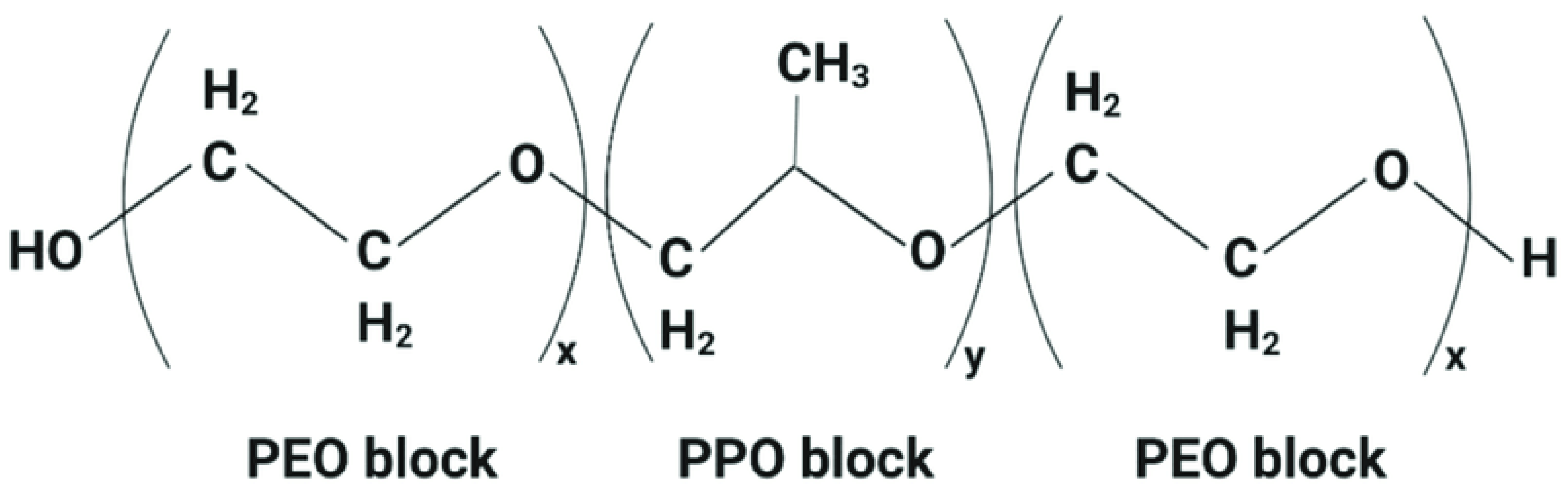
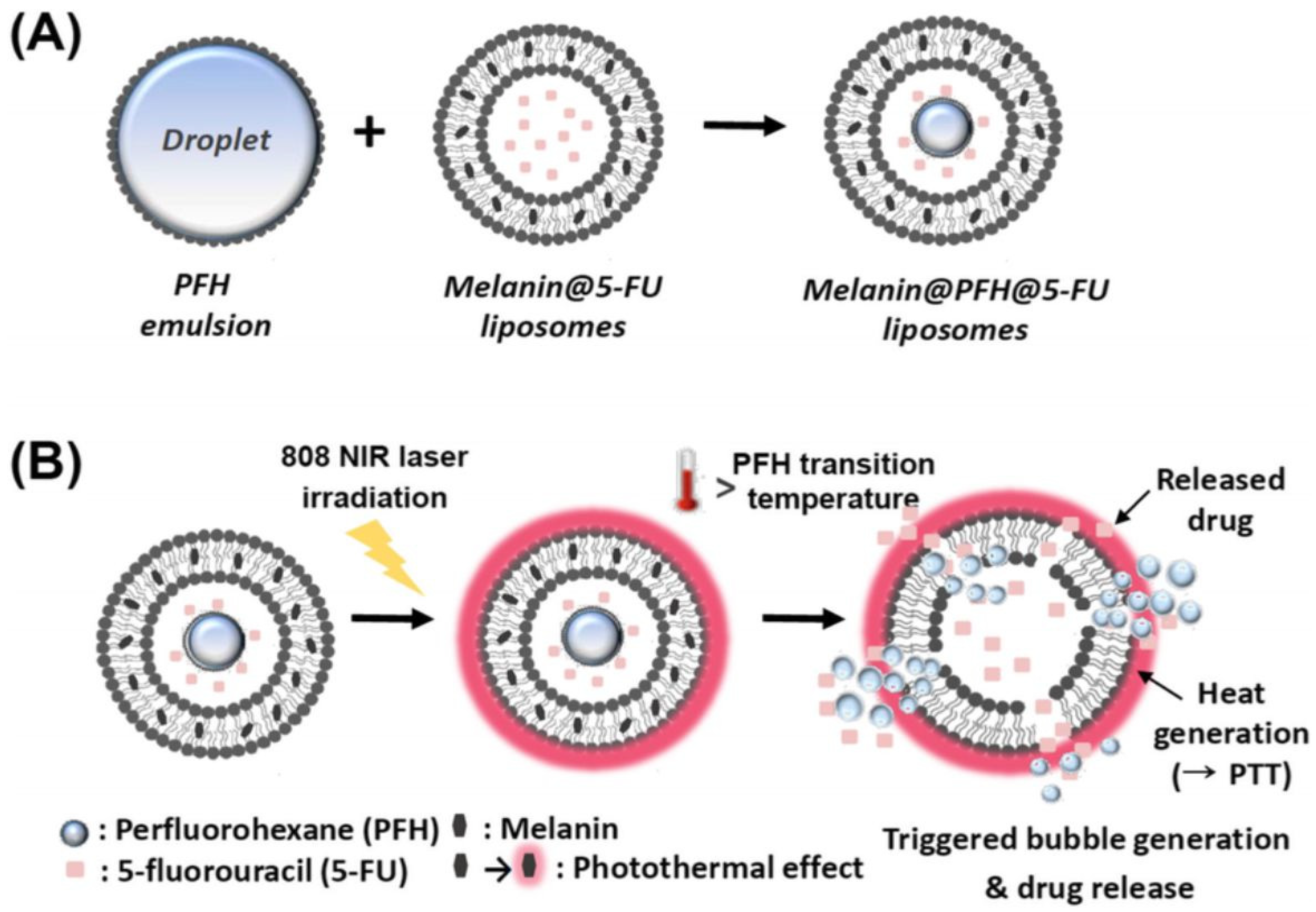
| Lipid/Polymer/Moiety Introduced for pH Sensitivity | Liposomal Composition | Drug | Refs. |
|---|---|---|---|
| Folate–PEG3350-CHEMS | HSPC/CHOL/mPEG2000-DSPE/folate–PEG3350-CHEMS (55:40:4:1) | Imatinib | [39] |
| OA | PE/CHOL/OA (3:2:3 w/w) | Docetaxel | [40] |
| Hydrazone | mPEG2000-Hz-CHEMS (90:10:3:3) | Paclitaxel | [41] |
| PDPA | HSPC or DOPC, Chol, and PEGm-PDPAn-PEGm in various molar ratios | Doxorubicin | [42] |
| PAA | DPPC/DOPG/cholesterol (18.048:1.152:12.8 μmol) + 10 mol% of Chol-PAA | Doxorubicin | [43] |
| Vinyl ether | DOPE:mPEG-VE-DOG (90:10 mol%) | Calcein | [44] |
| PIVE | PEG-PIVE/DOPE (2:98, 5:95, and 12:88 mPEG-PIVE/DOPE) | Calcein | [45] |
| Material | Liposomal Composition | Refs. |
|---|---|---|
| pH-Responsive Liposomes | ||
| CHEMS | CHEM/CH/tween 20/DSPE-PEG2000 (60:20:10:10 molar ratio) | [33] |
| SMA | DSPC/SMA (20:1 molar ratio) | [34] |
| DOPE | DOTAP/DOPE/des PEG/HA (1:1:1:0.5 molar ratio) | [35] |
| Chitosan | HSPC/CH/DPPG (7:2:1 molar ratio) + CS, miR and siRNA, CS, and HA layers onto the surface | [37] |
| Enzyme-Responsive Liposomes | ||
| sPLA2 enzyme | OFZG/CH/tween 80 (2:1:0.1 molar ratio) | [52] |
| Thermosensitive Liposomes | ||
| DPPC | DPPC/CH/DSPE-PEG (90:5:5 mol%) | [61] |
| Fe3O4 | Fe3O4/PC (3:4 mass ratio) | [62] |
