Recent Review on Biological Barriers and Host–Material Interfaces in Precision Drug Delivery: Advancement in Biomaterial Engineering for Better Treatment Therapies
Abstract
:1. Introduction
2. Biological Barriers
3. Microenvironmental Barriers
4. Barriers to NP Delivery and Biodistribution
5. Nanoparticles (NPs) in Precision Medicine
Local NP Distribution
| Formulation | Targeted Molecule | Advancement | Uses | Refs. |
|---|---|---|---|---|
| LNPs | mRNA | Charge | Cancer and autoimmune immunotherapies | [49] |
| Small molecule, photothermal agent | Metastatic breast cancer | [50] | ||
| mRNA | Melanoma, HPV, anaemia, and acute lymphoblastic leukaemia and retinal problems | [51,52] | ||
| Cyclic dinucleotide | Responsivity | Lung metastasis of melanoma and breast cancer | [53] | |
| mRNA, protein | Charge and responsibility | Lung and spleen diseases | [54] | |
| siRNA | Surface modification | Pulmonary diseases | [55] | |
| pDNA | Surface modification and responsivity | Osteoporosis | [56] | |
| siRNA | Charge and surface modification | Hepatocellular carcinoma | [57] | |
| NA; observed distribution | Shape | Neuroinflammatory disorders; cervical cancer | [36,58] | |
| Polymer NPs | Small molecule | Responsivity | Non-small-cell lung cancer, lung carcinoma | [59,60] |
| Protein | Diabetes | [61,62] | ||
| Protein, small molecule | Breast cancer immunotherapies | [63,64] | ||
| Protein, gRNA | Eye monogenetic diseases | [65] | ||
| Protein, ssDNA | Influenza A H1N1 vaccine | [66] | ||
| Anti-sense RNA | Mitochondrial disorders | [67] | ||
| Cyclic dinucleotide | Pancreatic adenocarcinomas; glioblastoma | [68] | ||
| siRNA, small molecule | Cancer | [69] | ||
| siRNA | Metastatic melanoma | [70] | ||
| Small molecule | Surface modification | TNBC; SLE; myocardial ischaemia reperfusion damage; breast cancer | [71] | |
| mRNA | Liver disease, ovarian cancer, melanoma, glioblastoma | [72,73] | ||
| mRNA, DNA | Cystic fibrosis | [74] | ||
| Dyes | Glioblastoma | [75] | ||
| NA; observed distribution | Osteoarthritis; nuclear delivery | [76,77] | ||
| Small molecule | Surface alteration and response | Ovarian, breast, and hepatocellular carcinoma cancer | [77] | |
| Small molecule, peptide, protein | Colorectal cancer | [78] | ||
| siRNA, pDNA | Hepatocellular carcinoma | [79] | ||
| Antibody, miRNA | Colorectal cancer | [80] | ||
| Antibody, photosensitiser | Metastatic breast cancer | [81] | ||
| Inorganic NPs | Imaging agent, small molecule | Responsivity | Breast cancer | [81] |
| Neoantigen, adjuvant, photosensor | Colon carcinoma, melanoma | [82] | ||
| Photosensitiser | Surface modification | Squamous cell oral cancer | [83] | |
| siRNA | Breast cancer | [84] | ||
| miRNA | TNBC | [85] | ||
| NA; observed distribution | Neurological disorders, glioblastoma | [37,86] | ||
| Protein, antibody | Surface modification and responsivity | Dysfunctions of mitochondria | [87] | |
| Small molecule | TNBC | [88] | ||
| NPs for magnetic hyperthermia | Surface modification and size | Breast cancer | [89] | |
| Small molecule | Surface modification and shape | NSCC lung cancer | [90] |
6. Biomaterial
6.1. Biomaterials in Targeted Drug Delivery
6.1.1. Passive Targeting
6.1.2. Nanomaterial-Induced Endothelial Leakiness
6.1.3. Stimuli-Responsive DDS
6.1.4. Antibodies or Ligands Target Actively
6.1.5. Biomaterials in Vaccine Development
6.1.6. Liposomes
6.1.7. Virus-like Particles (VLPs)
6.1.8. Inorganic Particles
6.1.9. Polymeric Particles
6.1.10. Outer-Membrane Vesicles (OMVs)
6.1.11. Immunostimulating Complexes (ISCOMs)
7. Biomaterials in Gene Therapy
7.1. Delivery of miRNA
7.2. Delivery of Encoding DNA
7.3. Therapeutic Drug–Nucleic Acid Co-Delivery
7.4. Delivery of CAR Gene
8. Biomaterials in Stem Cell Therapy
Stem Cell Carriers
9. NPs for Cancer Therapy
9.1. Active Targeting to Cancer Cells
9.2. NPs for Immunotherapy
9.3. Immune Activation
9.4. Immune Suppression
9.5. Tumour Microenvironment Adaptation
9.6. Bacteria as a Transport Vehicle
9.7. Carrier-Free Nanomedicine Delivery Systems
10. Prospects and Advancement
11. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lu, C.Y.; Terry, V.; Thomas, D.M. Precision medicine: Affording the successes of science. npj Precis. Oncol. 2023, 7, 3. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Gao, P.; Cummings, J.; Toschi, N.; Thompson, P.M.; Hu, Y.; Cho, M.; Vergallo, A. The foundation and architecture of precision medicine in neurology and psychiatry. Trends Neurosci. 2023, 46, 176–198. [Google Scholar] [CrossRef] [PubMed]
- Ezike, T.C.; Okpala, U.S.; Onoja, U.L.; Nwike, C.P.; Ezeako, E.C.; Okpara, O.J.; Okoroafor, C.C.; Eze, S.C.; Kalu, O.L.; Odoh, E.C.; et al. Advances in drug delivery systems, challenges and future directions. Heliyon 2023, 9, e17488. [Google Scholar] [CrossRef] [PubMed]
- Salahshoori, I.; Golriz, M.; Nobre, M.A.L.; Mahdavi, S.; Eshaghi Malekshah, R.; Javdani-Mallak, A. Simulation-based approaches for drug delivery systems: Navigating advancements, opportunities, and challenges. J. Mol. Liq. 2024, 395, 123888. [Google Scholar] [CrossRef]
- Desai, P.; Dasgupta, A.; Sofias, A.M.; Peña, Q.; Göstl, R.; Slabu, I.; Schwaneberg, U.; Stiehl, T.; Wagner, W.; Jockenhövel, S.; et al. Transformative Materials for Interfacial Drug Delivery. Adv. Healthc. Mater. 2023, 12, 2301062. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.; Wei, T.; Goldberg, H.; Wang, W.; Cullion, K.; Kohane, D.S. Getting Drugs Across Biological Barriers. Adv. Mater. 2017, 29, 1606596. [Google Scholar] [CrossRef] [PubMed]
- Paramshetti, S.; Angolkar, M.; Al Fatease, A.; Alshahrani, S.M.; Hani, U.; Garg, A.; Ravi, G.; Osmani, R.A.M. Revolutionizing Drug Delivery and Therapeutics: The Biomedical Applications of Conductive Polymers and Composites-Based Systems. Pharmaceutics 2023, 15, 1204. [Google Scholar] [CrossRef] [PubMed]
- Chacin Ruiz, E.A.; Swindle-Reilly, K.E.; Ford Versypt, A.N. Experimental and mathematical approaches for drug delivery for the treatment of wet age-related macular degeneration. J. Control. Release 2023, 363, 464–483. [Google Scholar] [CrossRef] [PubMed]
- Gašparovič, M.; Jungová, P.; Tomášik, J.; Mriňáková, B.; Hirjak, D.; Timková, S.; Danišovič, Ľ.; Janek, M.; Bača, Ľ.; Peciar, P.; et al. Evolving Strategies and Materials for Scaffold Development in Regenerative Dentistry. Appl. Sci. 2024, 14, 2270. [Google Scholar] [CrossRef]
- Han, X.; Alu, A.; Liu, H.; Shi, Y.; Wei, X.; Cai, L.; Wei, Y. Biomaterial-assisted biotherapy: A brief review of biomaterials used in drug delivery, vaccine development, gene therapy, and stem cell therapy. Bioact. Mater. 2022, 17, 29–48. [Google Scholar] [CrossRef]
- Huang, Y.; Guo, X.; Wu, Y.; Chen, X.; Feng, L.; Xie, N.; Shen, G. Nanotechnology’s frontier in combatting infectious and inflammatory diseases: Prevention and treatment. Signal Transduct. Target. Ther. 2024, 9, 34. [Google Scholar] [CrossRef]
- Soprano, E.; Polo, E.; Pelaz, B.; Del Pino, P. Biomimetic cell-derived nanocarriers in cancer research. J. Nanobiotechnol. 2022, 20, 538. [Google Scholar] [CrossRef]
- Rocha, C.V.; Gonçalves, V.; da Silva, M.C.; Bañobre-López, M.; Gallo, J. PLGA-Based Composites for Various Biomedical Applications. Int. J. Mol. Sci. 2022, 23, 2034. [Google Scholar] [CrossRef] [PubMed]
- Geevarghese, R.; Sajjadi, S.S.; Hudecki, A.; Sajjadi, S.; Jalal, N.R.; Madrakian, T.; Ahmadi, M.; Włodarczyk-Biegun, M.K.; Ghavami, S.; Likus, W.; et al. Biodegradable and Non-Biodegradable Biomaterials and Their Effect on Cell Differentiation. Int. J. Mol. Sci. 2022, 23, 16185. [Google Scholar] [CrossRef]
- Shiroud Heidari, B.; Ruan, R.; Vahabli, E.; Chen, P.; De-Juan-Pardo, E.M.; Zheng, M.; Doyle, B. Natural, synthetic and commercially-available biopolymers used to regenerate tendons and ligaments. Bioact. Mater. 2023, 19, 179–197. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.-H.; He, C.; Riviere, J.E.; Monteiro-Riviere, N.A.; Lin, Z. Meta-analysis of nanoparticle delivery to tumors using a physiologically based pharmacokinetic modeling and simulation approach. ACS Nano 2020, 14, 3075–3095. [Google Scholar] [CrossRef] [PubMed]
- Nag, O.K.; Delehanty, J.B. Active cellular and subcellular targeting of nano- particles for drug delivery. Pharmaceutics 2019, 11, 543. [Google Scholar] [CrossRef]
- Oliva, N.; Carcole, M.; Beckerman, M.; Seliktar, S.; Hayward, A.; Stanley, J.; Anne Parry, N.M.; Edelman, E.R.; Artzi, N. Regulation of dendrimer/dextran material performance by altered tissue microenvironment in inflammation and neoplasia. Sci. Transl. Med. 2015, 7, 272ra11. [Google Scholar] [CrossRef] [PubMed]
- Ensign, L.M.; Cone, R.; Hanes, J. Oral drug delivery with polymeric nanoparticles: The gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012, 64, 557–570. [Google Scholar] [CrossRef]
- Fernandes, T.; Daniel-da-Silva, A.L.; Trindade, T. Metal-dendrimer hybrid nanomaterials for sensing applications. Coord. Chem. Rev. 2022, 460, 214483. [Google Scholar] [CrossRef]
- Cooley, M.; Sarode, A.; Hoore, M.; Fedosov, D.A.; Mitragotri, S.; Sen Gupta, A. Influence of particle size and shape on their margination and wall-adhesion: Implications in drug delivery vehicle design across nano-to-micro scale. Nanoscale 2018, 10, 15350–15364. [Google Scholar] [CrossRef] [PubMed]
- Pieter, S.; Mahmoudi, M. Drug Delivery Systems; World Scientific: Singapore, 2017. [Google Scholar]
- Blanco, E.; Shen, H.; Ferrari, M. Principles of nanoparticle design for overcoming biological barriers to drug delivery. Nat. Biotechnol. 2015, 33, 941–951. [Google Scholar] [CrossRef]
- Wilhelm, S.; Tavares, A.J.; Dai, Q.; Ohta, S.; Audet, J.; Dvorak, H.F.; Chan, W. Analysis of nanoparticle delivery to tumours. Nat. Rev. Mater. 2016, 1, 16014. [Google Scholar] [CrossRef]
- von Roemeling, C.; Jiang, W.; Chan, C.K.; Weissman, I.L.; Kim, B.Y.S. Breaking down the barriers to precision cancer nanomedicine. Trends Biotechnol. 2017, 35, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Hoshyar, N.; Gray, S.; Han, H.; Bao, G. The effect of nanoparticle size on in vivo pharmacokinetics and cellular interaction. Nanomedicine 2016, 11, 673–692. [Google Scholar] [CrossRef] [PubMed]
- Zhong, Q.; Merkel, O.M.; Reineke, J.J.; da Rocha, S.R.P. Effect of the route of administration and PEGylation of poly(amidoamine) dendrimers on their systemic and lung cellular biodistribution. Mol. Pharm. 2016, 13, 1866–1878. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, L.; Panciani, P.P.; Muntoni, E.; Capucchio, M.T.; Biasibetti, E.; De Bonis, P.; Mioletti, S.; Fontanella, M.; Swaminathan, S. Lipid nanoparticles for intranasal administration: Application to nose-to-brain delivery. Expert Opin. Drug Deliv. 2018, 15, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Dölen, Y.; Valente, M.; Tagit, O.; Jäger, E.; Van Dinther, E.A.W.; van Riessen, N.K.; Hruby, M.; Gileadi, U.; Cerundolo, V.; Figdor, C.G. Nanovaccine administration route is critical to obtain pertinent iNKt cell help for robust anti-tumor T and B cell responses. Oncoimmunology 2020, 9, 1738813. [Google Scholar] [CrossRef] [PubMed]
- McLennan, D.N.; Porter CJ, H.; Charman, S.A. Subcutaneous drug delivery and the role of the lymphatics. Drug Discov. Today Technol. 2005, 2, 89–96. [Google Scholar] [CrossRef]
- Dong, W.; Ye, J.; Zhou, J.; Wang, W.; Wang, H.; Zheng, X.; Yang, Y.; Xia, X.; Liu, Y. Comparative study of mucoadhesive and mucus-penetrative nanoparticles based on phospholipid complex to overcome the mucus barrier for inhaled delivery of baicalein. Acta Pharm. Sin. B 2019, 10, 1576–1585. [Google Scholar] [CrossRef]
- Cone, R.A. Barrier properties of mucus. Adv. Drug Deliv. Rev. 2009, 61, 75–85. [Google Scholar] [CrossRef]
- Saraiva, C.; Praça, C.; Ferreira, R.; Santos, T.; Ferreira, L.; Bernardino, L. Nanoparticle-mediated brain drug delivery: Overcoming blood–brain barrier to treat neurodegenerative diseases. J. Control. Release 2016, 235, 34–47. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Shao, S.; Wang, J.; Xu, C.; Xiang, J.; Piao, Y.; Zhou, Z.; Yu, Q.; Tang, J.; Liu, X.; et al. Enzyme-activatable polymer–drug conjugate augments tumour penetration and treatment efficacy. Nat. Nanotechnol. 2019, 14, 799–809. [Google Scholar] [CrossRef]
