Emerging Trends and Innovations in the Treatment and Diagnosis of Atherosclerosis and Cardiovascular Disease: A Comprehensive Review towards Healthier Aging
Abstract
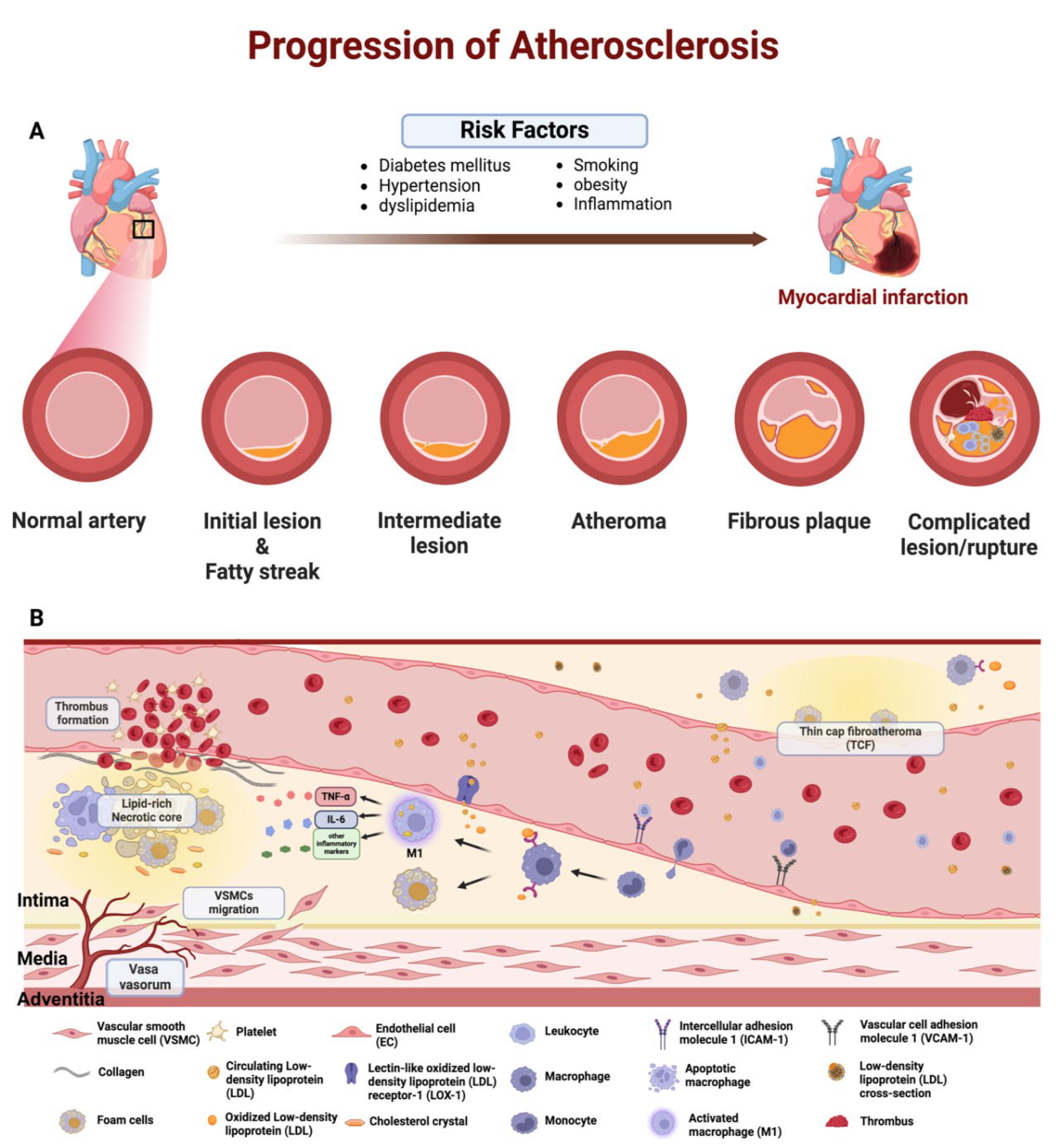
1. Introduction
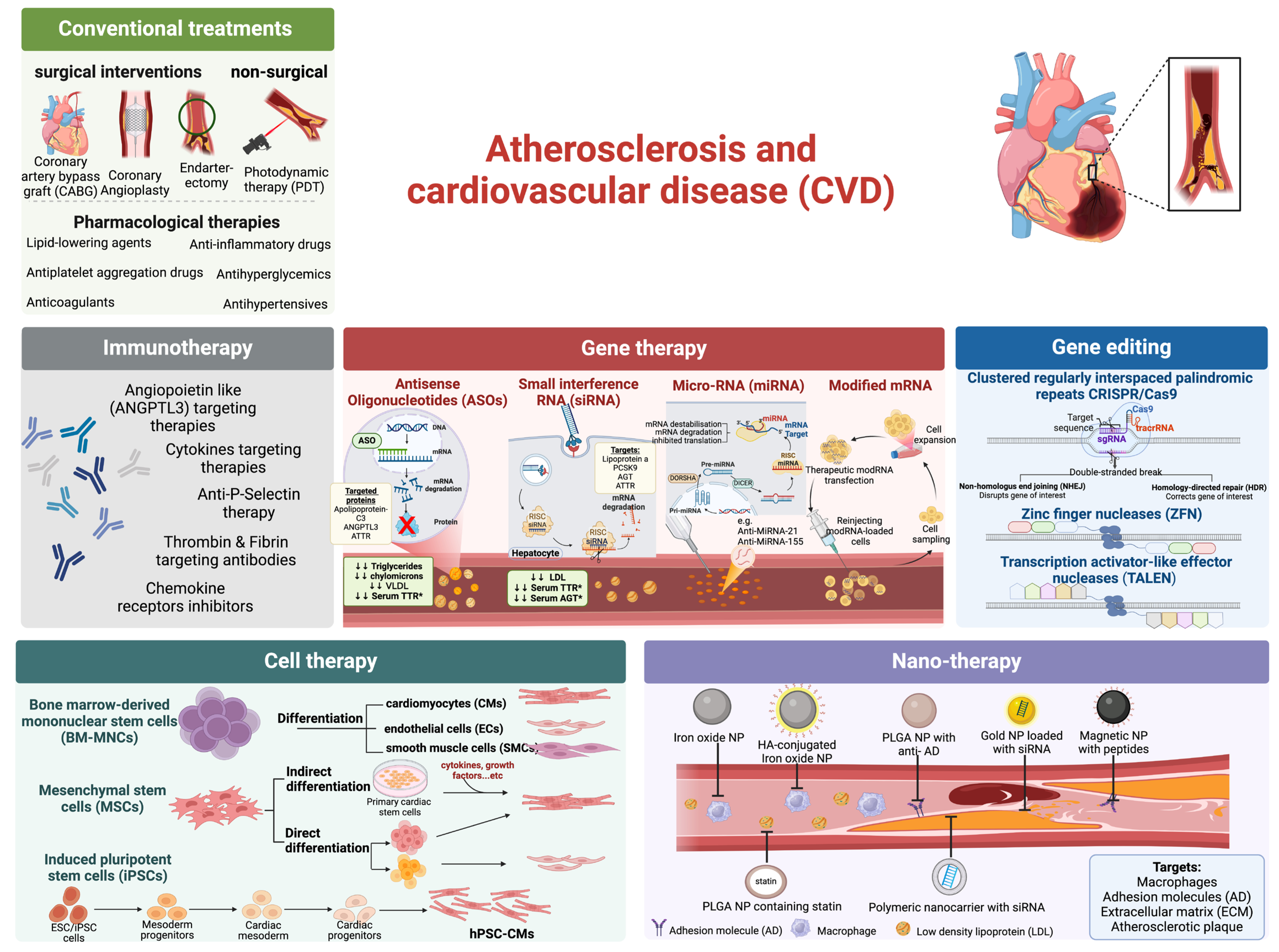
2. Atherosclerotic Treatment
2.1. Lipid Metabolism Drugs
2.2. Antiplatelet Aggregation Drugs
2.3. Antihypertensive Drugs
2.4. Anticoagulant Drugs
2.5. Sugar Metabolism Drugs
2.6. Cytokine-Targeting Therapy
2.7. Anti-P-Selectin Therapy
2.8. Angiopoietin like (ANGPTL3) Targeting Agents
2.9. Targeting Plaque-Weakening Proteases
2.10. Targeting Thrombin and Fibrin
2.11. Photodynamic Therapy (PDT)
3. Recent Advancements in Cell Therapy
4. Recent Advancements in Gene Therapy
4.1. Antisense Oligonucleotide (ASO)
4.1.1. ASO Targeting Apolipoprotein C-III
4.1.2. ASO Targeting Angiopoietin-like 3
4.1.3. ASO Targeting Transthyretin Amyloidosis
4.2. Small Interfering RNA (siRNA)
4.2.1. siRNA Targeting Proprotein Convertase Subtilisin/Kexin Type 9 (PCSK9)
4.2.2. siRNA Targeting Transthyretin Amyloidosis
4.2.3. siRNA Targeting Lipoprotein(a)
4.2.4. siRNA Targeting Angiotensinogen
4.3. microRNA Modulating Therapeutics
4.4. Modified mRNA Therapeutics
5. Recent Advancements in Gene Editing Therapeutics
5.1. Editing PCSK9 and ANGPTL3 Genes
5.2. Editing ApoC-III Gene
5.3. Editing TTR Gene
5.4. Challenges in the Development of Gene-Editing Pharmaceuticals
6. Recent Advancements in Immunotherapy
7. Nanotechnology for Targeted Therapy of Atherosclerosis
7.1. Strategies for Active Targeting of Atherosclerosis
7.1.1. Active Targeting of Macrophages
7.1.2. Active Targeting through Adhesion Molecules
7.1.3. Active Targeting of Extracellular Matrix
7.1.4. High-Density Lipoprotein Nanoparticles for Atherosclerotic Plaques Targeting
7.1.5. Hyaluronic Acid (HA) Based Nanoparticles for Atherosclerotic Plaques Targeting
7.1.6. Solid Lipid Nanoparticles for Atherosclerotic Plaques Targeting
8. Recent Advancements in Diagnosis
8.1. Diagnostic Application of Targeted Nanomedicine
8.2. Diagnostic Application of Nanotheranostics
9. Ongoing Clinical Trial on Atherosclerosis Diagnosis and Treatment
10. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Krist, A.H.; Davidson, K.W.; Mangione, C.M.; Barry, M.J.; Cabana, M.; Caughey, A.B.; Donahue, K.; Doubeni, C.A.; Epling, J.W.; Kubik, M. Behavioral counseling interventions to promote a healthy diet and physical activity for cardiovascular disease prevention in adults with cardiovascular risk factors: US Preventive Services Task Force recommendation statement. JAMA 2020, 324, 2069–2075. [Google Scholar] [PubMed]
- Falk, E. Pathogenesis of atherosclerosis. J. Am. Coll. Cardiol. 2006, 47, C7–C12. [Google Scholar] [CrossRef] [PubMed]
- Jinnouchi, H.; Guo, L.; Sakamoto, A.; Torii, S.; Sato, Y.; Cornelissen, A.; Kuntz, S.; Paek, K.H.; Fernandez, R.; Fuller, D. Diversity of macrophage phenotypes and responses in atherosclerosis. Cell. Mol. Life Sci. 2020, 77, 1919–1932. [Google Scholar] [CrossRef]
- Virmani, R.; Kolodgie, F.D.; Burke, A.P.; Farb, A.; Schwartz, S.M. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler. Thromb. Vasc. Biol. 2000, 20, 1262–1275. [Google Scholar] [CrossRef] [PubMed]
- Biswas, I.; Khan, G.A. Endothelial dysfunction in cardiovascular diseases. In Basic and Clinical Understanding of Microcirculation; Intech Open: London, UK, 2020; Volume 10. [Google Scholar]
- Theofilis, P.; Sagris, M.; Oikonomou, E.; Antonopoulos, A.S.; Siasos, G.; Tsioufis, C.; Tousoulis, D. Inflammatory mechanisms contributing to endothelial dysfunction. Biomedicines 2021, 9, 781. [Google Scholar] [CrossRef] [PubMed]
- Khatana, C.; Saini, N.K.; Chakrabarti, S.; Saini, V.; Sharma, A.; Saini, R.V.; Saini, A.K. Mechanistic insights into the oxidized low-density lipoprotein-induced atherosclerosis. Oxidative Med. Cell. Longev. 2020, 2020, 5245308. [Google Scholar] [CrossRef]
- Mushenkova, N.V.; Bezsonov, E.E.; Orekhova, V.A.; Popkova, T.V.; Starodubova, A.V.; Orekhov, A.N. Recognition of Oxidized Lipids by Macrophages and Its Role in Atherosclerosis Development. Biomedicines 2021, 9, 915. [Google Scholar] [CrossRef] [PubMed]
- Yurdagul, A. Crosstalk between Macrophages and Vascular Smooth Muscle Cells in Atherosclerotic Plaque Stability. Arterioscler. Thromb. Vasc. Biol. 2022, 42, 372–380. [Google Scholar] [CrossRef]
- Gerhardt, T.; Haghikia, A.; Stapmanns, P.; Leistner, D.M. Immune mechanisms of plaque instability. Front. Cardiovasc. Med. 2022, 8, 797046. [Google Scholar] [CrossRef]
- Mantella, L.E.; Liblik, K.; Johri, A.M. Vascular imaging of atherosclerosis: Strengths and weaknesses. Atherosclerosis 2021, 319, 42–50. [Google Scholar] [CrossRef] [PubMed]
- D’Abate, F.; Vitale, C. Carotid Atherosclerotic Plaque Ultrasound Assessment: A Pocket Guide for Ultrasound Practitioners; ABC Vascular: Atlanta, GA, USA, 2023. [Google Scholar]
- Blanchard, I.; Vootukuru, N.; Bhattaru, A.; Patil, S.; Rojulpote, C. PET Radiotracers in Atherosclerosis: A Review. Curr. Probl. Cardiol. 2023, 48, 101925. [Google Scholar] [CrossRef] [PubMed]
- Roth, G.A.; Mensah, G.A.; Fuster, V. The global burden of cardiovascular diseases and risks: A compass for global action. J. Am. Coll. Cardiol. 2020, 76, 2980–2981. [Google Scholar] [CrossRef] [PubMed]
- Safiri, S.; Karamzad, N.; Singh, K.; Carson-Chahhoud, K.; Adams, C.; Nejadghaderi, S.A.; Almasi-Hashiani, A.; Sullman, M.J.; Mansournia, M.A.; Bragazzi, N.L. Burden of ischemic heart disease and its attributable risk factors in 204 countries and territories, 1990–2019. Eur. J. Prev. Cardiol. 2022, 29, 420–431. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Alonso, A.; Aparicio, H.J.; Benjamin, E.J.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Cheng, S.; Delling, F.N.; et al. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation 2021, 143, e254–e743. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, X.; Shi, H.; Yu, Y.; Yu, Y.; Li, M.; Chen, R. NLRP3 inflammasome, an immune-inflammatory target in pathogenesis and treatment of cardiovascular diseases. Clin. Transl. Med. 2020, 10, 91–106. [Google Scholar] [CrossRef] [PubMed]
- Pala, R.; Anju, V.T.; Dyavaiah, M.; Busi, S.; Nauli, S.M. Nanoparticle-Mediated Drug Delivery for the Treatment of Cardiovascular Diseases. Int. J. Nanomed. 2020, 15, 3741–3769. [Google Scholar] [CrossRef] [PubMed]
- Prilepskii, A.Y.; Serov, N.S.; Kladko, D.V.; Vinogradov, V.V. Nanoparticle-Based Approaches towards the Treatment of Atherosclerosis. Pharmaceutics 2020, 12, 1056. [Google Scholar] [CrossRef] [PubMed]
- Meng, Q.; Liu, H.; Liu, J.; Pang, Y.; Liu, Q. Advances in immunotherapy modalities for atherosclerosis. Front. Pharmacol. 2022, 13, 1079185. [Google Scholar] [CrossRef]
- Hashimoto, H.; Olson, E.N.; Bassel-Duby, R. Therapeutic approaches for cardiac regeneration and repair. Nat. Rev. Cardiol. 2018, 15, 585–600. [Google Scholar] [CrossRef]
- Abdelnour, S.A.; Xie, L.; Hassanin, A.A.; Zuo, E.; Lu, Y. The Potential of CRISPR/Cas9 Gene Editing as a Treatment Strategy for Inherited Diseases. Front. Cell Dev. Biol. 2021, 9, 699597. [Google Scholar] [CrossRef] [PubMed]
- Grundy, S.M.; Stone, N.J.; Bailey, A.L.; Beam, C.; Birtcher, K.K.; Blumenthal, R.S.; Braun, L.T.; Ferranti, S.d.; Faiella-Tommasino, J.; Forman, D.E.; et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA Guideline on the Management of Blood Cholesterol: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 139, e1046–e1081. [Google Scholar] [CrossRef] [PubMed]
- Mach, F.; Baigent, C.; Catapano, A.L.; Koskinas, K.C.; Casula, M.; Badimon, L.; Chapman, M.J.; De Backer, G.G.; Delgado, V.; Ference, B.A.; et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: Lipid modification to reduce cardiovascular risk: The Task Force for the management of dyslipidaemias of the European Society of Cardiology (ESC) and European Atherosclerosis Society (EAS). Eur. Heart J. 2019, 41, 111–188. [Google Scholar] [CrossRef] [PubMed]
