Abstract
Cytokines are immune modulators which can enhance the immune response and have been proven to be an effective class of immunotherapy. Nevertheless, the clinical use of cytokines in cancer treatment has faced several challenges associated with poor pharmacokinetic properties and the occurrence of adverse effects. Immunocytokines (ICKs) have emerged as a promising approach to overcome the pharmacological limitations observed with cytokines. ICKs are fusion proteins designed to deliver cytokines in the tumor microenvironment by taking advantage of the stability and specificity of immunoglobulin-based scaffolds. Several technological approaches have been developed. This review focuses on ICKs designed with the most impactful cytokines in the cancer field: IL-2, TNFα, IL-10, IL-12, IL-15, IL-21, IFNγ, GM-CSF, and IFNα. An overview of the pharmacological effects of the naked cytokines and ICKs tested for cancer therapy is detailed. A particular emphasis is given on the immunomodulatory effects of ICKs associated with their technological design. In conclusion, this review highlights active ways of development of ICKs. Their already promising results observed in clinical trials are likely to be improved with the advances in targeting technologies such as cytokine/linker engineering and the design of multispecific antibodies with tumor targeting and immunostimulatory functional properties.
1. Introduction
Cytokines represent a class of low-molecular-weight proteins secreted by various cell types, including immune cells, endothelial cells, and stromal cells [1]. Cytokines bind to specific receptors on target cells, triggering a variety of intracellular signaling pathways that ultimately govern gene expression and cellular behavior. Cytokines play a pivotal role in orchestrating immune responses, modulating cell differentiation, and facilitating intercellular communication within biological systems [2]. They serve as indispensable regulators of the immune system and play important roles in numerous diseases, including cancer, autoimmune disorders, infectious diseases, and inflammatory conditions [1,2].
The genesis of cytokine research traces back to the early 20th century when investigators unveiled the presence of a factor within leukocytes capable of inducing the proliferation and differentiation of neighboring cells [1]. It was not until the 1950s and 1960s that a series of experiments revealed the existence of an intricate network of signaling molecules finely regulating immune responses [3]. This led to the identification of the first cytokine, interleukin(IL)-1, in 1979 [4]. Since then, the field of cytokine research has exploded, with over one hundred cytokines and their receptors identified and characterized [1]. Cytokines have been systematically categorized into various families, predicated on shared structural and functional attributes, with prominent examples encompassing the interleukin (IL), tumor necrosis factor (TNF), interferon (IFN), and chemokine families [1]. As potent immune modulators, cytokines can have pathological implications in certain disorders such as cancer, where their dysregulated production or action can drive tumor growth and impede the immune system’s efficacy in eradicating malignant cells [1]. The immune cell population within the tumor microenvironment (TME) can be categorized into two distinct subtypes: pro-inflammatory and anti-inflammatory cells, as illustrated in Figure 1. Pro-inflammatory cells include CD8+ T cells, M1 macrophages, natural killer (NK) cells, and dendritic cells (DCs). Among these, macrophages, NK cells and CD8+ T cells exhibit the capability to directly engage with tumor cells and eliminate them [5,6]. DCs, macrophages, and neutrophils exert their tumor cell-killing activity by releasing cytokines such as TNFα, IL-6, and IFNγ [7,8,9]. Neutrophils also exert their antitumor function through degranulation [10]. Another subset of pro-inflammatory cells comprises the CD4+ T helper 1 (Th1) T lymphocytes and B cells. These cells are characterized as pro-inflammatory due to their capacity to trigger the activation of CD8+ T cells through the secretion of IFNγ and IL-2 [11,12]. Consequently, IFNγ and IL-2 are categorized as Th1 cytokines. The anti-inflammatory cells that can be found in the TME include M2 macrophages, myeloid-derived suppressor cells (MDSCs), CD4+ T regulatory cells (Tregs) and CD4+ T helper 2 (Th2) lymphocytes. These cells possess the capability to release cytokines such as IL-10 and IL-4 [13,14]. Notably, these cytokines play a role in suppressing the Th1 immune response within the tumor milieu. Ultimately, cytokines, as potent immune modulators, can exert potent beneficial but also detrimental effects upon the natural antitumor response. For instance, cytokines typically classified as immune response-inhibitory cannot be categorically labeled as pro-tumoral cytokines, as exemplified by the case of IL-10, which has demonstrated the ability to induce both tumor rejection and foster long-lasting tumor immunity [14]. Conversely, TNFα, which was originally named based on its direct cytotoxic activity on tumor cells, has been shown to participate in both the initiation and progression of cancer [15,16,17]. Finally, transforming growth factor β (TGFβ) is another double-edged sword cytokine which can have anti-tumor activity via its direct growth inhibition activity on cancer cells but which can also exert anti-inflammatory and immunosuppressive functions contributing to the immunosurveillance escape and oncogenesis [18].
Beyond their role in the immune system, cytokines are also able to exert a direct influence on pre-malignant and cancerous cells. While certain cytokines, such as members of the IFN family, can directly induce apoptosis in tumor cells [19,20], some cytokines such as IL-6 [15,21], TNFα [22], and TGFβ [23] can enhance cell proliferation and resistance to induced cell death, ultimately fostering tumor growth and progression [24].
The understanding of cytokine functions on cancer and immune cells has led to an increasing interest in their use as drugs. The administration of recombinant IFNα was shown to trigger the phagocytosis of tumor cells by macrophages and extended the survival of mice in multiple xenograft tumor models [25], and recombinant IFNα was the first cytokine-based drug approved for clinical use in 1986 for hairy cell leukemia, followed by IFNγ in 1991 for chronic granulomatous disease [26]. This study showed the potential of recombinant cytokines as therapeutics for cancer and the approval of recombinant IL-2 (Proleukin) for metastatic renal cancer quickly followed [27].
Although the immunotherapeutic potential of cytokines has proven to be widely useful in various diseases including hepatitis B and hepatitis C, chronic granulomatous disease, multiple sclerosis, rheumatoid arthritis, psoriasis, Crohn’s disease, and ulcerative colitis [28], the success of cytokine therapies in cancer has been limited by their poor bioavailability, inadequate pharmacokinetics (PK), and toxicity profile at therapeutic doses [29]. Therefore, maintaining therapeutically active concentrations in patients without inducing significant side effects remains a current challenge in the field of cytokine therapy.
To address the poor bioavailability issues observed with cytokine treatment, numerous vectorization technologies have been developed. PEGylation, which aims to prolong the half-life of cytokines, has been one of the most frequent approaches to improve the pharmacological profile of cytokines. PEGylation is clinically approved for IFNα2a [30,31] and is currently under evaluation for IL-2 [32], IL-10 [33], and IL-15 [34,35]. However, PEGylation is often accompanied by an unwanted effect on bioactivity, particularly resulting from the amphiphilic nature of PEG, as exemplified by PEGylated IFNα2a that displays only 7 % in vitro activity of the native cytokine [36]. Other approaches to improve the pharmacological profile of therapeutic cytokines include fusing them to antibody Fc-domains [37,38,39], albumin [37,40], or collagen binding lumican [41] resulting in extended half-lives of cytokines. However, novel approaches are still critically needed to reduce the toxicity of cytokine treatment and to improve their efficacy.
Among the promising technologies, antibody–cytokine fusion is one of the most advanced vectorization techniques showing anti-cancer efficacy. This review is focused on antibody–cytokine fusion proteins, also named immunocytokines (ICKs), developed for cancer treatment with a focus on the most impactful cytokines IL-2, TNFα, IL-10, IL-12, IL-15, IL-21, IFNγ, GM-CSF, and IFNα, with a particular emphasis on their immunomodulatory effects associated with their technological design. An overview of the pharmacological effects of the naked cytokines and ICKs tested for cancer therapy is detailed. Finally, some perspectives on this exciting field of biopharmaceuticals are discussed.
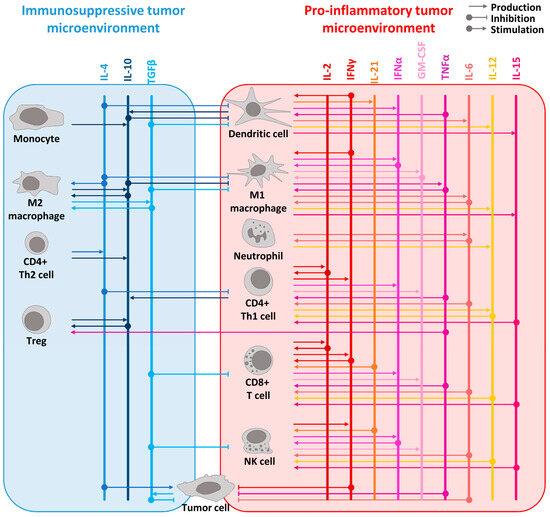
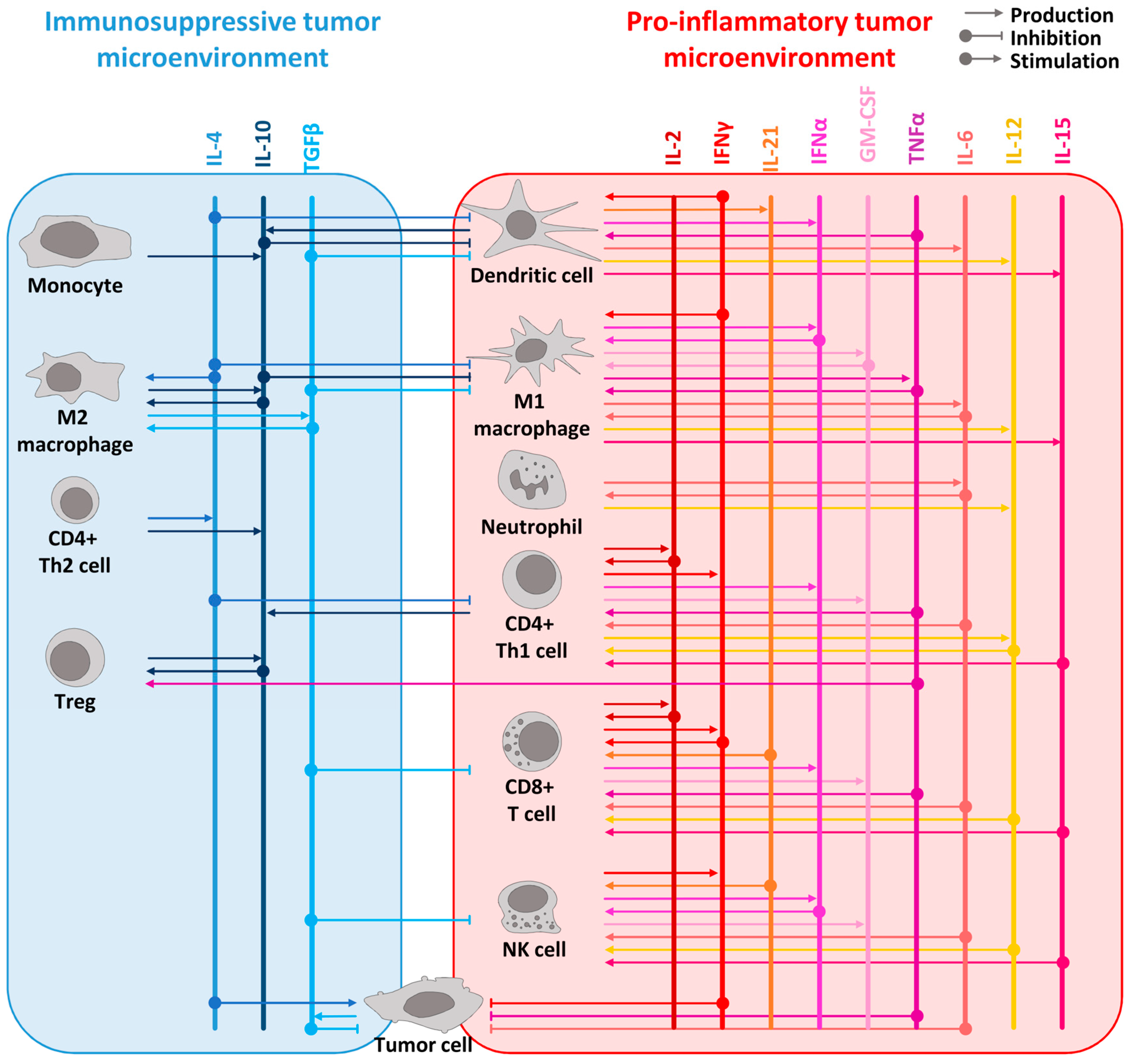
Figure 1.
Overview of the cytokine network in pro-inflammatory and immunosuppressive tumor microenvironment. The principal immunosuppressive cytokines are listed in blue shades at the top of the left panel whereas the main pro-inflammatory cytokines are in red shades at the top of the right panel. The cytokines are represented as vertical lines. Arrows from cells to the cytokines represent the production of a cytokine. Arrows from the cytokines to the cells represent its stimulatory or inhibitory effect on the designated cells. For example, pro-inflammatory cytokines such as IL-2, IL-12, IL-15, IL-21, IFNα, and TNFα are known to stimulate lymphocyte proliferation and activation, while IFNγ can activate both lymphocytes and myeloid cells. IL-15 is produced by dendritic cells and macrophages, like IL-12, which is also produced by neutrophils. IFNα is produced by pro-inflammatory immune cells such as lymphocytes, macrophages, and dendritic cells. TNFα, mainly produced by macrophages, acts as a proinflammatory cytokine through its receptor TNFR1 but leads to anti-inflammatory effects by stimulating Tregs through its receptor TNFR2. GM-CSF, which is produced by macrophages, T cells, and NK cells, elicits an anti-tumor immune response by specifically stimulating myeloid cells and, notably, macrophages. Additionally, IL-10 promotes an anti-tumor response by inducing the expression of IFNγ and enhancing CD8+ T cell activity. To simplify the scheme, direct effects of cytokines on tumor cells are not represented. However, many cytokines have a direct effect on cancer cells such as TGFβ which acts as an anti-tumoral factor in the early stages of tumorigenesis [42] or IL-4 with its pro-tumoral effect [43].
Figure 1.
Overview of the cytokine network in pro-inflammatory and immunosuppressive tumor microenvironment. The principal immunosuppressive cytokines are listed in blue shades at the top of the left panel whereas the main pro-inflammatory cytokines are in red shades at the top of the right panel. The cytokines are represented as vertical lines. Arrows from cells to the cytokines represent the production of a cytokine. Arrows from the cytokines to the cells represent its stimulatory or inhibitory effect on the designated cells. For example, pro-inflammatory cytokines such as IL-2, IL-12, IL-15, IL-21, IFNα, and TNFα are known to stimulate lymphocyte proliferation and activation, while IFNγ can activate both lymphocytes and myeloid cells. IL-15 is produced by dendritic cells and macrophages, like IL-12, which is also produced by neutrophils. IFNα is produced by pro-inflammatory immune cells such as lymphocytes, macrophages, and dendritic cells. TNFα, mainly produced by macrophages, acts as a proinflammatory cytokine through its receptor TNFR1 but leads to anti-inflammatory effects by stimulating Tregs through its receptor TNFR2. GM-CSF, which is produced by macrophages, T cells, and NK cells, elicits an anti-tumor immune response by specifically stimulating myeloid cells and, notably, macrophages. Additionally, IL-10 promotes an anti-tumor response by inducing the expression of IFNγ and enhancing CD8+ T cell activity. To simplify the scheme, direct effects of cytokines on tumor cells are not represented. However, many cytokines have a direct effect on cancer cells such as TGFβ which acts as an anti-tumoral factor in the early stages of tumorigenesis [42] or IL-4 with its pro-tumoral effect [43].
2. Methods
PubMed was used to find published studies. The search was focused on selected cytokines known to exert an important role in cancer: IL-2, IL-15, TNFα, IL-10, IL-12, IL-21, GM-CSF, IFNγ, and IFNα. The two following key word associations were used:
(1) (Immunocytokine [Title/Abstract]) AND (cancer [Title/Abstract]);
(2) (“cancer” [Title/Abstract] AND “cytokine name” [Title] AND (“immune cytokine” [Title/Abstract] OR “immunocytokine” [Title/Abstract] OR “fusion protein” [Title/Abstract] OR “antibody cytokine fusion” [Title/Abstract] OR “antibody fusion” [Title/Abstract])).
The obtained results were then filtered to focus on ICKs designed to target the cytokines in the TME and tested in vivo.
Clinicaltrials.gov was used to find all clinical trials involving ICKs. The following keywords were used to search for trials: “cytokine name” fusion protein, “cytokine name” immune cytokine, “cytokine name” monoclonal antibody–cytokine fusion, “cytokine name” antibody fusion, “cytokine name” antibody–cytokine fusion. Among the search results, only ICKs for the treatment of cancer were selected.
A list of abbreviations is given in Table S1.
3. Immunocytokines
ICKs are biotherapeutics using the advantageous properties of immunoglobulins by genetically fusing full antibody or antibody fragment coding sequences with a cytokine gene to limit the side effects and improve the poor PK observed with naked cytokine administration. They can be categorized into two types. The first type consists of large fusion proteins with cytokines fused to an intact immunoglobulin (Ig), while the second type comprises smaller fusion proteins based on antibody fragments such as single-chain variable fragment (scFv), F(ab’), F(ab’)2, tandem diabody (also called single-chain diabody), and tribody [44] (Figure 2). ICKs under clinical development are mostly fused to intact IgG and scFv but emerging diabodies [45] and tribodies [46,47] ICKs have been tested in preclinical models. The smaller fusion proteins display significantly reduced molecular masses, typically below 100 kDa in contrast to the Ig fusions, which have molecular weights in the range of 175 kDa [48]. Both the antibody format and cytokine composition play a significant role in determining the size, efficacy, and PK/pharmacodynamic (PD) profile of the resulting ICKs [49,50]. While Ig-based ICKs have several strengths such as a long serum half-life or high target avidity, their elevated size is associated with low tumor penetration resulting in low target exposure. Conversely, low-molecular-weight ICKs benefit from high tumor penetration but a shorter half-life.