- Sindhwani, S.; Syed, A.M.; Ngai, J.; Kingston, B.R.; Maiorino, L.; Rothschild, J.; MacMillan, P.; Zhang, Y.; Rajesh, N.U.; Hoang, T.; et al. The entry of nanoparticles into solid tumours. Nat. Mater. 2020, 19, 566–575. [Google Scholar] [CrossRef]
- Da Silva-Candal, A.; Brown, T.; Krishnan, V.; Lopez-Loureiro, I.; Ávila-Gómez, P.; Pusuluri, A.; Pérez-Díaz, A.; Correa-Paz, C.; Hervella, P.; Castillo, J.; et al. Shape effect in active targeting of nanoparticles to inflamed cerebral endothelium under static and flow conditions. J. Control. Release 2019, 309, 94–105. [Google Scholar] [CrossRef]
- Johnsen, K.B.; Bak, M.; Melander, F.; Thomsen, M.S.; Burkhart, A.; Kempen, P.J.; Andresen, T.L.; Moos, T. Modulating the antibody density changes the uptake and transport at the blood–brain barrier of both transferrin receptor-targeted gold nanoparticles and liposomal cargo. J. Control. Release 2019, 295, 237–249. [Google Scholar] [CrossRef]
- Sousa, F.; Dhaliwal, H.K.; Gattacceca, F.; Sarmento, B.; Amiji, M.M. Enhanced anti-angiogenic effects of bevacizumab in glioblastoma treatment upon intranasal administration in polymeric nanoparticles. J. Control. Release 2019, 309, 37–47. [Google Scholar] [CrossRef]
- Musumeci, T.; Bonaccorso, A.; Puglisi, G. Epilepsy disease and nose-to-brain delivery of polymeric nanoparticles: An overview. Pharmaceutics 2019, 11, 118. [Google Scholar] [CrossRef]
- Bruinsmann, F.A.; Richter Vaz, G.; de Cristo Soares Alves, A.; Aguirre, T.; Raffin Pohlmann, A.; Stanisçuaski Guterres, S.; Sonvico, F. Nasal drug delivery of anticancer drugs for the treatment of glioblastoma: Preclinical and clinical trials. Molecules 2019, 24, 4312. [Google Scholar] [CrossRef] [PubMed]
- Sykes, E.A.; Dai, Q.; Sarsons, C.D.; Chen, J.; Rocheleau, J.V.; Hwang, D.M.; Zheng, G.; Cramb, D.T.; Rinker, K.D.; Chan, W.C. Tailoring nanoparticle designs to target cancer based on tumor pathophysiology. Proc. Natl. Acad. Sci. USA 2016, 113, E1142–E1151. [Google Scholar] [CrossRef]
- Zan, Y.; Dai, Z.; Liang, L.; Deng, Y.; Dong, L. Co-delivery of plantamajoside and sorafenib by a multi-functional nanoparticle to combat the drug resistance of hepatocellular carcinoma through reprograming the tumor hypoxic microenvironment. Drug Deliv. 2019, 26, 1080–1091. [Google Scholar] [CrossRef]
- Dai, Q.; Wilhelm, S.; Ding, D.; Syed, A.M.; Sindhwani, S.; Zhang, Y.; Chen, Y.Y.; MacMillan, P.; Chan, W.C.W. Quantifying the ligand-coated nanoparticle delivery to cancer cells in solid tumors. ACS Nano 2018, 12, 8423–8435. [Google Scholar] [CrossRef]
- Witten, J.; Ribbeck, K. The particle in the spider’s web: Transport through biological hydrogels. Nanoscale 2017, 9, 8080–8095. [Google Scholar] [CrossRef]
- Nagel, G.; Sousa-Herves, A.; Wedepohl, S.; Calderón, M. Matrix metalloproteinase-sensitive multistage nanogels promote drug transport in 3D tumor model. Theranostics 2020, 10, 91–108. [Google Scholar] [CrossRef]
- Price LS, L.; Stern, S.T.; Deal, A.M.; Kabanov, A.V.; Zamboni, W.C. A reanalysis of nanoparticle tumor delivery using classical pharmacokinetic metrics. Sci. Adv. 2020, 6, eaay9249. [Google Scholar] [CrossRef] [PubMed]
- Fahy, J.V.; Dickey, B.F. Airway mucus function and dysfunction. N. Engl. J. Med. 2010, 363, 2233–2247. [Google Scholar] [CrossRef] [PubMed]
- Durán-Lobato, M.; Niu, Z.; Alonso, M.J. Oral delivery of biologics for precision medicine. Adv. Mater. 2019, 32, e1901935. [Google Scholar] [CrossRef]
- Firdessa-Fite, R.; Creusot, R.J. Nanoparticles versus dendritic cells as vehicles to deliver mRNA encoding multiple epitopes for immunotherapy. Mol. Ther. Methods Clin. Dev. 2019, 16, 50–62. [Google Scholar] [CrossRef]
- Tan, T.; Hu, H.; Wang, H.; Li, J.; Wang, Z.; Wang, J.; Wang, S.; Zhang, Z.; Li, Y. Bioinspired lipoproteins-mediated photothermia remodels tumor stroma to improve cancer cell accessibility of second nanoparticles. Nat. Commun. 2019, 10, 3322. [Google Scholar] [CrossRef]
- Miao, L.; Li, L.; Huang, Y.; Delcassian, D.; Chahal, J.; Han, J.; Shi, Y.; Sadtler, K.; Gao, W.; Anderson, D.G. Delivery of mRNA vaccines with heterocyclic lipids increases anti-tumor efficacy by STING-mediated immune cell activation. Nat. Biotechnol. 2019, 37, 1174–1185. [Google Scholar] [CrossRef]
- Maugeri, M.; Nawaz, M.; Papadimitriou, A.; Angerfors, A.; Camponeschi, A.; Na, M.; Hölttä, M.; Skantze, P.; Johansson, S.; Sundqvist, M.; et al. Linkage between endosomal escape of LNP-mRNA and loading into EVs for transport to other cells. Nat. Commun. 2019, 10, 4333. [Google Scholar] [CrossRef]
- Liu, Y.; Crowe, W.N.; Wang, L.; Lu, Y.; Petty, W.J.; Habib, A.A.; Zhao, D. An inhalable nanoparticulate STING agonist synergizes with radiotherapy to confer long- term control of lung metastases. Nat. Commun. 2019, 10, 5108. [Google Scholar] [CrossRef]
- Cheng, Q.; Wei, T.; Farbiak, L.; Johnson, L.T.; Dilliard, S.A.; Siegwart, D.J. Selective organ targeting (SORT) nanoparticles for tissue-specific mRNA delivery and CRISPR–Cas gene editing. Nat. Nanotechnol. 2020, 15, 313–320. [Google Scholar] [CrossRef]
- Almeida, A.P.B.; Damaceno, G.B.R.; Carneiro, A.F.; Bohr, A.; Gonçalves, H.R.; Valadares, M.C.; Nascimento, T.L.; Lima, E.M. Mucopenetrating lipoplexes modified with PEG and hyaluronic acid for CD44- targeted local siRNA delivery to the lungs. J. Biomater. Appl. 2019, 34, 617–630. [Google Scholar] [CrossRef]
- Vhora, I.; Lalani, R.; Bhatt, P.; Patil, S.; Misra, A. Lipid-nucleic acid nanoparticles of novel ionizable lipids for systemic BMP-9 gene delivery to bone- marrow mesenchymal stem cells for osteoinduction. Int. J. Pharm. 2019, 563, 324–336. [Google Scholar] [CrossRef]
- Chen, D.; Parayath, N.; Ganesh, S.; Wang, W.; Amiji, M. The role of apolipoprotein- and vitronectin-enriched protein corona on lipid nanoparticles for vivo targeted delivery and transfection of oligonucleotides in murine tumor models. Nanoscale 2019, 11, 18806–18824. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, Y.; Yang, D.; Huang, W.; Hao, P.; Feng, S.; Appelhans, D.; Zhang, T.; Zan, X. Shape effect of nanoparticles on tumor penetration in monolayers versus spheroids. Mol. Pharm. 2019, 16, 2902–2911. [Google Scholar] [CrossRef]
- Shi, H.; Xu, M.; Zhu, J.; Li, Y.; He, Z.; Zhang, Y.; Liu, Y. Programmed co-delivery of platinum nanodrugs and gemcitabine by a clustered nanocarrier for precision chemotherapy for NSCLC tumors. J. Mater. Chem. B 2020, 8, 332–342. [Google Scholar] [CrossRef]
- Guo, F.; Fu, Q.; Zhou, K.; Jin, C.; Wu, W.; Ji, X.; Yang, G. Matrix metalloprotein-triggered cell penetrating peptide-modified star-shaped nanoparticles for tumor targeting and cancer therapy. J. Nanobiotechnol. 2020, 18, 48. [Google Scholar] [CrossRef]
- Liu, X.; Li, C.; Lv, J.; Huang, F.; An, Y.; Shi, L.; Ma, R. Glucose and H2O2 dual-responsive polymeric micelles for the self-regulated release of insulin. ACS Appl. Bio Mater. 2020, 3, 1598–1606. [Google Scholar] [CrossRef]
- Volpatti, L.R.; Daniel, K.B.; Langer, R.; Anderson, D.G. Glucose-responsive nanoparticles for rapid and extended self-regulated insulin delivery. ACS Nano 2020, 14, 488–497. [Google Scholar] [CrossRef]
- Lim, W.Q.; Phua, S.Z.F.; Zhao, Y. Redox-responsive polymeric nanocomplex for delivery of cytotoxic protein and chemotherapeutics. ACS Appl. Mater. Interfaces 2019, 11, 31638–31648. [Google Scholar] [CrossRef]
- Wei, L.; Zhao, Y.; Hu, X.; Tang, L. Redox-responsive polycondensate neoepitope for enhanced personalized cancer vaccine. ACS Cent. Sci. 2020, 6, 404–412. [Google Scholar] [CrossRef]
- Chen, G.; Abdeen, A.A.; Wang, Y.; Shahi, P.K.; Robertson, S.; Xie, R.; Suzuki, M.; Pattnaik, B.R.; Saha, K.; Gong, S. A biodegradable nanocapsule delivers a Cas9 ribonucleoprotein complex for in vivo genome editing. Nat. Nanotechnol. 2019, 14, 974–980. [Google Scholar] [CrossRef]
- Knight, F.C.; Gilchuk, P.; Kumar, A.; Becker, K.W.; Sevimli, S.; Jacobson, M.E.; Suryadevara, N.; Wang-Bishop, L.; Boyd, K.L.; Crowe, J.E., Jr.; et al. Mucosal immunization with a pH-responsive nanoparticle vaccine induces protective CD8+ lung-resident memory T cells. ACS Nano 2019, 13, 10939–10960. [Google Scholar] [CrossRef]
- Yamada, Y.; Fukuda, Y.; Sasaki, D.; Maruyama, M.; Harashima, H. Development of a nanoparticle that releases nucleic acids in response to a mitochondrial environment. Mitochondrion 2020, 52, 67–74. [Google Scholar] [CrossRef]
- Strand, M.S.; Krasnick, B.A.; Pan, H.; Zhang, X.; Bi, Y.; Brooks, C.; Wetzel, C.; Sankpal, N.; Fleming, T.; Goedegebuure, S.P.; et al. Precision delivery of RAS-inhibiting siRNA to KRAS driven cancer via peptide-based nanoparticles. Oncotarget 2019, 10, 4761–4775. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Jia, L.; Wang, Q.; Hu, H.; Zhao, X.; Chen, D.; Qiao, M. pH/redox dual-responsive polyplex with effective endosomal escape for codelivery of siRNA and doxorubicin against drug-resistant cancer cells. ACS Appl. Mater. Interfaces 2019, 11, 16296–16310. [Google Scholar] [CrossRef] [PubMed]
- Shae, D.; Becker, K.W.; Christov, P.; Yun, D.S.; Lytton-Jean, A.K.R.; Sevimli, S.; Ascano, M.; Kelley, M.; Johnson, D.B.; Balko, J.M.; et al. Endosomolytic polymersomes increase the activity of cyclic dinucleotide STING agonists to enhance cancer immunotherapy. Nat. Nanotechnol. 2019, 14, 269–278. [Google Scholar] [CrossRef]
- Rao, L.; Yu, G.-T.; Meng, Q.-F.; Bu, L.-L.; Tian, R.; Lin, L.-S.; Deng, H.; Yang, W.; Zan, M.; Ding, J.; et al. Cancer cell membrane-coated nanoparticles for personalized therapy in patient- derived xenograft models. Adv. Funct. Mater. 2019, 29, 1905671. [Google Scholar] [CrossRef]
- Zhang, F.; Parayath, N.N.; Ene, C.I.; Stephan, S.B.; Koehne, A.L.; Coon, M.E.; Stephan, M.T. Genetic programming of macrophages to perform anti-tumor functions using targeted mRNA nanocarriers. Nat. Commun. 2019, 10, 3974. [Google Scholar] [CrossRef]
- Fornaguera, C.; Guerra-Rebollo, M.; Lázaro, M.Á.; Cascante, A.; Rubio, N.; Blanco, J.; Borrós, S. In vivo retargeting of poly(β- aminoester) (OM-PBAE) nanoparticles is influenced by protein corona. Adv. Healthc. Mater. 2019, 8, 1–11. [Google Scholar] [CrossRef]
- Guan, S.; Munder, A.; Hedtfeld, S.; Braubach, P.; Glage, S.; Zhang, L.; Lienenklaus, S.; Schultze, A.; Hasenpusch, G.; Garrels, W.; et al. Self-assembled peptide–poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat. Nanotechnol. 2019, 14, 287–297. [Google Scholar] [CrossRef]
- Wadajkar, A.S.; Dancy, J.G.; Roberts, N.B.; Connolly, N.P.; Strickland, D.K.; Winkles, J.A.; Woodworth, G.F.; Kim, A.J. Decreased non-specific adhesivity receptor targeted (DART) nanoparticles exhibit improved dispersion cellular uptake tumor retention in invasive gliomas. J. Control. Release 2017, 267, 144–153. [Google Scholar] [CrossRef]
- Brown, S.B.; Wang, L.; Jungels, R.R.; Sharma, B. Effects of cartilage-targeting moieties on nanoparticle biodistribution in healthy and osteoarthritic joints. Acta Biomater. 2019, 101, 469–483. [Google Scholar] [CrossRef]
- Zelmer, C.; Zweifel, L.P.; Kapinos, L.E.; Craciun, I.; Güven, Z.P.; Palivan, C.G.; Lim, R.Y.H. Organelle-specific targeting of polymersomes into the cell nucleus. Proc. Natl. Acad. Sci. USA 2020, 117, 2770–2778. [Google Scholar] [CrossRef]
- Clegg, J.R.; Irani, A.S.; Ander, E.W.; Ludolph, C.M.; Venkataraman, A.K.; Zhong, J.X.; Peppas, N.A. Synthetic networks with tunable responsiveness, biodegradation, and molecular recognition for precision medicine applications. Sci. Adv. 2019, 5, eaax7946. [Google Scholar] [CrossRef]
- Huang, K.-W.; Hsu, F.F.; Qiu, J.T.; Chern, G.J.; Lee, Y.A.; Chang, C.C.; Huang, Y.T.; Sung, Y.C.; Chiang, C.C.; Huang, R.L.; et al. Highly efficient and tumor-selective nanoparticles for dual-targeted immunogene therapy against cancer. Sci. Adv. 2020, 6, eaax5032. [Google Scholar] [CrossRef]