- Piepoli, M.F.; Hoes, A.W.; Agewall, S.; Albus, C.; Brotons, C.; Catapano, A.L.; Cooney, M.T.; Corrà, U.; Cosyns, B.; Deaton, C.; et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur. Heart J. 2016, 37, 2315–2381. [Google Scholar] [CrossRef] [PubMed]
- Omidi, F.; Rahmannia, M.; Shahidi Bonjar, A.H.; Mohammadsharifi, P.; Nasiri, M.J.; Sarmastzadeh, T. Ezetimibe and atherosclerotic cardiovascular disease: A systematic review and meta-analysis. Front. Cardiovasc. Med. 2023, 10, 1269172. [Google Scholar] [CrossRef] [PubMed]
- Schwartz Gregory, G.; Steg, P.G.; Szarek, M.; Bhatt Deepak, L.; Bittner Vera, A.; Diaz, R.; Edelberg Jay, M.; Goodman Shaun, G.; Hanotin, C.; Harrington Robert, A.; et al. Alirocumab and Cardiovascular Outcomes after Acute Coronary Syndrome. N. Engl. J. Med. 2018, 379, 2097–2107. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Wright, R.S.; Kallend, D.; Koenig, W.; Leiter, L.A.; Raal, F.J.; Bisch, J.A.; Richardson, T.; Jaros, M.; Wijngaard, P.L.J.; et al. Two Phase 3 Trials of Inclisiran in Patients with Elevated LDL Cholesterol. N. Engl. J. Med. 2020, 382, 1507–1519. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Saver, J.L.; Towfighi, A.; Chow, J.; Ovbiagele, B. Efficacy of fibrates for cardiovascular risk reduction in persons with atherogenic dyslipidemia: A meta-analysis. Atherosclerosis 2011, 217, 492–498. [Google Scholar] [CrossRef] [PubMed]
- Capodanno, D.; Angiolillo, D.J. Management of antiplatelet therapy in patients with coronary artery disease requiring cardiac and noncardiac surgery. Circulation 2013, 128, 2785–2798. [Google Scholar] [CrossRef]
- Arnett, D.K.; Blumenthal, R.S.; Albert, M.A.; Buroker, A.B.; Goldberger, Z.D.; Hahn, E.J.; Himmelfarb, C.D.; Khera, A.; Lloyd-Jones, D.; McEvoy, J.W.; et al. 2019 ACC/AHA Guideline on the Primary Prevention of Cardiovascular Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2019, 140, e596–e646. [Google Scholar] [CrossRef]
- Ancion, A.; Tridetti, J.; Nguyen Trung, M.L.; Oury, C.; Lancellotti, P. A Review of the Role of Bradykinin and Nitric Oxide in the Cardioprotective Action of Angiotensin-Converting Enzyme Inhibitors: Focus on Perindopril. Cardiol. Ther. 2019, 8, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Herman, L.L.; Padala, S.A.; Ahmed, I.; Bashir, K. Angiotensin-Converting Enzyme Inhibitors (ACEI). In StatPearls; StatPearls Publishing Copyright © 2024, StatPearls Publishing LLC.: Treasure Island, FL, USA, 2024. [Google Scholar]
- Reynolds, H.R.; Adhikari, S.; Pulgarin, C.; Troxel, A.B.; Iturrate, E.; Johnson, S.B.; Hausvater, A.; Newman, J.D.; Berger, J.S.; Bangalore, S.; et al. Renin-Angiotensin-Aldosterone System Inhibitors and Risk of COVID-19. N. Engl. J. Med. 2020, 382, 2441–2448. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Daniels, G.H.; Brown-Frandsen, K.; Kristensen, P.; Mann, J.F.; Nauck, M.A.; Nissen, S.E.; Pocock, S.; Poulter, N.R.; Ravn, L.S.; et al. Liraglutide and Cardiovascular Outcomes in Type 2 Diabetes. N. Engl. J. Med. 2016, 375, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Zinman, B.; Wanner, C.; Lachin, J.M.; Fitchett, D.; Bluhmki, E.; Hantel, S.; Mattheus, M.; Devins, T.; Johansen, O.E.; Woerle, H.J.; et al. Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N. Engl. J. Med. 2015, 373, 2117–2128. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.K.; La Lee, Y.; Jung, C.H. The Cardiovascular Effect of Tirzepatide: A Glucagon-Like Peptide-1 and Glucose-Dependent Insulinotropic Polypeptide Dual Agonist. J. Lipid Atheroscler. 2023, 12, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Tardif, J.C.; Kouz, S.; Waters, D.D.; Bertrand, O.F.; Diaz, R.; Maggioni, A.P.; Pinto, F.J.; Ibrahim, R.; Gamra, H.; Kiwan, G.S.; et al. Efficacy and Safety of Low-Dose Colchicine after Myocardial Infarction. N. Engl. J. Med. 2019, 381, 2497–2505. [Google Scholar] [CrossRef] [PubMed]
- Aprotosoaie, A.C.; Costache, A.D.; Costache, I.I. Therapeutic Strategies and Chemoprevention of Atherosclerosis: What Do We Know and Where Do We Go? Pharmaceutics 2022, 14, 722. [Google Scholar] [CrossRef]
- Soehnlein, O.; Libby, P. Targeting inflammation in atherosclerosis—from experimental insights to the clinic. Nat. Rev. Drug Discov. 2021, 20, 589–610. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.W.; Huang, L.H.; Randolph, G.J. Cytokine Circuits in Cardiovascular Disease. Immunity 2019, 50, 941–954. [Google Scholar] [CrossRef]
- Weber, C.; Habenicht, A.J.R.; von Hundelshausen, P. Novel mechanisms and therapeutic targets in atherosclerosis: Inflammation and beyond. Eur. Heart J. 2023, 44, 2672–2681. [Google Scholar] [CrossRef]
- Ridker, P.M.; Everett, B.M.; Thuren, T.; MacFadyen, J.G.; Chang, W.H.; Ballantyne, C.; Fonseca, F.; Nicolau, J.; Koenig, W.; Anker, S.D.; et al. Antiinflammatory Therapy with Canakinumab for Atherosclerotic Disease. N. Engl. J. Med. 2017, 377, 1119–1131. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Trankle, C.R.; Buckley, L.F.; Lipinski, M.J.; Appleton, D.; Kadariya, D.; Canada, J.M.; Carbone, S.; Roberts, C.S.; Abouzaki, N.; et al. Interleukin-1 Blockade Inhibits the Acute Inflammatory Response in Patients With ST-Segment-Elevation Myocardial Infarction. J. Am. Heart Assoc. 2020, 9, e014941. [Google Scholar] [CrossRef]
- Rakocevic, J.; Dobric, M.; Borovic, M.L.; Milutinovic, K.; Milenkovic, S.; Tomasevic, M. Anti-Inflammatory Therapy in Coronary Artery Disease: Where Do We Stand? RCM 2023, 24, 10. [Google Scholar] [CrossRef]
- Wada, Y.; Jensen, C.; Meyer, A.S.P.; Zonoozi, A.A.M.; Honda, H. Efficacy and safety of interleukin-6 inhibition with ziltivekimab in patients at high risk of atherosclerotic events in Japan (RESCUE-2): A randomized, double-blind, placebo-controlled, phase 2 trial. J. Cardiol. 2023, 82, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Ridker, P.M.; Everett, B.M.; Pradhan, A.; MacFadyen, J.G.; Solomon, D.H.; Zaharris, E.; Mam, V.; Hasan, A.; Rosenberg, Y.; Iturriaga, E.; et al. Low-Dose Methotrexate for the Prevention of Atherosclerotic Events. N. Engl. J. Med. 2019, 380, 752–762. [Google Scholar] [CrossRef] [PubMed]
- Nidorf, S.M.; Eikelboom, J.W.; Budgeon, C.A.; Thompson, P.L. Low-dose colchicine for secondary prevention of cardiovascular disease. J. Am. Coll. Cardiol. 2013, 61, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Dong, Z.M.; Brown, A.A.; Wagner, D.D. Prominent role of P-selectin in the development of advanced atherosclerosis in ApoE-deficient mice. Circulation 2000, 101, 2290–2295. [Google Scholar] [CrossRef] [PubMed]
- Burger, P.C.; Wagner, D.D. Platelet P-selectin facilitates atherosclerotic lesion development. Blood 2003, 101, 2661–2666. [Google Scholar] [CrossRef] [PubMed]
- Ye, Z.; Zhong, L.; Zhu, S.; Wang, Y.; Zheng, J.; Wang, S.; Zhang, J.; Huang, R. The P-selectin and PSGL-1 axis accelerates atherosclerosis via activation of dendritic cells by the TLR4 signaling pathway. Cell Death Dis. 2019, 10, 507. [Google Scholar] [CrossRef]
- Tanguay, J.F.; Geoffroy, P.; Sirois, M.G.; Libersan, D.; Kumar, A.; Schaub, R.G.; Merhi, Y. Prevention of in-stent restenosis via reduction of thrombo-inflammatory reactions with recombinant P-selectin glycoprotein ligand-1. Thromb. Haemost. 2004, 91, 1186–1193. [Google Scholar] [CrossRef]
- Phillips, J.W.; Barringhaus, K.G.; Sanders, J.M.; Hesselbacher, S.E.; Czarnik, A.C.; Manka, D.; Vestweber, D.; Ley, K.; Sarembock, I.J. Single injection of P-selectin or P-selectin glycoprotein ligand-1 monoclonal antibody blocks neointima formation after arterial injury in apolipoprotein E-deficient mice. Circulation 2003, 107, 2244–2249. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tardif, J.C.; Tanguay, J.F.; Wright, S.R.; Duchatelle, V.; Petroni, T.; Gregoire, J.C.; Ibrahim, R.; Heinonen, T.M.; Robb, S.; Bertrand, O.F.; et al. Effects of the P-selectin antagonist inclacumab on myocardial damage after percutaneous coronary intervention for non-ST-segment elevation myocardial infarction: Results of the SELECT-ACS trial. J. Am. Coll. Cardiol. 2013, 61, 2048–2055. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-Y.; Gao, W.-Y.; Liou, J.-W.; Lin, C.-Y.; Wu, M.-J.; Yen, J.-H. Angiopoietin-Like Protein 3 (ANGPTL3) Modulates Lipoprotein Metabolism and Dyslipidemia. Int. J. Mol. Sci. 2021, 22, 7310. [Google Scholar] [CrossRef]
