Biomineralized MnO2 Nanoparticle-Constituted Hydrogels Promote Spinal Cord Injury Repair by Modulating Redox Microenvironment and Inhibiting Ferroptosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Method
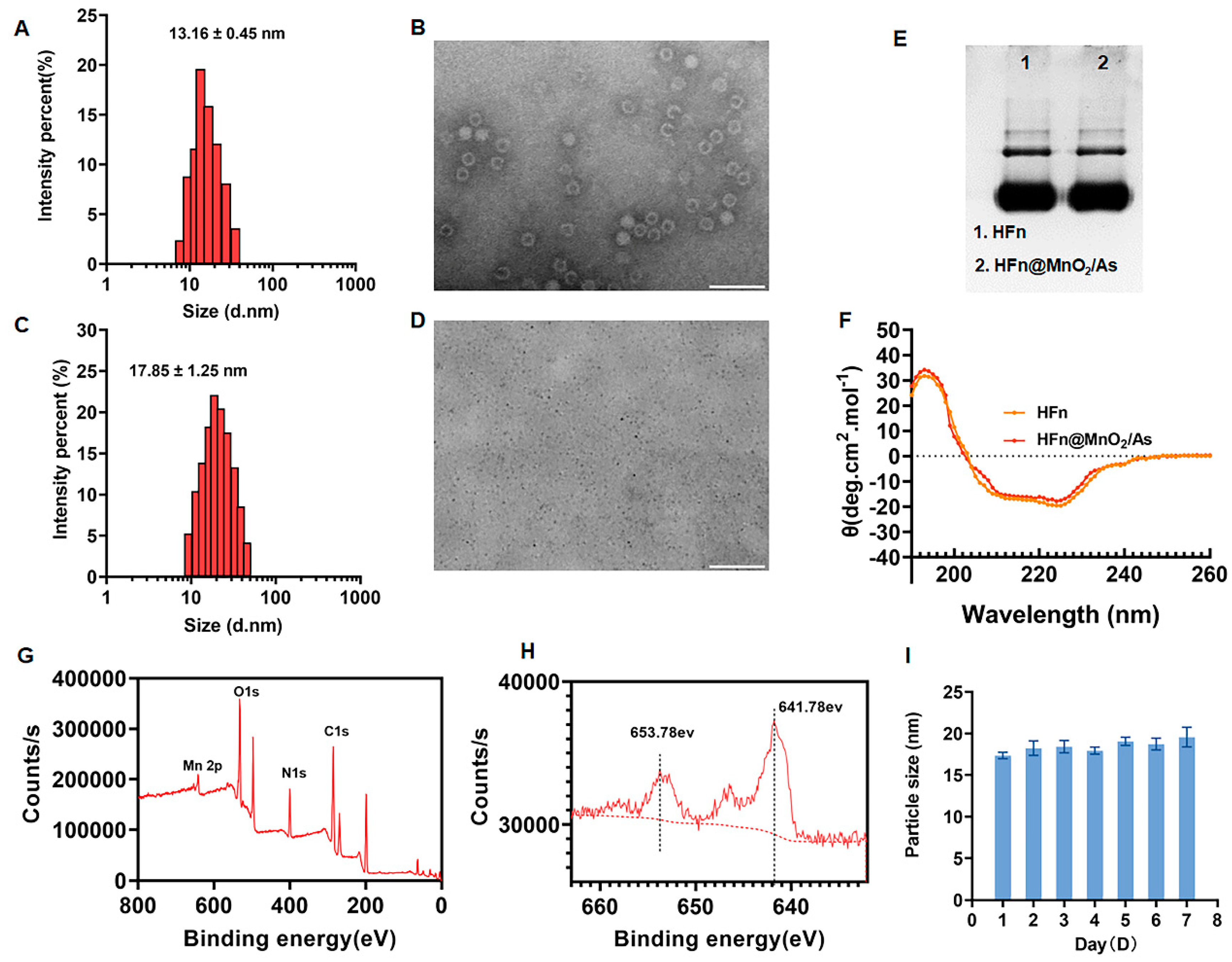
2.2.1. Preparation and Characterization of HFn@MnO2/AS
Expression and Purification of HFn Protein
Synthesis of HFn@MnO2 Using Biomineralization
Preparation and Characterization of HFn@MnO2/AS NPs
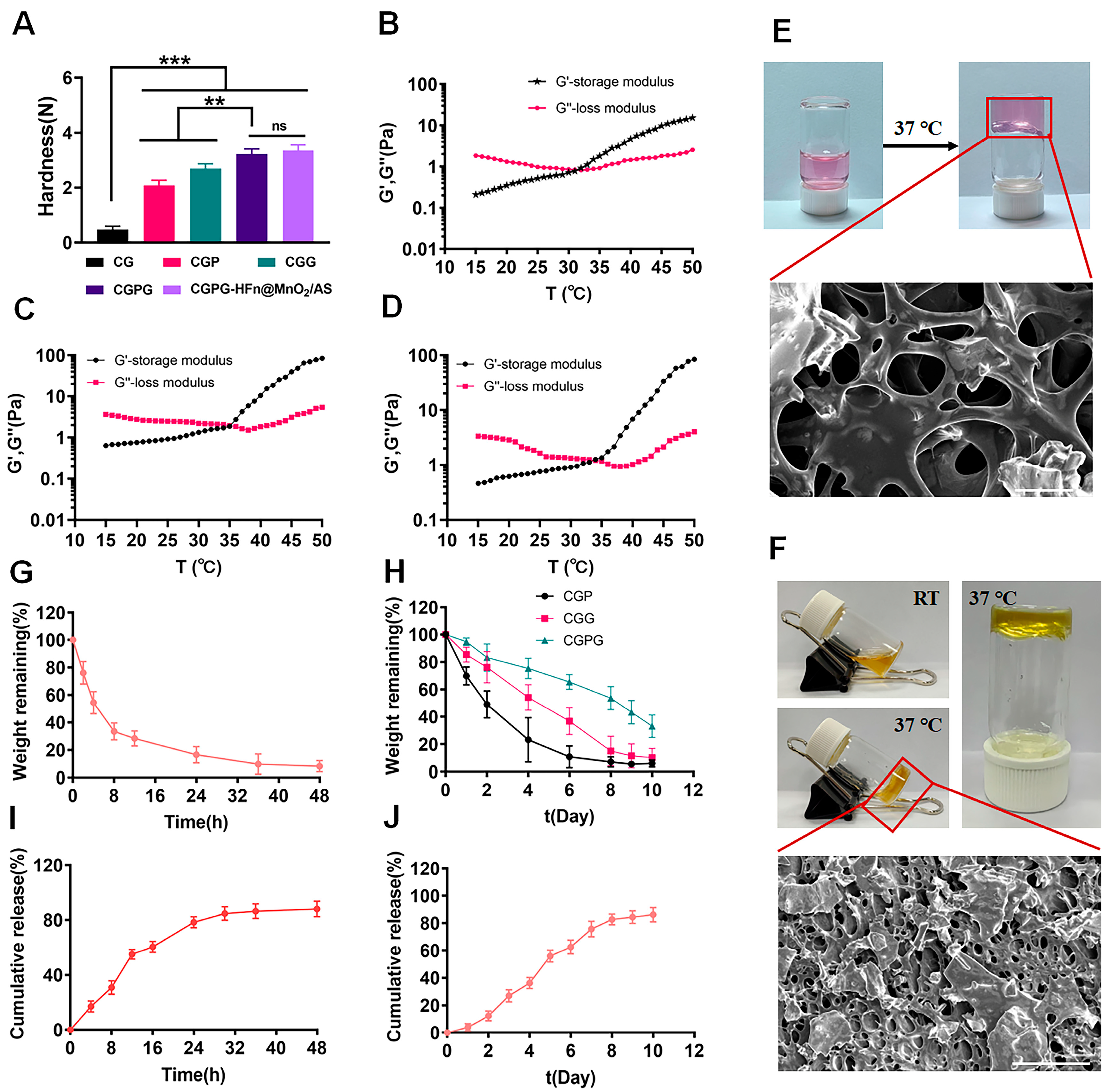
2.2.2. Preparation and Characterization of CGPG Hydrogel
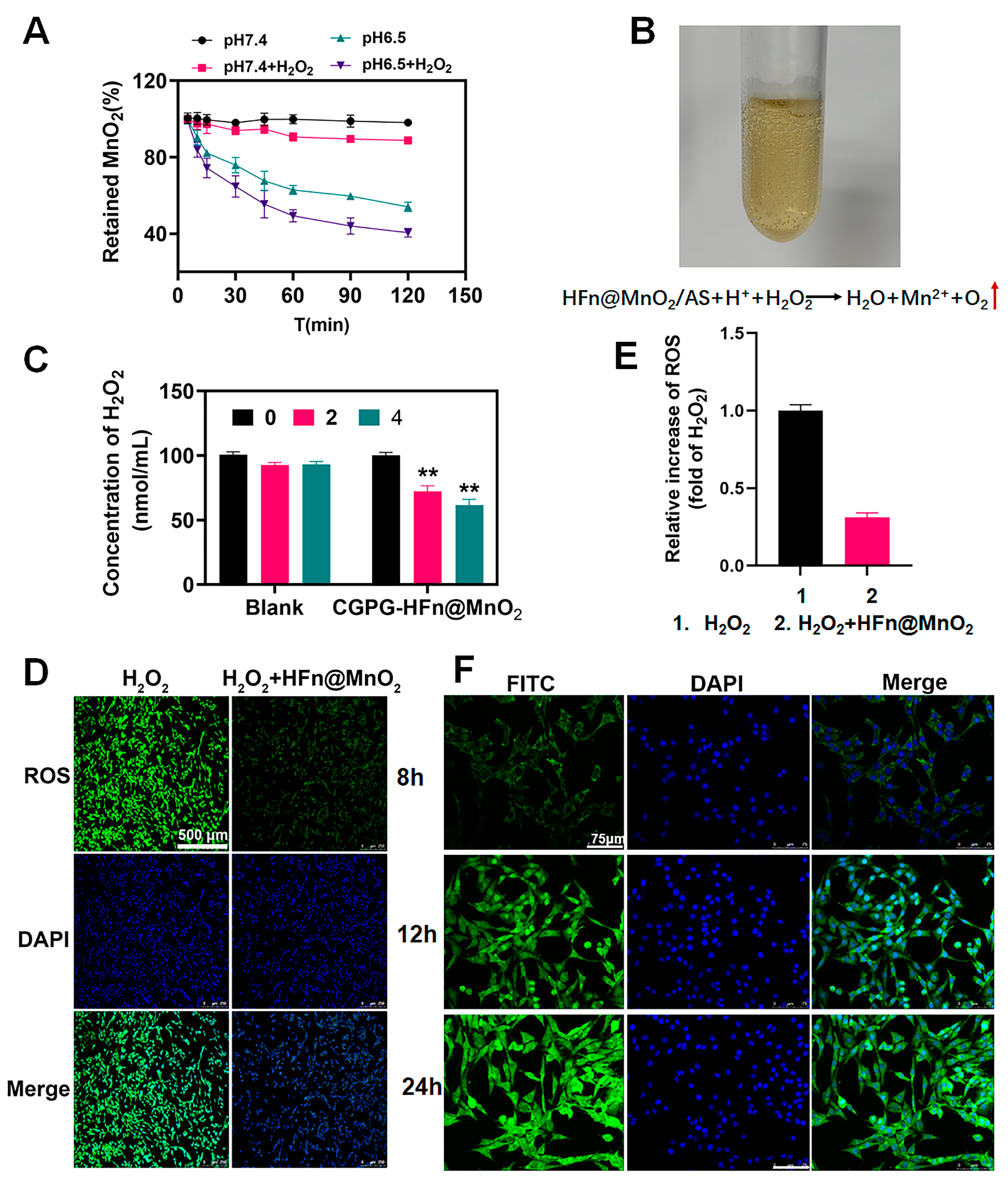
2.2.3. Characterization of MnO2 Related Properties
The Degradation Behavior of HFn@MnO2/AS Nanoparticles
The Antioxidant Ability of CGPG-HFn@MnO2/AS Hydrogels
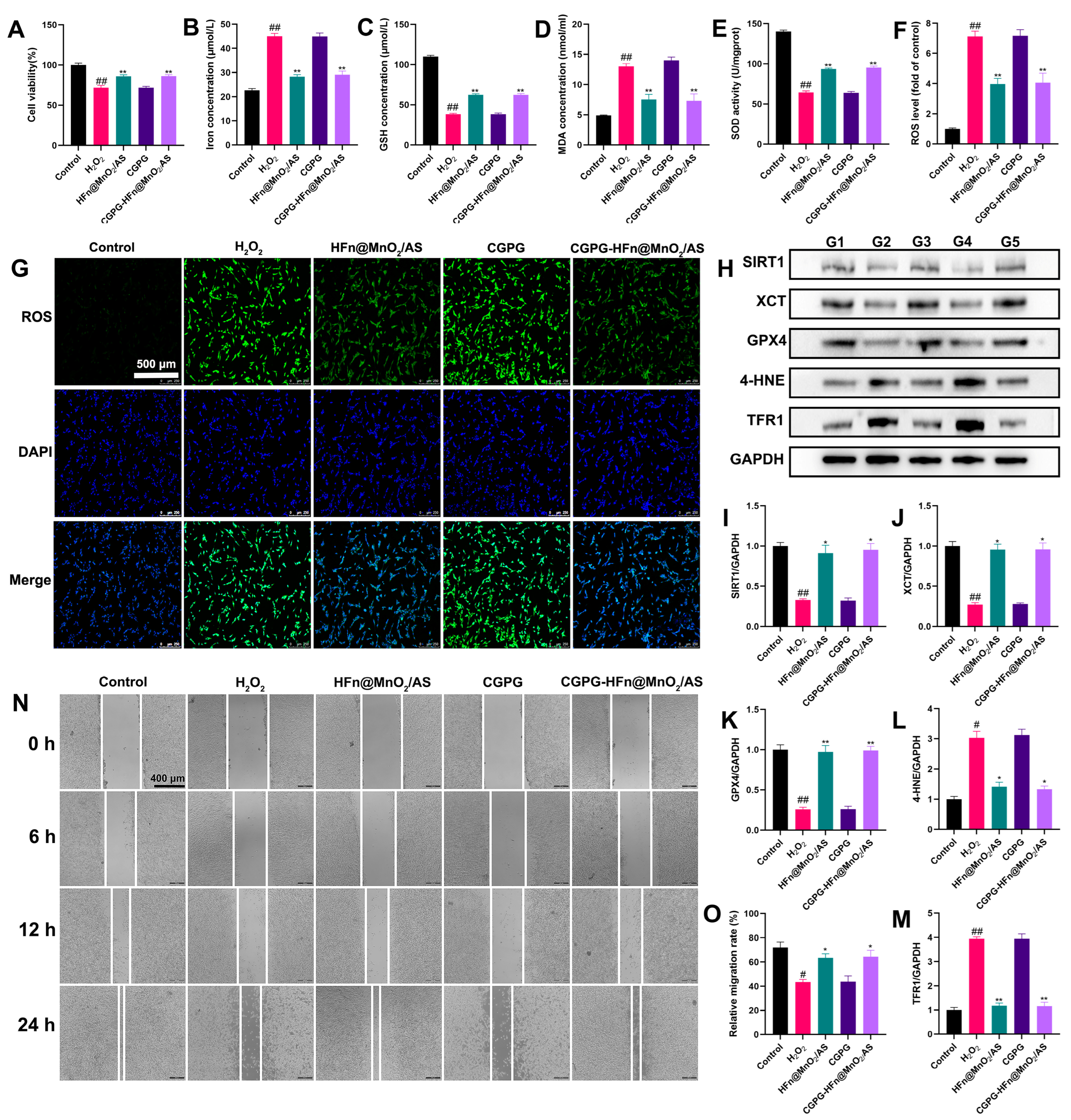
2.2.4. Cell Culture and Treatment
Cell Uptake
Cell Viability Assay
Cell Migration Assay
Detection of Cellular Iron Level
Measurements of Antioxidant Levels
Detection of ROS
2.2.5. Animals and Experimental Design
SCI Modeling
Behavioral Testing
H&E Staining
Western Blotting
2.2.6. Safety Profiles
2.2.7. Statistical Analysis
3. Results and Discussion
3.1. Preparation and Characterization of HFn@MnO2/AS
3.2. Characterization of CGPG Hydrogels
3.3. Characterization of MnO2-Related Properties
3.3.1. The Degradation Behavior of HFn@MnO2/AS Nanoparticles
3.3.2. The Antioxidant Ability of CGPG-HFn@MnO2/AS Hydrogels
3.4. Cellular Uptake
3.5. The Neuroprotective Effect of CGPG-HFn@MnO2/AS and Its Underlying Mechanism on PC12 Cells Stimulated by H2O2
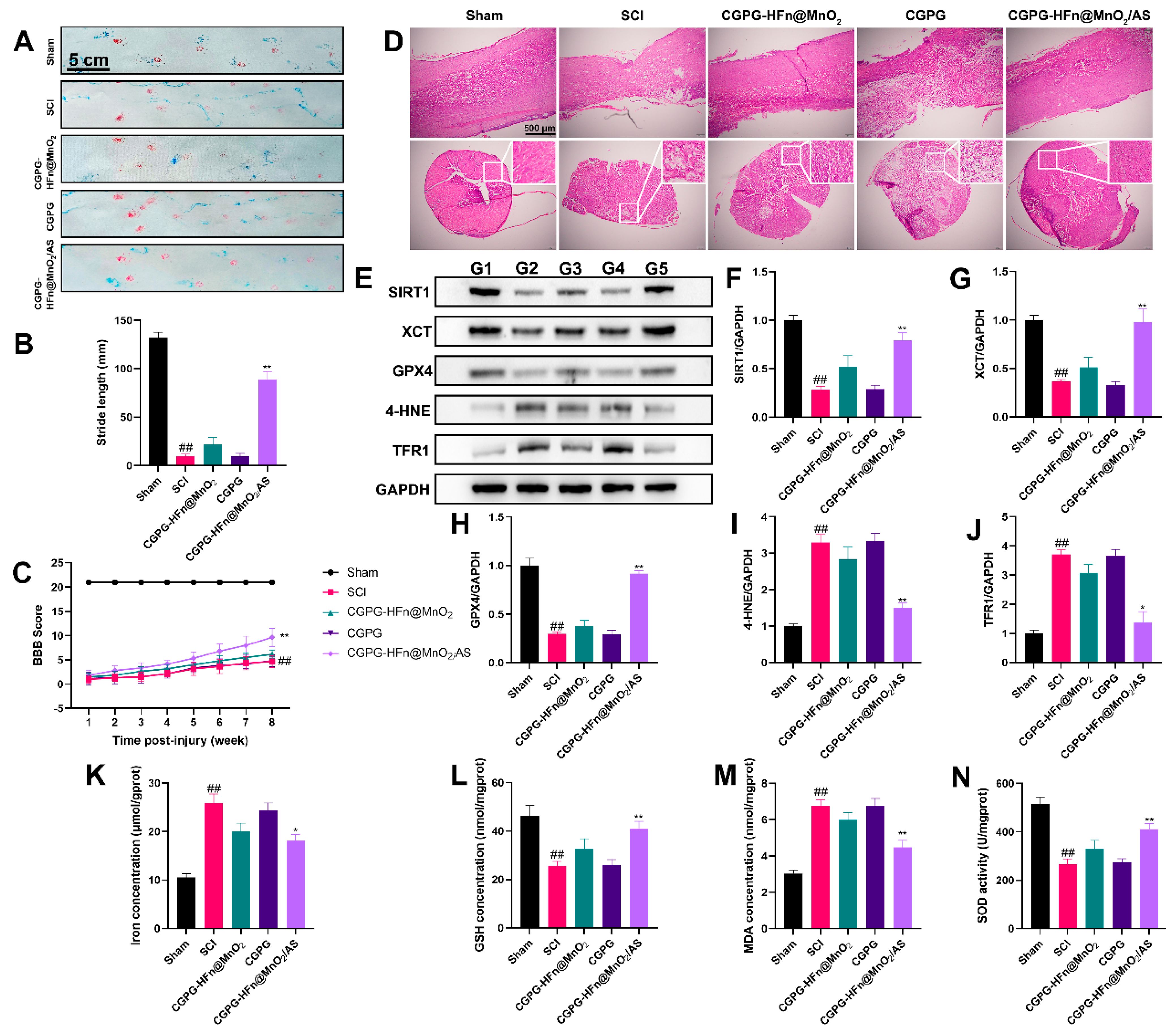
3.6. The Repair Effect and Mechanism of CGPG-HFn@MnO2/AS on Spinal Cord Injury in Rats