The longer half-life observed with Ig-fused ICKs is mostly explained by the fragment crystallizable (Fc) region of the antibody used, which is responsible for the non-specific binding to Fc receptors (FcRs) expressed on immune cells [51]. The catabolic elimination rate of Ig-fused ICKs depends on the Ig class, which is largely influenced by the binding to a specific FcR, the neonatal FcR (FcRn). IgG1, IgG2, and IgG4 isotypes have very long half-lives of 18–21 days whereas IgG3, IgM, and IgA have shorter half-lives of 5–8 days [1]. The non-specific binding of the Fc region to FcγRI, FcγRII, and FcγRIII is responsible for IgG effector functions such as antibody-dependent cellular cytotoxicity (ADCC) or complement-dependent cytotoxicity (CDC) involving the immune cells. On the one hand, ADCC and CDC can increase the antitumor efficacy of an ICK, but on the other hand, they can also trigger off-target toxicity which can constitute a significant weakness of IgG-fused ICKs. Beyond the advantage of being able to modulate the cytokines’ PK profile and to engage immune cells through effector functions, ICKs offer the possibility to vectorize a payload toward the tumor site by targeting tumor-associated antigens (TAAs) or tumor-specific antigens (TSAs) through their variable region. The affinity of the ICKs for the TAAs also impacts the PK through target-mediated drug disposition. The differential expression of the TAAs and TSAs in healthy versus tumor tissues is a critical parameter to ensure a higher accumulation of the ICKs at the tumor site and limit the cytokine-mediated toxicity in healthy organs.
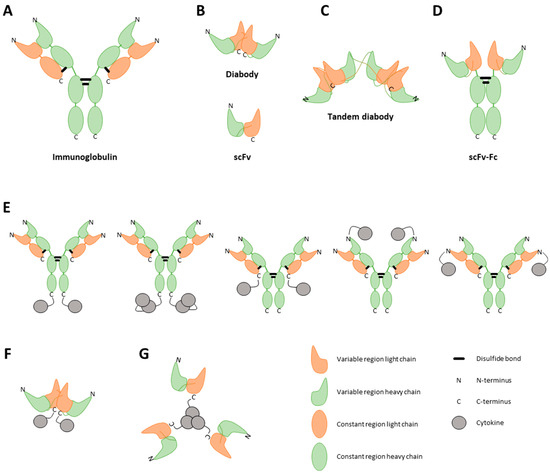
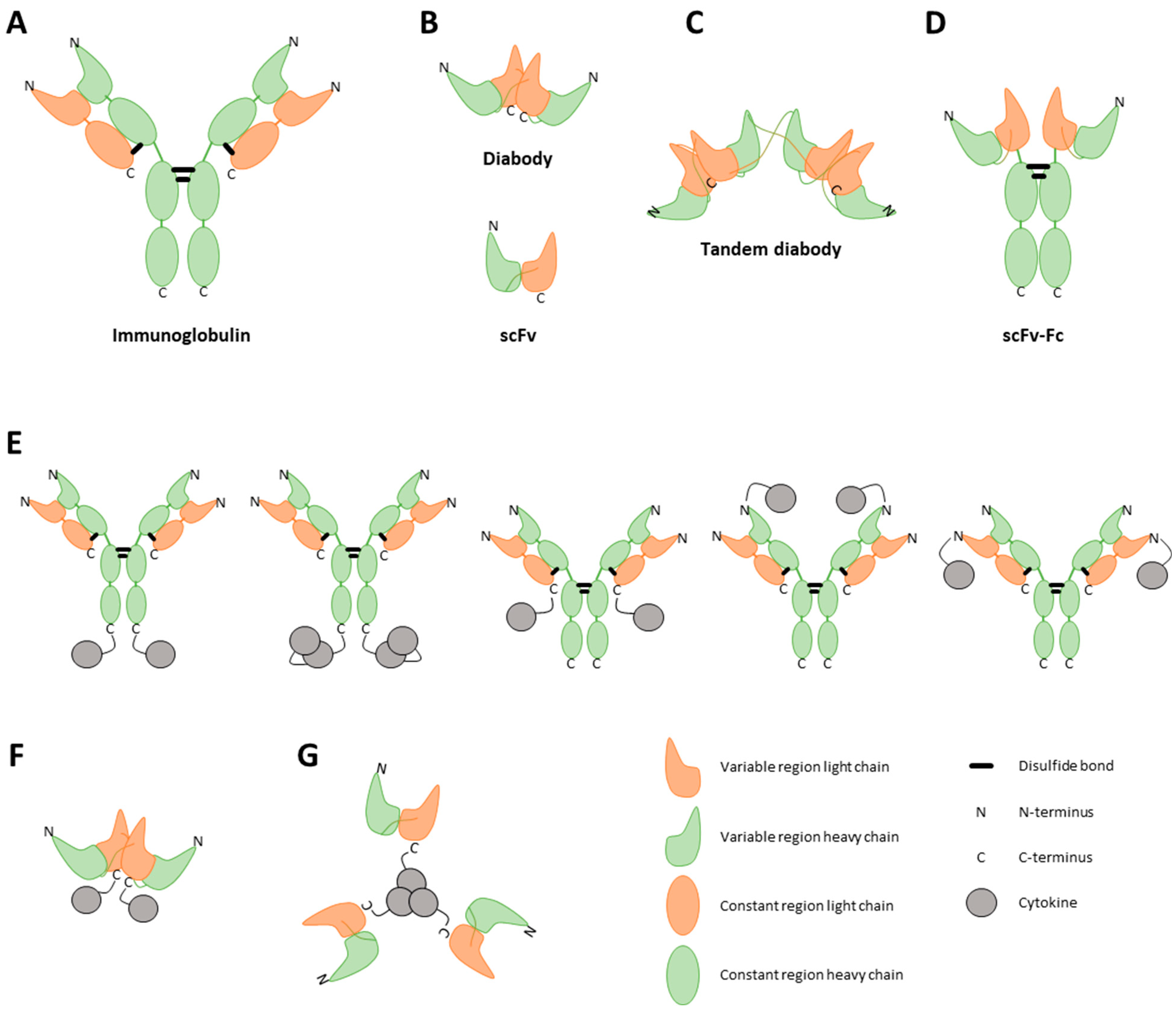
Figure 2.
Visual representation of different immunocytokine (ICK) formats. ICKs can consist of a full immunoglobulin (A), a single-chain variable fragment (scFv) (B, bottom), a diabody (B, top), or a tandem diabody, also called a single-chain diabody (C). An scFv consists of the variable regions of a heavy and light chain. A diabody consists of two connected scFvs. To enhance the half-life of the construct, Fc regions can be strategically linked to antibody fragments (D). Furthermore, cytokines can be incorporated into the fusion protein as either monomers, dimers, or trimers, tethered to either the C-terminal or N-terminal regions of both the heavy and light chains of the antibody (E) or of the diabody (F) [52]. The choice of cytokine fused to the antibody profoundly influences the final structural conformation of the fusion protein. As an illustrative example, TNF, known to naturally assemble into trimers, when combined with an scFv, results in a final fusion protein adopting a trimeric configuration (G) [47].
Figure 2.
Visual representation of different immunocytokine (ICK) formats. ICKs can consist of a full immunoglobulin (A), a single-chain variable fragment (scFv) (B, bottom), a diabody (B, top), or a tandem diabody, also called a single-chain diabody (C). An scFv consists of the variable regions of a heavy and light chain. A diabody consists of two connected scFvs. To enhance the half-life of the construct, Fc regions can be strategically linked to antibody fragments (D). Furthermore, cytokines can be incorporated into the fusion protein as either monomers, dimers, or trimers, tethered to either the C-terminal or N-terminal regions of both the heavy and light chains of the antibody (E) or of the diabody (F) [52]. The choice of cytokine fused to the antibody profoundly influences the final structural conformation of the fusion protein. As an illustrative example, TNF, known to naturally assemble into trimers, when combined with an scFv, results in a final fusion protein adopting a trimeric configuration (G) [47].
The ICKs described hereafter have been developed using different cytokines, various technologies, and several TAAs have been utilized to deliver the cytokines to the TME (Table S2). The antibodies used for ICKs are chosen based on the cancer indication of interest and the TAAs/TSAs which were found differentially expressed in the different given tumor type. While some TAAs/TSAs do not have any intrinsic function and are only used to vectorize the ICKs to the TME, some other antigens can exert direct or indirect tumorigenic functions. They enable to synergize the therapeutic potential of the blocking antibody with the immunostimulatory effects of the cytokine delivery in the TME, which constitutes a critical advantage of the newly developed ICKs.
4. IL-2
IL-2 is a pivotal factor in the growth, survival, and differentiation processes of T lymphocytes and remains one of the most extensively investigated cytokines [53,54,55,56]. IL-2 has a molecular size of 15 kDa and assumes a structural conformation comprising four α helices [53]. Functionally, IL-2 operates as a monomer, primarily being released from CD4+ T cells. Originally, IL-2 was called T cell growth factor [1] as it stimulates proliferation of antigen-stimulated T cells and NK cells by binding to the β and γ receptor chain of the IL-2 receptor (IL-2R) [57]. IL-2 is also able to stimulate Tregs by binding to the IL-2Rα chain [53]. Cancer cells from solid tumors lack the expression of IL-2R, signifying that the therapeutic impact of administered IL-2 can be primarily attributed to its immunological effects that promote the cytolytic activity of both NK and CD8+ cell types [58].
Recombinant IL-2, Proleukin, was approved for clinical use in 1985 for the treatment of metastatic renal carcinoma and melanoma [59,60,61]. Proleukin experiences rapid clearance resulting in a short half-life (±7 min) and achieving therapeutically active concentrations requires high doses or extended administration at low doses. High doses are frequently accompanied with severe side effects such as fever, malaise, arthralgias, hypotension, capillary leak syndrome, and severe influenza-like symptoms [62]. Low doses are accompanied by extended administration and long hospitalization periods. The clinical use of recombinant IL-2 is therefore in decline due to its poor PK and toxicity [63], so ICKs have been tested.
4.1. IL-2 Ig-Fused ICKs
The first ICK ever to be investigated in clinical trials to overcome recombinant IL-2-related issues is the hu14.18–IL-2 fusion protein [64,65,66]. The fusion consists of two IL-2 molecules linked to the C-terminal domain of the heavy chain of the humanized 14.18 IgG1 monoclonal antibody (mAb) that recognizes disialoganglioside (GD2) expressed on neuroblastoma and melanoma cells. Hu14.18–IL-2 is the humanized version of ch14.18–IL-2, that showed antitumor activity in murine models [66,67,68]. The fusion of IL-2 to 14.18 was supported by a clinical trial demonstrating the benefit of combining free recombinant IL-2 with the anti-GD2 14.G2a and was rationalized mechanistically by the stimulatory effect of IL-2 on NK cells and the resulting ADCC-mediated effect through the anti-GD2 mAb [69]. Four phase II clinical trials have been performed to assess the activity of the construct against melanoma and neuroblastoma [70,71,72,73]. Around 20% of the neuroblastoma patients responded to the treatment. In melanoma, hu14.18–IL-2 induced immune activation with some antitumor activity [74], particularly in patients with pre-existing tumor immune gene signatures [75].
The cytokine fusion to the C-terminal domain of the Ig heavy chain can impact the FcR and FcRn binding of the ICK that may decrease its effector function and half-life. Interestingly, IL-2 fusion to the light chain or N-terminal domain of the heavy chain is an option to overcome this issue. IL-2 fusion to the light chain C-terminal of the anti-GD2 14.18 antibody has been shown to improve the ICK half-life following subcutaneous administration and improved effector activities of ADCC and CDC in animal models [76].
In B cell lymphoma, promising data were obtained in both preclinical models and in the clinic with administration of IL-2 plus rituximab (anti-CD20) combination [77,78,79]. Based on these results, a B cell-targeting ICK was developed by fusing IL-2 to an anti-CD20 (DI-Leu16-IL-2) IgG antibody for this indication [79]. Interestingly, DI-Leu16-IL-2 was more effective than combinations of anti-CD20 antibodies and IL-2 in the preclinical model [79,80,81]. In addition, these studies concluded that the therapeutic effect of DI-Leu16-IL-2 was mainly due to the delivery of active IL-2 rather than mediated by ADCC. Phase I/II studies, conducted on patients with B cell lymphoma, showed lymphocyte expansion [81], and partial responses were observed in five out of 13 patients, while three out of 13 patients exhibited complete responses [80] when treated with DI-Leu16-IL-2.
Tucotuzumab celmoleukin (EMD 273066/huKS–IL-2) consists of two IL-2 molecules fused to the Fc part of a humanized IgG mAb-targeting epithelial cell adhesion molecule (EpCAM or KSA). EpCAM is highly expressed on many epithelial cancer types among which are prostate, colon, breast, and lung cancer [64,82]. Two phase I trials have been performed using huKS–IL-2 in several solid tumors [83,84,85,86]. Most patients showed a transient immune response to huKS–IL-2 and 38% of all patients showed a stable disease as best response [85]. In subjects with extensive-disease small cell lung cancer, huKS–IL-2 in combination with cyclophosphamide was well tolerated but did not show progression free survival (PFS) or overall survival (OS) benefits compared with best supportive care alone in a phase II study [87].
A different IL-2-based construct was designed to target phosphatidylserine (PS). PS is a type of phospholipid externalized on the outside of tumor cell and tumor endothelial cell membranes. Exposed PS induces the production of multiple mediators in the TME. Furthermore, 2aG4–IL-2 is an anti-PS antibody (2aG4) fused to IL-2 which takes advantage of IL-2 immunostimulatory activities and the several modes of action of the 2aG4 antibody. This antibody demonstrated tumor growth inhibition efficacy as a single agent in multiple tumor models by destroying tumor vessels through ADCC against the tumor vascular endothelium and by blocking the immunosuppressive signals from PS [88]. Mice treated with 2aG4–IL-2 had a longer survival rate compared to the non-tumor targeted IL-2 control group in the context of vaccination [89].
Carcinoembryonic antigen (CEA) is a highly expressed TSA in the majority of colon cancers, as well as many other solid tumors such as ovarian, breast, and pancreatic tumors [90,91,92,93]. M5A–IL-2 is an anti-CEA human IgG1 antibody fused to IL-2. Even though M5A–IL-2 did not improve the antitumor efficacy compared to IL-2–Fc fusion protein, higher tumor uptake and slower blood clearance were observed in M5A–IL-2-treated mice [94]. Of note, the fusion of IL-2 to Fc-derived immunoglobulins with high effector function (such as mouse IgG2 or human IgG1) showed constructs with low toxicity and high antitumor activity supported by Fc-mediated Treg depletion and moderate systemic immune cell activation [95,96]. This demonstrates the importance of selecting appropriate IgG subtypes for ICK design.
Prostate-specific membrane antigen (PSMA) is expressed in all forms of prostate tissue, including carcinomas, and is an attractive target for treatment of prostate cancer [97]. KM2812 is a C-terminus IL-2-fused anti-PSMA mouse–human chimeric IgG1 ICK. KM2812 was only tested in a xenograft model in which it exhibited antitumor activity [98].
Human epidermal growth factor receptor 2 (HER2/neu) is a well-recognized TAA in breast and ovarian cancers which are treated with the anti-HER2 mAb trastuzumab. Anti-HER2/neu IgG3–(IL-2) has been tested in preclinical models with the aim to improve the partial success of trastuzumab observed in the clinic. This fusion protein demonstrated promising efficacy associated with an increased antigen presentation efficacy in mouse models [99] but no further development was reported, possibly because of the limitation of using wild-type IL-2 described hereafter.
Although the above-mentioned constructs enabled to drive IL-2 targeting to the TME, some adverse effects of IL-2 were still observed with some ICKs such as hu14.18–IL-2 or DI-Leu16-IL-2. The characterization of the IL-2 signaling pathway enabled to better understand the underlying molecular mechanisms responsible for IL-2-related toxicity and led to the development of several engineered IL-2 cytokines which have been used for ICK design.
4.2. Engineered IL-2 Ig-Fused ICKs
Hu14.18–IL-2 has been associated with fever, chills, fatigue, pruritus, and myalgias, and severe toxicities, especially those of the vascular compartment such as capillary leak syndrome [100,101,102]. The activation of cells expressing the intermediate-affinity IL-2 receptor in the vascular compartment was proposed to be responsible for the vascular toxicity by inducing inflammatory cytokine release by NK and other cells in response to hu14.18–IL-2 [103,104]. To overcome this issue, IL-2 has been engineered with D20T mutation (IL-2LT) and fused to the C-terminal part of the heavy chain of an NHS76 IgG2 antibody, recognizing DNA structures in the necrotic core of a tumor [105]. The IL-2LT molecule demonstrated a reduction in weight loss and an extended time interval before the onset of toxic symptoms in mice, as compared to recombinant IL-2 [105,106]. The resulting construct selectikine (NHS-IL-2LT) was assessed for patients with metastatic, locally advanced solid tumors, and B cell lymphoma in two phase I clinical trials and a phase II clinical trial which was terminated due to discontinuation of the program by the leading pharmaceutical firm [107,108,109]. Although the efficacy was left unknown, NHS-IL-2LT induced strong activation of both CD4+ and CD8+ T cells and only weak NK cell activation in line with a reduced activation of the intermediate-affinity IL-2 receptor on NK cells [106].
Another undesirable activity of IL-2-based ICKs is the induction of Treg expansion through the binding of IL-2 to IL-2Rα observed in patients dosed with NHS-IL-2LT [106] and DI-Leu16-IL-2 [81]. Since Tregs are well known for their immunosuppressive activity favoring tumor progression, development efforts have been made to engineer a recombinant IL-2 with low stimulating activity on Tregs. This led to the discovery of IL-2 variant (IL-2v) harboring a mutation enabling the preferential activation of T cells through IL-2Rβγ heterodimeric receptor without activating IL-2Rα (CD25) and, consequentially, not stimulating Tregs [110,111,112,113,114,115]. IL-2v has been used to generate ICKs targeting several antigens which all have reached the clinical phases: CEA (CEA-IL-2v, RG7813, cergutuzumab amunaleukin), fibroblast activation protein α (FAP) (FAP-IL-2v, RO6874281/RG7461, simlukafusp alfa), and PD-1 (RO7284755, RG6279, eciskafusp alfa).
CEA-IL-2v is composed of IL-2v fused to the C-terminus of a CEA-specific antibody devoid of Fc-mediated effector functions [116]. Consistently with the reduced binding of IL-2v to CD25, CEA-IL-2v induced a CD8+:CD4+ ratio toward CD8+ T cells both in the periphery and in the tumor associated with single-agent and combination efficacy in syngeneic models.
FAP-IL-2v is an IL-2v cytokine fused to the human IgG1 antibody 4B9 with abolished FcγR binding but functional FcRn binding to retain the advantage of IgG-like antibody half-life [117]. FAP-IL-2v demonstrated improved tumor targeting compared to FAP-IL-2wt and showed antitumor efficacy in combination with anti-PD-L1 immune checkpoint blocker [118].
