Exploring the Therapeutic Potential of 5-Fluorouracil-Loaded Calcium Carbonate Nanoparticles Combined with Natural Compound Thymoquinone for Colon Cancer Treatment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of CaCO3np from Cockle Shells
2.3. Synthesis and Optimization of 5FU-CaCO3np
2.4. Characterization of 5FU-CaCO3np
2.4.1. Morphology, Size, and Surface Charge
2.4.2. Crystalline Nature and Chemical Property
2.4.3. In Vitro Drug Release Study
2.4.4. Hemocompatibility Analysis
2.4.5. Toxicity Analysis in Brine Shrimp
2.4.6. Cytotoxicity Analysis
2.5. In Vitro Cell Experiments of 5FU-CaCO3np Combined with Thymoquinone
2.5.1. 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium Bromide Assay
2.5.2. Synergism Analysis
2.5.3. Colony Formation Assay
2.5.4. Acridine Orange/Propidium Iodide Staining
2.5.5. Cell Apoptosis Analysis
2.5.6. Wound Healing Assay
2.5.7. Three-Dimensional Spheroid Inhibition Assay
2.6. Molecular Docking
2.7. Statistical Analysis
3. Results and Discussion
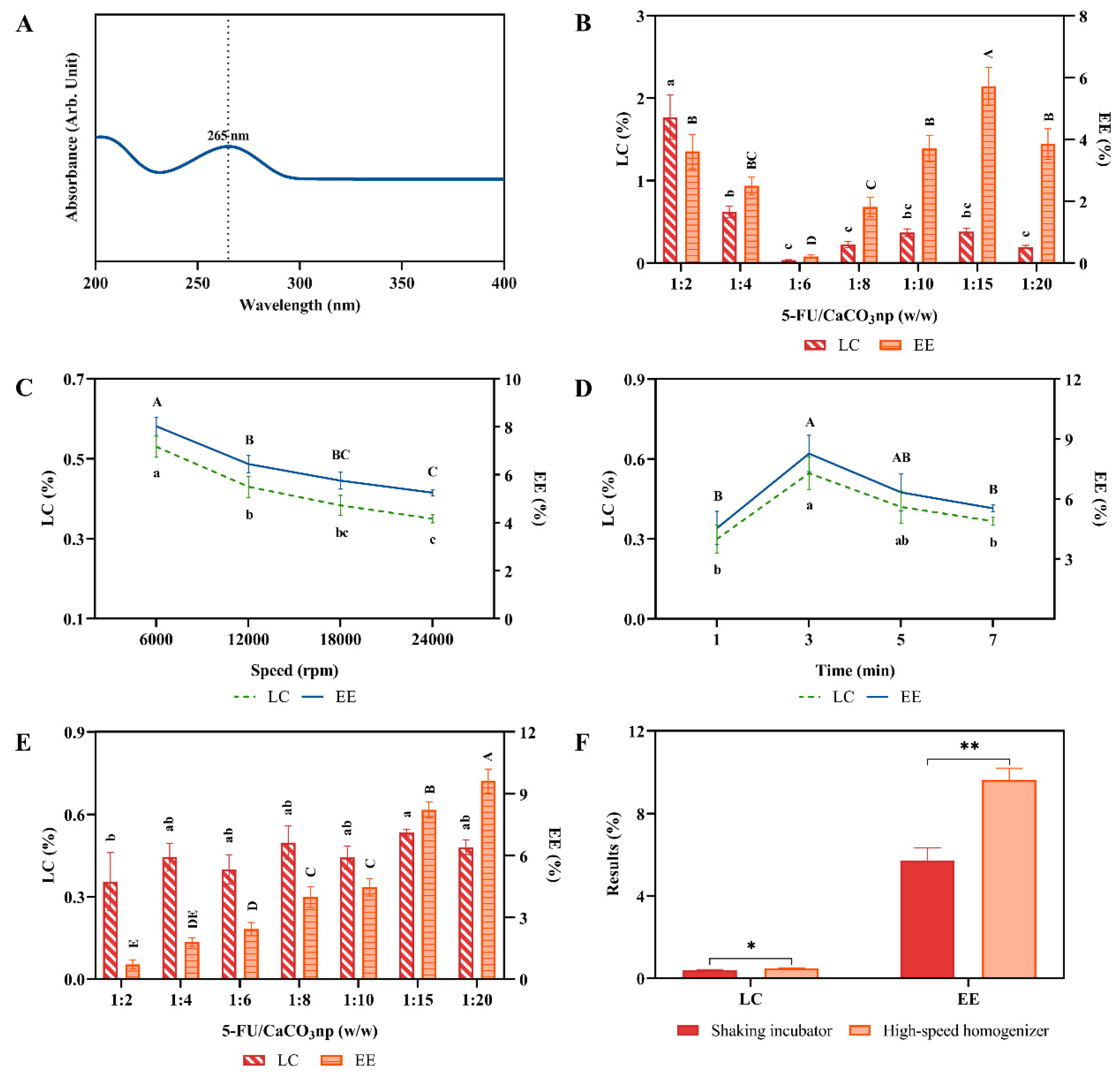
3.1. Preparation of 5FU-CaCO3np
3.2. Physicochemical Characterization and In Vitro Drug Release Profile of 5FU-CaCO3np
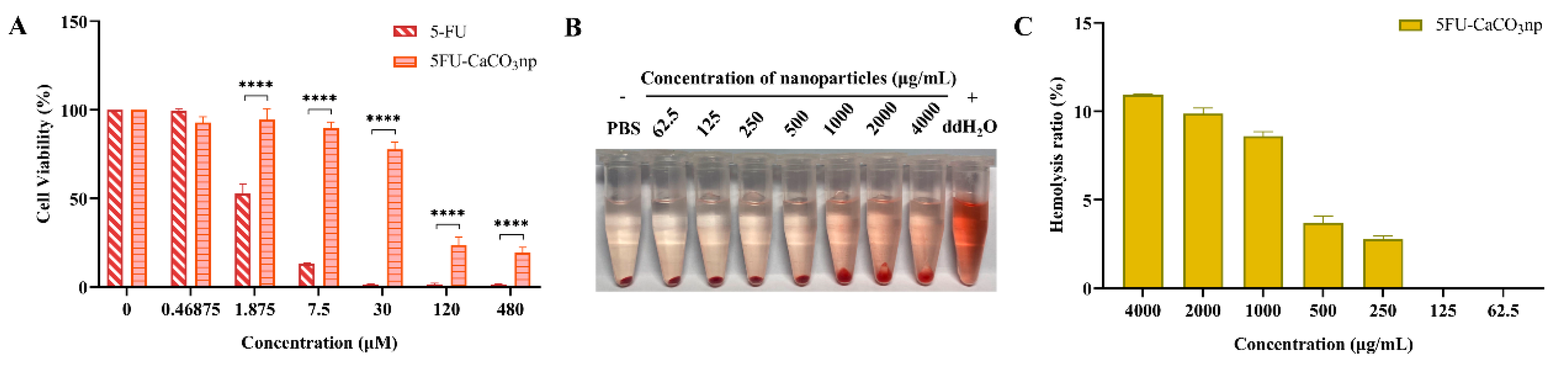
3.3. Biocompatibility of 5FU-CaCO3np
3.4. Inhibitory Effects of 5FU-CaCO3np Combined with Thymoquinone on CT26 Cell Proliferation
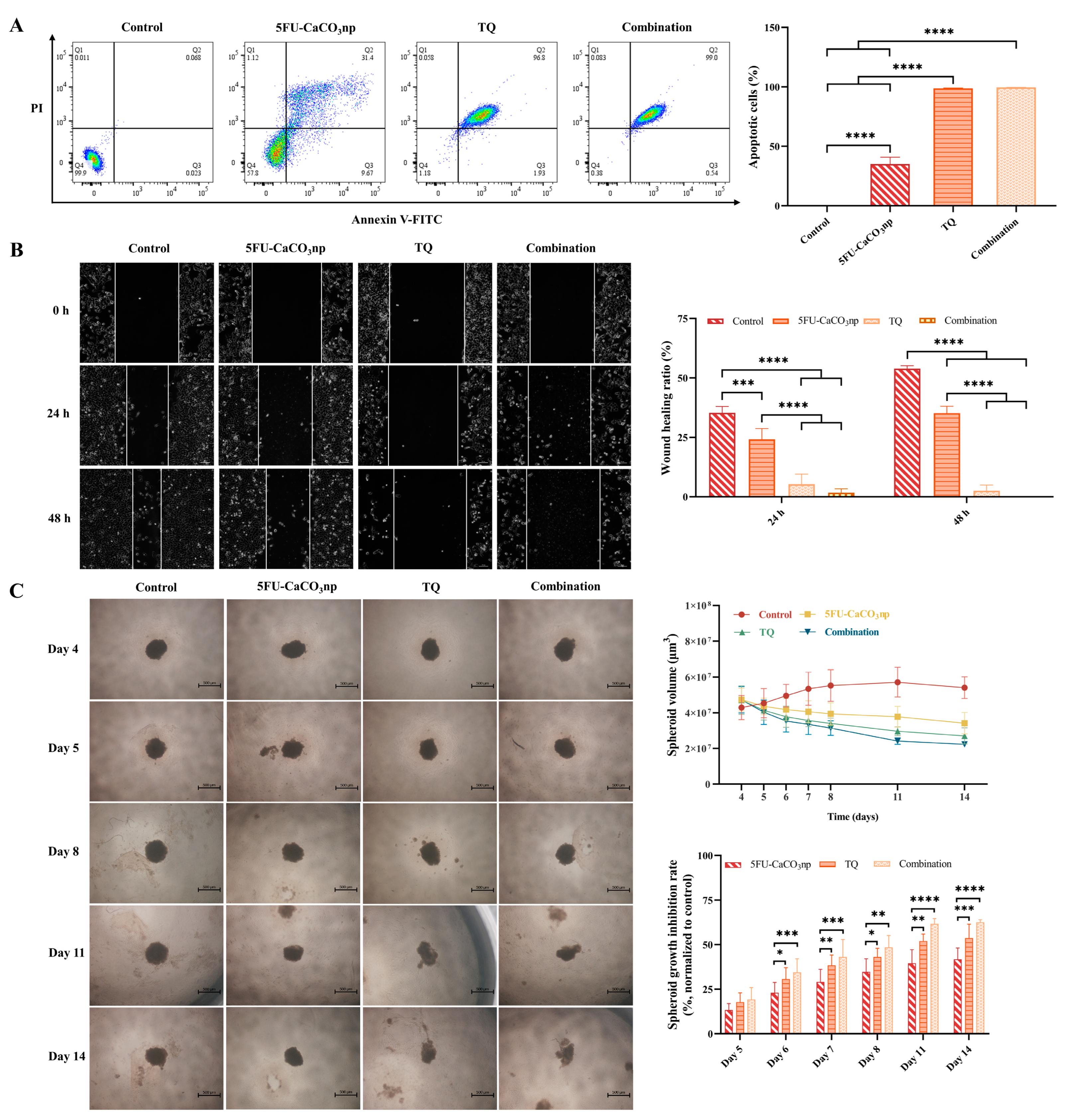
3.5. 5FU-CaCO3np Combined with Thymoquinone Induces Apoptosis and Inhibits the Migration of CT26 Cells
3.6. 5FU-CaCO3np Combined with Thymoquinone Inhibits the Growth of CT26 Spheroids
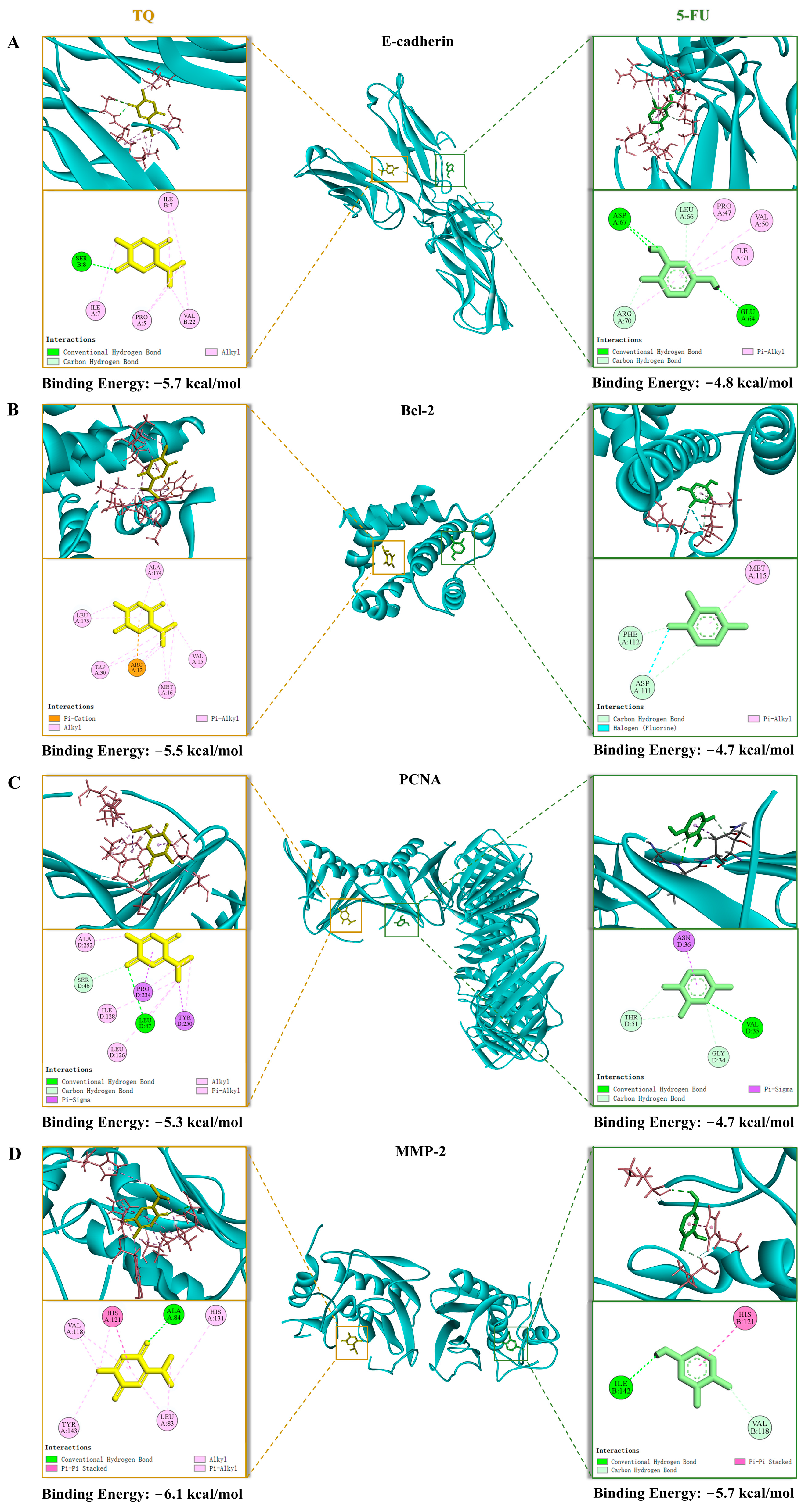
3.7. Molecular Docking
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global Cancer Statistics 2022: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef] [PubMed]
- Vodenkova, S.; Buchler, T.; Cervena, K.; Veskrnova, V.; Vodicka, P.; Vymetalkova, V. 5-Fluorouracil and Other Fluoropyrimidines in Colorectal Cancer: Past, Present and Future. Pharmacol. Ther. 2020, 206, 107447. [Google Scholar] [CrossRef] [PubMed]
