Dopamine and Citicoline-Co-Loaded Solid Lipid Nanoparticles as Multifunctional Nanomedicines for Parkinson’s Disease Treatment by Intranasal Administration
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of DA-CIT-SLNs-60
2.3. Quantification of DA and CIT
2.4. Physicochemical Characterization of SLNs
2.5. Solid-State Studies
2.5.1. Fourier Transform Infrared (FT-IR) Spectroscopy
2.5.2. Differential Scanning Calorimetry (DSC)
2.5.3. X-ray Powder Diffraction (XRPD)
2.6. Physical Stability of DA-CIT-SLNs
2.7. In Vitro Release Tests
2.8. Cytotoxicity Assessment in RPMI 2650 and in SH-SY5Y Cell Model Lines
2.9. Permeation Studies across RPMI-2650 Cell Monolayer
2.10. DPPH Assay for In Vitro Antioxidant Activity Evaluation
2.11. OxyBlot Assay
2.12. Statistics
3. Results
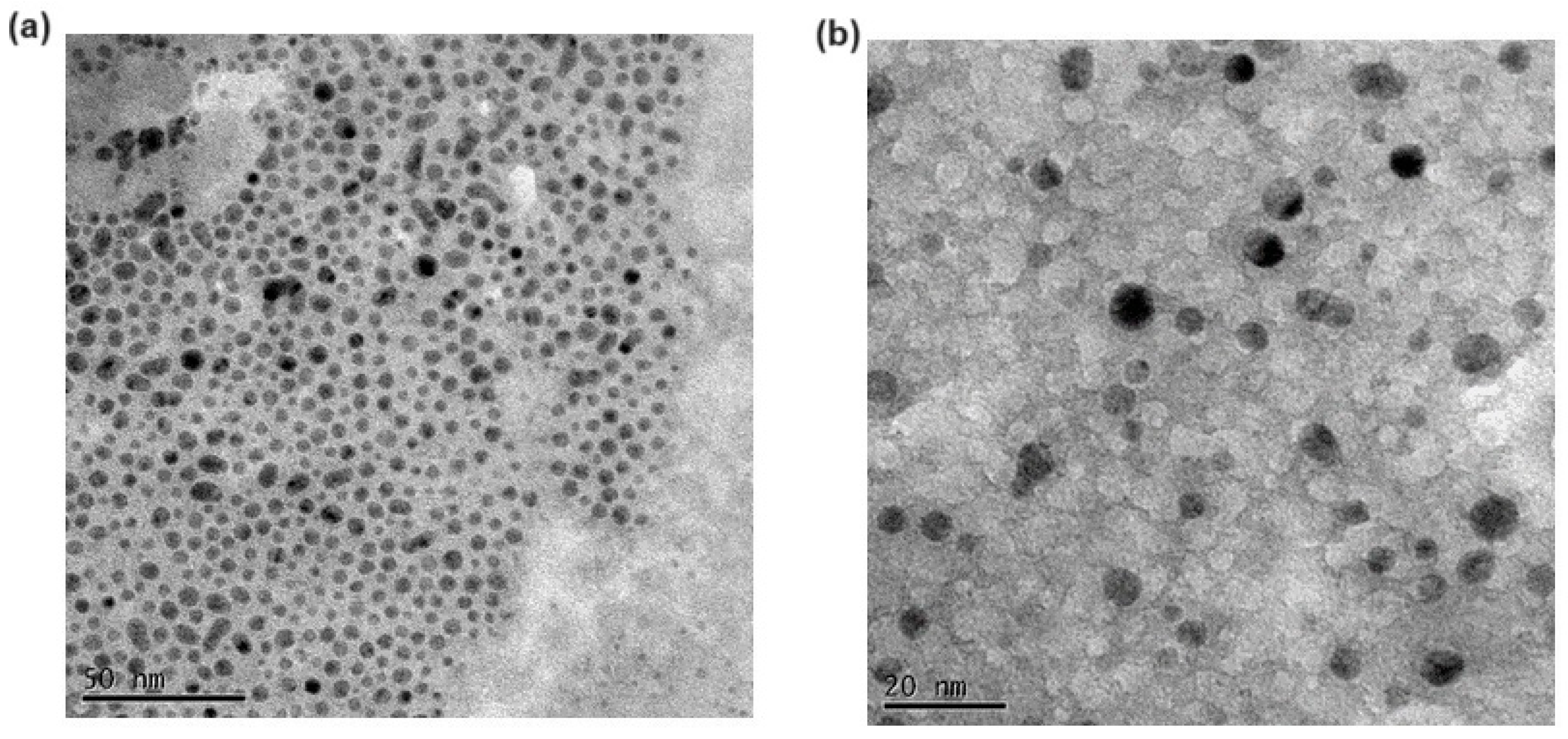
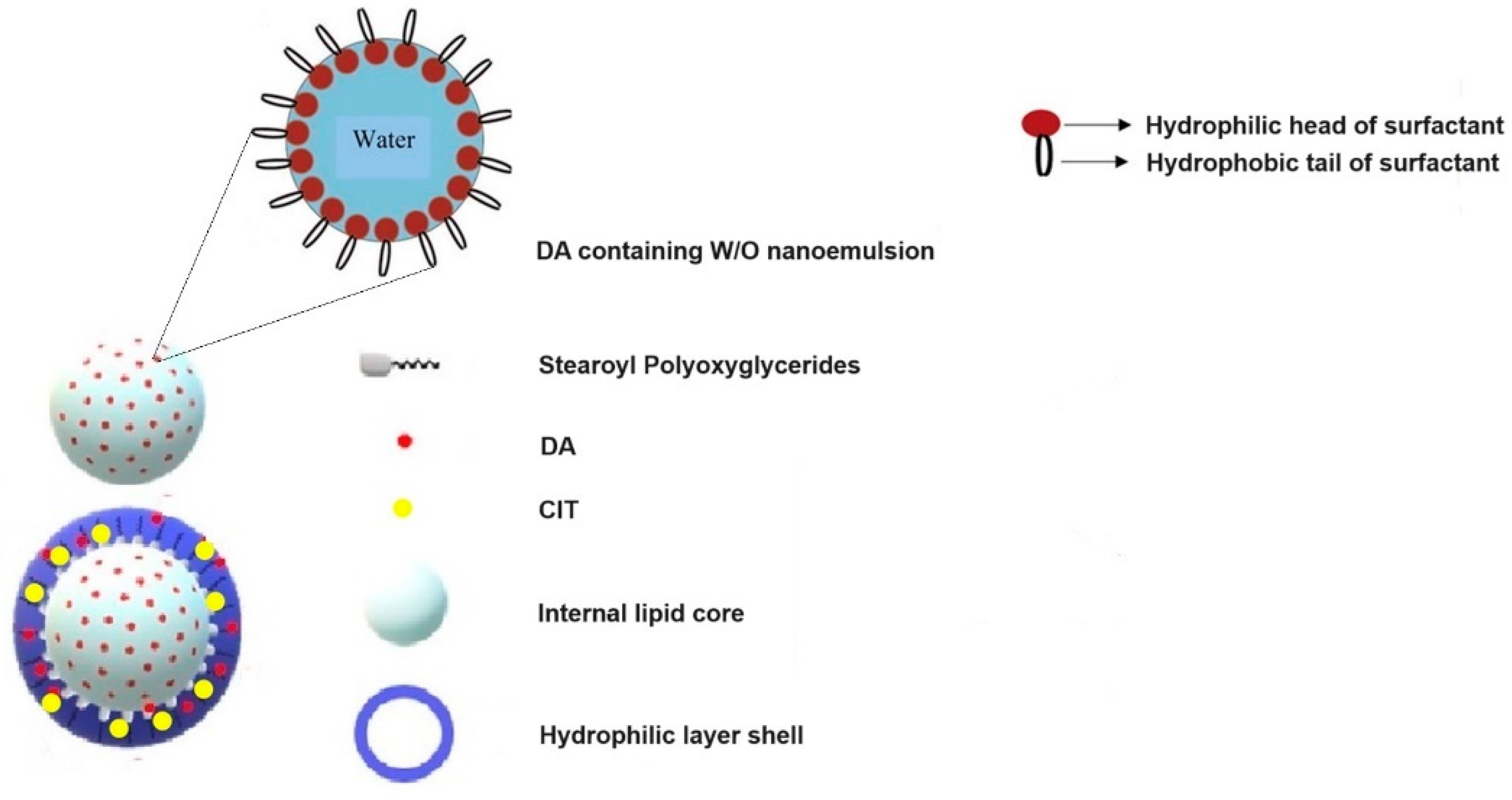
3.1. Preparation and Characterization of SLNs
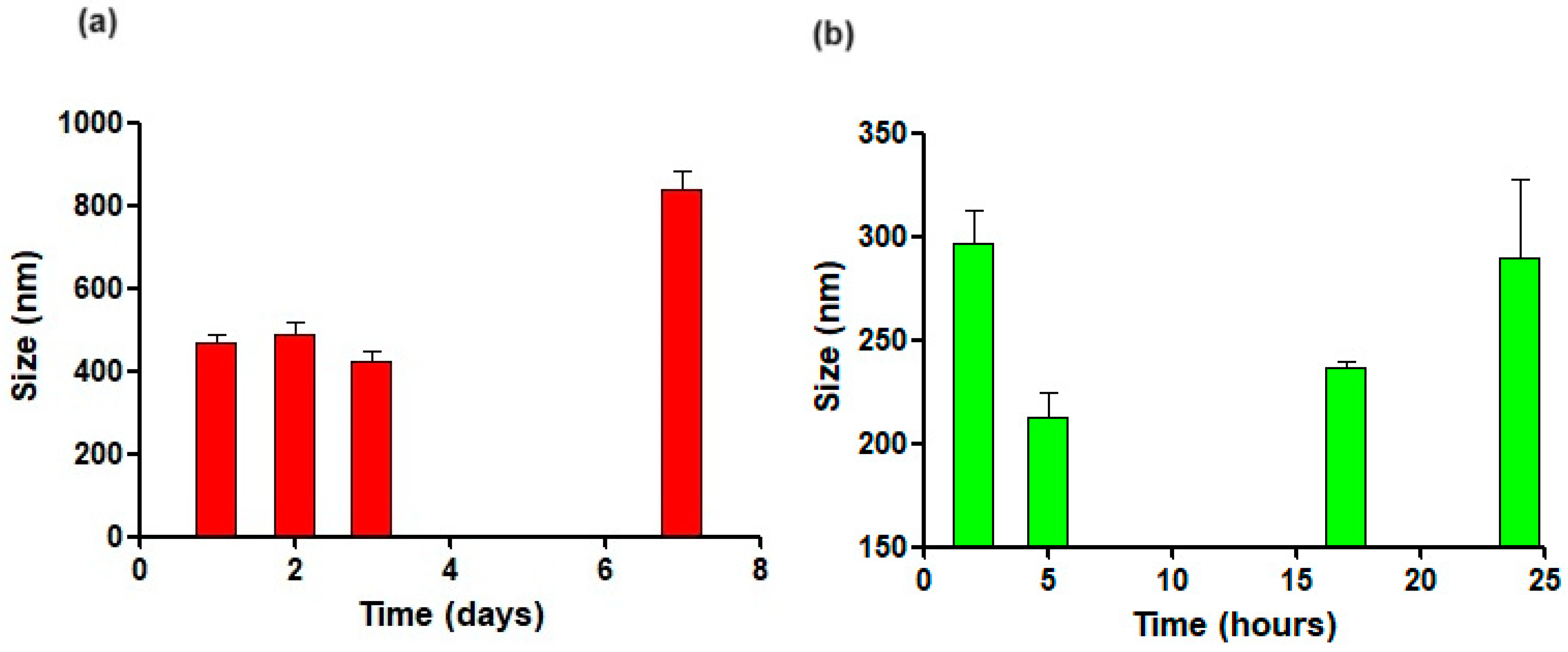
3.2. Physical Stability
3.3. Solid-State Studies
3.3.1. FT-IR Spectroscopy
3.3.2. DSC Analysis
3.3.3. X-ray Diffraction Spectra
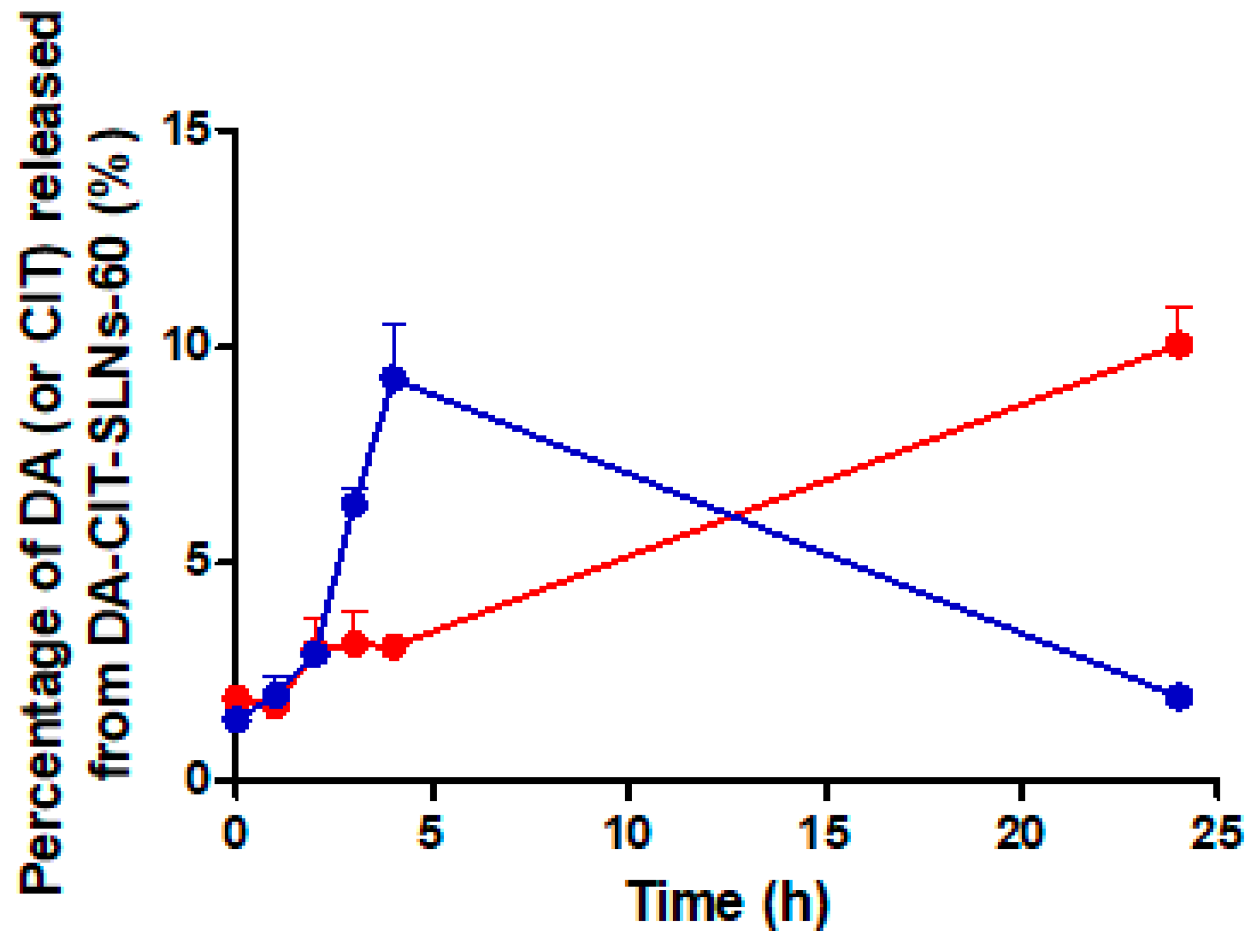
3.4. In Vitro Release Studies of DA-CIT-SLNs-60
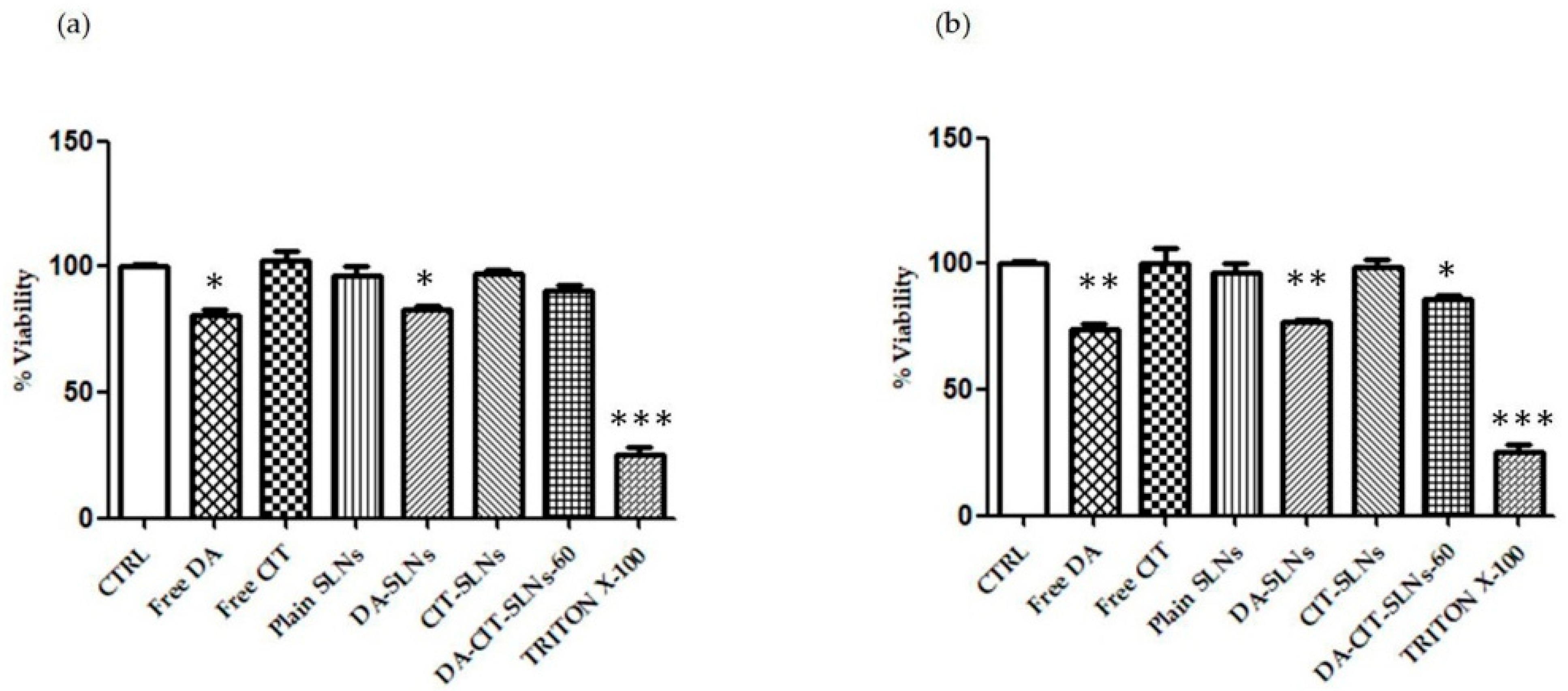
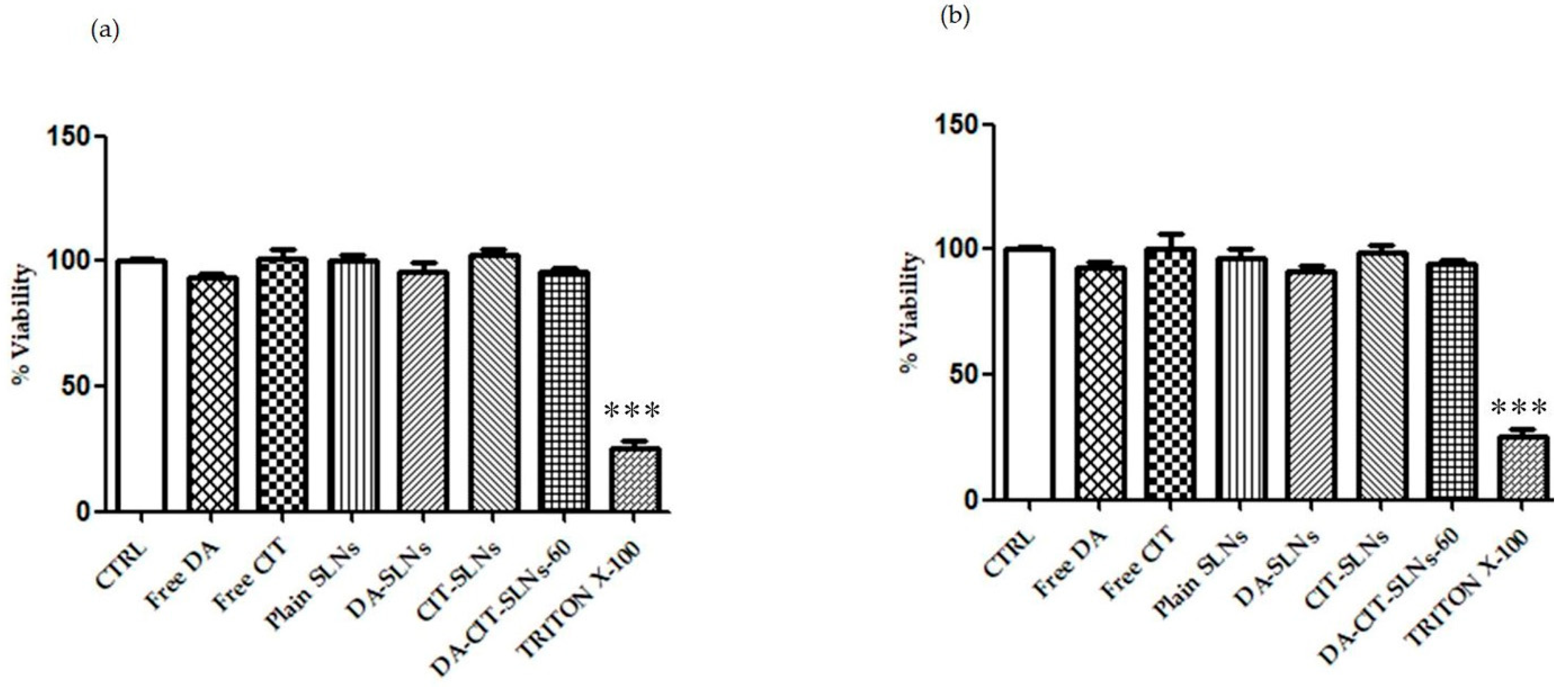
3.5. Cytobiocompatibility in SH-SY5Y and RPMI 2650 Cells
3.6. Permeation Studies across RPMI-2650 Cell Monolayer
3.7. DPPH Test and Antioxidant Activity
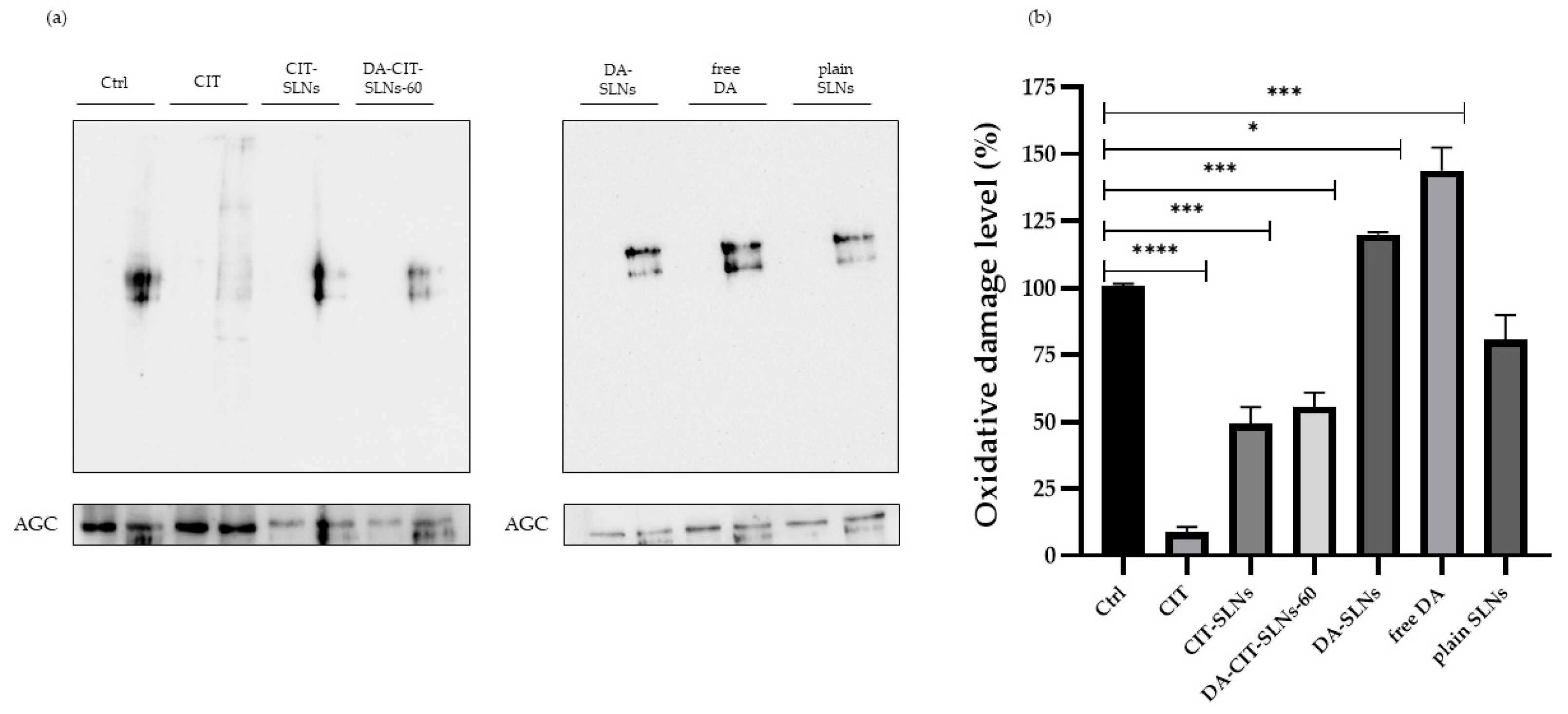
3.8. OxyBlot Assay in SHSY-5Y Cell Line
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Al-Kuraishy, H.M.; Al-Gareeb, A.I. Citicoline Improves Human Vigilance and Visual Working Memory: The Role of Neuronal Activation and Oxidative Stress. Basic Clin. Neurosci. 2020, 11, 423–432. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Salam, O.M.E.; Youness, E.R.; Mohammed, N.A.; Abd El-Moneim, O.M.; Shaffie, N. Citicoline Protects against Tramadol-Induced Oxidative Stress and Organ Damage. React. Oxyg. Species 2019, 7, 106–120. [Google Scholar] [CrossRef]
- Mozafari, N.; Farjadian, F.; Mohammadi Samani, S.; Azadi, S.; Azadi, A. Simvastatin-chitosan-citicoline conjugates nanoparticles as the co-delivery system in Alzheimer susceptible patients. Int. J. Biol. Macromol. 2020, 156, 1396–1407. [Google Scholar] [CrossRef] [PubMed]
- Que, D.S.; Jamora, R.D.G. Citicoline as Adjuvant Therapy in Parkinson’s Disease: A Systematic Review. Clin. Ther. 2021, 43, e19–e31. [Google Scholar] [CrossRef] [PubMed]