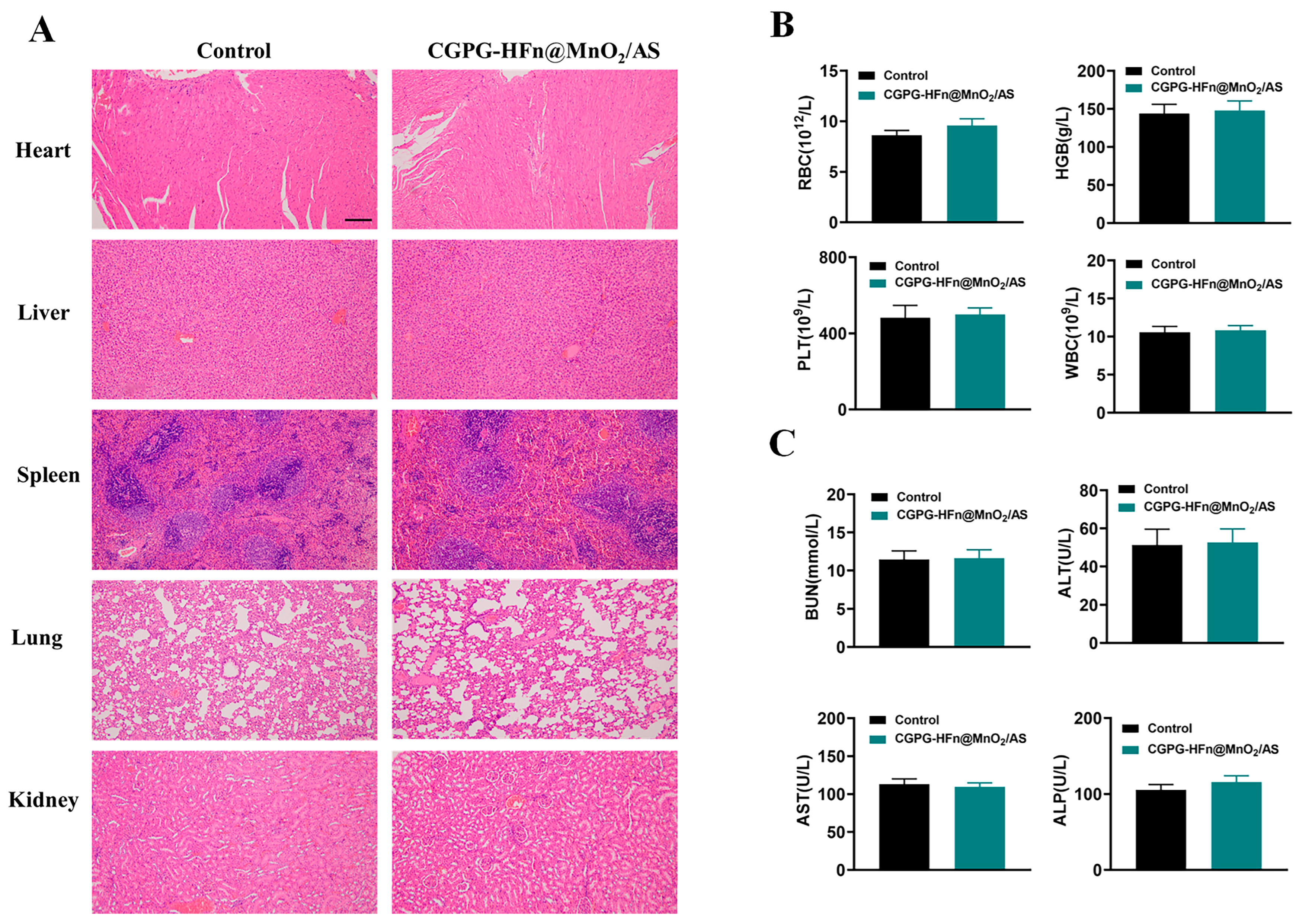
3.7. Safety Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sun, C.; Deng, J.; Ma, Y.; Meng, F.; Cui, X.; Li, M.; Li, J.; Li, J.; Yin, P.; Kong, L.; et al. The dual role of microglia in neuropathic pain after spinal cord injury: Detrimental and protective effects. Exp. Neurol. 2023, 370, 114570. [Google Scholar] [CrossRef] [PubMed]
- Ceruti, S.; Villa, G.; Genovese, T.; Mazzon, E.; Longhi, R.; Rosa, P.; Bramanti, P.; Cuzzocrea, S.; Abbracchio, M.P. The P2Y-like receptor GPR17 as a sensor of damage and a new potential target in spinal cord injury. Brain 2009, 132, 2206–2218. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Xiao, B.; Mu, J.; Zhang, Y.; Zhang, C.; Cao, H.; Chen, R.; Patra, H.K.; Yang, B.; Feng, S.; et al. A MnO(2) Nanoparticle-Dotted Hydrogel Promotes Spinal Cord Repair via Regulating Reactive Oxygen Species Microenvironment and Synergizing with Mesenchymal Stem Cells. ACS Nano 2019, 13, 14283–14293. [Google Scholar] [CrossRef] [PubMed]
- Sofroniew, M.V. Dissecting spinal cord regeneration. Nature 2018, 557, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Dalbayrak, S.; Yaman, O.; Yılmaz, T. Current and future surgery strategies for spinal cord injuries. World J. Orthop. 2015, 6, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Dietz, V.; Fouad, K. Restoration of sensorimotor functions after spinal cord injury. Brain 2014, 137, 654–667. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.C.; Fong, T.H.; Hsu, P.W.; Chiu, W.T. Multifaceted effects of rapamycin on functional recovery after spinal cord injury in rats through autophagy promotion, anti-inflammation, and neuroprotection. J. Surg. Res. 2013, 179, e203–e210. [Google Scholar] [CrossRef]
