Polyphenolic Nanomedicine Regulating Mitochondria REDOX for Innovative Cancer Treatment
Abstract
:1. Introduction
| Polyphenols | Mechanisms on REDOXResponsiveness | Mitochondrial Damage | Mitochondrial Apoptosis Pathway | Cancer Cell | Ref. |
|---|---|---|---|---|---|
| EGCG | ROS generation | Altered mitochondrial transmembrane potentials | Altered Bcl-2 family proteins, cytochrome C release, and activation of caspase 3 and caspase 9 | Hepatocarcinoma SMMC7721 cells | [36] |
| Curcumin | superoxide anion O2- production | Mitochondrial DNA damage, disruption of mitochondrial membrane potential | Cytochrome C release into the cytosol | HepG2 hepatocellular carcinoma cells | [37] |
| Dimethoxycurcumin | ROS generation | Reduced mitochondrial membrane potential | Decrease in cellular energy status (ATP/ADP) | Human breast carcinoma MCF7 cells | [38] |
| Resveratrol | ROS generation | Mitochondrial membrane potential loss | Decrease in glutathione levels, along with reduced mRNA expression and activity of superoxide dismutase | T cell leukemia Jurkat cells | [39] |
| trans-Resveratrol | Superoxide anions generation | — | Increasing in caspase 3-like activity | Human colorectal carcinoma HT-29 cells | [40] |
| Resveratrol derivatives | ROS generation | Mitochondrial depolarization | — | Colon cancer CT-26 cell | [41] |
| Quercetin | ROS generation | — | Elevated expression and activity of caspase 9 | Human glioblastoma A172 cell | [42] |
| Oroxylin A | ROS generation | — | Elevated levels of SIRT3 in mitochondria, and the detachment of mitochondrial hexokinase II and the inhibition of glycolysis | Human breast carcinoma cell | [43] |
| Kaempferol | ROS generation | Altered mitochondrial membrane potential | Decreased expression of Bcl-2, elevated active caspase 3 and cleaved poly (ADP-ribose) polymerase expression | Human glioblastoma cells | [44] |
| Flavonoid LW-214 | ROS generation | Mitochondrial membrane potential loss | Increased Bax/Bcl-2 ratio, caspase 9 activation, degradation of poly (ADP-ribose) polymerase (PARP), cytochrome C release and apoptosis-inducing factor transposition | Human breast cancer MCF-7 cells | [45] |
| Novel isoflavone derivative, NV-128 | Superoxide and hydrogen peroxide generation | — | Decreasing in ATP, Cox-I, and Cox-IV levels | CD44+/MyD88+ ovarian cancer stem cells | [46] |
| Hesperidin | ROS generation | Mitochondrial membrane potential loss | Enhanced cytochrome C and apoptosis-inducing factor release from mitochondria, and caspase 3 activation. | HeLa cells | [47] |
| Naringenin | ROS generation | Mitochondrial depolarization | — | human epidermoid carcinoma A431 cells | [48] |
| Apigenin | ROS generation | Disruption of mitochondrial membrane potential | Glutathione depletion, cytosolic release of cytochrome C | Human prostate cancer 22Rv1 cells | [49] |
| luteolin | ROS generation | Mitochondrial membrane potential loss, and mitochondrial swelling | Release of cytochrome C | Hepatocellular carcinoma cells | [50] |
| Hesperetin | ROS generation | Reduced mitochondrial membrane potential | Increase in cytochrome C | Colon adenocarcinoma HT-29 cells | [51] |
| Chrysophanol | ROS generation | Reduced mitochondrial membrane potential | — | A549 human lung cancer cells | [52] |
| Agrimoniin | ROS generation | Disruption of mitochondrial membrane potential | — | Pancreatic cancer cells | [53] |
| 3-deoxysappanchalcone | ROS generation | Mitochondrial membrane potential depolarization | — | Esophageal Squamous cell carcinoma ESCC cells | [54] |
| Calycosin | ROS generation | Reduced mitochondrial membrane potential | Decreased the expression of Bcl-2 and increased the expression of Bax, caspase 3, and poly (ADP ribose) polymerase | HepG2 hepatocellular carcinoma cells | [55] |
| Chlorogenic Acid | Superoxide (O2•-) | Reduced mitochondrial membrane potential, changes in mitochondrial morphology | — | MCF-7, MDA-MB-231, and HCC1419 breast cancer cells | [56] |
| Genistein | ROS generation | Decreased mitochondrial membrane potential, decrease mitochondrial activity | Up-regulated expression cytochrome C and Bax, decreased the expression of Bcl-2 | Non-small lung cancer A549 and 95D cells | [57] |
| Gallic acid | ROS generation | Mitochondrial respiratory inhibition | Reduced ATP levels | Acute myeloid leukemia cells | [58] |
| Tannic acid | ROS generation | Reduced mitochondrial membrane potential | Reduced ATP levels, the activation of the death ligand TRAIL | Human embryonic carcinoma cells | [59] |
| Gossypol | ROS generation | Mitochondrial membrane potential loss, | Release of cytochrome C and apoptosis-inducing factor from mitochondria to the cytoplasm | human colorectal carcinoma cells | [60] |
2. Polyphenolic Nanomedicine
2.1. Polyphenol Self-Assembly Nanomedicine
2.2. Metal-Phenolic Network Nanomedicine

2.3. Polyphenol–Protein Nanomedicine
2.4. Polyphenol-Hydrogel Nanomedicine
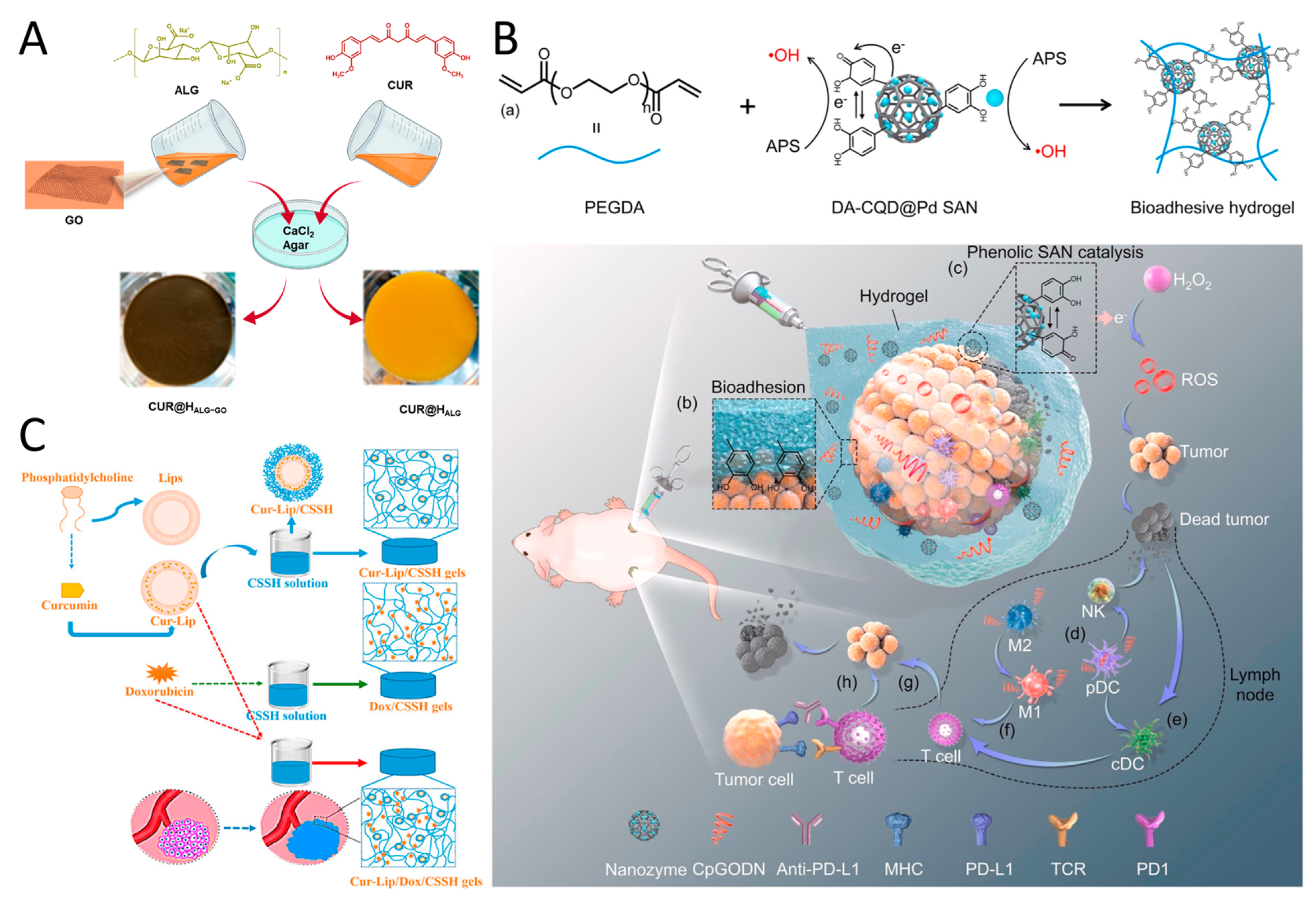
2.5. Polyphenol–Chitosan Nanomedicine
