A Method for the Colorimetric Quantification of Sodium Lauryl Sulphate in Tablets: A Proof of Concept
Abstract
1. Introduction
2. Experimental Part
2.1. Chemicals and Materials
2.2. Sample Preparation
2.3. Calibration Solutions
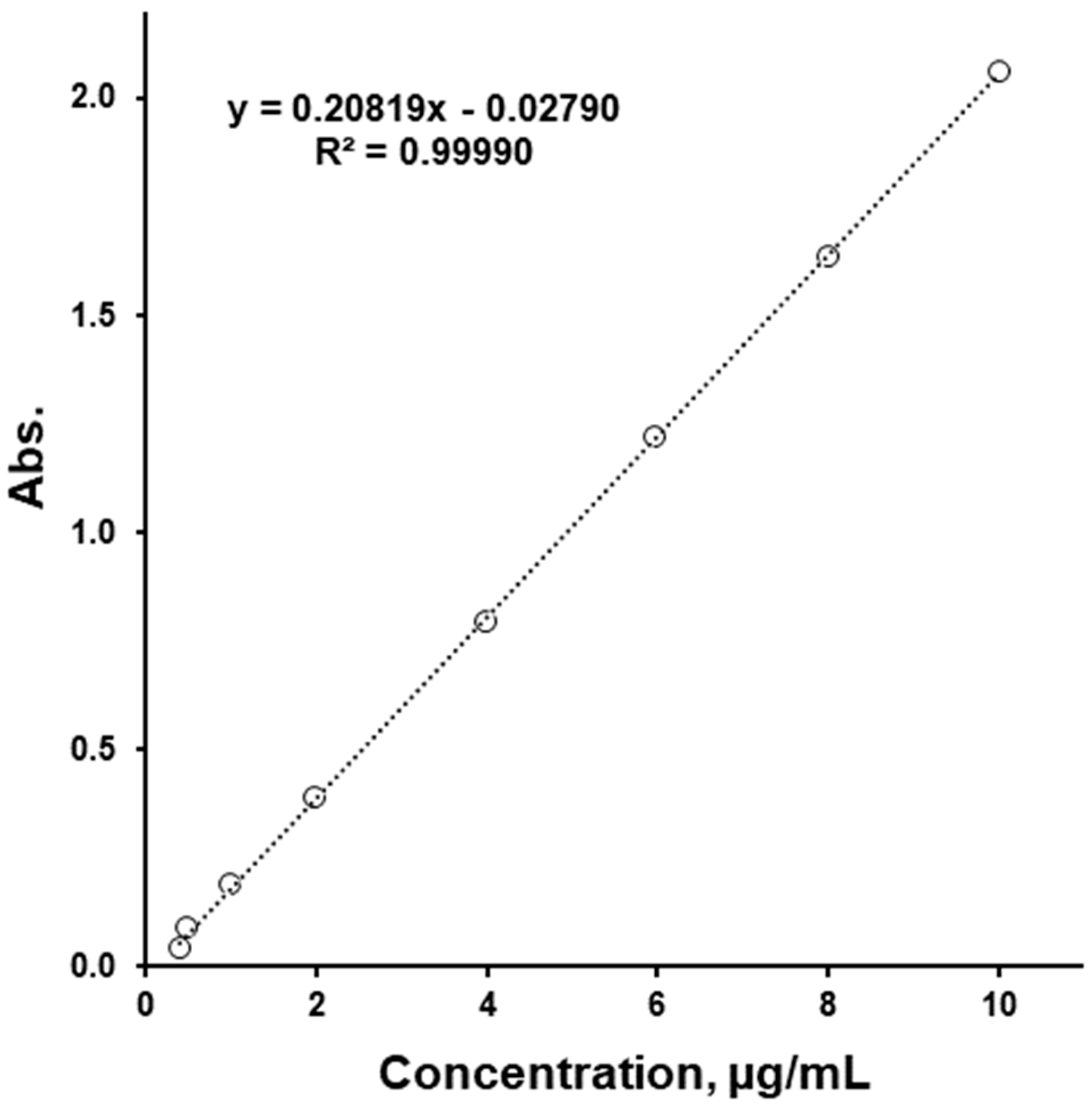
2.4. Spectrophotometry
2.5. Testing of the Method
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davit, B.; Braddy, A.C.; Conner, D.P.; Yu, L.X. International guidelines for bioequivalence of systemically available orally administered generic drug products: A survey of similarities and differences. AAPS J. 2013, 15, 974–990. [Google Scholar] [CrossRef] [PubMed]
- Inactive Ingredient Search for Approved Drug Products (Quarterly Renewed); Office of Pharmaceutical Quality, Center for Drug Evaluation and Research, U.S. FDA: Silver Spring, MD, USA, 2024. Available online: https://www.accessdata.fda.gov/scripts/cder/iig/index.cfm (accessed on 17 August 2024).