- Hong, S.T.; Lin, H.; Wang, C.S.; Chang, C.H.; Lin, A.M.; Yang, J.C.; Lo, Y.L. Improving the anticancer effect of afatinib and microRNA by using lipid polymeric nanoparticles conjugated with dual pH-responsive and targeting peptides. J. Nanobiotechnol. 2019, 17, 89. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Wang, T.; Yu, H.; Feng, B.; Zhou, L.; Zhou, F.; Li, Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 2019, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Nam, J.; Hong, H.; Xu, Y.; Moon, J.J. Positron emission tomography-guided photodynamic therapy with biodegradable mesoporous silica nanoparticles for personalized cancer immunotherapy. ACS Nano 2019, 13, 12148–12161. [Google Scholar] [CrossRef]
- Yeo, E.L.L.; Cheah, J.U.J.; Thong, P.S.P.; Soo, K.C.; Kah, J.C.Y. Gold nanorods coated with apolipoprotein E protein corona for drug delivery. ACS Appl. Nano Mater. 2019, 2, 6220–6229. [Google Scholar]
- Yi, Y.; Kim, H.J.; Zheng, M.; Mi, P.; Naito, M.; Kim, B.S.; Min, H.S.; Hayashi, K.; Perche, F.; Toh, K.; et al. Glucose-linked sub-50-nm unimer polyion complex-assembled gold nanoparticles for targeted siRNA delivery to glucose transporter 1-overexpressing breast cancer stem-like cells. J. Control. Release 2019, 295, 268–277. [Google Scholar] [CrossRef]
- Ramchandani, D.; Lee, S.K.; Yomtoubian, S.; Han, M.S.; Tung, C.H.; Mittal, V. Nanoparticle delivery of miR- 708 mimetic impairs breast cancer metastasis. Mol. Cancer Ther. 2019, 18, 579–591. [Google Scholar] [CrossRef]
- Stephen, Z.R.; Chiarelli, P.A.; Revia, R.A.; Wang, K.; Kievit, F.; Dayringer, C.; Jeon, M.; Ellenbogen, R.; Zhang, M. Time-resolved MRI assessment of convection-enhanced delivery by targeted and nontargeted nanoparticles in a human glioblastoma mouse model. Cancer Res. 2019, 79, 4776–4786. [Google Scholar] [CrossRef]
- Yuan, P.; Mao, X.; Wu, X.; Liew, S.S.; Li, L.; Yao, S.Q. Mitochondria-targeting, intracellular delivery of native proteins using biodegradable silica nanoparticles. Angew. Chem. 2019, 131, 7739–7743. [Google Scholar] [CrossRef]
- Li, K.; Lu, L.; Xue, C.; Liu, J.; He, Y.; Zhou, J.; Cai, K. Polarization of tumor-associated macrophage phenotype via porous hollow iron nanoparticles for tumor immunotherapy in vivo. Nanoscale 2020, 12, 130–144. [Google Scholar] [CrossRef]
- Du, Y.; Liu, X.; Liang, Q.; Liang, X.J.; Tian, J. Optimization and design of magnetic ferrite nanoparticles with uniform tumor distribution for highly sensitive MRI/MPI performance and improved magnetic hyperthermia therapy. Nano Lett. 2019, 19, 3618–3626. [Google Scholar] [CrossRef]
- Zhang, L.; Su, H.; Wang, H.; Li, Q.; Li, X.; Zhou, C.; Xu, J.; Chai, Y.; Liang, X.; Xiong, L.; et al. Tumor chemo-radiotherapy with rod-shaped and spherical gold nano probes: Shape and active targeting both matter. Theranostics 2019, 9, 1893–1908. [Google Scholar] [CrossRef]
- Yun, Y.H.; Lee, B.K.; Park, K. Controlled Drug Delivery: Historical perspective for the next generation. J. Control. Release 2015, 219, 2–7. [Google Scholar] [CrossRef]
- Allen, T.M.; Cullis, P.R. Drug delivery systems: Entering the mainstream. Science 2004, 303, 1818–1822. [Google Scholar] [CrossRef]
- Langer, R. Drug delivery and targeting. Nature 1998, 392 (Suppl. S6697), 5–10. [Google Scholar]
- Langer, R. New methods of drug delivery. Science 1990, 249, 1527–1533. [Google Scholar] [CrossRef]
- Hoare, T.; Kohane, D.; Hoare, T.R.; Kohane, D.S. Hydrogels in drug delivery: Progress and challenges. Polymer 2008, 49, 1993–2007. [Google Scholar] [CrossRef]
- Anselmo, A.C.; Mitragotri, S. An overview of clinical and commercial impact of drug delivery systems. J. Control. Release 2014, 190, 15–28. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, A.; Chen, X.; Xiang, G.; Jiang, T.; Zhao, X. Barnacle inspired strategy combined with solvent exchange for enhancing wet adhesion of hydrogels to promote seawater-immersed wound healing. Bioact. Mater. 2024, 41, 46–60. [Google Scholar] [CrossRef]
- Riley, R.S.; June, C.H.; Langer, R.; Mitchell, M.J. Delivery technologies for cancer immunotherapy. Nat. Rev. Drug Discov. 2019, 18, 175–196. [Google Scholar] [CrossRef]
- Lin, Y.X.; Wang, Y.; An, H.W.; Qi, B.; Wang, J.; Wang, L.; Wang, H. Peptide-based autophagic gene and cisplatin Co-delivery systems enable improved chemotherapy resistance. Nano Lett. 2019, 19, 2968–2978. [Google Scholar] [CrossRef]
- Shamay, Y.; Elkabets, M.; Li, H.; Shah, J.; Brook, S.; Wang, F.; Adler, K.; Baut, E.; Scaltriti, M.; Jena, P.V.; et al. P-selectin is a nanotherapeutic delivery target in the tumor microenvironment. Sci. Transl. Med. 2016, 8, 345ra387. [Google Scholar] [CrossRef]
- Zhang, Y.; Hsu, B.Y.; Ren, C.; Li, X.; Wang, J. Silica-based nanocapsules: Synthesis, structure control and biomedical applications. Chem. Soc. Rev. 2015, 44, 315–335. [Google Scholar] [CrossRef]
- Jia, Q.; Li, Z.; Guo, C.; Huang, X.; Song, Y.; Zhou, N.; Du, M. A gamma-cyclodextrin- based metal-organic framework embedded with graphene quantum dots and modified with PEGMA via SI-ATRP for anticancer drug delivery and therapy. Nanoscale 2019, 11, 20956–20967. [Google Scholar] [CrossRef]
- Pitek, A.S.; Hu, H.; Shukla, S.; Steinmetz, N.F. Cancer theranostic applications of albumin-coated tobacco mosaic virus nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 39468–39477. [Google Scholar] [CrossRef]
- Liang, X.; Gao, J.; Jiang, L.; Luo, J.; Jing, L.; Li, X.; Jin, Y.; Dai, Z. Nanohybrid liposomal cerasomes with good physiological stability and rapid temperature responsiveness for high intensity focused ultrasound triggered local chemotherapy of cancer. ACS Nano 2015, 9, 1280–1293. [Google Scholar] [CrossRef]
- Ngambenjawong, C.; Gustafson, H.H.; Pun, S.H. Progress in tumor-associated macrophage (TAM)-targeted therapeutics. Adv. Drug Deliv. Rev. 2017, 114, 206–221. [Google Scholar] [CrossRef]
- Roy, A.; Ernsting, M.J.; Undzys, E.; Li, S.D. A highly tumor-targeted nanoparticle of podophyllotoxin penetrated tumor core and regressed multidrug resistant tumors. Biomaterials 2015, 52, 335–346. [Google Scholar] [CrossRef]
- Yu, S.S.; Lau, C.M.; Thomas, S.N.; Jerome, W.G.; Maron, D.J.; Dickerson, J.H.; Hubbell, J.A.; Giorgio, T.D. Size- and charge-dependent non-specific uptake of PEGylated nanoparticles by macrophages. Int. J. Nanomed. 2012, 7, 799–813. [Google Scholar] [CrossRef]
- Perni, S.; Prokopovich, P. Poly-beta-amino-esters nano-vehicles based drug delivery system for cartilage. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 539–548. [Google Scholar] [CrossRef]
- El-Sawy, H.S.; Al-Abd, A.M.; Ahmed, T.A.; El-Say, K.M.; Torchilin, V.P. Stimuli- responsive nano-architecture drug-delivery systems to solid tumor micromilieu: Past, present, and future perspectives. ACS Nano 2018, 12, 10636–10664. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Chia, S.L.; Goh, S.L.; Fang, W.; Neo, M.J.; Chong, H.C.; Tan, S.M.; Loo, S.C.; Ng, K.W.; et al. Titanium dioxide nanomaterials cause endothelial cell leakiness by disrupting the homophilic interaction of VE-cadherin. Nat. Commun. 2013, 4, 1673. [Google Scholar] [CrossRef]
- Tee, J.K.; Yip, L.X.; Tan, E.S.; Santitewagun, S.; Prasath, A.; Ke, P.C. Nanoparticles’ interactions with vasculature in diseases. Chem. Soc. Rev. 2019, 48, 5381–5407. [Google Scholar] [CrossRef]
- Setyawati, M.I.; Tay, C.Y.; Bay, B.H.; Leong, D.T. Gold nanoparticles induced endothelial leakiness depends on particle size and endothelial cell origin. ACS Nano 2017, 11, 5020–5030. [Google Scholar] [CrossRef]
- Peng, F.; Setyawati, M.I.; Tee, J.K.; Ding, X.; Wang, J.; Nga, M.E.; Ho, H.K.; Leong, D.T. Nanoparticles promote in vivo breast cancer cell intravasation and extravasation by inducing endothelial leakiness. Nat. Nanotechnol. 2019, 14, 279–286. [Google Scholar] [CrossRef]
- Wang, X.; Li, X.; Mao, Y.; Wang, D.; Zhao, Q.; Wang, S. Multi-stimuli responsive nanosystem modified by tumor-targeted carbon dots for chemophototherapy synergistic therapy. J. Colloid Interface Sci. 2019, 552, 639–650. [Google Scholar] [CrossRef]
- Kanamala, M.; Wilson, W.R.; Yang, M.; Palmer, B.D.; Wu, Z. Mechanisms and biomaterials in pH-responsive tumour targeted drug delivery: A review. Biomaterials 2016, 85, 152–167. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, Y.; Wang, Q.; Liu, T.; Sun, J.; Zhang, R. Remote light-responsive nanocarriers for controlled drug delivery: Advances and perspectives. Small 2019, 15, e1903060. [Google Scholar] [CrossRef]
- Chen, J.; Dai, S.; Liu, L.; Maitz, M.F.; Liao, Y.; Cui, J.; Wang, Y. Photo-functionalized TiO2 nanotubes decorated with multifunctional Ag nanoparticles for enhanced vascular biocompatibility. Bioact. Mater. 2021, 6, 45–54. [Google Scholar] [CrossRef]
- Bazak, R.; Houri, M.; El Achy, S.; Kamel, S.; Refaat, T. Cancer active targeting by nanoparticles: A comprehensive review of literature. J. Cancer Res. Clin. Oncol. 2015, 141, 769–784. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, X.; Duan, C.; Li, J.; Tong, R.; Fan, Y.; Feng, J.; Cao, R.; Zhong, W.; Feng, X.; et al. Identification and characterization of mammaglobin-A epitope in heterogenous breast cancers for enhancing tumor-targeting therapy. Signal Transduct. Target. Ther. 2020, 5, 82. [Google Scholar] [CrossRef]
- Alshaer, W.; Hillaireau, H.; Fattal, E. Aptamer-guided nanomedicines for anticancer drug delivery. Adv. Drug Deliv. Rev. 2018, 134, 122–137. [Google Scholar] [CrossRef]
- Wang, S.H.; Yu, J. Structure-based design for binding peptides in anti-cancer therapy. Biomaterials 2018, 156, 1–15. [Google Scholar] [CrossRef]
- Cai, L.L.; Yang, C.Y.; Jia, W.F.; Liu, Y.W.; Xie, R.; Lei, T. Endo/lysosome- escapable delivery Depot for improving BBB transcytosis and neuron targeted therapy of alzheimer’s disease. Adv. Funct. Mater. 2020, 30, 1909999. [Google Scholar] [CrossRef]
- Du, X.; Yin, S.; Wang, Y.; Gu, X.; Wang, G.; Li, J. Hyaluronic acid-functionalized half-generation of sectorial dendrimers for anticancer drug delivery and enhanced biocompatibility. Carbohydr. Polym. 2018, 202, 513–522. [Google Scholar] [CrossRef]
- Zhang, Y.; Deng, C.; Liu, S.; Wu, J.; Chen, Z.; Li, C.; Lu, W. Active targeting of tumors through conformational epitope imprinting. Angew. Chem. 2015, 54, 5157–5160. [Google Scholar] [CrossRef]
- Hrkach, J.; Von Hoff, D.; Ali, M.M.; Andrianova, E.; Auer, J.; Campbell, T. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 2012, 4, 128ra139. [Google Scholar] [CrossRef]
- Schmid, D.; Park, C.G.; Hartl, C.A.; Subedi, N.; Cartwright, A.N.; Puerto, R.B.; Goldberg, M.S. T cell-targeting nanoparticles focus delivery of immunotherapy to improve antitumor immunity. Nat. Commun. 2017, 8, 1747. [Google Scholar] [CrossRef]
- Trombetta, E.S.; Mellman, I. Cell biology of antigen processing in vitro and in vivo. Annu. Rev. Immunol. 2005, 23, 975–1028. [Google Scholar] [CrossRef]
- Irvine, D.J.; Aung, A.; Silva, M. Controlling timing and location in vaccines. Adv. Drug Deliv. Rev. 2020, 158, 91–115. [Google Scholar] [CrossRef]
- Bonifaz, L.; Bonnyay, D.; Mahnke, K.; Rivera, M.; Nussenzweig, M.C.; Steinman, R. M Efficient targeting of protein antigen to the dendritic cell receptor DEC-205 in the steady state leads to antigen presentation on major histocompatibility complex class I products and peripheral CD8+ T cell tolerance. J. Exp. Med. 2002, 196, 1627–1638. [Google Scholar] [CrossRef]
- Reddy, S.T.; van der Vlies, A.J.; Simeoni, E.; Angeli, V.; Randolph, G.J.; O’Neil, C.P. Exploiting lymphatic transport and complement activation in nanoparticle vaccines. Nat. Biotechnol. 2007, 25, 1159–1164. [Google Scholar] [CrossRef]
- Rodell, C.B.; Arlauckas, S.P.; Cuccarese, M.F.; Garris, C.S.; Li, R.; Ahmed, M.S. TLR7/8-agonist-loaded nanoparticles promote the polarization of tumour- associated macrophages to enhance cancer immunotherapy. Nat. Biomed. Eng. 2018, 2, 578–588. [Google Scholar] [CrossRef]
- Kew, O.M.; Sutter, R.W.; de Gourville, E.M.; Dowdle, W.R.; Pallansch, M.A. Vaccine- derived polioviruses and the endgame strategy for global polio eradication. Annu. Rev. Microbiol. 2005, 59, 587–635. [Google Scholar] [CrossRef]
- Fenner, F. Risks and benefits of vaccinia vaccine use in the worldwide smallpox eradication campaign. Res. Virol. 1989, 140, 465–466, discussion 487–491. [Google Scholar] [CrossRef]