2.6. Polyphenol–Liposome Nanomedicine
2.7. Other Polyphenolic Nanomedicine
3. Conclusions and Outlooks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Gong, L.; Zhang, Y.; Liu, C.; Zhang, M.; Han, S. Application of Radiosensitizers in Cancer Radiotherapy. Int. J. Nanomed. 2021, 16, 1083–1102. [Google Scholar] [CrossRef]
- Mahoney, K.M.; Rennert, P.D.; Freeman, G.J. Combination cancer immunotherapy and new immunomodulatory targets. Nat. Rev. Drug Discov. 2015, 14, 561–584. [Google Scholar] [CrossRef] [PubMed]
- Keklikoglou, I.; Cianciaruso, C.; Güç, E.; Squadrito, M.L.; Spring, L.M.; Tazzyman, S.; Lambein, L.; Poissonnier, A.; Ferraro, G.B.; Baer, C.; et al. Chemotherapy elicits pro-metastatic extracellular vesicles in breast cancer models. Nat. Cell Biol. 2019, 21, 190–202. [Google Scholar] [CrossRef]
- Shi, J.; Kantoff, P.W.; Wooster, R.; Farokhzad, O.C. Cancer nanomedicine: Progress, challenges and opportunities. Nat. Rev. Cancer 2017, 17, 20–37. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, V.P.; Jain, R.K. Strategies for advancing cancer nanomedicine. Nat. Mater. 2013, 12, 958–962. [Google Scholar] [CrossRef]
- Wicki, A.; Witzigmann, D.; Balasubramanian, V.; Huwyler, J. Nanomedicine in cancer therapy: Challenges, opportunities, and clinical applications. J. Control. Release 2015, 200, 138–157. [Google Scholar] [CrossRef] [PubMed]
- Lei, H.; Pei, Z.; Jiang, C.; Cheng, L. Recent progress of metal-based nanomaterials with anti-tumor biological effects for enhanced cancer therapy. Exploration 2023, 3, 20220001. [Google Scholar] [CrossRef]
- Feng, Y.; Wang, J.; Cao, J.; Cao, F.; Chen, X. Manipulating calcium homeostasis with nanoplatforms for enhanced cancer therapy. Exploration 2023, 4, 20230019. [Google Scholar] [CrossRef]
- He, H.; Liu, L.; Morin, E.E.; Liu, M.; Schwendeman, A. Survey of Clinical Translation of Cancer Nanomedicines-Lessons Learned from Successes and Failures. Acc. Chem. Res. 2019, 52, 2445–2461. [Google Scholar] [CrossRef]
- Gogvadze, V.; Orrenius, S.; Zhivotovsky, B. Mitochondria in cancer cells: What is so special about them? Trends Cell Biol. 2008, 18, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Raut, G.K.; Chakrabarti, M.; Pamarthy, D.; Bhadra, M.P. Glucose starvation-induced oxidative stress causes mitochondrial dysfunction and apoptosis via Prohibitin 1 upregulation in human breast cancer cells. Free Radic. Biol. Med. 2019, 145, 428–441. [Google Scholar] [CrossRef]
- DeNicola, G.M.; Karreth, F.A.; Humpton, T.J.; Gopinathan, A.; Wei, C.; Frese, K.; Mangal, D.; Yu, K.H.; Yeo, C.J.; Calhoun, E.S.; et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature 2011, 475, 106–109. [Google Scholar] [CrossRef] [PubMed]
- Ippolito, L.; Giannoni, E.; Chiarugi, P.; Parri, M. Mitochondrial Redox Hubs as Promising Targets for Anticancer Therapy. Front. Oncol. 2020, 10, 256. [Google Scholar] [CrossRef]
- Kroemer, G.; Galluzzi, L.; Brenner, C. Mitochondrial membrane permeabilization in cell death. Physiol. Rev. 2007, 87, 99–163. [Google Scholar] [CrossRef] [PubMed]
- Neuzil, J.; Dong, L.F.; Rohlena, J.; Truksa, J.; Ralph, S.J. Classification of mitocans, anti-cancer drugs acting on mitochondria. Mitochondrion 2013, 13, 199–208. [Google Scholar] [CrossRef] [PubMed]
- Cheng, G.; Zielonka, J.; Ouari, O.; Lopez, M.; McAllister, D.; Boyle, K.; Barrios, C.S.; Weber, J.J.; Johnson, B.D.; Hardy, M.; et al. Mitochondria-Targeted Analogues of Metformin Exhibit Enhanced Antiproliferative and Radiosensitizing Effects in Pancreatic Cancer Cells. Cancer Res. 2016, 76, 3904–3915. [Google Scholar] [CrossRef] [PubMed]
- Tao, L.; Forester, S.C.; Lambert, J.D. The role of the mitochondrial oxidative stress in the cytotoxic effects of the green tea catechin, (-)-epigallocatechin-3-gallate, in oral cells. Mol. Nutr. Food Res. 2014, 58, 665–676. [Google Scholar] [CrossRef] [PubMed]
- Satoh, M.; Takemura, Y.; Hamada, H.; Sekido, Y.; Kubota, S. EGCG induces human mesothelioma cell death by inducing reactive oxygen species and autophagy. Cancer Cell Int. 2013, 13, 19. [Google Scholar] [CrossRef]
- Hsu, S.; Bollag, W.B.; Lewis, J.; Huang, Q.; Singh, B.; Sharawy, M.; Yamamoto, T.; Schuster, G. Green tea polyphenols induce differentiation and proliferation in epidermal keratinocytes. J. Pharmacol. Exp. Ther. 2003, 306, 29–34. [Google Scholar] [CrossRef]
- Tao, L.; Park, J.Y.; Lambert, J.D. Differential prooxidative effects of the green tea polyphenol, (-)-epigallocatechin-3-gallate, in normal and oral cancer cells are related to differences in sirtuin 3 signaling. Mol. Nutr. Food Res. 2015, 59, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, T.; Hsu, S.; Lewis, J.; Wataha, J.; Dickinson, D.; Singh, B.; Bollag, W.B.; Lockwood, P.; Ueta, E.; Osaki, T.; et al. Green tea polyphenol causes differential oxidative environments in tumor versus normal epithelial cells. J. Pharmacol. Exp. Ther. 2003, 307, 230–236. [Google Scholar] [CrossRef]
