Nano-Liposomal Beetroot Phyto-Pigment in Photodynamic Therapy as a Prospective Green Approach for Cancer Management: In Vitro Evaluation and Molecular Dynamic Simulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Plant Material and Preparation of the Beetroot Juice
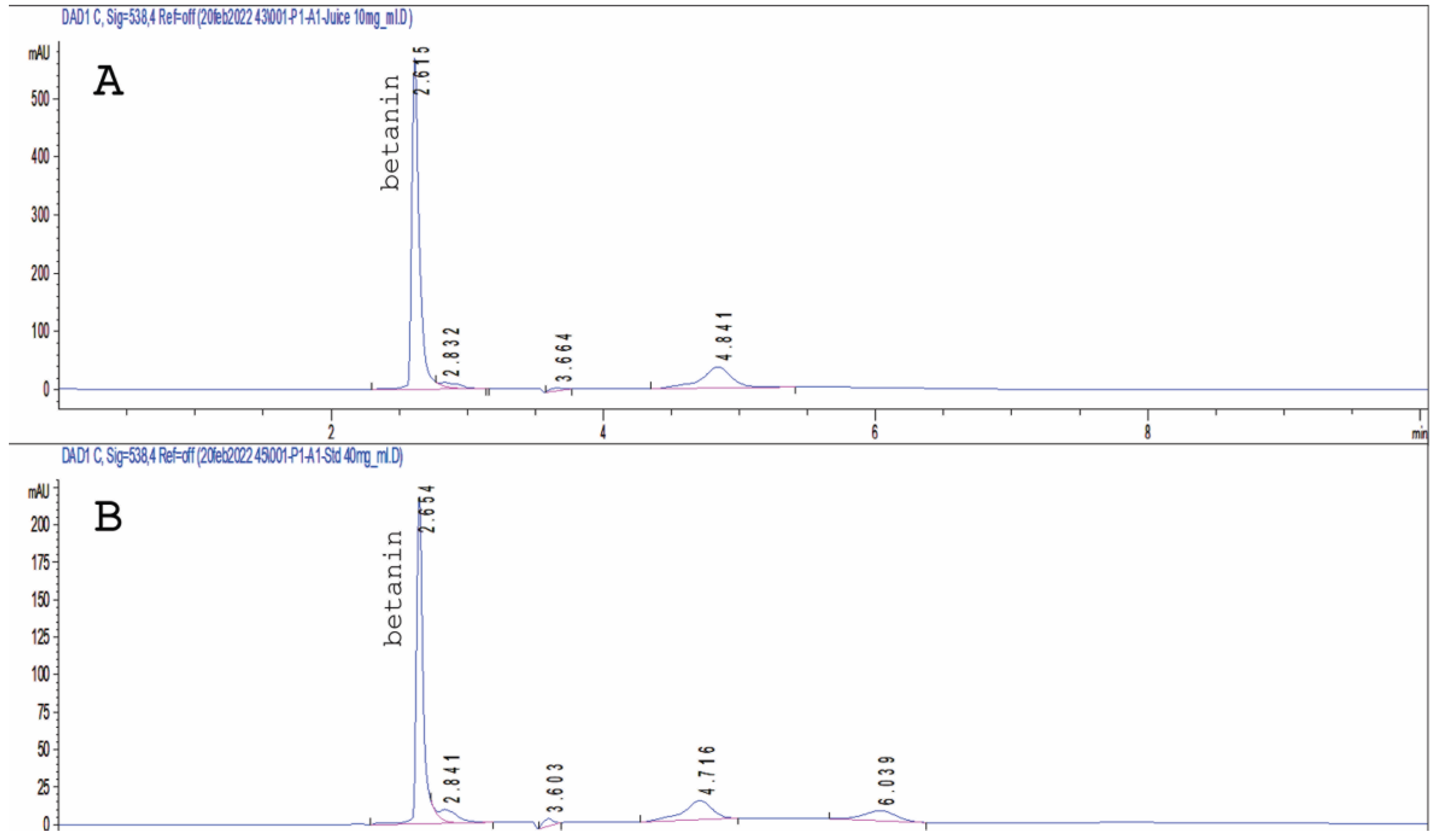
2.3. HPLC Analysis
2.4. Spectroscopic Properties of BRJ and Standard Betanin
2.5. Evaluation of Singlet Oxygen Generation
2.6. Molecular Dynamic Simulation Study (MD)
2.7. Preparation of Liposomes Loaded with BRJ (Lip-BRJ) and with Standard Betanin (Lip-Bet)
2.8. Characterization of the Prepared Liposomes
2.9. In Vitro Assessment of Photodynamic Activity
2.9.1. Cell Lines and Cell Culture
2.9.2. Dark Cytotoxicity
2.9.3. Photocytotoxicity
Statistical Analysis
3. Results
3.1. HPLC of Betanin in Beetroot Extract
3.2. Spectroscopic Properties of BRJ and Betanin
3.3. Singlet Oxygen Generation
3.4. Molecular Docking and Molecular Dynamic Simulation
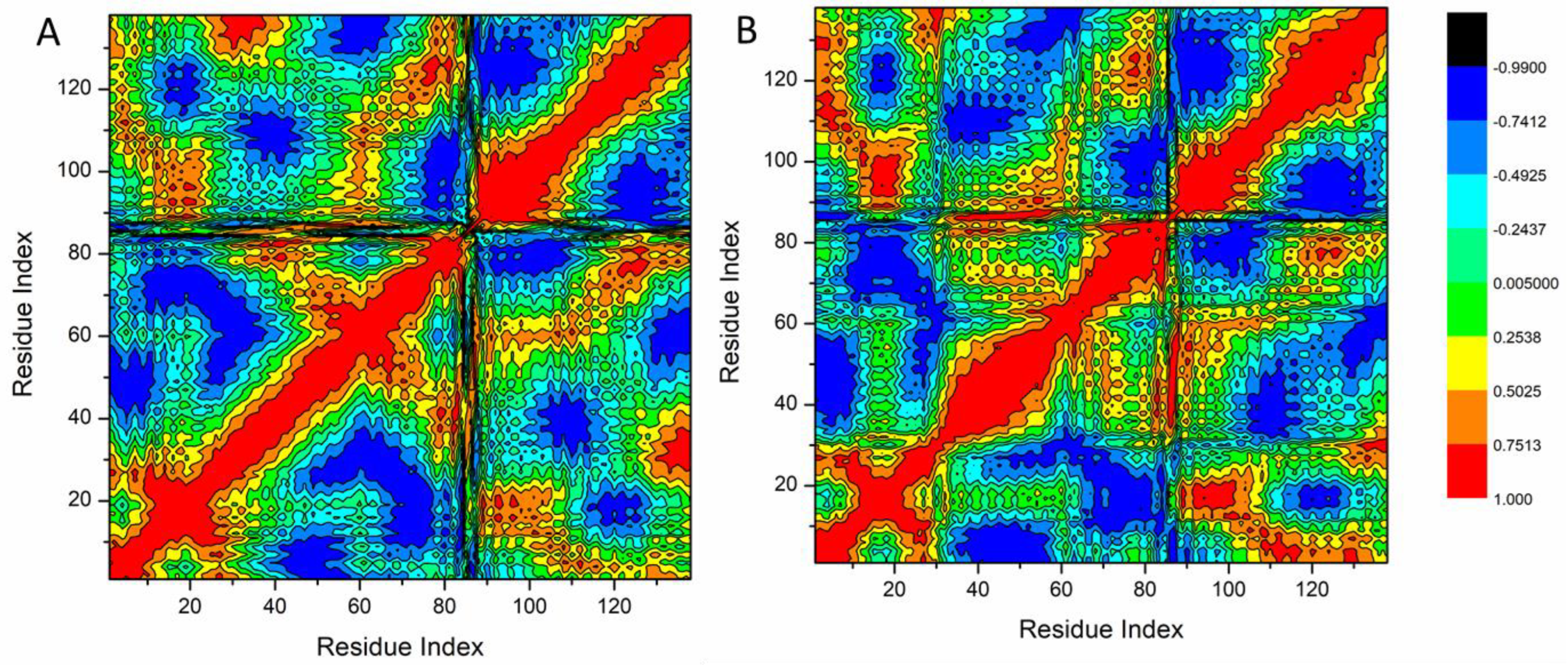
3.4.1. Molecular Dynamic and System Stability
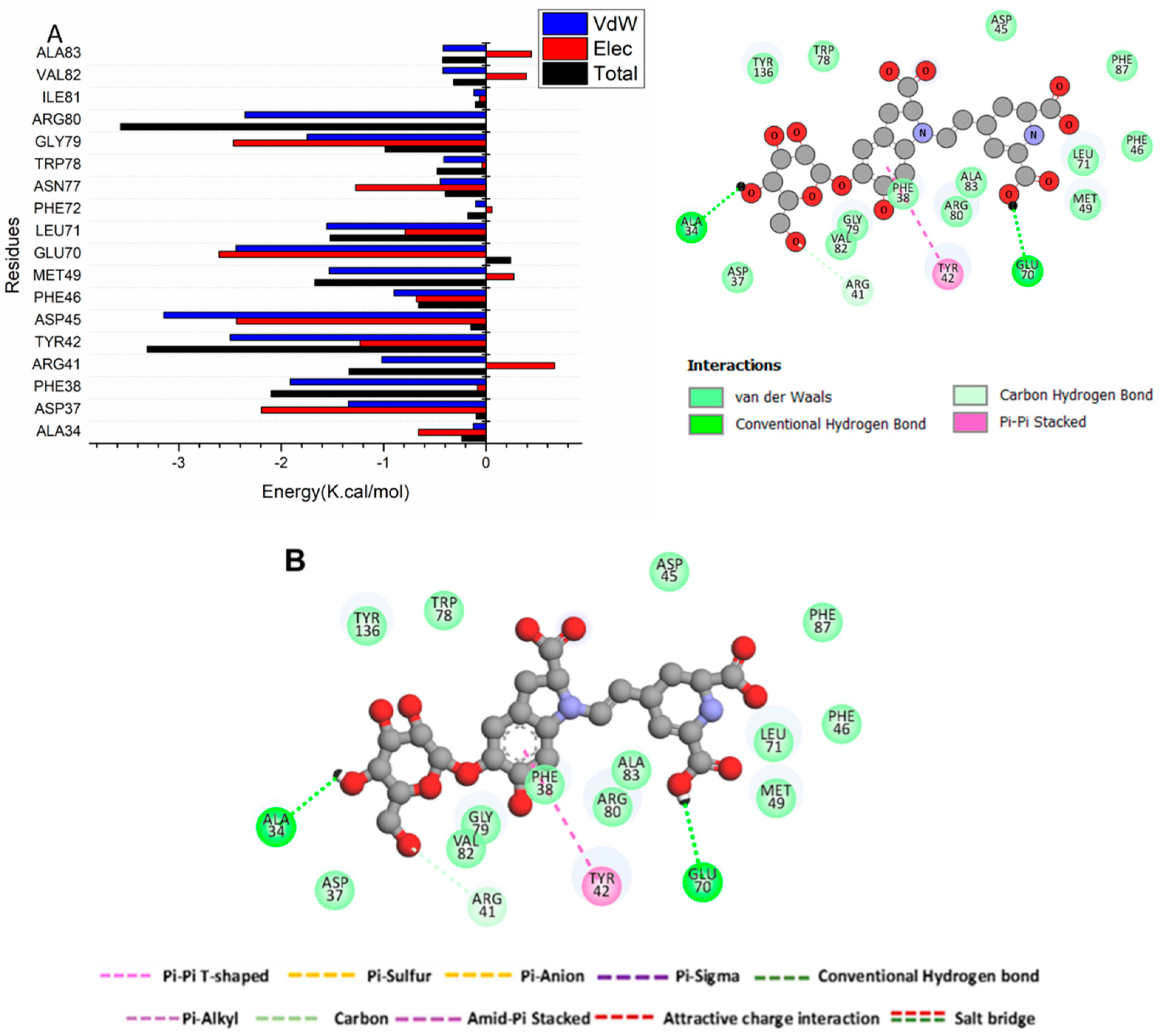
3.4.2. The Binding Free Energy Thermodynamic Calculations
3.4.3. Identification of the Residues Involved in Betanin Binding to Bcl2 Receptors and Interaction Network Profiles
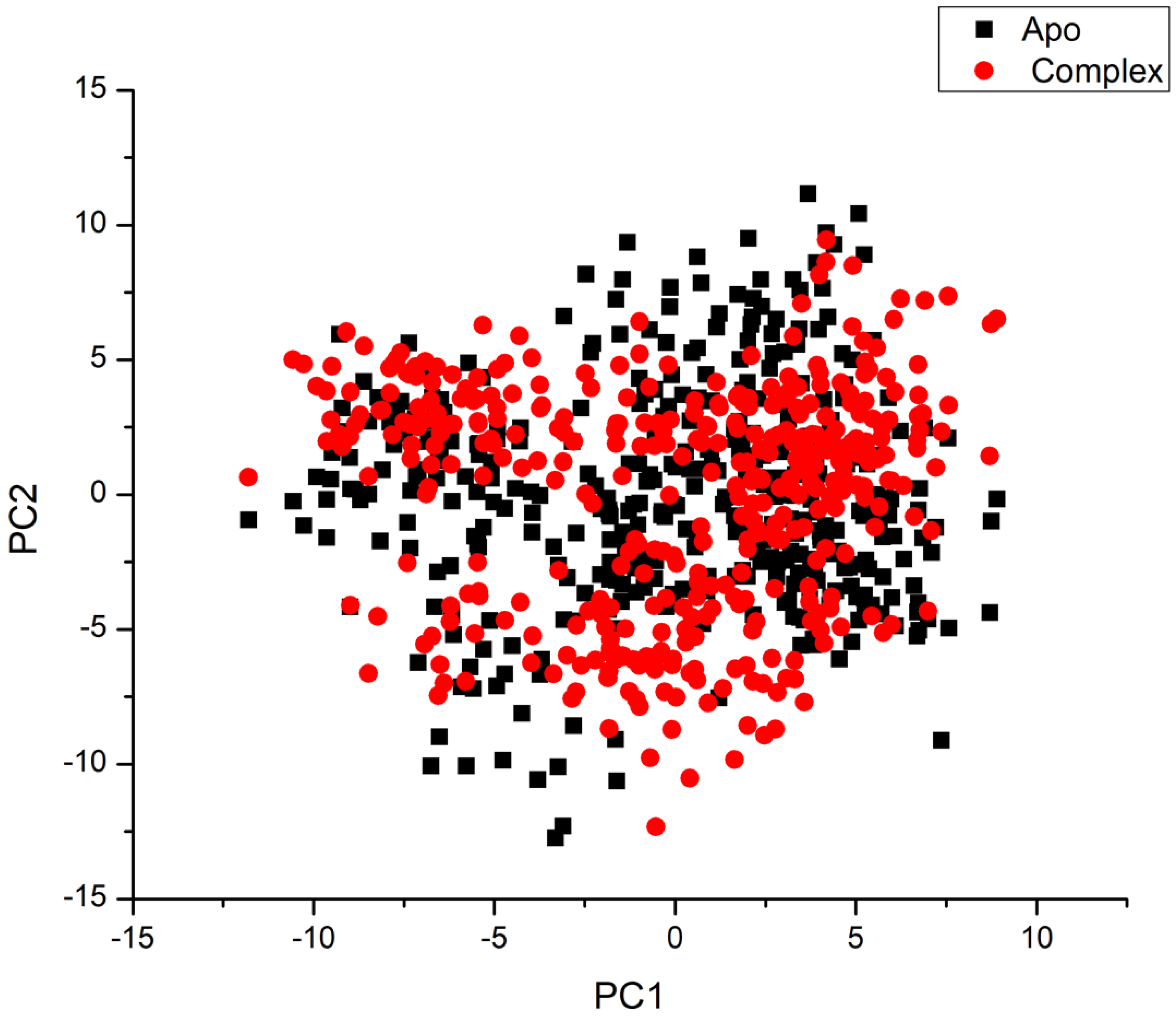
3.4.4. Principal Component Analysis (PCA)
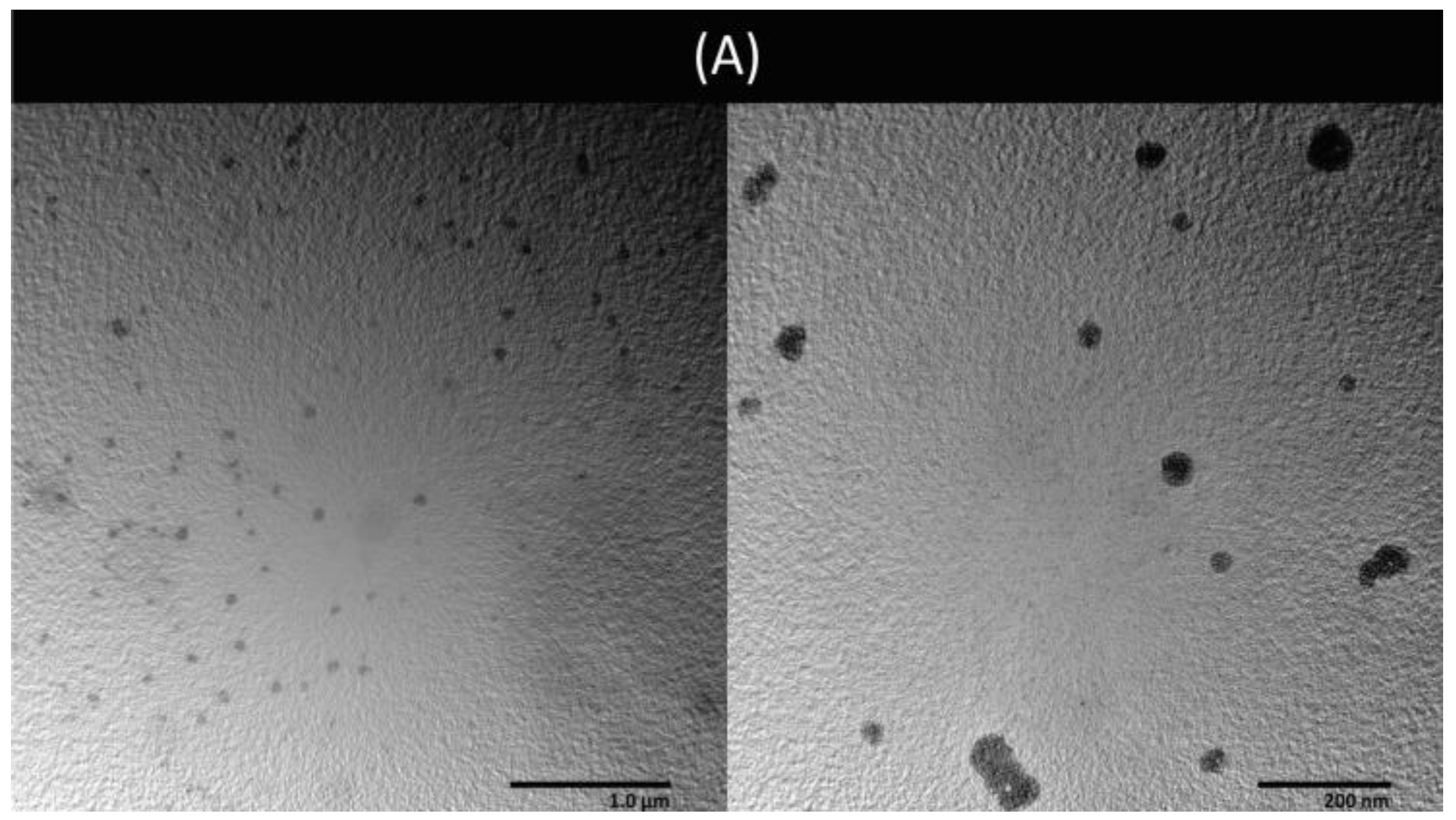
3.5. Preparation and Characterization of Liposomes
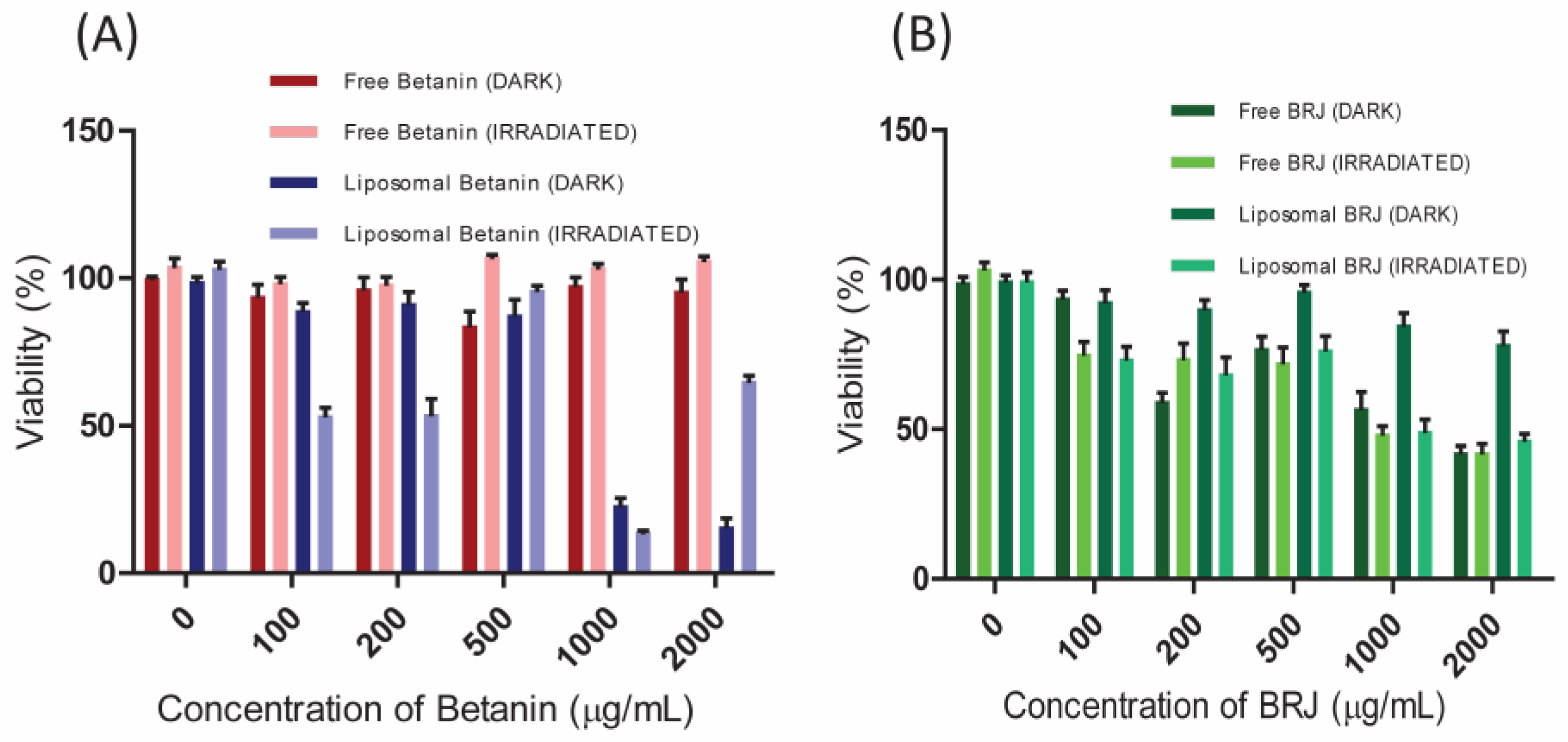
3.6. In Vitro Photodynamic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fahmy, S.A.; Azzazy, H.M.E.S.; Schaefer, J. Liposome Photosensitizer Formulations for Effective Cancer Photodynamic Therapy. Pharmaceutics 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Foresto, E.; Gilardi, P.; Ibarra, L.E.; Cogno, I.S. Light-Activated Green Drugs: How We Can Use Them in Photodynamic Therapy and Mass-Produce Them with Biotechnological Tools. Phytomed. Plus 2021, 1, 100044. [Google Scholar] [CrossRef]
