Maximizing the Use of Ivermectin Transethosomal Cream in the Treatment of Scabies
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Experimental Design
2.2.2. Preparation of IVM-Loaded TESMs Formulations
2.2.3. Evaluation of IVM-Loaded TESMs Formulations
Measurement of Entrapment Efficiency (EE%)
Measurement of Particle Size (PS), Polydispersity Index (PDI) and Zeta Potential (ZP)
Measurement of the Cumulative Percentage of Drug Released after 6 h (Q6h)
Kinetic Study of Drug Release
2.2.4. Statistical Analysis, Optimization and Validation
2.2.5. Characterization of the Optimized IVM-Loaded TESMs Formulation
Transmission Electron Microscopy (TEM)
Differential Scanning Calorimetry (DSC)
Fourier Transform Infrared Spectroscopy (FTIR)
Stability Studies
2.2.6. Preparation of IVM-Loaded Transethosomal Cream
2.2.7. Evaluation of the Prepared IVM-Loaded Transethosomal Cream
Physical Inspection
Determination of pH Value
Spreadability
Viscosity Measurement
2.2.8. In Vivo Experimental Study
Animals and Ethical Approval
Skin Irritation Test
Histopathological Study
2.2.9. Statistical Analysis
3. Results and Discussion
3.1. Preliminary Studies for Preparation of IVM-Loaded TESMs
3.2. Evaluation of IVM-Loaded TESMs Formulations
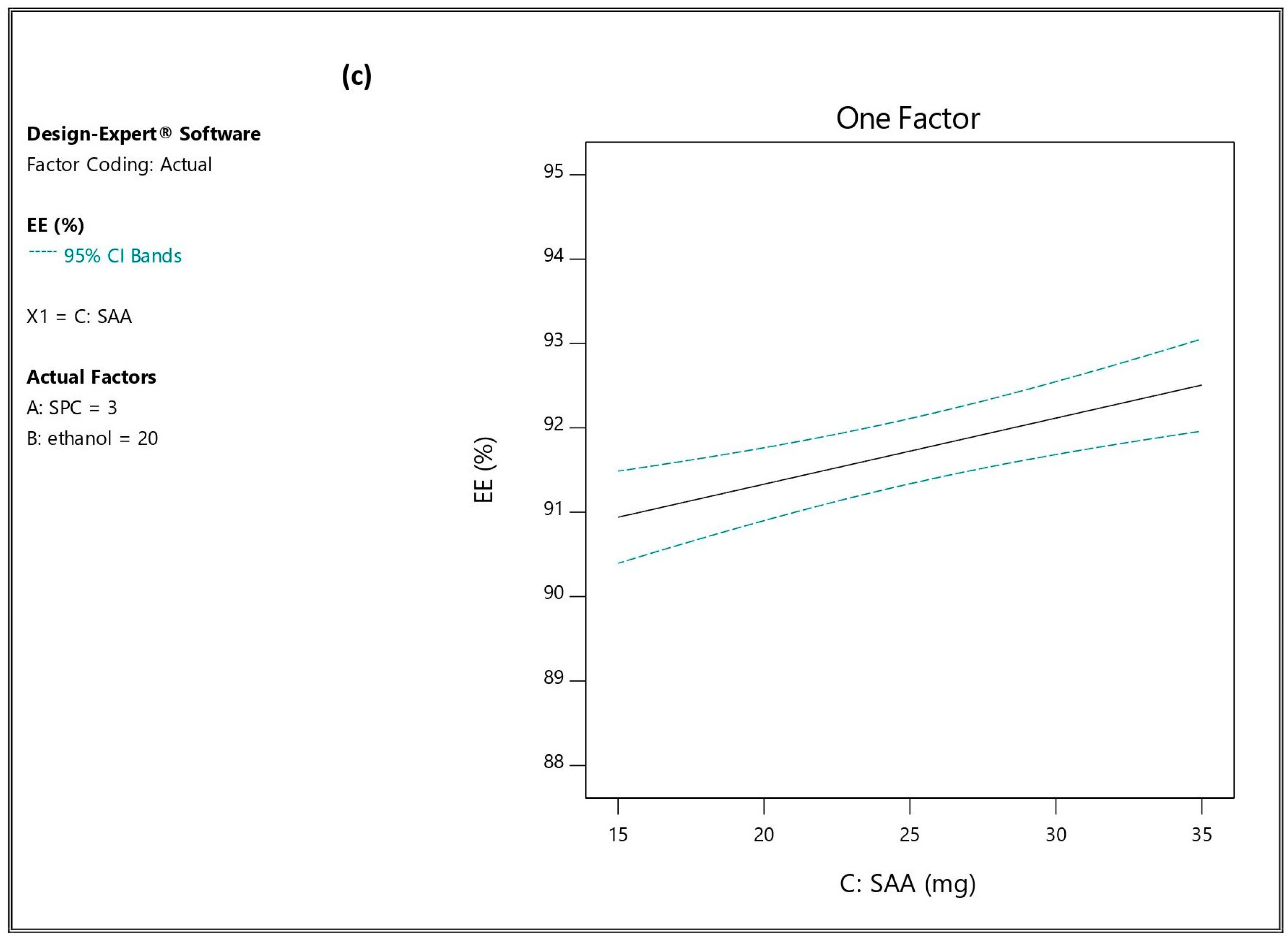
3.2.1. Influence of the Independent Variables on Entrapment Efficiency (EE%; Y1)
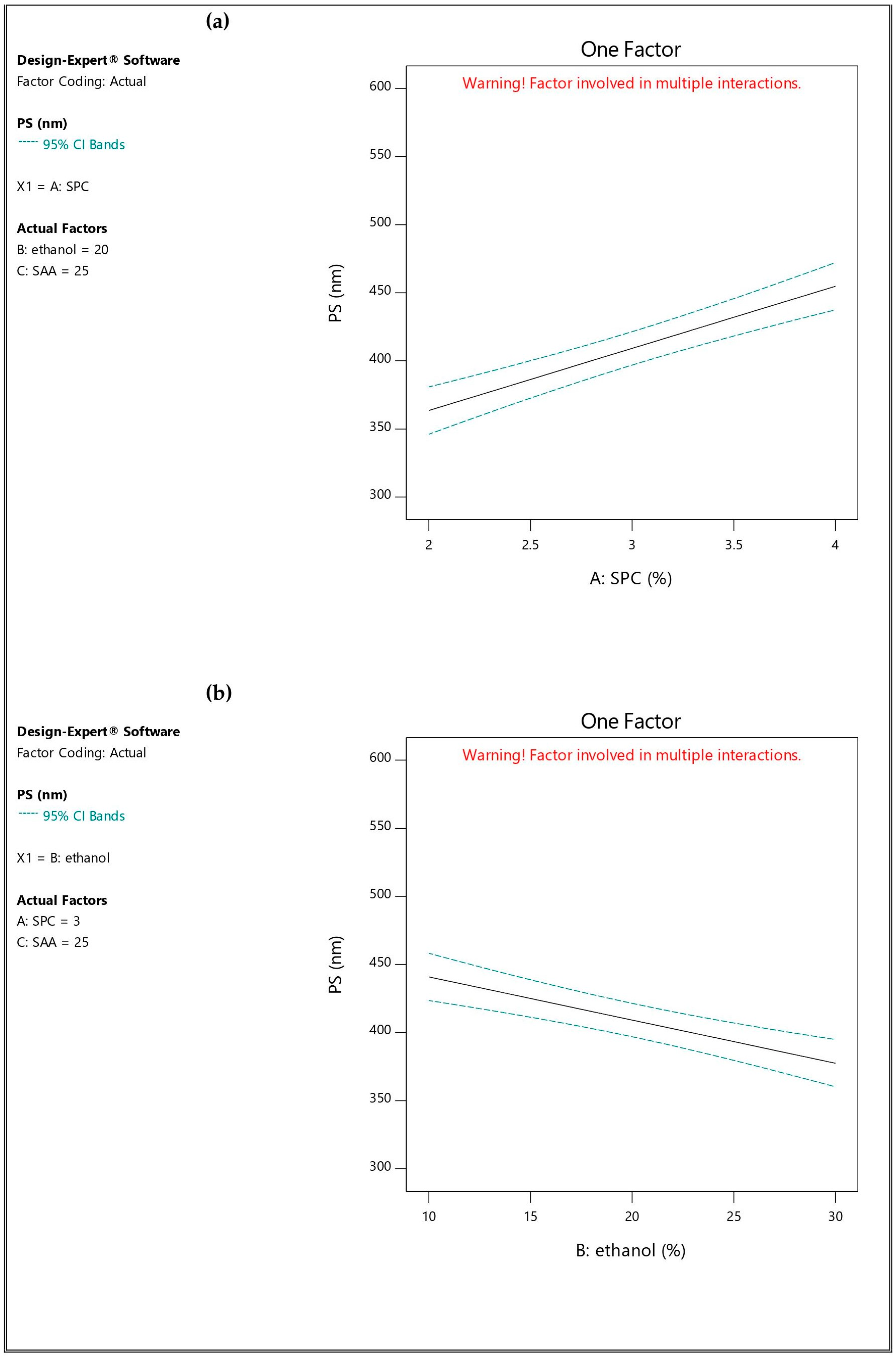
3.2.2. Influence of the Independent Variables on Particle Size (PS; Y2), Polydispersity Index (PDI; Y3) and Zeta Potential (ZP; Y4)
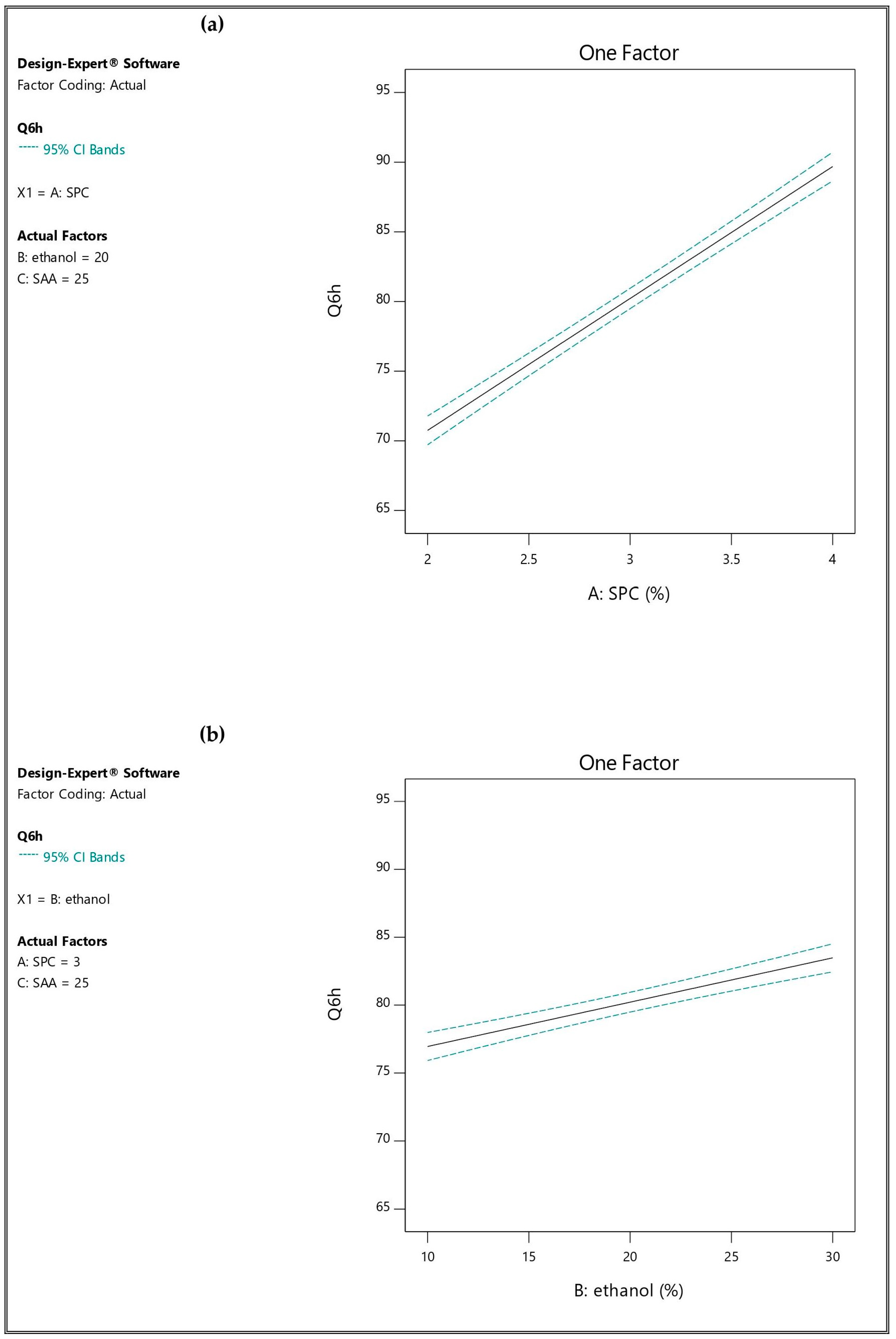
3.2.3. Influence of the Independent Variables on Percentage of Drug Released after 6 h (Q6h; Y5)
3.2.4. Kinetic Study of Drug Release
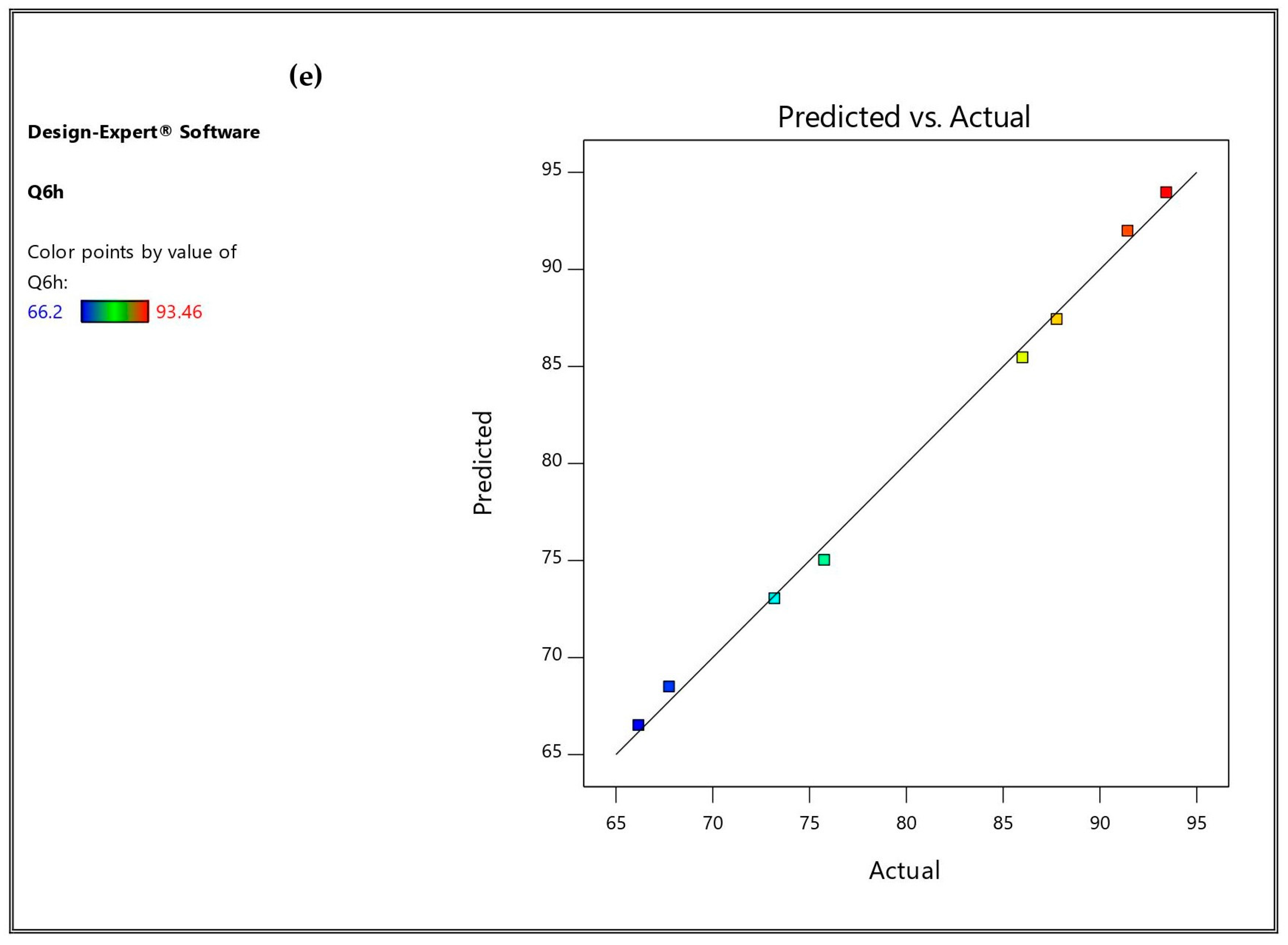
3.3. Optimization and Validation
3.4. Characterization of the Optimized IVM-Loaded TESMs
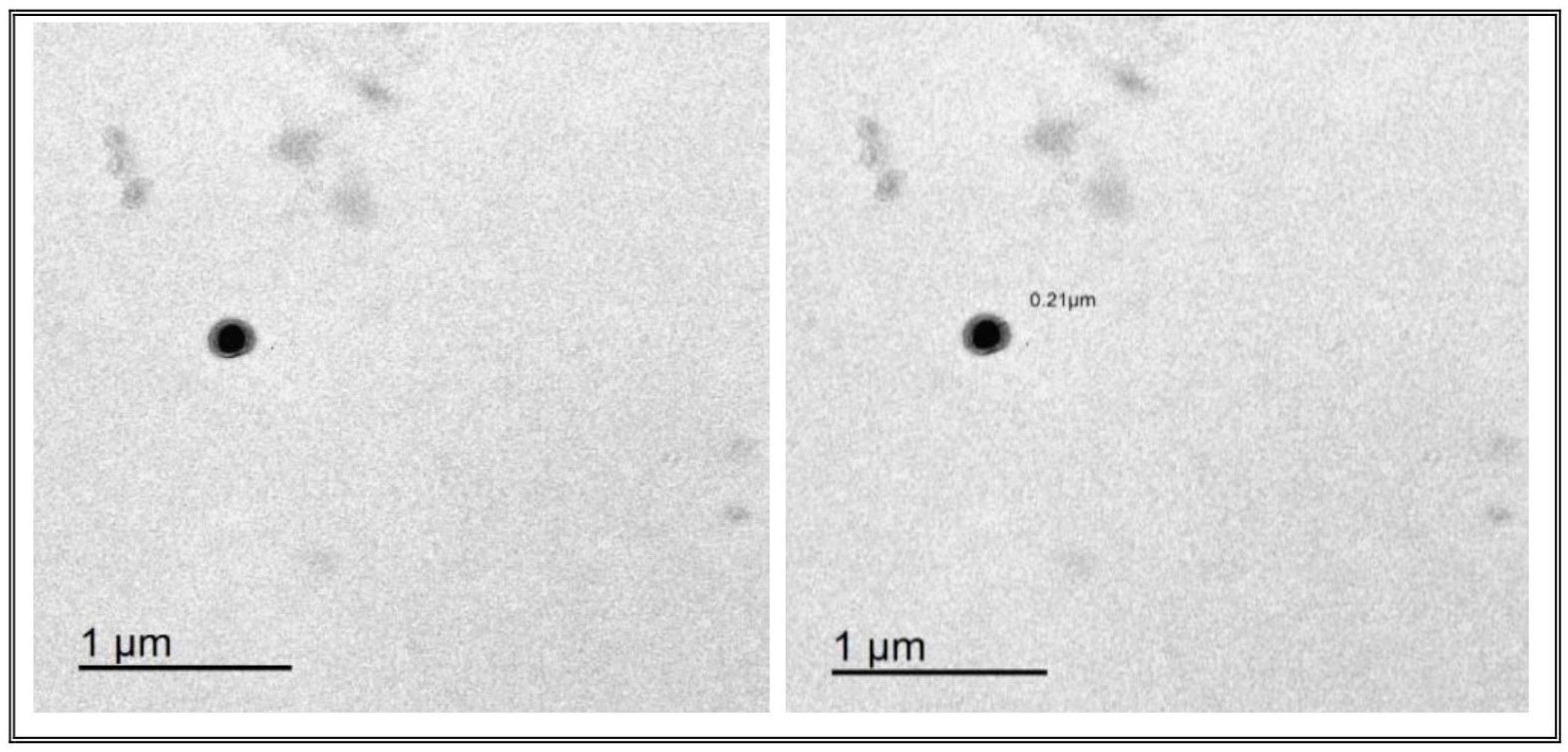
3.4.1. Transmission Electron Microscopy (TEM)
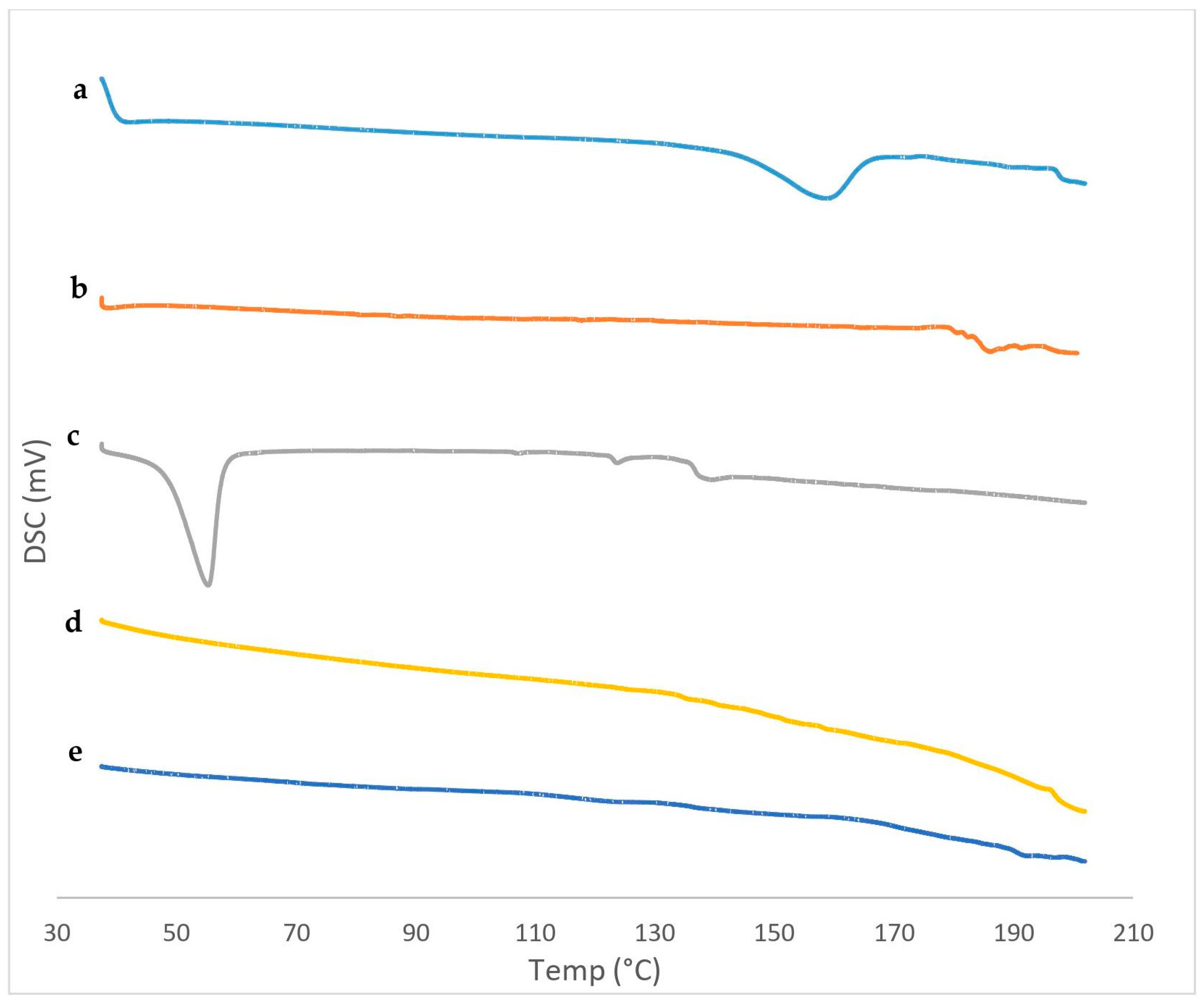
3.4.2. Differential Scanning Calorimetry (DSC)
3.4.3. Fourier Transform Infrared Spectroscopy (FTIR)
3.4.4. Stability Study
3.5. Evaluation of the Prepared IVM-Loaded Transethosomal Cream
3.6. In Vivo Experimental Study
3.6.1. Skin Irritation Test
3.6.2. Histopathological Study
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ribeiro, F.D.A.Q.; Taciro, E.; Guerra, M.R.M.; Eckley, C.A. Oral Ivermectin for the Treatment and Prophylaxis of Scabies in Prison. J. Dermatol. Treat. 2005, 16, 138–141. [Google Scholar] [CrossRef] [PubMed]
- Mohebbipour, A.; Saleh, P.; Goldust, M.; Amirnia, M.; Zadeh, Y.J.; Mohamad, R.M.; Rezaee, E. Comparison of Oral Ivermectin vs. Lindane Lotion 1% for the Treatment of Scabies. Clin. Exp. Dermatol. 2013, 38, 719–723. [Google Scholar] [CrossRef] [PubMed]
- Hong, M.; Lee, C.; Chuang, M.; Chao, S.; Tsai, M.; Chi, C. Factors Related to Missed Diagnosis of Incidental Scabies Infestations in Patients Admitted Through the Emergency Department to Inpatient Services. Acad. Emerg. Med. 2010, 17, 958–964. [Google Scholar] [CrossRef]
- Goldust, M.; Rezaee, E.; Raghiafar, R. Topical Ivermectin versus Crotamiton Cream 10% for the Treatment of Scabies. Int. J. Dermatol. 2014, 53, 904–908. [Google Scholar] [CrossRef]