- Llana-Ruiz-Cabello, M.; Gutiérrez-Praena, D.; Puerto, M.; Pichardo, S.; Jos, Á.; Cameán, A.M. In vitro pro-oxidant/antioxidant role of carvacrol, thymol and their mixture in the intestinal Caco-2 cell line. Toxicol. Vitro 2015, 29, 647–656. [Google Scholar] [CrossRef]
- Liang, W.Z.; Lu, C.H. Carvacrol-induced [Ca2+]i rise and apoptosis in human glioblastoma cells. Life Sci. 2012, 90, 703–711. [Google Scholar] [CrossRef] [PubMed]
- Günes-Bayir, A.; Kocyigit, A.; Güler, E.M.; Bilgin, M.G.; Ergün, İ.S.; Dadak, A. Effects of carvacrol on human fibroblast (WS-1) and gastric adenocarcinoma (AGS) cells in vitro and on Wistar rats in vivo. Mol. Cell. Biochem. 2018, 448, 237–249. [Google Scholar] [CrossRef] [PubMed]
- Hou, Z.; Sang, S.; You, H.; Lee, M.J.; Hong, J.; Chin, K.V.; Yang, C.S. Mechanism of action of (-)-epigallocatechin-3-gallate: Auto-oxidation-dependent inactivation of epidermal growth factor receptor and direct effects on growth inhibition in human esophageal cancer KYSE 150 cells. Cancer Res. 2005, 65, 8049–8056. [Google Scholar] [CrossRef] [PubMed]
- Li, G.X.; Chen, Y.K.; Hou, Z.; Xiao, H.; Jin, H.; Lu, G.; Lee, M.J.; Liu, B.; Guan, F.; Yang, Z.; et al. Pro-oxidative activities and dose-response relationship of (-)-epigallocatechin-3-gallate in the inhibition of lung cancer cell growth: A comparative study in vivo and in vitro. Carcinogenesis 2010, 31, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.Y.; Liao, J.; Li, C.; Chung, J.; Yurkow, E.J.; Ho, C.T.; Yang, C.S. Effect of black and green tea polyphenols on c-jun phosphorylation and H2O2 production in transformed and non-transformed human bronchial cell lines: Possible mechanisms of cell growth inhibition and apoptosis induction. Carcinogenesis 2000, 21, 2035–2039. [Google Scholar] [CrossRef]
- Yang, Q.Q.; Wei, X.L.; Fang, Y.P.; Gan, R.Y.; Wang, M.; Ge, Y.Y.; Zhang, D.; Cheng, L.Z.; Corke, H. Nanochemoprevention with therapeutic benefits: An updated review focused on epigallocatechin gallate delivery. Crit. Rev. Food Sci. Nutr. 2020, 60, 1243–1264. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Gámez-Meza, N.; Molina-Domínguez, C.C.; Hayano-Kanashiro, C.; Medina-Juárez, L.A. Simulated Gastrointestinal Digestion, Bioaccessibility and Antioxidant Capacity of Polyphenols from Red Chiltepin (Capsicum annuum L. Var. glabriusculum) Grown in Northwest Mexico. Plant Foods Hum. Nutr. 2018, 73, 116–121. [Google Scholar] [CrossRef]
- Dai, Q.; Geng, H.; Yu, Q.; Hao, J.; Cui, J. Polyphenol-Based Particles for Theranostics. Theranostics 2019, 9, 3170–3190. [Google Scholar] [CrossRef]
- Jia, W.; Zhou, L.; Li, L.; Zhou, P.; Shen, Z. Nano-Based Drug Delivery of Polyphenolic Compounds for Cancer Treatment: Progress, Opportunities, and Challenges. Pharmaceuticals 2023, 16, 101. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; He, J.; Qiao, L.; Wang, C.; Wang, Y.; Yu, R.; Xu, W.; Wang, F.; Yang, S.; Zhang, X.; et al. Multifunctional Dual Network Hydrogel Loaded with Novel Tea Polyphenol Magnesium Nanoparticles Accelerates Wound Repair of MRSA Infected Diabetes. Adv. Funct. Mater. 2024. [Google Scholar] [CrossRef]
- Ding, Y.; Wang, Y.; Hu, Q. Recent advances in overcoming barriers to cell-based delivery systems for cancer immunotherapy. Exploration 2022, 2, 20210106. [Google Scholar] [CrossRef] [PubMed]
- Corma, A.; Botella, P.; Rivero-Buceta, E. Silica-Based Stimuli-Responsive Systems for Antitumor Drug Delivery and Controlled Release. Pharmaceutics 2022, 14, 110. [Google Scholar] [CrossRef]
- Li, W.; Nie, S.; Yu, Q.; Xie, M. (-)-Epigallocatechin-3-gallate induces apoptosis of human hepatoma cells by mitochondrial pathways related to reactive oxygen species. J. Agric. Food Chem. 2009, 57, 6685–6691. [Google Scholar] [CrossRef]
- Cao, J.; Liu, Y.; Jia, L.; Zhou, H.M.; Kong, Y.; Yang, G.; Jiang, L.P.; Li, Q.J.; Zhong, L.F. Curcumin induces apoptosis through mitochondrial hyperpolarization and mtDNA damage in human hepatoma G2 cells. Free Radic. Biol. Med. 2007, 43, 968–975. [Google Scholar] [CrossRef] [PubMed]
- Kunwar, A.; Jayakumar, S.; Srivastava, A.K.; Priyadarsini, K.I. Dimethoxycurcumin-induced cell death in human breast carcinoma MCF7 cells: Evidence for pro-oxidant activity, mitochondrial dysfunction, and apoptosis. Arch. Toxicol. 2012, 86, 603–614. [Google Scholar] [CrossRef]
- Kucinska, M.; Piotrowska, H.; Luczak, M.W.; Mikula-Pietrasik, J.; Ksiazek, K.; Wozniak, M.; Wierzchowski, M.; Dudka, J.; Jäger, W.; Murias, M. Effects of hydroxylated resveratrol analogs on oxidative stress and cancer cells death in human acute T cell leukemia cell line: Prooxidative potential of hydroxylated resveratrol analogs. Chem. Biol. Interact. 2014, 209, 96–110. [Google Scholar] [CrossRef]
- Juan, M.E.; Wenzel, U.; Daniel, H.; Planas, J.M. Resveratrol induces apoptosis through ROS-dependent mitochondria pathway in HT-29 human colorectal carcinoma cells. J. Agric. Food Chem. 2008, 56, 4813–4818. [Google Scholar] [CrossRef]
