New Approaches for Basophil Activation Tests Employing Dendrimeric Antigen–Silica Nanoparticle Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Nps Chemical Studies
2.1.1. Np Synthesis
- 20dNps
- 30dNps
- 50dNps
- 50Nps
2.1.2. Np Surface Modification
- 20dNpNH2, 30dNpNH2, and 50dNpNH2
- 50NpNH2, 50Np0.1NH2 and 50Np0.01NH2
- 20dNpCO2H, 30dNpCO2H, and 50dNpCO2H
- 50NpCO2H, 50Np0.1NH2CO2H, or 50Np0.01NH2CO2H
- 20dNpDe, 30dNpDe, and 50dNpDe
- 50NpDe, 50Np0.1De, 50Np0.01De, 50Np0.1NH2De, or 50Np0.01NH2De
- 20dNpDeAXO, 30dNpDeAXO, and 50dNpDeAXO
- 50NpDeAXO, 50Np0.1DeDeAXO, 50Np0.01DeDeAXO, 50Np0.1NH2DeAXO, or 50Np0.01NH2DeAXO
2.1.3. Np Characterization
2.2. Patients Selection and Allergological Work Up
2.3. Cell Viability Measurement
2.4. Basophil Activation Test (BAT) with Different Nanoparticles (Nps)
2.5. Statistical Analyses
3. Results and Discussion
3.1. Synthesis and Characterization of Nps
3.2. Evaluation of Basophil Activation Capacity of Nanoparticles: 20dNpDeAXO, 30dNpDeAXO, 50dNpDeAXO, and 50NpDeAXO
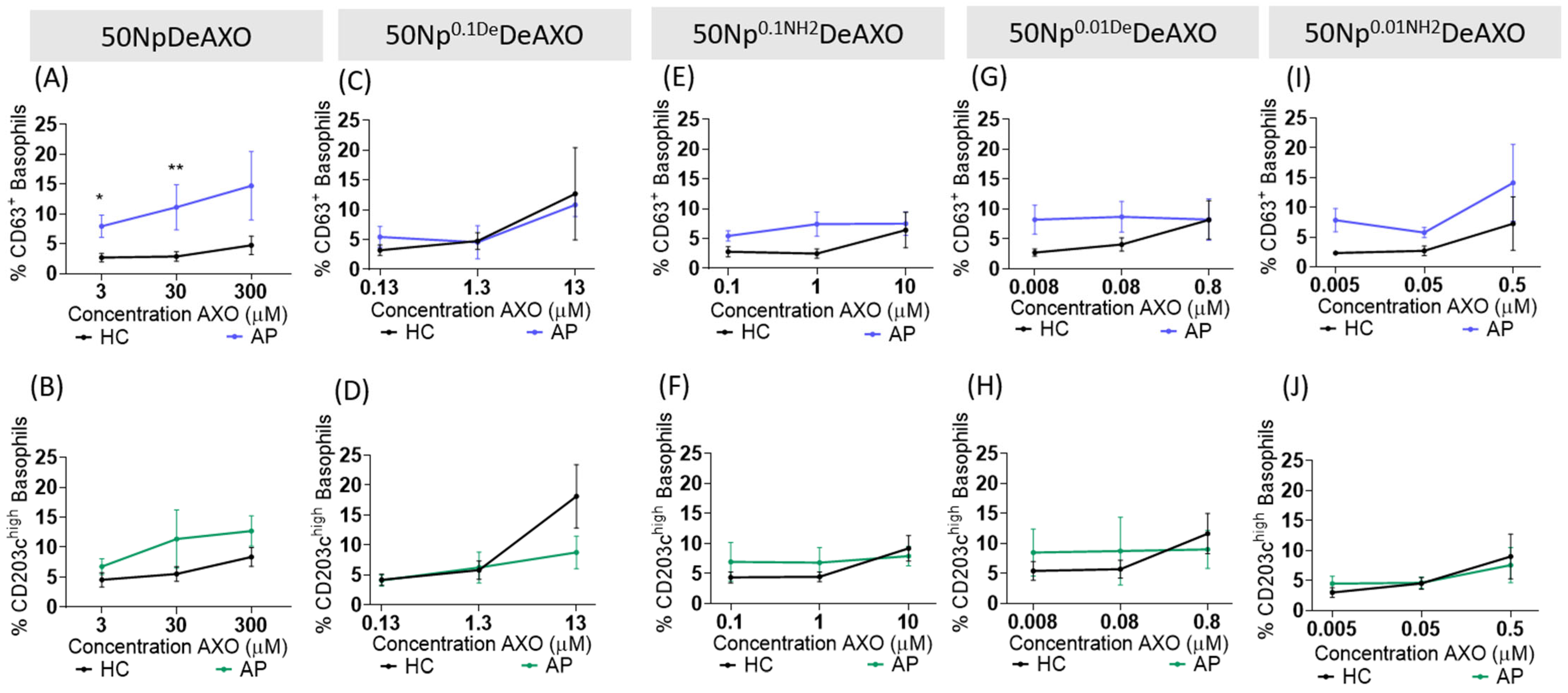
3.3. Effect of AXO Density on the Np Basophil Activation Capacity
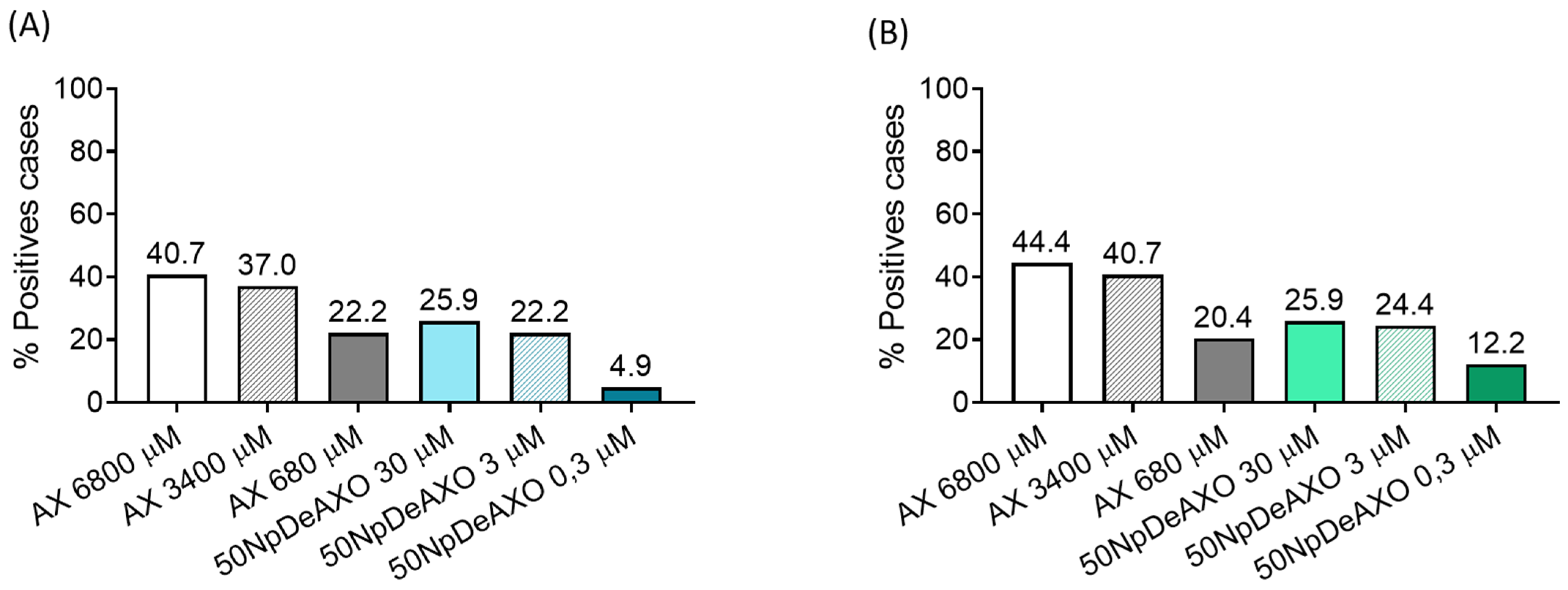
3.4. AXO Displayed in Nps is more Potent Than Free AX in Inducing Specific Basophil Activation
3.5. Evaluation of the Discrimination Capacity of Allergic Patients and Healthy Controls by 50NpDeAXO
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brunetti, V.; Bouchet, L.M.; Strumia, M.C. Nanoparticle-Cored Dendrimers: Functional Hybrid Nanocomposites as a New Platform for Drug Delivery Systems. Nanoscale 2015, 7, 3808–3816. [Google Scholar] [CrossRef]
- Shen, M.; Shi, X. Dendrimer-Based Organic/Inorganic Hybrid Nanoparticles in Biomedical Applications. Nanoscale 2010, 2, 1596–1610. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Perez-Inestrosa, E.; Molina, N.; Montañez, M.I. Development of Nanostructures in the Diagnosis of Drug Hypersensitivity Reactions. Curr. Opin. Allergy Clin. Immunol. 2016, 16, 300–307. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, C.; Perez-Inestrosa, E.; Rojo, J.; Ferrer, M.; Montañez, M.I. Role of Nanostructures in Allergy: Diagnostics, Treatments and Safety. Allergy 2021, 76, 3292–3306. [Google Scholar] [CrossRef]