PD-1-IL-2v is an anti-programmed cell death 1 (PD-1) antibody fused to IL-2v via its C-terminal. PD-1-IL-2v differentiates from CEA-IL-2v and FAP-IL-2v ICK from a targeting point of view since PD-1 is expressed on immune cells, particularly T lymphocytes, and has been well characterized as an immune checkpoint involved in T cell exhaustion and immunosuppression. PD-1 binds PD-L1 expressed on tumor cells or myeloid suppressive immune cells, resulting ultimately in tumor immunosurveillance escape [119]. The discovery of this mechanism, that also involves other immune checkpoint proteins, revolutionized the therapy of cancer with the development of immune checkpoint blockers such as anti-PD-1 antibodies. Therefore, the primary objective supporting the PD-1-IL-2v design was to obtain a construct with high immunostimulatory properties by taking advantage of both PD-1 blockade and IL-2 functions. RO7284755 treatment in combination with anti-PD-L1 elicited efficacious antitumor immunity by promoting pre-existing stem-like and tumor-reactive CD8+ T cells and remodeling immunosuppressive tumor-associated macrophages [115]. These data led to evaluation of PD-1-IL-2v in a phase I clinical trial to assess its safety profile across various dosage levels and to compare its efficacy both as a standalone treatment and in combination with anti-PD-L1 therapy, for the management of advanced and metastatic solid tumors.
IBI363, another anti-PD-1 antibody fused to a modified IL-2 (IL-2m) engineered to restore some CD25-mediated antitumor activities with a better safety profile than IL-2v [120], is also in clinical development.
SumIL-2 is another example of IL-2 engineering strategy designed to decrease CD25 binding while preferentially increasing IL-2Rβ binding to allow more-efficient cytotoxic T lymphocyte targeting in the TME. SumIL-2 has been fused to cetuximab (Erb-sumIL-2) to target epithelial growth factor receptor (EGFR) or to trastuzumab (a-HER2-sumIL-2) to target HER2/neu. Erb-sumIL-2 featuring an anti-human EGFR in one arm and a sumIL-2-Fc monomer in the second arm showed strong antitumor efficacy dependent on the induction of tumor-infiltrating CD8+ lymphocytes in animal models [110]. In the same study, the a-HER2-sumlL2 showed efficacy in combination with the tyrosine kinase inhibitor afatinib, although its effect on the antitumor immune functions was not documented.
4.3. Other IL-2 Fusion Proteins
Non-Ig ICK formats have been developed in order to take advantage of lower-molecular-weight proteins to facilitate the ICK penetration in the TME. Darleukin (L19-IL-2) and Teleukin (F16-IL-2) are two scFv diabody type IL-2 fusion ICK-targeting extracellular matrix components [50] that are still being clinically assessed. L19-IL-2 is composed of an antibody targeting the extracellular domain B (EDB) of fibronectin fused to two IL-2 molecules on the C-terminal domain of the heavy chains. L19-IL-2 has been assessed in clinical trials for the treatment of advanced solid tumors as monotherapy or in combination with existing chemotherapies. A two-armed phase II clinical trial in 69 patients suffering from stage IV melanoma concluded a significant increase in progression-free survival for patients receiving the L19-IL-2 plus dacarbazine combination therapy in comparison to chemotherapy alone [121]. F16-IL-2 uses another antibody (F16) which targets the Tenascin-C glycoprotein found in the extracellular matrix. F16-IL-2 showed beneficial signs and acceptable tolerability in combination with chemotherapy for the treatment of solid tumors and metastatic breast cancer [122]. F16-IL-2 has also shown promising anti-cancer effects and safety in patients suffering from acute myelogenous leukemia relapsed after allogeneic hematopoietic stem cell transplantation [123]. The most recent clinical trial investigates F16-IL-2 in combination with anti-PD-1 or chemotherapy.
Among the tumor antigens, some cell-surface proteins such as p53 or melanoma-associated antigen recognized by T cells (MART-1) are hardly druggable by classical antibodies because they require recognition in the context of major histocompatibility complex (MHC) class I presentation. T cell receptor (TCR) or antibody-like TCR have been designed to overcome this limitation. However, only few TCR-based ICKs have been reported, most likely because TCR exhibits overall reduced affinity of antigen binding and has slower on-rate binding which might not favor ICK accumulation in the TME compared to Ig-fused ICKs.
P53 tumor suppressor protein is overexpressed in a broad range of human malignancies. In addition, 264scTCR/IL-2 is a soluble single-chain TCR fusion protein constituted of IL-2 and a three-domain HLA-A2-restricted TCR specific for a peptide epitope of the human p53. Moreover, 264scTCR/IL-2 promoted NK cell infiltration into tumors [124] and reduced lung metastases in a mouse model [125].
MART-1scTCR-IL-2 is another example of a TCR-based ICK that has a similar design than 264scTCR/IL-2 but recognizes MART-1 antigen which is found on the surface of melanocytes and can be used to diagnose melanoma. MART-1scTCR-IL-2 has not been tested in a MART-1-positive tumor model but showed partial antitumor efficacy in a lung cancer model tumor in which the increased half-life of IL-2 was propose as a supporting factor underlying efficacy [124].
Finally, GI-101 is a non-Ig ICK which consists of the extracellular domain of CD80 acting as a cytotoxic T lymphocyte-associated protein 4 (CTLA-4) inhibitor linked to IL-2v through an IgG4 Fc fragment. CTLA-4 is constitutively expressed by Tregs but only upregulated in conventional T cells after activation. It binds to CD80 or CD86 expressed on the surface of antigen-presenting cells, a phenomenon which is particularly observed in cancers [126]. GI-101 inhibited tumor growth and induced a robust increase in M1 macrophages and CD8+ central memory T and NK cells, but not Tregs, in the TME of a syngeneic tumor model [127].
5. IL-15
IL-15 is a 13 kDa protein that exhibits effects similar to those of IL-2 [1]. This resemblance is attributed to the fact that IL-15 utilizes the same β and γ IL-2R chains for signaling as IL-2 but does not activate the IL-2Rα on Tregs [128]. IL-15 also diverges in its exclusive receptor, IL-15Rα [128]. Despite its comparable impact, IL-15 has garnered notably less attention in the field of ICKs compared to IL-2. IL-15 functions as a heterodimer cytokine complexed with IL-15Rα for stability [128] and promotes the growth and functions of NK cells and cytotoxic CD8+ T cells [129,130]. The antitumor effect of the cytokine directly depends on its ability to activate these two cell types. Compared to IL-2, toxicity of IL-15 is lower due to its ability to stimulate cytotoxic T cells without stimulating Tregs [131]. However, high dose recombinant IL-15 is associated to reduced appetite, diarrhea, and weight loss, without autoimmune manifestations or infections [132]. In addition, the half-life of IL-15 is around 1 h, making it hard to meet a therapeutic window [133]. Use of a truncated IL-15Rα composed of the sushi domain of the receptor has been successfully employed. Also called receptor-linker-IL-15 (RLI) (SO-C101), the fusion molecule of human IL-15 covalently linked to the human IL-15Rα sushi+ domain was employed for retaining IL-15 stability and binding capabilities [134].
5.1. IL-15 Ig-Fused ICKs
The anti-GD2-RLI and hu14.18–IL-15 are two ICKs designed to target IL-15 to GD2-positive tumors. The anti-GD2-RLI consists of an IgG1 anti-GD2 antibody fused to the N-terminal domain of human IL-15Rα linked via a 20-amino-acid sequence to human IL-15. This construct showed antibody effector functions and displayed strong antitumor activities in syngeneic cancer models. Interestingly, the anti-GD2-RLI had a higher therapeutic potency than those of IL-15Rα/IL-15 and anti-GD2 alone or in combination [135]. Hu14.18–IL-15 has a similar design to hu14.18–IL-2 described above but demonstrated higher efficacy in syngeneic mouse models with orthotopic neuroblastoma despite showing equivalent ADCC [136].
Combination therapy with recombinant IL-15 and blocking antibodies against CTLA-4 has been shown to increase survival and reduce tumor growth in murine tumor models, as compared to one of the treatments alone [137]. This led to the development of JK08 which is an ICK targeting the immune checkpoint family on T cells through a CTLA-4-targeting antibody connected to an IL-15/sushi domain fusion peptide. JK08 can elicit ADCC-mediated killing of CTLA-4-expressing cells such as Tregs enhancing the antitumor responses through depletion of Tregs and stimulation of NK and CD8+ T cells through IL-15 signaling [138]. JK08 is being evaluated in a phase I clinical trial.
KD033 and its murine cross-reactive surrogate srKD033 are anti-PD-L1/IL-15 fusion proteins engineered to have minimal effector functions via the introduction of the LALA mutation in the Fc portion which minimize the ADCC and CDC. IL-15 was linked to the C-terminal Fc domain with the intention to likely preserve high-affinity binding to PD-L1, favored by the opposite sites of binding, on either tumor or immune cells of the TME. Consistent with this mode of action, srKD033 demonstrated high retention in the TME and showed efficacy in several cancer models, including tumors known to be non-responsive to immune checkpoint blockers, such as the B16-F10 melanoma model [139].
GT-00AxIL-15 is an IL-15-based ICK targeting the tumor-specific, glycosylated epitope of MUC1 (TA-MUC1). TA-MUC1 is a carbohydrate–protein epitope expressed on a variety of tumor types but which is absent on normal cells. The targeting moiety of GT-00AxIL-15 is the humanized IgG1 mAb GT-00A (gatipotuzumab) clinically developed for treatment of solid tumors [140]. GT-00AxIL-15 is constituted of two human wild-type IL-15 molecules linked to the Fc domain of GT-00A which are not precomplexed with the IL-15Rα sushi domain. This construct exhibited a favorable tumor accumulation with a 13-day half-life in mouse serum and mediated antitumor effects associated with robust immune activation and expansion in a mouse model [141].
5.2. Engineered IL-15-Fused ICKs
IL-15N72D is an IL-15 mutein engineered to exhibit superagonist activity through improved binding ability to the human IL-15Rβ chain. N-803/ALT803 is an IL-15N72D associated to IL-15Rα sushi domain fused to a fully human IgG1 fragment [142]. 2B8T2M ICK was generated comprising four single-chain anti-human CD20 Fv domain of rituximab linked to the N-termini of the IL-15N72D and IL-15RαSu-Fc of the N-803 fusion protein [143]. With its IgG1 domain, 2B8T2M displays a tri-specific binding activity to CD20, IL-15Rβγc, and FcγR [143]. Treatment of tumor-bearing mice with 2B8T2M was more effective than rituximab [144].
The N-803 mutein was also fused to an anti-PD-L1 double scFv fusion protein to form an ICK named N-809. N-809 had improved efficacy versus N-803 + anti-PD-L1 combination and induced enhanced CD8+ T and NK cell tumor infiltration, activation, and function in the tumor model [145].
Another IL-15 mutein was engineered through the insertion of targeted mutations (N1G-D30N-E46G-V49R-E64Q) to eliminate IL-15Rα binding and reduce IL-2Rβ/γ affinity. This IL-15 mutein was linked monovalently to the C-terminus of an anti-PD-1 IgG1 having reduced FcγR binding through a flexible “GGGGSGGGGSGGGG” linker. The anti-PD-1-IL-15m (PF-07209960) was designed to deliver PD-1-mediated and avidity-driven IL-2/15 receptor stimulation to PD-1 positive tumor-infiltrating lymphocytes while minimally affecting circulating peripheral NK and T cells. This construct is currently being assessed in clinical trials after having shown the induction of exhausted CD8+ T cell expansion in tumors and higher efficacy, without aggravation of body weight loss, compared to single-agent treatments with an IL-15 superagonist, an anti-PD-1, or the combination thereof in a mouse model [146].
5.3. Other IL-15 Fusion Proteins
Lymphocyte-activation gene 3 (LAG-3) is a cell surface molecule expressed on activated T and NK cells. Similarly to PD-1 and CTLA-4, LAG-3 can be considered as a TAA involved in the negative regulation of the immune system. Anti-LAG-3 antibodies have been developed on the same rationale as other immune checkpoint blockers [147]. An anti-LAG-3-IL-15 ICK has been designed aiming to synergize the immunostimulatory effects of both the LAG-3 blockade and IL-15. This construct contains a single-chain IL-15/IL-15Rα and LAG-3-targeting arms attached to a heterodimeric Fc region. Interestingly, treatment with the anti-LAG-3-IL-15, combined productively with anti-PD-1, promoted T cell expansion and showed minimal peripheral activity [148].
PFC-1 (BJ-001) is a fusion protein targeting tumor cells that overexpress integrins such as alpha v beta 3 (avb3), alpha v beta 5 (avb5), and alpha v beta 6 (avb6). BJ-001 is composed of an integrin-targeting fusion protein, an Fc domain (IgG1), and an IL-15/IL-15Rα complex. BJ-001 was brought to clinical trial in 2020 after demonstrating impressive antitumor and immunostimulatory activities in mouse models [39,149].
Several IL-15-fused ICKs targeting the antigen FAP mentioned above have been generated. First, scFv_RD_IL-15 is an ICK featuring IL-15, an extended IL-15Rα sushi domain, and an anti-FAP antibody scFv moiety. The construct demonstrated strong tumor growth inhibitory effect in the B16 metastasis model compared to untargeted IL-15 or compared to the construct without the sushi domain [134]. Based on this construct, trifunctional antibody-fusion proteins have been tested by incorporating an additional arm with binding affinity for costimulatory members of the TNFR superfamily expressed on T and NK cells, CD137 (4-1BB), glucocorticoid-induced TNF (GITR), or CD134 (OX40), to functionally stimulate the immune response in the TME. These constructs have demonstrated promising potential to improve T cell activation and antitumor activities [150,151].
Similarly to Darleukin, a first IL-15 fusion protein with the L19 antibody in diabody format was designed but showed a suboptimal biodistribution profile compared to previously tested L19 ICKs [152]. Novel ICKs termed F8-F8-IL-15 and F8-F8-SD-IL-15, recognizing the alternatively spliced extra-domain A (EDA) of fibronectin, rather than its extra-domain B (EDB) like the L19 antibody fragment, have been developed with IL-15. F8-F8-IL-15 and F8-F8-SD-IL-15 are similar scFv diabodies except that F8-F8-SD-IL-15 is constituted of the IL-15Rα sushi domain in addition of IL-15. Despite a strong preferential uptake in solid tumors and their capacity to increase the CD8+/CD4+ T cell ratio in the TME, these two constructs did not show strong antitumor efficacy as monotherapies in primary tumors [153]. However, in the same study, both products were active in combination with targeted TNF therapy in mouse models and the F8-F8-SD-IL-15 was more efficient in reducing tumor metastasis formation in the lung thanks to the presence of the sushi domain.
The activating receptor natural killer group 2, member D (NKG2D) is an activating receptor belonging to the NKG2 family of C-type lectin-like receptors which is mostly expressed by NK cells and controls both adaptive and innate immunities [154]. Interaction of NKGD2 with its ligands (NKGD2L) leads to NK cell-mediated cytotoxicity. However, it was demonstrated that high serum concentrations of soluble NKG2DLs may suppress tumor immunity and NK cell activity via downregulation, contributing to an escape from the tumor immunosurveillance machinery [155,156]. DsNKG2D-IL-15 is an ICK comprising double NKG2D extracellular domains fused to IL-15 designed to target and stimulate NK cell activities. DsNKG2D-IL-15 administration increased the frequencies of NK but also CD8+ T cells in the TME and retarded the tumor growth in colon cancer models [157].
HCW9218 is a heterodimeric bifunctional fusion molecule consisting of the extracellular domains of the human TGFβ receptor II and the human interleukin-15 (IL-15)/IL-15 receptor α complex [158]. TGFβ exerts tumor-promoting effects via several mechanisms including immune suppression. Treatment with HCW9218 could block TGFβ functions through sequestration and lead to the reduction of the immunosuppressive TME, enhancing immune cell infiltration and tumor growth inhibition in mouse models [158,159]. HCW9218 is currently being tested in a phase Ib/II clinical trial.
5.4. Attenuated IL-15 with Tumor Cleavable Masking Systems
Similarly to N-809 or KD033, LH05 is also an anti-PD-L1 ICK fused to IL-15. LH05 differentiates from N-809 mainly based on the structure and the linker which were designed to attenuate IL-15 activity when fused to the ICK, and to release the active IL-15/IL-15Rα sushi domain in a proteolytic cleavage-dependent manner in the TME. This was achieved by incorporating a protease-cleavable linker between the antibody and the IL-15/IL-15Rα sushi domain which can mask IL-15 activity by steric hindrance caused by the Fc fragment and the sushi domain. In that sense, LH05 can be consider as an anti-PD-L1/IL-15 prodrug in which IL-15 is attenuated until reaching the TME, thereby preventing the “cytokine sink” effect and systemic IL-15 activities. The antitumor efficacy of LH05 was shown in an animal model and was associated with NK and CD8+ T cells recruitment/activation in cold tumors and demonstrated reduced systemic toxicity when compared to non-cleavable anti-PD-L1/IL-15 [160].
6. TNF
Tumor necrosis factor α (TNFα) was first identified in the 1970s, and a diverse range of functions were revealed [161,162] following the cloning of the gene in 1984. TNFα is predominantly synthesized in its full, membrane-bound form, organized as homotrimers [163]. The biologically active soluble form of TNF, measuring 17 kDa, is liberated through proteolytic cleavage by the metalloprotease TNFα-converting enzyme [164]. TNF’s role in tumor immunology is akin to a double-edged sword. On the one hand, systemic administration of recombinant TNFα (Beromun) has been approved for the treatment of patients suffering from soft-tissue sarcoma. TNFα inhibits tumor growth through several mechanisms. Firstly, the protein is directly cytotoxic for tumor cells. Secondly, TNFα promotes the expansion of activated B and T lymphocytes and facilitates the generation of cytotoxic T cells. In addition, it triggers the activation of monocytes/macrophages and granulocytes, resulting in heightened phagocytic activity, respiratory burst, degranulation, and enhanced adherence to endothelial cells [165]. However, this cytokine treatment is also limited in its therapeutic use due to its substantial side effects, like shock and organ failure, and short half-life [166,167]. On the other hand, in cancer resistant to TNFα-induced cytotoxicity, TNFα has the capacity to incite proliferation, enhance cancer cell survival, promote migration, and stimulate angiogenesis, resulting in tumor promotion [165].
Apart from an ICK named ZME-TNF, which binds to the glycoprotein 240 antigen and was the only TNF-IgG fused construct tested [168,169] but poorly documented, the scFv format was mostly used to design TNF-ICK as it favors the formation of trimeric TNF like endogenous homotrimers [47]. In addition, the smaller scFv and diabodies are more suited to decrease systemic cytokine levels [170].
A clinically advanced construct carrying TNF is the L19-TNF construct (Fibromun) which was designed using the same scFv antibody as Darleukin. Initially, monotherapy showed promising results in preclinical studies using mouse models of fibrosarcoma and colorectal cancer [171]. Tumors showed inhibited growth when compared to untargeted TNF and most tumors became necrotic upon a single i.v. administration of L19-TNF. However, the monotreatment in patients suffering from advanced solid tumors in a phase I/II clinical trial did not lead to any objective response [172]. In contrast, a combination treatment with chemotherapy induced an objective response in 89% of the patients suffering from advanced extremity melanoma [173].