- Wendel, K.; Rompalo, A. Scabies and Pediculosis Pubis: An Update of Treatment Regimens and General Review. Clin. Infect. Dis. 2002, 35, S146–S151. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sunderkötter, C.; Wohlrab, J.; Hamm, H. Scabies: Epidemiology, Diagnosis, and Treatment. Dtsch. Arztebl. Int. 2021, 118, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Arlian, L.G.; Runyan, R.A.; Sorlie, L.B.; Estes, S.A. Host-Seeking Behavior of Sarcoptes Scabiei. J. Am. Acad. Dermatol. 1984, 11, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Chandler, D.J.; Fuller, L.C. A Review of Scabies: An Infestation More than Skin Deep. Dermatology 2019, 235, 79–90. [Google Scholar] [CrossRef] [PubMed]
- Campillo, J.T.; Boussinesq, M.; Bertout, S.; Faillie, J.-L.; Chesnais, C.B. Serious Adverse Reactions Associated with Ivermectin: A Systematic Pharmacovigilance Study in Sub-Saharan Africa and in the Rest of the World. PLoS Negl. Trop. Dis. 2021, 15, e0009354. [Google Scholar] [CrossRef] [PubMed]
- Gazewood, J.D.; Johnson, K. Ivermectin 1% Cream (Soolantra) for Inflammatory Lesions of Rosacea. Am. Fam. Physician 2016, 94, 512–513. [Google Scholar]
- Castro, G.A.; Oliveira, C.A.; Mahecha, G.A.B.; Ferreira, L.A.M. Comedolytic Effect and Reduced Skin Irritation of a New Formulation of All-Trans Retinoic Acid-Loaded Solid Lipid Nanoparticles for Topical Treatment of Acne. Arch. Dermatol. Res. 2011, 303, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Ashtikar, M.; Nagarsekar, K.; Fahr, A. Transdermal Delivery from Liposomal Formulations—Evolution of the Technology over the Last Three Decades. J. Control. Release 2016, 242, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Schlich, M.; Musazzi, U.M.; Campani, V.; Biondi, M.; Franzé, S.; Lai, F.; De Rosa, G.; Sinico, C.; Cilurzo, F. Design and Development of Topical Liposomal Formulations in a Regulatory Perspective. Drug Deliv. Transl. Res. 2022, 12, 1811–1828. [Google Scholar] [CrossRef] [PubMed]
- Cevc, G.; Blume, G. Lipid Vesicles Penetrate into Intact Skin Owing to the Transdermal Osmotic Gradients and Hydration Force. Biochim. Biophys. Acta. 1992, 1104, 226–232. [Google Scholar] [CrossRef] [PubMed]
- AL Shuwaili, A.H.; Rasool, B.K.A.; Abdulrasool, A.A. Optimization of Elastic Transfersomes Formulations for Transdermal Delivery of Pentoxifylline. Eur. J. Pharm. Biopharm. 2016, 102, 101–114. [Google Scholar] [CrossRef]
- Touitou, E.; Dayan, N.; Bergelson, L.; Godin, B.; Eliaz, M. Ethosomes—Novel Vesicular Carriers for Enhanced Delivery: Characterization and Skin Penetration Properties. J. Control. Release 2000, 65, 403–418. [Google Scholar] [CrossRef]
- Maheshwari, R.G.S.; Tekade, R.K.; Sharma, P.A.; Darwhekar, G.; Tyagi, A.; Patel, R.P.; Jain, D.K. Ethosomes and Ultradeformable Liposomes for Transdermal Delivery of Clotrimazole: A Comparative Assessment. Saudi Pharm. J. 2012, 20, 161–170. [Google Scholar] [CrossRef]
- Chen, Z.X.; Li, B.; Liu, T.; Wang, X.; Zhu, Y.; Wang, L.; Wang, X.H.; Niu, X.; Xiao, Y.; Sun, Q. Evaluation of Paeonol-Loaded Transethosomes as Transdermal Delivery Carriers. Eur. J. Pharm. Sci. 2017, 99, 240–245. [Google Scholar] [CrossRef]
