The Role of Pseudomonas aeruginosa in the Pathogenesis of Corneal Ulcer, Its Associated Virulence Factors, and Suggested Novel Treatment Approaches
Abstract
:1. Introduction
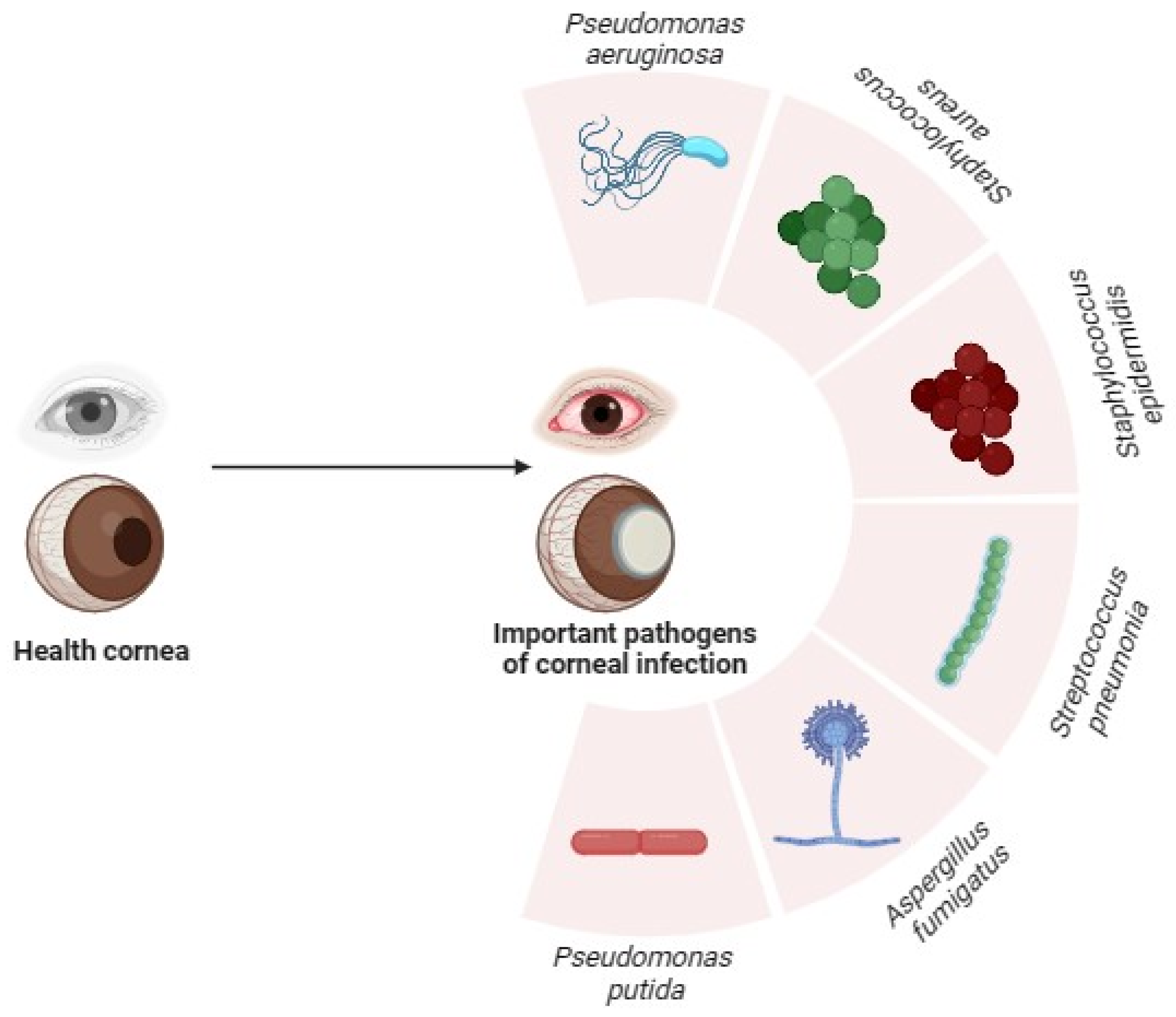
2. Overview of Pseudomonas aeruginosa and Corneal Infections
2.1. Pseudomonas aeruginosa Associated Corneal Infections in Contact Lens Wearers
2.2. Uncommon Species of Pseudomonas in Corneal Infections
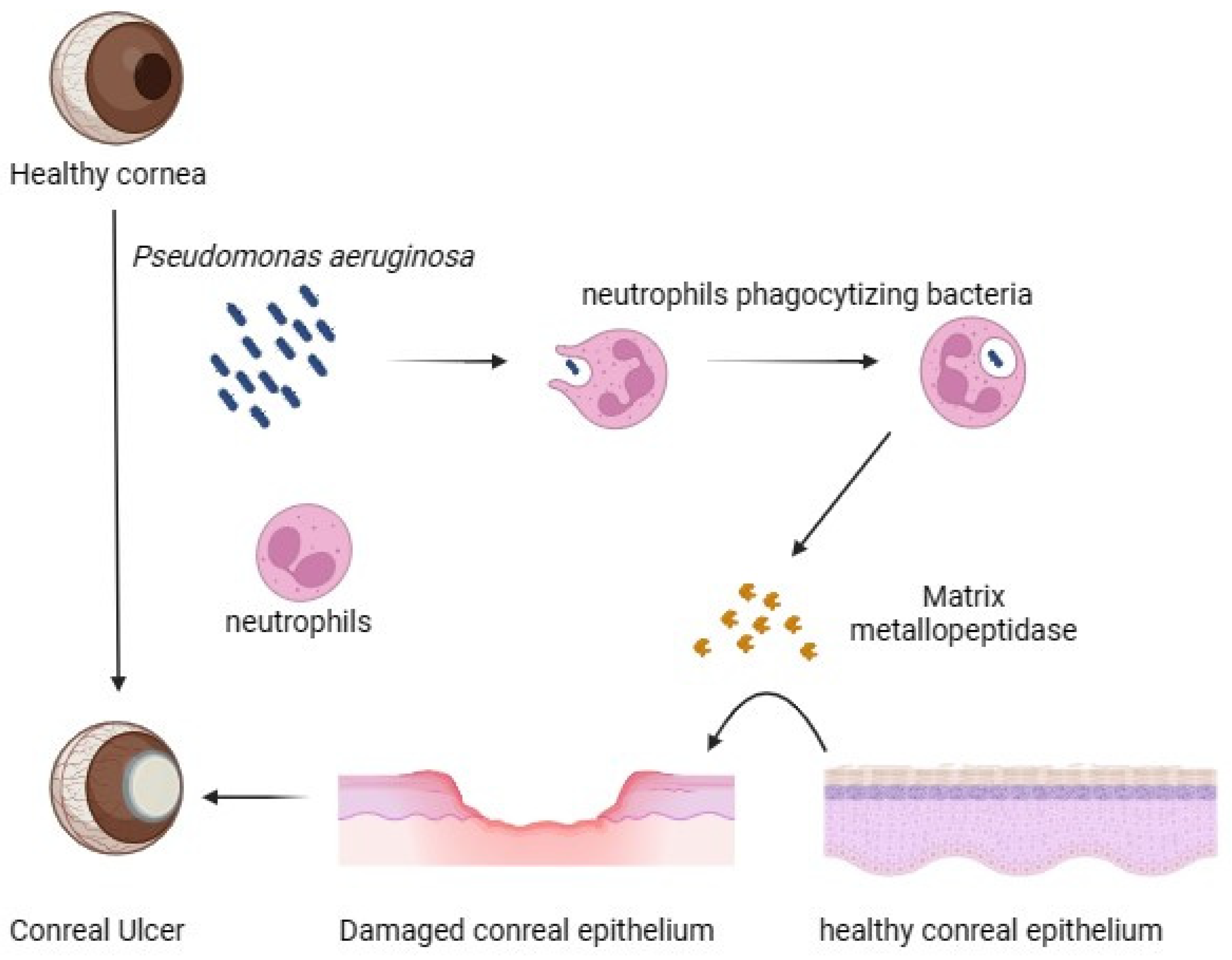
3. Modulation of Corneal Protein and Host Defence during Pseudomonas aeruginosa Pathogenicity
4. Virulence Factors Associated with P. aeruginosa in Ocular Infections
4.1. Type III Secretion System (T3SS) of P. aeruginosa
4.2. The Role of Condensins in P. aeruginosa CL Infections
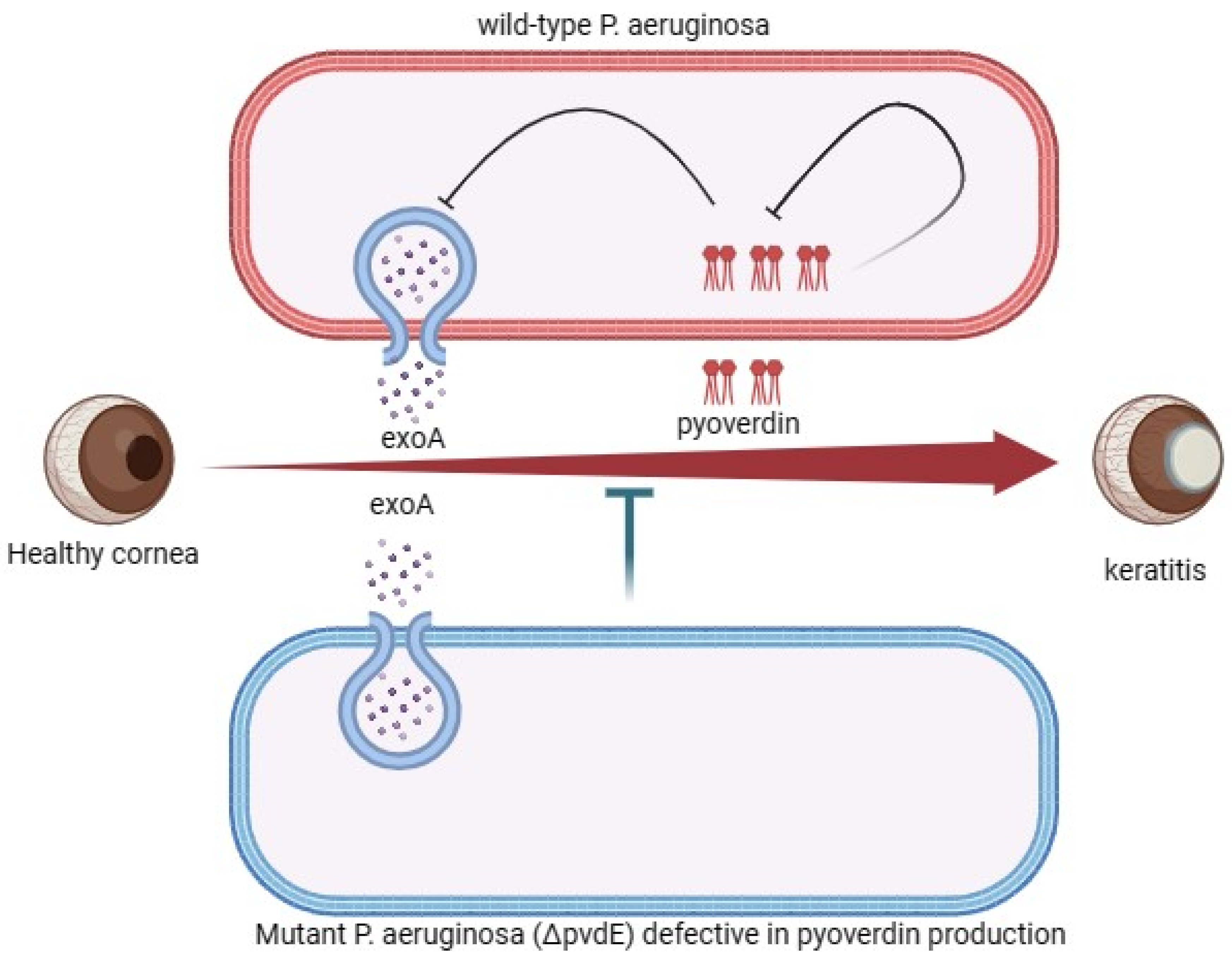
4.3. Exoenzymes’ Pathogenesis Associated with P. aeruginosa Corneal Infection
5. Cytotoxic Strains of P. aeruginosa
6. Genetic and Phenotypic Features of P. aeruginosa
7. Drug-Resistance and Treatment Options of Pseudomonas aeruginosa in Corneal Infection
8. Drug Delivery in the Treatment of P. aeruginosa Cornea Infection
8.1. Use of Predatory Prokaryote as Antimicrobial
8.2. Bacteriophages as Phage Therapy
9. Potential Drug Targets in P. aeruginosa Keratitis
10. Nanomedicine in Keratitis
10.1. Benefits of Nanomedicine in Keratitis
10.2. Levofloxacin
10.3. Ciprofloxacin
11. Future Research Directions
12. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| OS | Ocular surface |
| TCU | Traumatic corneal ulcer |
| HC | Healthy control |
| PAK | P. aeruginosa keratitis |
| CLWs | Contact lens wearers |
| VA | Visual acuity |
| PMN | Polymorphonuclear neutrophils |
| MMP | Matrix metalloproteinases |
| HIF1A | Hypoxia-inducible factor 1-alpha |
| NO | Nitric Oxide |
| IL1B | Interleukin-1beta |
| NE | Norepinephrine |
| CL | Contact lens |
| IgA | Immunoglobulin A |
| LPS- sIgA | Soluble IgA against lipopolysaccharide |
| ETA-sIgA | Soluble IgA against exotoxin A |
| Exo | Exotoxin |
| EF-2 | Elongation factor 2 |
| HCEC | Human cultured corneal epithelial cells |
| T3SS | Type III Secretion System |
| c-di-GMP | Cyclic diguanylate |
| GSH | Glutathione |
| GSSG | Oxidized glutathione |
| PPK1 | Polyphosphate kinase 1 |
| PLC | Phospholipase C |
| PASP | P. aeruginosa small protease |
| FAS | Factor activating Exoenzyme S |
| ADPr | ADP-ribosyltransferase |
| TER | Transepithelial Resistance |
| SOD | Superoxide dismutase |
| XDR | Extensively drug-resistant |
| MPC | Methacryloyloxyethyl phosphorylcholine |
| MK | Microbial keratitis |
| CXL | Corneal collagen cross-linking |
| M-CXL | Modified CXL |
| FI | Frequent instillations |
| HCLE | Human corneal-limbal epithelial |
| TNF | Tumour necrosis factor |
| TEM | Transmission electron microscopy |
Appendix A
| Sl. No | Resistant Antibiotic | Sensitive Antibiotic | Treatment Duration | County | Reference |
|---|---|---|---|---|---|
| 1 | Amikacin, Aztreonam, Cefepime, Ceftazidime, Ciprofloxacin, Gentamicin, Imipenem, Levofloxacin, Meropenem, Piperacillin/Tazobactam and Tobramycin | Colistin | 5 weeks | Peru | [121] |
| 2 | Fluoroquinolones, aminoglycosides, beta-lactams, macrolides, sulphonamides, and tetracycline | Not reported | - | Australia and India | [122] |
| 3 | Ciprofloxacin | Not reported | - | India | [123] |
| 4 | Ceftriaxone and ceftazidime | Piperacillin or imipenem | - | India | [124] |
| 5 | All β-lactam antibiotics, fluoroquinolones and gentamicin | Tobramycin and amikacin | - | German | [125] |
| 6 | Penicillin, amoxicillin and flucloxacillin | Gentamicin, amikacin, ciprofloxacin and levofloxacin | - | United Kingdom | [126] |
| 7 | Cephotaxime, cefuroxime, ceftriaxone, ceftazidime, chloramphenicol, vancomycin, amikacin, tobramycin, ciprofloxacin, moxifloxacin, co-trimoxazole, and piperacillin-tazobactam | Imipenem | - | India | [127] |
| 8 | Cefalotin, chloramphenicol, ciprofloxacin and vancomycin | Gentamicin or tobramycin | - | Australia | [128] |
| 9 | Ampicillin, piperacillin/tazobactam, ceftazidime, ceftriaxone, cefepime, imipenem, meropenem, gentamicin, tobramycin, ciprofloxacin, moxifloxacin and trimethoprim/sulfamethoxazole | Ceftazidime, vancomycin and moxifloxacin | - | Mexico | [129] |
| 10 | Minocycline, trimethoprim/sulfamethoxazole and chloramphenicol | Piperacillin/tazobactam | - | USA | [130] |
| 11 | Trimethoprim and Erythromycin | Bacitracin, Vancomycin, Moxifloxacin and Ofloxacin | - | USA | [131] |
| 12 | Ciprofloxacin and gentamicin | Ceftazidime, tobramycin, and piperacillin/tazobactam | - | Canada | [132] |
| 13 | Ciprofloxacin, levofloxacin, trimethoprim/sulfamethoxazole | Ceftazidime plus gentamicin | 3 days | Portugal | [133] |
| 14 | Amikacin, gentamicin, vancomycin, cephazolin, ceftazidime, ciprofloxacin, gatifloxacin, and chloramphenicol | Piperacillin/tazobactam | 3 days | India | [134] |
| 15 | Ceftazidime, gentamicin, ofloxacin and ciprofloxacin | Not reported | - | Saudi Arabia | [135] |
| 16 | Amikacin, Chloramphenicol, Ceftazidime, Ciprofloxacin, Cefazolin, Gatifloxacin, Gentamicin, Imipenem, Moxifloxacin, Ofloxacin, and Tobramycin | Imipenem/cilastatin, Colistin and Colistimethate | 3 to 7 days | India | [83] |
| 17 | Amikacin, Gentamicin, Tobramycin, minocycline, Ciprofloxacin, levofloxacin, Imipenem, meropenem and Ceftazidime | 1.5% levofloxacin | 3 days | Japan | [136] |