- Kennedy, R.B.; Ovsyannikova, I.G.; Palese, P.; Poland, G.A. Current challenges in vaccinology. Front. Immunol. 2020, 11, 1181. [Google Scholar] [CrossRef] [PubMed]
- Bookstaver, M.L.; Tsai, S.J.; Bromberg, J.S.; Jewell, C.M. Improving vaccine and immunotherapy design using biomaterials. Trends Immunol. 2018, 39, 135–150. [Google Scholar] [CrossRef]
- Chaudhary, N.; Weissman, D.; Whitehead, K.A. mRNA vaccines for infectious diseases: Principles, delivery and clinical translation. Nat. Rev. Drug Discov. 2021, 20, 817–838. [Google Scholar] [CrossRef] [PubMed]
- Papahadjopoulos, D.; Nir, S.; Oki, S. Permeability properties of phospholipid membranes: Effect of cholesterol and temperature. Biochim. Biophys. Acta 1972, 266, 561–583. [Google Scholar] [CrossRef]
- Papahadjopoulos, D.; Cowden, M.; Kimelberg, H. Role of cholesterol in membranes. Effects on phospholipid-protein interactions, membrane permeability and enzymatic activity. Biochim. Biophys. Acta 1973, 330, 8–26. [Google Scholar] [CrossRef]
- Li, M.; Du, C.; Guo, N.; Teng, Y.; Meng, X.; Sun, H. Composition design and medical application of liposomes. Eur. J. Med. Chem. 2019, 164, 640–653. [Google Scholar] [CrossRef]
- Simo, S.; Filipe, A.; Faneca, H.; Mano, M.; Penacho, N.; Düzgünes, N. Cationic liposomes for gene delivery. Expet Opin. Drug Deliv. 2005, 2, 237–254. [Google Scholar]
- Butts, C.; Socinski, M.A.; Mitchell, P.L.; Thatcher, N.; Havel, L.; Krzakowski, M. Tecemotide (L-BLP25) versus placebo after chemoradiotherapy for stage III non- small-cell lung cancer (START): A randomised, double-blind, phase 3 trial. Lancet Oncol. 2014, 15, 59–68. [Google Scholar] [CrossRef]
- Kranz, L.M.; Diken, M.; Haas, H.; Kreiter, S.; Loquai, C.; Reuter, K.C. Systemic RNA delivery to dendritic cells exploits antiviral defence for cancer immunotherapy. Nature 2016, 534, 396–401. [Google Scholar] [CrossRef]
- Qiao, C.; Liu, J.; Yang, J.; Li, Y.; Weng, J.; Shao, Y. Enhanced non- inflammasome mediated immune responses by mannosylated zwitterionic-based cationic liposomes for HIV DNA vaccines. Biomaterials 2016, 85, 1–17. [Google Scholar] [CrossRef]
- Morrison, C. Landmark green light for Mosquirix malaria vaccine. Nat. Biotechnol. 2015, 33, 1015–1016. [Google Scholar] [CrossRef]
- RTS,S Clinical Trials Partnership. Efficacy and safety of RTS,S/AS01 malaria vaccine with or without a booster dose in infants and children in Africa: Final results of a phase 3, individually randomised, controlled trial. Lancet 2015, 386, 31–45. [Google Scholar] [CrossRef] [PubMed]
- Ward, B.J.; Gobeil, P.; Séguin, A.; Atkins, J.; Boulay, I.; Charbonneau, P.Y. Phase 1 randomized trial of a plant-derived virus-like particle vaccine for COVID-19. Nat. Med. 2021, 27, 1071–1078. [Google Scholar] [CrossRef]
- Mekaru, H.; Lu, J.; Tamanoi, F. Development of mesoporous silica-based nanoparticles with controlled release capability for cancer therapy. Adv. Drug Deliv. Rev. 2015, 95, 40–49. [Google Scholar] [CrossRef]
- An, M.; Li, M.; Xi, J.; Liu, H. Silica nanoparticle as a lymph node targeting platform for vaccine delivery. ACS Appl. Mater. Interfaces 2017, 9, 23466–23475. [Google Scholar] [CrossRef]
- Wang, T.; Jiang, H.; Zhao, Q.; Wang, S.; Zou, M.; Cheng, G. Enhanced mucosal and systemic immune responses obtained by porous silica nanoparticles used as an oral vaccine adjuvant: Effect of silica architecture on immunological properties. Int. J. Pharm. 2012, 436, 351–358. [Google Scholar] [CrossRef]
- Super, M.; Doherty, E.J.; Cartwright, M.J.; Seiler, B.T.; Langellotto, F.; Dimitrakakis, N.; Mooney, D.J. Biomaterial vaccines capturing pathogen-associated molecular patterns protect against bacterial infections and septic shock. Nat. Biomed. Eng. 2021, 6, 8–18. [Google Scholar] [CrossRef]
- Wang, T.; Zou, M.; Jiang, H.; Ji, Z.; Gao, P.; Cheng, G. Synthesis of a novel kind of carbon nanoparticle with large mesopores and macropores and its application as an oral vaccine adjuvant. Eur. J. Pharmaceut. Sci. 2011, 44, 653–659. [Google Scholar] [CrossRef]
- Villa, C.H.; Dao, T.; Ahearn, I.; Fehrenbacher, N.; Casey, E.; Rey, D.A.; Scheinberg, D.A. Single- walled carbon nanotubes deliver peptide antigen into dendritic cells and enhance IgG responses to tumor-associated antigens. ACS Nano 2011, 5, 5300–5311. [Google Scholar] [CrossRef]
- Niikura, K.; Matsunaga, T.; Suzuki, T.; Kobayashi, S.; Yamaguchi, H.; Orba, Y.; Sawa, H. Gold nanoparticles as a vaccine platform: Influence of size and shape on immunological responses in vitro and in vivo. ACS Nano 2013, 7, 3926–3938. [Google Scholar] [CrossRef]
- Tao, W.; Ziemer, K.S.; Gill, H.S. Gold nanoparticle-M2e conjugate coformulated with CpG induces protective immunity against influenza A virus. Nanomedicine 2014, 9, 237–251. [Google Scholar] [CrossRef]
- Xu, L.; Liu, Y.; Chen, Z.; Li, W.; Liu, Y.; Wang, L.; Chen, C. Surface-engineered gold nanorods: Promising DNA vaccine adjuvant for HIV-1 treatment. Nano Lett. 2012, 12, 2003–2012. [Google Scholar] [CrossRef]
- Yen, H.J.; Hsu, S.H.; Tsai, C.L. Cytotoxicity and immunological response of gold and silver nanoparticles of different sizes. Small 2009, 5, 1553–1561. [Google Scholar] [CrossRef] [PubMed]
- Wibowo, D.; Jorritsma, S.H.T.; Gonzaga, Z.J.; Evert, B.; Chen, S.; Rehm, B.H.A. Polymeric nanoparticle vaccines to combat emerging and pandemic threats. Biomaterials 2021, 268, 120597. [Google Scholar] [CrossRef]
- Kasturi, S.P.; Skountzou, I.; Albrecht, R.A.; Koutsonanos, D.; Hua, T.; Nakaya, H.I.; Pulendran, B. Programming the magnitude and persistence of antibody responses with innate immunity. Nature 2011, 470, 543–547. [Google Scholar] [CrossRef]
- Thomas, C.; Rawat, A.; Hope-Weeks, L.; Ahsan, F. Aerosolized PLA and PLGA nanoparticles enhance humoral, mucosal and cytokine responses to hepatitis B vaccine. Mol. Pharm. 2011, 8, 405–415. [Google Scholar] [CrossRef]
- Rahimian, S.; Fransen, M.F.; Kleinovink, J.W.; Christensen, J.R.; Amidi, M.; Hennink, W.E.; Ossendorp, F. Polymeric nanoparticles for co-delivery of synthetic long peptide antigen and poly IC as therapeutic cancer vaccine formulation. J. Control. Release 2015, 203, 16–22. [Google Scholar] [CrossRef]
- Zhao, F.; Zhang, X.; Liu, S.; Zeng, T.; Yu, J.; Gu, W.; Wu, Y. Assessment of the immune responses to Treponema pallidum Gpd DNA vaccine adjuvanted with IL-2 and chitosan nanoparticles before and after Treponema pallidum challenge in rabbits. Sci. China Life Sci. 2013, 56, 174–180. [Google Scholar] [CrossRef]
- Borges, O.; Cordeiro-da-Silva, A.; Tavares, J.; Santar, N.; de Sousa, A.; Borchard, G.; Junginger, H.E. Immune response by nasal delivery of hepatitis B surface antigen and codelivery of a CpG ODN in alginate coated chitosan nanoparticles. Eur. J. Pharm. Biopharm. 2008, 69, 405–416. [Google Scholar] [CrossRef]
- Gong, X.; Gao, Y.; Shu, J.; Zhang, C.; Zhao, K. Chitosan-Based Nanomaterial as Immune Adjuvant and Delivery Carrier for Vaccines. Vaccines 2022, 10, 1906. [Google Scholar] [CrossRef]
- Eskandari, S.; Guerin, T.; Toth, I.; Stephenson, R.J. Recent advances in self- assembled peptides: Implications for targeted drug delivery and vaccine engineering. Adv. Drug Deliv. Rev. 2017, 110–111, 169–187. [Google Scholar] [CrossRef]
- Gordon, D.; Kelley, P.; Heinzel, S.; Cooper, P.; Petrovsky, N. Immunogenicity and safety of Advax™, a novel polysaccharide adjuvant based on delta inulin, when formulated with hepatitis B surface antigen: A randomized controlled Phase 1 study. Vaccine 2014, 32, 6469–6477. [Google Scholar] [CrossRef] [PubMed]
- Gordon, D.L.; Sajkov, D.; Honda-Okubo, Y.; Wilks, S.H.; Aban, M.; Barr, I.G.; Petrovsky, N. Human Phase 1 trial of low-dose inactivated seasonal influenza vaccine formulated with Advax™ delta inulin adjuvant. Vaccine 2016, 34, 3780–3786. [Google Scholar] [CrossRef]
- Green, J.J.; Langer, R.; Anderson, D.G. A combinatorial polymer library approach yields insight into nonviral gene delivery. Acc. Chem. Res. 2008, 41, 749–759. [Google Scholar] [CrossRef]
- Liu, Y.; Busscher, H.J.; Zhao, B.; Li, Y.; Zhang, Z.; van der Mei, H.C.; Shi, L. Surface- adaptive, antimicrobially loaded, micellar nanocarriers with enhanced penetration and killing efficiency in staphylococcal biofilms. ACS Nano 2016, 10, 4779–4789. [Google Scholar] [CrossRef]
- Kaparakis-Liaskos, M.; Ferrero, R.L. Immune modulation by bacterial outer membrane vesicles. Nat. Rev. Immunol. 2015, 15, 375–387. [Google Scholar] [CrossRef]
- Chatterjee, S.N.; Das, J. Electron microscopic observations on the excretion of cell- wall material by Vibrio cholerae. J. Gen. Microbiol. 1967, 49, 1–11. [Google Scholar] [CrossRef]
- Devoe, I.W.; Gilchrist, J.E. Release of endotoxin in the form of cell wall blebs during in vitro growth of Neisseria meningitidis. J. Exp. Med. 1973, 138, 1156–1167. [Google Scholar] [CrossRef]
- Arigita, C.; Jiskoot, W.; Westdijk, J.; van Ingen, C.; Hennink, W.E.; Crommelin, D.J.; Kersten, G.F. Stability of mono- and trivalent meningococcal outer membrane vesicle vaccines. Vaccine 2004, 22, 629–642. [Google Scholar] [CrossRef]
- Baker, J.L.; Chen, L.; Rosenthal, J.A.; Putnam, D.; DeLisa, M.P. Microbial biosynthesis of designer outer membrane vesicles. Curr. Opin. Biotechnol. 2014, 29, 76–84. [Google Scholar] [CrossRef]
- Ellis, T.N.; Kuehn, M.J. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol. Mol. Biol. Rev. 2010, 74, 81–94. [Google Scholar] [CrossRef]
- O’Ryan, M.; Stoddard, J.; Toneatto, D.; Wassil, J.; Dull, P.M. A multi-component meningococcal serogroup B vaccine (4CMenB): The clinical development program. Drugs 2014, 74, 15–30. [Google Scholar] [CrossRef]
- Gerritzen, M.J.H.; Martens, D.E.; Wijffels, R.H.; van der Pol, L.; Stork, M. Bioengineering bacterial outer membrane vesicles as vaccine platform. Biotechnol. Adv. 2017, 35, 565–574. [Google Scholar] [CrossRef]
- Morein, B.; Sundquist, B.; Höglund, S.; Dalsgaard, K.; Osterhaus, A. Iscom, a novel structure for antigenic presentation of membrane proteins from enveloped viruses. Nature 1984, 308, 457–460. [Google Scholar] [CrossRef]
- Pabreja, S.; Garg, T.; Rath, G.; Goyal, A.K. Mucosal vaccination against tuberculosis using Ag85A-loaded immunostimulating complexes. Artif. Cells Nanomed. Biotechnol. 2016, 44, 532–539. [Google Scholar] [CrossRef]
- Pearse, M.J.; Drane, D. ISCOMATRIX adjuvant for antigen delivery. Adv. Drug Deliv. Rev. 2005, 57, 465–474. [Google Scholar] [CrossRef]
- Brito, L.A.; Malyala, P.; O’Hagan, D.T. Vaccine adjuvant formulations: A pharmaceutical perspective. Semin. Immunol. 2013, 25, 130–145. [Google Scholar] [CrossRef]
- Morelli, A.B.; Becher, D.; Koernig, S.; Silva, A.; Drane, D.; Maraskovsky, E. ISCOMATRIX: A novel adjuvant for use in prophylactic and therapeutic vaccines against infectious diseases. J. Med. Microbiol. 2012, 61, 935–943. [Google Scholar] [CrossRef]
- Nicholaou, T.; Ebert, L.M.; Davis, I.D.; McArthur, G.A.; Jackson, H.; Dimopoulos, N.; Cebon, J. Regulatory T-cell-mediated attenuation of T-cell responses to the NY-ESO-1 ISCOMATRIX vaccine in patients with advanced malignant melanoma. Clin. Cancer Res. 2009, 15, 2166–2173. [Google Scholar] [CrossRef]
- Kulkarni, J.A.; Witzigmann, D.; Thomson, S.B.; Chen, S.; Leavitt, B.R.; Cullis, P.R.; van der Meel, R. The current landscape of nucleic acid therapeutics. Nat. Nanotechnol. 2021, 16, 630–643. [Google Scholar] [CrossRef]
- Zeng, M.; Xu, Q.; Zhou, D.; Sigen, A.; Alshehri, F.; Lara-Saez, I.; Wang, W. Highly branched poly(beta-amino ester)s for gene delivery in hereditary skin diseases. Adv. Drug Deliv. Rev. 2021, 176, 113842. [Google Scholar] [CrossRef]
- Saunders, N.R.M.; Paolini, M.S.; Fenton, O.S.; Poul, L.; Devalliere, J.; Mpambani, F.; Darmon, A.; Bergère, M.; Jibault, O.; Germain, M.; et al. A Nanoprimer To Improve the Systemic Delivery of siRNA and mRNA. Nano Lett. 2020, 20, 4264–4269. [Google Scholar] [CrossRef]
- Wen, P.; Ke, W.; Dirisala, A.; Toh, K.; Tanaka, M.; Li, J. Stealth and pseudo-stealth nanocarriers. Adv. Drug Deliv. Rev. 2023, 198, 114895. [Google Scholar] [CrossRef]
- Phua, K.K.; Leong, K.W.; Nair, S.K. Transfection efficiency and transgene expression kinetics of mRNA delivered in naked and nanoparticle format. J. Control. Release 2013, 166, 227–233. [Google Scholar] [CrossRef]