- Jaffer, H.; Andrabi, S.S.; Petro, M.; Kuang, Y.; Steinmetz, M.P.; Labhasetwar, V. Catalytic antioxidant nanoparticles mitigate secondary injury progression and promote functional recovery in spinal cord injury model. J. Control Release 2023, 364, 109–123. [Google Scholar] [CrossRef]
- Torregrossa, F.; Sallì, M.; Grasso, G. Emerging Therapeutic Strategies for Traumatic Spinal Cord Injury. World Neurosurg. 2020, 140, 591–601. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Jiang, W.; Li, W.; Zhu, S.; Liao, C.; Zou, L.; Ding, R.; Chen, J. Advanced oxidation protein products induce microglia-mediated neuroinflammation via MAPKs-NF-κB signaling pathway and pyroptosis after secondary spinal cord injury. J. Neuroinflamm. 2020, 17, 90. [Google Scholar] [CrossRef]
- Endale, H.T.; Tesfaye, W.; Mengstie, T.A. ROS induced lipid peroxidation and their role in ferroptosis. Front. Cell Dev. Biol. 2023, 11, 1226044. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Jin, D.; Liu, M.; Dai, Y.; Li, L.; Zheng, X.; Wang, L.; Shen, A.; Yu, J.; Wu, S.; et al. Oxygen Self-Supply Engineering-Ferritin for the Relief of Hypoxia in Tumors and the Enhancement of Photodynamic Therapy Efficacy. Small 2022, 18, e2200116. [Google Scholar] [CrossRef] [PubMed]
- Bai, X.Y.; Liu, X.L.; Deng, Z.Z.; Wei, D.M.; Zhang, D.; Xi, H.L.; Wang, Q.Y.; He, M.Z.; Yang, Y.L. Ferroptosis is a new therapeutic target for spinal cord injury. Front. Neurosci. 2023, 17, 1136143. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, J.; Zhu, Y.; Huang, Q.; Gu, Q.; Tian, S.; Ge, J.; Lin, X.; Sha, W. Guanine-rich RNA sequence binding factor 1 regulates neuronal ferroptosis after spinal cord injury in rats via the GPX4 signaling pathway. Brain Res. 2023, 1818, 148497. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Zhao, Y.; Chen, S.; Chen, L.; Sun, L.; Cao, M.; Li, C.; Zhou, X. Astragaloside IV Alleviates Ammonia-Induced Apoptosis and Oxidative Stress in Bovine Mammary Epithelial Cells. Int. J. Mol. Sci. 2019, 20, 600. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Li, Z.; Dong, X.; Wu, Y.; Li, B.; Kuang, B.; Chen, G.; Zhang, L. Astragaloside IV attenuates myocardial dysfunction in diabetic cardiomyopathy rats through downregulation of CD36-mediated ferroptosis. Phytother. Res. 2023, 37, 3042–3056. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Sha, K.; Veroniaina, H.; Wu, Z.; Wu, Z.; Qi, X. Ca(2+) participating self-assembly of an apoferritin nanostructure for nucleic acid drug delivery. Nanoscale 2020, 12, 7347–7357. [Google Scholar] [CrossRef] [PubMed]
- Song, N.; Zhang, J.; Zhai, J.; Hong, J.; Yuan, C.; Liang, M. Ferritin: A Multifunctional Nanoplatform for Biological Detection, Imaging Diagnosis, and Drug Delivery. ACC Chem. Res. 2021, 54, 3313–3325. [Google Scholar] [CrossRef]
- Domínguez-Vera, J.M.; Fernández, B.; Gálvez, N. Native and synthetic ferritins for nanobiomedical applications: Recent advances and new perspectives. Future Med. Chem. 2010, 2, 609–618. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.K.; Zhu, Z.J.; Liang, Z.W.; Li, P.H.; Ma, Y.G.; Ding, T.; Li, K.; Zuo, X.S.; Ju, C.; et al. Photobiomodulation provides neuroprotection through regulating mitochondrial fission imbalance in the subacute phase of spinal cord injury. Neural Regen. Res. 2023, 18, 2005–2010. [Google Scholar]