- Ciardiello, F.; Ciardiello, D.; Martini, G.; Napolitano, S.; Tabernero, J.; Cervantes, A. Clinical Management of Metastatic Colorectal Cancer in the Era of Precision Medicine. CA Cancer J. Clin. 2022, 72, 372–401. [Google Scholar] [CrossRef] [PubMed]
- Longley, D.B.; Harkin, D.P.; Johnston, P.G. 5-Fluorouracil: Mechanisms of Action and Clinical Strategies. Nat. Rev. Cancer 2003, 3, 330–338. [Google Scholar] [CrossRef]
- Sethy, C.; Kundu, C.N. 5-Fluorouracil (5-FU) Resistance and the New Strategy to Enhance the Sensitivity against Cancer: Implication of DNA Repair Inhibition. Biomed. Pharmacother. 2021, 137, 111285. [Google Scholar] [CrossRef] [PubMed]
- Wahab, S.; Alshahrani, M.Y.; Ahmad, M.F.; Abbas, H. Current Trends and Future Perspectives of Nanomedicine for the Management of Colon Cancer. Eur. J. Pharmacol. 2021, 910, 174464. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.H.; Chen, Y.X.; Fang, J.Y. Comprehensive Review of Targeted Therapy for Colorectal Cancer. Signal Transduct. Target. Ther. 2020, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Dang, Y.; Guan, J. Nanoparticle-Based Drug Delivery Systems for Cancer Therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Senapati, S.; Mahanta, A.K.; Kumar, S.; Maiti, P. Controlled Drug Delivery Vehicles for Cancer Treatment and Their Performance. Signal Transduct. Target. Ther. 2018, 3, 7. [Google Scholar] [CrossRef]
- Sun, L.; Liu, H.; Ye, Y.; Lei, Y.; Islam, R.; Tan, S.; Tong, R.; Miao, Y.B.; Cai, L. Smart Nanoparticles for Cancer Therapy. Signal Transduct. Target. Ther. 2023, 8, 418. [Google Scholar] [CrossRef]
- Kumari, S.; Kondapi, A.K. Lactoferrin Nanoparticle Mediated Targeted Delivery of 5-Fluorouracil for Enhanced Therapeutic Efficacy. Int. J. Biol. Macromol. 2017, 95, 232–237. [Google Scholar] [CrossRef] [PubMed]
- Pan, G.; Jia, T.T.; Huang, Q.X.; Qiu, Y.Y.; Xu, J.; Yin, P.H.; Liu, T. Mesoporous Silica Nanoparticles (MSNs)-Based Organic/Inorganic Hybrid Nanocarriers Loading 5-Fluorouracil for the Treatment of Colon Cancer with Improved Anticancer Efficacy. Colloids Surf. B Biointerfaces 2017, 159, 375–385. [Google Scholar] [CrossRef] [PubMed]