- Siewert, B.; Stuppner, H. The Photoactivity of Natural Products—An Overlooked Potential of Phytomedicines? Phytomedicine 2019, 60, 152985. [Google Scholar] [CrossRef]
- Cheng, X.; Gao, J.; Ding, Y.; Lu, Y.; Wei, Q.; Cui, D.; Fan, J.; Li, X.; Zhu, E.; Lu, Y. Multi-functional Liposome: A Powerful Theranostic Nano-platform Enhancing Photodynamic Therapy. Adv. Sci. 2021, 8, 2100876. [Google Scholar] [CrossRef]
- Mittal, S.; Roy, S.; Srivastava, J.N. Fungicidal Response of a Novel Natural Photosensitizer (Beta vulgaris) on Candida albicans with Low-Power Laser Radiation. Laser Phys. 2013, 23, 055606. [Google Scholar] [CrossRef]
- Sulaiman, C.; George, B.P.; Balachandran, I.; Abrahamse, H. Photoactive Herbal Compounds: A Green Approach to Photodynamic Therapy. Molecules 2022, 27, 5084. [Google Scholar] [CrossRef]
- Sengupta, D.; Mondal, B.; Mukherjee, K. Visible Light Absorption and Photo-Sensitizing Properties of Spinach Leaves and Beetroot Extracted Natural Dyes. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2015, 148, 85–92. [Google Scholar] [CrossRef]
- Lechner, J.F.; Stoner, G.D. Red Beetroot and Betalains as Cancer Chemopreventative Agents. Molecules 2019, 24, 1602. [Google Scholar] [CrossRef]
- Chhikara, N.; Kushwaha, K.; Jaglan, S.; Sharma, P.; Panghal, A. Nutritional, Physicochemical, and Functional Quality of Beetroot (Beta vulgaris L.) Incorporated Asian Noodles. Cereal Chem. 2019, 96, 154–161. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, Y.; Hu, Z.; Wu, S.; Jin, C. Beetroot as a Functional Food with Huge Health Benefits: Antioxidant, Antitumor, Physical Function, and Chronic Metabolomics Activity. Food Sci. Nutr. 2021, 9, 6406–6420. [Google Scholar] [CrossRef]
- Tesoriere, L.; Gentile, C.; Angileri, F.; Attanzio, A.; Tutone, M.; Allegra, M.; Livrea, M.A. Trans-Epithelial Transport of the Betalain Pigments Indicaxanthin and Betanin across Caco-2 Cell Monolayers and Influence of Food Matrix. Eur. J. Nutr. 2013, 52, 1077–1087. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Biological Properties and Applications of Betalains. Molecules 2021, 26, 2520. [Google Scholar] [CrossRef]