- Garg, V.; Singh, H.; Bhatia, A.; Raza, K.; Singh, S.K.; Singh, B.; Beg, S. Systematic Development of Transethosomal Gel System of Piroxicam: Formulation Optimization, In Vitro Evaluation, and Ex Vivo Assessment. AAPS PharmSciTech 2017, 18, 58–71. [Google Scholar] [CrossRef]
- Albash, R.; Abdelbary, A.A.; Refai, H.; El-Nabarawi, M.A. Use of Transethosomes for Enhancing the Transdermal Delivery of Olmesartan Medoxomil: In Vitro, Ex Vivo, and in Vivo Evaluation. Int. J. Nanomed. 2019, 14, 1953–1968. [Google Scholar] [CrossRef]
- Zaki, R.M.; Alfadhel, M.M.; Alossaimi, M.A.; Elsawaf, L.A.; Seshadri, V.D.; Almurshedi, A.S.; Yusif, R.M.; Said, M. Central Composite Optimization of Glycerosomes for the Enhanced Oral Bioavailability and Brain Delivery of Quetiapine Fumarate. Pharmaceuticals 2022, 15, 940. [Google Scholar] [CrossRef]
- Said, M.; Elsayed, I.; Aboelwafa, A.A.; Elshafeey, A.H. A Novel Concept of Overcoming the Skin Barrier Using Augmented Liquid Nanocrystals: Box-Behnken Optimization, Ex Vivo and In Vivo Evaluation. Colloid. Surf. B-Biointerfaces 2018, 170, 258–265. [Google Scholar] [CrossRef]
- Zaki, R.M.; Seshadri, V.D.; Mutayran, A.S.; Elsawaf, L.A.; Hamad, A.M.; Almurshedi, A.S.; Yusif, R.M.; Said, M. Wound Healing Efficacy of Rosuvastatin Transethosomal Gel, I Optimal Optimization, Histological and In Vivo Evaluation. Pharmaceutics 2022, 14, 2521. [Google Scholar] [CrossRef]
- Abdallah, M.H.; Abu Lila, A.S.; Shawky, S.M.; Almansour, K.; Alshammari, F.; Khafagy, E.; Makram, T.S. Experimental Design and Optimization of Nano-Transfersomal Gel to Enhance the Hypoglycemic Activity of Silymarin. Polymers 2022, 14, 508. [Google Scholar] [CrossRef]
- Sheshala, R.; Hong, G.C.; Yee, W.P.; Meka, V.S.; Thakur, R.R.S. In Situ Forming Phase-Inversion Implants for Sustained Ocular Delivery of Triamcinolone Acetonide. Drug Deliv. Transl. Res. 2019, 9, 534–542. [Google Scholar] [CrossRef]
- Mazyed, E.A.; Abdelaziz, A.E. Fabrication of Transgelosomes for Enhancing the Ocular Delivery of Acetazolamide: Statistical Optimization, In Vitro Characterization, and In Vivo Study. Pharmaceutics 2020, 12, 465. [Google Scholar] [CrossRef]
- Alyami, M.H.; Musallam, A.A.; Ibrahim, T.M.; Mahdy, M.A.; Elnahas, H.M.; Aldeeb, R.A. The Exploitation of PH-Responsive Eudragit-Coated Mesoporous Silica Nanostructures in the Repurposing of Terbinafine Hydrochloride for Targeted Colon Cancer Inhibition: Design Optimization, In Vitro Characterization, and Cytotoxicity Assessment. Pharmaceutics 2023, 15, 2677. [Google Scholar] [CrossRef]
- Badria, F.; Mazyed, E. Formulation of Nanospanlastics as a Promising Approach for Improving the Topical Delivery of a Natural Leukotriene Inhibitor (3-Acetyl-11-Keto—β-Boswellic Acid): Statistical Optimization, in Vitro Characterization, and Ex Vivo Permeation Study. Drug Des. Devel. Ther. 2020, 14, 3697–3721. [Google Scholar] [CrossRef]
- Salem, H.F.; Kharshoum, R.M.; El-ela, F.I.A.; F, A.G.; Abdellatif, K.R.A. Evaluation and Optimization of PH—Responsive Niosomes as a Carrier for Efficient Treatment of Breast Cancer. Drug Deliv. Transl. Res. 2018, 8, 633–644. [Google Scholar] [CrossRef]
- Abdellatif, M.M.; Khalil, I.A.; Khalil, M.A.F. Sertaconazole Nitrate Loaded Nanovesicular Systems for Targeting Skin Fungal Infection: In-Vitro, Ex-Vivo and in-Vivo Evaluation. Int. J. Pharm. 2017, 527, 1–11. [Google Scholar] [CrossRef]