- Vergaro, V.; Pisano, I.; Grisorio, R.; Baldassarre, F.; Mallamaci, R.; Santoro, A.; Suranna, G.P.; Papadia, P.; Fanizzi, F.P.; Ciccarella, G. CaCO3 as an Environmentally Friendly Renewable Material for Drug Delivery Systems: Uptake of HSA-CaCO3 Nanocrystals Conjugates in Cancer Cell Lines. Materials 2019, 12, 1481. [Google Scholar] [CrossRef] [PubMed]
- Borrego-Sánchez, A.; Sánchez-Espejo, R.; Albertini, B.; Passerini, N.; Cerezo, P.; Viseras, C.; Sainz-Díaz, C.I. Ground Calcium Carbonate as a Low Cost and Biosafety Excipient for Solubility and Dissolution Improvement of Praziquantel. Pharmaceutics 2019, 11, 533. [Google Scholar] [CrossRef] [PubMed]
- Shi, H.; Li, L.; Zhang, L.; Wang, T.; Wang, C.; Zhu, D.; Su, Z. Designed Preparation of Polyacrylic Acid/Calcium Carbonate Nanoparticles with High Doxorubicin Payload for Liver Cancer Chemotherapy. CrystEngComm 2015, 17, 4768–4773. [Google Scholar] [CrossRef]
- Almajali, B.; Al-Jamal, H.A.N.; Taib, W.R.W.; Ismail, I.; Johan, M.F.; Doolaanea, A.A.; Ibrahim, W.N. Thymoquinone, as a Novel Therapeutic Candidate of Cancers. Pharmaceuticals 2021, 14, 369. [Google Scholar] [CrossRef] [PubMed]
- Kundu, J.; Choi, B.Y.; Jeong, C.H.; Kundu, J.K.; Chun, K.S. Thymoquinone Induces Apoptosis in Human Colon Cancer HCT116 Cells through Inactivation of STAT3 by Blocking JAK2- and Src-Mediated Phosphorylation of EGF Receptor Tyrosine Kinase. Oncol. Rep. 2014, 32, 821–828. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Bai, Y.; Yang, Y. Thymoquinone Chemosensitizes Colon Cancer Cells through Inhibition of NF-ΚB. Oncol. Lett. 2016, 12, 2840–2845. [Google Scholar] [CrossRef]
- Chen, M.C.; Lee, N.H.; Hsu, H.H.; Ho, T.J.; Tu, C.C.; Chen, R.J.; Lin, Y.M.; Viswanadha, V.P.; Kuo, W.W.; Huang, C.Y. Inhibition of NF-ΚB and Metastasis in Irinotecan (CPT-11)-Resistant LoVo Colon Cancer Cells by Thymoquinone via JNK and P38. Environ. Toxicol. 2017, 32, 669–678. [Google Scholar] [CrossRef] [PubMed]