| 18 | Moxifloxacin | Ciprofloxacin and Gatifloxacin | - | USA | [137] |
| 19 | Chloramphenicol, ceftazidime, gentamicin, and ciprofloxacin | Not reported | - | Malaysia | [138] |
| 20 | Moxifloxacin | Not reported | - | India | [72] |
| 21 | Ciprofloxacin | Vancomycin and tobramycin | 6 days | USA | [139] |
| 22 | Cefazolin, Neomycin and Chloramphenicol | Ofloxacin, Ceftazidime, Gentamicin and Polymyxin B | - | Saudi Arabia | [140] |
| 23 | Gentamicin and tobramycin | Ceftazidime | 21 days | Israel | [141] |
| 24 | Imipenem, ciprofloxacin, tobramycin, piperacillin, levofloxacin, ceftazidime, gentamicin and polymyxin B | Not reported | - | Australia and India | [142] |
References
- Al-Mujaini, A.; Al-Kharusi, N.; Thakral, A.; Wali, U.K. Bacterial Keratitis: Perspective on Epidemiology, Clinico-Pathogenesis, Diagnosis and Treatment. Sultan Qaboos Univ. Med. J. 2009, 9, 184–195. [Google Scholar] [PubMed]
- Panjwani, N.; Clark, B.; Cohen, M.; Barza, M.; Baum, J. Differential Binding of P. aeruginosa and S. aureus to Corneal Epithelium in Culture. Investig. Ophthalmol. Vis. Sci. 1990, 31, 696–701. [Google Scholar]
- Ormerod, L.D.; Hertzmark, E.; Gomez, D.S.; Stabiner, R.G.; Schanzlin, D.J.; Smith, R.E. Epidemiology of Microbial Keratitis in Southern California. Ophthalmology 1987, 94, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.; Zhang, H.; Hu, M.; Ma, Y.; Chen, P.; Zhao, Z.; Li, J.; Ye, Y.; Zheng, M.; Lou, Y. Alterations in the Ocular Surface Microbiome in Traumatic Corneal Ulcer Patients. Investig. Ophthalmol. Vis. Sci. 2020, 61, 35. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.P. The Bhaktapur Eye Study: Ocular Trauma and Antibiotic Prophylaxis for the Prevention of Corneal Ulceration in Nepal. Br. J. Ophthalmol. 2001, 85, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Eltis, M. Contact-Lens-Related Microbial Keratitis: Case Report and Review. J. Optom. 2011, 4, 122–127. [Google Scholar] [CrossRef]
- Lim, C.H.L.; Carnt, N.A.; Farook, M.; Lam, J.; Tan, D.T.; Mehta, J.S.; Stapleton, F. Risk Factors for Contact Lens-Related Microbial Keratitis in Singapore. Eye 2016, 30, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Carney, J.M.; Holloway, F.A.; Modrow, H.E. Discriminative Stimulus Properties of Methylxanthines and Their Metabolites in Rats. Life Sci. 1985, 36, 913–920. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Cairns, J.; Gopal, B.P.; Ho, C.S.; Krstic, L.; Elsahn, A.; Lister, M.; Said, D.G.; Dua, H.S. Risk Factors, Clinical Outcomes, and Prognostic Factors of Bacterial Keratitis: The Nottingham Infectious Keratitis Study. Front. Med. 2021, 8, 715118. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, M.P.; Srinivasan, M.; Whitcher, J.P. Diagnosing and Managing Microbial Keratitis. Community Eye Health 2015, 28, 3–6. [Google Scholar] [PubMed]
- Austin, A.; Lietman, T.; Rose-Nussbaumer, J. Update on the Management of Infectious Keratitis. Ophthalmology 2017, 124, 1678–1689. [Google Scholar] [CrossRef] [PubMed]
- Khdair, A.I.; Khdair, S.I.; Abu-Rumman, G.A. Dataset on Some Soil Properties Improvement by the Addition of Olive Pomace. Data Br. 2019, 24, 103878. [Google Scholar] [CrossRef]
- Slusher, M.M.; Myrvik, Q.N.; Lewis, J.C.; Gristina, A.G. Extended-Wear Lenses, Biofilm, and Bacterial Adhesion. Arch. Ophthalmol. 1987, 105, 110–115. [Google Scholar] [CrossRef] [PubMed]
- Stellwagen, A.; MacGregor, C.; Kung, R.; Konstantopoulos, A.; Hossain, P. Personal Hygiene Risk Factors for Contact Lens-Related Microbial Keratitis. BMJ Open Ophthalmol. 2020, 5, e000476. [Google Scholar] [CrossRef] [PubMed]
- Perzia, B.; Enzor, R.; Kowalski, R.P.; Jhanji, V. Bilateral Pseudomonas aeruginosa Keratitis in 7 Patients. Eye Contact Lens Sci. Clin. Pract. 2021, 47, 476–479. [Google Scholar] [CrossRef] [PubMed]
- Enzor, R.; Bowers, E.M.R.; Perzia, B.; Perera, C.; Palazzolo, L.; Mammen, A.; Dhaliwal, D.K.; Kowalski, R.P.; Jhanji, V. Comparison of Clinical Features and Treatment Outcomes of Pseudomonas aeruginosa Keratitis in Contact Lens and Non–Contact Lens Wearers. Am. J. Ophthalmol. 2021, 227, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thamizhselvi, S.; Pooja, A.; Prajna, L.; Rameshkumar, G.; Prajna, N.V.; Karpagam, R. Incidence, Clinical Profile, and Management of Keratitis Caused by Uncommon Species of Pseudomonas at a Tertiary Eye Care Center. Cornea 2023, 42, 359–364. [Google Scholar] [CrossRef] [PubMed]
- Deepika, J. Pseudomonas Gessardii—A Novel Pathogenic Bacterium Associated with the Cases of Corneal Ulcers and Producing Virulent Pyoverdine Pigment. J. Appl. Biol. Biotechnol. 2022, 10, 68–73. [Google Scholar] [CrossRef]
- Khoo, L.W.; Srinivasan, S.S.; Henriquez, F.L.; Bal, A.M. A Rare Case of Mixed Infectious Keratitis Caused by Pseudomonas Koreensis and Aspergillus Fumigatus. Case Rep. Ophthalmol. 2020, 11, 600–605. [Google Scholar] [CrossRef]
- Steuhl, K.P.; Döring, G.; Henni, A.; Thiel, H.J.; Botzenhart, K. Relevance of Host-Derived and Bacterial Factors in Pseudomonas aeruginosa Corneal Infections. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1559–1568. [Google Scholar]
- Chidambaram, J.D.; Kannambath, S.; Srikanthi, P.; Shah, M.; Lalitha, P.; Elakkiya, S.; Bauer, J.; Prajna, N.V.; Holland, M.J.; Burton, M.J. Persistence of Innate Immune Pathways in Late Stage Human Bacterial and Fungal Keratitis: Results from a Comparative Transcriptome Analysis. Front. Cell. Infect. Microbiol. 2017, 7, 193. [Google Scholar] [CrossRef] [PubMed]