- Uetrecht, J. Immune-Mediated Adverse Drug Reactions. Chem. Res. Toxicol. 2009, 22, 24–34. [Google Scholar] [CrossRef]
- Uetrecht, J. Idiosyncratic Drug Reactions: Past, Present, and Future. Chem. Res. Toxicol. 2008, 21, 84–92. [Google Scholar] [CrossRef] [PubMed]
- Doña, I.; Labella, M.; Bogas, G.; Sáenz de Santa María, R.; Salas, M.; Ariza, A.; Torres, M.J. Antibiotic Allergy De-Labeling: A Pathway against Antibiotic Resistance. Antibiotics 2022, 11, 1055. [Google Scholar] [CrossRef]
- Macy, E.; Contreras, R. Health Care Use and Serious Infection Prevalence Associated with Penicillin “Allergy” in Hospitalized Patients: A Cohort Study. J. Allergy Clin. Immunol. 2014, 133, 790–796. [Google Scholar] [CrossRef]
- Doña, I.; Torres, M.J.; Montañez, M.I.; Fernández, T.D. In Vitro Diagnostic Testing for Antibiotic Allergy. Allergy. Asthma Immunol. Res. 2017, 9, 288–298. [Google Scholar] [CrossRef]
- Mayorga, C.; Celik, G.; Rouzaire, P.; Whitaker, P.; Bonadonna, P.; Rodrigues-Cernadas, J.; Vultaggio, A.; Brockow, K.; Caubet, J.C.; Makowska, J.; et al. In Vitro Tests for Drug Hypersensitivity Reactions: An ENDA/EAACI Drug Allergy Interest Group Position Paper. Allergy 2016, 71, 1103–1134. [Google Scholar] [CrossRef]
- Ebo, D.G.; Bridts, C.H.; Mertens, C.H.; Sabato, V. Principles, Potential, and Limitations of Ex Vivo Basophil Activation by Flow Cytometry in Allergology: A Narrative Review. J. Allergy Clin. Immunol. 2021, 147, 1143–1153. [Google Scholar] [CrossRef] [PubMed]
- Santos, A.F.; Alpan, O.; Hoffmann, H.J. Basophil Activation Test: Mechanisms and Considerations for Use in Clinical Trials and Clinical Practice. Allergy 2021, 76, 2420–2432. [Google Scholar] [CrossRef] [PubMed]
- Paar, J.M.; Harris, N.T.; Holowka, D.; Baird, B. Bivalent Ligands with Rigid Double-Stranded DNA Spacers Reveal Structural Constraints on Signaling by FcεRI. J. Immunol. 2002, 169, 856–864. [Google Scholar] [CrossRef]