- Rejhová, A.; Opattová, A.; Čumová, A.; Slíva, D.; Vodička, P. Natural Compounds and Combination Therapy in Colorectal Cancer Treatment. Eur. J. Med. Chem. 2018, 144, 582–594. [Google Scholar] [CrossRef]
- Kensara, O.A.; El-Shemi, A.G.; Mohamed, A.M.; Refaat, B.; Idris, S.; Ahmad, J. Thymoquinone Subdues Tumor Growth and Potentiates the Chemopreventive Effect of 5-Fluorouracil on the Early Stages of Colorectal Carcinogenesis in Rats. Drug Des. Dev. Ther. 2016, 10, 2239–2253. [Google Scholar] [CrossRef]
- Chou, T.C. Preclinical versus Clinical Drug Combination Studies. Leuk. Lymphoma 2008, 49, 2059–2080. [Google Scholar] [CrossRef] [PubMed]
- Shen, S.; Wu, Y.; Liu, Y.; Wu, D. High Drug-Loading Nanomedicines: Progress, Current Status, and Prospects. Int. J. Nanomed. 2017, 12, 4085–4109. [Google Scholar] [CrossRef]
- Sharma, N.; Madan, P.; Lin, S. Effect of Process and Formulation Variables on the Preparation of Parenteral Paclitaxel-Loaded Biodegradable Polymeric Nanoparticles: A Co-Surfactant Study. Asian J. Pharm. Sci. 2016, 11, 404–416. [Google Scholar] [CrossRef]
- Safavi, M.S.; Shojaosadati, S.A.; Dorkoosh, F.A.; Jo, H.J.; Kwon, Y.; Lee, K.C.; Yang, H.G.; Park, E.J.; Na, D.H. The Synthesis of Tamoxifen-Loaded Albumin Nanoparticles by Homogenizers: Optimization and in Vitro Characterization. J. Drug Deliv. Sci. Technol. 2017, 41, 20–30. [Google Scholar] [CrossRef]
- Dey, D.; Kumar, P.; Samantaray, S. A Review of Nanofluid Preparation, Stability, and Thermo-Physical Properties. Heat Transf.-Asian Res. 2017, 46, 1413–1442. [Google Scholar] [CrossRef]
- Di, J.; Gao, X.; Du, Y.; Zhang, H.; Gao, J.; Zheng, A. Size, Shape, Charge and “Stealthy” Surface: Carrier Properties Affect the Drug Circulation Time In Vivo. Asian J. Pharm. Sci. 2021, 16, 444–458. [Google Scholar] [CrossRef] [PubMed]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of Nanoparticle Design for Overcoming Biological Barriers to Drug Delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef] [PubMed]