- Zhang, H.; Hu, Y.M.; Wang, Y.J.; Zhou, Y.; Zhu, Z.J.; Chen, M.H.; Wang, Y.J.; Xu, H.; Wang, Y.H. Macrophage migration inhibitory factor facilitates astrocytic production of the CCL2 chemokine following spinal cord injury. Neural Regen. Res. 2023, 18, 1802–1808. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Zhuang, Y.; Wenwen, L.; Dong, T.; Jingjing, Z. Modulation of Hypoxia in Solid Tumor Microenvironment with MnO2 Nanoparticles to Enhance Photodynamic Therapy. Adv. Funct. Mater. 2016, 26, 5490–5498. [Google Scholar]
- Voniatis, C.; Závoti, O.; Manikion, K.; Budavári, B.; Hajdu, A.J. Fabrication of Mechanically Enhanced, Suturable, Fibrous Hydrogel Membranes. Membranes 2023, 13, 116. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Zhao, X.; Xu, C.; Wang, L.; Xia, Y. Progress in the mechanical enhancement of hydrogels: Fabrication strategies and underlying mechanisms. J. Polym. Sci. 2022, 60, 2525–2542. [Google Scholar] [CrossRef]
- Tang, Y.; Du, Y.; Li, Y.; Wang, X.; Hu, X. A thermosensitive chitosan/poly(vinyl alcohol) hydrogel containing hydroxyapatite for protein delivery. J. Biomed. Mater. Res. A 2009, 91, 953–963. [Google Scholar] [CrossRef] [PubMed]
- Zan, J.; Chen, H.; Jiang, G.; Lin, Y.; Ding, F.J. Preparation and properties of crosslinked chitosan thermosensitive hydrogel for injectable drug delivery systems. J. Appl. Polym. Sci. 2006, 101, 1892–1898. [Google Scholar] [CrossRef]