- Berger, E.A.; McClellan, S.A.; Vistisen, K.S.; Hazlett, L.D. HIF-1α Is Essential for Effective PMN Bacterial Killing, Antimicrobial Peptide Production and Apoptosis in Pseudomonas aeruginosa Keratitis. PLoS Pathog. 2013, 9, e1003457. [Google Scholar] [CrossRef] [PubMed]
- McClellan, S.A.; Huang, X.; Barrett, R.P.; Lighvani, S.; Zhang, Y.; Richiert, D.; Hazlett, L.D. Matrix Metalloproteinase-9 Amplifies the Immune Response to Pseudomonas aeruginosa Corneal Infection. Investig. Ophthalmol. Vis. Sci. 2006, 47, 256–264. [Google Scholar] [CrossRef] [PubMed]
- McElvanney, A.M. Doxycycline in the Management of Pseudomonas Corneal Melting. Eye Contact Lens Sci. Clin. Pract. 2003, 29, 258–261. [Google Scholar] [CrossRef] [PubMed]
- Gao, N.; Kumar, A.; Yu, F.-S.X. Matrix Metalloproteinase-13 as a Target for Suppressing Corneal Ulceration Caused by Pseudomonas aeruginosa Infection. J. Infect. Dis. 2015, 212, 116–127. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Ma, X.; Zhao, L.; Li, Y.; Zhou, Q.; Du, X. Extended Contact Lens Wear Promotes Corneal Norepinephrine Secretion and Pseudomonas aeruginosa Infection in Mice. Investig. Ophthalmol. Vis. Sci. 2020, 61, 17. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Wang, Q.; Song, F.; Li, Y.; Li, J.; Dou, S.; Xie, L.; Zhou, Q. Corneal Epithelial Injury-Induced Norepinephrine Promotes Pseudomonas aeruginosa Keratitis. Exp. Eye Res. 2020, 195, 108048. [Google Scholar] [CrossRef] [PubMed]
- Masinick, S.A.; Montgomery, C.P.; Montgomery, P.C.; Hazlett, L.D. Secretory IgA Inhibits Pseudomonas aeruginosa Binding to Cornea and Protects against Keratitis. Investig. Ophthalmol. Vis. Sci. 1997, 38, 910–918. [Google Scholar]
- Nakashima, M.; Inada, N.; Kato, H.; Shoji, J.; Sawa, M. Evaluation of Anti-Pseudomonas aeruginosa Specific Secretory IgA Antibodies in Tears of Patients with Pseudomonas aeruginosa Keratitis. Nippon Ganka Gakkai Zasshi 2013, 117, 996–1003. [Google Scholar] [PubMed]
- Siegall, C.B.; Chaudhary, V.K.; FitzGerald, D.J.; Pastan, I. Functional Analysis of Domains II, Ib, and III of Pseudomonas Exotoxin. J. Biol. Chem. 1989, 264, 14256–14261. [Google Scholar] [CrossRef] [PubMed]
- Twining, S.S.; Kirschner, S.E.; Mahnke, L.A.; Frank, D.W. Effect of Pseudomonas aeruginosa Elastase, Alkaline Protease, and Exotoxin A on Corneal Proteinases and Proteins. Investig. Ophthalmol. Vis. Sci. 1993, 34, 2699–2712. [Google Scholar]
- Pillar, C.M.; Hobden, J.A. Pseudomonas aeruginosa Exotoxin A and Keratitis in Mice. Investig. Ophthalmol. Vis. Sci. 2002, 43, 1437–1444. [Google Scholar]
- Suzuki, T.; Okamoto, S.; Oka, N.; Hayashi, N.; Gotoh, N.; Shiraishi, A. Role of PvdE Pyoverdine Synthesis in Pseudomonas aeruginosa Keratitis. Cornea 2018, 37, S99–S105. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Liu, Q.; Song, F.; Huang, Y. Differentially Expressed Genes of Pseudomonas aeruginosa Isolates from Eyes with Keratitis and Healthy Conjunctival Sacs. Infect. Drug Resist. 2022, 15, 4495–4506. [Google Scholar] [CrossRef] [PubMed]
- Yahr, T.L.; Goranson, J.; Frank, D.W. Exoenzyme S of Pseudomonas aeruginosa Is Secreted by a Type III Pathway. Mol. Microbiol. 1996, 22, 991–1003. [Google Scholar] [CrossRef] [PubMed]
- Kroken, A.R.; Klein, K.A.; Mitchell, P.S.; Nieto, V.; Jedel, E.J.; Evans, D.J.; Fleiszig, S.M.J. Intracellular Replication of Pseudomonas aeruginosa in Epithelial Cells Requires Suppression of the Caspase-4 Inflammasome. bioRxiv Prepr. Serv. Biol. 2023, 24, e00351-23. [Google Scholar] [CrossRef]
- Toska, J.; Sun, Y.; Carbonell, D.A.; Foster, A.N.-S.; Jacobs, M.R.; Pearlman, E.; Rietsch, A. Diversity of Virulence Phenotypes among Type III Secretion Negative Pseudomonas aeruginosa Clinical Isolates. PLoS ONE 2014, 9, e86829. [Google Scholar] [CrossRef]
- Yam, J.K.H.; Aung, T.T.; Chua, S.L.; Cheng, Y.; Kohli, G.S.; Zhou, J.; Constancias, F.; Liu, Y.; Cai, Z.; Salido, M.M.S.; et al. Elevated C-Di-GMP Levels and Expression of the Type III Secretion System Promote Corneal Infection by Pseudomonas aeruginosa. Infect. Immun. 2022, 90, e00061-22. [Google Scholar] [CrossRef]
- Zhao, H.; Clevenger, A.L.; Ritchey, J.W.; Zgurskaya, H.I.; Rybenkov, V.V. Pseudomonas aeruginosa Condensins Support Opposite Differentiation States. J. Bacteriol. 2016, 198, 2936–2944. [Google Scholar] [CrossRef]
- Zhao, H.; Clevenger, A.L.; Coburn, P.S.; Callegan, M.C.; Rybenkov, V.V. Condensins Are Essential for Pseudomonas aeruginosa Corneal Virulence through Their Control of Lifestyle and Virulence Programs. Mol. Microbiol. 2022, 117, 937–957. [Google Scholar] [CrossRef]
- O’Malley, Y.Q.; Reszka, K.J.; Spitz, D.R.; Denning, G.M.; Britigan, B.E. Pseudomonas aeruginosa Pyocyanin Directly Oxidizes Glutathione and Decreases Its Levels in Airway Epithelial Cells. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2004, 287, L94–L103. [Google Scholar] [CrossRef] [PubMed]
- Parks, Q.M.; Hobden, J.A. Polyphosphate Kinase 1 and the Ocular Virulence of Pseudomonas aeruginosa. Investig. Ophthalmol. Vis. Sci. 2005, 46, 248–251. [Google Scholar] [CrossRef] [PubMed]
- Domenech, C.E.; Garrido, M.N.; Lisa, T.A. Pseudomonas aeruginosa Cholinesterase and Phosphorylcholine Phosphatase: Two Enzymes Contributing to Corneal Infection. FEMS Microbiol. Lett. 1991, 66, 131–135. [Google Scholar] [CrossRef] [PubMed]