- Miyake, K.; Shibata, S.; Yoshikawa, S.; Karasuyama, H. Basophils and Their Effector Molecules in Allergic Disorders. Allergy 2021, 76, 1693–1706. [Google Scholar] [CrossRef] [PubMed]
- Ariza, A.; Collado, D.; Vida, Y.; Montañez, M.I.; Pérez-Inestrosa, E.; Blanca, M.; Torres, M.J.; Cañada, F.J.; Pérez-Sala, D. Study of Protein Haptenation by Amoxicillin Through the Use of a Biotinylated Antibiotic. PLoS ONE 2014, 9, e90891. [Google Scholar] [CrossRef]
- Torres, M.J.; Montañez, M.I.; Ariza, A.; Salas, M.; Fernandez, T.D.; Barbero, N.; Mayorga, C.; Blanca, M. The Role of IgE Recognition in Allergic Reactions to Amoxicillin and Clavulanic Acid. Clin. Exp. Allergy 2016, 46, 264–274. [Google Scholar] [CrossRef]
- Montañez, M.I.; Perez-Inestrosa, E.; Suau, R.; Mayorga, C.; Torres, M.J.; Blanca, M. Dendrimerized Cellulose as a Scaffold for Artificial Antigens with Applications in Drug Allergy Diagnosis. Biomacromolecules 2008, 9, 1461–1466. [Google Scholar] [CrossRef]
- Montañez, M.I.; Najera, F.; Perez-Inestrosa, E. NMR Studies and Molecular Dynamic Simulation of Synthetic Dendritic Antigens. Polymers 2011, 3, 1533–1553. [Google Scholar] [CrossRef]
- Montañez, M.I.; Najera, F.; Mayorga, C.; Ruiz-Sanchez, A.J.; Vida, Y.; Collado, D.; Blanca, M.; Torres, M.J.; Perez-Inestrosa, E. Recognition of Multiepitope Dendrimeric Antigens by Human Immunoglobulin E. Nanomed. Nanotechnol. Biol. Med. 2015, 11, 579–588. [Google Scholar] [CrossRef]
- Molina, N.; Martin-Serrano, A.; Fernandez, T.D.; Tesfaye, A.; Najera, F.; Torres, M.J.; Mayorga, C.; Vida, Y.; Montanez, M.I.; Perez-Inestrosa, E. Dendrimeric Antigens for Drug Allergy Diagnosis: A New Approach for Basophil Activation Tests. Molecules 2018, 23, 997. [Google Scholar] [CrossRef]
- Huang, Y.F.; Liu, H.; Xiong, X.; Chen, Y.; Tan, W. Nanoparticle-Mediated IgE-Receptor Aggregation and Signaling in RBL Mast Cells. J. Am. Chem. Soc. 2009, 131, 17328–17334. [Google Scholar] [CrossRef] [PubMed]