- Patel, A.K.; Kaczmarek, J.C.; Bose, S.; Kauffman, K.J.; Mir, F.; Heartlein, M.W.; Anderson, D.G. Inhaled nanoformulated mRNA polyplexes for protein production in lung epithelium. Adv. Mater. 2019, 31, e1805116. [Google Scholar] [CrossRef]
- Hoy, S.M. Patisiran: First global approval. Drugs 2018, 78, 1625–1631. [Google Scholar] [CrossRef]
- Hajj, K.A.; Whitehead, K.A. Tools for translation: Non-viral materials for therapeutic mRNA delivery. Nat. Rev. Mater. 2017, 2, 17056. [Google Scholar] [CrossRef]
- Dong, Y.; Siegwart, D.J.; Anderson, D.G. Strategies; design, and chemistry in siRNA delivery systems. Adv. Drug Deliv. Rev. 2019, 144, 133–147. [Google Scholar] [CrossRef]
- Fernandez-Pin, I.; Badiola, I.; Sanchez, A. Nanocarriers for microRNA delivery in cancer medicine. Biotechnol. Adv. 2017, 35, 350–360. [Google Scholar] [CrossRef]
- Li, R.Q.; Wu, Y.; Zhi, Y.; Yang, X.; Li, Y.; Xua, F.J.; Du, J. PGMA-based star-like polycations with plentiful hydroxyl groups act as highly efficient miRNA delivery nanovectors for effective applications in heart diseases. Adv. Mater. 2016, 28, 7204–7212. [Google Scholar] [CrossRef]
- Nguyen, M.A.; Wyatt, H.; Susser, L.; Geoffrion, M.; Rasheed, A.; Duchez, A.C.; Rayner, K.J. Delivery of MicroRNAs by chitosan nanoparticles to functionally alter macrophage cholesterol efflux in vitro and in vivo. ACS Nano 2019, 13, 6491–6505. [Google Scholar] [CrossRef]
- Chan, C.; Guo, N.; Duan, X.; Han, W.; Xue, L.; Bryan, D.; Lin, W. Systemic miRNA delivery by nontoxic nanoscale coordination polymers limits epithelial-to- mesenchymal transition and suppresses liver metastases of colorectal cancer. Biomaterials 2019, 210, 94–104. [Google Scholar] [CrossRef]
- Di Ianni, T.; Bose, R.J.C.; Sukumar, U.K.; Bachawal, S.; Wang, H.; Telichko, A.; Dahl, J.D. Ultrasound/microbubble-mediated targeted delivery of anticancer microRNA- loaded nanoparticles to deep tissues in pigs. J. Contr. Release 2019, 309, 1–10. [Google Scholar] [CrossRef]
- Bejerano, T.; Etzion, S.; Elyagon, S.; Etzion, Y.; Cohen, S. Nanoparticle delivery of miRNA-21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett. 2018, 18, 5885–5891. [Google Scholar] [CrossRef]
- Varshney, A.; Panda, J.J.; Singh, A.K.; Yadav, N.; Bihari, C.; Biswas, S.; Chauhan, V.S. Targeted delivery of microRNA-199a-3p using self-assembled dipeptide nanoparticles efficiently reduces hepatocellular carcinoma in mice. Hepatology 2018, 67, 1392–1407. [Google Scholar] [CrossRef] [PubMed]
- Farooqi, A.A.; Desai, N.N.; Qureshi, M.Z.; Librelotto, D.R.N.; Gasparri, M.L.; Bishayee, A.; Daglia, M. Exosome biogenesis, bioactivities and functions as new delivery systems of natural compounds. Biotechnol. Adv. 2018, 36, 328–334. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.; Wei, M.; Gao, X.; Zhao, L.; Shi, R.; Yuan, L. In vitro and in vivo RNA inhibition by CD9-HuR functionalized exosomes encapsulated with miRNA or CRISPR/dCas9. Nano Lett. 2019, 19, 19–28. [Google Scholar] [CrossRef]
- Kim, H.J.; Yi, S.W.; Oh, H.J.; Lee, J.S.; Park, J.S.; Park, K.H. Transfection of gene regulation nanoparticles complexed with pDNA and shRNA controls multilineage differentiation of hMSCs. Biomaterials 2018, 177, 1–13. [Google Scholar] [CrossRef]
- Wang, Y.; Xie, Y.; Kilchrist, K.V.; Li, J.; Duvall, C.L.; Oupický, D. Endosomolytic and tumor-penetrating mesoporous silica nanoparticles for siRNA/miRNA combination cancer therapy. ACS Appl. Mater. Interfaces 2020, 12, 4308–4322. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Kanasty, R.L.; Eltoukhy, A.A.; Vegas, A.J.; Dorkin, J.R.; Anderson, D.G. Non- viral vectors for gene-based therapy. Nat. Rev. Genet. 2014, 15, 541–555. [Google Scholar] [CrossRef]
- Buck, J.; Grossen, P.; Cullis, P.R.; Huwyler, J.; Witzigmann, D. Lipid-based DNA therapeutics: Hallmarks of non-viral gene delivery. ACS Nano 2019, 13, 3754–3782. [Google Scholar] [CrossRef]
- Rajala, A.; Wang, Y.; Zhu, Y.; Ranjo-Bishop, M.; Ma, J.X.; Mao, C.; Rajala, R.V. Nanoparticle- assisted targeted delivery of eye-specific genes to eyes significantly improves the vision of blind mice in vivo. Nano Lett. 2014, 14, 5257–5263. [Google Scholar] [CrossRef]
- Zhou, Z.; Liu, X.; Zhu, D.; Wang, Y.; Zhang, Z.; Zhou, X.; Shen, Y. Nonviral cancer gene therapy: Delivery cascade and vector nanoproperty integration. Adv. Drug Deliv. Rev. 2017, 115, 115–154. [Google Scholar] [CrossRef]
- Zeng, M.; Alshehri, F.; Zhou, D.; Lara-Sáez, I.; Wang, X.; Li, X.; Wang, W. Efficient and robust highly branched poly(β-amino ester)/minicircle COL7A1 polymeric nanoparticles for gene delivery to recessive dystrophic epidermolysis bullosa keratinocytes. ACS Appl. Mater. Interfaces 2019, 11, 30661–30672. [Google Scholar] [CrossRef]
- Lv, H.; Zhang, S.; Wang, B.; Cui, S.; Yan, J. Toxicity of cationic lipids and cationic polymers in gene delivery. J. Control. Release 2006, 114, 100–109. [Google Scholar] [CrossRef]
- Zhou, D.; Cutlar, L.; Gao, Y.; Wang, W.; O’Keeffe-Ahern, J.; McMahon, S.; Wang, W. The transition from linear to highly branched poly(β-amino ester)s: Branching matters for gene delivery. Sci. Adv. 2016, 2, e1600102. [Google Scholar] [CrossRef]
- Zeng, M.; Zhou, D.; Alshehri, F.; Lara-Sáez, I.; Lyu, Y.; Creagh-Flynn, J.; Wang, W. Manipulation of transgene expression in fibroblast cells by a multifunctional linear-branched hybrid poly(β-amino ester) synthesized through an oligomer combination approach. Nano Lett. 2019, 19, 381–391. [Google Scholar] [CrossRef]
- Tockary, T.A.; Foo, W.; Dirisala, A.; Chen, Q.; Uchida, S.; Osawa, S.; Kataoka, K. Single- stranded DNA-packaged polyplex micelle as adeno-associated-virus-inspired compact vector to systemically target stroma-rich pancreatic cancer. ACS Nano 2019, 13, 12732–12742. [Google Scholar] [CrossRef]
- Tabatabaei, S.N.; Derbali, R.M.; Yang, C.; Superstein, R.; Hamel, P.; Chain, J.L.; Hardy, P. Co-delivery of miR-181a and melphalan by lipid nanoparticles for treatment of seeded retinoblastoma. J. Control. Release 2019, 298, 177–185. [Google Scholar] [CrossRef]
- Yu, Q.; Zhang, M.; Chen, Y.; Chen, X.; Shi, S.; Sun, K.; Peng, J. Self-assembled nanoparticles prepared from low-molecular-weight PEI and low-generation PAMAM for EGFRvIII-chimeric antigen receptor gene loading and T-cell transient modification. Int. J. Nanomed. 2020, 15, 483–495. [Google Scholar] [CrossRef]
- Billingsley, M.M.; Singh, N.; Ravikumar, P.; Zhang, R.; June, C.H.; Mitchell, M.J. Ionizable lipid nanoparticle-mediated mRNA delivery for human CAR T cell engineering. Nano Lett. 2020, 20, 1578–1589. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Han, J.H.; Park, J.H.; Kim, H.K.; Choi, S.H.; Kim, G.R.; Park, K.S. Multifunctional nanoparticles for genetic engineering and bioimaging of natural killer (NK) cell therapeutics. Biomaterials 2019, 221, 119418. [Google Scholar] [CrossRef]
- Parayath, N.N.; Stephan, M.T. In situ programming of CAR T cells. Annu. Rev. Biomed. Eng. 2021, 23, 385–405. [Google Scholar] [CrossRef]
- Smith, T.T.; Stephan, S.B.; Moffett, H.F.; McKnight, L.E.; Ji, W.; Reiman, D.; Bonagofski, E.; Wohlfahrt, M.E.; Pillai, S.P.S.; Stephan, M.T. In situ programming of leukaemia-specific T cells using synthetic DNA nanocarriers. Nat. Nanotechnol. 2017, 12, 813–820. [Google Scholar] [CrossRef]
- Parayath, N.N.; Stephan, S.B.; Koehne, A.L.; Nelson, P.S.; Stephan, M.T. In vitro- transcribed antigen receptor mRNA nanocarriers for transient expression in circulating T cells in vivo. Nat. Commun. 2020, 11, 6080. [Google Scholar] [CrossRef]
- Allemani, C.; Matsuda, T.; Di Carlo, V.; Harewood, R.; Matz, M.; Nik, M.; Hood, M. Global surveillance of trends in cancer survival 2000-14 (CONCORD-3): Analysis of individual records for 37513025patients diagnosed with one of 18 cancers from 322 population -basedregistries in 71 countries. Lancet 2018, 391, 1023–1075. [Google Scholar] [CrossRef]
- Liu, Z.; Tang, M.; Zhao, J.; Chai, R.; Kang, J. Looking into the future: Toward advanced 3D biomaterials for stem-cell-based regenerative medicine. Adv. Mater. 2018, 30, e1705388. [Google Scholar] [CrossRef]
- Tam, R.Y.; Yockell-Leli, J.; Smith, L.J.; Julian, L.M.; Baker, A.E.G.; Choey, C.; Shoichet, M.S. Rationally designed 3D hydrogels model invasive lung diseases enabling high- content drug screening. Adv. Mater. 2019, 31, e1806214. [Google Scholar] [CrossRef]
- Shadish, J.A.; Benuska, G.M.; DeForest, C.A. Bioactive site-specifically modified proteins for 4D patterning of gel biomaterials. Nat. Mater. 2019, 18, 1005–1014. [Google Scholar] [CrossRef]
- Caiazzo, M.; Okawa, Y.; Ranga, A.; Piersigilli, A.; Tabata, Y.; Lutolf, M.P. Defined three-dimensional microenvironments boost induction of pluripotency. Nat. Mater. 2016, 15, 344–352. [Google Scholar] [CrossRef]
- Chaudhuri, O.; Gu, L.; Klumpers, D.; Darnell, M.; Bencherif, S.A.; Weaver, J.C.; Mooney, D.J. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016, 15, 326–334. [Google Scholar] [CrossRef]
- Huebsch, N.; Lippens, E.; Lee, K.; Mehta, M.; Koshy, S.T.; Darnell, M.C.; Mooney, D.J. Matrix elasticity of void-forming hydrogels controls transplanted-stem-cell-mediated bone formation. Nat. Mater. 2015, 14, 1269–1277. [Google Scholar] [CrossRef]
- Das, R.K.; Gocheva, V.; Hammink, R.; Zouani, O.F.; Rowan, A.E. Stress-stiffening- mediated stem-cell commitment switch in soft responsive hydrogels. Nat. Mater. 2016, 15, 318–325. [Google Scholar] [CrossRef]
- Madl, C.M.; LeSavage, B.L.; Dewi, R.E.; Dinh, C.B.; Stowers, R.S.; Khariton, M.; Heilshorn, S.C. Maintenance of neural progenitor cell stemness in 3D hydrogels requires matrix remodeling. Nat. Mater. 2017, 16, 1233–1242. [Google Scholar] [CrossRef]
- Ho, M.; Raute, K.; Hummel, B.; Madl, J.; Creusen, G.; Thomas, O.S.; Weber, W. Phytochrome-based extracellular matrix with reversibly tunable mechanical properties. Adv. Mater. 2019, 31, e1806727. [Google Scholar]
- Jia, X.; Minami, K.; Uto, K.; Chang, A.C.; Hill, J.P.; Nakanishi, J.; Ariga, K. Adaptive liquid interfacially assembled protein nanosheets for guiding mesenchymal stem cell fate. Adv. Mater. 2020, 32, e1905942. [Google Scholar] [CrossRef]
- Yang, L.; Conley, B.M.; Cerqueira, S.R.; Pongkulapa, T.; Wang, S.; Lee, J.K.; Lee, K.B. Effective modulation of CNS inhibitory microenvironment using bioinspired hybrid-nanoscaffold-based therapeutic interventions. Adv. Mater. 2020, 32, e2002578. [Google Scholar] [CrossRef]
- Facklam, A.L.; Volpatti, L.R.; Anderson, D.G. Biomaterials for personalized cell therapy. Adv. Mater. 2020, 32, e1902005. [Google Scholar] [CrossRef]
- Roche, E.T.; Hastings, C.L.; Lewin, S.A.; Shvartsman, D.; Brudno, Y.; Vasilyev, N.V.; Mooney, D.J. Comparison of biomaterial delivery vehicles for improving acute retention of stem cells in the infarcted heart. Biomaterials 2014, 35, 6850–6858. [Google Scholar] [CrossRef]
- Gao, L.; Kupfer, M.E.; Jung, J.P.; Yang, L.; Zhang, P.; Da Sie, Y.; Zhang, J. Myocardial tissue engineering with cells derived from human-induced pluripotent stem cells and a native-like, high-resolution, 3-dimensionally printed scaffold. Circ. Res. 2017, 120, 1318–1325. [Google Scholar] [CrossRef]
- Gao, L.; Gregorich, Z.R.; Zhu, W.; Mattapally, S.; Oduk, Y.; Lou, X.; Zhang, J. Large cardiac muscle patches engineered from human induced-pluripotent stem cell- derived cardiac cells improve recovery from myocardial infarction in swine. Circulation 2018, 137, 1712–1730. [Google Scholar] [CrossRef]
- Sharma, R.; Khristov, V.; Rising, A.; Jha, B.S.; Dejene, R.; Hotaling, N.; Bharti, K. Clinical- grade stem cell-derived retinal pigment epithelium patch rescues retinal degeneration in rodents and pigs. Sci. Transl. Med. 2019, 11, eaat5580. [Google Scholar] [CrossRef]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Coffey, P.J. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef]
- American Cancer Society. Cancer Facts & Figures. ACS, 2019. Available online: https://www.cancer.org/research/cancer-facts-statistics/all-cancer-facts-figures/cancer-facts-figures-2019.html#:~:text=The%20Facts%20%26%20Figures%20annual%20report,deaths%20in%20the%20United%20States (accessed on 3 June 2024).