- Marquart, M.E.; Caballero, A.R.; Chomnawang, M.; Thibodeaux, B.A.; Twining, S.S.; O’Callaghan, R.J. Identification of a Novel Secreted Protease from Pseudomonas aeruginosa That Causes Corneal Erosions. Investig. Ophthalmol. Vis. Sci. 2005, 46, 3761–3768. [Google Scholar] [CrossRef] [PubMed]
- Thibodeaux, B.A.; Caballero, A.R.; Marquart, M.E.; Tommassen, J.; O’Callaghan, R.J. Corneal Virulence of Pseudomonas aeruginosa Elastase B and Alkaline Protease Produced by Pseudomonas Putida. Curr. Eye Res. 2007, 32, 373–386. [Google Scholar] [CrossRef]
- Qin, S.; Xiao, W.; Zhou, C.; Pu, Q.; Deng, X.; Lan, L.; Liang, H.; Song, X.; Wu, M. Pseudomonas aeruginosa: Pathogenesis, Virulence Factors, Antibiotic Resistance, Interaction with Host, Technology Advances and Emerging Therapeutics. Signal Transduct. Target. Ther. 2022, 7, 199. [Google Scholar] [CrossRef] [PubMed]
- Iglewski, B.H.; Sadoff, J.; Bjorn, M.J.; Maxwell, E.S. Pseudomonas aeruginosa Exoenzyme S: An Adenosine Diphosphate Ribosyltransferase Distinct from Toxin A. Proc. Natl. Acad. Sci. USA 1978, 75, 3211–3215. [Google Scholar] [CrossRef] [PubMed]
- Hritonenko, V.; Metruccio, M.; Evans, D.; Fleiszig, S. Epithelial Cell Lysates Induce ExoS Expression and Secretion by Pseudomonas aeruginosa. FEMS Microbiol. Lett. 2018, 365, fny053. [Google Scholar] [CrossRef] [PubMed]
- Coburn, J.; Kane, A.V.; Feig, L.; Gill, D.M. Pseudomonas aeruginosa Exoenzyme S Requires a Eukaryotic Protein for ADP-Ribosyltransferase Activity. J. Biol. Chem. 1991, 266, 6438–6446. [Google Scholar] [CrossRef] [PubMed]
- Angus, A.A.; Lee, A.A.; Augustin, D.K.; Lee, E.J.; Evans, D.J.; Fleiszig, S.M.J. Pseudomonas aeruginosa Induces Membrane Blebs in Epithelial Cells, Which Are Utilized as a Niche for Intracellular Replication and Motility. Infect. Immun. 2008, 76, 1992–2001. [Google Scholar] [CrossRef] [PubMed]
- Hritonenko, V.; Evans, D.J.; Fleiszig, S.M.J. Translocon-Independent Intracellular Replication by Pseudomonas aeruginosa Requires the ADP-Ribosylation Domain of ExoS. Microbes Infect. 2012, 14, 1366–1373. [Google Scholar] [CrossRef] [PubMed]
- Heimer, S.R.; Evans, D.J.; Stern, M.E.; Barbieri, J.T.; Yahr, T.; Fleiszig, S.M.J. Pseudomonas aeruginosa Utilizes the Type III Secreted Toxin ExoS to Avoid Acidified Compartments within Epithelial Cells. PLoS ONE 2013, 8, e73111. [Google Scholar] [CrossRef] [PubMed]
- Angus, A.A.; Evans, D.J.; Barbieri, J.T.; Fleiszig, S.M.J. The ADP-Ribosylation Domain of Pseudomonas aeruginosa ExoS Is Required for Membrane Bleb Niche Formation and Bacterial Survival within Epithelial Cells. Infect. Immun. 2010, 78, 4500–4510. [Google Scholar] [CrossRef] [PubMed]
- Fleiszig, S.M.; Zaidi, T.S.; Preston, M.J.; Grout, M.; Evans, D.J.; Pier, G.B. Relationship between Cytotoxicity and Corneal Epithelial Cell Invasion by Clinical Isolates of Pseudomonas aeruginosa. Infect. Immun. 1996, 64, 2288–2294. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.J.; Evans, D.J.; Fleiszig, S.M.J. Role of Pseudomonas aeruginosa ExsA in Penetration through Corneal Epithelium in a Novel In Vivo Model. Investig. Ophthalmol. Vis. Sci. 2003, 44, 5220–5227. [Google Scholar] [CrossRef] [PubMed]
- Evans, D.J.; Frank, D.W.; Finck-Barbançon, V.; Wu, C.; Fleiszig, S.M.J. Pseudomonas aeruginosa Invasion and Cytotoxicity Are Independent Events, Both of Which Involve Protein Tyrosine Kinase Activity. Infect. Immun. 1998, 66, 1453–1459. [Google Scholar] [CrossRef] [PubMed]
- Yahr, T.L.; Barbieri, J.T.; Frank, D.W. Genetic Relationship between the 53- and 49-Kilodalton Forms of Exoenzyme S from Pseudomonas aeruginosa. J. Bacteriol. 1996, 178, 1412–1419. [Google Scholar] [CrossRef] [PubMed]
- Cowell, B.A.; Chen, D.Y.; Frank, D.W.; Vallis, A.J.; Fleiszig, S.M.J. ExoT of Cytotoxic Pseudomonas aeruginosa Prevents Uptake by Corneal Epithelial Cells. Infect. Immun. 2000, 68, 403–406. [Google Scholar] [CrossRef] [PubMed]
- Finck-Barbançon, V.; Goranson, J.; Zhu, L.; Sawa, T.; Wiener-Kronish, J.P.; Fleiszig, S.M.J.; Wu, C.; Mende-Mueller, L.; Frank, D.W. ExoU Expression by Pseudomonas aeruginosa Correlates with Acute Cytotoxicity and Epithelial Injury. Mol. Microbiol. 1997, 25, 547–557. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Wu, S.; Sun, Y.; Zou, X.; Zheng, L.; Duan, S.; Wang, J.; Yu, B.; Sui, R.; Xu, F. Bacteria-Targeting Photodynamic Nanoassemblies for Efficient Treatment of Multidrug-Resistant Biofilm Infected Keratitis. Adv. Funct. Mater. 2022, 32, 2111066. [Google Scholar] [CrossRef]
- Ramirez, J.C.; Fleiszig, S.M.J.; Sullivan, A.B.; Tam, C.; Borazjani, R.; Evans, D.J. Traversal of Multilayered Corneal Epithelia by Cytotoxic Pseudomonas aeruginosa Requires the Phospholipase Domain of ExoU. Investig. Ophthalmol. Vis. Sci. 2012, 53, 448–453. [Google Scholar] [CrossRef] [PubMed]
- Sato, H.; Feix, J.B.; Frank, D.W. Identification of Superoxide Dismutase as a Cofactor for the Pseudomonas Type III Toxin, ExoU. Biochemistry 2006, 45, 10368–10375. [Google Scholar] [CrossRef] [PubMed]
- Karthikeyan, R.S.; Priya, J.L.; Leal, S.M.; Toska, J.; Rietsch, A.; Prajna, V.; Pearlman, E.; Lalitha, P. Host Response and Bacterial Virulence Factor Expression in Pseudomonas aeruginosa and Streptococcus Pneumoniae Corneal Ulcers. PLoS ONE 2013, 8, e64867. [Google Scholar] [CrossRef] [PubMed]