- Tesfaye, A.; Rodríguez-Nogales, A.; Benedé, S.; Fernández, T.D.; Paris, J.L.; Rodriguez, M.J.; Jiménez-Sánchez, I.M.; Bogas, G.; Mayorga, C.; Torres, M.J.; et al. Nanoarchitectures for Efficient IgE Cross-Linking on Effector Cells to Study Amoxicillin Allergy. Allergy 2021, 76, 3183–3193. [Google Scholar] [CrossRef] [PubMed]
- Baird, E.J.; Holowka, D.; Coates, G.W.; Baird, B. Highly Effective Poly(Ethylene Glycol) Architectures for Specific Inhibition of Immune Receptor Activation. Biochemistry 2003, 42, 12739–12748. [Google Scholar] [CrossRef] [PubMed]
- Gieras, A.; Linhart, B.; Roux, K.H.; Dutta, M.; Khodoun, M.; Zafred, D.; Cabauatan, C.R.; Lupinek, C.; Weber, M.; Focke-Tejkl, M.; et al. IgE Epitope Proximity Determines Immune Complex Shape and Effector Cell Activation Capacity. J. Allergy Clin. Immunol. 2016, 137, 1557–1565. [Google Scholar] [CrossRef] [PubMed]
- Gil-Ocaña, V.; Jimenez, I.M.; Mayorga, C.; Doña, I.; Céspedes, J.A.; Montañez, M.I.; Vida, Y.; Torres, M.J.; Perez-Inestrosa, E. Multiepitope Dendrimeric Antigen-Silica Particle Composites as Nano-Based Platforms for Specific Recognition of IgEs. Front. Immunol. 2021, 12, 750109. [Google Scholar] [CrossRef]
- Vida, Y.; Montañez, M.I.; Collado, D.; Najera, F.; Ariza, A.; Blanca, M.; Torres, M.J.; Mayorga, C.; Perez-Inestrosa, E. Dendrimeric Antigen–Silica Particle Composites: An Innovative Approach for IgE Quantification. J. Mater. Chem. B 2013, 1, 3044–3050. [Google Scholar] [CrossRef] [PubMed]
- Pascal, M.; Edelman, S.M.; Nopp, A.; Möbs, C.; Geilenkeuser, W.J.; Knol, E.F.; Ebo, D.G.; Mertens, C.; Shamji, M.H.; Santos, A.F.; et al. EAACI Task Force Report: A Consensus Protocol for the Basophil Activation Test for Collaboration and External Quality Assurance. Allergy 2024, 79, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Moore, S. Amino Acid Analysis: Aqueous Dimethyl Sulfoxide as Solvent for the Ninhydrin Reaction. J. Biol. Chem. 1968, 243, 6281–6283. [Google Scholar] [CrossRef]