- Sassi, N.; Mattarei, A.; Azzolini, M.; Bernardi, P.; Szabo, I.; Paradisi, C.; Zoratti, M.; Biasutto, L. Mitochondria-targeted resveratrol derivatives act as cytotoxic pro-oxidants. Curr. Pharm. Des. 2014, 20, 172–179. [Google Scholar] [CrossRef] [PubMed]
- Kusaczuk, M.; Krętowski, R.; Naumowicz, M.; Stypułkowska, A.; Cechowska-Pasko, M. A Preliminary Study of the Effect of Quercetin on Cytotoxicity, Apoptosis, and Stress Responses in Glioblastoma Cell Lines. Int. J. Mol. Sci. 2022, 23, 1345. [Google Scholar] [CrossRef]
- Wei, L.; Zhou, Y.; Dai, Q.; Qiao, C.; Zhao, L.; Hui, H.; Lu, N.; Guo, Q.L. Oroxylin A induces dissociation of hexokinase II from the mitochondria and inhibits glycolysis by SIRT3-mediated deacetylation of cyclophilin D in breast carcinoma. Cell Death Dis. 2013, 4, e601. [Google Scholar] [CrossRef] [PubMed]
- Sharma, V.; Joseph, C.; Ghosh, S.; Agarwal, A.; Mishra, M.K.; Sen, E. Kaempferol induces apoptosis in glioblastoma cells through oxidative stress. Mol. Cancer Ther. 2007, 6, 2544–2553. [Google Scholar] [CrossRef]
- Pan, D.; Li, W.; Miao, H.; Yao, J.; Li, Z.; Wei, L.; Zhao, L.; Guo, Q. LW-214, a newly synthesized flavonoid, induces intrinsic apoptosis pathway by down-regulating Trx-1 in MCF-7 human breast cells. Biochem. Pharmacol. 2014, 87, 598–610. [Google Scholar] [CrossRef] [PubMed]
- Alvero, A.B.; Montagna, M.K.; Holmberg, J.C.; Craveiro, V.; Brown, D.; Mor, G. Targeting the mitochondria activates two independent cell death pathways in ovarian cancer stem cells. Mol. Cancer Ther. 2011, 10, 1385–1393. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, H.; Zhang, J.; Gao, J.; Ge, X.; Lou, G. Hesperidin inhibits HeLa cell proliferation through apoptosis mediated by endoplasmic reticulum stress pathways and cell cycle arrest. BMC Cancer 2015, 15, 682. [Google Scholar] [CrossRef]
- Ahamad, M.S.; Siddiqui, S.; Jafri, A.; Ahmad, S.; Afzal, M.; Arshad, M. Induction of apoptosis and antiproliferative activity of naringenin in human epidermoid carcinoma cell through ROS generation and cell cycle arrest. PLoS ONE 2014, 9, e110003. [Google Scholar] [CrossRef]
- Shukla, S.; Gupta, S. Apigenin-induced prostate cancer cell death is initiated by reactive oxygen species and p53 activation. Free Radic. Biol. Med. 2008, 44, 1833–1845. [Google Scholar] [CrossRef]
- Seydi, E.; Salimi, A.; Rasekh, H.R.; Mohsenifar, Z.; Pourahmad, J. Selective Cytotoxicity of Luteolin and Kaempferol on Cancerous Hepatocytes Obtained from Rat Model of Hepatocellular Carcinoma: Involvement of ROS-Mediated Mitochondrial Targeting. Nutr. Cancer 2018, 70, 594–604. [Google Scholar] [CrossRef]
- Sivagami, G.; Vinothkumar, R.; Bernini, R.; Preethy, C.P.; Riyasdeen, A.; Akbarsha, M.A.; Menon, V.P.; Nalini, N. Role of hesperetin (a natural flavonoid) and its analogue on apoptosis in HT-29 human colon adenocarcinoma cell line—A comparative study. Food Chem. Toxicol. 2012, 50, 660–671. [Google Scholar] [CrossRef] [PubMed]
- Ni, C.H.; Yu, C.S.; Lu, H.F.; Yang, J.S.; Huang, H.Y.; Chen, P.Y.; Wu, S.H.; Ip, S.W.; Chiang, S.Y.; Lin, J.G.; et al. Chrysophanol-induced cell death (necrosis) in human lung cancer A549 cells is mediated through increasing reactive oxygen species and decreasing the level of mitochondrial membrane potential. Environ. Toxicol. 2014, 29, 740–749. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, Y.; Wang, Y.; Zhang, H.; Wang, X.; Tang, H.; Huang, H.; Zhou, Z.; Chen, B.; Sun, L. Agrimoniin sensitizes pancreatic cancer to apoptosis through ROS-mediated energy metabolism dysfunction. Phytomedicine 2022, 96, 153807. [Google Scholar] [CrossRef] [PubMed]
- Kwak, A.W.; Lee, M.J.; Lee, M.H.; Yoon, G.; Cho, S.S.; Chae, J.I.; Shim, J.H. The 3-deoxysappanchalcone induces ROS-mediated apoptosis and cell cycle arrest via JNK/p38 MAPKs signaling pathway in human esophageal cancer cells. Phytomedicine 2021, 86, 153564. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Piao, X.J.; Xu, W.T.; Zhang, Y.; Zhang, T.; Xue, H.; Li, Y.N.; Zuo, W.B.; Sun, G.; Fu, Z.R.; et al. Calycosin induces mitochondrial-dependent apoptosis and cell cycle arrest, and inhibits cell migration through a ROS-mediated signaling pathway in HepG2 hepatocellular carcinoma cells. Toxicol. Vitro 2021, 70, 105052. [Google Scholar] [CrossRef]
- Schuster, C.; Wolpert, N.; Moustaid-Moussa, N.; Gollahon, L.S. Combinatorial Effects of the Natural Products Arctigenin, Chlorogenic Acid, and Cinnamaldehyde Commit Oxidation Assassination on Breast Cancer Cells. Antioxidants 2022, 11, 591. [Google Scholar] [CrossRef] [PubMed]
- Chan, L.; Pang, Y.; Wang, Y.; Zhu, D.; Taledaohan, A.; Jia, Y.; Zhao, L.; Wang, W. Genistein-induced mitochondrial dysfunction and FOXO3a/PUMA expression in non-small lung cancer cells. Pharm. Biol. 2022, 60, 1876–1883. [Google Scholar] [CrossRef]
- Gu, R.; Zhang, M.; Meng, H.; Xu, D.; Xie, Y. Gallic acid targets acute myeloid leukemia via Akt/mTOR-dependent mitochondrial respiration inhibition. Biomed. Pharmacother. 2018, 105, 491–497. [Google Scholar] [CrossRef]