- Mo, R.; Gu, Z. Tumor Microenvironment and Intracellular Signal-Activated Nanomaterials for Anticancer Drug Delivery. Mater. Today 2016, 19, 274–283. [Google Scholar] [CrossRef]
- Sun, L.; Chen, Y.; Zhou, Y.; Guo, D.; Fan, Y.; Guo, F.; Zheng, Y.; Chen, W. Preparation of 5-Fluorouracil-Loaded Chitosan Nanoparticles and Study of the Sustained Release in Vitro and in Vivo. Asian J. Pharm. Sci. 2017, 12, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Adepu, S.; Ramakrishna, S. Controlled Drug Delivery Systems: Current Status and Future Directions. Molecules 2021, 26, 5905. [Google Scholar] [CrossRef] [PubMed]
- Costa, P.; Sousa Lobo, J.M. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef] [PubMed]
- Dobrovolskaia, M.A.; McNeil, S.E. Understanding the Correlation between in Vitro and in Vivo Immunotoxicity Tests for Nanomedicines. J. Control. Release 2013, 172, 456–466. [Google Scholar] [CrossRef] [PubMed]
- Amin, K.; Dannenfelser, R.M. In Vitro Hemolysis: Guidance for the Pharmaceutical Scientist. J. Pharm. Sci. 2006, 95, 1173–1176. [Google Scholar] [CrossRef] [PubMed]
- Rajabi, S.; Ramazani, A.; Hamidi, M.; Naji, T. Artemia Salina as a Model Organism in Toxicity Assessment of Nanoparticles. DARU J. Pharm. Sci. 2015, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Pinheiro, S.K.D.P.; Lima, A.K.M.; Miguel, T.B.A.R.; Filho, A.G.S.; Ferreira, O.P.; Pontes, M.D.S.; Grillo, R.; Miguel, E.D.C. Assessing Toxicity Mechanism of Silver Nanoparticles by Using Brine Shrimp (Artemia salina) as Model. Chemosphere 2023, 347, 140673. [Google Scholar] [CrossRef] [PubMed]
- Clarkson, C.; Maharaj, V.J.; Crouch, N.R.; Grace, O.M.; Pillay, P.; Matsabisa, M.G.; Bhagwandin, N.; Smith, P.J.; Folb, P.I. In Vitro Antiplasmodial Activity of Medicinal Plants Native to or Naturalised in South Africa. J. Ethnopharmacol. 2004, 92, 177–191. [Google Scholar] [CrossRef] [PubMed]