- Garg, P.; Sharma, S.; Rao, G.N. Ciprofloxacin-Resistant Pseudomonas Keratitis. Ophthalmology 1999, 106, 1319–1323. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, M.; Mohajernezhadfard, Z.; Khodabande, A.; Vahedi, P. Antibiotic Susceptibility Patterns of Pseudomonas Corneal Ulcers in Contact Lens Wearers. Middle East Afr. J. Ophthalmol. 2011, 18, 228–231. [Google Scholar] [CrossRef] [PubMed]
- Dave, A.; Samarth, A.; Karolia, R.; Sharma, S.; Karunakaran, E.; Partridge, L.; MacNeil, S.; Monk, P.N.; Garg, P.; Roy, S. Characterization of Ocular Clinical Isolates of Pseudomonas aeruginosa from Non-Contact Lens Related Keratitis Patients from South India. Microorganisms 2020, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Morelli, M.K.; Kloosterboer, A.; Fulton, S.A.; Furin, J.; Newman, N.; Omar, A.F.; Rojas, L.J.; Marshall, S.H.; Yasmin, M.; Bonomo, R.A. Investigating and Treating a Corneal Ulcer Due to Extensively Drug-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2023, 67, e00277-23. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Steen, D.; Amin, S. Successful Treatment of an Extensively Drug-Resistant Pseudomonal Ulcer Associated with Contaminated Artificial Tears. Am. J. Ophthalmol. Case Rep. 2023, 32, 101909. [Google Scholar] [CrossRef] [PubMed]
- Campardelli, R.; Trucillo, P.; Reverchon, E. Supercritical Assisted Process for the Efficient Production of Liposomes Containing Antibiotics for Ocular Delivery. J. CO2 Util. 2018, 25, 235–241. [Google Scholar] [CrossRef]
- Rezvani Ghomi, E.; Nourbakhsh, N.; Akbari Kenari, M.; Zare, M.; Ramakrishna, S. Collagen-based Biomaterials for Biomedical Applications. J. Biomed. Mater. Res. Part B Appl. Biomater. 2021, 109, 1986–1999. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, M.; Vira, D.; Medikonda, R.; Kumar, N. Extensively and Pan-Drug Resistant Pseudomonas aeruginosa Keratitis: Clinical Features, Risk Factors, and Outcome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Guo, D.; Liu, X.; Jin, X.; Shi, Y.; Wang, Y.; Zhang, N.; Zhang, H. Ocular Pathogens and Antibiotic Resistance in Microbial Keratitis over Three Years in Harbin, Northeast China. Acta Ophthalmol. 2021, 99, 909–915. [Google Scholar] [CrossRef] [PubMed]
- Lalitha, P.; Manoharan, G.; Karpagam, R.; Prajna, N.V.; Srinivasan, M.; Mascarenhas, J.; Das, M.; Porco, T.C.; Lietman, T.M.; Cevallos, V.; et al. Trends in Antibiotic Resistance in Bacterial Keratitis Isolates from South India. Br. J. Ophthalmol. 2017, 101, 108–113. [Google Scholar] [CrossRef] [PubMed]
- Oldenburg, C.E.; Lalitha, P.; Srinivasan, M.; Rajaraman, R.; Ravindran, M.; Mascarenhas, J.; Borkar, D.S.; Ray, K.J.; Zegans, M.E.; McLeod, S.D.; et al. Emerging Moxifloxacin Resistance in Pseudomonas aeruginosa Keratitis Isolates in South India. Ophthalmic Epidemiol. 2013, 20, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Vazirani, J.; Wurity, S.; Ali, M.H. Multidrug-Resistant Pseudomonas aeruginosa Keratitis. Ophthalmology 2015, 122, 2110–2114. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Elofsson, M.; Roy, S. Attenuation of Pseudomonas aeruginosa Infection by INP0341, a Salicylidene Acylhydrazide, in a Murine Model of Keratitis. Virulence 2020, 11, 795–804. [Google Scholar] [CrossRef] [PubMed]
- Zaidi, T.S.; Zaidi, T.; Pier, G.B.; Priebe, G.P. Topical Neutralization of Interleukin-17 during Experimental Pseudomonas aeruginosa Corneal Infection Promotes Bacterial Clearance and Reduces Pathology. Infect. Immun. 2012, 80, 3706–3712. [Google Scholar] [CrossRef] [PubMed]
- Me, R.; Gao, N.; Dai, C.; Yu, F.X. IL-17 Promotes Pseudomonas aeruginosa Keratitis in C57BL/6 Mouse Corneas. J. Immunol. 2020, 204, 169–179. [Google Scholar] [CrossRef]
- Suryawanshi, A.; Cao, Z.; Thitiprasert, T.; Zaidi, T.S.; Panjwani, N. Galectin-1–Mediated Suppression of Pseudomonas aeruginosa –Induced Corneal Immunopathology. J. Immunol. 2013, 190, 6397–6409. [Google Scholar] [CrossRef]
- Xu, S.; Liu, X.; Liu, X.; Shi, Y.; Jin, X.; Zhang, N.; Li, X.; Zhang, H. Wedelolactone Ameliorates Pseudomonas aeruginosa-Induced Inflammation and Corneal Injury by Suppressing Caspase-4/5/11/GSDMD-Mediated Non-Canonical Pyroptosis. Exp. Eye Res. 2021, 211, 108750. [Google Scholar] [CrossRef] [PubMed]
- Ramadan, A.; Cao, Z.; Gadjeva, M.; Zaidi, T.S.; Rathinam, V.A.; Panjwani, N. The NLRP3 Inflammasome Is Required for Protection Against Pseudomonas Keratitis. Investig. Ophthalmol. Vis. Sci. 2023, 64, 11. [Google Scholar] [CrossRef]
- Jain, R.; Murthy, S.I.; Motukupally, S.R. Clinical Outcomes of Corneal Graft Infections Caused by Multi–Drug Resistant Pseudomonas aeruginosa. Cornea 2014, 33, 22–26. [Google Scholar] [CrossRef]
- Hobden, J.A. Treatment of Experimental Pseudomonas Keratitis Using Collagen Shields Containing Tobramycin. Arch. Ophthalmol. 1988, 106, 1605–1607. [Google Scholar] [CrossRef]
- del Mar Cendra, M.; Christodoulides, M.; Hossain, P. Effect of Different Antibiotic Chemotherapies on Pseudomonas aeruginosa Infection In Vitro of Primary Human Corneal Fibroblast Cells. Front. Microbiol. 2017, 8, 1614. [Google Scholar] [CrossRef]