- Sylvers-Davie, K.L.; Segura-Roman, A.; Salvi, A.M.; Schache, K.J.; Davies, B.S.J. Angiopoietin-like 3 inhibition of endothelial lipase is not modulated by angiopoietin-like 8. J. Lipid Res. 2021, 62, 100112. [Google Scholar] [CrossRef] [PubMed]
- Dewey, F.E.; Gusarova, V.; Dunbar, R.L.; O’Dushlaine, C.; Schurmann, C.; Gottesman, O.; McCarthy, S.; Van Hout, C.V.; Bruse, S.; Dansky, H.M.; et al. Genetic and Pharmacologic Inactivation of ANGPTL3 and Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 211–221. [Google Scholar] [CrossRef] [PubMed]
- Stitziel, N.O.; Khera, A.V.; Wang, X.; Bierhals, A.J.; Vourakis, A.C.; Sperry, A.E.; Natarajan, P.; Klarin, D.; Emdin, C.A.; Zekavat, S.M.; et al. ANGPTL3 Deficiency and Protection Against Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 2054–2063. [Google Scholar] [CrossRef]
- Kersten, S. Angiopoietin-like 3 in lipoprotein metabolism. Nat. Rev. Endocrinol. 2017, 13, 731–739. [Google Scholar] [CrossRef] [PubMed]
- Gusarova, V.; Banfi, S.; Alexa-Braun, C.A.; Shihanian, L.M.; Mintah, I.J.; Lee, J.S.; Xin, Y.; Su, Q.; Kamat, V.; Cohen, J.C.; et al. ANGPTL8 Blockade With a Monoclonal Antibody Promotes Triglyceride Clearance, Energy Expenditure, and Weight Loss in Mice. Endocrinology 2017, 158, 1252–1259. [Google Scholar] [CrossRef]
- Raal, F.J.; Rosenson, R.S.; Reeskamp, L.F.; Hovingh, G.K.; Kastelein, J.J.P.; Rubba, P.; Ali, S.; Banerjee, P.; Chan, K.C.; Gipe, D.A.; et al. Evinacumab for Homozygous Familial Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 711–720. [Google Scholar] [CrossRef]
- Rosenson, R.S.; Burgess, L.J.; Ebenbichler, C.F.; Baum, S.J.; Stroes, E.S.G.; Ali, S.; Khilla, N.; Hamlin, R.; Pordy, R.; Dong, Y.; et al. Evinacumab in Patients with Refractory Hypercholesterolemia. N. Engl. J. Med. 2020, 383, 2307–2319. [Google Scholar] [CrossRef]
- Gaudet, D.; Karwatowska-Prokopczuk, E.; Baum, S.J.; Hurh, E.; Kingsbury, J.; Bartlett, V.J.; Figueroa, A.L.; Piscitelli, P.; Singleton, W.; Witztum, J.L.; et al. Vupanorsen, an N-acetyl galactosamine-conjugated antisense drug to ANGPTL3 mRNA, lowers triglycerides and atherogenic lipoproteins in patients with diabetes, hepatic steatosis, and hypertriglyceridaemia. Eur. Heart J. 2020, 41, 3936–3945. [Google Scholar] [CrossRef] [PubMed]
- Bergmark, B.A.; Marston, N.A.; Bramson, C.R.; Curto, M.; Ramos, V.; Jevne, A.; Kuder, J.F.; Park, J.G.; Murphy, S.A.; Verma, S.; et al. Effect of Vupanorsen on Non-High-Density Lipoprotein Cholesterol Levels in Statin-Treated Patients With Elevated Cholesterol: TRANSLATE-TIMI 70. Circulation 2022, 145, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Hafiane, A. Vulnerable Plaque, Characteristics, Detection, and Potential Therapies. J. Cardiovasc. Dev. Dis. 2019, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Zia, A.; Wu, Y.; Nguyen, T.; Wang, X.; Peter, K.; Ta, H.T. The choice of targets and ligands for site-specific delivery of nanomedicine to atherosclerosis. Cardiovasc. Res. 2020, 116, 2055–2068. [Google Scholar] [CrossRef] [PubMed]
- Newby, A.C. Metalloproteinases and vulnerable atherosclerotic plaques. Trends Cardiovasc. Med. 2007, 17, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, A.; Romero-Perez, D.; Jacobsen, J.A.; Villarreal, F.J.; Cohen, S.M. Zinc-binding groups modulate selective inhibition of MMPs. ChemMedChem 2008, 3, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Hugenberg, V.; Behrends, M.; Wagner, S.; Hermann, S.; Schäfers, M.; Kolb, H.C.; Szardenings, K.; Walsh, J.C.; Gomez, L.F.; Kopka, K.; et al. Synthesis, radiosynthesis, in vitro and first in vivo evaluation of a new matrix metalloproteinase inhibitor based on γ-fluorinated α-sulfonylaminohydroxamic acid. EJNMMI Radiopharm. Chem. 2018, 3, 10. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.P.; Mackman, N. Tissue Factor. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 709–725. [Google Scholar] [CrossRef] [PubMed]
- Mann, K.G.; Brummel, K.; Butenas, S. What is all that thrombin for? J. Thromb. Haemost. 2003, 1, 1504–1514. [Google Scholar] [CrossRef]
- Ząbczyk, M.; Natorska, J.; Undas, A. Fibrin Clot Properties in Atherosclerotic Vascular Disease: From Pathophysiology to Clinical Outcomes. J. Clin. Med. 2021, 10, 2999. [Google Scholar] [CrossRef]
- Borissoff, J.I.; Spronk, H.M.H.; ten Cate, H. The Hemostatic System as a Modulator of Atherosclerosis. N. Engl. J. Med. 2011, 364, 1746–1760. [Google Scholar] [CrossRef] [PubMed]
- Finn, A.V.; Nakano, M.; Narula, J.; Kolodgie, F.D.; Virmani, R. Concept of Vulnerable/Unstable Plaque. Arterioscler. Thromb. Vasc. Biol. 2010, 30, 1282–1292. [Google Scholar] [CrossRef] [PubMed]
- Tung, C.H.; Gerszten, R.E.; Jaffer, F.A.; Weissleder, R. A novel near-infrared fluorescence sensor for detection of thrombin activation in blood. Chembiochem 2002, 3, 207–211. [Google Scholar] [CrossRef] [PubMed]
- Ta, H.T.; Arndt, N.; Wu, Y.; Lim, H.J.; Landeen, S.; Zhang, R.; Kamato, D.; Little, P.J.; Whittaker, A.K.; Xu, Z.P. Activatable magnetic resonance nanosensor as a potential imaging agent for detecting and discriminating thrombosis. Nanoscale 2018, 10, 15103–15115. [Google Scholar] [CrossRef] [PubMed]
- Yusof, N.N.M.; McCann, A.; Little, P.J.; Ta, H.T. Non-invasive imaging techniques for the differentiation of acute and chronic thrombosis. Thromb. Res. 2019, 177, 161–171. [Google Scholar] [CrossRef] [PubMed]
- Raut, S.; Gaffney, P.J. Evaluation of the fibrin binding profile of two anti-fibrin monoclonal antibodies. Thromb. Haemost. 1996, 76, 56–64. [Google Scholar] [CrossRef]
- Putelli, A.; Kiefer, J.D.; Zadory, M.; Matasci, M.; Neri, D. A fibrin-specific monoclonal antibody from a designed phage display library inhibits clot formation and localizes to tumors in vivo. J. Mol. Biol. 2014, 426, 3606–3618. [Google Scholar] [CrossRef] [PubMed]
- Spokojny, A.M.; Serur, J.R.; Skillman, J.; Richard Spears, J. Uptake of hematoporphyrin derivative by atheromatous plaques: Studies in human in vitro and rabbit in vivo. J. Am. Coll. Cardiol. 1986, 8, 1387–1392. [Google Scholar] [CrossRef] [PubMed]
- Wooten, R.S.; Smith, K.C.; Ahlquist, D.A.; Muller, S.A.; Balm, R.K. Prospective study of cutaneous phototoxicity after systemic hematoporphyrin derivative. Lasers Surg. Med. 1988, 8, 294–300. [Google Scholar] [CrossRef]
- Vincent, G.M.; Fox, J.; Charlton, G.; Hill, J.S.; McClane, R.; Spikes, J.D. Presence of blood significantly decreases transmission of 630 nm laser light. Lasers Surg. Med. 1991, 11, 399–403. [Google Scholar] [CrossRef]
- Baskaran, R.; Lee, J.; Yang, S.G. Clinical development of photodynamic agents and therapeutic applications. Biomater. Res. 2018, 22, 25. [Google Scholar] [CrossRef] [PubMed]
- Houthoofd, S.; Vuylsteke, M.; Mordon, S.; Fourneau, I. Photodynamic therapy for atherosclerosis. The potential of indocyanine green. Photodiagnosis Photodyn. Ther. 2020, 29, 101568. [Google Scholar] [CrossRef] [PubMed]
- Hsiang, Y.N.; Todd, M.E.; Bower, R.D. Determining light dose for photodynamic therapy of atherosclerotic lesions in the Yucatan miniswine. J. Endovasc. Surg. 1995, 2, 365–371. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.M.; Woodburn, K.W.; Cheong, W.-F.; Lacy, S.A.; Sudhir, K.; Adelman, D.C.; Wahr, D. Photodynamic therapy: Applications in atherosclerotic vascular disease with motexafin lutetium. Catheter. Cardiovasc. Interv. 2002, 57, 387–394. [Google Scholar] [CrossRef] [PubMed]
- Vaka, R.; Davis, D.R. State-of-play for cellular therapies in cardiac repair and regeneration. Stem Cells 2021, 39, 1579–1588. [Google Scholar] [CrossRef]