- Amjadi, S.; Ghorbani, M.; Hamishehkar, H.; Roufegarinejad, L. Improvement in the Stability of Betanin by Liposomal Nanocarriers: Its Application in Gummy Candy as a Food Model. Food Chem. 2018, 256, 156–162. [Google Scholar] [CrossRef]
- Hadipour, E.; Taleghani, A.; Tayarani-Najaran, N.; Tayarani-Najaran, Z. Biological Effects of Red Beetroot and Betalains: A Review. Phytother. Res. 2020, 34, 1847–1867. [Google Scholar] [CrossRef]
- Amjadi, S.; Mesgari Abbasi, M.; Shokouhi, B.; Ghorbani, M.; Hamishehkar, H. Enhancement of Therapeutic Efficacy of Betanin for Diabetes Treatment by Liposomal Nanocarriers. J. Funct. Foods 2019, 59, 119–128. [Google Scholar] [CrossRef]
- Amjadi, S.; Hamishehkar, H.; Ghorbani, M. A Novel Smart PEGylated Gelatin Nanoparticle for Co-Delivery of Doxorubicin and Betanin: A Strategy for Enhancing the Therapeutic Efficacy of Chemotherapy. Mater. Sci. Eng. C 2019, 97, 833–841. [Google Scholar] [CrossRef]
- Abdel Fadeel, D.A.; Hanafy, M.S.; Kelany, N.A.; Elywa, M.A. Novel Greenly Synthesized Titanium Dioxide Nanoparticles Compared to Liposomes in Drug Delivery: In Vivo Investigation on Ehrlich Solid Tumor Model. Heliyon 2021, 7, e07370. [Google Scholar] [CrossRef]
- Rahman, H.S.; Othman, H.H.; Hammadi, N.I.; Yeap, S.K.; Amin, K.M.; Samad, N.A.; Alitheen, N.B. Novel Drug Delivery Systems for Loading of Natural Plant Extracts and Their Biomedical Applications. Int. J. Nanomed. 2020, 15, 2439–2483. [Google Scholar] [CrossRef]
- Dias, S.; Castanheira, E.M.S.; Fortes, A.G.; Pereira, D.M.; Rodrigues, A.R.O.; Pereira, R.; Gonçalves, M.S.T. Application of Natural Pigments in Ordinary Cooked Ham. Molecules 2020, 25, 2241. [Google Scholar] [CrossRef]
- Kaloni, D.; Diepstraten, S.T.; Strasser, A.; Kelly, G.L. BCL-2 Protein Family: Attractive Targets for Cancer Therapy. Apoptosis 2023, 28, 20–38. [Google Scholar] [CrossRef]
- Herman, J.; Neal, S.L. Efficiency Comparison of the Imidazole plus RNO Method for Singlet Oxygen Detection in Biorelevant Solvents. Anal. Bioanal. Chem. 2019, 411, 5287–5296. [Google Scholar] [CrossRef] [PubMed]