- Ewert de Oliveira, B.; Junqueira Amorim, O.H.; Lima, L.L.; Rezende, R.A.; Mestnik, N.C.; Bagatin, E.; Leonardi, G.R. 5-Fluorouracil, Innovative Drug Delivery Systems to Enhance Bioavailability for Topical Use. J. Drug Deliv. Sci. Technol. 2021, 61, 102155. [Google Scholar] [CrossRef]
- Chou, T.C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Xie, X.; Li, Y.; Lian, S.; Lu, Y.; Jia, L. Cancer Metastasis Chemoprevention Prevents Circulating Tumour Cells from Germination. Signal Transduct. Target. Ther. 2022, 7, 341. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.G.; Sanders, A.J.; Katoh, M.; Ungefroren, H.; Gieseler, F.; Prince, M.; Thompson, S.K.; Zollo, M.; Spano, D.; Dhawan, P.; et al. Tissue Invasion and Metastasis: Molecular, Biological and Clinical Perspectives. Semin. Cancer Biol. 2015, 35, S244–S275. [Google Scholar] [CrossRef] [PubMed]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D Tumor Spheroids: An Overview on the Tools and Techniques Used for Their Analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Chatzinikolaidou, M. Cell Spheroids: The New Frontiers in in Vitro Models for Cancer Drug Validation. Drug Discov. Today 2016, 21, 1553–1560. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.B.; Le, V.M.; Gu, C.H.; Zheng, Y.H.; Lang, M.D.; Lu, Y.H.; Liu, J.W. Overcoming Multidrug Resistance in 2D and 3D Culture Models by Controlled Drug Chitosan-Graft Poly(Caprolactone)-Based Nanoparticles. J. Pharm. Sci. 2014, 103, 1064–1074. [Google Scholar] [CrossRef] [PubMed]
- Virgone-Carlotta, A.; Lemasson, M.; Mertani, H.C.; Diaz, J.J.; Monnier, S.; Dehoux, T.; Delanoë-Ayari, H.; Rivière, C.; Rieu, J.P. In-Depth Phenotypic Characterization of Multicellular Tumor Spheroids: Effects of 5-Fluorouracil. PLoS ONE 2017, 12, e0188100. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, H.; Fryknäs, M.; Larsson, R.; Nygren, P. Loss of Cancer Drug Activity in Colon Cancer HCT-116 Cells during Spheroid Formation in a New 3-D Spheroid Cell Culture System. Exp. Cell Res. 2012, 318, 1577–1585. [Google Scholar] [CrossRef] [PubMed]