- Agulla, J.; Brea, D.; Campos, F.; Sobrino, T.; Argibay, B.; Al-Soufi, W.; Blanco, M.; Castillo, J.; Ramos-Cabrer, P. In vivo theranostics at the peri-infarct region in cerebral ischemia. Theranostics 2013, 4, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Wold Health Organization (WHO). Over 1 in 3 People Affected by Neurological Conditions, the Leading Cause of Illness and Disability Worldwid. Available online: http://www.who.int. (accessed on 9 July 2024).
- Kaur, G.; Arora, M.; Ravi Kumar, M.N.V. Oral Drug Delivery Technologies-A Decade of Developments. J. Pharmacol. Exp. Ther. 2019, 370, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Trapani, G.; Franco, M.; Trapani, A.; Lopedota, A.; Latrofa, A.; Gallucci, E.; Micelli, S.; Liso, G. Frog intestinal sac: A new in vitro method for the assessment of intestinal permeability. J. Pharm. Sci. 2004, 93, 2909–2919. [Google Scholar] [CrossRef] [PubMed]
- Keller, L.A.; Merkel, O.; Popp, A. Intranasal drug delivery: Opportunities and toxicologic challenges during drug development. Drug Deliv. Transl. Res. 2022, 12, 735–757. [Google Scholar] [CrossRef] [PubMed]
- Crowe, T.P.; Hsu, W.H. Evaluation of Recent Intranasal Drug Delivery Systems to the Central Nervous System. Pharmaceutics 2022, 14, 629. [Google Scholar] [CrossRef]
- Koo, J.; Lim, C.; Oh, K.T. Recent Advances in Intranasal Administration for Brain-Targeting Delivery: A Comprehensive Review of Lipid-Based Nanoparticles and Stimuli-Responsive Gel Formulations. Int. J. Nanomed. 2024, 19, 1767–1807. [Google Scholar] [CrossRef]
- Samal, J.; Rebelo, A.L.; Pandit, A. A window into the brain: Tools to assess pre-clinical efficacy of biomaterials-based therapies on central nervous system disorders. Adv. Drug Deliv. Rev. 2019, 148, 68–145. [Google Scholar] [CrossRef]
- Singh, A.; Kutscher, H.L.; Bulmahn, J.C.; Mahajan, S.D.; He, G.S.; Prasad, P.N. Laser ablation for pharmaceutical nanoformulations: Multi-drug nanoencapsulation and theranostics for HIV. Nanomedicine 2020, 25, 102172. [Google Scholar] [CrossRef]
- Ancona, A.; Sportelli, M.; Trapani, A.; Picca, R.A.; Palazzo, C.; Bonerba, E.; Mezzapesa, F.; Tantillo, G.; Trapani, G.; Cioffi, N. Synthesis and Characterization of Hybrid Copper-Chitosan Nano-antimicrobials by Femtosecond Laser-Ablation in Liquids. Mater. Lett. 2014, 136, 397–400. [Google Scholar] [CrossRef]
- Faria, P.; Pacheco, C.; Moura, R.P.; Sarmento, B.; Martins, C. Multifunctional nanomedicine strategies to manage brain diseases. Drug Deliv. Transl. Res. 2023, 13, 1322–1342. [Google Scholar] [CrossRef]
- Park, M.W.; Cha, H.W.; Kim, J.; Kim, J.H.; Yang, H.; Yoon, S.; Boonpraman, N.; Yi, S.S.; Yoo, I.D.; Moon, J.S. NOX4 promotes ferroptosis of astrocytes by oxidative stress-induced lipid peroxidation via the impairment of mitochondrial metabolism in Alzheimer’s diseases. Redox Biol. 2021, 41, 101947. [Google Scholar] [CrossRef]