However, the most clinically advanced TNF ICK treatment is a combination therapy with L19-TNF and L19-IL-2 called Nidlegy. The L19-IL-2/L19-TNF combination showed a complete response of some melanoma lesions upon intralesional administration [174]. In addition, around 50% of the non-injected lesions showed a complete response suggesting a systemic response [174]. The most advanced clinical trial, the phase III NeoDREAM trial, assesses the effect of L19-IL-2 and L19-TNF combination as neoadjuvant therapy in stage IIIB/C melanoma patients. As of today, no results of the trial have been published yet. The most recent clinical trial, assessing this combination, started in April 2022. The phase II, open-label, multicentric, proof-of-principle basket trial is recruiting 70 patients suffering from malignant tumors of the skin amenable to intra-tumoral injection.
The above-mentioned F8 scFv, targeting fibronectin as well, was fused to murine TNF (F8-TNF) [175]. The construct has been tested as a mono-treatment, or in combination with chemotherapy, in murine models of sarcoma in pre-clinical trials [175,176]. When used as a mono-therapy, F8-TNF demonstrated significant inhibition of tumor growth compared to the control group and resulted in complete cures for 40% of the animals [175]. Moreover, when combined with doxorubicin, F8-TNF exhibited an even stronger antitumor effect and led to complete eradication of tumors [175]. Further experiments revealed that when mice were rechallenged again with WEHI-164, a fibrosarcoma model, or heterologous C51 or CT26 colon cancer models, tumors did not grow showing immune memory. Using a depletion strategy, the authors showed that this immune memory was mediated by CD8+ T lymphocytes [176].
Carbonic anhydrase IX (CA-IX) is a TAA induced by hypoxia, implicated in cancer invasiveness, and correlated with therapeutic resistance [177]. cG250-TNF is an ICK comprising IgG CH2/CH3 truncated chimeric G250 antibody directed against CA-IX and fused to TNF. cG250-TNF demonstrated antitumor activity which could be synergized by combination treatment of low doses of nontargeted IFNγ. Administration of cG250-TNF and IFNγ resulted in significant synergistic tumoricidal activity in a renal cancer model [178].
Human TNF was engineered to generate an attenuated TNF-ICK that could reduce systemic toxicity through the substitution of isoleucine for alanine (TNFI97A) and was fused to the L19 antibody fragment (L19-TNFI97A). L19-TNFI97A showed decreased activity and binding on TNFR1 when compared to wild-type L19-TNF [171]. Interestingly, the biological activity of L19-TNFI97A on TNFR1 was restored upon binding to the target antigen EDB. L19-TNFI97A showed a potent antitumor effect without apparent toxicity compared with the wild-type protein.
A recombinant human TNFα (rmhTNFα) was fused to the GX1 peptide designed to target the gastric cancer vasculature [179,180]. The fusion protein that binds to Transglutaminase 2 (TGM2) was selectively delivered to targeted tumor sites, significantly improving the antitumor activity and decreasing the systemic toxicity of rmhTNFα.
7. IL-12
IL-12 is a cytokine consisting of heterodimeric subunits encoded by distinct genes: IL-12A (p35) and IL-12B (p40). The two separate proteins are linked through the formation of three disulfide bridges, resulting in the creation of the P70 fragment [181]. Functionally, this cytokine is classified as pro-inflammatory, as it elicits the differentiation of CD4+ Th1 T cells, promotes the development of cytotoxic CD8+ T cells, induces NK cells and NK T cells, and concurrently suppresses tumor-associated macrophages [182,183,184]. In addition, IL-12 induces the release of IFNγ [185] and has exhibited notable antitumor efficacy in numerous pre-clinical cancer models [186]. However, the efficacy of tolerable dose recombinant IL-12 in clinical trials showed no significant benefit [187,188,189,190]. As it is often the case with cytokines, the systemic administration of recombinant IL-12 is accompanied by pronounced adverse events [187,191]. Systemic IL-12 administration, in humans, is linked to a surge in systemic levels of IFNγ, TNFα, and IL-6 associated with a reduction in peripheral blood lymphocytes, monocytes, and neutrophils [187,191]. Since IL-12 can activate both the innate and the adaptive components of the immune system [186], it represents an ideal payload for ICK-based immunotherapies.
7.1. IL-12 Ig-Fused ICKs
Recently, IL-12 constructs have also been introduced to clinical trials. The most well-established IL-12 construct used in clinical trials is NHS-IL-12, which is composed of the previously mentioned NHS76 antibodies fused to the P70 heterodimeric form of IL-12 [192]. The construct showed an increase in host immunity with a reduction in immunosuppressive myeloid cells in the TME leading to a significant reduction in tumor growth in a murine bladder cancer model [193,194]. Since 2020, NHS-IL-12 entered in 10 clinical trials for the treatment of patients suffering from several types of cancers including breast, cervical, and pancreatic cancer. Early results show treatment-related adverse events including a decrease in circulating lymphocytes, an increase in transaminases, and flu-like symptoms, but most adverse events were low-grade [195]. In addition, an increase in frequencies of activated and mature NK cells and NK T cells was witnessed. Around 50% of the patients experienced a stable disease but no objective antitumor response was observed [195,196].
IL-12 has also been fused to another antibody, named BC1, targeting the extra-domain B (EDB) of fibronectin [197,198,199]. BC1-IL-12 (AS1409) showed antitumor activity in several human tumor models in SCID mice [200]. SCID mice lack functional T and B cells, suggesting that the therapeutic effect is NK cell-driven rather than B or T cell-driven. The construct was evaluated in patients with melanoma and renal cell carcinoma for its safety, efficacy, markers of IL-12-mediated immune response (IFNγ levels), and pharmacokinetics [197]. BC1-IL-12 was well tolerated and IFNγ levels were increased in all patients. In six out of 11 patients a stable disease was witnessed [197].
The chTNT-3/huIL-12 ICK was constructed by fusing IL-12 with the necrosis (single-stranded DNA)-targeting antibody chTNT-3. This fusion protein induced reduction in five prostatic tumors in a humanized mouse model [201].
7.2. Other IL-12-Fused ICKs
A novel antibody targeting FAP, named 7NP2, was recently fused to IL-12 in an scFv diabody format. This construct showed a potent antitumor activity in mouse models, both as a single agent and in combination with an immune checkpoint blocker. In non-human primates, the fully human construct was tolerated [202].
Another ICK carrying IL-12 is based around the L19 antibody, described above for IL-2 delivery, called IL-12-L19L19 (Dodekin). The construct is a product of a great extent of protein engineering for optimal IL-12 delivery [203,204,205,206,207]. IL-12 is fused to a single chain of the L19 diabody through its C-terminus by a 15-amino-acid linker [203]. The single chain still comprises two fused heavy chains and two light chains [208]. Linker optimization studies revealed that a 15-amino-acid linker (GSADGGSSAGGSDAG) displayed the best tumor-targeting properties [207]. Dodekin is currently being tested in a phase I clinical trial in patients with solid tumors.
The von Willebrand factor A3 domain has been shown to serve as a collagen-binding domain (CBD) enabling binding of fusion proteins to exposed collagen in disordered tumor vasculature [209]. A CBD-IL-12 ICK has been shown to remodel the TME of immunologically “cold” murine tumor models and to cooperate with immune checkpoint blockers to induce tumor eradication [190,210].
8. IL-21
IL-21 is a heterodimeric cytokine described for its pleiotropic effects [1]. In the context of tumor biology, its most pivotal role lies in the induction of cytotoxic T cells [211]. IL-21 alone does not initiate the development of CD8+ T cells, but it exhibits robust synergy with IL-7 or IL-15, bolstering CD8+ proliferation, enhancing functional responses, and promoting the production of IFNγ in mice [212]. Despite being evaluated in clinical trials, recombinant IL-21 has also encountered challenges related to toxicity and half-life [213]. This necessitates a critical focus on strategies such as half-life extension and localized delivery to optimize IL-21 antitumor efficacy.
Currently an IL-21 carrying human serum albumin (HSA)-targeting nanobody (JS014) is in a phase I clinical trial. HSA is one of the most abundant proteins in serum and has a half-life of around 19 days which makes it a good candidate for half-life extension strategies [214]. This strategy resulted in the half-life of JS014 to be 10-fold of that of recombinant IL-21 [215]. The construct showed strong antitumor efficacy in murine MC38 tumors. In addition, JS014 increased the antitumor effect of PD-1 and T cell immunoglobulin and ITIM domain (TIGIT) blockades in MC38 tumors [215,216].
A different construct, still in pre-clinical development, is an EGFR-targeting antibody (Erbitux) carrying IL-21 [217]. The construct was compared to anti-EGFR-IL-2 and showed much lower toxicity and improved antitumor efficacy in MC38 tumors bearing a chimeric EGFR, in which six amino acids of mouse EGFR were replaced by the residues found in human EGFR. The efficacy was achieved by expanding existing intra-tumoral functional CD8+ T cells rather than recruiting new lymphocytes. Additionally, EGFR-IL-21 could overcome checkpoint blockade resistance in mice with advanced MC38 tumors [217].
IL-21 was also fused to the fibronectin-targeting antibody F8 in a single-chain diabody format at the C-terminus [218]. Biodistribution experiments showed that this construct did not preferentially accumulate in the tumor site due to the peripheral binding of IL-21, highlighing the need to engineer IL-21 to improve its binding and activity at the tumor site.
In line with this concept, another IL-21-carrying construct is PD-1-targeted (AMG256) and was in a phase I trial in 2020 to evaluate its safety, tolerability, pharmacokinetics, and pharmacodynamics in patients with advanced solid tumors [219]. The rationale behind the construct was to reactivate the intra-tumoral CD8+ T cells by targeting them using anti-PD-1. The AMG256 construct does this by delivering a highly attenuated IL-21 mutant that was created by using structure-guided protein engineering. The mutant cytokine was designed to bind its receptor only when the antibody is already bound to PD-1 [220]. This design was used to restrict the activity of the cytokine to target cells and thereby improve the safety profile properties of the drug [220]. The fusion protein inhibited cancer growth in a humanized melanoma model compared to PD-1 treatment [220].
IL-21 was also fused to hu14.18. The resulting construct hu14.18–IL-21 ICK stimulated CD8+ T cells and M1 tumor-associated macrophages, decreased the recruitment of immunosuppressive cells to the TME, and mediated potent antitumor cytotoxicity against neuroblastoma more efficiently than hu14.18–IL-2 [136].
9. IL-10
IL-10 is recognized as an anti-inflammatory cytokine that operates as a dimer [1]. Its primary functions include the downregulation of antigen presentation and the inhibition of the release of pro-inflammatory mediators [1]. The role of IL-10 in cancer remains a subject of controversy. IL-10 is known to exert inhibitory effects on macrophages and the pro-inflammatory CD4+ Th17 T cell response [221]. Notably, mice and humans deficient in IL-10 display spontaneous development of inflammatory bowel disease [222]. The anti-inflammatory properties foster the pro-tumor image of IL-10. Nonetheless, individuals in whom proper IL-10 signaling is compromised, whether mice or humans, display a markedly heightened susceptibility to spontaneous tumor development [223,224]. In contrast, IL-10 treatment has surprisingly demonstrated antitumor efficacy, as shown with PEGylated IL-10, which induced the expression of IFNγ and granzymes in the tumor and induced the CD8+ T cell-mediated rejection of breast cancer models in mice. When challenged after 8 months, the mice rejected the tumor, suggesting durable immune memory induced by the treatment [225]. The immunostimulatory functions of the PEGylated form of IL-10 (pegilodecakin), that extend the cytokine lifetime, were confirmed in cancer patients [226], but it did not improve the outcome of patients with gemcitabine-refractory metastatic pancreatic [227] or metastatic lung cancer [228]. This led to the discontinuation of the pegilodecakin program, despite evidence of IL-10 biological effect in peripheral blood.
Due to its unexpected and relatively lately uncovered antitumor activity, IL-10 has also recently begun to receive attention in the ICK field. The F8 antibody carrying IL-10 is clinically being tested for the treatment of rheumatoid arthritis or ulcerative colitis [229] and would be an interesting construct to assess for cancer therapy. To date, there have been no ICKs incorporating IL-10 that have advanced to clinical trials for cancer treatment.
CmAb-(IL-10) is an anti-EGFR antibody (cetuximab)-based IL-10 ICK which displayed enhanced antitumor responses on an EGFR-positive tumor mouse model associated with a reduced toxicity. The authors draw the conclusion that this construct effectively hinders dendritic cell-mediated apoptosis of CD8+ T cells [230].
The anti-CSF1R-IL-10 is a fusion protein consisting of a murine immunoglobulin G2a (IgG2a) isotype anti-mouse colony-stimulating factor-1 receptor (CSF1R) antibody fused to the heavy-chain C-terminus via a linker sequence fused to IL-10. This construct intends to better deliver IL-10 in tumor-associated macrophages (TAMs) since CSF1R is highly expressed in TAMs. Treatment with this ICK demonstrated a reduction in TAMs and significant antitumor activity in preclinical models [231].
10. GM-CSF
Granulocyte-macrophage colony-stimulating factor (GM-CSF) is a glycoprotein secreted by immune cells, endothelial cells, and fibroblasts which drives the generation of myeloid cell subsets including monocytes, neutrophils, macrophages, and DCs in response to infections and cancers. The recombinant form of GM-CSF (sargramostim) has potent immunostimulant functions and is primarily used for myeloid reconstitution after bone marrow transplantation. In cancer, GM-CSF has been considered as a double-edged sword in the field of immunotherapy as it can, depending on the context, polarize TAMs toward M1 or M2 phenotypes, induce inflammatory neutrophils but enhance immunosuppressive MDSCs, increase pro-inflammatory DCs but also render them tolerogenic, and, finally, stimulate both effector T cells and Tregs [232]. Administration of GM-CSF has shown to provide clinical benefit in patients with several cancers [233,234,235,236]. In the field of anti-cancer vaccines, although GM-CSF has been widely used, it is unclear to which extent it contributed to the efficacy of treatment in these studies [237,238,239]. A critical factor influencing whether GM-CSF exerts anti- or pro-tumorigenic effects is the administrated dose [240] and the administration route. For example, the MDSC immunosuppressive effect of GM-CSF is seen only during its systemic administration but not when GM-CSF secretion is localized to the TME [241]. Therefore, targeted delivery of GM-CSF to the TME may be more beneficial than its systemic administration to promote DC maturation and enhance the presentation of tumor antigens.
Notably, anti-HER2/neu IgG3-(GM-CSF) is a full IgG ICK fused to GM-CSF that accumulated in the tumor and spleen in animal models and was able to enhance both CD4+ Th1- and Th2-mediated immune responses and reduce tumor growth in anti-HER2-resistant tumors [242].
More recently, interesting studies with the fusion protein rlipoE7m-MoGM were published. rlipoE7m-MoGM is a bifunctional protein which combines Toll-like receptor 2 (TLR2) agonist and GM-CSF activities. TLR members are expressed in myeloid cells such as DCs and are known to stimulate antigen presentation [243]. rlipoE7m is a recombinant lipoprotein engineered with a mutated HPV16 E7 tumor antigen that binds to TLR2 and can activate DCs and eradicate tumor progression in mice [244]. rlipoE7m-MoGM demonstrated strong antitumor immune response, both local and systemic, in mouse models [245].
11. IFNγ
IFNγ is a key immunoregulatory pleotropic cytokine that belongs to the type II class of interferons. IFNγ is secreted by immune cells themselves and dimerizes to induce strong innate and adaptive immune responses through the binding to IFNGR1 and IFNGR2. Like GM-CSF, IFNγ has a dual role in cancer as it can favor the antitumor immune response through ischemia induction, antigen presentation cell activation, macrophage repolarization, induction of Tregs fragility, and MDSC inhibition, but it also favors tumor escape via the induction of tolerant molecules expression (such as CTLA-4 and PD-L1), stimulating angiogenesis and promoting tumor progression [246].
In the clinic, administration of recombinant IFNγ (IFNγ1b, Actimmune) in combination with chemotherapy has shown controversial results [247,248,249]. Despite a high number of ongoing clinical trials, this cytokine has not been approved by the FDA to treat patients with cancers, except malignant osteoporosis, possibly because of the contribution of IFNγ to tumor evasion and the induction of adverse events [249].
Surprisingly, IFNγ has only attracted minor interest in the field of ICKs so far, likely because this cytokine is prone to an important “cytokine sink” effect due to its cognate receptors found in different organs which may limit its accumulation in the TME. IFNγ was fused to L19 and F8 antibodies as described above. The tumor targeting ability of the L19-IFNγ ICK was influenced by the number of IFNγ receptors expressed in the mouse and the F8-IFNγ ICK did not localized preferentially in the tumor as it could have been expected [250,251]. One recently published construct with improved tumor-homing properties is L19-IFNγ KRG. This ICK is an IgG4 format of the L19 antibody fused at the heavy-chain C-terminal domain to a truncated version of IFNγ designed to be protected against proteolytic digestion at its C-terminus and to show reduced affinity to its receptor [252]. L19-IFNγ KRG selectively localized to neoplastic lesions, led to tumor growth inhibition, and increased the intra-tumoral T and NK cells in combination with anti-PD-1.
chTNT-3/muIFNγ is another IFNγ-based ICK designed with the necrosis-targeting antibody chTNT-3. This ICK showed significant intra-tumoral retention and was able to decreased the number of metastatic tumors in an animal model without causing any observable toxicity, but its activity was not associated with any modulation of tumor-infiltrating cytotoxic T cells [253].
12. IFNα
Interferon alfa 2 (IFNα) is the most extensively characterized cytokine of the IFN type I family. IFNα modulates both innate and adaptive immune responses via activating monocytes and antigen-presenting cells. IFNα is used to treat several types of cancer, including hairy cell leukemia, melanoma, and renal cell carcinoma [254]. Mechanistically, IFNα2 exhibits direct cytotoxicity and anti-proliferative activities on tumor cells [255]. In addition, IFNα stimulates the antitumor immune function by activating dendritic cells [256]. However, the frequent administration of IFNα required to compensate for its short half-life produces toxic side effects including severe depression, fever, and headache leading to the abandonment of the treatment in many patients [257].
IFNα fused to rituximab demonstrated efficacy in lymphoma models though a molecular mechanism involving the binding of the construct to CD20 and the expression of IFNα receptor on tumor cells, suggesting a direct antitumor effect of this construct. However, the activity of this construct on the immune system was not described [258].