- Riccò, R.; Nizzero, S.; Penna, E.; Meneghello, A.; Cretaio, E.; Enrichi, F. Ultra-Small Dye-Doped Silica Nanoparticles via Modified Sol-Gel Technique. J. Nanoparticle Res. 2018, 20, 117. [Google Scholar] [CrossRef]
- Cornejo-Monroy, D.; Sánchez-Ramírez, J.F.; Pescador Rojas, J.A.; Herrera-Pérez, J.L.; González- Araoz, M.P.; Guarneros, C. Nanoesferas Monodispersas de SiO2: Síntesis Controlada y Caracterización. Superf. y Vacío 2009, 22, 44–48. [Google Scholar]
- Jafarzadeh, M.; Rahman, I.A.; Sipaut, C.S. Synthesis of Silica Nanoparticles by Modified Sol-Gel Process: The Effect of Mixing Modes of the Reactants and Drying Techniques. J. Sol-Gel Sci. Technol. 2009, 50, 328–336. [Google Scholar] [CrossRef]
- Jia, G.; Ji, Z.; Wang, H.; Chen, R. Preparation and Properties of Five-Layer Graded-Refractive-Index Antireflection Coating Nanostructured by Solid and Hollow Silica Particles. Thin Solid Films 2017, 642, 174–181. [Google Scholar] [CrossRef]
- Ruiz-Sanchez, A.J.; Montañez, M.I.; Mayorga, C.; Torres, M.J.; Kehr, N.S.; Vida, Y.; Collado, D.; Najera, F.; De Cola, L.; Perez-Inestrosa, E. Dendrimer-Modified Solid Supports: Nanostructured Materials with Potential Drug Allergy Diagnostic Applications. Curr. Med. Chem. 2012, 19, 4942–4954. [Google Scholar] [CrossRef] [PubMed]
- Romano, A.; Atanaskovic-Markovic, M.; Barbaud, A.; Bircher, A.J.; Brockow, K.; Caubet, J.C.; Celik, G.; Cernadas, J.; Chiriac, A.M.; Demoly, P.; et al. Towards a More Precise Diagnosis of Hypersensitivity to Beta-Lactams—An EAACI Position Paper. Allergy 2020, 75, 1300–1315. [Google Scholar] [CrossRef]
- Brown, S.G.A. Clinical Features and Severity Grading of Anaphylaxis. J. Allergy Clin. Immunol. 2004, 114, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Aberer, W.; Bircher, A.; Romano, A.; Blanca, M.; Campi, P.; Fernandez, J.; Brockow, K.; Pichler, W.J.; Demoly, P. Drug Provocation Testing in the Diagnosis of Drug Hypersensitivity Reactions: General Considerations. Allergy 2003, 58, 854–863. [Google Scholar] [CrossRef] [PubMed]