- Infield, D.T.; Rasouli, A.; Galles, G.D.; Chipot, C.; Tajkhorshid, E.; Ahern, C.A. Cation-π Interactions and Their Functional Roles in Membrane Proteins: Cation-π Interactions in Membrane Proteins. J. Mol. Biol. 2021, 433, 167035. [Google Scholar] [CrossRef]
- Sheikh, T.; Maqbool, S.; Mandal, P.; Nag, A. Introducing Intermolecular Cation-π Interactions for Water-Stable Low Dimensional Hybrid Lead Halide Perovskites. Angew. Chem.-Int. Ed. 2021, 60, 18271. [Google Scholar] [CrossRef] [PubMed]
- Sawtell, R.M.; Rew, D.A.; Stradling, R.N.; Wilson, G.D. Pan Cycle Expression of Proliferating Cell Nuclear Antigen in Human Colorectal Cancer and Its Proliferative Correlations. Cytometry 1995, 22, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Lemech, C.R.; Kichenadasse, G.; Marschner, J.P.; Alevizopoulos, K.; Otterlei, M.; Millward, M. ATX-101, a Cell-Penetrating Protein Targeting PCNA, Can Be Safely Administered as Intravenous Infusion in Patients and Shows Clinical Activity in a Phase 1 Study. Oncogene 2023, 42, 541–544. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Medema, J.P. BCL-2 Family Deregulation in Colorectal Cancer: Potential for BH3 Mimetics in Therapy. Apoptosis 2020, 25, 305–320. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, P.; Lannagan, T.R.M.; Jackstadt, R.; Atencia Taboada, L.; Lansu, N.; Wirapati, P.; van Hooff, S.R.; Dekker, D.; Pritchard, J.; Kirov, A.B.; et al. BCL-XL Is Crucial for Progression through the Adenoma-to-Carcinoma Sequence of Colorectal Cancer. Cell Death Differ. 2021, 28, 3282–3296. [Google Scholar] [CrossRef]
- Han, S.J.; Kwon, S.; Kim, K.S. Challenges of Applying Multicellular Tumor Spheroids in Preclinical Phase. Cancer Cell Int. 2021, 21, 152. [Google Scholar] [CrossRef] [PubMed]
- Langers, A.M.J.; Verspaget, H.W.; Hawinkels, L.J.A.C.; Kubben, F.J.G.M.; Van Duijn, W.; Van Der Reijden, J.J.; Hardwick, J.C.H.; Hommes, D.W.; Sier, C.F.M. MMP-2 and MMP-9 in Normal Mucosa Are Independently Associated with Outcome of Colorectal Cancer Patients. Br. J. Cancer 2012, 106, 1495–1498. [Google Scholar] [CrossRef] [PubMed]