- Bruncko, M.; Oost, T.K.; Belli, B.A.; Ding, H.; Joseph, M.K.; Kunzer, A.; Martineau, D.; McClellan, W.J.; Mitten, M.; Ng, S.-C. Studies Leading to Potent, Dual Inhibitors of Bcl-2 and Bcl-XL. J. Med. Chem. 2007, 50, 641–662. [Google Scholar] [CrossRef] [PubMed]
- El Malah, T.; El-Mageid, R.E.S.A.; Shamroukh, A.H.; Rashad, A.E.; El-Rashedy, A.A.; Awad, H.M.; Abdel-Megeid, F.M.E.; Hegab, M.I. Click Synthesis, Anticancer and Molecular Docking Evaluation of Some Hexahydro-6H-Indolo[2,3-b]Quinoxalines Incorporated Triazole Moiety. J. Mol. Struct. 2024, 1303, 137573. [Google Scholar] [CrossRef]
- Lee, T.-S.; Cerutti, D.S.; Mermelstein, D.; Lin, C.; LeGrand, S.; Giese, T.J.; Roitberg, A.; Case, D.A.; Walker, R.C.; York, D.M. GPU-Accelerated Molecular Dynamics and Free Energy Methods in Amber18: Performance Enhancements and New Features. J. Chem. Inf. Model. 2018, 58, 2043–2050. [Google Scholar] [CrossRef]
- Tuccinardi, T. What Is the Current Value of MM/PBSA and MM/GBSA Methods in Drug Discovery? Expert Opin. Drug Discov. 2021, 16, 1233–1237. [Google Scholar] [CrossRef] [PubMed]
- Ylilauri, M.; Pentikäinen, O.T. MMGBSA as a Tool to Understand the Binding Affinities of Filamin–Peptide Interactions. J. Chem. Inf. Model. 2013, 53, 2626–2633. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Lian, J.; Xu, Z.; Ning, Y.; Shi, M.; Zhao, Z.; Zhang, Z. Chitosan-Coated Nanoliposomes for Efficient Delivery of Betanin with Enhanced Stability and Bioavailability. Food Hydrocoll. 2022, 132, 107871. [Google Scholar] [CrossRef]
- Koli, P.; Pareek, R.K.; Dayma, Y.; Kumar, R. Beetroot’s Crude Aqueous Extract Photosensitizer-Formic Acid-Sodium Lauryl Sulphate Photogalvanic Electrolyte: Solar Power and Storage. Bioresour. Technol. Rep. 2022, 18, 101083. [Google Scholar] [CrossRef]
- Hasanin, M.; Hashem, A.H.; El-Rashedy, A.A.; Kamel, S. Synthesis of Novel Heterocyclic Compounds Based on Dialdehyde Cellulose: Characterization, Antimicrobial, Antitumor Activity, Molecular Dynamics Simulation and Target Identification. Cellulose 2021, 28, 8355–8374. [Google Scholar] [CrossRef]