- Chen, X.; Cui, J.; Zhai, X.; Zhang, J.; Gu, Z.; Zhi, X.; Weng, W.; Pan, P.; Cao, L.; Ji, F.; et al. Inhalation of Hydrogen of Different Concentrations Ameliorates Spinal Cord Injury in Mice by Protecting Spinal Cord Neurons from Apoptosis, Oxidative Injury and Mitochondrial Structure Damages. Cell Physiol. Biochem. 2018, 47, 176–190. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Song, X.; Geng, Z.; Xu, Y.; Xiao, L.; Chen, Y.; Li, B.; Shi, J.; Wang, L.; Wang, Y.; et al. IL-11 ameliorates oxidative stress damage in neurons after spinal cord injury by activating the JAK/STAT signaling pathway. Int. Immunopharmacol. 2023, 127, 111367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, K.; Zhu, X.; Sun, S.; Yang, W.; Liu, S.; Tang, Z.; Zhang, R.; Li, J.; Shen, T.; Hei, M. Inhibition of TLR4 prevents hippocampal hypoxic-ischemic injury by regulating ferroptosis in neonatal rats. Exp. Neurol. 2021, 345, 113828. [Google Scholar] [CrossRef]
- Cheng, L.; Liang, R.; Li, Z.; Ren, J.; Yang, S.; Bai, J.; Niu, Q.; Yu, H.; Zhang, H.; Xia, N.; et al. Aluminum maltolate triggers ferroptosis in neurons: Mechanism of action. Toxicol. Mech. Methods 2021, 31, 33–42. [Google Scholar] [CrossRef]
- Zhang, X.S.; Wu, Q.; Wu, L.Y.; Ye, Z.N.; Jiang, T.W.; Li, W.; Zhuang, Z.; Zhou, M.L.; Zhang, X.; Hang, C.H. Sirtuin 1 activation protects against early brain injury after experimental subarachnoid hemorrhage in rats. Cell Death Dis. 2016, 7, e2416. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.S.; Fonseca-Kelly, Z.; Callinan, C.; Zuo, L.; Sachdeva, M.M.; Shindler, K.S. SIRT1 activating compounds reduce oxidative stress and prevent cell death in neuronal cells. Front. Cell Neurosci. 2012, 6, 63. [Google Scholar] [CrossRef] [PubMed]