- Rodriguez-Nogales, C.; Garbayo, E.; Carmona-Abellan, M.M.; Luquin, M.R.; Blanco-Prieto, M.J. Brain aging and Parkinson’s disease: New therapeutic approaches using drug delivery systems. Maturitas 2016, 84, 25–31. [Google Scholar] [CrossRef]
- Bonferoni, M.C.; Rassu, G.; Gavini, E.; Sorrenti, M.; Catenacci, L.; Giunchedi, P. Nose-to-Brain Delivery of Antioxidants as a Potential Tool for the Therapy of Neurological Diseases. Pharmaceutics 2020, 12, 1246. [Google Scholar] [CrossRef]
- Qadri, R.; Goyal, V.; Behari, M.; Subramanian, A.; Datta, S.K.; Mukhopadhyay, A.K. Alteration of Mitochondrial Function in Oxidative Stress in Parkinsonian Neurodegeneration: A Cross-Sectional Study. Ann. Indian Acad. Neurol. 2021, 24, 506–512. [Google Scholar]
- Wen, P.; Ren, C. Research progress on intranasal treatment for Parkinson’s disease. Neuroprotection 2024, 2, 79–99. [Google Scholar] [CrossRef]
- Trapani, A.; Castellani, S.; Guerra, L.; De Giglio, E.; Fracchiolla, G.; Corbo, F.; Cioffi, N.; Passantino, G.; Poeta, M.L.; Montemurro, P.; et al. Combined Dopamine and Grape Seed Extract-Loaded Solid Lipid Nanoparticles: Nasal Mucosa Permeation, and Uptake by Olfactory Ensheathing Cells and Neuronal SH-SY5Y Cells. Pharmaceutics 2023, 15, 881. [Google Scholar] [CrossRef]
- Margari, A.; Monteduro, A.G.; Rizzato, S.; Capobianco, L.; Crestini, A.; Rivabene, R.; Piscopo, P.; D‘Onofrio, M.; Manzini, V.; Trapani, G.; et al. The Encapsulation of Citicoline within Solid Lipid Nanoparticles Enhances Its Capability to Counteract the 6-Hydroxydopamine-Induced Cytotoxicity in Human Neuroblastoma SH-SY5Y Cells. Pharmaceutics 2022, 14, 1827. [Google Scholar] [CrossRef]
- Satapathy, M.K.; Yen, T.L.; Jan, J.S.; Tang, R.D.; Wang, J.Y.; Taliyan, R.; Yang, C.H. Solid Lipid Nanoparticles (SLNs): An Advanced Drug Delivery System Targeting Brain through BBB. Pharmaceutics 2021, 13, 1183. [Google Scholar] [CrossRef]
- Trapani, A.; Mandracchia, D.; Tripodo, G.; Di Gioia, S.; Castellani, S.; Cioffi, N.; Ditaranto, N.; Esteban, M.A.; Conese, M. Solid lipid nanoparticles made of self-emulsifying lipids for efficient encapsulation of hydrophilic substances. In AIP Conference Proceedings; AIP Publishing: College Park, MD, USA, 2019; p. 020004. [Google Scholar]
- Trapani, A.; De Giglio, E.; Cometa, S.; Bonifacio, M.A.; Dazzi, L.; Di Gioia, S.; Hossain, M.N.; Pellitteri, R.; Antimisiaris, S.G.; Conese, M. Dopamine-loaded lipid based nanocarriers for intranasal administration of the neurotransmitter: A comparative study. Eur. J. Pharm. Biopharm. 2021, 167, 189–200. [Google Scholar] [CrossRef]