JZA01 and JZA02 are two IFNα-based ICKs in an Ig format fused to an anti-VEGFR2 designed to target solid tumors. JZA01 showed efficacy in tumor-bearing mice despite its reduced IFNα activity observed in vitro compared to native IFNα [259]. JZA02 has the same design as JZA01 but carries a mutated version of IFNα aiming to optimize affinity to its receptor which was reduced when fused as an ICK. Treatment with this construct showed improved antitumor efficacy compared to JZA01 and demonstrated enhanced dendritic cell activity and increased CD8+ T cell infiltration in the TME [260].
13. Summary and Perspectives
The cytokine field has expanded significantly over the last few decades, but their therapeutic use has faced several challenges, as described in this review. The growing number of published ICKs entering the clinical phase demonstrates the relevance of this technology, which provides several advantages and opportunities for improvement.
ICKs often increase the cytokine half-life, which was one of the main issues observed with naked cytokine administration. Depending on the format choice, ICKs offer the possibility to modulate tumor penetration and engage, or not, the binding to selected Fc receptors. This modulation affects pharmacokinetics and the contribution of antibody-mediated effector functions, which can impact the antitumor immune response and the extent of side effects simultaneously. The first ICKs were designed to only deliver cytokines to the TME. The advances in cancer biology understanding, associated with the discovery of therapeutic mAb, having antitumor activity on their own, enabled the generation of ICKs that combine several modes of action. The antibody moieties of ICKs have targeted a broad range of tumor-associated/specific antigens, initially mostly expressed by tumor cells, the extracellular matrix, and tumor-associated vessels. With the recent breakthrough of cancer immunotherapy, an increasing number of ICKs were designed to target immune cells, enabling, for example, the combination of immunostimulatory modes of action of the selected cytokine with immune checkpoint blockades. Although very promising and despite many completed clinical trials, none of the developed ICKs have been approved for use in the treatment of cancer so far [261] and there are still several ways of improvement to be considered before these molecules can translate into clinical use.
In many cases, the adverse effects linked to the use of the ICK format resemble those observed with free cytokines, and this is because the cytokine is selected for its ability to retain functionality when fused to an antibody. A possible future direction for ICKs is tumor-activatable cytokines. This solution would reduce the systemic activity of the cytokines and thereby most of their side effects. Activation mechanisms of a cap have been explored using enzymes that are highly expressed in the TME compared to healthy tissue. Such a construct could combine a cytokine capping or activation system in combination with an ICK [262]. Tumor-activatable cytokines can be achieved by using amino acid sequences, sensitive to tumor-enriched proteases, to fuse the cytokine with an mAb. This emerging approach was successful in preclinical models with the LH05 ICK (anti-PD-L1-IL-15) [160]. In addition, ICK fusions such as the anti-VEGFR2-INFα can exhibit a partial reduction in cytokine activity [259]. This might not only be a result of the nature of the cytokine but also of the cytokine position on the antibody or the type of fusion, which constitutes another way, potentially combinable with a tumor-activatable technology, to attenuate the cytokine and minimize both the peripheric “cytokine sink” effect and the cytokine systemic toxicity. Cytokine engineering is also an option to attenuate cytokine functions, as shown with the L19-TNFI97A [171]. Activity-on-Target cytokines (AcTakines) are ICKs combining a targeting antibody and an engineered cytokine mutant with reduced receptor affinity. In AcTakines, the wild-type cytokine is replaced by a mutant version with strongly reduced affinity for its receptor. Consequently, they are inactive until they regain full activity on the targeted cells by local avidity-driven receptor binding [263]. Although this technology can reduce systemic toxicity, such constructs contain foreign epitopes that can induce immunogenicity, as reported in a phase I clinical trial with the Hu14.18-IL-2 ICK [264]. Nevertheless, the number of ICKs using the concept is progressing in clinical trials, demonstrating the promise of this therapeutic approach.
For ICK monotherapy that did not lead to the expected clinical efficacy, multiple cytokine-carrier ICKs may be considered. While Tripokin, a fully-human immunostimulatory product consisting of a trimeric TNF/IL-2/L19 ICK seems promising [265], an mDCH fusion protein, composed of an anti-epithelial cell adhesion molecule (EpCam) fused to IL-2 and GM-CSF, did not significantly synergize in tumor rejection [266]. The number of dual-cytokine ICKs is still limited but the scale and the variety of the cytokine repertoire open the path to many possible combinations which, if rationally selected, may have synergetic effects in vivo.
Finally, the increasing number of bispecific antibodies developed opens the possibility to design ICKs with additional modes of action via the engagement of two antigens such as the bispecific PD-1-IL-2v and anti-PD-L1 ICK [115]. The design of bispecific-antibody ICKs can also enable to further stimulate the antitumor immune response through the targeting of two cell types, similarly to the bispecific T cell engagers (BiTEs) [267].
In conclusion, multiple solutions are being developed around the ICK concept to fully optimize the therapeutic potential of cytokines. ICKs have not been approved yet, but the constantly evolving protein engineering technologies enabling a tumor-specific activation of the cytokines, together with the right selection of optimal combinations of cytokines with antibodies targeting tumor immunosuppressive mechanisms, might have synergic functional properties on the anti-tumor immune system which should translate to novel therapeutic options for patients with cancer.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics16080974/s1, Table S1: Abreviations list03.06.2024, Table S2: ICK 03062024.
Author Contributions
Conception: B.B. and A.P.; search, analyses, and interpretation of literature: B.B., H.P. and A.P.; figures: H.P.; writing: B.B. and A.P.; revision of the manuscript: all authors. All authors have read and agreed to the published version of the manuscript.
Funding
The APC was funded by a I-Site Cap 20-25 from the Université Clermont Auvergne (IR24POMMIER).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Acknowledgments
The authors would like to thank Peter Lowe for his critical comments and advice on the manuscript.
Conflicts of Interest
The authors declare no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Abbas, A.K.; Lichtman, A.H.; Pillai, S.; Baker, D.L. Basic Immunology: Functions and Disorders of the Immune System, 8th ed.; Elsevier, Saunders: Philadelphia, PA, USA, 2015. [Google Scholar]
- Dinarello, C.A. Overview of the IL-1 Family in Innate Inflammation and Acquired Immunity. Immunol. Rev. 2018, 281, 8–27. [Google Scholar] [CrossRef]
- Dinarello, C.A. Historical Review of Cytokines. Eur. J. Immunol. 2007, 37 (Suppl. S1), S34–S45. [Google Scholar] [CrossRef] [PubMed]
- Rosenwasser, L.J.; Dinarello, C.A.; Rosenthal, A.S. Adherent Cell Function in Murine T-Lymphocyte Antigen Recognition. IV. Enhancement of Murine T-Cell Antigen Recognition by Human Leukocytic Pyrogen. J. Exp. Med. 1979, 150, 709–714. [Google Scholar] [CrossRef]
- Kiessling, R.; Klein, E.; Wigzell, H. “Natural” Killer Cells in the Mouse. I. Cytotoxic Cells with Specificity for Mouse Moloney Leukemia Cells. Specificity and Distribution According to Genotype. Eur. J. Immunol. 1975, 5, 112–117. [Google Scholar] [CrossRef]
- Slingluff, C.L.; Cox, A.L.; Stover, J.M.; Moore, M.M.; Hunt, D.F.; Engelhard, V.H. Cytotoxic T-Lymphocyte Response to Autologous Human Squamous Cell Cancer of the Lung: Epitope Reconstitution with Peptides Extracted from HLA-Aw68. Cancer Res. 1994, 54, 2731–2737. [Google Scholar]
- Chan, C.W.; Housseau, F. The ‘Kiss of Death’ by Dendritic Cells to Cancer Cells. Cell Death Differ. 2008, 15, 58–69. [Google Scholar] [CrossRef]
- Xiong, S.; Dong, L.; Cheng, L. Neutrophils in Cancer Carcinogenesis and Metastasis. J. Hematol. Oncol. 2021, 14, 173. [Google Scholar] [CrossRef]
- Matlung, H.L.; Babes, L.; Zhao, X.W.; van Houdt, M.; Treffers, L.W.; van Rees, D.J.; Franke, K.; Schornagel, K.; Verkuijlen, P.; Janssen, H.; et al. Neutrophils Kill Antibody-Opsonized Cancer Cells by Trogoptosis. Cell Rep. 2018, 23, 3946–3959.e6. [Google Scholar] [CrossRef]
- Wu, L.; Saxena, S.; Singh, R.K. Neutrophils in the Tumor Microenvironment. Adv. Exp. Med. Biol. 2020, 1224, 1–20. [Google Scholar] [CrossRef]
- Sarvaria, A.; Madrigal, J.A.; Saudemont, A. B Cell Regulation in Cancer and Anti-Tumor Immunity. Cell Mol. Immunol. 2017, 14, 662–674. [Google Scholar] [CrossRef]
- Jorgovanovic, D.; Song, M.; Wang, L.; Zhang, Y. Roles of IFN-γ in Tumor Progression and Regression: A Review. Biomark. Res. 2020, 8, 49. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, L.; Qin, Z. Paradoxical Roles of IL-4 in Tumor Immunity. Cell Mol. Immunol. 2009, 6, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Oft, M. IL-10: Master Switch from Tumor-Promoting Inflammation to Antitumor Immunity. Cancer Immunol. Res. 2014, 2, 194–199. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic Inflammation and Cytokines in the Tumor Microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed]
- Mumm, J.B.; Oft, M. Cytokine-Based Transformation of Immune Surveillance into Tumor-Promoting Inflammation. Oncogene 2008, 27, 5913–5919. [Google Scholar] [CrossRef] [PubMed]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a Tumor Promoter in Cancer Induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Massagué, J.; Blain, S.W.; Lo, R.S. TGFβ Signaling in Growth Control, Cancer, and Heritable Disorders. Cell 2000, 103, 295–309. [Google Scholar] [CrossRef] [PubMed]
- Chawla-Sarkar, M.; Leaman, D.W.; Borden, E.C. Preferential Induction of Apoptosis by Interferon (IFN)-Beta Compared with IFN-Alpha2: Correlation with TRAIL/Apo2L Induction in Melanoma Cell Lines. Clin. Cancer Res. 2001, 7, 1821–1831. [Google Scholar] [PubMed]
- Steen, H.C.; Gamero, A.M. Interferon-Lambda as a Potential Therapeutic Agent in Cancer Treatment. J. Interferon Cytokine Res. 2010, 30, 597–602. [Google Scholar] [CrossRef]
- Heichler, C.; Scheibe, K.; Schmied, A.; Geppert, C.I.; Schmid, B.; Wirtz, S.; Thoma, O.-M.; Kramer, V.; Waldner, M.J.; Büttner, C.; et al. STAT3 Activation through IL-6/IL-11 in Cancer-Associated Fibroblasts Promotes Colorectal Tumour Development and Correlates with Poor Prognosis. Gut 2020, 69, 1269–1282. [Google Scholar] [CrossRef]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Ségui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef] [PubMed]
- Baba, A.B.; Rah, B.; Bhat, G.R.; Mushtaq, I.; Parveen, S.; Hassan, R.; Hameed Zargar, M.; Afroze, D. Transforming Growth Factor-Beta (TGF-β) Signaling in Cancer-A Betrayal Within. Front. Pharmacol. 2022, 13, 791272. [Google Scholar] [CrossRef] [PubMed]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, Inflammation, and Cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Gresser, I.; Bourali, C. Antitumor Effects of Interferon Preparations in Mice. JNCI J. Natl. Cancer Inst. 1970, 45, 365–376. [Google Scholar] [CrossRef] [PubMed]
- Borden, E.C.; Sen, G.C.; Uze, G.; Silverman, R.H.; Ransohoff, R.M.; Foster, G.R.; Stark, G.R. Interferons at Age 50: Past, Current and Future Impact on Biomedicine. Nat. Rev. Drug Discov. 2007, 6, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Jiang, T.; Zhou, C.; Ren, S. Role of IL-2 in Cancer Immunotherapy. Oncoimmunology 2016, 5, e1163462. [Google Scholar] [CrossRef] [PubMed]
- Zídek, Z.; Anzenbacher, P.; Kmoníčková, E. Current Status and Challenges of Cytokine Pharmacology. Br. J. Pharmacol. 2009, 157, 342–361. [Google Scholar] [CrossRef] [PubMed]
- Boersma, B.; Jiskoot, W.; Lowe, P.; Bourquin, C. The Interleukin-1 Cytokine Family Members: Role in Cancer Pathogenesis and Potential Therapeutic Applications in Cancer Immunotherapy. Cytokine Growth Factor. Rev. 2021, 62, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gowin, K.; Jain, T.; Kosiorek, H.; Tibes, R.; Camoriano, J.; Palmer, J.; Mesa, R. Pegylated Interferon Alpha-2a Is Clinically Effective and Tolerable in Myeloproliferative Neoplasm Patients Treated off Clinical Trial. Leuk. Res. 2017, 54, 73–77. [Google Scholar] [CrossRef]
- Sunela, K.L.; Koskinen, S.; Kellokumpu-Lehtinen, P.-L. A Phase-II Study of Combination of Pegylated Interferon Alfa-2a and Capecitabine in Locally Advanced or Metastatic Renal Cell Cancer. Cancer Chemother. Pharmacol. 2010, 66, 59–67. [Google Scholar] [CrossRef]
- Bentebibel, S.-E.; Hurwitz, M.E.; Bernatchez, C.; Haymaker, C.; Hudgens, C.W.; Kluger, H.M.; Tetzlaff, M.T.; Tagliaferri, M.A.; Zalevsky, J.; Hoch, U.; et al. A First-in-Human Study and Biomarker Analysis of NKTR-214, a Novel IL2Rβγ-Biased Cytokine, in Patients with Advanced or Metastatic Solid Tumors. Cancer Discov. 2019, 9, 711–721. [Google Scholar] [CrossRef] [PubMed]
- Mattos, A.; de Jager-Krikken, A.; de Haan, M.; Beljaars, L.; Poelstra, K. PEGylation of Interleukin-10 Improves the Pharmacokinetic Profile and Enhances the Antifibrotic Effectivity in CCl4-Induced Fibrogenesis in Mice. J. Control. Release 2012, 162, 84–91. [Google Scholar] [CrossRef]
- Pettit, D.K.; Bonnert, T.P.; Eisenman, J.; Srinivasan, S.; Paxton, R.; Beers, C.; Lynch, D.; Miller, B.; Yost, J.; Grabstein, K.H.; et al. Structure-Function Studies of Interleukin 15 Using Site-Specific Mutagenesis, Polyethylene Glycol Conjugation, and Homology Modeling. J. Biol. Chem. 1997, 272, 2312–2318. [Google Scholar] [CrossRef] [PubMed]
- Robinson, T.O.; Hegde, S.M.; Chang, A.; Gangadharan, A.; Rivas, S.; Madakamutil, L.; Zalevsky, J.; Miyazaki, T.; Schluns, K.S. NKTR-255 Is a Polymer-Conjugated IL-15 with Unique Mechanisms of Action on T and Natural Killer Cells. J. Clin. Investig. 2021, 131, e144365. [Google Scholar] [CrossRef]
- Podobnik, B.; Helk, B.; Smilović, V.; Škrajnar, Š.; Fidler, K.; Jevševar, S.; Godwin, A.; Williams, P. Conjugation of PolyPEG to Interferon Alpha Extends Serum Half-Life While Maintaining Low Viscosity of the Conjugate. Bioconjugate Chem. 2015, 26, 452–459. [Google Scholar] [CrossRef]
- Joung, C.-H.; Shin, J.-Y.; Koo, J.-K.; Lim, J.J.; Wang, J.-S.; Lee, S.-J.; Tan, H.-K.; Kim, S.-L.; Lim, S.-M. Production and Characterization of Long-Acting Recombinant Human Albumin-EPO Fusion Protein Expressed in CHO Cell. Protein Expr. Purif. 2009, 68, 137–145. [Google Scholar] [CrossRef]
- Xu, W.; Jones, M.; Liu, B.; Zhu, X.; Johnson, C.B.; Edwards, A.C.; Kong, L.; Jeng, E.K.; Han, K.; Marcus, W.D.; et al. Efficacy and Mechanism-of-Action of a Novel Superagonist Interleukin-15: Interleukin-15 Receptor αSu/Fc Fusion Complex in Syngeneic Murine Models of Multiple Myeloma. Cancer Res. 2013, 73, 3075–3086. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Huang, Q.; Liu, J.; Xing, J.; Zhang, N.; Liu, Y.; Wang, Z.; Li, Q. A Targeted IL-15 Fusion Protein with Potent Anti-Tumor Activity. Cancer Biol. Ther. 2015, 16, 1415–1421. [Google Scholar] [CrossRef][Green Version]
- Zhao, J.; Si, Y.; Cheng, M.; Yang, Y.; Niu, Y.; Li, X.; Liu, X.; Yang, W. Albumin Fusion of Interleukin-28B: Production and Characterization of Its Biological Activities and Protein Stability. PLoS ONE 2013, 8, e64301. [Google Scholar] [CrossRef]
- Momin, N.; Mehta, N.K.; Bennett, N.R.; Ma, L.; Palmeri, J.R.; Chinn, M.M.; Lutz, E.A.; Kang, B.; Irvine, D.J.; Spranger, S.; et al. Anchoring of Intratumorally Administered Cytokines to Collagen Safely Potentiates Systemic Cancer Immunotherapy. Sci. Transl. Med. 2019, 11, eaaw2614. [Google Scholar] [CrossRef]
- Chen, B.; Mu, C.; Zhang, Z.; He, X.; Liu, X. The Love-Hate Relationship Between TGF-β Signaling and the Immune System During Development and Tumorigenesis. Front. Immunol. 2022, 13. [Google Scholar] [CrossRef]
- Kwaśniak, K.; Czarnik-Kwaśniak, J.; Maziarz, A.; Aebisher, D.; Zielińska, K.; Karczmarek-Borowska, B.; Tabarkiewicz, J. Scientific Reports Concerning the Impact of Interleukin 4, Interleukin 10 and Transforming Growth Factor β on Cancer Cells. Cent. Eur. J. Immunol. 2019, 44, 190–200. [Google Scholar] [CrossRef]
- Gout, D.Y.; Groen, L.S.; van Egmond, M. The Present and Future of Immunocytokines for Cancer Treatment. Cell. Mol. Life Sci. 2022, 79, 509. [Google Scholar] [CrossRef]
- Lyu, M.-A.; Kurzrock, R.; Rosenblum, M.G. The Immunocytokine scFv23/TNF Targeting HER-2/Neu Induces Synergistic Cytotoxic Effects with 5-Fluorouracil in TNF-Resistant Pancreatic Cancer Cell Lines. Biochem. Pharmacol. 2008, 75, 836–846. [Google Scholar] [CrossRef]
- Menssen, H.D.; Harnack, U.; Erben, U.; Neri, D.; Hirsch, B.; Dürkop, H. Antibody-Based Delivery of Tumor Necrosis Factor (L19-TNFα) and Interleukin-2 (L19-IL2) to Tumor-Associated Blood Vessels Has Potent Immunological and Anticancer Activity in the Syngeneic J558L BALB/c Myeloma Model. J. Cancer Res. Clin. Oncol. 2018, 144, 499–507. [Google Scholar] [CrossRef]
- Schwager, K.; Hemmerle, T.; Aebischer, D.; Neri, D. The Immunocytokine L19–IL2 Eradicates Cancer When Used in Combination with CTLA-4 Blockade or with L19-TNF. J. Investig. Dermatol. 2013, 133, 751–758. [Google Scholar] [CrossRef]
- Villa, A.; Ongaro, T. Il2 Immunoconjugates. EP3660039A1, 3 June 2020. Available online: https://patents.google.com/patent/EP3660039A1/en (accessed on 20 September 2023).