- Abdelbary, A.A.; Al-mahallawi, A.M.; Abdelrahim, M.E.; Ali, A.M. Preparation, Optimization, and in Vitro Simulated Inhalation Delivery of Carvedilol Nanoparticles Loaded on a Coarse Carrier Intended for Pulmonary Administration. Int. J. Nanomed. 2015, 10, 6339–6353. [Google Scholar] [CrossRef]
- Mazyed, E.A.; Zakaria, S. Enhancement of Dissolution Characteristics of Clopidogrel Bisulphate by Proniosomes. Int. J. Appl. Pharm. 2019, 11, 77–85. [Google Scholar] [CrossRef]
- Chen, M.X.; Alexander, K.S.; Baki, G. Formulation and Evaluation of Antibacterial Creams and Gels Containing Metal Ions for Topical Application. J. Pharm. 2016, 2016, 5754349. [Google Scholar] [CrossRef]
- Dantas, M.G.B.; Reis, S.A.G.B.; Damasceno, C.M.D.; Rolim, L.A.; Rolim-Neto, P.J.; Carvalho, F.O.; Quintans-Junior, L.J.; Almeida, J.R.G.d.S. Development and Evaluation of Stability of a Gel Formulation Containing the Monoterpene Borneol. Sci. World J. 2016, 2016, 7394685. [Google Scholar] [CrossRef]
- Shah, A.; Boldhane, S.; Pawar, A.; Bothiraja, C. Advanced Development of a Non-Ionic Surfactant and Cholesterol Material Based Niosomal Gel Formulation for the Topical Delivery of Anti-Acne Drugs. Mater. Adv. 2020, 1, 1763–1774. [Google Scholar] [CrossRef]
- Aziz, D.E.; Abdelbary, A.A.; Elassasy, A.I. Investigating Superiority of Novel Bilosomes over Niosomes in the Transdermal Delivery of Diacerein: In Vitro Characterization, Ex Vivo Permeation and in Vivo Skin Deposition Study. J. Liposome Res. 2019, 29, 73–85. [Google Scholar] [CrossRef]
- Yeo, L.K.; Chaw, C.S.; Elkordy, A.A. Effects of Preparation Methods on the Characteristics of Niosomes. Br. J. Pharm. 2019, 4, S24–S25. [Google Scholar] [CrossRef]
- Abdallah, M.H. Transferosomes as a Transdermal Drug Delivery System for Enhancement the Antifungal Activity of Nystatin. Int. J. Pharm. Pharm. Sci. 2013, 5, 560–567. [Google Scholar]
- Said, M.; Aboelwafa, A.A.; Elshafeey, A.H.; Elsayed, I. Central Composite Optimization of Ocular Mucoadhesive Cubosomes for Enhanced Bioavailability and Controlled Delivery of Voriconazole. J. Drug Deliv. Sci. Technol. 2020, 61, 102075. [Google Scholar] [CrossRef]
- Awad, H.; Rawas-qalaji, M.; El Hosary, R.; Jagal, J.; Ahmed, I.S. Formulation and Optimization of Ivermectin Nanocrystals for Enhanced Topical Delivery. Int. J. Pharm. X 2023, 6, 100210. [Google Scholar] [CrossRef]
- Faisal, W.; Soliman, G.M.; Hamdan, A.M. Enhanced Skin Deposition and Delivery of Voriconazole Using Ethosomal Preparations Waleed. J. Liposome Res. 2018, 28, 14–21. [Google Scholar] [CrossRef]
- Ahad, A.; Raish, M.; Al-mohizea, A.M.; Al-jenoobi, F.I.; Alam, M.A. Enhanced Anti-Inflammatory Activity of Carbopol Loaded Meloxicam Nanoethosomes Gel. Int. J. Biol. Macromol. 2014, 67, 99–104. [Google Scholar] [CrossRef]
- Peram, M.R.; Jalalpure, S.; Kumbar, V.; Patil, S.; Joshi, S.; Bhat, K.; Diwan, P. Factorial Design Based Curcumin Ethosomal Nanocarriers for the Skin Cancer Delivery: In Vitro Evaluation. J. Liposome Res. 2019, 29, 291–311. [Google Scholar] [CrossRef]