- Kasai-Brunswick, T.H.; Carvalho, A.B.; Campos de Carvalho, A.C. Stem cell therapies in cardiac diseases: Current status and future possibilities. World J. Stem Cells 2021, 13, 1231–1247. [Google Scholar] [CrossRef] [PubMed]
- Hosseinpour, A.; Kheshti, F.; Kazemi, A.; Attar, A. Comparing the effect of bone marrow mono-nuclear cells with mesenchymal stem cells after acute myocardial infarction on improvement of left ventricular function: A meta-analysis of clinical trials. Stem Cell Res. Ther. 2022, 13, 203. [Google Scholar] [CrossRef]
- Gilbert, G. Approaches to Optimize Stem Cell-Derived Cardiomyocyte Maturation and Function. Curr. Stem Cell Rep. 2021, 7, 140–160. [Google Scholar] [CrossRef]
- Lin, Y.; Zhu, W.; Chen, X. The involving progress of MSCs based therapy in atherosclerosis. Stem Cell Res. Ther. 2020, 11, 216. [Google Scholar] [CrossRef]
- Meng, W.T.; Guo, H.D. Small Extracellular Vesicles Derived from Induced Pluripotent Stem Cells in the Treatment of Myocardial Injury. Int. J. Mol. Sci. 2023, 24, 4577. [Google Scholar] [CrossRef]
- Wang, J.; An, M.; Haubner, B.J.; Penninger, J.M. Cardiac regeneration: Options for repairing the injured heart. Front. Cardiovasc. Med. 2022, 9, 981982. [Google Scholar] [CrossRef] [PubMed]
- Laflamme, M.A.; Chen, K.Y.; Naumova, A.V.; Muskheli, V.; Fugate, J.A.; Dupras, S.K.; Reinecke, H.; Xu, C.; Hassanipour, M.; Police, S.; et al. Cardiomyocytes derived from human embryonic stem cells in pro-survival factors enhance function of infarcted rat hearts. Nat. Biotechnol. 2007, 25, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, S.; Naumova, A.V.; Zhu, W.Z.; Laflamme, M.A.; Gold, J.; Murry, C.E. Human embryonic stem cell-derived cardiomyocytes engraft but do not alter cardiac remodeling after chronic infarction in rats. J. Mol. Cell Cardiol. 2010, 49, 941–949. [Google Scholar] [CrossRef] [PubMed]
- Miyagawa, S.; Kainuma, S.; Kawamura, T.; Suzuki, K.; Ito, Y.; Iseoka, H.; Ito, E.; Takeda, M.; Sasai, M.; Mochizuki-Oda, N.; et al. Case report: Transplantation of human induced pluripotent stem cell-derived cardiomyocyte patches for ischemic cardiomyopathy. Front. Cardiovasc. Med. 2022, 9, 950829. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.V.A.; Hwang, J.W.; Lee, J.H.; Park, H.J.; Ban, K. Challenges and Limitations of Strategies to Promote Therapeutic Potential of Human Mesenchymal Stem Cells for Cell-Based Cardiac Repair. Korean Circ. J. 2021, 51, 97–113. [Google Scholar] [CrossRef] [PubMed]
- Liew, L.C.; Ho, B.X.; Soh, B.S. Mending a broken heart: Current strategies and limitations of cell-based therapy. Stem Cell Res. Ther. 2020, 11, 138. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, R.E.; Anzai, T.; Chanthra, N.; Uosaki, H. A Brief Review of Current Maturation Methods for Human Induced Pluripotent Stem Cells-Derived Cardiomyocytes. Front. Cell Dev. Biol. 2020, 8, 178. [Google Scholar] [CrossRef] [PubMed]
- Sacchetto, C.; Vitiello, L.; de Windt, L.J.; Rampazzo, A.; Calore, M. Modeling Cardiovascular Diseases with hiPSC-Derived Cardiomyocytes in 2D and 3D Cultures. Int. J. Mol. Sci. 2020, 21, 3404. [Google Scholar] [CrossRef]
- Yang, X.; Rodriguez, M.; Pabon, L.; Fischer, K.A.; Reinecke, H.; Regnier, M.; Sniadecki, N.J.; Ruohola-Baker, H.; Murry, C.E. Tri-iodo-l-thyronine promotes the maturation of human cardiomyocytes-derived from induced pluripotent stem cells. J. Mol. Cell Cardiol. 2014, 72, 296–304. [Google Scholar] [CrossRef]
- Cring, M.R.; Sheffield, V.C. Gene therapy and gene correction: Targets, progress, and challenges for treating human diseases. Gene Ther. 2022, 29, 3–12. [Google Scholar] [CrossRef]
- Bejar, N.; Tat, T.T.; Kiss, D.L. RNA Therapeutics: The Next Generation of Drugs for Cardiovascular Diseases. Curr. Atheroscler. Rep. 2022, 24, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Vermersch, E.; Jouve, C.; Hulot, J.-S. CRISPR/Cas9 gene-editing strategies in cardiovascular cells. Cardiovasc. Res. 2020, 116, 894–907. [Google Scholar] [CrossRef] [PubMed]
- Bennett, C.F. Therapeutic Antisense Oligonucleotides Are Coming of Age. Annu. Rev. Med. 2019, 70, 307–321. [Google Scholar] [CrossRef] [PubMed]
- Biessen, E.A.L.; Van Berkel, T.J.C. N-Acetyl Galactosamine Targeting: Paving the Way for Clinical Application of Nucleotide Medicines in Cardiovascular Diseases. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2855–2865. [Google Scholar] [CrossRef] [PubMed]
- Crooke, S.T. Molecular Mechanisms of Antisense Oligonucleotides. Nucleic Acid. Ther. 2017, 27, 70–77. [Google Scholar] [CrossRef] [PubMed]
- Landmesser, U.; Poller, W.; Tsimikas, S.; Most, P.; Paneni, F.; Lüscher, T.F. From traditional pharmacological towards nucleic acid-based therapies for cardiovascular diseases. Eur. Heart J. 2020, 41, 3884–3899. [Google Scholar] [CrossRef]
- Ramms, B.; Patel, S.; Nora, C.; Pessentheiner, A.R.; Chang, M.W.; Green, C.R.; Golden, G.J.; Secrest, P.; Krauss, R.M.; Metallo, C.M.; et al. ApoC-III ASO promotes tissue LPL activity in the absence of apoE-mediated TRL clearance. J. Lipid Res. 2019, 60, 1379–1395. [Google Scholar] [CrossRef]
- Gouni-Berthold, I.; Alexander, V.J.; Yang, Q.; Hurh, E.; Steinhagen-Thiessen, E.; Moriarty, P.M.; Hughes, S.G.; Gaudet, D.; Hegele, R.A.; O’Dea, L.S.L.; et al. Efficacy and safety of volanesorsen in patients with multifactorial chylomicronaemia (COMPASS): A multicentre, double-blind, randomised, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2021, 9, 264–275. [Google Scholar] [CrossRef]
- Witztum, J.L.; Gaudet, D.; Freedman, S.D.; Alexander, V.J.; Digenio, A.; Williams, K.R.; Yang, Q.; Hughes, S.G.; Geary, R.S.; Arca, M.; et al. Volanesorsen and Triglyceride Levels in Familial Chylomicronemia Syndrome. N. Engl. J. Med. 2019, 381, 531–542. [Google Scholar] [CrossRef]
- Lazarte, J.; Hegele, R.A. Volanesorsen for treatment of familial chylomicronemia syndrome. Expert. Rev. Cardiovasc. Ther. 2021, 19, 685–693. [Google Scholar] [CrossRef]
- Gaudet, D.; Brisson, D.; Tremblay, K.; Alexander, V.J.; Singleton, W.; Hughes, S.G.; Geary, R.S.; Baker, B.F.; Graham, M.J.; Crooke, R.M.; et al. Targeting APOC3 in the familial chylomicronemia syndrome. N. Engl. J. Med. 2014, 371, 2200–2206. [Google Scholar] [CrossRef] [PubMed]
- Alexander, V.J.; Xia, S.; Hurh, E.; Hughes, S.G.; O’Dea, L.; Geary, R.S.; Witztum, J.L.; Tsimikas, S. N-acetyl galactosamine-conjugated antisense drug to APOC3 mRNA, triglycerides and atherogenic lipoprotein levels. Eur. Heart J. 2019, 40, 2785–2796. [Google Scholar] [CrossRef] [PubMed]
- Karwatowska-Prokopczuk, E.; Tardif, J.-C.; Gaudet, D.; Ballantyne, C.M.; Shapiro, M.D.; Moriarty, P.M.; Baum, S.J.; Amour, E.S.; Alexander, V.J.; Xia, S.; et al. Effect of olezarsen targeting APOC-III on lipoprotein size and particle number measured by NMR in patients with hypertriglyceridemia. J. Clin. Lipidol. 2022, 16, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, Y.; Jiao, X.; Yu, H.; Du, Y.; Zhang, M.; Hu, C.; Wei, Y.; Qin, Y. The Clinical Role of Angiopoietin-Like Protein 3 in Evaluating Coronary Artery Disease in Patients with Obstructive Sleep Apnea. Cardiovasc. Drugs Ther. 2020, 34, 773–780. [Google Scholar] [CrossRef]
- Minicocci, I.; Santini, S.; Cantisani, V.; Stitziel, N.; Kathiresan, S.; Arroyo, J.A.; Martí, G.; Pisciotta, L.; Noto, D.; Cefalù, A.B.; et al. Clinical characteristics and plasma lipids in subjects with familial combined hypolipidemia: A pooled analysis. J. Lipid Res. 2013, 54, 3481–3490. [Google Scholar] [CrossRef] [PubMed]
- Akcea, T. A Phase 2 Open-Label Study to Assess the Pharmacodynamics, Pharmacokinetics, Safety and Tolerability of AKCEA-ANGPTL3-LRx (ISIS 703802) Administered Subcutaneously to Patients with Familial Chylomicronemia Syndrome (FCS); NCT03360747; clinicaltrials.gov: 2020/12/11/2020. Available online: https://clinicaltrials.gov/study/NCT03360747 (accessed on 27 May 2024).