- Chatterjee, S.; Agrawal, D. Multi-Drug Resistant Pseudomonas aeruginosa Keratitis and Its Effective Treatment with Topical Colistimethate. Indian J. Ophthalmol. 2016, 64, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Harris, V.; Pifer, R.; Shannon, P.; Crary, M. Comparative Evaluation of Pseudomonas aeruginosa Adhesion to a Poly-(2-Methacryloyloxyethyl Phosphorylcholine)-Modified Silicone Hydrogel Contact Lens. Vision 2023, 7, 27. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, K.; Fukazawa, K.; Sharma, V.; Liang, S.; Shows, A.; Dunbar, D.C.; Zheng, Y.; Ge, J.; Zhang, S.; Hong, Y.; et al. Antifouling Silicone Hydrogel Contact Lenses with a Bioinspired 2-Methacryloyloxyethyl Phosphorylcholine Polymer Surface. ACS Omega 2021, 6, 7058–7067. [Google Scholar] [CrossRef] [PubMed]
- Willcox, M.D.P.; Hume, E.B.H.; Aliwarga, Y.; Kumar, N.; Cole, N. A Novel Cationic-Peptide Coating for the Prevention of Microbial Colonization on Contact Lenses. J. Appl. Microbiol. 2008, 105, 1817–1825. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Comparative Mode of Action of the Antimicrobial Peptide Melimine and Its Derivative Mel4 against Pseudomonas aeruginosa. Sci. Rep. 2019, 9, 7063. [Google Scholar] [CrossRef]
- Dutta, D.; Zhao, T.; Cheah, K.B.; Holmlund, L.; Willcox, M.D.P. Activity of a Melimine Derived Peptide Mel4 against Stenotrophomonas, Delftia, Elizabethkingia, Burkholderia and Biocompatibility as a Contact Lens Coating. Contact Lens Anterior Eye 2017, 40, 175–183. [Google Scholar] [CrossRef]
- Yasir, M.; Dutta, D.; Willcox, M.D.P. Mode of Action of the Antimicrobial Peptide Mel4 Is Independent of Staphylococcus aureus Cell Membrane Permeability. PLoS ONE 2019, 14, e0215703. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Vijay, A.K.; Kumar, N.; Willcox, M.D.P. Melimine-Coated Antimicrobial Contact Lenses Reduce Microbial Keratitis in an Animal Model. Investig. Ophthalmol. Vis. Sci. 2016, 57, 5616–5624. [Google Scholar] [CrossRef] [PubMed]
- Dutta, D.; Ozkan, J.; Willcox, M.D.P. Biocompatibility of Antimicrobial Melimine Lenses. Optom. Vis. Sci. 2014, 91, 570–581. [Google Scholar] [CrossRef] [PubMed]
- Spoerl, E.; Huhle, M.; Seiler, T. Induction of Cross-Links in Corneal Tissue. Exp. Eye Res. 1998, 66, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Schrier, A.; Greebel, G.; Attia, H.; Trokel, S.; Smith, E.F. In Vitro Antimicrobial Efficacy of Riboflavin and Ultraviolet Light on Staphylococcus aureus, Methicillin-Resistant Staphylococcus aureus, and Pseudomonas aeruginosa. J. Refract. Surg. 2009, 25, S799–S802. [Google Scholar] [CrossRef] [PubMed]
- Saad, Z.A.; Elnashar, H.; Negm, S.; Elsayed, H.A.; Abdallah, M.G.; Abuamara, T.M.M.; Abd-Elhay, W.M.; Elghonemy, H.M. Collagen Cross-Linking as Monotherapy in Experimentally Induced Corneal Abscess in Rabbits. BMC Ophthalmol. 2023, 23, 266. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Yin, Y.; Hu, T.; Du, K.; Fu, Y.; Xiang, A.; Fu, Q.; Wu, X.; Li, Y.; Wen, D. Polymicrobial Keratitis after Accelerated Corneal Collagen Cross-Linking in Keratoconus: Case Reports and Literature Review. Eur. J. Ophthalmol. 2022, 32, 1375–1385. [Google Scholar] [CrossRef] [PubMed]
- Kasparova, E.A.; Fedorov, A.A.; Kasparova, E.A.; Yang, B. Modified Crosslinking in the Treatment of Purulent Keratitis and Corneal Ulcers. J. EuCornea 2019, 3, 13–21. [Google Scholar] [CrossRef]
- Knyazer, B.; Krakauer, Y.; Tailakh, M.A.; Achiron, A.; Hecht, I.; Lifshitz, T.; Torres-Netto, E.A.; Hafezi, N.L.; Hafezi, F. Accelerated Corneal Cross-Linking as an Adjunct Therapy in the Management of Presumed Bacterial Keratitis: A Cohort Study. J. Refract. Surg. 2020, 36, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Shanks, R.M.Q.; Davra, V.R.; Romanowski, E.G.; Brothers, K.M.; Stella, N.A.; Godboley, D.; Kadouri, D.E. An Eye to a Kill: Using Predatory Bacteria to Control Gram-Negative Pathogens Associated with Ocular Infections. PLoS ONE 2013, 8, e66723. [Google Scholar] [CrossRef] [PubMed]
- Romanowski, E.G.; Gupta, S.; Pericleous, A.; Kadouri, D.E.; Shanks, R.M.Q. Clearance of Gram-Negative Bacterial Pathogens from the Ocular Surface by Predatory Bacteria. Antibiotics 2021, 10, 810. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, K.; Ishida, W.; Uchiyama, J.; Rashel, M.; Kato, S.; Morita, T.; Muraoka, A.; Sumi, T.; Matsuzaki, S.; Daibata, M.; et al. Pseudomonas aeruginosa Keratitis in Mice: Effects of Topical Bacteriophage KPP12 Administration. PLoS ONE 2012, 7, e47742. [Google Scholar] [CrossRef] [PubMed]
- Wannasrichan, W.; Htoo, H.H.; Suwansaeng, R.; Pogliano, J.; Nonejuie, P.; Chaikeeratisak, V. Phage-Resistant Pseudomonas aeruginosa against a Novel Lytic Phage JJ01 Exhibits Hypersensitivity to Colistin and Reduces Biofilm Production. Front. Microbiol. 2022, 13, 1004733. [Google Scholar] [CrossRef] [PubMed]
- Majdani, R.; Ghahfarokhi, E.S. Isolation and Characterization of Lytic Bacteriophages against Pseudomonas aeruginosa Isolates from Human Infections in the North-West of Iran. Iran. J. Microbiol. 2022, 14, 203–213. [Google Scholar] [CrossRef] [PubMed]
- Sanya, D.R.A.; Onésime, D.; Vizzarro, G.; Jacquier, N. Recent Advances in Therapeutic Targets Identification and Development of Treatment Strategies towards Pseudomonas aeruginosa Infections. BMC Microbiol. 2023, 23, 86. [Google Scholar] [CrossRef] [PubMed]
- AbuSamra, D.B.; Argüeso, P. Lectin-Glycan Interactions in Corneal Infection and Inflammation. Front. Immunol. 2018, 9, 2338. [Google Scholar] [CrossRef] [PubMed]