- Dalby, M.; Cree, I.A.; Challoner, B.R.; Ghosh, S.; Thurston, D.E. The precision medicine approach to cancer therapy: Part 1—solid tumours. Pharm. J. 2019. [Google Scholar] [CrossRef]
- Hauschild, A.; Ascierto, P.A.; Schadendorf, D.; Grob, J.J.; Ribas, A.; Kiecker, F.; Chapman, P.B. Long-term outcomes in patients with BRAFV600-mutant metastatic melanoma receiving dabrafenib monotherapy: Analysis from phase, 2 and 3 clinical trials. Eur. J. Cancer 2020, 125, 114–120. [Google Scholar] [CrossRef]
- Chapman, P.B.; Hauschild, A.; Robert, C.; Haanen, J.B.; Ascierto, P.; Larkin, J.; McArthur, G.A. Improved survival with vemurafenib in melanoma with BRAFV600E mutation. N. Engl. J. Med. 2011, 364, 2507–2516. [Google Scholar] [CrossRef]
- Ou, W.; Thapa, R.K.; Jiang, L.; Soe, Z.C.; Gautam, M.; Chang, J.H.; Kim, J.O. Regulatory T cell-targeted hybrid nanoparticles combined with immuno-checkpoint blockage for cancer immunotherapy. J. Control. Release 2018, 281, 84–96. [Google Scholar] [CrossRef]
- Lyon, R.P.; Setter, J.R.; Bovee, T.D.; Doronina, S.O.; Hunter, J.H.; Anderson, M.E.; Senter, P.D. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody–drug conjugates. Nat. Biotechnol. 2014, 32, 1059–1062. [Google Scholar] [CrossRef]
- Chen, W.H.; Luo, G.F.; Zhang, X.Z. Recent advances in subcellular targeted cancer therapy based on functional materials. Adv. Mater. 2019, 31, 1–39. [Google Scholar] [CrossRef]
- Goswami, U.; Dutta, A.; Raza, A.; Kandimalla, R.; Kalita, S.; Ghosh, S.S.; Chattopadhyay, A. Transferrin–copper nanocluster– doxorubicin nanoparticles as targeted theranostic cancer nanodrug. ACS Appl. Mater. Interfaces 2018, 10, 3282–3294. [Google Scholar] [CrossRef]
- Qiao, Y.; Wan, J.; Zhou, L.; Ma, W.; Yang, Y.; Luo, W.; Wang, H. Stimuli-responsive nanotherapeutics for precision drug delivery and cancer therapy. Rev. Nanomed. Nanobiotechnol. 2019, 11, 1–20. [Google Scholar] [CrossRef]
- Jin, Q.; Deng, Y.; Chen, X.; Ji, J. Rational design of cancer nanomedicine for simultaneous stealth surface and enhanced cellular uptake. ACS Nano 2019, 13, 954–977. [Google Scholar] [CrossRef]
- Fang, Y.; Xue, J.; Gao, S.; Lu, A.; Yang, D.; Jiang, H.; Shi, K. Cleavable PEGylation: A strategy for overcoming the “PEG dilemma” in efficient drug delivery. Drug Deliv. 2017, 24, 22–32. [Google Scholar] [CrossRef]
- Eno, J. Immunotherapy through the years. J. Adv. Pract. Oncol. 2017, 8, 747–753. [Google Scholar]
- Dvorak, H.F.; Brown, L.F.; Detmar, M.; Dvorak, A.M. Vascular permeability factor/vascular endothelial growth factor, microvascular hyperpermeability, and angiogenesis. Am. J. Pathol. 1995, 146, 1029–1039. [Google Scholar]
- Urbanavicius, D.; Alvarez, T.; Such, G.K.; Johnston AP, R.; Mintern, J.D. The potential of nanoparticle vaccines as a treatment for cancer. Mol. Immunol. 2018, 98, 2–7. [Google Scholar] [CrossRef]
- Truex, N.L.; Holden, R.L.; Wang, B.Y.; Chen, P.G.; Hanna, S.; Hu, Z.; Pentelute, B.L. Automated flow synthesis of tumor neoantigen peptides for personalized immunotherapy. Sci. Rep. 2020, 10, 723. [Google Scholar] [CrossRef]
- Guo, Y.; Lei, K.; Tang, L. Neoantigen vaccine delivery for personalized anticancer immunotherapy. Front. Immunol. 2018, 9, 1499. [Google Scholar] [CrossRef]
- Dombroski, J.A.; Jyotsana, N.; Crews, D.W.; Zhang, Z.; King, M.R. Fabrication and characterization of tumor nano-lysate as a preventative vaccine for breast cancer. Langmuir 2020, 36, 6531–6539. [Google Scholar] [CrossRef]
- Gu, P.; Wusiman, A.; Zhang, Y.; Liu, Z.; Bo, R.; Hu, Y.; Wang, D. Rational design of PLGA nanoparticle vaccine delivery systems to improve immune responses. Mol. Pharm. 2019, 16, 5000–5012. [Google Scholar] [CrossRef]
- Soema, P.C.; Willems, G.J.; Jiskoot, W.; Amorij, J.P.; Kersten, G.F. Predicting the influence of liposomal lipid composition on liposome size, zeta potential and liposome-induced dendritic cell maturation using a design of experiments approach. Eur. J. Pharm. Biopharm. 2015, 94, 427–435. [Google Scholar] [CrossRef]
- Cao, F.; Yan, M.; Liu, Y.; Liu, L.; Ma, G. Photothermally controlled MHC class I restricted CD8+ T-cell responses elicited by hyaluronic acid decorated gold nanoparticles as a vaccine for cancer immunotherapy. Adv. Healthc. Mater. 2018, 7, 1–12. [Google Scholar]
- Fontana, F.; Liu, D.; Hirvonen, J.; Santos, H.A. Delivery of therapeutics with nanoparticles: What’s new in cancer immunotherapy? Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2017, 9, 1–26. [Google Scholar] [CrossRef]
- Tran, T.H.; Tran, T.T.P.; Nguyen, H.T.; Dai Phung, C.; Jeong, J.H.; Stenzel, M.H.; Kim, J.O. Nanoparticles for dendritic cell-based immunotherapy. Int. J. Pharm. 2018, 542, 253–265. [Google Scholar] [CrossRef]
- Grippin, A.J.; Sayour, E.J.; Mitchell, D.A. Translational nanoparticle engineering for cancer vaccines. Oncoimmunology 2017, 6, e1290036. [Google Scholar] [CrossRef]
- Liu, J.; Miao, L.; Sui, J.; Hao, Y.; Huang, G. Nanoparticle cancer vaccines: Design considerations and recent advances. Asian J. Pharm. Sci. 2019, 15, 576–590. [Google Scholar] [CrossRef]
- Tostanoski, L.H.; Gosselin, E.A.; Jewell, C.M. Engineering tolerance using biomaterials to target and control antigen presenting cells. Discov. Med. 2016, 21, 403–410. [Google Scholar]
- Zupančič, E.; Curato, C.; Paisana, M.; Rodrigues, C.; Porat, Z.; Viana, A.S.; Florindo, H.F. Rational design of nanoparticles towards targeting antigen-presenting cells and improved T cell priming. J. Control. Release 2017, 258, 182–195. [Google Scholar] [CrossRef]
- Macri, C.; Dumont, C.; Johnston AP, R.; Mintern, J.D. Targeting dendritic cells: A promising strategy to improve vaccine effectiveness. Clin. Transl. Immunol. 2016, 5, e66–e68. [Google Scholar] [CrossRef] [PubMed]
- Chavez-Santoscoy, A.V.; Roychoudhury, R.; Pohl, N.L.; Wannemuehler, M.J.; Narasimhan, B.; Ramer-Tait, A.E. Tailoring the immune response by targeting C-type lectin receptors on alveolar macrophages using ‘pathogen-like’ amphiphilic polyanhydride nanoparticles. Biomaterials 2012, 33, 4762–4772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Shi, G.; Zhang, J.; Song, H.; Niu, J.; Shi, S.; Kong, D. Targeted antigen delivery to dendritic cell via functionalized alginate nanoparticles for cancer immunotherapy. J. Control. Release 2017, 256, 170–181. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.C.; Kawasaki, N.; Nycholat, C.M.; Han, S.; Pilotte, J.; Crocker, P.R.; Paulson, J.C. Antigen delivery to macrophages using liposomal nanoparticles targeting sialoadhesin/CD169. PLoS ONE 2012, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tatar, A.S.; Jurj, A.; Tomuleasa, C.; Florea, A.; Berindan-Neagoe, I.; Cialla-May, D.; Boca, S. CD19-targeted, Raman tagged gold nanourchins as theranostic agents against acute lymphoblastic leukemia. Colloids Surf. B Biointerfaces 2019, 184, 110478. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Jin, H.; Qiao, S.; Dai, Y.; Huang, C.; Lu, L.; Zhang, Z. Targeting dendritic cells in lymph node with an antigen peptide-based nanovaccine for cancer immunotherapy. Biomaterials 2016, 98, 171–183. [Google Scholar] [CrossRef]
- Pearson, R.M.; Casey, L.M.; Hughes, K.R.; Miller, S.D.; Shea, L.D. In vivo reprogramming of immune cells: Technologies for induction of antigen-specific tolerance. Adv. Drug Deliv. Rev. 2017, 114, 240–255. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Peppas, N.A. Hydrogels and scaffolds for immunomodulation. Adv. Mater. 2014, 26, 6530–6541. [Google Scholar] [CrossRef]