- De Giglio, E.; Bakowsky, U.; Engelhardt, K.; Caponio, A.; La Pietra, M.; Cometa, S.; Castellani, S.; Guerra, L.; Fracchiolla, G.; Poeta, M.L.; et al. Solid Lipid Nanoparticles Containing Dopamine and Grape Seed Extract: Freeze-Drying with Cryoprotection as a Formulation Strategy to Achieve Nasal Powders. Molecules 2023, 28, 7706. [Google Scholar] [CrossRef]
- Cometa, S.; Bonifacio, M.A.; Trapani, G.; Di Gioia, S.; Dazzi, L.; De Giglio, E.; Trapani, A. In vitro investigations on dopamine loaded Solid Lipid Nanoparticles. J. Pharm. Biomed. Anal. 2020, 185, 113257. [Google Scholar] [CrossRef]
- Trapani, A.; De Giglio, E.; Cafagna, D.; Denora, N.; Agrimi, G.; Cassano, T.; Gaetani, S.; Cuomo, V.; Trapani, G. Characterization and evaluation of chitosan nanoparticles for dopamine brain delivery. Int. J. Pharm. 2011, 419, 296–307. [Google Scholar] [CrossRef]
- Trapani, A.; Cometa, S.; De Giglio, E.; Corbo, F.; Cassano, R.; Di Gioia, M.L.; Trombino, S.; Hossain, M.N.; Di Gioia, S.; Trapani, G.; et al. Novel Nanoparticles Based on N,O-Carboxymethyl Chitosan-Dopamine Amide Conjugate for Nose-to-Brain Delivery. Pharmaceutics 2022, 14, 147. [Google Scholar] [CrossRef]
- Trapani, A.; Corbo, F.; Agrimi, G.; Ditaranto, N.; Cioffi, N.; Perna, F.; Quivelli, A.; Stefano, E.; Lunetti, P.; Muscella, A.; et al. Oxidized Alginate Dopamine Conjugate: In Vitro Characterization for Nose-to-Brain Delivery Application. Materials 2021, 14, 3495. [Google Scholar] [CrossRef]
- Stephenson, A.P.; Schneider, J.A.; Nelson, B.C.; Atha, D.H.; Jain, A.; Soliman, K.F.; Aschner, M.; Mazzio, E.; Renee Reams, R. Manganese-induced oxidative DNA damage in neuronal SH-SY5Y cells: Attenuation of thymine base lesions by glutathione and N-acetylcysteine. Toxicol. Lett. 2013, 218, 299–307. [Google Scholar] [CrossRef]
- Wan, X.; Wang, W.; Liang, Z. Epigallocatechin-3-gallate inhibits the growth of three-dimensional in vitro models of neuroblastoma cell SH-SY5Y. Mol. Cell. Biochem. 2021, 476, 3141–3148. [Google Scholar] [CrossRef]
- Yamashita, K.; Kiyonari, S.; Tsubota, S.; Kishida, S.; Sakai, R.; Kadomatsu, K. Thymidylate synthase inhibitor raltitrexed can induce high levels of DNA damage in MYCN-amplified neuroblastoma cells. Cancer Sci. 2020, 111, 2431–2439. [Google Scholar] [CrossRef]
- Mercier, C.; Hodin, S.; He, Z.; Perek, N.; Delavenne, X. Pharmacological Characterization of the RPMI 2650 Model as a Relevant Tool for Assessing the Permeability of Intranasal Drugs. Mol. Pharm. 2018, 15, 2246–2256. [Google Scholar] [CrossRef]