- Lim, G.B. ANGPTL3: A therapeutic target for atherosclerosis. Nat. Rev. Cardiol. 2017, 14, 381. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Zheng, W.; Zheng, S.; Yuan, Y.; Wen, W.; Cui, W.; Xue, L.; Sun, X.; Shang, H.; Zhang, H.; et al. Targeting ANGPTL3 by GalNAc-conjugated siRNA ANGsiR10 lowers blood lipids with long-lasting and potent efficacy in mice and monkeys. Mol. Ther.-Nucleic Acids 2023, 31, 68–77. [Google Scholar] [CrossRef] [PubMed]
- Akoumianakis, I.; Zvintzou, E.; Kypreos, K.; Filippatos, T.D. ANGPTL3 and Apolipoprotein C-III as Novel Lipid-Lowering Targets. Curr. Atheroscler. Rep. 2021, 23, 20. [Google Scholar] [CrossRef] [PubMed]
- Di Stefano, V.; Fava, A.; Gentile, L.; Guaraldi, P.; Leonardi, L.; Poli, L.; Tagliapietra, M.; Vastola, M.; Fanara, S.; Ferrero, B.; et al. Italian Real-Life Experience of Patients with Hereditary Transthyretin Amyloidosis Treated with Patisiran. Pharmacogenomics Pers. Med. 2022, 15, 499–514. [Google Scholar] [CrossRef]
- Viney, N.J.; Guo, S.; Tai, L.-J.; Baker, B.F.; Aghajan, M.; Jung, S.W.; Yu, R.Z.; Booten, S.; Murray, H.; Machemer, T.; et al. Ligand conjugated antisense oligonucleotide for the treatment of transthyretin amyloidosis: Preclinical and phase 1 data. ESC Heart Fail. 2021, 8, 652–661. [Google Scholar] [CrossRef]
- Merlo, M.; Pagura, L.; Porcari, A.; Cameli, M.; Vergaro, G.; Musumeci, B.; Biagini, E.; Canepa, M.; Crotti, L.; Imazio, M.; et al. Unmasking the prevalence of amyloid cardiomyopathy in the real world: Results from Phase 2 of the AC-TIVE study, an Italian nationwide survey. Eur. J. Heart Fail. 2022, 24, 1377–1386. [Google Scholar] [CrossRef] [PubMed]
- Benson, M.D.; Waddington-Cruz, M.; Berk, J.L.; Polydefkis, M.; Dyck, P.J.; Wang, A.K.; Planté-Bordeneuve, V.; Barroso, F.A.; Merlini, G.; Obici, L.; et al. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N. Engl. J. Med. 2018, 379, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Ackermann, E.J.; Guo, S.; Benson, M.D.; Booten, S.; Freier, S.; Hughes, S.G.; Kim, T.-W.; Jesse Kwoh, T.; Matson, J.; Norris, D.; et al. Suppressing transthyretin production in mice, monkeys and humans using 2nd-Generation antisense oligonucleotides. Amyloid 2016, 23, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, C.; Sharma, A.R.; Sharma, G.; Doss, C.G.P.; Lee, S.-S. Therapeutic miRNA and siRNA: Moving from Bench to Clinic as Next Generation Medicine. Mol. Ther.-Nucleic Acids 2017, 8, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Zhong, L.; Weng, Y.; Peng, L.; Huang, Y.; Zhao, Y.; Liang, X.-J. Therapeutic siRNA: State of the art. Signal Transduct. Target. Ther. 2020, 5, 101. [Google Scholar] [CrossRef] [PubMed]
- Ranasinghe, P.; Addison, M.L.; Dear, J.W.; Webb, D.J. Small interfering RNA: Discovery, pharmacology and clinical development—An introductory review. Br. J. Pharmacol. 2023, 180, 2697–2720. [Google Scholar] [CrossRef] [PubMed]
- Iwakawa, H.-o.; Tomari, Y. Life of RISC: Formation, action, and degradation of RNA-induced silencing complex. Mol. Cell 2022, 82, 30–43. [Google Scholar] [CrossRef] [PubMed]
- Burnett, J.C.; Rossi, J.J.; Tiemann, K. Current progress of siRNA/shRNA therapeutics in clinical trials. Biotechnol. J. 2011, 6, 1130–1146. [Google Scholar] [CrossRef] [PubMed]
- Stoekenbroek, R.M.; Kallend, D.; Wijngaard, P.L.J.; Kastelein, J.J.P. Inclisiran for the treatment of cardiovascular disease: The ORION clinical development program. Future Cardiol. 2018, 14, 433–442. [Google Scholar] [CrossRef]
- Sarzani, R.; Spannella, F.; Di Pentima, C.; Giulietti, F.; Landolfo, M.; Allevi, M. Molecular Therapies in Cardiovascular Diseases: Small Interfering RNA in Atherosclerosis, Heart Failure, and Hypertension. Int. J. Mol. Sci. 2024, 25, 328. [Google Scholar] [CrossRef]
- Leiter, L.A.; Teoh, H.; Kallend, D.; Wright, R.S.; Landmesser, U.; Wijngaard, P.L.J.; Kastelein, J.J.P.; Ray, K.K. Inclisiran Lowers LDL-C and PCSK9 Irrespective of Diabetes Status: The ORION-1 Randomized Clinical Trial. Diabetes Care 2018, 42, 173–176. [Google Scholar] [CrossRef] [PubMed]
- Catapano, A.L.; Pirillo, A.; Norata, G.D. Insights from ORION studies: Focus on inclisiran safety. Cardiovasc. Res. 2021, 117, 24–26. [Google Scholar] [CrossRef] [PubMed]
- Koenig, W.; Conde, L.G.; Landmesser, U.; Leiter, L.A.; Ray, K.K.; Schwartz, G.G.; Wright, R.S.; Han, J.; Raal, F.J. Efficacy and Safety of Inclisiran in Patients with Polyvascular Disease: Pooled, Post Hoc Analysis of the ORION-9, ORION-10, and ORION-11 Phase 3 Randomized Controlled Trials. Cardiovasc. Drugs Ther. 2022, 38, 493–503. [Google Scholar] [CrossRef] [PubMed]
- Brandts, J.; Ray, K.K. Clinical implications and outcomes of the ORION Phase III trials. Future Cardiol. 2021, 17, 769–777. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, D.; Bonfioli, G.B.; Aimo, A.; Adamo, M.; Canepa, M.; Inciardi, R.M.; Lombardi, C.M.; Nardi, M.; Pagnesi, M.; Riccardi, M.; et al. Treating amyloid transthyretin cardiomyopathy: Lessons learned from clinical trials. Front. Cardiovasc. Med. 2023, 10, 1154594. [Google Scholar] [CrossRef] [PubMed]
- Kristen, A.V.; Ajroud-Driss, S.; Conceição, I.; Gorevic, P.; Kyriakides, T.; Obici, L. Patisiran, an RNAi therapeutic for the treatment of hereditary transthyretin-mediated amyloidosis. Neurodegener. Dis. Manag. 2019, 9, 5–23. [Google Scholar] [CrossRef] [PubMed]
- Janas, M.M.; Schlegel, M.K.; Harbison, C.E.; Yilmaz, V.O.; Jiang, Y.; Parmar, R.; Zlatev, I.; Castoreno, A.; Xu, H.; Shulga-Morskaya, S.; et al. Selection of GalNAc-conjugated siRNAs with limited off-target-driven rat hepatotoxicity. Nat. Commun. 2018, 9, 723. [Google Scholar] [CrossRef] [PubMed]
- Akinc, A.; Maier, M.A.; Manoharan, M.; Fitzgerald, K.; Jayaraman, M.; Barros, S.; Ansell, S.; Du, X.; Hope, M.J.; Madden, T.D.; et al. The Onpattro story and the clinical translation of nanomedicines containing nucleic acid-based drugs. Nat. Nanotechnol. 2019, 14, 1084–1087. [Google Scholar] [CrossRef]
- Niemietz, C.; Nadzemova, O.; Zibert, A.; Schmidt, H.H.J. APOE polymorphism in ATTR amyloidosis patients treated with lipid nanoparticle siRNA. Amyloid 2020, 27, 45–51. [Google Scholar] [CrossRef]
- Coelho, T.; Adams, D.; Conceição, I.; Waddington-Cruz, M.; Schmidt, H.H.; Buades, J.; Campistol, J.; Berk, J.L.; Polydefkis, M.; Wang, J.J.; et al. A phase II, open-label, extension study of long-term patisiran treatment in patients with hereditary transthyretin-mediated (hATTR) amyloidosis. Orphanet J. Rare Dis. 2020, 15, 179. [Google Scholar] [CrossRef]
- Kamstrup, P.R.; Tybjærg-Hansen, A.; Steffensen, R.; Nordestgaard, B.G. Genetically Elevated Lipoprotein(a) and Increased Risk of Myocardial Infarction. JAMA 2009, 301, 2331–2339. [Google Scholar] [CrossRef]
- Thanassoulis, G.; Campbell, C.Y.; Owens, D.S.; Smith, J.G.; Smith, A.V.; Peloso, G.M.; Kerr, K.F.; Pechlivanis, S.; Budoff, M.J.; Harris, T.B.; et al. Genetic Associations with Valvular Calcification and Aortic Stenosis. N. Engl. J. Med. 2013, 368, 503–512. [Google Scholar] [CrossRef]
- Reyes-Soffer, G.; Ginsberg, H.N.; Berglund, L.; Duell, P.B.; Heffron, S.P.; Kamstrup, P.R.; Lloyd-Jones, D.M.; Marcovina, S.M.; Yeang, C.; Koschinsky, M.L.; et al. Lipoprotein(a): A Genetically Determined, Causal, and Prevalent Risk Factor for Atherosclerotic Cardiovascular Disease: A Scientific Statement From the American Heart Association. Arterioscler. Thromb. Vasc. Biol. 2022, 42, e48–e60. [Google Scholar] [CrossRef] [PubMed]
- Nordestgaard, B.G.; Chapman, M.J.; Ray, K.; Borén, J.; Andreotti, F.; Watts, G.F.; Ginsberg, H.; Amarenco, P.; Catapano, A.; Descamps, O.S.; et al. Lipoprotein(a) as a cardiovascular risk factor: Current status. Eur. Heart J. 2010, 31, 2844–2853. [Google Scholar] [CrossRef] [PubMed]
- Koren, M.J.; Moriarty, P.M.; Baum, S.J.; Neutel, J.; Hernandez-Illas, M.; Weintraub, H.S.; Florio, M.; Kassahun, H.; Melquist, S.; Varrieur, T.; et al. Preclinical development and phase 1 trial of a novel siRNA targeting lipoprotein(a). Nat. Med. 2022, 28, 96–103. [Google Scholar] [CrossRef]
- Nissen, S.E.; Linnebjerg, H.; Shen, X.; Wolski, K.; Ma, X.; Lim, S.; Michael, L.F.; Ruotolo, G.; Gribble, G.; Navar, A.M.; et al. Lepodisiran, an Extended-Duration Short Interfering RNA Targeting Lipoprotein(a): A Randomized Dose-Ascending Clinical Trial. JAMA 2023, 330, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Matsusaka, T.; Niimura, F.; Shimizu, A.; Pastan, I.; Saito, A.; Kobori, H.; Nishiyama, A.; Ichikawa, I. Liver Angiotensinogen Is the Primary Source of Renal Angiotensin II. J. Am. Soc. Nephrol. 2012, 23, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Surma, S.; Oparil, S. Zilebesiran—the first siRNA-based drug in hypertensiology: Why is it needed, and will it change the treatment approach of hypertension? Arter. Hypertens. 2024. [Google Scholar]
- Desai, A.S.; Webb, D.J.; Taubel, J.; Casey, S.; Cheng, Y.; Robbie, G.J.; Foster, D.; Huang, S.A.; Rhyee, S.; Sweetser, M.T.; et al. Zilebesiran, an RNA Interference Therapeutic Agent for Hypertension. N. Engl. J. Med. 2023, 389, 228–238. [Google Scholar] [CrossRef]
- Poller, W.; Dimmeler, S.; Heymans, S.; Zeller, T.; Haas, J.; Karakas, M.; Leistner, D.-M.; Jakob, P.; Nakagawa, S.; Blankenberg, S.; et al. Non-coding RNAs in cardiovascular diseases: Diagnostic and therapeutic perspectives. Eur. Heart J. 2018, 39, 2704–2716. [Google Scholar] [CrossRef]
- Gebert, L.F.R.; MacRae, I.J. Regulation of microRNA function in animals. Nat. Rev. Mol. Cell Biol. 2019, 20, 21–37. [Google Scholar] [CrossRef] [PubMed]
- Samanta, S.; Balasubramanian, S.; Rajasingh, S.; Patel, U.; Dhanasekaran, A.; Dawn, B.; Rajasingh, J. MicroRNA: A new therapeutic strategy for cardiovascular diseases. Trends Cardiovasc. Med. 2016, 26, 407–419. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Zeller, T. microRNA-based diagnostics and therapy in cardiovascular disease—Summing up the facts. Cardiovasc. Diagn. Ther. 2015, 5, 17–36. [Google Scholar] [CrossRef] [PubMed]
- Gallant-Behm, C.L.; Piper, J.; Dickinson, B.A.; Dalby, C.M.; Pestano, L.A.; Jackson, A.L. A synthetic microRNA-92a inhibitor (MRG-110) accelerates angiogenesis and wound healing in diabetic and nondiabetic wounds. Wound Repair. Regen. 2018, 26, 311–323. [Google Scholar] [CrossRef] [PubMed]
- Täubel, J.; Hauke, W.; Rump, S.; Viereck, J.; Batkai, S.; Poetzsch, J.; Rode, L.; Weigt, H.; Genschel, C.; Lorch, U.; et al. Novel antisense therapy targeting microRNA-132 in patients with heart failure: Results of a first-in-human Phase 1b randomized, double-blind, placebo-controlled study. Eur. Heart J. 2021, 42, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://classic.clinicaltrials.gov/ProvidedDocs/20/NCT03514420/Prot_SAP_000.pdf (accessed on 27 May 2024).