- Kontermann, R.E. Antibody–Cytokine Fusion Proteins. Arch. Biochem. Biophys. 2012, 526, 194–205. [Google Scholar] [CrossRef]
- Murer, P.; Neri, D. Antibody-Cytokine Fusion Proteins: A Novel Class of Biopharmaceuticals for the Therapy of Cancer and of Chronic Inflammation. New Biotechnol. 2019, 52, 42–53. [Google Scholar] [CrossRef]
- Ghetie, V.; Popov, S.; Borvak, J.; Radu, C.; Matesoi, D.; Medesan, C.; Ober, R.J.; Ward, E.S. Increasing the Serum Persistence of an IgG Fragment by Random Mutagenesis. Nat. Biotechnol. 1997, 15, 637–640. [Google Scholar] [CrossRef]
- Siegemund, M.; Seifert, O.; Zarani, M.; Džinić, T.; De Leo, V.; Göttsch, D.; Münkel, S.; Hutt, M.; Pfizenmaier, K.; Kontermann, R.E. An Optimized Antibody-Single-Chain TRAIL Fusion Protein for Cancer Therapy. MAbs 2016, 8, 879–891. [Google Scholar] [CrossRef]
- Bachmann, M.F.; Oxenius, A. Interleukin 2: From Immunostimulation to Immunoregulation and Back Again. EMBO Rep. 2007, 8, 1142–1148. [Google Scholar] [CrossRef] [PubMed]
- Gillis, S.; Baker, P.E.; Ruscetti, F.W.; Smith, K.A. Long-Term Culture of Human Antigen-Specific Cytotoxic T-Cell Lines. J. Exp. Med. 1978, 148, 1093–1098. [Google Scholar] [CrossRef] [PubMed]
- Gillis, S.; Smith, K.A. Long Term Culture of Tumour-Specific Cytotoxic T Cells. Nature 1977, 268, 154–156. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.A.; Ruscetti, F.W.; Gallo, R. Selective In Vitro Growth of T Lymphocytes from Normal Human Bone Marrows. Science 1976, 193, 1007–1008. [Google Scholar] [CrossRef] [PubMed]
- Orozco Valencia, A.; Camargo Knirsch, M.; Suavinho Ferro, E.; Antonio Stephano, M. Interleukin-2 as Immunotherapeutic in the Autoimmune Diseases. Int. Immunopharmacol. 2020, 81, 106296. [Google Scholar] [CrossRef] [PubMed]
- Siegel, J.P.; Sharon, M.; Smith, P.L.; Leonard, W.J. The IL-2 Receptor Beta Chain (P70): Role in Mediating Signals for LAK, NK, and Proliferative Activities. Science 1987, 238, 75–78. [Google Scholar] [CrossRef] [PubMed]
- Atkins, M.B.; Lotze, M.T.; Dutcher, J.P.; Fisher, R.I.; Weiss, G.; Margolin, K.; Abrams, J.; Sznol, M.; Parkinson, D.; Hawkins, M.; et al. High-Dose Recombinant Interleukin 2 Therapy for Patients with Metastatic Melanoma: Analysis of 270 Patients Treated between 1985 and 1993. J. Clin. Oncol. 1999, 17, 2105–2116. [Google Scholar] [CrossRef] [PubMed]
- Klapper, J.A.; Downey, S.G.; Smith, F.O.; Yang, J.C.; Hughes, M.S.; Kammula, U.S.; Sherry, R.M.; Royal, R.E.; Steinberg, S.M.; Rosenberg, S. High-Dose Interleukin-2 for the Treatment of Metastatic Renal Cell Carcinoma: A Retrospective Analysis of Response and Survival in Patients Treated in the Surgery Branch at the National Cancer Institute between 1986 and 2006. Cancer 2008, 113, 293–301. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, S.A. IL-2: The First Effective Immunotherapy for Human Cancer. J. Immunol. 2014, 192, 5451–5458. [Google Scholar] [CrossRef]
- Lotze, M.T.; Matory, Y.L.; Ettinghausen, S.E.; Rayner, A.A.; Sharrow, S.O.; Seipp, C.A.; Custer, M.C.; Rosenberg, S.A. In Vivo Administration of Purified Human Interleukin 2. II. Half Life, Immunologic Effects, and Expansion of Peripheral Lymphoid Cells In Vivo with Recombinant IL 2. J. Immunol. 1985, 135, 2865–2875. [Google Scholar] [CrossRef]
- Baldo, B.A. Side Effects of Cytokines Approved for Therapy. Drug Saf. 2014, 37, 921–943. [Google Scholar] [CrossRef] [PubMed]
- Xiang, R.; Lode, H.N.; Dolman, C.S.; Dreier, T.; Varki, N.M.; Qian, X.; Lo, K.M.; Lan, Y.; Super, M.; Gillies, S.D.; et al. Elimination of Established Murine Colon Carcinoma Metastases by Antibody-Interleukin 2 Fusion Protein Therapy. Cancer Res. 1997, 57, 4948–4955. [Google Scholar] [PubMed]
- Harvill, E.T.; Morrison, S.L. An IgG3-IL2 Fusion Protein Activates Complement, Binds Fc Gamma RI, Generates LAK Activity and Shows Enhanced Binding to the High Affinity IL-2R. Immunotechnology 1995, 1, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Sabzevari, H.; Gillies, S.D.; Mueller, B.M.; Pancook, J.D.; Reisfeld, R.A. A Recombinant Antibody-Interleukin 2 Fusion Protein Suppresses Growth of Hepatic Human Neuroblastoma Metastases in Severe Combined Immunodeficiency Mice. Proc. Natl. Acad. Sci. USA 1994, 91, 9626–9630. [Google Scholar] [CrossRef] [PubMed]
- Pancook, J.D.; Becker, J.C.; Gillies, S.D.; Reisfeld, R.A. Eradication of Established Hepatic Human Neuroblastoma Metastases in Mice with Severe Combined Immunodeficiency by Antibody-Targeted Interleukin-2. Cancer Immunol. Immunother. 1996, 42, 88–92. [Google Scholar] [CrossRef] [PubMed]
- Becker, J.C.; Pancook, J.D.; Gillies, S.D.; Furukawa, K.; Reisfeld, R.A. T Cell-Mediated Eradication of Murine Metastatic Melanoma Induced by Targeted Interleukin 2 Therapy. J. Exp. Med. 1996, 183, 2361–2366. [Google Scholar] [CrossRef] [PubMed]
- Munn, D.H.; Cheung, N.K. Interleukin-2 Enhancement of Monoclonal Antibody-Mediated Cellular Cytotoxicity against Human Melanoma. Cancer Res. 1987, 47 Pt 1, 6600–6605. [Google Scholar] [PubMed]
- Children’s Oncology Group. A Phase II Study of Hu14.18-IL2 in Children with Recurrent or Refractory Neuroblastoma; Clinical Trial Registration NCT00082758; clinicaltrials.gov; Children’s Oncology Group: Monrovia, CA, USA, 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT00082758 (accessed on 23 March 2023).
- University of Wisconsin, Madison. A Pilot Trial of HU14.18-IL2 (EMD273063) in Subjects with Completely Resectable Recurrent Stage III or Stage IV Melanoma; Clinical Trial Registration NCT00590824; clinicaltrials.gov; University of Wisconsin, Madison: Madison, WI, USA, 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT00590824 (accessed on 23 March 2023).
- Children’s Oncology Group. Feasibility/Phase II Study of Hu14.18-IL2 Immunocytokine + GM-CSF and Isotretinoin in Patients with Relapsed or Refractory Neuroblastoma; Clinical Trial Registration NCT01334515; clinicaltrials.gov; Children’s Oncology Group: Monrovia, CA, USA, 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT01334515 (accessed on 23 March 2023).
- University of Wisconsin, Madison. Phase II Trial of Hu14.18-IL2 (EMD 273063) in Subjects with Advanced Melanoma; Clinical Trial Registration NCT00109863; clinicaltrials.gov; University of Wisconsin, Madison: Madison, WI, USA, 2019. Available online: https://clinicaltrials.gov/ct2/show/NCT00109863 (accessed on 23 March 2023).
- King, D.M.; Albertini, M.R.; Schalch, H.; Hank, J.A.; Gan, J.; Surfus, J.; Mahvi, D.; Schiller, J.H.; Warner, T.; Kim, K.; et al. Phase I Clinical Trial of the Immunocytokine EMD 273063 in Melanoma Patients. JCO 2004, 22, 4463–4473. [Google Scholar] [CrossRef] [PubMed]
- Yang, R.K.; Kuznetsov, I.B.; Ranheim, E.A.; Wei, J.S.; Sindiri, S.; Gryder, B.E.; Gangalapudi, V.; Song, Y.K.; Patel, V.; Hank, J.A.; et al. Outcome-Related Signatures Identified by Whole Transcriptome Sequencing of Resectable Stage III/IV Melanoma Evaluated after Starting Hu14.18-IL2. Clin. Cancer Res. 2020, 26, 3296–3306. [Google Scholar] [CrossRef]
- Gillies, S.D. A New Platform for Constructing Antibody-Cytokine Fusion Proteins (Immunocytokines) with Improved Biological Properties and Adaptable Cytokine Activity. Protein Eng. Des. Sel. 2013, 26, 561–569. [Google Scholar] [CrossRef]
- Friedberg, J.W.; Neuberg, D.; Gribben, J.G.; Fisher, D.C.; and Koval, M.; Poor, C.M.; Green, L.M.; Daley, J.; Soiffer, R.; Ritz, J.; et al. Combination Immunotherapy with Rituximab and Interleukin 2 in Patients with Relapsed or Refractory Follicular Non-Hodgkin’s Lymphoma. Br. J. Haematol. 2002, 117, 828–834. [Google Scholar] [CrossRef]
- Gluck, W.L.; Hurst, D.; Yuen, A.; Levine, A.M.; Dayton, M.A.; Gockerman, J.P.; Lucas, J.; Denis-Mize, K.; Tong, B.; Navis, D.; et al. Phase I Studies of Interleukin (IL)-2 and Rituximab in B-Cell Non-Hodgkin’s Lymphoma: IL-2 Mediated Natural Killer Cell Expansion Correlations with Clinical Response. Clin. Cancer Res. 2004, 10, 2253–2264. [Google Scholar] [CrossRef]
- Gillies, S.D.; Lan, Y.; Williams, S.; Carr, F.; Forman, S.; Raubitschek, A.; Lo, K.-M. An Anti-CD20-IL-2 Immunocytokine Is Highly Efficacious in a SCID Mouse Model of Established Human B Lymphoma. Blood 2005, 105, 3972–3978. [Google Scholar] [CrossRef]
- Bachanova, V.; Lansigan, F.; Quick, D.P.; Vlock, D.; Gillies, S.; Nakamura, R. Remission Induction in a Phase I/II Study of an Anti-CD20-Interleukin-2 Immunocytokine DI-Leu16-IL2 in Patients with Relapsed B-Cell Lymphoma. Blood 2015, 126, 1533. [Google Scholar] [CrossRef]
- Lansigan, F.; Nakamura, R.; Quick, D.; Vlock, D.; Raubitschek, A.; Gillies, S.; Bachanova, V. DI-Leu16-IL2, an Anti-CD20-Interleukin-2 Immunocytokine, Is Safe and Active in Patients with Relapsed and Refractory B-Cell Lymphoma: A Report of Maximum Tolerated Dose, Optimal Biologic Dose, and Recommended Phase 2 Dose. Blood 2016, 128, 620. [Google Scholar] [CrossRef]
- Holden, S.A.; Lan, Y.; Pardo, A.M.; Wesolowski, J.S.; Gillies, S.D. Augmentation of Antitumor Activity of an Antibody-Interleukin 2 Immunocytokine with Chemotherapeutic Agents. Clin. Cancer Res. 2001, 7, 2862–2869. [Google Scholar]
- EMD Serono. Phase I Dose-Escalation Study of the Pharmacokinetic, Safety, Tolerability, and Biologic Activity of huKS-IL-2 Administered Daily as a 1-Hour Intravenous Infusion for Five Consecutive Days for Treatment of Refractory Epithelial Cancer; Clinical Trial Registration NCT00016237; clinicaltrials.gov; EMD Serono: Rockland, MA, USA, 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT00016237 (accessed on 23 March 2023).
- EMD Serono. A Study to Find the Highest Dose of Biological Study Drug (EMD 273066) That Can Be Given Safely to Patients with Recurrent EpCAM Positive Ovarian, Prostate, Colorectal or Non-Small Cell Lung Cancers When First Given a Low Dose of Cyclophosphamide; Clinical Trial Registration NCT00132522; clinicaltrials.gov; EMD Serono: Rockland, MA, USA, 2013. Available online: https://clinicaltrials.gov/ct2/show/NCT00132522 (accessed on 23 March 2023).
- Connor, J.P.; Cristea, M.C.; Lewis, N.L.; Lewis, L.D.; Komarnitsky, P.B.; Mattiacci, M.R.; Felder, M.; Stewart, S.; Harter, J.; Henslee-Downey, J.; et al. A Phase 1b Study of Humanized KS-Interleukin-2 (huKS-IL2) Immunocytokine with Cyclophosphamide in Patients with EpCAM-Positive Advanced Solid Tumors. BMC Cancer 2013, 13, 20. [Google Scholar] [CrossRef]
- Ko, Y.-J.; Bubley, G.J.; Weber, R.; Redfern, C.; Gold, D.P.; Finke, L.; Kovar, A.; Dahl, T.; Gillies, S.D. Safety, Pharmacokinetics, and Biological Pharmacodynamics of the Immunocytokine EMD 273066 (huKS-IL2): Results of a Phase I Trial in Patients with Prostate Cancer. J. Immunother. 2004, 27, 232. [Google Scholar] [CrossRef]
- Gladkov, O.; Ramlau, R.; Serwatowski, P.; Milanowski, J.; Tomeczko, J.; Komarnitsky, P.B.; Kramer, D.; Krzakowski, M.J. Cyclophosphamide and Tucotuzumab (huKS-IL2) Following First-Line Chemotherapy in Responding Patients with Extensive-Disease Small-Cell Lung Cancer. Anticancer Drugs 2015, 26, 1061–1068. [Google Scholar] [CrossRef]
- Ran, S.; He, J.; Huang, X.; Soares, M.; Scothorn, D.; Thorpe, P.E. Antitumor Effects of a Monoclonal Antibody That Binds Anionic Phospholipids on the Surface of Tumor Blood Vessels in Mice. Clin. Cancer Res. 2005, 11, 1551–1562. [Google Scholar] [CrossRef]
- Huang, X.; Ye, D.; Thorpe, P.E. Enhancing the Potency of a Whole-Cell Breast Cancer Vaccine in Mice with an Antibody-IL-2 Immunocytokine That Targets Exposed Phosphatidylserine. Vaccine 2011, 29, 4785–4793. [Google Scholar] [CrossRef]
- Duffy, M.J. Carcinoembryonic Antigen as a Marker for Colorectal Cancer: Is It Clinically Useful? Clin. Chem. 2001, 47, 624–630. [Google Scholar] [CrossRef]
- Goldstein, M.J.; Mitchell, E.P. Carcinoembryonic Antigen in the Staging and Follow-up of Patients with Colorectal Cancer. Cancer Investig. 2005, 23, 338–351. [Google Scholar] [CrossRef]
- Lee, J.S.; Park, S.; Park, J.M.; Cho, J.H.; Kim, S.I.; Park, B.-W. Elevated Levels of Preoperative CA 15-3 and CEA Serum Levels Have Independently Poor Prognostic Significance in Breast Cancer. Ann. Oncol. 2013, 24, 1225–1231. [Google Scholar] [CrossRef]
- Shao, Y.; Sun, X.; He, Y.; Liu, C.; Liu, H. Elevated Levels of Serum Tumor Markers CEA and CA15-3 Are Prognostic Parameters for Different Molecular Subtypes of Breast Cancer. PLoS ONE 2015, 10, e0133830. [Google Scholar] [CrossRef]
- Williams, L.; Li, L.; Yazaki, P.J.; Wong, P.; Hong, T.; Poku, E.K.; Hui, S.; Ghimire, H.; Shively, J.E.; Kujawski, M. Comparison of IL-2-Antibody to IL-2-Fc with or without Stereotactic Radiation Therapy in CEA Immunocompetent Mice with CEA Positive Tumors. Cancer Med. 2024, 13, e6909. [Google Scholar] [CrossRef]
- Vazquez-Lombardi, R.; Loetsch, C.; Zinkl, D.; Jackson, J.; Schofield, P.; Deenick, E.K.; King, C.; Phan, T.G.; Webster, K.E.; Sprent, J.; et al. Potent Antitumour Activity of Interleukin-2-Fc Fusion Proteins Requires Fc-Mediated Depletion of Regulatory T-Cells. Nat. Commun. 2017, 8, 15373. [Google Scholar] [CrossRef]
- Williams, L.; Li, L.; Yazaki, P.J.; Wong, P.; Miller, A.; Hong, T.; Poku, E.K.; Bhattacharya, S.; Shively, J.E.; Kujawski, M. Generation of IL-2-Fc-Antibody Conjugates by Click Chemistry. Biotechnol. J. 2023, 18, 2300115. [Google Scholar] [CrossRef]
- Chang, S.S. Overview of Prostate-Specific Membrane Antigen. Rev. Urol. 2004, 6 (Suppl. S10), S13–S18. [Google Scholar]
- Sugimoto, Y.; Hirota, M.; Yoshikawa, K.; Sumitomo, M.; Nakamura, K.; Ueda, R.; Niwa, R.; Suzawa, T.; Yamasaki, M.; Shitara, K.; et al. The Therapeutic Potential of a Novel PSMA Antibody and Its IL-2 Conjugate in Prostate Cancer. Anticancer Res. 2014, 34, 89–97. [Google Scholar]
- Penichet, M.L.; Dela Cruz, J.S.; Shin, S.-U.; Morrison, S.L. A Recombinant IgG3-(IL-2) Fusion Protein for the Treatment of Human HER2/Neu Expressing Tumors. HAB 2001, 10, 43–49. [Google Scholar] [CrossRef]
- Shusterman, S.; London, W.B.; Gillies, S.D.; Hank, J.A.; Voss, S.D.; Seeger, R.C.; Reynolds, C.P.; Kimball, J.; Albertini, M.R.; Wagner, B.; et al. Antitumor Activity of Hu14.18-IL2 in Patients with Relapsed/Refractory Neuroblastoma: A Children’s Oncology Group (COG) Phase II Study. JCO 2010, 28, 4969–4975. [Google Scholar] [CrossRef]
- Albertini, M.R.; Yang, R.K.; Ranheim, E.A.; Hank, J.A.; Zuleger, C.L.; Weber, S.; Neuman, H.; Hartig, G.; Weigel, T.; Mahvi, D.; et al. Pilot Trial of the Hu14.18-IL2 Immunocytokine in Patients with Completely Resectable Recurrent Stage III or Stage IV Melanoma. Cancer Immunol. Immunother. 2018, 67, 1647–1658. [Google Scholar] [CrossRef]
- Albertini, M.R.; Hank, J.A.; Gadbaw, B.; Kostlevy, J.; Haldeman, J.; Schalch, H.; Gan, J.; Kim, K.; Eickhoff, J.; Gillies, S.D.; et al. Phase II Trial of Hu14.18-IL2 for Patients with Metastatic Melanoma. Cancer Immunol. Immunother. 2012, 61, 2261–2271. [Google Scholar] [CrossRef]
- Epstein, A.L.; Mizokami, M.M.; Li, J.; Hu, P.; Khawli, L.A. Identification of a Protein Fragment of Interleukin 2 Responsible for Vasopermeability. JNCI J. Natl. Cancer Inst. 2003, 95, 741–749. [Google Scholar] [CrossRef] [PubMed]
- Shanafelt, A.B.; Lin, Y.; Shanafelt, M.-C.; Forte, C.P.; Dubois-Stringfellow, N.; Carter, C.; Gibbons, J.A.; Cheng, S.; Delaria, K.A.; Fleischer, R.; et al. A T-Cell-Selective Interleukin 2 Mutein Exhibits Potent Antitumor Activity and Is Well Tolerated In Vivo. Nat. Biotechnol. 2000, 18, 1197–1202. [Google Scholar] [CrossRef]
- Gillies, S.D.; Lan, Y.; Hettmann, T.; Brunkhorst, B.; Sun, Y.; Mueller, S.O.; Lo, K.-M. A Low-Toxicity IL-2-Based Immunocytokine Retains Antitumor Activity despite Its High Degree of IL-2 Receptor Selectivity. Clin. Cancer Res. 2011, 17, 3673–3685. [Google Scholar] [CrossRef]
- Laurent, J.; Touvrey, C.; Gillessen, S.; Joffraud, M.; Vicari, M.; Bertrand, C.; Ongarello, S.; Liedert, B.; Gallerani, E.; Beck, J.; et al. T-Cell Activation by Treatment of Cancer Patients with EMD 521873 (Selectikine), an IL-2/Anti-DNA Fusion Protein. J. Transl. Med. 2013, 11, 5. [Google Scholar] [CrossRef]
- EMD Serono. A Safety Study for MSB0010445 in Combination with Stereotactic Body Radiation in Advanced Melanoma Subjects Following Prior Treatment with Ipilimumab; Clinical Trial Registration NCT01973608; clinicaltrials.gov; EMD Serono: Rockland, MA, USA, 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT01973608 (accessed on 23 March 2023).