- Kreft, M.E.; Jerman, U.D.; Lasic, E.; Lanisnik Rizner, T.; Hevir-Kene, N.; Peternel, L.; Kristan, K. The characterization of the human nasal epithelial cell line RPMI 2650 under different culture conditions and their optimization for an appropriate in vitro nasal model. Pharm. Res. 2015, 32, 665–679. [Google Scholar] [CrossRef]
- Fir, M.; Milivojevic, L.; Prosek, M.; Smidovnik, A. Properties Studies of Coenzyme Q10-Cyclodextrins complexes. Acta Chim. Slov. 2009, 56, 885–891. [Google Scholar]
- Aresta, A.; Calvano, C.D.; Trapani, A.; Cellamare, S.; Zambonin, C.G.; De Giglio, E. Development and analytical characterization of vitamin(s)-loaded chitosan nanoparticles for potential food packaging applications. J. Nanopart. Res. 2013, 15, 1592. [Google Scholar] [CrossRef]
- Lunetti, P.; Di Giacomo, M.; Vergara, D.; De Domenico, S.; Maffia, M.; Zara, V.; Capobianco, L.; Ferramosca, A. Metabolic reprogramming in breast cancer results in distinct mitochondrial bioenergetics between luminal and basal subtypes. FEBS J. 2019, 286, 688–709. [Google Scholar] [CrossRef]
- Trapani, A.; Tripodo, G.; Mandracchia, D.; Cioffi, N.; Ditaranto, N.; De Leo, V.; Cordero, H.; Estebane, M.A. Glutathione-loaded solid lipid nanoparticles based on Gelucire® 50/13: Spectroscopic characterization and interactions with fish cells. J. Drug Deliv. Sci. Technol. 2018, 47, 359–366. [Google Scholar] [CrossRef]
- Di Gioia, S.; Trapani, A.; Cassano, R.; Di Gioia, M.L.; Trombino, S.; Cellamare, S.; Bolognino, I.; Hossain, M.N.; Sanna, E.; Trapani, G.; et al. Nose-to-brain delivery: A comparative study between carboxymethyl chitosan based conjugates of dopamine. Int. J. Pharm. 2021, 599, 120453. [Google Scholar] [CrossRef]
- Trapani, A.; Mandracchia, D.; Tripodo, G.; Cometa, S.; Cellamare, S.; De Giglio, E.; Klepetsanis, P.; Antimisiaris, S.G. Protection of dopamine towards autoxidation reaction by encapsulation into non-coated- or chitosan- or thiolated chitosan-coated-liposomes. Colloids Surf. B Biointerfaces 2018, 170, 11–19. [Google Scholar] [CrossRef]
- Trapani, A.; Guerra, L.; Corbo, F.; Castellani, S.; Sanna, E.; Capobianco, L.; Monteduro, A.G.; Manno, D.E.; Mandracchia, D.; Di Gioia, S.; et al. Cyto/Biocompatibility of Dopamine Combined with the Antioxidant Grape Seed-Derived Polyphenol Compounds in Solid Lipid Nanoparticles. Molecules 2021, 26, 916. [Google Scholar] [CrossRef]
- Lerch, H.G. Hyperhydrated Citicoline, Process and Use. U.S. Patent US006057301A, 2 May 2000. [Google Scholar]