- Wilson, V.; Siram, K.; Rajendran, S.; Sankar, V. Development and Evaluation of Finasteride Loaded Ethosomes for Targeting to the Pilosebaceous Unit. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1892–1901. [Google Scholar] [CrossRef]
- Ahad, A.; Aqil, M.; Kohli, K.; Sultana, Y.; Mujeeb, M. Enhanced Transdermal Delivery of an Anti-Hypertensive Agent via Nanoethosomes: Statistical Optimization, Characterization and Pharmacokinetic Assessment. Int. J. Pharm. 2013, 443, 26–38. [Google Scholar] [CrossRef]
- Mbah, C.C.; Builders, P.F.; Attama, A.A. Nanovesicular Carriers as Alternative Drug Delivery Systems: Ethosomes in Focus. Expert Opin. Drug Deliv. 2014, 11, 45–59. [Google Scholar] [CrossRef]
- Teaima, M.; Abdelmonem, R.; Adel, Y.A.; El-Nabarawi, M.A.; El-Nawawy, T.M. Transdermal Delivery of Telmisartan: Formulation, in Vitro, Ex Vivo, Iontophoretic Permeation Enhancement and Comparative Pharmacokinetic Study in Rats. Drug Des. Devel. Ther. 2021, 15, 4603–4614. [Google Scholar] [CrossRef]
- Khalil, R.M.; Abdelbary, G.A.; Basha, M.; Awad, G.E.A.; El-Hashemy, H.A. Enhancement of Lomefloxacin Hcl Ocular Efficacy via Niosomal Encapsulation: In Vitro Characterization and in Vivo Evaluation. J. Liposome Res. 2017, 27, 312–323. [Google Scholar] [CrossRef]
- Nasri, S.; Ebrahimi-Hosseinzadeh, B.; Rahaie, M.; Hatamian-Zarmi, A.; Sahraeian, R. Thymoquinone—Loaded Ethosome with Breast Cancer Potential: Optimization, in Vitro and Biological Assessment. J. Nanostructure Chem. 2020, 10, 19–31. [Google Scholar] [CrossRef]
- Kandil, S.M.; Soliman, I.I.; Diab, H.M.; Bedair, N.I.; Mahrous, M.H.; Abdoua, E.M. Magnesium Ascorbyl Phosphate Vesicular Carriers for Topical Delivery; Preparation, in-Vitro and Ex-Vivo Evaluation, Factorial Optimization and Clinical Assessment in Melasma Patients. Drug Deliv. 2022, 29, 534–547. [Google Scholar] [CrossRef]
- Jain, S.; Patel, N.; Madan, P.; Lin, S. Quality by Design Approach for Formulation, Evaluation and Statistical Optimization of Diclofenac-Loaded Ethosomes via Transdermal Route. Pharm. Dev. Technol. 2015, 20, 473–489. [Google Scholar] [CrossRef]
- Hinze, W.L.; Pramauro, E. A Critical Review of Surfactant-Mediated Phase Separations (Cloud-Point Extractions): Theory and Applications. Crit. Rev. Anal. Chem. 1993, 24, 133–177. [Google Scholar] [CrossRef]
- Olejnik, A.; Kapuscinska, A.; Schroeder, G.; Nowak, I. Physico—Chemical Characterization of Formulations Containing Endomorphin—2 Derivatives. Amino Acids 2017, 49, 1719–1731. [Google Scholar] [CrossRef]
- Hassan, A.S.; Hofni, A.; Abourehab, M.A.S.; Abdel-Rahman, I.A. Ginger Extract—Loaded Transethosomes for Effective Transdermal Permeation and Anti- Inflammation in Rat Model. Int. J. Nanomed. 2023, 18, 1259–1280. [Google Scholar] [CrossRef]
- Costa, P.; Lobo, J.M.S. Modeling and Comparison of Dissolution Profiles. Eur. J. Pharm. Sci. 2001, 13, 123–133. [Google Scholar] [CrossRef]
- Sabry, S.A.; Abd El Razek, A.M.; Nabil, M.; Khedr, S.M.; El-nahas, H.M. Brain-Targeted Delivery of Valsartan Using Solid Lipid Nanoparticles Labeled with Rhodamine B; a Promising Technique for Mitigating the Negative Effects of Stroke. Drug Deliv. 2023, 30, 2179127. [Google Scholar] [CrossRef]