- Ren, P.; Chan, T.; Yang, W.-C.; Frost, M.; Wang, Y.; Luke, M.; Kim, M.-J.; Lionberger, R.; Zhang, Y. Effect of the Similarity of Formulations and Excipients of Approved Generic Drug Products on In Vivo Bioequivalence for Putative Biopharmaceutics Classification System Class III Drugs. Pharmaceutics 2023, 15, 2366. [Google Scholar] [CrossRef] [PubMed]
- Sabbatini, B.; Perinelli, D.R.; Palmieri, G.F.; Cespi, M.; Bonacucina, G. Sodium lauryl sulfate as lubricant in tablets formulations: Is it worth? Int. J. Pharm. 2023, 643, 123265. [Google Scholar] [PubMed]
- Zhang, Y. Essential Elements of BCS III-Based Biowaiver Request; Office of Research and Standards, Office of Generic Drugs, CDER, U.S. FDA: Silver Spring, MD, USA, 2022. Available online: https://www.fda.gov/media/166153/download (accessed on 17 August 2024).
- Ates, M.; Kaynak, M.S.; Sahin, S. Effect of permeability enhancers on paracellular permeability of acyclovir. J. Pharm. Pharmacol. 2016, 68, 781–790. [Google Scholar] [CrossRef] [PubMed]
- Anderberg, E.K.; Artursson, P. Epithelial transport of drugs in cell culture. VIII: Effects of sodium dodecyl sulfate on cell membrane and tight junction permeability in human intestinal epithelial (Caco-2) cells. J. Pharm. Sci. 1993, 82, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Siegel, I.A.; Gordon, H.P. Effects of surfactants on the permeability of canine oral mucosa in vitro. Toxicol. Lett. 1985, 26, 153–158. [Google Scholar] [CrossRef] [PubMed]
- Gulsun, T.; Izat, N.; Sahin, S. Influence of permeability enhancers on the paracellular permeability of metformin hydrochloride and furosemide across Caco-2 cells. Can. J. Physiol. Pharmacol. 2023, 101, 185–199. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, H. Gas chromatographic determination of sodium dodecyl sulfate. Anal. Biochem. 1974, 57, 564–568. [Google Scholar] [CrossRef] [PubMed]
- Shende, N.; Karale, A.; Marathe, P.; Chakraborty, S.; Mallya, A.D.; Dhere, R.M. Quantitation of residual sodium dodecyl sulfate in meningococcal polysaccharide by gas chromatography-mass spectrometry. Biologicals 2019, 60, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Campbell, R.; Winkler, M.A.; Wu, H. Quantification of sodium dodecyl sulfate in microliter biochemical samples by gas chromatography. Anal. Biochem. 2004, 335, 98–102. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Ramos, C.; Ballesteros, O.; Blanc, R.; Zafra-Gómez, A.; Jiménez-Díaz, I.; Navalón, A.; Vílchez, J. Determination of alcohol sulfates in wastewater treatment plant influents and effluents by gas chromatography-mass spectrometry. Talanta 2012, 98, 166–171. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, K.; Wojciechowski, K. Precursor ion approach for simultaneous determination of nonethoxylated and ethoxylated alkylsulfate surfactants. J. Chromatogr. A 2021, 1653, 462421. [Google Scholar] [CrossRef] [PubMed]
- Arand, M.; Friedberg, T.; Oesch, F. Colorimetric quantitation of trace amounts of sodium lauryl sulfate in the presence of nucleic acids and proteins. Anal. Biochem. 1992, 207, 73–75. [Google Scholar] [CrossRef] [PubMed]