- Mirzaei, S.; Eisvand, F.; Hadizadeh, F.; Mosaffa, F.; Ghasemi, A.; Ghodsi, R. Design, Synthesis and Biological Evaluation of Novel 5, 6, 7-Trimethoxy-N-Aryl-2-Styrylquinolin-4-Amines as Potential Anticancer Agents and Tubulin Polymerization Inhibitors. Bioorg. Chem. 2020, 98, 103711. [Google Scholar] [CrossRef]
- Pourhajibagher, M. Molecular Modeling and Simulation Analysis of Antimicrobial Photodynamic Therapy Potential for Control of COVID-19. Sci. World J. 2022, 2022, 7089576. [Google Scholar] [CrossRef] [PubMed]
- Taghvaei, S.; Sabouni, F.; Minuchehr, Z.; Taghvaei, A. Identification of Novel Anti-Cancer Agents, Applying in Silico Method for SENP1 Protease Inhibition. J. Biomol. Struct. Dyn. 2022, 40, 6228–6242. [Google Scholar] [CrossRef] [PubMed]
- Roggia, I.; Dalcin, A.J.F.; Ourique, A.F.; da Cruz, I.B.M.; Ribeiro, E.E.; Mitjans, M.; Vinardell, M.P.; Gomes, P. Protective Effect of Guarana-Loaded Liposomes on Hemolytic Activity. Colloids Surf. B Biointerfaces 2020, 187, 110636. [Google Scholar] [CrossRef]
- Li, Y.; Xu, P.; He, D.; Xu, B.; Tu, J.; Shen, Y. Long-Circulating Thermosensitive Liposomes for the Targeted Drug Delivery of Oxaliplatin. Int. J. Nanomed. 2020, 15, 6721–6734. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.; Li, D.; Jin, Y.; Zhang, W.; Teng, L.; Bunt, C.; Wen, J. Deformable Liposomes by Reverse-Phase Evaporation Method for an Enhanced Skin Delivery of (+)-Catechin. Drug Dev. Ind. Pharm. 2014, 40, 260–265. [Google Scholar] [CrossRef]
- Yu, S.; Li, D.; Shi, A.; Long, Y.; Deng, J.; Ma, Y.; Li, X.; Wen, J.; Hu, Y.; He, X. Multidrug-Loaded Liposomes Prevent Ischemic Stroke through Intranasal Administration. Biomed. Pharmacother. 2023, 162, 114542. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, C.; Tao, Y.; Hou, X.; Liu, Y.; Sang, H.; Jiang, G. Near-Infrared Light-Triggered Thermosensitive Liposomes Modified with Membrane Peptides for the Local Chemo/Photothermal Therapy of Melanoma. OncoTargets Ther. 2021, 14, 1317–1329. [Google Scholar] [CrossRef] [PubMed]