- Merck KGaA. A Phase 1, Open-Label, Two-Group, Dose- Escalation Study to Investigate the Safety, Tolerability, Pharmacokinetics, Biological and Clinical Activity of EMD 521873 Alone and in Combination with Fixed Low Doses of Cyclophosphamide in Patients with Metastatic or Locally Advanced Solid Tumors or B-Cell Non-Hodgkin Lymphoma; Clinical Trial Registration NCT01032681; clinicaltrials.gov; Merck KGaA: Darmstadt, Germany, 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT01032681 (accessed on 23 March 2023).
- Merck KGaA. An Open-Label, Phase Ib, Dose-Escalation Trial on the Safety, Tolerability, Pharmacokinetics, Immunogenicity, Biological Effects and Antitumor Activity of EMD 521873 in Combination With Local Irradiation (20 Gy) of Primary Tumors or Metastases in Subjects with Non-Small Cell Lung Cancer Stage IIIb with Malignant Pleural Effusion or Stage IV with Disease Control (Partial Response or Stable Disease) after Application of 4 Cycles of Platinum-Based, First-Line Chemotherapy; Clinical Trial Registration NCT00879866; clinicaltrials.gov; Merck KGaA: Darmstadt, Germany, 2014. Available online: https://clinicaltrials.gov/ct2/show/NCT00879866 (accessed on 23 March 2023).
- Sun, Z.; Ren, Z.; Yang, K.; Liu, Z.; Cao, S.; Deng, S.; Xu, L.; Liang, Y.; Guo, J.; Bian, Y.; et al. A Next-Generation Tumor-Targeting IL-2 Preferentially Promotes Tumor-Infiltrating CD8+ T-Cell Response and Effective Tumor Control. Nat. Commun. 2019, 10, 3874. [Google Scholar] [CrossRef]
- Spolski, R.; Li, P.; Leonard, W.J. Biology and Regulation of IL-2: From Molecular Mechanisms to Human Therapy. Nat. Rev. Immunol. 2018, 18, 648–659. [Google Scholar] [CrossRef]
- Levin, A.M.; Bates, D.L.; Ring, A.M.; Krieg, C.; Lin, J.T.; Su, L.; Moraga, I.; Raeber, M.E.; Bowman, G.R.; Novick, P.; et al. Exploiting a Natural Conformational Switch to Engineer an Interleukin-2 ‘Superkine’. Nature 2012, 484, 529–533. [Google Scholar] [CrossRef]
- Klein, C.; Waldhauer, I.; Nicolini, V.; Dunn, C.; Freimoser-Grundschober, A.; Danny, G.; Boerman, O.; Nayak, T.; Herter, S.; Van Puijenbroek, E.; et al. Novel Tumor-Targeted, Engineered IL-2 Variant (IL2v)-Based Immunocytokines for Immunotherapy of Cancer. Blood 2013, 122, 2278. [Google Scholar] [CrossRef]
- Chen, X.; Ai, X.; Wu, C.; Wang, H.; Zeng, G.; Yang, P.; Liu, G. A Novel Human IL-2 Mutein with Minimal Systemic Toxicity Exerts Greater Antitumor Efficacy than Wild-Type IL-2. Cell Death Dis. 2018, 9, 989. [Google Scholar] [CrossRef]
- Tichet, M.; Wullschleger, S.; Chryplewicz, A.; Fournier, N.; Marcone, R.; Kauzlaric, A.; Homicsko, K.; Deak, L.C.; Umaña, P.; Klein, C.; et al. Bispecific PD1-IL2v and Anti-PD-L1 Break Tumor Immunity Resistance by Enhancing Stem-like Tumor-Reactive CD8+ T Cells and Reprogramming Macrophages. Immunity 2023, 56, 162–179.e6. [Google Scholar] [CrossRef]
- Klein, C.; Waldhauer, I.; Nicolini, V.G.; Freimoser-Grundschober, A.; Nayak, T.; Vugts, D.J.; Dunn, C.; Bolijn, M.; Benz, J.; Stihle, M.; et al. Cergutuzumab Amunaleukin (CEA-IL2v), a CEA-Targeted IL-2 Variant-Based Immunocytokine for Combination Cancer Immunotherapy: Overcoming Limitations of Aldesleukin and Conventional IL-2-Based Immunocytokines. OncoImmunology 2017, 6, e1277306. [Google Scholar] [CrossRef]
- Kuo, T.T.; Aveson, V.G. Neonatal Fc Receptor and IgG-Based Therapeutics. mAbs 2011, 3, 422–430. [Google Scholar] [CrossRef]
- Waldhauer, I.; Gonzalez-Nicolini, V.; Freimoser-Grundschober, A.; Nayak, T.K.; Fahrni, L.; Hosse, R.J.; Gerrits, D.; Geven, E.J.W.; Sam, J.; Lang, S.; et al. Simlukafusp Alfa (FAP-IL2v) Immunocytokine Is a Versatile Combination Partner for Cancer Immunotherapy. mAbs 2021, 13, 1913791. [Google Scholar] [CrossRef]
- Doroshow, D.B.; Bhalla, S.; Beasley, M.B.; Sholl, L.M.; Kerr, K.M.; Gnjatic, S.; Wistuba, I.I.; Rimm, D.L.; Tsao, M.S.; Hirsch, F.R. PD-L1 as a Biomarker of Response to Immune-Checkpoint Inhibitors. Nat. Rev. Clin. Oncol. 2021, 18, 345–362. [Google Scholar] [CrossRef]
- Wu, W.; Chia, T.; Lu, J.; Li, X.; Guan, J.; Li, Y.; Fu, F.; Zhou, S.; Feng, Y.; Deng, J.; et al. IL-2Rα-Biased Agonist Enhances Antitumor Immunity by Invigorating Tumor-Infiltrating CD25+CD8+ T Cells. Nat. Cancer 2023, 4, 1309–1325. [Google Scholar] [CrossRef]
- Weide, B.; Eigentler, T.; Catania, C.; Ascierto, P.A.; Cascinu, S.; Becker, J.C.; Hauschild, A.; Romanini, A.; Danielli, R.; Dummer, R.; et al. A Phase II Study of the L19IL2 Immunocytokine in Combination with Dacarbazine in Advanced Metastatic Melanoma Patients. Cancer Immunol. Immunother. 2019, 68, 1547–1559. [Google Scholar] [CrossRef]
- Catania, C.; Maur, M.; Berardi, R.; Rocca, A.; Giacomo, A.M.D.; Spitaleri, G.; Masini, C.; Pierantoni, C.; González-Iglesias, R.; Zigon, G.; et al. The Tumor-Targeting Immunocytokine F16-IL2 in Combination with Doxorubicin: Dose Escalation in Patients with Advanced Solid Tumors and Expansion into Patients with Metastatic Breast Cancer. Cell Adh. Migr. 2015, 9, 14–21. [Google Scholar] [CrossRef]
- Schliemann, C.; Gutbrodt, K.L.; Kerkhoff, A.; Pohlen, M.; Wiebe, S.; Silling, G.; Angenendt, L.; Kessler, T.; Mesters, R.M.; Giovannoni, L.; et al. Targeting Interleukin-2 to the Bone Marrow Stroma for Therapy of Acute Myeloid Leukemia Relapsing after Allogeneic Hematopoietic Stem Cell Transplantation. Cancer Immunol. Res. 2015, 3, 547–556. [Google Scholar] [CrossRef]
- Wen, J.; Zhu, X.; Liu, B.; You, L.; Kong, L.; Lee, H.; Han, K.; Wong, J.L.; Rhode, P.R.; Wong, H.C. Targeting Activity of a TCR/IL-2 Fusion Protein against Established Tumors. Cancer Immunol. Immunother. 2008, 57, 1781–1794. [Google Scholar] [CrossRef]
- Card, K.F.; Price-Schiavi, S.A.; Liu, B.; Thomson, E.; Nieves, E.; Belmont, H.; Builes, J.; Jiao, J.; Hernandez, J.; Weidanz, J.; et al. A Soluble Single-Chain T-Cell Receptor IL-2 Fusion Protein Retains MHC-Restricted Peptide Specificity and IL-2 Bioactivity. Cancer Immunol. Immunother. 2004, 53, 345–357. [Google Scholar] [CrossRef]
- Syn, N.L.; Teng, M.W.L.; Mok, T.S.K.; Soo, R.A. De-Novo and Acquired Resistance to Immune Checkpoint Targeting. Lancet Oncol. 2017, 18, e731–e741. [Google Scholar] [CrossRef]
- Pyo, K.-H.; Koh, Y.J.; Synn, C.-B.; Kim, J.H.; Byeon, Y.; Jo, H.N.; Kim, Y.S.; Lee, W.; Kim, D.H.; Lee, S.; et al. Abstract 1826: Comprehensive Preclinical Study on GI-101, a Novel CD80-IgG4-IL2 Variant Protein, as a Therapeutic Antibody Candidate with Bispecific Immuno-Oncology Target. Cancer Res. 2021, 81 (Suppl. S13), 1826. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Chang, C.J.; Karger, A.; Keller, M.; Pfaff, F.; Wangkahart, E.; Wang, T.; Secombes, C.J.; Kimoto, A.; Furihata, M.; et al. Ancient Cytokine Interleukin 15-Like (IL-15L) Induces a Type 2 Immune Response. Front. Immunol. 2020, 11, 549319. [Google Scholar] [CrossRef]
- Waldmann, T.A. The Shared and Contrasting Roles of IL2 and IL15 in the Life and Death of Normal and Neoplastic Lymphocytes: Implications for Cancer Therapy. Cancer Immunol. Res. 2015, 3, 219–227. [Google Scholar] [CrossRef]
- Conlon, K.C.; Lugli, E.; Welles, H.C.; Rosenberg, S.A.; Fojo, A.T.; Morris, J.C.; Fleisher, T.A.; Dubois, S.P.; Perera, L.P.; Stewart, D.M.; et al. Redistribution, Hyperproliferation, Activation of Natural Killer Cells and CD8 T Cells, and Cytokine Production during First-in-Human Clinical Trial of Recombinant Human Interleukin-15 in Patients with Cancer. J. Clin. Oncol. 2015, 33, 74–82. [Google Scholar] [CrossRef]
- Berger, C.; Berger, M.; Hackman, R.C.; Gough, M.; Elliott, C.; Jensen, M.C.; Riddell, S.R. Safety and Immunologic Effects of IL-15 Administration in Nonhuman Primates. Blood 2009, 114, 2417–2426. [Google Scholar] [CrossRef]
- Conlon, K.C.; Potter, E.L.; Pittaluga, S.; Lee, C.-C.R.; Miljkovic, M.D.; Fleisher, T.A.; Dubois, S.; Bryant, B.R.; Petrus, M.; Perera, L.P.; et al. IL15 by Continuous Intravenous Infusion to Adult Patients with Solid Tumors in a Phase I Trial Induced Dramatic NK-Cell Subset Expansion. Clin. Cancer Res. 2019, 25, 4945–4954. [Google Scholar] [CrossRef] [PubMed]
- Waldmann, T.A.; Lugli, E.; Roederer, M.; Perera, L.P.; Smedley, J.V.; Macallister, R.P.; Goldman, C.K.; Bryant, B.R.; Decker, J.M.; Fleisher, T.A.; et al. Safety (Toxicity), Pharmacokinetics, Immunogenicity, and Impact on Elements of the Normal Immune System of Recombinant Human IL-15 in Rhesus Macaques. Blood 2011, 117, 4787–4795. [Google Scholar] [CrossRef] [PubMed]
- Kermer, V.; Baum, V.; Hornig, N.; Kontermann, R.E.; Müller, D. An Antibody Fusion Protein for Cancer Immunotherapy Mimicking IL-15 Trans-Presentation at the Tumor Site. Mol. Cancer Ther. 2012, 11, 1279–1288. [Google Scholar] [CrossRef] [PubMed]
- Vincent, M.; Bessard, A.; Cochonneau, D.; Teppaz, G.; Solé, V.; Maillasson, M.; Birklé, S.; Garrigue-Antar, L.; Quéméner, A.; Jacques, Y. Tumor Targeting of the IL-15 Superagonist RLI by an Anti-GD2 Antibody Strongly Enhances Its Antitumor Potency. Int. J. Cancer 2013, 133, 757–765. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Zhang, X.; Sun, M.; Abbas, S.; Seibert, C.; Kelly, M.C.; Shern, J.F.; Thiele, C.J. Anti-GD2 Antibodies Conjugated to IL15 and IL21 Mediate Potent Antitumor Cytotoxicity against Neuroblastoma. Clin. Cancer Res. 2022, 28, 3785–3796. [Google Scholar] [CrossRef] [PubMed]
- Kim, P.S.; Kwilas, A.R.; Xu, W.; Alter, S.; Jeng, E.K.; Wong, H.C.; Schlom, J.; Hodge, J.W. IL-15 Superagonist/IL-15RαSushi-Fc Fusion Complex (IL-15SA/IL-15RαSu-Fc; ALT-803) Markedly Enhances Specific Subpopulations of NK and Memory CD8+ T Cells, and Mediates Potent Anti-Tumor Activity against Murine Breast and Colon Carcinomas. Oncotarget 2016, 7, 16130–16145. [Google Scholar] [CrossRef] [PubMed]
- Kotecki, N.; Rottey, S.; Delafontaine, B.; Garralda, E.; Galvao, V.; Miguel, M.d.; Saavedra, O.; Boni, V.; McEwen, A.; Singhal, S.; et al. 728 A Phase 1/2 Study of JK08, an IL-15 Antibody Fusion Protein Targeting CTLA-4, in Patients with Advanced Solid Tumors. J. Immunother. Cancer 2023, 11 (Suppl. S1), A823. [Google Scholar] [CrossRef]
- Martomo, S.A.; Lu, D.; Polonskaya, Z.; Luna, X.; Zhang, Z.; Feldstein, S.; Lumban-Tobing, R.; Almstead, D.K.; Miyara, F.; Patel, J. Single-Dose Anti–PD-L1/IL-15 Fusion Protein KD033 Generates Synergistic Antitumor Immunity with Robust Tumor-Immune Gene Signatures and Memory Responses. Mol. Cancer Ther. 2021, 20, 347–356. [Google Scholar] [CrossRef] [PubMed]
- Ledermann, J.A.; Zurawski, B.; Raspagliesi, F.; De Giorgi, U.; Arranz Arija, J.; Romeo Marin, M.; Lisyanskaya, A.; Póka, R.L.; Markowska, J.; Cebotaru, C.; et al. Maintenance Therapy of Patients with Recurrent Epithelial Ovarian Carcinoma with the Anti-Tumor-Associated-Mucin-1 Antibody Gatipotuzumab: Results from a Double-Blind, Placebo-Controlled, Randomized, Phase II Study. ESMO Open 2022, 7, 100311. [Google Scholar] [CrossRef] [PubMed]
- Gellert, J.; Jäkel, A.; Danielczyk, A.; Goletz, C.; Lischke, T.; Flechner, A.; Dix, L.; Günzl, A.; Kehler, P. GT-00AxIL15, a Novel Tumor-Targeted IL-15-Based Immunocytokine for the Treatment of TA-MUC1-Positive Solid Tumors: Preclinical In Vitro and In Vivo Pharmacodynamics and Biodistribution Studies. Int. J. Mol. Sci. 2024, 25, 1406. [Google Scholar] [CrossRef]
- Zhu, X.; Marcus, W.D.; Xu, W.; Lee, H.; Han, K.; Egan, J.O.; Yovandich, J.L.; Rhode, P.R.; Wong, H.C. Novel Human Interleukin-15 Agonists. J. Immunol. 2009, 183, 3598–3607. [Google Scholar] [CrossRef] [PubMed]
- Alter, S.; Rhode, P.R.; Jeng, E.K.; Wong, H.C. Targeted IL-15-Based Protein Fusion Complexes as Cancer Immunotherapy Approaches. J. Immunol. Sci. 2018, 2, 15–18. [Google Scholar] [CrossRef]
- Liu, B.; Kong, L.; Marcus, W.D.; Chen, X.; Han, K.; Rhode, P.R.; Wong, H.C. Evaluation of a Novel CD20-Targeted IL-15 Immunotherapeutic with Potent Activity against B Cell Lymphoma. J. Immunother. Cancer 2014, 2, P122. [Google Scholar] [CrossRef][Green Version]
- Hicks, K.C.; Knudson, K.M.; Ozawa, Y.; Schlom, J.; Gameiro, S.R. 1222P—Evaluation of the Anti-Tumour Efficacy and Immune Effects of N-809, a Novel IL-15 Superagonist/Anti-PD-L1 Bispecific Agent. Ann. Oncol. 2019, 30, v500. [Google Scholar] [CrossRef]
- Xu, Y.; Carrascosa, L.C.; Yeung, Y.A.; Chu, M.L.-H.; Yang, W.; Djuretic, I.; Pappas, D.C.; Zeytounian, J.; Ge, Z.; de Ruiter, V.; et al. An Engineered IL15 Cytokine Mutein Fused to an Anti-PD1 Improves Intratumoral T-Cell Function and Antitumor Immunity. Cancer Immunol. Res. 2021, 9, 1141–1157. [Google Scholar] [CrossRef]