- Marloye, M.; Lawler, S.E.; Berger, G. Current patent and clinical status of stimulator of interferon genes (STING) agonists for cancer immunotherapy. Pharm. Pat. Anal. 2019, 8, 87–90. [Google Scholar] [CrossRef]
- Cheng, N.; Watkins-Schulz, R.; Junkins, R.D.; David, C.N.; Johnson, B.M.; Montgomery, S.A.; Ting, J.P. A nanoparticle-incorporated STING activator enhances antitumor immunity in PD- L1-insensitive models of triple-negative breast cancer. JCI Insight 2018, 3, 1–20. [Google Scholar] [CrossRef]
- Wilson, D.R.; Sen, R.; Sunshine, J.C.; Pardoll, D.M.; Green, J.J.; Kim, Y.J. Biodegradable STING agonist nanoparticles for enhanced cancer immunotherapy. Nanomed. Nanotechnol. Biol. Med. 2018, 14, 237–246. [Google Scholar] [CrossRef]
- Wang, C.; Ye, Y.; Hochu, G.M.; Sadeghifar, H.; Gu, Z. Enhanced cancer immunotherapy by microneedle patch-assisted delivery of anti-PD1 antibody. Nano Lett. 2016, 16, 2334–2340. [Google Scholar] [CrossRef]
- Wang, C.; Sun, W.; Wright, G.; Wang, A.Z.; Gu, Z. Inflammation-triggered cancer immunotherapy by programmed delivery of CpG and Anti-PD1 antibody. Adv. Mater. 2016, 28, 8912–8920. [Google Scholar] [CrossRef]
- Deng, H.; Zhang, Z. The application of nanotechnology in immune checkpoint blockade for cancer treatment. J. Control. Release 2018, 290, 28–45. [Google Scholar] [CrossRef]
- Dunn, Z.S.; Mac, J.; Wang, P. T cell immunotherapy enhanced by designer biomaterials. Biomaterials 2019, 217, 119265. [Google Scholar] [CrossRef]
- Hickey, J.W.; Vicente, F.P.; Howard, G.P.; Mao, H.Q.; Schneck, J.P. Biologically inspired design of nanoparticle artificial antigen-presenting cells for immunomodulation. Nano Lett. 2017, 17, 7045–7054. [Google Scholar] [CrossRef]
- Sunshine, J.C.; Perica, K.; Schneck, J.P.; Green, J.J. Particle shape dependence of CD8+ T cell activation by artificial antigen presenting cells. Biomaterials 2014, 35, 269–277. [Google Scholar] [CrossRef]
- Pozsgay, J.; Szekanecz, Z.; Sármay, G. Antigen- specific immunotherapies in rheumatic diseases. Nat. Rev. Rheumatol. 2017, 13, 525–537. [Google Scholar] [CrossRef]
- Jung, H.H.; Kim, S.H.; Moon, J.H.; Jeong, S.U.; Jang, S.; Park, C.S.; Lee, C.K. Polymeric nanoparticles containing both antigen and vitamin D3 induce antigen-specific immune suppression. Immune Netw. 2019, 19, 1–12. [Google Scholar] [CrossRef]
- Pang, L.; Macauley, M.S.; Arlian, B.M.; Nycholat, C.M.; Paulson, J.C. Encapsulating an immunosuppressant enhances tolerance induction by Siglec-engaging tolerogenic liposomes. ChemBioChem 2017, 18, 1226–1233. [Google Scholar] [CrossRef] [PubMed]
- Horwitz, D.A.; Bickerton, S.; Koss, M.; Fahmy, T.M.; La Cava, A. Suppression of murine lupus by CD4+ and CD8+ Treg cells induced by T cell–targeted nanoparticles loaded with interleukin-2 and transforming growth factor β. Arthritis Rheumatol. 2019, 71, 632–640. [Google Scholar] [CrossRef]
- Hu, T.; Hu, W.; Ma, L.; Zeng, X.; Liu, J.; Cheng, B.; Yang, P.; Qiu, S.; Yang, G.; Chen, D.; et al. pVAX1-A20 alleviates colitis in mice by promoting regulatory T cells. Dig. Liver Dis. 2019, 51, 790–797. [Google Scholar] [CrossRef]
- Shahzad, K.A.; Naeem, M.; Zhang, L.; Wan, X.; Song, S.; Pei, W.; Zhao, C.; Jin, X.; Shen, C. Design and optimization of PLGA particles to deliver immunomodulatory drugs for the prevention of skin allograft rejection. Immunol. Investig. 2019, 49, 840–857. [Google Scholar] [CrossRef]
- Li, R.; Liang, J.; He, Y.; Qin, J.; He, H.; Lee, S.; Pang, Z.; Wang, J. Sustained release of immunosuppressant by nanoparticle-anchoring hydrogel scaffold improved the survival of transplanted stem cells and tissue regeneration. Theranostics 2018, 8, 878–893. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, S.; Zhao, G.; Xu, C.F.; Zhang, H.B.; Luo, Y.L.; Cao, Z.T.; Shi, J.; Zhao, Z.B.; Lian, Z.X.; et al. In situ repurposing of dendritic cells with CRISPR/Cas9-based nanomedicine to induce transplant tolerance. Biomaterials 2019, 217, 119302. [Google Scholar] [CrossRef]
- Roma-Rodrigues, C.; Mendes, R.; Baptista, P.V.; Fernandes, A.R. Targeting tumor microenvironment for cancer therapy. Int. J. Mol. Sci. 2019, 20, 840. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Zhang, Y.; Feng, N. Cell membrane-coated nanosized active targeted drug delivery systems homing to tumor cells: A review. Mater. Sci. Eng. C. 2020, 106, 110298. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Luan, Z.; Zhao, C.; Bai, C.; Yang, K. Target delivery selective CSF-1R inhibitor to tumor-associated macrophages via erythrocyte-cancer cell hybrid membrane camouflaged pH-responsive copolymer micelle for cancer immunotherapy. Eur. J. Pharm. Sci. 2020, 142, 105136. [Google Scholar] [CrossRef]
- Li, R.; He, Y.; Zhang, S.; Qin, J.; Wang, J. Cell membrane-based nanoparticles: A new biomimetic platform for tumor diagnosis and treatment. Acta Pharm. Sin. B 2018, 8, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Karlsson, J.; Rui, Y.; Kozielski, K.L.; Placone, A.L.; Choi, O.; Tzeng, S.Y.; Kim, J.; Keyes, J.J.; Bogorad, M.I.; Gabrielson, K.; et al. Engineered nanoparticles for systemic siRNA delivery to malignant brain tumours. Nanoscale 2019, 11, 20045–20057. [Google Scholar] [CrossRef] [PubMed]
- Jeevanandam, J.; Kiew, S.F.; Boakye-Ansah, S.; Lau, S.Y.; Barhoum, A.; Danquah, M.K. Green approaches for the synthesis of metal and metal oxide nanoparticles using microbial and plant extracts. Nanoscale 2022, 14, 2534–2571. [Google Scholar] [CrossRef] [PubMed]
- Jain, R.K.; Stylianopoulos, T. Delivering nanomedicine to solid tumors. Nat. Rev. Clin. Oncol. 2010, 7, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Divyashree, M.; Prakash, S.K.; Aditya, V.; Aljabali, A.A.A.; Alzahrani, K.J.; Azevedo, V. Bugs as drugs: Neglected but a promising future therapeutic strategy in cancer. Future Oncol. 2022, 18, 1609–1626. [Google Scholar] [CrossRef] [PubMed]
- Taherkhani, S.; Mohammadi, M.; Daoud, J.; Martel, S.; Tabrizian, M. Covalent binding of nanoliposomes to the surface of magnetotactic bacteria for the synthesis of self-propelled therapeutic agents. ACS Nano 2014, 8, 5049–5060. [Google Scholar] [CrossRef] [PubMed]
- Boegh, M.; Nielsen, H.M. Mucus as a barrier to drug delivery–understanding and mimicking the barrier properties. Basic Clin. Pharmacol. Toxicol. 2015, 116, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Mei, H.; Cai, S.; Huang, D.; Gao, H.; Cao, J.; He, B. Carrier-free nanodrugs with efficient drug delivery and release for cancer therapy: From intrinsic physicochemical properties to external modification. Bioact. Mater. 2022, 8, 220–240. [Google Scholar] [CrossRef]
- Zhao, Z.; Ukidve, A.; Krishnan, V.; Mitragotri, S. Effect of physicochemical and surface properties on in vivo fate of drug nanocarriers. Adv. Drug Deliv. Rev. 2019, 143, 3–21. [Google Scholar] [CrossRef] [PubMed]
- Barenholz, Y. Doxil®–the first FDA-approved nano-drug: Lessons learned. J. Control. Release 2012, 160, 117–134. [Google Scholar] [CrossRef] [PubMed]
- Soloman, R.; Gabizon, A.A. Clinical pharmacology of liposomal anthracyclines: Focus on pegylated liposomal Doxorubicin. Clin. Lymphoma Myeloma 2008, 8, 21–32. [Google Scholar] [CrossRef]