- Sp, N.; Kang, D.Y.; Jo, E.S.; Rugamba, A.; Kim, W.S.; Park, Y.M.; Hwang, D.Y.; Yoo, J.S.; Liu, Q.; Jang, K.J.; et al. Tannic Acid Promotes TRAIL-Induced Extrinsic Apoptosis by Regulating Mitochondrial ROS in Human Embryonic Carcinoma Cells. Cells 2020, 9, 282. [Google Scholar] [CrossRef]
- Ko, C.H.; Shen, S.C.; Yang, L.Y.; Lin, C.W.; Chen, Y.C. Gossypol reduction of tumor growth through ROS-dependent mitochondria pathway in human colorectal carcinoma cells. Int. J. Cancer 2007, 121, 1670–1679. [Google Scholar] [CrossRef]
- Li, Q.; Dong, Z.; Chen, M.; Feng, L. Phenolic molecules constructed nanomedicine for innovative cancer treatment. Coord. Chem. Rev. 2021, 439, 213912. [Google Scholar] [CrossRef]
- Chow, H.H.; Hakim, I.A. Pharmacokinetic and chemoprevention studies on tea in humans. Pharmacol. Res. 2011, 64, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Jithan, A.; Madhavi, K.; Madhavi, M.; Prabhakar, K. Preparation and characterization of albumin nanoparticles encapsulating curcumin intended for the treatment of breast cancer. Int. J. Pharm Investig. 2011, 1, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, W.; Guo, M.; Fu, F.; Wang, W.; Sung, T.; Zhang, M.; Zhong, Z.; Wu, C.; Pan, X.; et al. Inhalable iron redox cycling powered nanoreactor for amplified ferroptosis-apoptosis synergetic therapy of lung cancer. Nano Res. 2024, 17, 5435–5451. [Google Scholar] [CrossRef]
- Caddeo, C.; Gabriele, M.; Nácher, A.; Fernàndez-Busquets, X.; Valenti, D.; Maria Fadda, A.; Pucci, L.; Manconi, M. Resveratrol and artemisinin eudragit-coated liposomes: A strategy to tackle intestinal tumors. Int. J. Pharm. 2021, 592, 120083. [Google Scholar] [CrossRef]
- Kang, J.H.; Ko, Y.T. Enhanced Subcellular Trafficking of Resveratrol Using Mitochondriotropic Liposomes in Cancer Cells. Pharmaceutics 2019, 11, 423. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Yu, H.; Liang, L.; Wang, C.; Shi, D.; Zhang, X.; Ying, Y.; Cai, W.; Li, W.; Li, J.; et al. Redox Homeostasis Disruptors Based on Metal-Phenolic Network Nanoparticles for Chemo/Chemodynamic Synergistic Tumor Therapy through Activating Apoptosis and Cuproptosis. Adv. Healthc. Mater. 2023, 12, e2301346. [Google Scholar] [CrossRef]
- Wang, D.; Wang, T.; Yu, H.; Feng, B.; Zhou, L.; Zhou, F.; Hou, B.; Zhang, H.; Luo, M.; Li, Y. Engineering nanoparticles to locally activate T cells in the tumor microenvironment. Sci. Immunol. 2019, 4, eaau6584. [Google Scholar] [CrossRef]
- Nanda, S.S.; Yi, D.K. Recent Advances in Synergistic Effect of Nanoparticles and Its Biomedical Application. Int. J. Mol. Sci. 2024, 25, 3266. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Wu, X.; Wang, Y.; Wang, D.; Wang, Z.; Yang, M.; Yang, L.; Wang, F.; Wang, W.; Zhang, X. Formation of EGCG oxidation self-assembled nanoparticles and their antioxidant activity in vitro and hepatic REDOX regulation activity in vivo. Food Funct. 2024, 15, 2181–2196. [Google Scholar] [CrossRef]
- Peng, A.; Lin, L.; Zhao, M. Chemical basis and self-assembly mechanism of submicroparticles forming in chrysanthemum tea infusion. Food Chem. 2023, 427, 136745. [Google Scholar] [CrossRef]
- Bigaj-Józefowska, M.J.; Coy, E.; Załęski, K.; Zalewski, T.; Grabowska, M.; Jaskot, K.; Perrigue, P.; Mrówczyński, R.; Grześkowiak, B.F. Biomimetic theranostic nanoparticles for effective anticancer therapy and MRI imaging. J. Photochem. Photobiol. B. 2023, 249, 112813. [Google Scholar] [CrossRef]
- Nieto, C.; Vega, M.A.; Marcelo, G.; Martín Del Valle, E.M. Polydopamine nanoparticles kill cancer cells. RSC Adv. 2018, 8, 36201–36208. [Google Scholar] [CrossRef]
- Xing, Y.; Zhang, J.; Chen, F.; Liu, J.; Cai, K. Mesoporous polydopamine nanoparticles with co-delivery function for overcoming multidrug resistance via synergistic chemo-photothermal therapy. Nanoscale 2017, 9, 8781–8790. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Zhang, J.; Wang, Y.; Wang, C.; Xiao, J.; Zhang, Q.; Cheng, Y. Multi-responsive photothermal-chemotherapy with drug-loaded melanin-like nanoparticles for synergetic tumor ablation. Biomaterials 2016, 81, 114–124. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Wang, B.; Liu, Y.; Wan, Y.; Liu, Q.; Xu, H.; Liang, R.; Shi, Y.; Tu, P.; Wu, H.; et al. Mitochondria-targeting polydopamine-coated nanodrugs for effective photothermal- and chemo-synergistic therapies against lung cancer. Regen Biomater. 2022, 9, rbac051. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Ai, W.; Guo, X.; Li, Y.; Ma, Y.; Chen, L.; Zhang, H.; Wang, T.; Zhang, X.; Wang, Z. Mitochondria-Targeted Polydopamine Nanocomposite with AIE Photosensitizer for Image-Guided Photodynamic and Photothermal Tumor Ablation. Small 2019, 15, e1902352. [Google Scholar] [CrossRef]
- Zhang, B.; Jiang, J.; Wu, P.; Zou, J.; Le, J.; Lin, J.; Li, C.; Luo, B.; Zhang, Y.; Huang, R.; et al. A smart dual-drug nanosystem based on co-assembly of plant and food-derived natural products for synergistic HCC immunotherapy. Acta Pharm. Sin. B 2021, 11, 246–257. [Google Scholar] [CrossRef]