- Banerjee, K.; Munshi, S.; Sen, O.; Pramanik, V.; Roy Mukherjee, T.; Chakrabarti, S. Dopamine Cytotoxicity Involves Both Oxidative and Nonoxidative Pathways in SH-SY5Y Cells: Potential Role of Alpha-Synuclein Overexpression and Proteasomal Inhibition in the Etiopathogenesis of Parkinson’s Disease. Park. Dis. 2014, 2014, 878935. [Google Scholar] [CrossRef]
- Shavali, S.; Sens, D.A. Synergistic neurotoxic effects of arsenic and dopamine in human dopaminergic neuroblastoma SH-SY5Y cells. Toxicol. Sci. 2008, 102, 254–261. [Google Scholar] [CrossRef]
- Stadtman, E.R. Oxidation of free amino acids and amino acid residues in proteins by radiolysis and by metal-catalyzed reactions. Annu. Rev. Biochem. 1993, 62, 797–821. [Google Scholar] [CrossRef]
- Yuan, H.; Chen, C.Y.; Chai, G.H.; Du, Y.Z.; Hu, F.Q. Improved transport and absorption through gastrointestinal tract by PEGylated solid lipid nanoparticles. Mol. Pharm. 2013, 10, 1865–1873. [Google Scholar] [CrossRef]
- Matougui, N.; Boge, L.; Groo, A.C.; Umerska, A.; Ringstad, L.; Bysell, H.; Saulnier, P. Lipid-based nanoformulations for peptide delivery. Int. J. Pharm. 2016, 502, 80–97. [Google Scholar] [CrossRef]
- Garg, G.; Garg, S.; Patel, P.; Gupta, G.D.; Kurmi, B.D. Advances in solid-lipid nanoparticle chemistry as drug delivery vehicles. Int. J. Polym. Mater. Polym. Biomater. 2024, 1–15. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Ahmed, A.B.; Omar, M.A.; Derayea, S.M.; Abdelwahab, N.S. Development and validation of stability indicating chromatographic methods for simultaneous determination of citicoline and piracetam. J. Sep. Sci. 2020, 43, 2981–2988. [Google Scholar] [CrossRef]
- Umek, N.; Gersak, B.; Vintar, N.; Sostaric, M.; Mavri, J. Dopamine Autoxidation Is Controlled by Acidic pH. Front. Mol. Neurosci. 2018, 11, 467. [Google Scholar] [CrossRef]
- Monteduro, A.G.; Rizzato, S.; Caragnano, G.; Trapani, A.; Giannelli, G.; Maruccio, G. Organs-on-chips technologies—A guide from disease models to opportunities for drug development. Biosens. Bioelectron. 2023, 231, 115271. [Google Scholar] [CrossRef]
- Padilla-Godinez, F.J.; Ruiz-Ortega, L.I.; Guerra-Crespo, M. Nanomedicine in the Face of Parkinson’s Disease: From Drug Delivery Systems to Nanozymes. Cells 2022, 11, 3445. [Google Scholar] [CrossRef]
- Shakeri, M.; Ghobadi, R.; Sohrabvandi, S.; Khanniri, E.; Mollakhalili-Meybodi, N. Co-encapsulation of omega-3 and vitamin D(3) in beeswax solid lipid nanoparticles to evaluate physicochemical and in vitro release properties. Front. Nutr. 2024, 11, 1323067. [Google Scholar] [CrossRef]
- Grieb, P. Neuroprotective properties of citicoline: Facts, doubts and unresolved issues. CNS Drugs 2014, 28, 185–193. [Google Scholar] [CrossRef]