- Na, T.Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The Functional Activity of E-Cadherin Controls Tumor Cell Metastasis at Multiple Steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [CrossRef] [PubMed]


| Factor | Speed (rpm) | Time (min) |
|---|---|---|
| Speed | 6000 | 3 |
| 12,000 | 3 | |
| 18,000 | 3 | |
| 24,000 | 3 | |
| Time | 6000 | 1 |
| 6000 | 3 | |
| 6000 | 5 | |
| 6000 | 7 |
| Peak | Peak Assignment | Wavenumber (cm−1) |
|---|---|---|
| a | In-plane C-O bending | 710.68 |
| b | Out of plane C-O bending | 851.60 |
| c | Symmetric C-O stretching | 1082.21 |
| d | Asymmetric C-O stretching | 1440.93 |
| e | Symmetric C-O stretching; in-plane C-O bending | 1783.63 |
| pH | Zero-Order Model R2 | First-Order Model R2 | Higuchi Model R2 | Hixon–Crowell Model R2 | Korsmeyer–Peppas Model | |
|---|---|---|---|---|---|---|
| R2 | n | |||||
| 7.4 | 0.84579 | 0.92832 | 0.96651 | 0.86380 | 0.98258 | 0.38839 |
| 6.5 | 0.83112 | 0.90811 | 0.96204 | 0.86074 | 0.98988 | 0.34495 |
| 5.0 | 0.76977 | 0.86509 | 0.92401 | 0.83058 | 0.99084 | 0.27514 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Yang, Z.; Chan, K.W.; Abu Bakar, M.Z. Exploring the Therapeutic Potential of 5-Fluorouracil-Loaded Calcium Carbonate Nanoparticles Combined with Natural Compound Thymoquinone for Colon Cancer Treatment. Pharmaceutics 2024, 16, 1011. https://doi.org/10.3390/pharmaceutics16081011
Deng X, Yang Z, Chan KW, Abu Bakar MZ. Exploring the Therapeutic Potential of 5-Fluorouracil-Loaded Calcium Carbonate Nanoparticles Combined with Natural Compound Thymoquinone for Colon Cancer Treatment. Pharmaceutics. 2024; 16(8):1011. https://doi.org/10.3390/pharmaceutics16081011
Chicago/Turabian StyleDeng, Xi, Zhongming Yang, Kim Wei Chan, and Md Zuki Abu Bakar. 2024. "Exploring the Therapeutic Potential of 5-Fluorouracil-Loaded Calcium Carbonate Nanoparticles Combined with Natural Compound Thymoquinone for Colon Cancer Treatment" Pharmaceutics 16, no. 8: 1011. https://doi.org/10.3390/pharmaceutics16081011