- Guo, Z.; Liu, T.; Gao, W.; Iffelsberger, C.; Kong, B.; Pumera, M. Multi-Wavelength Light-Responsive Metal-Phenolic Network-Based Microrobots for Reactive Species Scavenging. Adv. Mater. 2023, 35, e2210994. [Google Scholar] [CrossRef]
- Mattos, B.D.; Zhu, Y.; Tardy, B.L.; Beaumont, M.; Ribeiro, A.C.R.; Missio, A.L.; Otoni, C.G.; Rojas, O.J. Versatile Assembly of Metal-Phenolic Network Foams Enabled by Tannin-Cellulose Nanofibers. Adv. Mater. 2023, 35, e2209685. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Ju, Y.; Rahim, M.A.; Richardson, J.J.; Caruso, F. Polyphenol-Mediated Assembly for Particle Engineering. Acc. Chem. Res. 2020, 53, 1269–1278. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, Z.; Penna, M.; Pan, S.; Ju, Y.; Li, S.; Han, Y.; Chen, J.; Lin, G.; Richardson, J.J.; et al. Particle engineering enabled by polyphenol-mediated supramolecular networks. Nat. Commun. 2020, 11, 4804. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Jiang, L.; Gao, H.; Liu, Z.; Mäkilä, E.; Wang, S.; Saiding, Q.; Xiang, L.; Tang, X.; Shi, M.; et al. A pH-Responsive Cluster Metal-Organic Framework Nanoparticle for Enhanced Tumor Accumulation and Antitumor Effect. Adv. Mater. 2022, 34, e2203915. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Gong, G.; Chen, M.; Zhang, H.; Zhang, Y.; Richardson, J.J.; Chan, W.Y.; He, Y.; Guo, J. Metal-Phenolic Nanocloaks on Cancer Cells Potentiate STING Pathway Activation for Synergistic Cancer Immunotherapy. Angew. Chem. Int. Ed. Engl. 2024, 63, e202314501. [Google Scholar] [CrossRef] [PubMed]
- Xu, W.; Lin, Z.; Pan, S.; Chen, J.; Wang, T.; Cortez-Jugo, C.; Caruso, F. Direct Assembly of Metal-Phenolic Network Nanoparticles for Biomedical Applications. Angew. Chem. Int. Ed. Engl. 2023, 62, e202312925. [Google Scholar] [CrossRef]
- Wang, H.; Wang, D.; Yu, J.; Zhang, Y.; Zhou, Y. Applications of metal-phenolic networks in nanomedicine: A review. Biomater. Sci. 2022, 10, 5786–5808. [Google Scholar] [CrossRef]
- Li, K.; Dai, Y.; Chen, W.; Yu, K.; Xiao, G.; Richardson, J.J.; Huang, W.; Guo, J.; Liao, X.; Shi, B. Self-Assembled Metal-Phenolic Nanoparticles for Enhanced Synergistic Combination Therapy against Colon Cancer. Adv. Biosyst. 2019, 3, e1800241. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Xiao, G.; Richardson, J.J.; Tardy, B.L.; Ejima, H.; Huang, W.; Guo, J.; Liao, X.; Shi, B. Targeted Therapy against Metastatic Melanoma Based on Self-Assembled Metal-Phenolic Nanocomplexes Comprised of Green Tea Catechin. Adv. Sci. 2019, 6, 1801688. [Google Scholar] [CrossRef]
- Meng, X.; Chen, L.; Lv, R.; Liu, M.; He, N.; Wang, Z. A metal-phenolic network-based multifunctional nanocomposite with pH-responsive ROS generation and drug release for synergistic chemodynamic/photothermal/chemo-therapy. J. Mater. Chem. B 2020, 8, 2177–2188. [Google Scholar] [CrossRef]
- Dai, Y.; Yang, Z.; Cheng, S.; Wang, Z.; Zhang, R.; Zhu, G.; Wang, Z.; Yung, B.C.; Tian, R.; Jacobson, O.; et al. Toxic Reactive Oxygen Species Enhanced Synergistic Combination Therapy by Self-Assembled Metal-Phenolic Network Nanoparticles. Adv. Mater. 2018, 30, 1704877. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Fan, J.X.; Zheng, D.W.; Liu, F.; Zeng, X.; Yan, G.P.; Zhang, X.Z. A multi-functional drug delivery system based on polyphenols for efficient tumor inhibition and metastasis prevention. Biomater. Sci. 2020, 8, 702–711. [Google Scholar] [CrossRef]
- Shan, L.; Gao, G.; Wang, W.; Tang, W.; Wang, Z.; Yang, Z.; Fan, W.; Zhu, G.; Zhai, K.; Jacobson, O.; et al. Self-assembled green tea polyphenol-based coordination nanomaterials to improve chemotherapy efficacy by inhibition of carbonyl reductase 1. Biomaterials 2019, 210, 62–69. [Google Scholar] [CrossRef]
- Zhang, N.; Mei, K.; Guan, P.; Hu, X.; Zhao, Y. Protein-Based Artificial Nanosystems in Cancer Therapy. Small 2020, 16, e1907256. [Google Scholar] [CrossRef] [PubMed]
- Ramalho, M.J.; Loureiro, J.A.; Coelho, M.A.N.; Pereira, M.C. Transferrin Receptor-Targeted Nanocarriers: Overcoming Barriers to Treat Glioblastoma. Pharmaceutics 2022, 14, 279. [Google Scholar] [CrossRef]
- Liu, C.; Shen, W.; Li, B.; Li, T.; Chang, H.; Cheng, Y. Natural Polyphenols Augment Cytosolic Protein Delivery by a Functional Polymer. Chem. Mater. 2019, 31, 1956–1965. [Google Scholar] [CrossRef]
- Qiao, H.; Fang, D.; Zhang, L.; Gu, X.; Lu, Y.; Sun, M.; Sun, C.; Ping, Q.; Li, J.; Chen, Z.; et al. Nanostructured Peptidotoxins as Natural Pro-Oxidants Induced Cancer Cell Death via Amplification of Oxidative Stress. ACS Appl. Mater. Interfaces 2018, 10, 4569–4581. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jin, P.; Zhang, X.; Ravichandran, N.; Ying, H.; Yu, C.; Ying, H.; Xu, Y.; Yin, J.; Wang, K.; et al. New epigallocatechin gallate (EGCG) nanocomplexes co-assembled with 3-mercapto-1-hexanol and β-lactoglobulin for improvement of antitumor activity. J. Biomed. Nanotechnol. 2017, 13, 805–814. [Google Scholar] [CrossRef]