- Varga, Z.; Fehér, B.; Kitka, D.; Wacha, A.; Bóta, A.; Berényi, S.; Pipich, V.; Fraikin, J.L. Size Measurement of Extracellular Vesicles and Synthetic Liposomes: The Impact of the Hydration Shell and the Protein Corona. Colloids Surf. B Biointerfaces 2020, 192, 111053. [Google Scholar] [CrossRef]
- Carreño, E.A.; Alberto, A.V.P.; de Souza, C.A.M.; de Mello, H.L.; Henriques-Pons, A.; Anastacio Alves, L. Considerations and Technical Pitfalls in the Employment of the MTT Assay to Evaluate Photosensitizers for Photodynamic Therapy. Appl. Sci. 2021, 11, 2603. [Google Scholar] [CrossRef]
- Nowacki, L.; Vigneron, P.; Rotellini, L.; Cazzola, H.; Merlier, F.; Prost, E.; Ralanairina, R.; Gadonna, J.P.; Rossi, C.; Vayssade, M. Betanin-Enriched Red Beetroot (Beta vulgaris L.) Extract Induces Apoptosis and Autophagic Cell Death in MCF-7 Cells. Phytother. Res. 2015, 29, 1964–1973. [Google Scholar] [CrossRef]
- Zhang, Q.; Pan, J.; Wang, Y.; Lubet, R.; You, M. Beetroot Red (Betanin) Inhibits Vinyl Carbamate- and Benzo(a)Pyrene-Induced Lung Tumorigenesis through Apoptosis. Mol. Carcinog. 2013, 52, 686–691. [Google Scholar] [CrossRef]
- Lee, E.J.; An, D.; Nguyen, C.T.T.; Patil, B.S.; Kim, J.; Yoo, K.S. Betalain and Betaine Composition of Greenhouse- or Field-Produced Beetroot (Beta vulgaris L.) and Inhibition of HepG2 Cell Proliferation. J. Agric. Food Chem. 2014, 62, 1324–1331. [Google Scholar] [CrossRef] [PubMed]
- Kapadia, G.J.; Rao, G.S.; Ramachandran, C.; Iida, A.; Suzuki, N.; Tokuda, H. Synergistic Cytotoxicity of Red Beetroot (Beta vulgaris L.) Extract with Doxorubicin in Human Pancreatic, Breast and Prostate Cancer Cell Lines. J. Complement. Integr. Med. 2013, 10, 113–122. [Google Scholar] [CrossRef] [PubMed]
- Romero, S.A.; Pavan, I.C.B.; Morelli, A.P.; Mancini, M.C.S.; da Silva, L.G.S.; Fagundes, I.; Silva, C.H.R.; Ponte, L.G.S.; Rostagno, M.A.; Bezerra, R.M.N.; et al. Anticancer Effects of Root and Beet Leaf Extracts (Beta vulgaris L.) in Cervical Cancer Cells (HeLa). Phytother. Res. 2021, 35, 6191–6203. [Google Scholar] [CrossRef] [PubMed]