- Secades, J.J.; Gareri, P. Citicoline: Pharmacological and clinical review, 2022 update. Rev. Neurol. 2022, 75, S1–S89. [Google Scholar]
- Ganguly, U.; Ganguly, A.; Sen, O.; Ganguly, G.; Cappai, R.; Sahoo, A.; Chakrabarti, S. Dopamine Cytotoxicity on SH-SY5Y Cells: Involvement of alpha-Synuclein and Relevance in the Neurodegeneration of Sporadic Parkinson’s Disease. Neurotox. Res. 2019, 35, 898–907. [Google Scholar] [CrossRef]
- Saladino, G.M.; Kilic, N.I.; Brodin, B.; Hamawandi, B.; Yazgan, I.; Hertz, H.M.; Toprak, M.S. Carbon Quantum Dots Conjugated Rhodium Nanoparticles as Hybrid Multimodal Contrast Agents. Nanomaterials 2021, 11, 2165. [Google Scholar] [CrossRef]
- Tincu, R.; Mihaila, M.; Bostan, M.; Teodorescu, F.; Istrati, D.; Badea, N.; Lacatusu, I. Novel Bovine Serum Albumin-Decorated-Nanostructured Lipid Carriers Able to Modulate Apoptosis and Cell-Cycle Response in Ovarian, Breast, and Colon Tumoral Cells. Pharmaceutics 2023, 15, 1125. [Google Scholar] [CrossRef]
- Lacatusu, I.; Badea, N.; Badea, G.; Mihaila, M.; Ott, C.; Stan, R.; Meghea, A. Advanced bioactive lipid nanocarriers loaded with natural and synthetic anti-inflammatory actives. Chem. Eng. Sci. 2019, 200, 113–126. [Google Scholar] [CrossRef]
- Costa, C.P.; Barreiro, S.; Moreira, J.N.; Silva, R.; Almeida, H.; Sousa Lobo, J.M.; Silva, A.C. In Vitro Studies on Nasal Formulations of Nanostructured Lipid Carriers (NLC) and Solid Lipid Nanoparticles (SLN). Pharmaceuticals 2021, 14, 711. [Google Scholar] [CrossRef]
- Qian, K.; Gu, Y.; Zhao, Y.; Li, Z.; Sun, M. Citicoline protects brain against closed head injury in rats through suppressing oxidative stress and calpain over-activation. Neurochem. Res. 2014, 39, 1206–1218. [Google Scholar] [CrossRef]
- Olufunmilayo, E.O.; Gerke-Duncan, M.B.; Holsinger, R.M.D. Oxidative Stress and Antioxidants in Neurodegenerative Disorders. Antioxidants 2023, 12, 517. [Google Scholar] [CrossRef]
- Oikawa, S.; Kobayashi, H.; Kitamura, Y.; Zhu, H.; Obata, K.; Minabe, Y.; Dazortsava, M.; Ohashi, K.; Tada-Oikawa, S.; Takahashi, H.; et al. Proteomic analysis of carbonylated proteins in the monkey substantia nigra after ischemia-reperfusion. Free Radic. Res. 2014, 48, 694–705. [Google Scholar] [CrossRef]
- Bellucci, S.; Fracchiolla, G.; Pannunzio, A.; Caponio, A.; Donghia, D.; Corbo, F.; Capobianco, L.; Muscella, A.; Manno, D.E.; Stefàno, E.; et al. Dopamine and Antioxidant Grape Seed Extract loaded chitosan nanoparticles: A preliminary in vitro characterization. Nano Med. Mater. 2023, 3, 40. [Google Scholar] [CrossRef]
| Formulation | Size (nm) | PDI b | Zeta Potential (mV) | E.E. DA (%) | E.E. CIT (%) |