- Gutiérrez-Saucedo, R.A.; Gómez-López, J.C.; Villanueva-Briseño, A.A.; Topete, A.; Soltero-Martínez, J.F.A.; Mendizábal, E.; Jasso-Gastinel, C.F.; Taboada, P.; Figueroa-Ochoa, E.B. Pluronic F127 and P104 Polymeric Micelles as Efficient Nanocarriers for Loading and Release of Single and Dual Antineoplastic Drugs. Polymers 2023, 15, 2249. [Google Scholar] [CrossRef]
- Madni, A.; Rahim, M.A.; Mahmood, M.A.; Jabar, A.; Rehman, M.; Shah, H.; Khan, A.; Tahir, N.; Shah, A. Enhancement of Dissolution and Skin Permeability of Pentazocine by Proniosomes and Niosomal Gel. AAPS PharmSciTech 2018, 19, 1544–1553. [Google Scholar] [CrossRef]
- Mohapatra, S.; Mirza, M.A.; Ahmad, S.; Farooq, U.; Ansari, M.J.; Kohli, K.; Iqbal, Z. Quality by Design Assisted Optimization and Risk Assessment of Black Cohosh Loaded Ethosomal Gel for Menopause: Investigating Different Formulation and Process Variables. Pharm. Artic. 2023, 15, 465. [Google Scholar] [CrossRef]
- Farooq, M.; Usman, F.; Zaib, S.; Shah, H.S.; Jamil, Q.A.; Sheikh, F.A.; Khan, A.; Rabea, S.; Hagras, S.A.A.; Batiha, G.E.-S.; et al. Fabrication and Evaluation of Voriconazole Loaded Transethosomal Gel for Enhanced Antifungal and Antileishmanial Activity. Mol. Artic. 2022, 27, 3347. [Google Scholar] [CrossRef]
- Asghar, Z.; Jamshaid, T.; Sajid-ur-rehman, M.; Jamshaid, U.; Gad, H.A. Novel Transethosomal Gel Containing Miconazole Nitrate; Development, Characterization, and Enhanced Antifungal Activity. Pharmaceutics 2023, 15, 2537. [Google Scholar] [CrossRef]
- Hani, U.; Osmani, R.A.M.; Alqahtani, A.; Ghazwani, M.; Rahamathulla, M.; Almordy, S.A.; Alsaleh, H.A. 23 Full Factorial Design for Formulation and Evaluation of Floating Oral In Situ Gelling System of Piroxicam. J. Pharm. Innov. 2021, 16, 528–536. [Google Scholar] [CrossRef]
- Abouelatta, S.M.; Aboelwafa, A.A.; El-gazayerly, O.N. Gastroretentive Raft Liquid Delivery System as a New Approach to Release Extension for Carrier-Mediated Drug. Drug Deliv. 2018, 25, 1161–1174. [Google Scholar] [CrossRef]
- El Zaafarany, G.M.; Awad, G.A.S.; Holayel, S.M.; Mortada, N.D. Role of Edge Activators and Surface Charge in Developing Ultradeformable Vesicles with Enhanced Skin Delivery. Int. J. Pharm. 2010, 397, 164–172. [Google Scholar] [CrossRef]
- Rolim, L.A.; dos Santos, F.C.M.; Chaves, L.L.; Gonc¸alves, M.L.C.M.; Freitas-Neto, J.L.; da Silva do Nascimento, A.L.; Soares-Sobrinho, J.L.; de Albuquerque, M.M.; do Carmo Alves de Lima, M.; Rolim-Neto, P.J. Preformulation Study of Ivermectin Raw Material. J. Therm. Anal. Calorim. 2015, 120, 807–816. [Google Scholar] [CrossRef]
- Nair, R.S.; Billa, N.; Mooi, L.Y.; Morris, A.P. Characterization and Ex Vivo Evaluation of Curcumin Nanoethosomes for Melanoma Treatment. Pharm. Dev. Technol. 2022, 27, 72–82. [Google Scholar] [CrossRef]
- Yanyu, X.; Yunmei, S.; Zhipeng, C.; Qineng, P. The Preparation of Silybin—Phospholipid Complex and the Study on Its Pharmacokinetics in Rats. Int. J. Pharm. 2006, 307, 77–82. [Google Scholar] [CrossRef]
- Ahmed, S.; Kassem, M.A.; Sayed, S. Bilosomes as Promising Nanovesicular Carriers for Improved Transdermal Delivery: Construction, in Vitro Optimization, Ex Vivo Permeation and in Vivo Evaluation. Int. J. Nanomed. 2020, 15, 9783–9798. [Google Scholar] [CrossRef]