- Thum, T.; Gross, C.; Fiedler, J.; Fischer, T.; Kissler, S.; Bussen, M.; Galuppo, P.; Just, S.; Rottbauer, W.; Frantz, S.; et al. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature 2008, 456, 980–984. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Suárez, Y.; Dávalos, A.; Parathath, S.; Fitzgerald, M.L.; Tamehiro, N.; Fisher, E.A.; Moore, K.J.; Fernández-Hernando, C. MiR-33 Contributes to the Regulation of Cholesterol Homeostasis. Science 2010, 328, 1570–1573. [Google Scholar] [CrossRef] [PubMed]
- Corsten, M.F.; Papageorgiou, A.; Verhesen, W.; Carai, P.; Lindow, M.; Obad, S.; Summer, G.; Coort, S.L.M.; Hazebroek, M.; van Leeuwen, R.; et al. MicroRNA Profiling Identifies MicroRNA-155 as an Adverse Mediator of Cardiac Injury and Dysfunction During Acute Viral Myocarditis. Circ. Res. 2012, 111, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Saenz-Pipaon, G.; Dichek, D.A. Targeting and delivery of microRNA-targeting antisense oligonucleotides in cardiovascular diseases. Atherosclerosis 2023, 374, 44–54. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, M.; Sun, S.; Li, B.; Du, D.; Sun, J.; Cao, F.; Li, H.; Jia, F.; Wang, T.; et al. The use of antibody modified liposomes loaded with AMO-1 to deliver oligonucleotides to ischemic myocardium for arrhythmia therapy. Biomaterials 2014, 35, 3697–3707. [Google Scholar] [CrossRef]
- Xue, X.; Shi, X.; Dong, H.; You, S.; Cao, H.; Wang, K.; Wen, Y.; Shi, D.; He, B.; Li, Y. Delivery of microRNA-1 inhibitor by dendrimer-based nanovector: An early targeting therapy for myocardial infarction in mice. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 619–631. [Google Scholar] [CrossRef] [PubMed]
- Talebi, A.; Rahnema, M.; Bigdeli, M.R. The Positive Effect of MiR1 Antagomir on Ischemic Neurological Disorders via Changing the Expression of Bcl-w and Bad Genes. Basic Clin. Neurosci. 2020, 11, 811–820. [Google Scholar] [CrossRef] [PubMed]
- Bellera, N.; Barba, I.; Rodriguez-Sinovas, A.; Ferret, E.; Asín, M.A.; Gonzalez-Alujas, M.T.; Pérez-Rodon, J.; Esteves, M.; Fonseca, C.; Toran, N.; et al. Single intracoronary injection of encapsulated antagomir-92a promotes angiogenesis and prevents adverse infarct remodeling. J. Am. Heart Assoc. 2014, 3, e000946. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.-Y.; Park, H.; Kim, H.; Mun, D.; Park, H.; Yun, N.; Joung, B. Human peripheral blood-derived exosomes for microRNA delivery. Int. J. Mol. Med. 2019, 43, 2319–2328. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ichikawa, T.; Villacorta, L.; Janicki, J.S.; Brower, G.L.; Yamamoto, M.; Cui, T. Nrf2 Protects Against Maladaptive Cardiac Responses to Hemodynamic Stress. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 1843–1850. [Google Scholar] [CrossRef] [PubMed]
- Hinkel, R.; Batkai, S.; Bähr, A.; Bozoglu, T.; Straub, S.; Borchert, T.; Viereck, J.; Howe, A.; Hornaschewitz, N.; Oberberger, L.; et al. AntimiR-132 Attenuates Myocardial Hypertrophy in an Animal Model of Percutaneous Aortic Constriction. J. Am. Coll. Cardiol. 2021, 77, 2923–2935. [Google Scholar] [CrossRef] [PubMed]
- Batkai, S.; Genschel, C.; Viereck, J.; Rump, S.; Bär, C.; Borchert, T.; Traxler, D.; Riesenhuber, M.; Spannbauer, A.; Lukovic, D.; et al. CDR132L improves systolic and diastolic function in a large animal model of chronic heart failure. Eur. Heart J. 2021, 42, 192–201. [Google Scholar] [CrossRef]
- Xu, M.; Zhang, K.; Song, J. Targeted Therapy in Cardiovascular Disease: A Precision Therapy Era. Front. Pharmacol. 2021, 12, 623674. [Google Scholar] [CrossRef] [PubMed]
- Zangi, L.; Lui, K.O.; von Gise, A.; Ma, Q.; Ebina, W.; Ptaszek, L.M.; Später, D.; Xu, H.; Tabebordbar, M.; Gorbatov, R.; et al. Modified mRNA directs the fate of heart progenitor cells and induces vascular regeneration after myocardial infarction. Nat. Biotechnol. 2013, 31, 898–907. [Google Scholar] [CrossRef]
- Gan, L.-M.; Lagerström-Fermér, M.; Carlsson, L.G.; Arfvidsson, C.; Egnell, A.-C.; Rudvik, A.; Kjaer, M.; Collén, A.; Thompson, J.D.; Joyal, J.; et al. Intradermal delivery of modified mRNA encoding VEGF-A in patients with type 2 diabetes. Nat. Commun. 2019, 10, 871. [Google Scholar] [CrossRef]
- Anttila, V.; Saraste, A.; Knuuti, J.; Jaakkola, P.; Hedman, M.; Svedlund, S.; Lagerström-Fermér, M.; Kjaer, M.; Jeppsson, A.; Gan, L.M. Synthetic mRNA Encoding VEGF-A in Patients Undergoing Coronary Artery Bypass Grafting: Design of a Phase 2a Clinical Trial. Mol. Ther. Methods Clin. Dev. 2020, 18, 464–472. [Google Scholar] [CrossRef] [PubMed]
- Musunuru, K. Moving toward genome-editing therapies for cardiovascular diseases. J. Clin. Investig. 2022, 132, e148555. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Yang, Y.; Hong, W.; Huang, M.; Wu, M.; Zhao, X. Applications of genome editing technology in the targeted therapy of human diseases: Mechanisms, advances and prospects. Signal Transduct. Target. Ther. 2020, 5, 1. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Kaur, H.; Kapoor, H.K.; Sharma, R.; Kaur, H.; Kyum, M. Genome Editing: A Review of the Challenges and Approaches. In Genome Editing: Current Technology Advances and Applications for Crop Improvement; Wani, S.H., Hensel, G., Eds.; Springer International Publishing: Cham, Germany, 2022; pp. 71–101. [Google Scholar]
- Roshanravan, N.; Tutunchi, H.; Najafipour, F.; Dastouri, M.; Ghaffari, S.; Jebeli, A. A glance at the application of CRISPR/Cas9 gene-editing technology in cardiovascular diseases. J. Cardiovasc. Thorac. Res. 2022, 14, 77–83. [Google Scholar] [CrossRef]
- Parsamanesh, N.; Kooshkaki, O.; Siami, H.; Santos, R.D.; Jamialahmadi, T.; Sahebkar, A. Gene and cell therapy approaches for familial hypercholesterolemia: An update. Drug Discov. Today 2023, 28, 103470. [Google Scholar] [CrossRef] [PubMed]
- Dong, M.; Liu, J.; Liu, C.; Wang, H.; Sun, W.; Liu, B. CRISPR/CAS9: A promising approach for the research and treatment of cardiovascular diseases. Pharmacol. Res. 2022, 185, 106480. [Google Scholar] [CrossRef] [PubMed]
- Scharenberg, A.M.; Duchateau, P.; Smith, J. Genome engineering with TAL-effector nucleases and alternative modular nuclease technologies. Curr. Gene Ther. 2013, 13, 291–303. [Google Scholar] [CrossRef]
- Bibikova, M.; Beumer, K.; Trautman, J.K.; Carroll, D. Enhancing Gene Targeting with Designed Zinc Finger Nucleases. Science 2003, 300, 764. [Google Scholar] [CrossRef] [PubMed]
- Enache, O.M.; Rendo, V.; Abdusamad, M.; Lam, D.; Davison, D.; Pal, S.; Currimjee, N.; Hess, J.; Pantel, S.; Nag, A.; et al. Cas9 activates the p53 pathway and selects for p53-inactivating mutations. Nat. Genet. 2020, 52, 662–668. [Google Scholar] [CrossRef]
- Dorn, L.E.; Tual-Chalot, S.; Stellos, K.; Accornero, F. RNA epigenetics and cardiovascular diseases. J. Mol. Cell. Cardiol. 2019, 129, 272–280. [Google Scholar] [CrossRef]
- Musunuru, K.; Chadwick, A.C.; Mizoguchi, T.; Garcia, S.P.; DeNizio, J.E.; Reiss, C.W.; Wang, K.; Iyer, S.; Dutta, C.; Clendaniel, V.; et al. In vivo CRISPR base editing of PCSK9 durably lowers cholesterol in primates. Nature 2021, 593, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Lopez, S.A.; Dominguez, F.; Lopes, L.R.; Ochoa, J.P.; Barriales, V.R.; Climent, V.; Linschoten, M.; Tiron, C.; Chiriatti, C.; Marques, N.; et al. Clinical Features and Natural History of PRKAG2 Variant Cardiac Glycogenosis. J. Am. Coll. Cardiol. 2020, 76, 186–197. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.G.; Mazzola, A.M.; Braun, M.C.; Platt, C.; Vafai, S.B.; Kathiresan, S.; Rohde, E.; Bellinger, A.M.; Khera, A.V. Efficacy and Safety of an Investigational Single-Course CRISPR Base-Editing Therapy Targeting PCSK9 in Nonhuman Primate and Mouse Models. Circulation 2023, 147, 242–253. [Google Scholar] [CrossRef]
- Rothgangl, T.; Dennis, M.K.; Lin, P.J.C.; Oka, R.; Witzigmann, D.; Villiger, L.; Qi, W.; Hruzova, M.; Kissling, L.; Lenggenhager, D.; et al. In vivo adenine base editing of PCSK9 in macaques reduces LDL cholesterol levels. Nat. Biotechnol. 2021, 39, 949–957. [Google Scholar] [CrossRef]
- Chadwick, A.C.; Evitt, N.H.; Lv, W.; Musunuru, K. Reduced Blood Lipid Levels With In Vivo CRISPR-Cas9 Base Editing of ANGPTL3. Circulation 2018, 137, 975–977. [Google Scholar] [CrossRef]
- Pechlaner, R.; Tsimikas, S.; Yin, X.; Willeit, P.; Baig, F.; Santer, P.; Oberhollenzer, F.; Egger, G.; Witztum, J.L.; Alexander, V.J.; et al. Very-Low-Density Lipoprotein–Associated Apolipoproteins Predict Cardiovascular Events and Are Lowered by Inhibition of APOC-III. J. Am. Coll. Cardiol. 2017, 69, 789–800. [Google Scholar] [CrossRef]
- Guo, M.; Xu, Y.; Dong, Z.; Zhou, Z.; Cong, N.; Gao, M.; Huang, W.; Wang, Y.; Liu, G.; Xian, X. Inactivation of ApoC3 by CRISPR/Cas9 Protects Against Atherosclerosis in Hamsters. Circ. Res. 2020, 127, 1456–1458. [Google Scholar] [CrossRef]
- Zha, Y.; Lu, Y.; Zhang, T.; Yan, K.; Zhuang, W.; Liang, J.; Cheng, Y.; Wang, Y. CRISPR/Cas9-mediated knockout of APOC3 stabilizes plasma lipids and inhibits atherosclerosis in rabbits. Lipids Health Dis. 2021, 20, 180. [Google Scholar] [CrossRef]
- Finn, J.D.; Smith, A.R.; Patel, M.C.; Shaw, L.; Youniss, M.R.; van Heteren, J.; Dirstine, T.; Ciullo, C.; Lescarbeau, R.; Seitzer, J.; et al. A Single Administration of CRISPR/Cas9 Lipid Nanoparticles Achieves Robust and Persistent In Vivo Genome Editing. Cell Rep. 2018, 22, 2227–2235. [Google Scholar] [CrossRef]
- Gillmore, J.D.; Gane, E.; Taubel, J.; Kao, J.; Fontana, M.; Maitland, M.L.; Seitzer, J.; O’Connell, D.; Walsh, K.R.; Wood, K.; et al. CRISPR-Cas9 In Vivo Gene Editing for Transthyretin Amyloidosis. N. Engl. J. Med. 2021, 385, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Zaib, S.; Saleem, M.A.; Khan, I. CRISPR-Cas9 Genome Engineering: Trends in Medicine and Health. Mini Rev. Med. Chem. 2022, 22, 410–421. [Google Scholar] [CrossRef] [PubMed]
- Bravo, J.P.K.; Liu, M.-S.; Hibshman, G.N.; Dangerfield, T.L.; Jung, K.; McCool, R.S.; Johnson, K.A.; Taylor, D.W. Structural basis for mismatch surveillance by CRISPR–Cas9. Nature 2022, 603, 343–347. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-H.; Tee, L.Y.; Wang, X.-G.; Huang, Q.-S.; Yang, S.-H. Off-target Effects in CRISPR/Cas9-mediated Genome Engineering. Mol. Ther.-Nucleic Acids 2015, 4, e264. [Google Scholar] [CrossRef] [PubMed]