- Passos da Silva, D.; Matwichuk, M.L.; Townsend, D.O.; Reichhardt, C.; Lamba, D.; Wozniak, D.J.; Parsek, M.R. The Pseudomonas aeruginosa Lectin LecB Binds to the Exopolysaccharide Psl and Stabilizes the Biofilm Matrix. Nat. Commun. 2019, 10, 2183. [Google Scholar] [CrossRef] [PubMed]
- Sommer, R.; Wagner, S.; Rox, K.; Varrot, A.; Hauck, D.; Wamhoff, E.-C.; Schreiber, J.; Ryckmans, T.; Brunner, T.; Rademacher, C.; et al. Glycomimetic, Orally Bioavailable LecB Inhibitors Block Biofilm Formation of Pseudomonas aeruginosa. J. Am. Chem. Soc. 2018, 140, 2537–2545. [Google Scholar] [CrossRef] [PubMed]
- Hazlett, L.; McClellan, S.; Somayajulu, M.; Bessert, D. Targeting Inflammation Driven by HMGB1 in Bacterial Keratitis—A Review. Pathogens 2021, 10, 1235. [Google Scholar] [CrossRef] [PubMed]
- Ekanayaka, S.A.; McClellan, S.A.; Peng, X.; Barrett, R.P.; Francis, R.; Hazlett, L.D. HMGB1 Antagonist, Box A, Reduces TLR4, RAGE, and Inflammatory Cytokines in the Cornea of P. aeruginosa-Infected Mice. J. Ocul. Pharmacol. Ther. 2018, 34, 659–669. [Google Scholar] [CrossRef]
- Hazlett, L.D.; McClellan, S.A.; Ekanayaka, S.A. Decreasing HMGB1 Levels Improves Outcome of Pseudomonas aeruginosa Keratitis in Mice. J. Rare Dis. Res. Treat. 2016, 1, 36–39. [Google Scholar] [CrossRef]
- Xue, J.; Suarez, J.S.; Minaai, M.; Li, S.; Gaudino, G.; Pass, H.I.; Carbone, M.; Yang, H. HMGB1 as a Therapeutic Target in Disease. J. Cell. Physiol. 2021, 236, 3406–3419. [Google Scholar] [CrossRef] [PubMed]
- Baig, M.S.; Ahad, A.; Aslam, M.; Imam, S.S.; Aqil, M.; Ali, A. Application of Box–Behnken Design for Preparation of Levofloxacin-Loaded Stearic Acid Solid Lipid Nanoparticles for Ocular Delivery: Optimization, In Vitro Release, Ocular Tolerance, and Antibacterial Activity. Int. J. Biol. Macromol. 2016, 85, 258–270. [Google Scholar] [CrossRef] [PubMed]
- Subrizi, A.; del Amo, E.M.; Korzhikov-Vlakh, V.; Tennikova, T.; Ruponen, M.; Urtti, A. Design Principles of Ocular Drug Delivery Systems: Importance of Drug Payload, Release Rate, and Material Properties. Drug Discov. Today 2019, 24, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Tsai, C.-H.; Wang, P.-Y.; Lin, I.-C.; Huang, H.; Liu, G.-S.; Tseng, C.-L. Ocular Drug Delivery: Role of Degradable Polymeric Nanocarriers for Ophthalmic Application. Int. J. Mol. Sci. 2018, 19, 2830. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles─From Liposomes to MRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Furneri, P.M.; Fresta, M.; Puglisi, G.; Tempera, G. Ofloxacin-Loaded Liposomes: In Vitro Activity and Drug Accumulation in Bacteria. Antimicrob. Agents Chemother. 2000, 44, 2458–2464. [Google Scholar] [CrossRef]
- Navaratnam, S.; Claridge, J. Primary Photophysical Properties of Ofloxacin ¶. Photochem. Photobiol. 2007, 72, 283–290. [Google Scholar] [CrossRef]
- Hassan, H.A.F.M.; Ali, A.I.; ElDesawy, E.M.; ElShafeey, A.H. Pharmacokinetic and Pharmacodynamic Evaluation of Gemifloxacin Chitosan Nanoparticles As an Antibacterial Ocular Dosage Form. J. Pharm. Sci. 2022, 111, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Mehanna, M.M.; Elmaradny, H.A.; Samaha, M.W. Mucoadhesive Liposomes as Ocular Delivery System: Physical, Microbiological, and in Vivo Assessment. Drug Dev. Ind. Pharm. 2010, 36, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Maticorena-Quevedo, J.; Patiño-Valderrama, L.; Arellano-Caro, K. Use of Topical Colistin in Bacterial Keratitis Caused by Extensively Drug-Resistant Pseudomonas aeruginosa: A Case Report. Arq. Bras. Oftalmol. 2022, 86, 384–387. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Stapleton, F.; Summers, S.; Rice, S.A.; Willcox, M.D.P. Antibiotic Resistance Characteristics of Pseudomonas aeruginosa Isolated from Keratitis in Australia and India. Antibiotics 2020, 9, 600. [Google Scholar] [CrossRef] [PubMed]
- Lomholt, J.A. Ciprofloxacin Susceptibility of Pseudomonas aeruginosa Isolates from Keratitis. Br. J. Ophthalmol. 2003, 87, 1238–1240. [Google Scholar] [CrossRef] [PubMed]
- Parchand, S.; Agrawal, D.; Chatterjee, S.; Gangwe, A.; Mishra, M.; Agrawal, D. Post-Cataract Surgery Cluster Endophthalmitis Due to Multidrug-Resistant Pseudomonas aeruginosa: A Retrospective Cohort Study of Six Clusters. Indian J. Ophthalmol. 2020, 68, 1424–1431. [Google Scholar] [CrossRef]
- Schubert, F.; Sekundo, W.; Paul, C. Therapierefraktäre 4-MRGN-Keratitis. Der Ophthalmol. 2021, 118, 486–489. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.J.; Ho, C.S.; Cairns, J.; Elsahn, A.; Al-Aqaba, M.; Boswell, T.; Said, D.G.; Dua, H.S. 12-Year Analysis of Incidence, Microbiological Profiles and in Vitro Antimicrobial Susceptibility of Infectious Keratitis: The Nottingham Infectious Keratitis Study. Br. J. Ophthalmol. 2021, 105, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Bawankar, P.; Bhattacharjee, H.; Barman, M.; Soibam, R.; Deka, H.; Chandra Kuri, G.; Medhi, J. Outbreak of Multidrug-Resistant Pseudomonas aeruginosa Endophthalmitis Due to Contaminated Trypan Blue Solution. J. Ophthalmic Vis. Res. 2019, 14, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Watson, S.; Cabrera-Aguas, M.; Khoo, P.; Pratama, R.; Gatus, B.J.; Gulholm, T.; El-Nasser, J.; Lahra, M.M. Keratitis Antimicrobial Resistance Surveillance Program, Sydney, Australia: 2016 Annual Report. Clin. Exp. Ophthalmol. 2019, 47, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Serna-Ojeda, J.C.; Pedro-Aguilar, L.; Rodriguez-Quintanilla, C.; Mejía-López, H.; Ponce-Angulo, D.G.; Navas, A.; Bautista-de Lucio, V.M.; Graue-Hernandez, E.O. Post-Keratoplasty Endophthalmitis by Multidrug-Resistant Pseudomonas aeruginosa with Positive Culture of the Contralateral Donor Cornea: A Case Report. Transplant. Proc. 2018, 50, 964–966. [Google Scholar] [CrossRef] [PubMed]