| Bismuth nanosheets/DPPC | DPPC/CH/DSPE-PEG2000 (86:10:4 molar ratio) + BiNSs | [63] |
| Magnetic Liposomes | ||
| Iron oxide I, II | DMPC/CH/DSPE-PEG (85:10:5) | [76] |
| Ultrasound-Responsive Liposomes | ||
| Crude soybean lecithin | Crude soybean lecithin (75 mg) and cholesterol (25 mg) | [85] |
| Photo-Triggerable Liposomes | ||
| Melanin | PC (10 mg), Chol (1.5 mg), DSPE-PEG (3 mg), and melanin (1 mg) mixed with PFH emulsion in a 1:1 ratio | [93] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alrbyawi, H. Stimuli-Responsive Liposomes of 5-Fluorouracil: Progressive Steps for Safe and Effective Treatment of Colorectal Cancer. Pharmaceutics 2024, 16, 966. https://doi.org/10.3390/pharmaceutics16070966
Alrbyawi H. Stimuli-Responsive Liposomes of 5-Fluorouracil: Progressive Steps for Safe and Effective Treatment of Colorectal Cancer. Pharmaceutics. 2024; 16(7):966. https://doi.org/10.3390/pharmaceutics16070966
Chicago/Turabian StyleAlrbyawi, Hamad. 2024. "Stimuli-Responsive Liposomes of 5-Fluorouracil: Progressive Steps for Safe and Effective Treatment of Colorectal Cancer" Pharmaceutics 16, no. 7: 966. https://doi.org/10.3390/pharmaceutics16070966
APA StyleAlrbyawi, H. (2024). Stimuli-Responsive Liposomes of 5-Fluorouracil: Progressive Steps for Safe and Effective Treatment of Colorectal Cancer. Pharmaceutics, 16(7), 966. https://doi.org/10.3390/pharmaceutics16070966