|---|---|---|---|---|---|
| DA-CIT-SLNs-60 | 131 ± 20 | 0.40 ± 0.04 | −10.2 ± 1.1 | 77 ± 7 | 75 ± 2 |
| DA-CIT-SLNs-120 | 405 ± 25 ** | 0.53 ± 0.01 | −7.8 ± 0.4 | 65 ± 3 | 59 ± 8 |
| DA-SLNs c | 171 ± 6 ** | 0.20 ± 0.01 | −2.0 ± 0.7 ** | 19 ± 3 | - |
| CIT-SLNs d | 201 ± 24 ** | 0.45 ± 0.08 | −2.2 ± 0.2 ** | - | 80 ± 7 |
| Plain SLNs e | 141 ± 11 | 0.35 ± 0.17 | −9.7 ± 0.8 | - | - |
| Formulation | Papp DA (cm/s) | Papp CIT (cm/s) |
|---|---|---|
| DA-CIT-SLNs-60 | 0.0369 (±0.041 × 10−4) | <LOQ |
| DA-SLNs | 0.0109 (±0.040 × 10−4) | - |
| CIT-SLNs | - | 0.0577 (±6 × 10−8) |
| Free-DA | NP a | |
| Free CIT | 0.0413 (±6 × 10−8) |
| Formulation | Antioxidant Activity (%) |
|---|---|
| CIT | 88.6 ± 0.6 |
| Gelucire®50/13 a | 55.0 ± 0.2 |
| Plain SLNs a | 72.8 ± 5.3 |
| CIT-SLNs | 93.2 ± 5.0 |
| DA-SLN a | 54.7 ± 2.5 |
| DA-CIT-SLNs-60 | 91.3 ± 1.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Castellani, S.; Iaconisi, G.N.; Tripaldi, F.; Porcelli, V.; Trapani, A.; Messina, E.; Guerra, L.; Di Franco, C.; Maruccio, G.; Monteduro, A.G.; et al. Dopamine and Citicoline-Co-Loaded Solid Lipid Nanoparticles as Multifunctional Nanomedicines for Parkinson’s Disease Treatment by Intranasal Administration. Pharmaceutics 2024, 16, 1048. https://doi.org/10.3390/pharmaceutics16081048
Castellani S, Iaconisi GN, Tripaldi F, Porcelli V, Trapani A, Messina E, Guerra L, Di Franco C, Maruccio G, Monteduro AG, et al. Dopamine and Citicoline-Co-Loaded Solid Lipid Nanoparticles as Multifunctional Nanomedicines for Parkinson’s Disease Treatment by Intranasal Administration. Pharmaceutics. 2024; 16(8):1048. https://doi.org/10.3390/pharmaceutics16081048
Chicago/Turabian StyleCastellani, Stefano, Giorgia Natalia Iaconisi, Francesca Tripaldi, Vito Porcelli, Adriana Trapani, Eugenia Messina, Lorenzo Guerra, Cinzia Di Franco, Giuseppe Maruccio, Anna Grazia Monteduro, and et al. 2024. "Dopamine and Citicoline-Co-Loaded Solid Lipid Nanoparticles as Multifunctional Nanomedicines for Parkinson’s Disease Treatment by Intranasal Administration" Pharmaceutics 16, no. 8: 1048. https://doi.org/10.3390/pharmaceutics16081048
APA StyleCastellani, S., Iaconisi, G. N., Tripaldi, F., Porcelli, V., Trapani, A., Messina, E., Guerra, L., Di Franco, C., Maruccio, G., Monteduro, A. G., Corbo, F., Di Gioia, S., Trapani, G., & Conese, M. (2024). Dopamine and Citicoline-Co-Loaded Solid Lipid Nanoparticles as Multifunctional Nanomedicines for Parkinson’s Disease Treatment by Intranasal Administration. Pharmaceutics, 16(8), 1048. https://doi.org/10.3390/pharmaceutics16081048