- Mishra, P.; Mittal, A.K.; Kalonia, H.; Madan, S.; Ghosh, S.; Sinha, J.K.; Rajput, S.K. SIRT1 Promotes Neuronal Fortification in Neurodegenerative Diseases through Attenuation of Pathological Hallmarks and Enhancement of Cellular Lifespan. Curr. Neuropharmacol. 2021, 19, 1019–1037. [Google Scholar] [PubMed]
- Li, W.; Zhao, X.; Zhang, R.; Liu, X.; Qi, Z.; Zhang, Y.; Yang, W.; Pang, Y.; Zhao, C.; Fan, B.; et al. Ferroptosis inhibition protects vascular endothelial cells and maintains integrity of the blood-spinal cord barrier after spinal cord injury. Neural Regen. Res. 2023, 18, 2474–2481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Ye, T.J.; DeCaro, E.; Buehler, B.; Stahl, Z.; Bonavita, G.; Daniels, M.; You, M. Intestinal SIRT1 Deficiency Protects Mice from Ethanol-Induced Liver Injury by Mitigating Ferroptosis. Am. J. Pathol. 2020, 190, 82–92. [Google Scholar] [CrossRef] [PubMed]
- Liao, Y.; Ke, B.; Long, X.; Xu, J.; Wu, Y. Abnormalities in the SIRT1-SIRT3 axis promote myocardial ischemia-reperfusion injury through ferroptosis caused by silencing the PINK1/Parkin signaling pathway. BMC Cardiovasc. Disord. 2023, 23, 582. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xiao, L.; Chen, Y.; Li, W.; Liu, Y.; Zhou, Y.; Tan, H. YT521-B homology domain containing 1 ameliorates mitochondrial damage and ferroptosis in sleep deprivation by activating the sirtuin 1/nuclear factor erythroid-derived 2-like 2/heme oxygenase 1 pathway. Brain Res. Bull. 2023, 197, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Zhong, X.; Fang, Z.; Li, J.; Li, H.; Liu, X.; Yuan, X.; Huang, W.; Huang, Z. Cardiac sirtuin1 deficiency exacerbates ferroptosis in doxorubicin-induced cardiac injury through the Nrf2/Keap1 pathway. Chem. Biol. Interact. 2023, 377, 110469. [Google Scholar] [CrossRef]