- El-ghannam, G.; Moawad, M.; Abo-Elfadl, M.T.; Elfeky, S.A. Beetroot Extract@chitosan Nanocomposite as a Promising Approach towards Cancer Therapy. Int. J. Biol. Macromol. 2024, 261, 129700. [Google Scholar] [CrossRef]
- Bashir, R.; Makhdoom, A.R.; Bilal, M.K.; Badar, M.A. Comparative Study of the Photovoltaic Behavior of Ruthenium and the Other Organic and Inorganic Dye-Sensitized Solar Cells (DSSC). Optik 2018, 157, 11–15. [Google Scholar] [CrossRef]
- Cogno, I.S.; Gilardi, P.; Comini, L.; Núñez-Montoya, S.C.; Cabrera, J.L.; Rivarola, V.A. Natural Photosensitizers in Photodynamic Therapy: In Vitro Activity against Monolayers and Spheroids of Human Colorectal Adenocarcinoma SW480 Cells. Photodiagn. Photodyn. Ther. 2020, 31, 101852. [Google Scholar] [CrossRef]
- Polat, E.; Kang, K. Natural Photosensitizers in Antimicrobial Photodynamic Therapy. Biomedicines 2021, 9, 584. [Google Scholar] [CrossRef]
- Ibrahim, N.A.; Abdel Fadeel, D.A.; Sadek, A.; Fadel, M.; Tawfik, A. Intralesional Vitamin D3 versus New Topical Photodynamic Therapy in Recalcitrant Palmoplanter Warts Randomized Comparative Controlled Study. Photodiagn. Photodyn. Ther. 2020, 32, 101979. [Google Scholar] [CrossRef]
- Fadeel, D.A.A.; Fadel, M.; Tawfik, A.; Omar, Y. Transfersomal Eosin Topical Delivery Assisted by Fractional CO2 Laser for Photodynamic Treatment of Palmar Hyperhidrosis: Case Study. Drug Deliv. Transl. Res. 2022, 12, 3000–3006. [Google Scholar] [CrossRef]
- Dhaini, B.; Wagner, L.; Moinard, M.; Daouk, J.; Arnoux, P.; Schohn, H.; Schneller, P.; Acherar, S.; Hamieh, T.; Frochot, C. Importance of Rose Bengal Loaded with Nanoparticles for Anti-Cancer Photodynamic Therapy. Pharmaceuticals 2022, 15, 1093. [Google Scholar] [CrossRef] [PubMed]
- Fadel, M.; Fadeel, D.A.; Tawfik, A.; El-Kholy, A.I.; Mosaad, Y.O. Rose Bengal-Gold-Polypyrrole Nanoparticles as a Photothermal/Photodynamic Dual Treatment of Recalcitrant Plantar Warts: Animal and Clinical Study. J. Drug Deliv. Sci. Technol. 2022, 69, 103095. [Google Scholar] [CrossRef]