- Kok, F.O.; Lawson, N.D. A Platform for Reverse Genetics in Endothelial Cells. Circ. Res. 2015, 117, 107–108. [Google Scholar] [CrossRef]
- Jiang, F.; Doudna, J.A. CRISPR–Cas9 Structures and Mechanisms. Annu. Rev. Biophys. 2017, 46, 505–529. [Google Scholar] [CrossRef] [PubMed]
- Komor, A.C.; Kim, Y.B.; Packer, M.S.; Zuris, J.A.; Liu, D.R. Programmable editing of a target base in genomic DNA without double-stranded DNA cleavage. Nature 2016, 533, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Gostimskaya, I. CRISPR–Cas9: A History of Its Discovery and Ethical Considerations of Its Use in Genome Editing. Biochem. Mosc. 2022, 87, 777–788. [Google Scholar] [CrossRef] [PubMed]
- Sasidharan Nair, V.; Elkord, E. Immune checkpoint inhibitors in cancer therapy: A focus on T-regulatory cells. Immunol. Cell Biol. 2018, 96, 21–33. [Google Scholar] [CrossRef] [PubMed]
- Poels, K.; van Leent, M.M.; Reiche, M.E.; Kusters, P.J.; Huveneers, S.; de Winther, M.P.; Mulder, W.J.; Lutgens, E.; Seijkens, T.T. Antibody-mediated inhibition of CTLA4 aggravates atherosclerotic plaque inflammation and progression in hyperlipidemic mice. Cells 2020, 9, 1987. [Google Scholar] [CrossRef]
- Poels, K.; van Leent, M.M.; Boutros, C.; Tissot, H.; Roy, S.; Meerwaldt, A.E.; Toner, Y.C.; Reiche, M.E.; Kusters, P.J.; Malinova, T. Immune checkpoint inhibitor therapy aggravates T cell–driven plaque inflammation in atherosclerosis. Cardio Oncol. 2020, 2, 599–610. [Google Scholar] [CrossRef]
- Bao, X.; Liang, Y.; Chang, H.; Cai, T.; Feng, B.; Gordon, K.; Zhu, Y.; Shi, H.; He, Y.; Xie, L. Targeting proprotein convertase subtilisin/kexin type 9 (PCSK9): From bench to bedside. Signal Transduct. Target. Ther. 2024, 9, 13. [Google Scholar] [CrossRef] [PubMed]
- Vroom, M.M.; Lu, H.; Lewis, M.; Thibodeaux, B.A.; Brooks, J.K.; Longo, M.S.; Ramos, M.M.; Sahni, J.; Wiggins, J.; Boyd, J.D. VXX-401, a novel anti-PCSK9 vaccine, reduces LDL-C in cynomolgus monkeys. J. Lipid Res. 2024, 65, 100497. [Google Scholar] [CrossRef] [PubMed]
- Ray, K.K.; Troquay, R.P.; Visseren, F.L.; Leiter, L.A.; Wright, R.S.; Vikarunnessa, S.; Talloczy, Z.; Zang, X.; Maheux, P.; Lesogor, A. Long-term efficacy and safety of inclisiran in patients with high cardiovascular risk and elevated LDL cholesterol (ORION-3): Results from the 4-year open-label extension of the ORION-1 trial. Lancet Diabetes Endocrinol. 2023, 11, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.-L.; Zhu, Q.-Q.; Zhu, L.; Chen, J.-Z.; Chen, Q.-H.; Li, G.-N.; Xie, J.; Kang, L.-N.; Xu, B. Safety and efficacy of anti-PCSK9 antibodies: A meta-analysis of 25 randomized, controlled trials. BMC Med. 2015, 13, 123. [Google Scholar] [CrossRef] [PubMed]
- Tsioufis, P.; Theofilis, P.; Tsioufis, K.; Tousoulis, D. The impact of cytokines in coronary atherosclerotic plaque: Current therapeutic approaches. Int. J. Mol. Sci. 2022, 23, 15937. [Google Scholar] [CrossRef] [PubMed]
- Soroureddin, Z.; Nouri-Vaskeh, M.; Maleki, M.; Baghbanzadeh, A.; Hajiasgharzadeh, K.; Sadeghi, M.T.; Baradaran, B. Targeted anti-inflammatory therapy is a new insight for reducing cardiovascular events: A review from physiology to the clinic. Life Sci. 2020, 253, 117720. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.; Sahu, A.; Hwang, Y.; Kim, G.B.; Nam, G.H.; Kim, I.-S.; Kwon, I.C.; Tae, G. Targeted delivery of anti-inflammatory cytokine by nanocarrier reduces atherosclerosis in Apo E−/-mice. Biomaterials 2020, 226, 119550. [Google Scholar] [CrossRef]
- Thompson, P.L.; Nidorf, S.M. Anti-inflammatory therapy with canakinumab for atherosclerotic disease: Lessons from the CANTOS trial. J. Thorac. Dis. 2018, 10, 695. [Google Scholar] [CrossRef]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef]
- Yan, Y.; Thakur, M.; van der Vorst, E.P.; Weber, C.; Döring, Y. Targeting the chemokine network in atherosclerosis. Atherosclerosis 2021, 330, 95–106. [Google Scholar] [CrossRef]
- Gilbert, J.; Lekstrom-Himes, J.; Donaldson, D.; Lee, Y.; Hu, M.; Xu, J.; Wyant, T.; Davidson, M.; Group, M.S. Effect of CC chemokine receptor 2 CCR2 blockade on serum C-reactive protein in individuals at atherosclerotic risk and with a single nucleotide polymorphism of the monocyte chemoattractant protein-1 promoter region. Am. J. Cardiol. 2011, 107, 906–911. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.-H.; He, L.-H.; Yu, X.-H.; Zhao, Z.-W.; Wang, G.; Zou, J.; Wen, F.-J.; Zhou, L.; Wan, X.-J.; Zhang, D.-W. CXCL12 promotes atherosclerosis by downregulating ABCA1 expression via the CXCR4/GSK3β/β-cateninT120/TCF21 pathway. J. Lipid Res. 2019, 60, 2020–2033. [Google Scholar] [CrossRef] [PubMed]
- Van Wanrooij, E.J.; de Jager, S.C.; van Es, T.; de Vos, P.; Birch, H.L.; Owen, D.A.; Watson, R.J.; Biessen, E.A.; Chapman, G.A.; van Berkel, T.J. CXCR3 antagonist NBI-74330 attenuates atherosclerotic plaque formation in LDL receptor–deficient mice. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 251–257. [Google Scholar] [CrossRef] [PubMed]
- Francisci, D.; Pirro, M.; Schiaroli, E.; Mannarino, M.R.; Cipriani, S.; Bianconi, V.; Alunno, A.; Bagaglia, F.; Bistoni, O.; Falcinelli, E. Maraviroc intensification modulates atherosclerotic progression in HIV-suppressed patients at high cardiovascular risk. A randomized, crossover pilot study. Proc. Open Forum Infect. Dis. 2019, 6, ofz112. [Google Scholar] [CrossRef] [PubMed]
- Oniszczuk, A.; Oniszczuk, T.; Gancarz, M.; Szymańska, J. Role of gut microbiota, probiotics and prebiotics in the cardiovascular diseases. Molecules 2021, 26, 1172. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, D.; Jia, Z.; Hui, J.; Xin, Q.; Zhou, Q.; Cong, W.; Xu, F. A bibliometric analysis of research on the links between gut microbiota and atherosclerosis. Front. Cardiovasc. Med. 2022, 9, 941607. [Google Scholar] [CrossRef] [PubMed]
- Jie, Z.; Xia, H.; Zhong, S.-L.; Feng, Q.; Li, S.; Liang, S.; Zhong, H.; Liu, Z.; Gao, Y.; Zhao, H. The gut microbiome in atherosclerotic cardiovascular disease. Nat. Commun. 2017, 8, 845. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Yang, S.; Wang, S.; Cao, Y.; Zhao, R.; Li, X.; Xing, Y.; Liu, L. Effect of berberine on atherosclerosis and gut microbiota modulation and their correlation in high-fat diet-fed ApoE−/− mice. Front. Pharmacol. 2020, 11, 223. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Zhang, Y.; Li, M.; Huang, Z.; Jiang, J.; Chen, Y.; Chen, J.; Jia, Y.; Zhang, L.; Zhou, F. Ferulic acid ameliorates atherosclerotic injury by modulating gut microbiota and lipid metabolism. Front. Pharmacol. 2021, 12, 621339. [Google Scholar] [CrossRef]
- Khongrum, J.; Yingthongchai, P.; Boonyapranai, K.; Wongtanasarasin, W.; Aobchecy, P.; Tateing, S.; Prachansuwan, A.; Sitdhipol, J.; Niwasabutra, K.; Thaveethaptaikul, P. Safety and Effects of Lactobacillus paracasei TISTR 2593 Supplementation on Improving Cholesterol Metabolism and Atherosclerosis-Related Parameters in Subjects with Hypercholesterolemia: A Randomized, Double-Blind, Placebo-Controlled Clinical Trial. Nutrients 2023, 15, 661. [Google Scholar] [CrossRef]
- Zendeboodi, F.; Khorshidian, N.; Mortazavian, A.M.; da Cruz, A.G. Probiotic: Conceptualization from a new approach. Curr. Opin. Food Sci. 2020, 32, 103–123. [Google Scholar] [CrossRef]
- Peters, L.J.F.; Jans, A.; Bartneck, M.; van der Vorst, E.P.C. Immunomodulatory Nanomedicine for the Treatment of Atherosclerosis. J. Clin. Med. 2021, 10, 3185. [Google Scholar] [CrossRef] [PubMed]
- Maruf, A.; Wang, Y.; Yin, T.; Huang, J.; Wang, N.; Durkan, C.; Tan, Y.; Wu, W.; Wang, G. Atherosclerosis Treatment with Stimuli-Responsive Nanoagents: Recent Advances and Future Perspectives. Adv. Healthc. Mater. 2019, 8, e1900036. [Google Scholar] [CrossRef] [PubMed]
- Dou, Y.; Chen, Y.; Zhang, X.; Xu, X.; Chen, Y.; Guo, J.; Zhang, D.; Wang, R.; Li, X.; Zhang, J. Non-proinflammatory and responsive nanoplatforms for targeted treatment of atherosclerosis. Biomaterials 2017, 143, 93–108. [Google Scholar] [CrossRef] [PubMed]
- Hossaini Nasr, S.; Huang, X. Nanotechnology for Targeted Therapy of Atherosclerosis. Front. Pharmacol. 2021, 12, 755569. [Google Scholar] [CrossRef] [PubMed]
- Lobatto, M.E.; Fayad, Z.A.; Silvera, S.; Vucic, E.; Calcagno, C.; Mani, V.; Dickson, S.D.; Nicolay, K.; Banciu, M.; Schiffelers, R.M.; et al. Multimodal clinical imaging to longitudinally assess a nanomedical anti-inflammatory treatment in experimental atherosclerosis. Mol. Pharm. 2010, 7, 2020–2029. [Google Scholar] [CrossRef] [PubMed]
- van der Valk, F.M.; Schulte, D.M.; Meiler, S.; Tang, J.; Zheng, K.H.; Van den Bossche, J.; Seijkens, T.; Laudes, M.; de Winther, M.; Lutgens, E.; et al. Liposomal prednisolone promotes macrophage lipotoxicity in experimental atherosclerosis. Nanomedicine 2016, 12, 1463–1470. [Google Scholar] [CrossRef]
- Luo, T.; Zhang, Z.; Xu, J.; Liu, H.; Cai, L.; Huang, G.; Wang, C.; Chen, Y.; Xia, L.; Ding, X.; et al. Atherosclerosis treatment with nanoagent: Potential targets, stimulus signals and drug delivery mechanisms. Front. Bioeng. Biotechnol. 2023, 11, 1205751. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zeng, Y.; Zhu, X.; Miao, L.; Liang, X.; Duan, J.; Li, H.; Tian, X.; Pang, L.; Wei, Y.; et al. Significant difference between sirolimus and paclitaxel nanoparticles in anti-proliferation effect in normoxia and hypoxia: The basis of better selection of atherosclerosis treatment. Bioact. Mater. 2021, 6, 880–889. [Google Scholar] [CrossRef]
- Modak, M.; Frey, M.A.; Yi, S.; Liu, Y.; Scott, E.A. Employment of targeted nanoparticles for imaging of cellular processes in cardiovascular disease. Curr. Opin. Biotechnol. 2020, 66, 59–68. [Google Scholar] [CrossRef]
- Fang, F.; Xiao, C.; Li, C.; Liu, X.; Li, S. Tuning macrophages for atherosclerosis treatment. Regen. Biomater. 2023, 10, rbac103. [Google Scholar] [CrossRef] [PubMed]