- Spierer, O.; Miller, D.; O’Brien, T.P. Comparative Activity of Antimicrobials against Pseudomonas aeruginosa, Achromobacter xylosoxidans and Stenotrophomonas maltophilia Keratitis Isolates. Br. J. Ophthalmol. 2018, 102, 708–712. [Google Scholar] [CrossRef]
- Peng, M.Y.; Cevallos, V.; McLeod, S.D.; Lietman, T.M.; Rose-Nussbaumer, J. Bacterial Keratitis: Isolated Organisms and Antibiotic Resistance Patterns in San Francisco. Cornea 2018, 37, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Tam, A.L.C.; Côté, E.; Saldanha, M.; Lichtinger, A.; Slomovic, A.R. Bacterial Keratitis in Toronto: A 16-Year Review of the Microorganisms Isolated and the Resistance Patterns Observed. Cornea 2017, 36, 1528–1534. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, C.S.; Figueira, L.; Moreira-Gonçalves, N.; Moreira, R.; Torrão, L.; Falcão-Reis, F. Clinical and Microbiological Profile of Bacterial Microbial Keratitis in a Portuguese Tertiary Referral Center—Where Are We in 2015? Eye Contact Lens Sci. Clin. Pract. 2018, 44, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Pathengay, A.; Mathai, A.; Shah, G.Y.; Ambatipudi, S. Intravitreal Piperacillin/Tazobactam in the Management of Multidrug-Resistant Pseudomonas aeruginosa Endophthalmitis. J. Cataract Refract. Surg. 2010, 36, 2210–2211. [Google Scholar] [CrossRef] [PubMed]
- Al-Dhaheri, H.S.; Al-Tamimi, M.D.; Khandekar, R.B.; Khan, M.; Stone, D.U. Ocular Pathogens and Antibiotic Sensitivity in Bacterial Keratitis Isolates at King Khaled Eye Specialist Hospital, 2011 to 2014. Cornea 2016, 35, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Tajima, K.; Miyake, T.; Koike, N.; Hattori, T.; Takahashi, H.; Matsumoto, T.; Fujita, K.; Kuroda, M.; Ito, N.; Goto, H. Two Different Concentrations of Topical Levofloxacin for the Treatment of Multidrug-Resistant Pseudomonas aeruginosa Keratitis. J. Ocul. Pharmacol. Ther. 2015, 31, 636–641. [Google Scholar] [CrossRef] [PubMed]
- Ni, N.; Nam, E.M.; Hammersmith, K.M.; Nagra, P.K.; Azari, A.A.; Leiby, B.E.; Dai, Y.; Cabrera, F.A.; Ma, J.F.; Lambert, C.E.; et al. Seasonal, Geographic, and Antimicrobial Resistance Patterns in Microbial Keratitis. Cornea 2015, 34, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Rahman, Z.A.; Harun, A.; Hasan, H.; Mohamed, Z.; Noor, S.S.M.; Deris, Z.Z.; Ismail, N.; Hassan, A.S.; Ahmad, F.; Yaakub, A. Ocular Surface Infections in Northeastern State of Malaysia. Eye Contact Lens Sci. Clin. Pract. 2013, 39, 355–360. [Google Scholar] [CrossRef] [PubMed]
- Moshirfar, M.; Mirzaian, G.; Feiz, V.; Kang, P.C. Fourth-Generation Fluoroquinolone-Resistant Bacterial Keratitis after Refractive Surgery. J. Cataract Refract. Surg. 2006, 32, 515–518. [Google Scholar] [CrossRef] [PubMed]
- Begum, N.N.; Al-Khattaf, A.S.; Al-Mansouri, S.M.; Yeboah, E.A.; Kambal, A.-M.M. A Study of Bacterial Isolates from Corneal Specimens and Their Antibiotic Resistance Profile. Saudi Med. J. 2006, 27, 41–45. [Google Scholar] [PubMed]
- Robinson, A.; Kremer, I.; Avisar, R.; Gaton, D.; Savir, H.; Yassur, Y. The Combination of Topical Ceftazidime and Aminoglycosides in the Treatment of Refractory Pseudomonal Keratitis. Graefe’s Arch. Clin. Exp. Ophthalmol. 1999, 237, 177–180. [Google Scholar] [CrossRef]
- Khan, M.; Ma, K.; Wan, I.; Willcox, M.D. Ciprofloxacin Resistance and Tolerance of Pseudomonas aeruginosa Ocular Isolates. Contact Lens Anterior Eye 2023, 46, 101819. [Google Scholar] [CrossRef]
 = represents activation process, X = denotes attenuation process.
= represents activation process, X = denotes attenuation process.
 = represents activation process, X = denotes attenuation process.
= represents activation process, X = denotes attenuation process.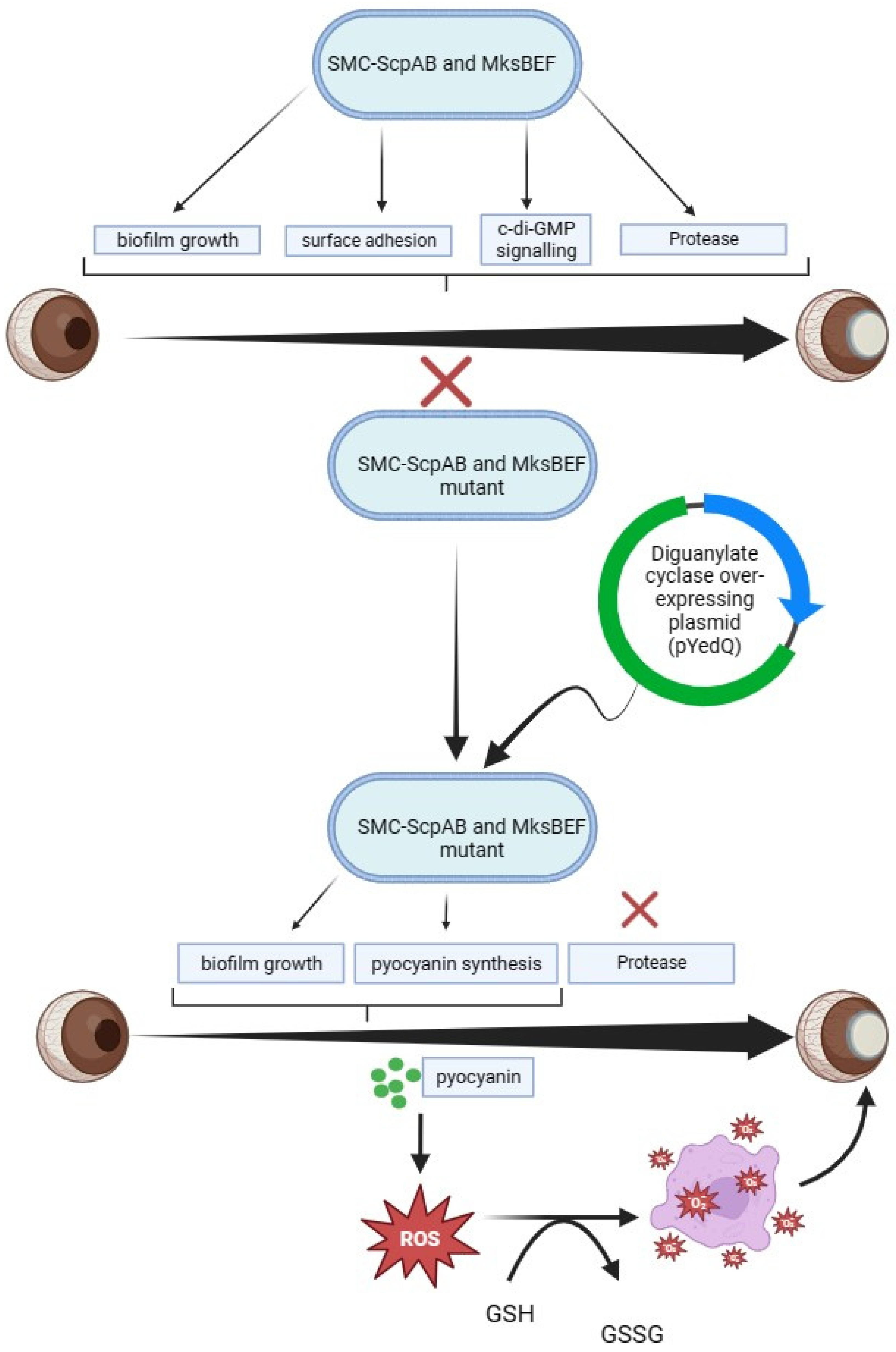
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Badger-Emeka, L.; Emeka, P.; Thirugnanasambantham, K.; Alatawi, A.S. The Role of Pseudomonas aeruginosa in the Pathogenesis of Corneal Ulcer, Its Associated Virulence Factors, and Suggested Novel Treatment Approaches. Pharmaceutics 2024, 16, 1074. https://doi.org/10.3390/pharmaceutics16081074
Badger-Emeka L, Emeka P, Thirugnanasambantham K, Alatawi AS. The Role of Pseudomonas aeruginosa in the Pathogenesis of Corneal Ulcer, Its Associated Virulence Factors, and Suggested Novel Treatment Approaches. Pharmaceutics. 2024; 16(8):1074. https://doi.org/10.3390/pharmaceutics16081074
Chicago/Turabian StyleBadger-Emeka, Lorina, Promise Emeka, Krishnaraj Thirugnanasambantham, and Abdulaziz S. Alatawi. 2024. "The Role of Pseudomonas aeruginosa in the Pathogenesis of Corneal Ulcer, Its Associated Virulence Factors, and Suggested Novel Treatment Approaches" Pharmaceutics 16, no. 8: 1074. https://doi.org/10.3390/pharmaceutics16081074
APA StyleBadger-Emeka, L., Emeka, P., Thirugnanasambantham, K., & Alatawi, A. S. (2024). The Role of Pseudomonas aeruginosa in the Pathogenesis of Corneal Ulcer, Its Associated Virulence Factors, and Suggested Novel Treatment Approaches. Pharmaceutics, 16(8), 1074. https://doi.org/10.3390/pharmaceutics16081074