- Chung, J.E.; Tan, S.; Gao, S.J.; Yongvongsoontorn, N.; Kim, S.H.; Lee, J.H.; Choi, H.S.; Yano, H.; Zhuo, L.; Kurisawa, M.; et al. Self-assembled micellar nanocomplexes comprising green tea catechin derivatives and protein drugs for cancer therapy. Nat. Nanotechnol. 2014, 9, 907–912. [Google Scholar] [CrossRef]
- Zhang, Y.S.; Khademhosseini, A. Advances in engineering hydrogels. Science 2017, 356, eaaf3627. [Google Scholar] [CrossRef]
- Dimatteo, R.; Darling, N.J.; Segura, T. In situ forming injectable hydrogels for drug delivery and wound repair. Adv. Drug Deliv. Rev. 2018, 127, 167–184. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, Z.; Chen, H.; Chen, K.; Tao, W.; Ouyang, X.K.; Mei, L.; Zeng, X. Polyphenol-based hydrogels: Pyramid evolution from crosslinked structures to biomedical applications and the reverse design. Bioact. Mater. 2022, 17, 49–70. [Google Scholar] [CrossRef] [PubMed]
- Teong, B.; Lin, C.Y.; Chang, S.J.; Niu, G.C.; Yao, C.H.; Chen, I.F.; Kuo, S.M. Enhanced anti-cancer activity by curcumin-loaded hydrogel nanoparticle derived aggregates on A549 lung adenocarcinoma cells. J. Mater. Sci. Mater. Med. 2015, 26, 5357. [Google Scholar] [CrossRef] [PubMed]
- Madeo, L.F.; Sarogni, P.; Cirillo, G.; Vittorio, O.; Voliani, V.; Curcio, M.; Shai-Hee, T.; Büchner, B.; Mertig, M.; Hampel, S. Curcumin and Graphene Oxide Incorporated into Alginate Hydrogels as Versatile Devices for the Local Treatment of Squamous Cell Carcinoma. Materials 2022, 15, 1648. [Google Scholar] [CrossRef] [PubMed]
- Jeong, D.I.; Kim, H.J.; Lee, S.Y.; Kim, S.; Huh, J.W.; Ahn, J.H.; Karmakar, M.; Kim, H.J.; Lee, K.; Lee, J.; et al. Hydrogel design to overcome thermal resistance and ROS detoxification in photothermal and photodynamic therapy of cancer. J. Control. Release 2024, 366, 142–159. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Fei, Z.; Guo, T.; Hou, Y.; Li, D.; Wang, K.; Ren, F.; Fan, K.; Zhou, D.; Xie, C.; et al. Bioadhesive injectable hydrogel with phenolic carbon quantum dot supported Pd single atom nanozymes as a localized immunomodulation niche for cancer catalytic immunotherapy. Biomaterials 2022, 280, 121272. [Google Scholar] [CrossRef] [PubMed]
- George, D.; Maheswari, P.U.; Begum, K. Chitosan-cellulose hydrogel conjugated with L-histidine and zinc oxide nanoparticles for sustained drug delivery: Kinetics and in-vitro biological studies. Carbohydr. Polym. 2020, 236, 116101. [Google Scholar] [CrossRef]
- Li, N.; Lin, J.; Liu, C.; Zhang, Q.; Li, R.; Wang, C.; Zhao, C.; Lu, L.; Zhou, C.; Tian, J.; et al. Temperature- and pH-responsive injectable chitosan hydrogels loaded with doxorubicin and curcumin as long-lasting release platforms for the treatment of solid tumors. Front. Bioeng. Biotechnol. 2022, 10, 1043939. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zhou, Z.; Huang, Y.; Liu, H.; He, N.; Zhu, X.; Han, X.; Zhou, D.; Duan, X.; Chen, X.; et al. A versatile 3D-printable hydrogel for antichondrosarcoma, antibacterial, and tissue repair. J. Mater. Sci. Technol. 2023, 136, 200–211. [Google Scholar] [CrossRef]
- Tien, N.D.; Lyngstadaas, S.P.; Mano, J.F.; Blaker, J.J.; Haugen, H.J. Recent Developments in Chitosan-Based Micro/Nanofibers for Sustainable Food Packaging, Smart Textiles, Cosmeceuticals, and Biomedical Applications. Molecules 2021, 26, 2683. [Google Scholar] [CrossRef]
- Toragall, V.; Jayapala, N.; Muthukumar, S.P.; Vallikanan, B. Biodegradable chitosan-sodium alginate-oleic acid nanocarrier promotes bioavailability and target delivery of lutein in rat model with no toxicity. Food Chem. 2020, 330, 127195. [Google Scholar] [CrossRef] [PubMed]
- Anisiei, A.; Rosca, I.; Sandu, A.I.; Bele, A.; Cheng, X.; Marin, L. Imination of Microporous Chitosan Fibers-A Route to Biomaterials with “On Demand” Antimicrobial Activity and Biodegradation for Wound Dressings. Pharmaceutics 2022, 14, 117. [Google Scholar] [CrossRef] [PubMed]
- Chanphai, P.; Tajmir-Riahi, H.A. Conjugation of tea catechins with chitosan nanoparticles. Food Hydrocoll. 2018, 84, 561–570. [Google Scholar] [CrossRef]
- Hu, Q.; Luo, Y. Polyphenol-chitosan conjugates: Synthesis, characterization, and applications. Carbohydr. Polym. 2016, 151, 624–639. [Google Scholar] [CrossRef] [PubMed]
- Hu, Q.; Luo, Y. Chitosan-based nanocarriers for encapsulation and delivery of curcumin: A review. Int. J. Biol. Macromol. 2021, 179, 125–135. [Google Scholar] [CrossRef] [PubMed]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef]
- Tsai, W.H.; Yu, K.H.; Huang, Y.C.; Lee, C.I. EGFR-targeted photodynamic therapy by curcumin-encapsulated chitosan/TPP nanoparticles. Int. J. Nanomed. 2018, 13, 903–916. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Bharali, D.J.; Adhami, V.M.; Siddiqui, I.A.; Cui, H.; Shabana, S.M.; Mousa, S.A.; Mukhtar, H. Oral administration of naturally occurring chitosan-based nanoformulated green tea polyphenol EGCG effectively inhibits prostate cancer cell growth in a xenograft model. Carcinogenesis 2014, 35, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Wang, Y.; Xie, M.; Hu, G.; Ma, F.; Zeng, X. Polymer nanoparticles composed with gallic acid grafted chitosan and bioactive peptides combined antioxidant, anticancer activities and improved delivery property for labile polyphenols. J. Funct. Foods 2015, 15, 593–603. [Google Scholar] [CrossRef]