- Sonder, S.U.; Plassmeyer, M.; Loizou, D.; Alpan, O. Towards Standardizing Basophil Identification by Flow Cytometry. Front. Allergy 2023, 4, 1133378. [Google Scholar] [CrossRef] [PubMed]
- Stöber, W.; Fink, A.; Bohn, E. Controlled Growth of Monodisperse Silica Spheres in the Micron Size Range. J. Colloid Interface Sci. 1968, 26, 62–69. [Google Scholar] [CrossRef]
- Tadanaga, K.; Morita, K.; Mori, K.; Tatsumisago, M. Synthesis of Monodispersed Silica Nanoparticles with High Concentration by the Stöber Process. J. Sol-Gel Sci. Technol. 2013, 68, 341–345. [Google Scholar] [CrossRef]




| Nps | Aspect | Diameter (nm) | AXO (µmolAXO/gNps) 1 |
|---|---|---|---|
| 20dNpDeAXO | Suspension | 20 | 290 |
| 30dNpDeAXO | Suspension | 30 | 270 2 |
| 50dNpDeAXO | Suspension | 50 | 270 2 |
| 50NpDeAXO | Solid | 50 | 300 |
| 50Np0.1DeDeAXO | Solid | 50 | 130 |
| 50Np0.01DeDeAXO | Solid | 50 | 80 |
| 50Np0.1NH2DeAXO | Solid | 50 | 100 |
| 50Np0.01NH2DeAXO | Solid | 50 | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Serrano, S.; Matamoros, E.; Céspedes, J.A.; Fernández-Santamaría, R.; Gil-Ocaña, V.; Perez-Inestrosa, E.; Frecha, C.; Montañez, M.I.; Vida, Y.; Mayorga, C.; et al. New Approaches for Basophil Activation Tests Employing Dendrimeric Antigen–Silica Nanoparticle Composites. Pharmaceutics 2024, 16, 1039. https://doi.org/10.3390/pharmaceutics16081039
Calvo-Serrano S, Matamoros E, Céspedes JA, Fernández-Santamaría R, Gil-Ocaña V, Perez-Inestrosa E, Frecha C, Montañez MI, Vida Y, Mayorga C, et al. New Approaches for Basophil Activation Tests Employing Dendrimeric Antigen–Silica Nanoparticle Composites. Pharmaceutics. 2024; 16(8):1039. https://doi.org/10.3390/pharmaceutics16081039
Chicago/Turabian StyleCalvo-Serrano, Silvia, Esther Matamoros, Jose Antonio Céspedes, Rubén Fernández-Santamaría, Violeta Gil-Ocaña, Ezequiel Perez-Inestrosa, Cecilia Frecha, Maria I. Montañez, Yolanda Vida, Cristobalina Mayorga, and et al. 2024. "New Approaches for Basophil Activation Tests Employing Dendrimeric Antigen–Silica Nanoparticle Composites" Pharmaceutics 16, no. 8: 1039. https://doi.org/10.3390/pharmaceutics16081039