- Jiang, T.; Qin, T.; Gao, P.; Tao, Z.; Wang, X.; Wu, M.; Gu, J.; Chu, B.; Zheng, Z.; Yi, J.; et al. SIRT1 attenuates blood-spinal cord barrier disruption after spinal cord injury by deacetylating p66Shc. Redox. Biol. 2023, 60, 102615. [Google Scholar] [CrossRef]
- Li, R.; Zhang, X.; Gu, L.; Yuan, Y.; Luo, X.; Shen, W.; Xie, Z. CDGSH iron sulfur domain 2 over-expression alleviates neuronal ferroptosis and brain injury by inhibiting lipid peroxidation via AKT/mTOR pathway following intracerebral hemorrhage in mice. J. Neurochem. 2023, 165, 426–444. [Google Scholar] [CrossRef]
- Kim, S.W.; Kim, Y.; Kim, S.E.; An, J.Y. Ferroptosis-Related Genes in Neurodevelopment and Central Nervous System. Biology 2021, 10, 35. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, Y.; Zhang, J.; Gu, Y.; Liu, T.; Chen, L. Biomineralized MnO2 Nanoparticle-Constituted Hydrogels Promote Spinal Cord Injury Repair by Modulating Redox Microenvironment and Inhibiting Ferroptosis. Pharmaceutics 2024, 16, 1057. https://doi.org/10.3390/pharmaceutics16081057
Sun Y, Zhang J, Gu Y, Liu T, Chen L. Biomineralized MnO2 Nanoparticle-Constituted Hydrogels Promote Spinal Cord Injury Repair by Modulating Redox Microenvironment and Inhibiting Ferroptosis. Pharmaceutics. 2024; 16(8):1057. https://doi.org/10.3390/pharmaceutics16081057
Chicago/Turabian StyleSun, Yuyu, Jinlong Zhang, Yong Gu, Tianqing Liu, and Liang Chen. 2024. "Biomineralized MnO2 Nanoparticle-Constituted Hydrogels Promote Spinal Cord Injury Repair by Modulating Redox Microenvironment and Inhibiting Ferroptosis" Pharmaceutics 16, no. 8: 1057. https://doi.org/10.3390/pharmaceutics16081057