- Wang, X.; Chen, Y.; Dahmani, F.Z.; Yin, L.; Zhou, J.; Yao, J. Amphiphilic carboxymethyl chitosan-quercetin conjugate with P-gp inhibitory properties for oral delivery of paclitaxel. Biomaterials 2014, 35, 7654–7665. [Google Scholar] [CrossRef]
- Mu, Y.; Wu, G.; Su, C.; Dong, Y.; Zhang, K.; Li, J.; Sun, X.; Li, Y.; Chen, X.; Feng, C. pH-sensitive amphiphilic chitosan-quercetin conjugate for intracellular delivery of doxorubicin enhancement. Carbohydr. Polym. 2019, 223, 115072. [Google Scholar] [CrossRef] [PubMed]
- Mu, M.; Liang, X.; Chuan, D.; Zhao, S.; Yu, W.; Fan, R.; Tong, A.; Zhao, N.; Han, B.; Guo, G. Chitosan coated pH-responsive metal-polyphenol delivery platform for melanoma chemotherapy. Carbohydr. Polym. 2021, 264, 118000. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154–155, 102–122. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Guo, X.; Zeng, S.; Wang, Y.; Hou, J.; Yang, D.; Zhou, S. A multifunctional liposomal nanoplatform co-delivering hydrophobic and hydrophilic doxorubicin for complete eradication of xenografted tumors. Nanoscale 2019, 11, 17759–17772. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; He, W.; Xia, Q.; Zhou, W.; Yao, C.; Li, X. Thioether Phosphatidylcholine Liposomes: A Novel ROS-Responsive Platform for Drug Delivery. ACS Appl. Mater. Interfaces 2019, 11, 37411–37420. [Google Scholar] [CrossRef] [PubMed]
- Mignet, N.; Seguin, J.; Chabot, G.G. Bioavailability of polyphenol liposomes: A challenge ahead. Pharmaceutics 2013, 5, 457–471. [Google Scholar] [CrossRef] [PubMed]
- Long, Q.; Xiel, Y.; Huang, Y.; Wu, Q.; Zhang, H.; Xiong, S.; Liu, Y.; Chen, L.; Wei, Y.; Zhao, X.; et al. Induction of apoptosis and inhibition of angiogenesis by PEGylated liposomal quercetin in both cisplatin-sensitive and cisplatin-resistant ovarian cancers. J. Biomed. Nanotechnol. 2013, 9, 965–975. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Zhong, Q.; Chen, L.J.; Qi, X.R.; Fu, A.F.; Yang, H.S.; Yang, F.; Lin, H.G.; Wei, Y.Q.; Zhao, X. Liposomal honokiol, a promising agent for treatment of cisplatin-resistant human ovarian cancer. J. Cancer Res. Clin. Oncol. 2008, 134, 937–945. [Google Scholar] [CrossRef] [PubMed]
- Ezzat, H.M.; Elnaggar, Y.S.R.; Abdallah, O.Y. Improved oral bioavailability of the anticancer drug catechin using chitosomes: Design, in-vitro appraisal and in-vivo studies. Int. J. Pharm. 2019, 565, 488–498. [Google Scholar] [CrossRef]
- Li, R.; Lin, Z.; Zhang, Q.; Zhang, Y.; Liu, Y.; Lyu, Y.; Li, X.; Zhou, C.; Wu, G.; Ao, N.; et al. Injectable and In Situ-Formable Thiolated Chitosan-Coated Liposomal Hydrogels as Curcumin Carriers for Prevention of In Vivo Breast Cancer Recurrence. ACS Appl. Mater. Interfaces 2020, 12, 17936–17948. [Google Scholar] [CrossRef]
- Singh, Y.; Meher, J.G.; Raval, K.; Khan, F.A.; Chaurasia, M.; Jain, N.K.; Chourasia, M.K. Nanoemulsion: Concepts, development and applications in drug delivery. J. Control. Release 2017, 252, 28–49. [Google Scholar] [CrossRef] [PubMed]
- Das, S.S.; Sarkar, A.; Chabattula, S.C.; Verma, P.R.P.; Nazir, A.; Gupta, P.K.; Ruokolainen, J.; Kesari, K.K.; Singh, S.K. Food-Grade Quercetin-Loaded Nanoemulsion Ameliorates Effects Associated with Parkinson’s Disease and Cancer: Studies Employing a Transgenic C. elegans Model and Human Cancer Cell Lines. Antioxidants 2022, 11, 1378. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Li, S.J.; Liu, Y.; Zhang, T.; Xue, P.; Kang, Y.; Sun, Z.J.; Xu, Z. Bioengineered nanogels for cancer immunotherapy. Chem. Soc. Rev. 2022, 51, 5136–5174. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.; Yu, S.; Wang, Z.; Huang, P.; Wang, W.; Xing, J. Nanogels loading curcumin in situ through microemulsion photopolymerization for enhancement of antitumor effects. J. Mater. Chem. B 2022, 10, 3293–3302. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, M.; He, Y.; Ni, Q.; Zhou, M.; Chen, H.; Li, G.; Yu, J.; Wu, X.; Zhang, X. Polyphenolic Nanomedicine Regulating Mitochondria REDOX for Innovative Cancer Treatment. Pharmaceutics 2024, 16, 972. https://doi.org/10.3390/pharmaceutics16080972
Yang M, He Y, Ni Q, Zhou M, Chen H, Li G, Yu J, Wu X, Zhang X. Polyphenolic Nanomedicine Regulating Mitochondria REDOX for Innovative Cancer Treatment. Pharmaceutics. 2024; 16(8):972. https://doi.org/10.3390/pharmaceutics16080972
Chicago/Turabian StyleYang, Mingchuan, Yufeng He, Qingqing Ni, Mengxue Zhou, Hongping Chen, Guangyun Li, Jizhong Yu, Ximing Wu, and Xiangchun Zhang. 2024. "Polyphenolic Nanomedicine Regulating Mitochondria REDOX for Innovative Cancer Treatment" Pharmaceutics 16, no. 8: 972. https://doi.org/10.3390/pharmaceutics16080972