- Dhanasekara, C.S.; Zhang, J.; Nie, S.; Li, G.; Fan, Z.; Wang, S. Nanoparticles target intimal macrophages in atherosclerotic lesions. Nanomedicine 2021, 32, 102346. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Vazquez-Prada, K.X.; Liu, Y.; Whittaker, A.K.; Zhang, R.; Ta, H.T. Recent Advances in the Development of Theranostic Nanoparticles for Cardiovascular Diseases. Nanotheranostics 2021, 5, 499–514. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Tian, X.Y.; Zhang, Y.; Mu, C.; Shen, H.; Bismuth, J.; Pownall, H.J.; Huang, Y.; Wong, W.T. E-selectin-targeting delivery of microRNAs by microparticles ameliorates endothelial inflammation and atherosclerosis. Sci. Rep. 2016, 6, 22910. [Google Scholar] [CrossRef] [PubMed]
- Khodabandehlou, K.; Masehi-Lano, J.J.; Poon, C.; Wang, J.; Chung, E.J. Targeting cell adhesion molecules with nanoparticles using in vivo and flow-based in vitro models of atherosclerosis. Exp. Biol. Med. 2017, 242, 799–812. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Centurion, F.; Misra, A.; Patel, S.; Gu, Z. Molecularly targeted nanomedicine enabled by inorganic nanoparticles for atherosclerosis diagnosis and treatment. Adv. Drug Deliv. Rev. 2023, 194, 114709. [Google Scholar] [CrossRef] [PubMed]
- Ou, L.C.; Zhong, S.; Ou, J.S.; Tian, J.W. Application of targeted therapy strategies with nanomedicine delivery for atherosclerosis. Acta Pharmacol. Sin. 2021, 42, 10–17. [Google Scholar] [CrossRef]
- Sanchez-Gaytan, B.L.; Fay, F.; Lobatto, M.E.; Tang, J.; Ouimet, M.; Kim, Y.; van der Staay, S.E.; van Rijs, S.M.; Priem, B.; Zhang, L.; et al. HDL-mimetic PLGA nanoparticle to target atherosclerosis plaque macrophages. Bioconjug Chem. 2015, 26, 443–451. [Google Scholar] [CrossRef]
- Hossaini Nasr, S.; Rashidijahanabad, Z.; Ramadan, S.; Kauffman, N.; Parameswaran, N.; Zinn, K.R.; Qian, C.; Arora, R.; Agnew, D.; Huang, X. Effective atherosclerotic plaque inflammation inhibition with targeted drug delivery by hyaluronan conjugated atorvastatin nanoparticles. Nanoscale 2020, 12, 9541–9556. [Google Scholar] [CrossRef]
- Ahmed, M.S.; Rodell, C.B.; Hulsmans, M.; Kohler, R.H.; Aguirre, A.D.; Nahrendorf, M.; Weissleder, R. A Supramolecular Nanocarrier for Delivery of Amiodarone Anti-Arrhythmic Therapy to the Heart. Bioconjug Chem. 2019, 30, 733–740. [Google Scholar] [CrossRef]
- Mehu, M.; Narasimhulu, C.A.; Singla, D.K. Inflammatory Cells in Atherosclerosis. Antioxidants 2022, 11, 233. [Google Scholar] [CrossRef] [PubMed]
- Tarin, C.; Carril, M.; Martin-Ventura, J.L.; Markuerkiaga, I.; Padro, D.; Llamas-Granda, P.; Moreno, J.A.; Garcia, I.; Genicio, N.; Plaza-Garcia, S.; et al. Targeted gold-coated iron oxide nanoparticles for CD163 detection in atherosclerosis by MRI. Sci. Rep. 2015, 5, 17135. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Schilperoort, M.; Cao, Y.; Shi, J.; Tabas, I.; Tao, W. Macrophage-targeted nanomedicine for the diagnosis and treatment of atherosclerosis. Nat. Rev. Cardiol. 2022, 19, 228–249. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.W.; Lewis, D.R.; Petersen, L.K.; Moghe, P.V.; Uhrich, K.E. Amphiphilic macromolecule nanoassemblies suppress smooth muscle cell proliferation and platelet adhesion. Biomaterials 2016, 84, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Lewis, D.R.; Petersen, L.K.; York, A.W.; Zablocki, K.R.; Joseph, L.B.; Kholodovych, V.; Prud’homme, R.K.; Uhrich, K.E.; Moghe, P.V. Sugar-based amphiphilic nanoparticles arrest atherosclerosis in vivo. Proc. Natl. Acad. Sci. USA 2015, 112, 2693–2698. [Google Scholar] [CrossRef] [PubMed]
- Kaur, G.; Sharma, D.; Bisen, S.; Mukhopadhyay, C.S.; Gurdziel, K.; Singh, N.K. Vascular cell-adhesion molecule 1 (VCAM-1) regulates JunB-mediated IL-8/CXCL1 expression and pathological neovascularization. Commun. Biol. 2023, 6, 516. [Google Scholar] [CrossRef] [PubMed]
- Bjorkegren, J.L.M.; Lusis, A.J. Atherosclerosis: Recent developments. Cell 2022, 185, 1630–1645. [Google Scholar] [CrossRef]
- Kim, M.; Sahu, A.; Kim, G.B.; Nam, G.H.; Um, W.; Shin, S.J.; Jeong, Y.Y.; Kim, I.S.; Kim, K.; Kwon, I.C.; et al. Comparison of in vivo targeting ability between cRGD and collagen-targeting peptide conjugated nano-carriers for atherosclerosis. J. Control Release 2018, 269, 337–346. [Google Scholar] [CrossRef]
- Allen, A.; Gau, D.; Roy, P. The role of profilin-1 in cardiovascular diseases. J. Cell Sci. 2021, 134, jcs249060. [Google Scholar] [CrossRef]
- Martinez-Campanario, M.C.; Cortes, M.; Moreno-Lanceta, A.; Han, L.; Ninfali, C.; Dominguez, V.; Andres-Manzano, M.J.; Farras, M.; Esteve-Codina, A.; Enrich, C.; et al. Atherosclerotic plaque development in mice is enhanced by myeloid ZEB1 downregulation. Nat. Commun. 2023, 14, 8316. [Google Scholar] [CrossRef]
- Li, M.; Sun, J.; Zhang, W.; Zhao, Y.; Zhang, S.; Zhang, S. Drug delivery systems based on CD44-targeted glycosaminoglycans for cancer therapy. Carbohydr. Polym. 2021, 251, 117103. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ. Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Hajipour, M.J.; Safavi-Sohi, R.; Sharifi, S.; Mahmoud, N.; Ashkarran, A.A.; Voke, E.; Serpooshan, V.; Ramezankhani, M.; Milani, A.S.; Landry, M.P.; et al. An Overview of Nanoparticle Protein Corona Literature. Small 2023, 19, e2301838. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Gu, C.; Xu, J.; He, J.; Li, S.; Zheng, H.; Pang, B.; Wen, Y.; Fang, Q.; Liu, W.; et al. Strategy for Avoiding Protein Corona Inhibition of Targeted Drug Delivery by Linking Recombinant Affibody Scaffold to Magnetosomes. Int. J. Nanomed. 2022, 17, 665–680. [Google Scholar] [CrossRef] [PubMed]
- Padin-Gonzalez, E.; Lancaster, P.; Bottini, M.; Gasco, P.; Tran, L.; Fadeel, B.; Wilkins, T.; Monopoli, M.P. Understanding the Role and Impact of Poly (Ethylene Glycol) (PEG) on Nanoparticle Formulation: Implications for COVID-19 Vaccines. Front. Bioeng. Biotechnol. 2022, 10, 882363. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, X.; Millican, R.; Sherwood, J.; Martin, S.; Jo, H.; Yoon, Y.S.; Brott, B.C.; Jun, H.W. Recent advances in nanomaterials for therapy and diagnosis for atherosclerosis. Adv. Drug Deliv. Rev. 2021, 170, 142–199. [Google Scholar] [CrossRef] [PubMed]
- Kratz, J.D.; Chaddha, A.; Bhattacharjee, S.; Goonewardena, S.N. Atherosclerosis and Nanotechnology: Diagnostic and Therapeutic Applications. Cardiovasc. Drugs Ther. 2016, 30, 33–39. [Google Scholar] [CrossRef]
- Lobatto, M.E.; Fuster, V.; Fayad, Z.A.; Mulder, W.J.M. Perspectives and opportunities for nanomedicine in the management of atherosclerosis. Nat. Rev. Drug Discov. 2011, 10, 835–852. [Google Scholar] [CrossRef]
- Clinical Trials. Available online: https://clinicaltrials.gov/ (accessed on 21 January 2024).
| Chemokine/Receptor Target | Clinical Trials | Outcomes | Further Work | References |
|---|---|---|---|---|
| CCL2/CCR2 | Phase 1, 112 patients (CCR2 antagonism (MLN1202)) | Safety outcome with reduction in serum C-reactive protein level | The efficacy of MLN1202 needs to be evaluated in atherosclerosis patients | [217] |
| CXCL12/CXCR4 | No current trials identified | Overexpression of CXCL12 is associated with increased macrophage infiltration in the plaques | Pre-clinical and clinical assessment of the potency and safety of CXCL12/CXCR4 modulation | [218] |
| CXCL10/CXCR3 | No current trials identified | In vivo, administration of the CXCR3 antagonist NBI-74330 demonstrates reduced atherogenesis | Further pre-clinical and clinical evaluations are needed to address concerns about specificity and potential impacts on ant-pathogen immunity | [219] |
| CCR5 | CCR5 antagonist (Maraviroc) on 22 patients | Several markers associated with cardiovascular risk showed improvement compared to a control group | More studies are needed to evaluate the efficacy, address concerns about off-target effects, and investigate potential long-term consequences. | [220] |
| Nanoparticles | Loaded Molecule | Target | Reference |
|---|---|---|---|
| Liposome | Prednisolone | Macrophages | [233] |
| Iron oxide | Anti-CD163 mAb | Macrophages | [236] |
| Iron oxide-HA | Anti-CD44 mAb | CD44 receptor | [237] |
| Phosphatidylcholine lipid | Anti-CD36 mAb | CD36 receptor | [238] |
| Iron oxide | Anti-osteopontin mAb | Osteopontin | [231] |
| SV40 | Hirulog peptide | Macrophages | [239] |
| Thioaptamer | miR-146a/-181b | Selectin | [240] |
| PLGA | Anti-ICAM-1 peptide | ICAM-1 | [231] |
| Magnetic | Anti-VCAM-1 peptide | VCAM-1 | [241] |
| Gold | Anti-profilin-1 siRNA | Profilin-1 | [242] |
| PLGA-PEG | IL-10 | Collagen IV | [243] |
| PLGA-HDL | Statins | Macrophages | [244] |
| PLGA-HA | Simvastatin | CD44 receptor | [231] |
| Polymer-HA | Atorvastatin | CD44 receptor | [245] |
| Solid lipid | Methotrexate | Proinflammatory cytokines | [231] |
| Liposome | Amiodarone | Proinflammatory cytokines | [246] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alradwan, I.; AL Fayez, N.; Alomary, M.N.; Alshehri, A.A.; Aodah, A.H.; Almughem, F.A.; Alsulami, K.A.; Aldossary, A.M.; Alawad, A.O.; Tawfik, Y.M.K.; et al. Emerging Trends and Innovations in the Treatment and Diagnosis of Atherosclerosis and Cardiovascular Disease: A Comprehensive Review towards Healthier Aging. Pharmaceutics 2024, 16, 1037. https://doi.org/10.3390/pharmaceutics16081037
Alradwan I, AL Fayez N, Alomary MN, Alshehri AA, Aodah AH, Almughem FA, Alsulami KA, Aldossary AM, Alawad AO, Tawfik YMK, et al. Emerging Trends and Innovations in the Treatment and Diagnosis of Atherosclerosis and Cardiovascular Disease: A Comprehensive Review towards Healthier Aging. Pharmaceutics. 2024; 16(8):1037. https://doi.org/10.3390/pharmaceutics16081037
Chicago/Turabian StyleAlradwan, Ibrahim, Nojoud AL Fayez, Mohammad N. Alomary, Abdullah A. Alshehri, Alhassan H. Aodah, Fahad A. Almughem, Khulud A. Alsulami, Ahmad M. Aldossary, Abdullah O. Alawad, Yahya M. K. Tawfik, and et al. 2024. "Emerging Trends and Innovations in the Treatment and Diagnosis of Atherosclerosis and Cardiovascular Disease: A Comprehensive Review towards Healthier Aging" Pharmaceutics 16, no. 8: 1037. https://doi.org/10.3390/pharmaceutics16081037
APA StyleAlradwan, I., AL Fayez, N., Alomary, M. N., Alshehri, A. A., Aodah, A. H., Almughem, F. A., Alsulami, K. A., Aldossary, A. M., Alawad, A. O., Tawfik, Y. M. K., & Tawfik, E. A. (2024). Emerging Trends and Innovations in the Treatment and Diagnosis of Atherosclerosis and Cardiovascular Disease: A Comprehensive Review towards Healthier Aging. Pharmaceutics, 16(8), 1037. https://doi.org/10.3390/pharmaceutics16081037