- De Vincenzo, R.; Conte, C.; Ricci, C.; Scambia, G.; Capelli, G. Long-term efficacy and safety of human papillomavirus vaccination. Int. J. Womens Health 2014, 6, 999–1010. [Google Scholar] [CrossRef]
- Schiller, J.T.; Castellsagu, X.; Garland, S.M. A review of clinical trials of human papillomavirus prophylactic vaccines. Vaccine 2012, 30 (Suppl. S5), F123–F138. [Google Scholar] [CrossRef] [PubMed]
- Eliasof, S.; Lazarus, D.; Peters, C.G.; Case, R.I.; Cole, R.O.; Hwang, J. Correlating preclinical animal studies and human clinical trials of a multifunctional, polymeric nanoparticle. Proc. Natl. Acad. Sci. USA 2013, 110, 15127–15132. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Reese, T.A. Making mouse models that reflect human immune responses. Trends Immunol. 2017, 38, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Mestas, J.; Hughes, C.C. Of mice and not men: Differences between mouse and human immunology. J. Immunol. 2004, 172, 2731–2738. [Google Scholar] [CrossRef] [PubMed]
- Jiang, W.; Wang, Y.; Wargo, J.A.; Lang, F.F.; Kim, B.Y.S. Considerations for designing preclinical cancer immune nanomedicine studies. Nat. Nanotechnol. 2021, 16, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Hua, S.; de Matos, M.B.C.; Metselaar, J.M.; Storm, G. Current trends and challenges in the clinical translation of nanoparticulate nanomedicines: Pathways for translational development and commercialization. Front. Pharmacol. 2018, 9, 790. [Google Scholar] [CrossRef]
- Castro, F.; Pinto, M.L.; Pereira, C.L.; Serre, K.; Barbosa, M.A.; Vermaelen, K. Chitosan/γ-PGA nanoparticles-based immunotherapy as adjuvant to radiotherapy in breast cancer. Biomaterials 2020, 257, 120218. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Yu, F.; Xu, J.; Hu, X. Knowledge gaps in immune response and immunotherapy involving nanomaterials: Databases and artificial intelligence for material design. Biomaterials 2021, 266, 120469. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, R.; Kosuri, S.; Tamasi, M.; Meyer, T.A.; Atta, S.; Webb, M.A. Automation and data-driven design of polymer therapeutics. Adv. Drug Deliv. Rev. 2021, 171, 1–28. [Google Scholar] [CrossRef]
- Ouyang, B.; Poon, W.; Zhang, Y.N.; Lin, Z.P.; Kingston, B.R.; Tavares, A.J. The dose threshold for nanoparticle tumour delivery. Nat. Mater. 2020, 19, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
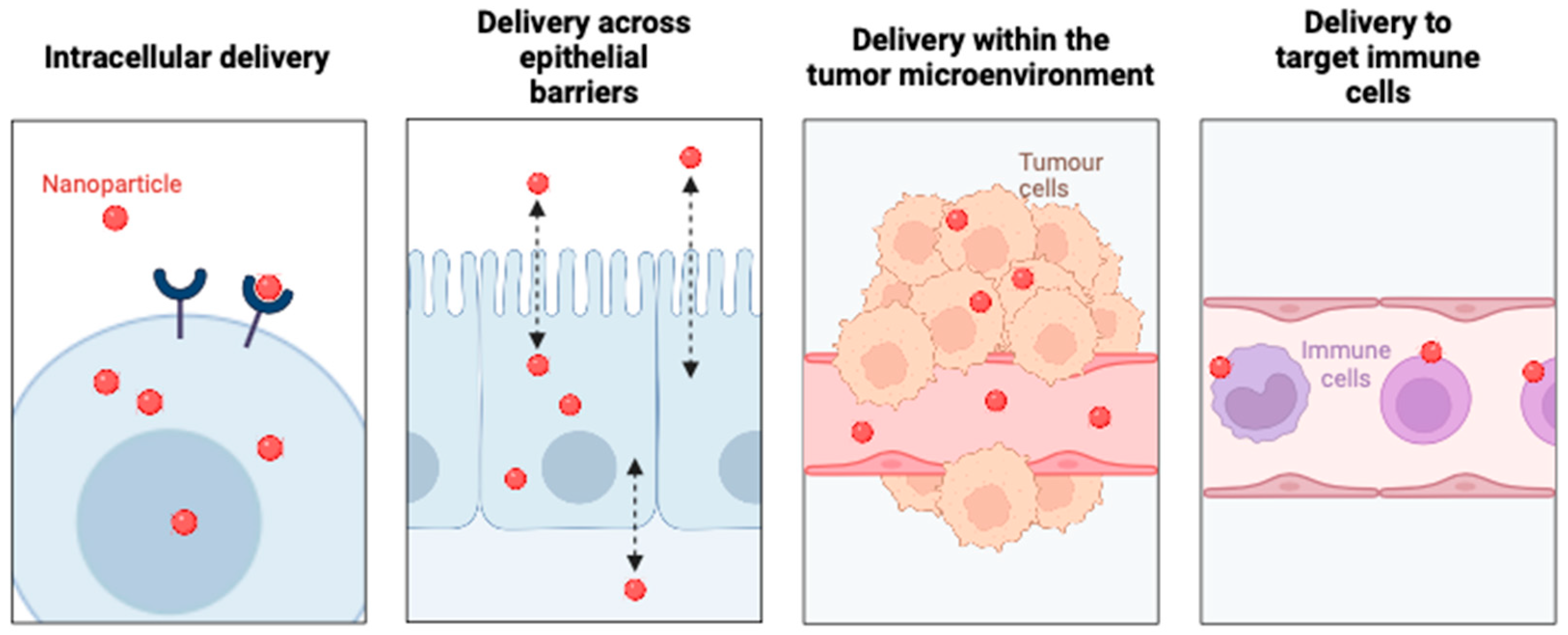

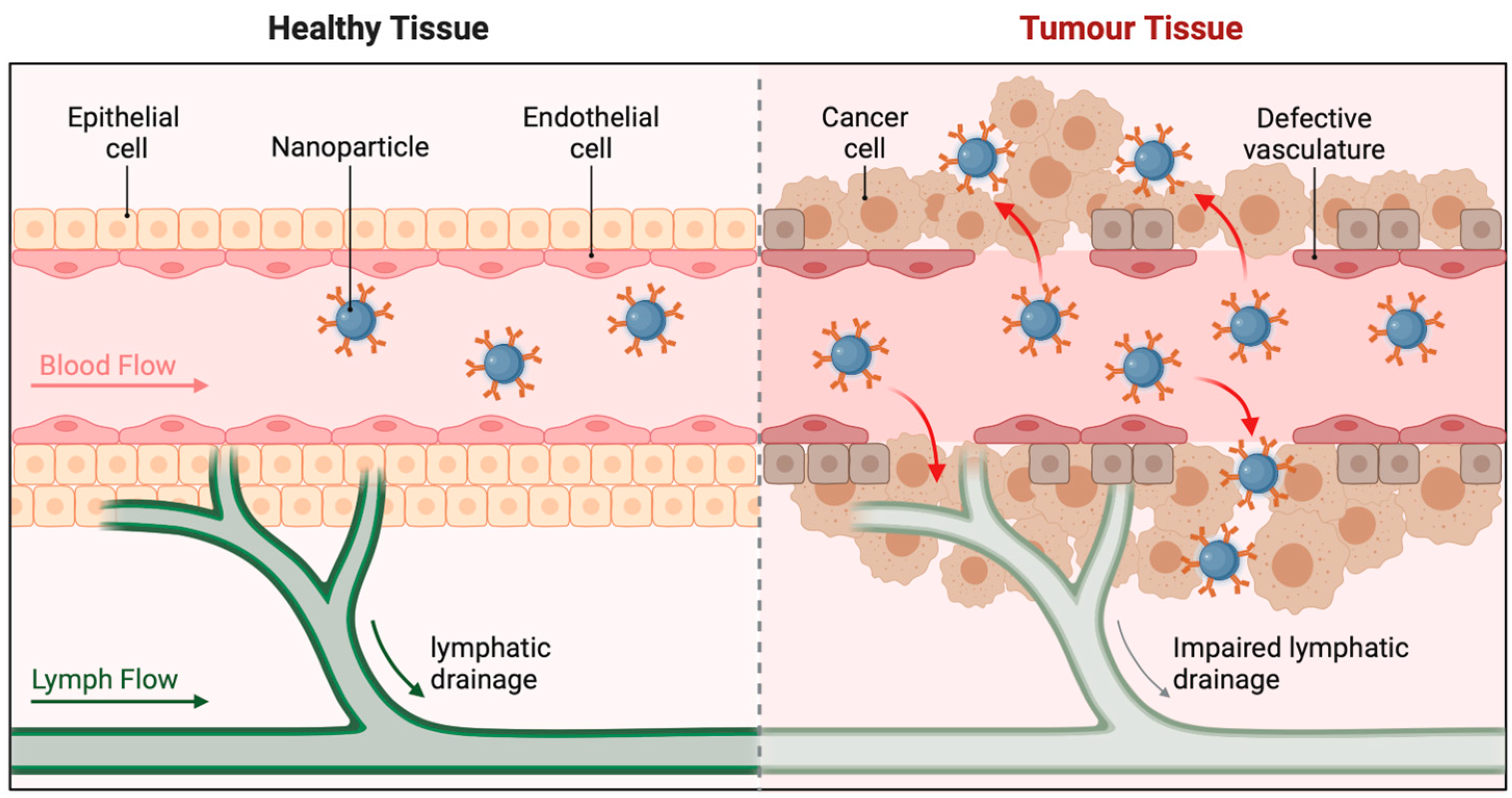

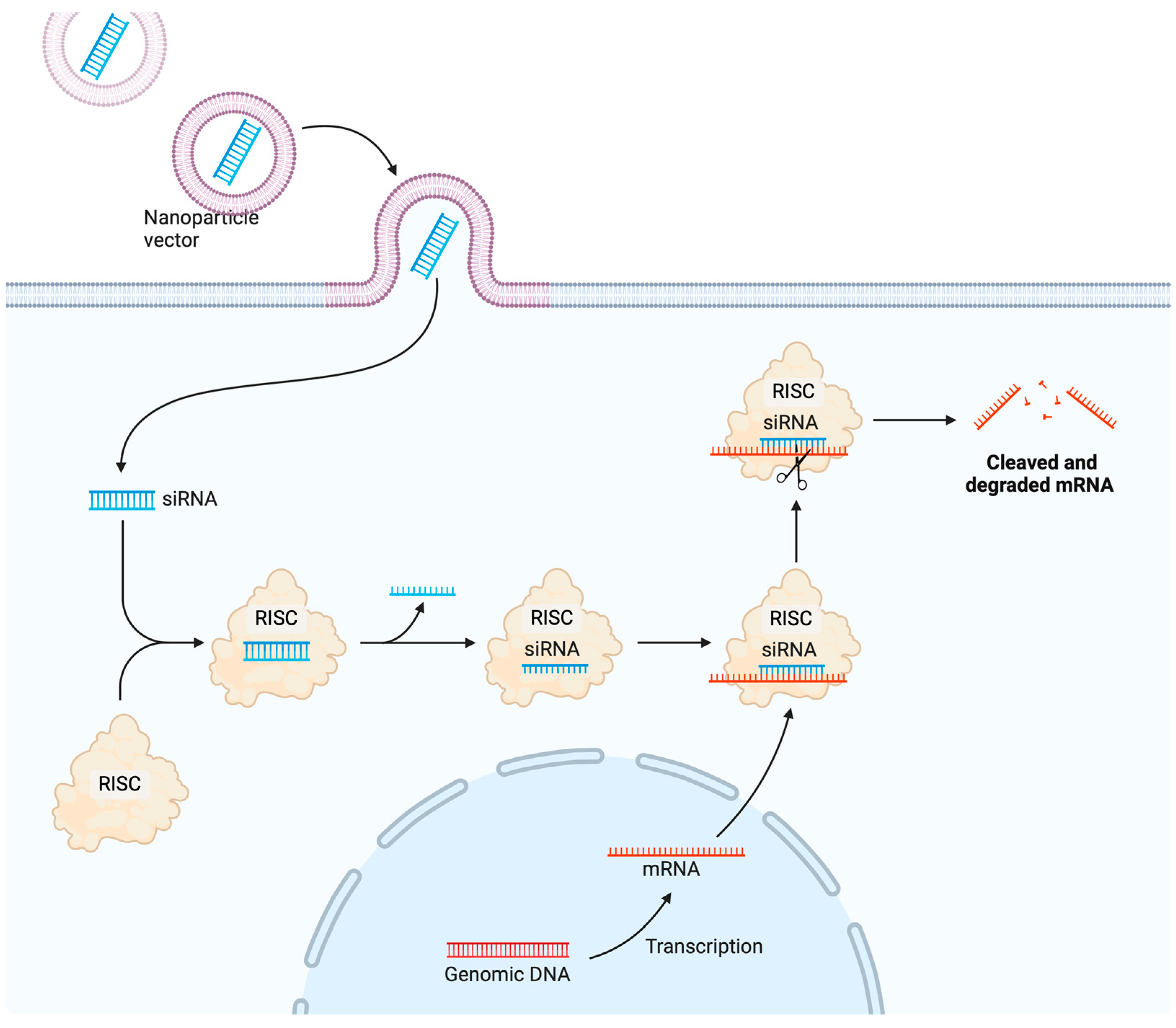
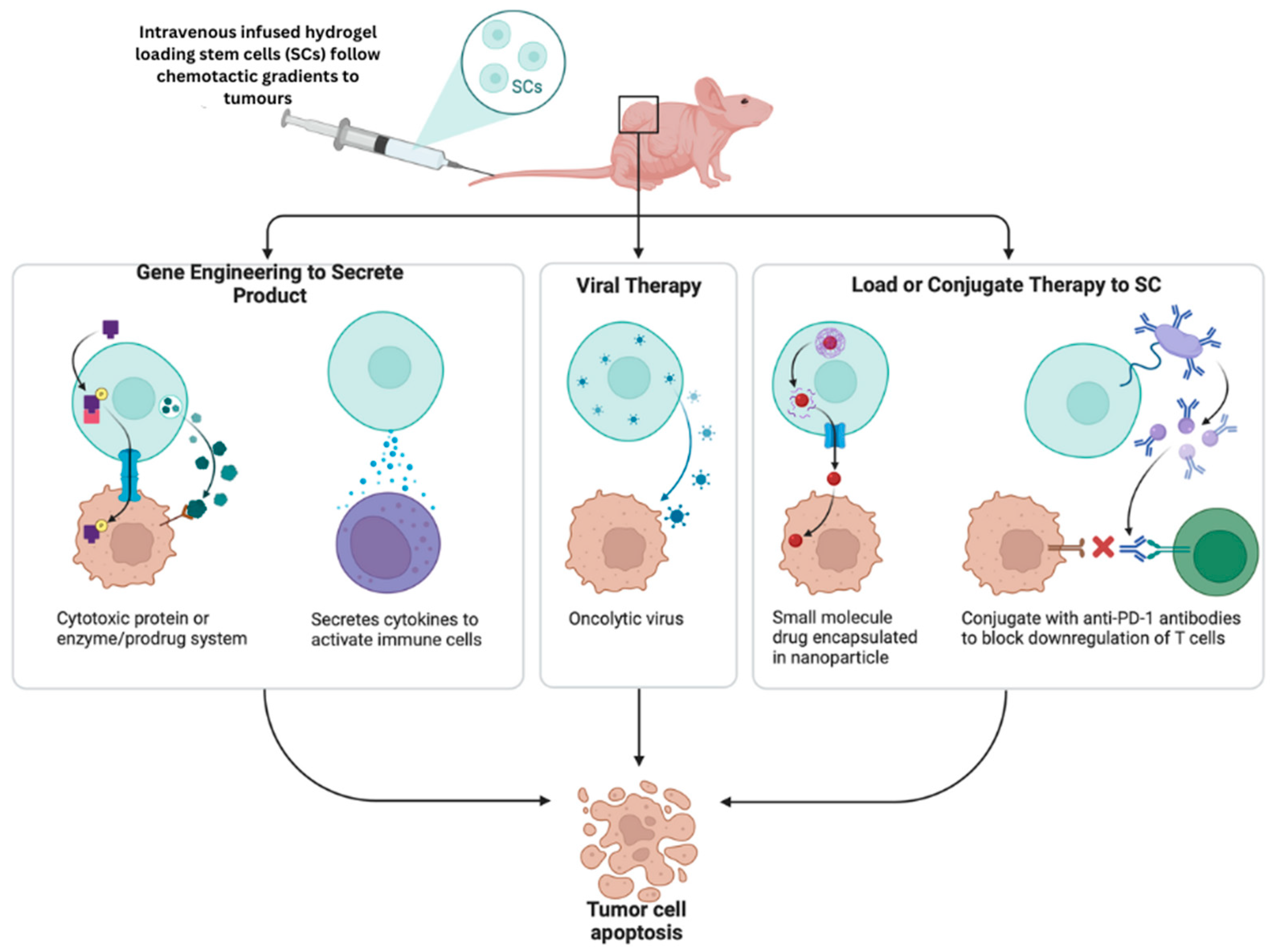

| Vaccine | Phase | Conditions | Project No. |
|---|---|---|---|
| VBI-2902a | I/II | COVID-19 | NCT04773665 |
| COVID-19 | II/III | SARS-CoV-2 infection | NCT04662697 |
| 9-Valent HPV Vaccine | III | HPV/cervical/vulvar/vaginal/genital warts | NCT00543543 |
| CHIKV VLP Vaccine | II | Chikungunya | NCT03483961 |
| NoV GI.1/GII.4 Bivalent VLP vaccine | II | Norovirus | NCT02153112 |
| V501 | III | Cervical cancer/genital warts | NCT00092534 |
| Quadrivalent VLP Influenza Vaccine | III | RNA virus/respiratory tract infections/virus illnesses | NCT03301051 |
| Novartis Meningococcal ACWY Conjugate Vaccine | III | Meningitis/HPV/pertussis/tetanus | NCT00518180 |
| RSV-F Protein Nanoparticle vaccine | II | RSV-F | NCT01960686 |
| A/H1N1 2009 Influenza VLP vaccine | II | Seasonal influenza | NCT01072799 |
| CYT006-AngQb | II | Mild essential hypertension/moderate essential hypertension | NCT00710372 |
| Formalin-inactivated EV71 vaccine | III | HFMD | NCT01569581 |
| VRC-ZKADNA090-00-VP | II | Infections include Zika, Virus, Flavivirus, Flaviviridae, and RNA viruses. | NCT03110770 |
| HIV p17 | I | HIV infections | NCT00001053 |
| Biomaterials | License Year | Commercial Name | Treatment | Indications |
|---|---|---|---|---|
| PLGA | 1999 | Neutropin subcutaneous | Microspheres | multiforme growth hormone deficiency |
| Lipid nanoparticles | 1995 | Doxil® | Chemotherapy (Doxorubicin) | multiple myeloma; breast neoplasms; ovarian neoplasms; Kaposi’s sarcoma |
| PCPP-SA | 1996 | Gliadel® | Intracranial wafer | |
| Poly (L-Lactide) | 2016 | Absorb GT1 | Vascular scaffold system | coronary artery syndrome |
| Virus-like particle | 2006 | Depot® Gardasil® | Vaccine delivery | cervical cancer |
| Polyethylene glycol-based | 2018 | Dextenza® | Insert (Dexamethasone) | amyloidosis in adults, ocular inflammation and pain |
| Lipid nanoparticles | 2018 | Onpattro® | Gene therapy (siRNA) | hereditary transthyretin-mediated polyneuropathy |
| PLGA microspheres | 1989 | Leupron Depot® | Hormone therapy | advanced prostate cancer |
| Hydrogel conjugated with fluorescein lipid nanoparticles | 2020 | Comirnaty® | Vaccine delivery | surgery SARS-CoV-2 infection |
| Crosslinked polydimethylsiloXane | 1990 | Norplant® implant | Implant (Levonorgestrel) | contraceptive |
| Drug | Date of First Approval | Application |
|---|---|---|
| Lipid-based | ||
| DaunoXome | 1996 | Kaposi’s sarcoma |
| Doxil | 1995 | Ovarian cancer, Kaposi’s sarcoma, multiple myeloma |
| Visudyne | 2000 | myopia, wet age-related macular degeneration, ocular histoplasmosis |
| AmBisome | 1997 | Fungal/protozoal infections |
| Onivyde | 2015 | Metastatic pancreatic cancer |
| Marqibo | 2012 | Acute lymphoblastic leukaemia |
| Onpattro | 2018 | Transthyretin-mediated amyloidosis |
| Vyxeos | 2017 | Acute myeloid leukaemia |
| Polymer-based | ||
| Copaxone | 1996 | Multiple sclerosis |
| Oncaspar | 1994 | Acute lymphoblastic leukaemia |
| Eligard | 2002 | Prostate cancer |
| PegIntron | 2001 | Hepatitis C infection |
| Abraxane | 2005 | Metastatic breast cancer, lung cancer, metastatic pancreatic cancer |
| Neulasta | 2002 | Neutropenia, chemotherapy induced |
| Plegridy | 2014 | Multiple sclerosis |
| Cimiza | 2008 | Rheumatoid arthritis, Crohn’s disease, psoriatic arthritis, ankylosing spondylitis |
| Adynovate | 2015 | Haemophilia |
| Inorganic | ||
| DexFerrum | 1996 | Iron-deficient anaemia |
| INFeD | 1992 | Iron-deficient anaemia |
| Venofer | 2000 | Iron deficiency in chronic kidney disease |
| Ferrlecit | 1999 | Iron deficiency in chronic kidney disease |
| Injectafer | 2013 | Iron-deficient anaemia |
| Feraheme | 2009 | Iron deficiency in chronic kidney disease |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deshmukh, R.; Sethi, P.; Singh, B.; Shiekmydeen, J.; Salave, S.; Patel, R.J.; Ali, N.; Rashid, S.; Elossaily, G.M.; Kumar, A. Recent Review on Biological Barriers and Host–Material Interfaces in Precision Drug Delivery: Advancement in Biomaterial Engineering for Better Treatment Therapies. Pharmaceutics 2024, 16, 1076. https://doi.org/10.3390/pharmaceutics16081076
Deshmukh R, Sethi P, Singh B, Shiekmydeen J, Salave S, Patel RJ, Ali N, Rashid S, Elossaily GM, Kumar A. Recent Review on Biological Barriers and Host–Material Interfaces in Precision Drug Delivery: Advancement in Biomaterial Engineering for Better Treatment Therapies. Pharmaceutics. 2024; 16(8):1076. https://doi.org/10.3390/pharmaceutics16081076
Chicago/Turabian StyleDeshmukh, Rohitas, Pranshul Sethi, Bhupendra Singh, Jailani Shiekmydeen, Sagar Salave, Ravish J. Patel, Nemat Ali, Summya Rashid, Gehan M. Elossaily, and Arun Kumar. 2024. "Recent Review on Biological Barriers and Host–Material Interfaces in Precision Drug Delivery: Advancement in Biomaterial Engineering for Better Treatment Therapies" Pharmaceutics 16, no. 8: 1076. https://doi.org/10.3390/pharmaceutics16081076