| Energy Components (kcal/mol) | |||||
|---|---|---|---|---|---|
| Protein Complex | ΔEvdW | ΔEelec | ΔGgas | ΔGsolv | ΔGbind |
| Betanin-Bcl2 | −42.42 ± 0.36 | −152.86 ± 1.63 | −195.28 ± 1.61 | 154.34 ± 1.37 | −40.94 ± 0.42 |
| Liposomes | Encapsulation Efficiency % | Loading Capacity % | Particle Size (nm) | Zeta Potential (mV) | PDI |
|---|---|---|---|---|---|
| Empty Liposomes | - | - | 210 ± 20 | −19 ± 6.0 | 0.3 |
| Lip-BRJ | 61.9 ± 5.3 | 12 ± 4.0 | 441.5 ± 160 | −43.7 ± 7.5 | 0.4 |
| Lip-Bet | 65.2 ± 3.0 | 14 ± 3.0 | 233.0 ± 60 | −23.0 ± 8.0 | 0.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fadeel, D.A.; Fadel, M.; El-Kholy, A.I.; El-Rashedy, A.A.; Mohsen, E.; Ezzat, M.I.; Issa, M.Y. Nano-Liposomal Beetroot Phyto-Pigment in Photodynamic Therapy as a Prospective Green Approach for Cancer Management: In Vitro Evaluation and Molecular Dynamic Simulation. Pharmaceutics 2024, 16, 1038. https://doi.org/10.3390/pharmaceutics16081038
Fadeel DA, Fadel M, El-Kholy AI, El-Rashedy AA, Mohsen E, Ezzat MI, Issa MY. Nano-Liposomal Beetroot Phyto-Pigment in Photodynamic Therapy as a Prospective Green Approach for Cancer Management: In Vitro Evaluation and Molecular Dynamic Simulation. Pharmaceutics. 2024; 16(8):1038. https://doi.org/10.3390/pharmaceutics16081038
Chicago/Turabian StyleFadeel, Doaa Abdel, Maha Fadel, Abdullah Ibrahim El-Kholy, Ahmed A. El-Rashedy, Engy Mohsen, Marwa I. Ezzat, and Marwa Y. Issa. 2024. "Nano-Liposomal Beetroot Phyto-Pigment in Photodynamic Therapy as a Prospective Green Approach for Cancer Management: In Vitro Evaluation and Molecular Dynamic Simulation" Pharmaceutics 16, no. 8: 1038. https://doi.org/10.3390/pharmaceutics16081038
APA StyleFadeel, D. A., Fadel, M., El-Kholy, A. I., El-Rashedy, A. A., Mohsen, E., Ezzat, M. I., & Issa, M. Y. (2024). Nano-Liposomal Beetroot Phyto-Pigment in Photodynamic Therapy as a Prospective Green Approach for Cancer Management: In Vitro Evaluation and Molecular Dynamic Simulation. Pharmaceutics, 16(8), 1038. https://doi.org/10.3390/pharmaceutics16081038