- Sar, S.K.; Verma, C.; Pandey, P.K.; Bhui, A. Reliable Technique for the Determination of Sodium Dodecyl Sulphate by Crystal Violet in Relation to the Effluents of Durg-Bhilai Region. J. Chin. Chem. Soc. 2013, 56, 1250–1256. [Google Scholar] [CrossRef]
- Kakalejčíková, S.; Bazeľ, Y. A combination of vortex-assisted liquid–liquid microextraction with fluorescence detection: An innovative approach for a green and highly sensitive determination of sodium dodecyl sulfate in water samples and pharmaceuticals. Microchem. J. 2024, 199, 110226. [Google Scholar] [CrossRef]
- Ghiasvand, A.R.; Taherimaslak, Z.; Allahyari, M. Sensitive and Selective Spectrophotometric and a new Adsorptive Stripping Voltammetric Determination of Sodium Dodecyl Sulfate in Nonaqueous Solution after its Extraction Using Toluidine Blue. Int. J. Electrochem. Sci. 2009, 4, 320–335. [Google Scholar] [CrossRef]
- Alizadeh, M.N.; Shayanfar, A.; Jouyban, A. Solubilization of drugs using sodium lauryl sulfate: Experimental data and modeling. J. Mol. Liquids 2018, 268, 410–414. [Google Scholar] [CrossRef]

| Ingredients | “50/1000” | “50/850” | “50/500” | |||
|---|---|---|---|---|---|---|
| mg | w/w % | mg | w/w % | mg | w/w % | |
| Sitagliptin phosphate monohydrate | 64.3 | 4.8 | 64.3 | 5.6 | 64.3 | 9.1 |
| Metformin hydrochloride | 1000.0 | 74.6 | 850.0 | 73.9 | 500.0 | 70.4 |
| Microcrystaline cellulose | ≈137.4 | ≈10.3 | ≈117.0 | ≈10.2 | ≈72.4 | ≈10.2 |
| Polyvinylpyrrolidone | ≈51.5 | ≈3.8 | 44.2 | ≈3.8 | ≈27.3 | ≈3.8 |
| Sodium lauryl sulphate | U | U | U | U | U | U |
| Sodium stearyl fumarate | ≈26.8 | ≈2.0 | ≈23.0 | ≈2.0 | ≈14.2 | ≈2.0 |
| Film coating | ≈53.6 | ≈4.0 | ≈46.0 | ≈4.0 | ≈28.4 | ≈4.0 |
| ∑ | 1340.0 | 100.0 | 1150.0 | 100.0 | 710.0 | 100.0 |
| Calibration Level | Concentration of the Stock Solution Used | Volume of Stock Solution Added | Volume of Water Added | Concentration of the Calibration Solution |
|---|---|---|---|---|
| µg/mL | µL | µL | µg/mL | |
| 1 | 50.00 (stock solution 2) | 80 | 9920 | 0.40 |
| 2 | 100 | 9900 | 0.50 | |
| 3 | 200 | 9800 | 1.00 | |
| 4 | 400 | 9600 | 2.00 | |
| 5 | 500.0 (stock solution 1) | 80 | 9920 | 4.00 |
| 6 | 120 | 9880 | 6.00 | |
| 7 | 160 | 9840 | 8.00 | |
| 8 | 200 | 9800 | 10.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paulausks, A.; Mazurs, A.; Mohylyuk, V. A Method for the Colorimetric Quantification of Sodium Lauryl Sulphate in Tablets: A Proof of Concept. Pharmaceutics 2024, 16, 1100. https://doi.org/10.3390/pharmaceutics16081100
Paulausks A, Mazurs A, Mohylyuk V. A Method for the Colorimetric Quantification of Sodium Lauryl Sulphate in Tablets: A Proof of Concept. Pharmaceutics. 2024; 16(8):1100. https://doi.org/10.3390/pharmaceutics16081100
Chicago/Turabian StylePaulausks, Artūrs, Austris Mazurs, and Valentyn Mohylyuk. 2024. "A Method for the Colorimetric Quantification of Sodium Lauryl Sulphate in Tablets: A Proof of Concept" Pharmaceutics 16, no. 8: 1100. https://doi.org/10.3390/pharmaceutics16081100
APA StylePaulausks, A., Mazurs, A., & Mohylyuk, V. (2024). A Method for the Colorimetric Quantification of Sodium Lauryl Sulphate in Tablets: A Proof of Concept. Pharmaceutics, 16(8), 1100. https://doi.org/10.3390/pharmaceutics16081100