- Meng, X.; Pan, Q.; Ding, Y.; Jiang, L. Rapid Determination of Phospholipid Content of Vegetable Oils by FTIR Spectroscopy Combined with Partial Least-Square Regression. Food Chem. 2014, 147, 272–278. [Google Scholar] [CrossRef]
- El-sayed, M.M.; Hussein, A.K.; Sarhan, H.A.; Mansour, H.F. Flurbiprofen-Loaded Niosomes-in-Gel System Improves the Ocular Bioavailability of Flurbiprofen in the Aqueous Humor. Drug Dev. Ind. Pharm. 2017, 43, 902–910. [Google Scholar] [CrossRef]
- Verma, P.; Pathak, K. Nanosized Ethanolic Vesicles Loaded with Econazole Nitrate for the Treatment of Deep Fungal Infections through Topical Gel Formulation. Nanomed. Nanotechnol. Biol. Med. 2012, 8, 489–496. [Google Scholar] [CrossRef]
- Ibrahim, T.M.; Abdallah, M.H.; El-Megrab, N.A.; El-Nahas, H.M. Transdermal Ethosomal Gel Nanocarriers; a Promising Strategy for Enhancement of Anti- Hypertensive Effect of Carvedilol. J. Liposome Res. 2019, 29, 215–228. [Google Scholar] [CrossRef]
- Kogan, A.; Garti, N. Microemulsions as Transdermal Drug Delivery Vehicles. Adv. Colloid Interface Sci. 2006, 126, 369–385. [Google Scholar] [CrossRef] [PubMed]










| Independent Factors | Factors Symbol | Unit | Factors Type | Factors Levels Low (−1) High (+1) | |
| SPC concentration | A | % w/v | Numeric | 2 | 4 |
| Ethanol concentration | B | % v/v | Numeric | 10 | 30 |
| Span 60 amount | C | mg | Numeric | 15 | 35 |
| Dependent responses | Responses symbol | Unit | Desirability constraints (goals) | ||
| Entrapment efficiency | Y1 | % | Maximize | ||
| Particle size | Y2 | nm | Minimize | ||
| Polydispersity index | Y3 | --- | Minimize | ||
| Zeta potential | Y4 | mV | Maximize (as absolute value) | ||
| Cumulative amount of drug release after 6 h | Y5 | % | Maximize | ||
| Formulation * | A (%) | B (%) | C (mg) | EE% (Y1) | PS (nm) (Y2) | PDI (Y3) | ZP (mV) (Y4) | Q6h (Y5) |
|---|---|---|---|---|---|---|---|---|
| TESM1 | 2 | 10 | 15 | 88.55 ± 0.576 | 363.267 ± 28.74 | 0.636 ± 0.096 | −54.20 ± 2.08 | 66.20 ± 0.30 |
| TESM2 | 2 | 30 | 15 | 90.71 ± 0.520 | 318.033 ± 45.61 | 0.559 ± 0.081 | −57.13 ± 0.12 | 73.22 ± 1.70 |
| TESM3 | 4 | 10 | 15 | 91.10 ± 0.721 | 423.567 ± 35.38 | 0.616 ± 0.113 | −56.23 ± 1.30 | 86.03 ± 0.27 |
| TESM4 | 4 | 30 | 15 | 93.40 ± 0.393 | 350.500 ± 43.25 | 0.328 ± 0.139 | −60.50 ± 2.34 | 91.46 ± 1.50 |
| TESM5 | 2 | 10 | 35 | 90.37 ± 0.416 | 415.200 ± 24.45 | 0.573 ± 0.257 | −54.13 ± 1.09 | 67.78 ± 1.01 |
| TESM6 | 2 | 30 | 35 | 92.34 ± 0.603 | 357.633 ± 31.81 | 0.671 ± 0.103 | −57.43 ± 1.01 | 75.79 ± 0.50 |
| TESM7 | 4 | 10 | 35 | 93.19 ± 0.363 | 561.400 ± 45.17 | 0.453 ± 0.164 | −55.47 ± 0.51 | 87.80 ± 0.68 |
| TESM8 | 4 | 30 | 35 | 94.13 ± 0.305 | 483.733 ± 37.99 | 0.526 ± 0.157 | −59.50 ± 0.73 | 93.46 ± 0.86 |
| Measured Responses | Y1 (%) | Y2 (nm) | Y3 | Y4 (mV) | Y5 (%) |
|---|---|---|---|---|---|
| Suggested model | linear | 2-FI | 2-FI | linear | linear |
| p-value | p-value | p-value | p-value | p-value | |
| Model A B C AB AC BC | 0.0012 * 0.0009 * 0.0027 * 0.0049 * --- --- --- | 0.024 * 0.0135 * 0.0194 * 0.0136 * 0.1018 0.0274 * 0.2727 | 0.4064 0.2202 0.4866 0.7299 0.4249 0.9522 0.2126 | 0.0015 * 0.0039 * 0.0006 * 0.3565 --- --- --- | <0.0001 * <0.0001 * 0.0002 * 0.0198 * --- --- --- |
| Significant factors | A, B, C | A, B, C, AC | --- | A, B | A, B, C |
| R2 | 0.9746 | 0.9998 | 0.9498 | 0.9712 | 0.9973 |
| Adequate precision | 21.0938 | 95.9126 | 5.5760 | 16.9278 | 52.147 |
| Adjusted R2 | 0.9556 | 0.9988 | 0.6484 | 0.9496 | 0.9952 |
| Predicted R2 | 0.8986 | 0.9895 | −2.2148 | 0.8848 | 0.9891 |
| Release Model | R2 | |||||||
|---|---|---|---|---|---|---|---|---|
| TESM1 | TESM2 | TESM3 | TESM4 | TESM5 | TESM6 | TESM7 | TESM8 | |
| Zero-order | −0.6073 | −0.5437 | −0.6545 | −0.5404 | −0.6271 | −0.5637 | −0.1609 | −0.7089 |
| First-order | 0.2456 | 0.4177 | 0.7239 | 0.8571 | 0.2252 | 0.4456 | 0.7882 | 0.9322 |
| Higuchi | 0.5096 | 0.5410 | 0.4751 | 0.5425 | 0.4919 | 0.5265 | 0.7311 | 0.4510 |
| Korsmeyer–Peppas | 0.9982 | 0.9959 | 0.9994 | 0.9992 | 0.9994 | 0.9992 | 0.9992 | 0.9971 |
| Hixson–Crowell | 0.0244 | 0.2121 | 0.4898 | 0.6218 | 0.0047 | 0.2545 | 0.6701 | 0.5899 |
| Best-fit model | Korsmeyer–Peppas | Korsmeyer–Peppas | Korsmeyer–Peppas | Korsmeyer–Peppas | Korsmeyer–Peppas | Korsmeyer–Peppas | Korsmeyer–Peppas | Korsmeyer–Peppas |
| n-value of Korsmeyer–Peppas | 0.417 | 0.453 | 0.438 | 0.430 | 0.370 | 0.381 | 0.383 | 0.379 |
| Y1 (%) | Y2 (nm) | Y3 | Y4 (mV) | Y5 (%) | |
|---|---|---|---|---|---|
| Predicted values | 93.093 | 351.467 | 0.351 | −59.932 | 91.963 |
| Observed values | 93.4 | 350.5 | 0.328 | −60.5 | 91.46 |
| % Relative error | 0.329 | 0.275 | 6.55 | 0.947 | 0.547 |
| Animal No. | Placebo Cream | Marketed IVM-Cream | IVM-Loaded Transethosomal Cream | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 24 h | 48 h | 72 h | 10 Days | 24 h | 48 h | 72 h | 10 Days | 24 h | 48 h | 72 h | 10 Days | |
| 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 1 | 1 | 2 | 2 | 0 | 1 | 1 | 1 |
| 3 | 0 | 0 | 0 | 0 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 1 |
| 4 | 0 | 0 | 0 | 0 | 1 | 2 | 2 | 1 | 0 | 0 | 0 | 0 |
| 5 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 3 | 0 | 0 | 0 | 0 |
| 6 | 0 | 0 | 0 | 0 | 2 | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
| PII* | 0 | 0 | 0 | 0 | 1.17 | 1.5 | 1.67 | 1.67 | 0.33 | 0.5 | 0.5 | 0.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alyami, M.H.; Alyami, H.S.; Abdo, A.M.; A. Sabry, S.; El-Nahas, H.M.; Ayoub, M.M. Maximizing the Use of Ivermectin Transethosomal Cream in the Treatment of Scabies. Pharmaceutics 2024, 16, 1026. https://doi.org/10.3390/pharmaceutics16081026
Alyami MH, Alyami HS, Abdo AM, A. Sabry S, El-Nahas HM, Ayoub MM. Maximizing the Use of Ivermectin Transethosomal Cream in the Treatment of Scabies. Pharmaceutics. 2024; 16(8):1026. https://doi.org/10.3390/pharmaceutics16081026
Chicago/Turabian StyleAlyami, Mohammad H., Hamad S. Alyami, Asmaa M. Abdo, Shereen A. Sabry, Hanan M. El-Nahas, and Margrit M. Ayoub. 2024. "Maximizing the Use of Ivermectin Transethosomal Cream in the Treatment of Scabies" Pharmaceutics 16, no. 8: 1026. https://doi.org/10.3390/pharmaceutics16081026
APA StyleAlyami, M. H., Alyami, H. S., Abdo, A. M., A. Sabry, S., El-Nahas, H. M., & Ayoub, M. M. (2024). Maximizing the Use of Ivermectin Transethosomal Cream in the Treatment of Scabies. Pharmaceutics, 16(8), 1026. https://doi.org/10.3390/pharmaceutics16081026