- Maruhashi, T.; Sugiura, D.; Okazaki, I.; Okazaki, T. LAG-3: From Molecular Functions to Clinical Applications. J. Immunother. Cancer 2020, 8, e001014. [Google Scholar] [CrossRef]
- Bernett, M.J.; Schubbert, S.; Hackett, M.; Ochyl, L.J.; Scott, L.E.; Bonzon, C.; Rashid, R.; Avery, K.N.; Leung, I.W.; Rodriguez, N.; et al. Abstract 2080: LAG3-Targeted IL15/IL15Rα-Fc (LAG3 x IL15) Fusion Proteins for Preferential TIL Expansion. Cancer Res. 2022, 82 (Suppl. S12), 2080. [Google Scholar] [CrossRef]
- Chung, K.Y.; Park, H.; Abdul-Karim, R.M.; Doroshow, D.B.; Chaves, J.; Coleman, T.A.; Nakai, K.; Patel, P.; Wang, J.; Zhang, H.; et al. Phase I Study of BJ-001, a Tumor-Targeting Interleukin-15 Fusion Protein, in Patients with Solid Tumor. JCO 2021, 39 (Suppl. S15), e14545. [Google Scholar] [CrossRef]
- Kermer, V.; Hornig, N.; Harder, M.; Bondarieva, A.; Kontermann, R.E.; Müller, D. Combining Antibody-Directed Presentation of IL-15 and 4-1BBL in a Trifunctional Fusion Protein for Cancer Immunotherapy. Mol. Cancer Ther. 2014, 13, 112–121. [Google Scholar] [CrossRef]
- Beha, N.; Harder, M.; Ring, S.; Kontermann, R.E.; Müller, D. IL15-Based Trifunctional Antibody-Fusion Proteins with Costimulatory TNF-Superfamily Ligands in the Single-Chain Format for Cancer Immunotherapy. Mol. Cancer Ther. 2019, 18, 1278–1288. [Google Scholar] [CrossRef]
- Kaspar, M.; Trachsel, E.; Neri, D. The Antibody-Mediated Targeted Delivery of Interleukin-15 and GM-CSF to the Tumor Neovasculature Inhibits Tumor Growth and Metastasis. Cancer Res. 2007, 67, 4940–4948. [Google Scholar] [CrossRef] [PubMed]
- Corbellari, R.; Stringhini, M.; Mock, J.; Ongaro, T.; Villa, A.; Neri, D.; De Luca, R. A Novel Antibody–IL15 Fusion Protein Selectively Localizes to Tumors, Synergizes with TNF-Based Immunocytokine, and Inhibits Metastasis. Mol. Cancer Ther. 2021, 20, 859–871. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, S.; Xin, J.; Wang, J.; Yao, C.; Zhang, Z. Role of NKG2D and Its Ligands in Cancer Immunotherapy. Am. J. Cancer Res. 2019, 9, 2064–2078. [Google Scholar] [PubMed]
- González, S.; López-Soto, A.; Xin, J.; Wang, J.; Yao, C.; Zhang, Z. NKG2D Ligands: Key Targets of the Immune Response. Trends Immunol. 2008, 29, 397–403. [Google Scholar] [CrossRef] [PubMed]
- Schmiedel, D.; Mandelboim, O. NKG2D Ligands–Critical Targets for Cancer Immune Escape and Therapy. Front. Immunol. 2018, 9, 2040. [Google Scholar] [CrossRef]
- Xia, Y.; Chen, B.; Shao, X.; Xiao, W.; Qian, L.; Ding, Y.; Ji, M.; Gong, W. Treatment with a Fusion Protein of the Extracellular Domains of NKG2D to IL-15 Retards Colon Cancer Growth in Mice. J. Immunother. 2014, 37, 257–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Zhu, X.; Kong, L.; Wang, M.; Spanoudis, C.; Chaturvedi, P.; George, V.; Jiao, J.; You, L.; Egan, J.O.; et al. Bifunctional TGF-β Trap/IL-15 Protein Complex Elicits Potent NK Cell and CD8+ T Cell Immunity against Solid Tumors. Mol. Ther. 2021, 29, 2949–2962. [Google Scholar] [CrossRef]
- Chaturvedi, P.; George, V.; Shrestha, N.; Wang, M.; Dee, M.J.; Zhu, X.; Liu, B.; Egan, J.; D’Eramo, F.; Spanoudis, C.; et al. Immunotherapeutic HCW9218 Augments Anti-Tumor Activity of Chemotherapy via NK Cell-Mediated Reduction of Therapy-Induced Senescent Cells. Mol. Ther. 2022, 30, 1171–1187. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Liu, N.; Liu, Z.; Yang, Y.; Zeng, Q.; Wang, Y.; Song, L.; Hu, F.; Fu, J.; Chen, J.; et al. Next-Generation Anti-PD-L1/IL-15 Immunocytokine Elicits Superior Antitumor Immunity in Cold Tumors with Minimal Toxicity. Cell Reports Medicine 2024, 5, 101531. [Google Scholar] [CrossRef]
- Matthews, N.; Watkins, J.F. Tumour-Necrosis Factor from the Rabbit. I. Mode of Action, Specificity and Physicochemical Properties. Br. J. Cancer 1978, 38, 302–309. [Google Scholar] [CrossRef]
- Green, S.; Dobrjansky, A.; Chiasson, M.A. Murine Tumor Necrosis-Inducing Factor: Purification and Effects on Myelomonocytic Leukemia Cells. J. Natl. Cancer Inst. 1982, 68, 997–1003. [Google Scholar] [PubMed]
- Kriegler, M.; Perez, C.; DeFay, K.; Albert, I.; Lu, S.D. A Novel Form of TNF/Cachectin Is a Cell Surface Cytotoxic Transmembrane Protein: Ramifications for the Complex Physiology of TNF. Cell 1988, 53, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Black, R.A.; Rauch, C.T.; Kozlosky, C.J.; Peschon, J.J.; Slack, J.L.; Wolfson, M.F.; Castner, B.J.; Stocking, K.L.; Reddy, P.; Srinivasan, S.; et al. A Metalloproteinase Disintegrin That Releases Tumour-Necrosis Factor-Alpha from Cells. Nature 1997, 385, 729–733. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Lin, Y. Tumor Necrosis Factor and Cancer, Buddies or Foes? Acta Pharmacol. Sin. 2008, 29, 1275–1288. [Google Scholar] [CrossRef] [PubMed]
- Gamm, H.; Lindemann, A.; Mertelsmann, R.; Herrmann, F. Phase I Trial of Recombinant Human Tumour Necrosis Factor Alpha in Patients with Advanced Malignancy. Eur. J. Cancer 1991, 27, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, N.; Niitsu, Y.; Umeno, H.; Kuriyama, H.; Neda, H.; Yamauchi, N.; Maeda, M.; Urushizaki, I. Toxic Effect of Tumor Necrosis Factor on Tumor Vasculature in Mice. Cancer Res. 1988, 48, 2179–2183. [Google Scholar] [PubMed]
- Rosenblum, M.G.; Cheung, L.; Mujoo, K.; Murray, J.L. An Antimelanoma Immunotoxin Containing Recombinant Human Tumor Necrosis Factor: Tissue Disposition, Pharmacokinetic, and Therapeutic Studies in Xenograft Models. Cancer Immunol. Immunother. 1995, 40, 322–328. [Google Scholar] [CrossRef] [PubMed]
- Rosenblum, M.G.; Cheung, L.; Murray, J.L.; Bartholomew, R. Antibody-Mediated Delivery of Tumor Necrosis Factor (TNF-Alpha): Improvement of Cytotoxicity and Reduction of Cellular Resistance. Cancer Commun. 1991, 3, 21–27. [Google Scholar] [PubMed]
- Fercher, C.; Keshvari, S.; McGuckin, M.A.; Barnard, R.T. Evolution of the Magic Bullet: Single Chain Antibody Fragments for the Targeted Delivery of Immunomodulatory Proteins. Exp. Biol. Med. 2018, 243, 166–183. [Google Scholar] [CrossRef]
- Dakhel, S.; Lizak, C.; Matasci, M.; Mock, J.; Villa, A.; Neri, D.; Cazzamalli, S. An Attenuated Targeted-TNF Localizes to Tumors In Vivo and Regains Activity at the Site of Disease. Int. J. Mol. Sci. 2021, 22, 10020. [Google Scholar] [CrossRef]
- Spitaleri, G.; Berardi, R.; Pierantoni, C.; De Pas, T.; Noberasco, C.; Libbra, C.; González-Iglesias, R.; Giovannoni, L.; Tasciotti, A.; Neri, D.; et al. Phase I/II Study of the Tumour-Targeting Human Monoclonal Antibody-Cytokine Fusion Protein L19-TNF in Patients with Advanced Solid Tumours. J. Cancer Res. Clin. Oncol. 2013, 139, 447–455. [Google Scholar] [CrossRef] [PubMed]
- Papadia, F.; Basso, V.; Patuzzo, R.; Maurichi, A.; Di Florio, A.; Zardi, L.; Ventura, E.; González-Iglesias, R.; Lovato, V.; Giovannoni, L.; et al. Isolated Limb Perfusion with the Tumor-Targeting Human Monoclonal Antibody–Cytokine Fusion Protein L19-TNF plus Melphalan and Mild Hyperthermia in Patients with Locally Advanced Extremity Melanoma. J. Surg. Oncol. 2013, 107, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Danielli, R.; Patuzzo, R.; Di Giacomo, A.M.; Gallino, G.; Maurichi, A.; Di Florio, A.; Cutaia, O.; Lazzeri, A.; Fazio, C.; Miracco, C.; et al. Intralesional Administration of L19-IL2/L19-TNF in Stage III or Stage IVM1a Melanoma Patients: Results of a Phase II Study. Cancer Immunol. Immunother. 2015, 64, 999–1009. [Google Scholar] [CrossRef] [PubMed]
- Hemmerle, T.; Probst, P.; Giovannoni, L.; Green, A.J.; Meyer, T.; Neri, D. The Antibody-Based Targeted Delivery of TNF in Combination with Doxorubicin Eradicates Sarcomas in Mice and Confers Protective Immunity. Br. J. Cancer 2013, 109, 1206–1213. [Google Scholar] [CrossRef] [PubMed]
- Probst, P.; Kopp, J.; Oxenius, A.; Colombo, M.P.; Ritz, D.; Fugmann, T.; Neri, D. Sarcoma Eradication by Doxorubicin and Targeted TNF Relies upon CD8+ T Cell Recognition of a Retroviral Antigen. Cancer Res. 2017, 77, 3644–3654. [Google Scholar] [CrossRef] [PubMed]
- Pastorekova, S.; Gillies, R.J. The Role of Carbonic Anhydrase IX in Cancer Development: Links to Hypoxia, Acidosis, and Beyond. Cancer Metastasis Rev. 2019, 38, 65–77. [Google Scholar] [CrossRef]
- Bauer, S.; Oosterwijk-Wakka, J.C.; Adrian, N.; Oosterwijk, E.; Fischer, E.; Wüest, T.; Stenner, F.; Perani, A.; Cohen, L.; Knuth, A.; et al. Targeted Therapy of Renal Cell Carcinoma: Synergistic Activity of cG250-TNF and IFNg. Int. J. Cancer 2009, 125, 115–123. [Google Scholar] [CrossRef] [PubMed]
- Zhi, M.; Wu, K.-C.; Dong, L.; Hao, Z.-M.; Deng, T.-Z.; Hong, L.; Liang, S.-H.; Zhao, P.-T.; Qiao, T.-D.; Wang, Y.; et al. Characterization of a Specific Phage-Displayed Peptide Binding to Vasculature of Human Gastric Cancer. Cancer Biol. Ther. 2004, 3, 1232–1235. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Cao, S.; Zhang, Y.; Wang, X.; Liu, J.; Hui, X.; Wan, Y.; Du, W.; Wang, L.; Wu, K.; et al. A Novel Peptide (GX1) Homing to Gastric Cancer Vasculature Inhibits Angiogenesis and Cooperates with TNF Alpha in Anti-Tumor Therapy. BMC Cell Biol. 2009, 10, 63. [Google Scholar] [CrossRef]
- Gubler, U.; Chua, A.O.; Schoenhaut, D.S.; Dwyer, C.M.; McComas, W.; Motyka, R.; Nabavi, N.; Wolitzky, A.G.; Quinn, P.M.; Familletti, P.C. Coexpression of Two Distinct Genes Is Required to Generate Secreted Bioactive Cytotoxic Lymphocyte Maturation Factor. Proc. Natl. Acad. Sci. USA 1991, 88, 4143–4147. [Google Scholar] [CrossRef]
- Zaharoff, D.A.; Hance, K.W.; Rogers, C.J.; Schlom, J.; Greiner, J.W. Intratumoral Immunotherapy of Established Solid Tumors with Chitosan/IL-12. J. Immunother. 2010, 33, 697. [Google Scholar] [CrossRef]
- Smyth, M.J.; Taniguchi, M.; Street, S.E.A. The Anti-Tumor Activity of IL-12: Mechanisms of Innate Immunity That Are Model and Dose Dependent1. J. Immunol. 2000, 165, 2665–2670. [Google Scholar] [CrossRef] [PubMed]
- Brunda, M.J.; Luistro, L.; Warrier, R.R.; Wright, R.B.; Hubbard, B.R.; Murphy, M.; Wolf, S.F.; Gately, M.K. Antitumor and Antimetastatic Activity of Interleukin 12 against Murine Tumors. J. Exp. Med. 1993, 178, 1223–1230. [Google Scholar] [CrossRef]
- Car, B.D.; Eng, V.M.; Schnyder, B.; LeHir, M.; Shakhov, A.N.; Woerly, G.; Huang, S.; Aguet, M.; Anderson, T.D.; Ryffel, B. Role of Interferon-Gamma in Interleukin 12-Induced Pathology in Mice. Am. J. Pathol. 1995, 147, 1693–1707. [Google Scholar] [PubMed]
- Lasek, W.; Zagożdżon, R.; Jakobisiak, M. Interleukin 12: Still a Promising Candidate for Tumor Immunotherapy? Cancer Immunol. Immunother. 2014, 63, 419–435. [Google Scholar] [CrossRef]
- Leonard, J.P.; Sherman, M.L.; Fisher, G.L.; Buchanan, L.J.; Larsen, G.; Atkins, M.B.; Sosman, J.A.; Dutcher, J.P.; Vogelzang, N.J.; Ryan, J.L. Effects of Single-Dose Interleukin-12 Exposure on Interleukin-12–Associated Toxicity and Interferon-γ Production. Blood 1997, 90, 2541–2548. [Google Scholar] [CrossRef]
- Hurteau, J.A.; Blessing, J.A.; DeCesare, S.L.; Creasman, W.T. Evaluation of Recombinant Human Interleukin-12 in Patients with Recurrent or Refractory Ovarian Cancer: A Gynecologic Oncology Group Study. Gynecol. Oncol. 2001, 82, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Motzer, R.J.; Rakhit, A.; Thompson, J.A.; Nemunaitis, J.; Murphy, B.A.; Ellerhorst, J.; Schwartz, L.H.; Berg, W.J.; Bukowski, R.M. Randomized Multicenter Phase II Trial of Subcutaneous Recombinant Human Interleukin-12 Versus Interferon-A2a for Patients with Advanced Renal Cell Carcinoma. J. Interferon Cytokine Res. 2001, 21, 257–263. [Google Scholar] [CrossRef]
- Mansurov, A.; Ishihara, J.; Hosseinchi, P.; Potin, L.; Marchell, T.M.; Ishihara, A.; Williford, J.-M.; Alpar, A.T.; Raczy, M.M.; Gray, L.T.; et al. Collagen-Binding IL-12 Enhances Tumour Inflammation and Drives the Complete Remission of Established Immunologically Cold Mouse Tumours. Nat. Biomed. Eng. 2020, 4, 531–543. [Google Scholar] [CrossRef]
- Portielje, J.E.A.; Lamers, C.H.J.; Kruit, W.H.J.; Sparreboom, A.; Bolhuis, R.L.H.; Stoter, G.; Huber, C.; Gratama, J.W. Repeated Administrations of Interleukin (IL)-12 Are Associated with Persistently Elevated Plasma Levels of IL-10 and Declining IFN-γ, Tumor Necrosis Factor-α, IL-6, and IL-8 Responses. Clin. Cancer Res. 2003, 9, 76–83. [Google Scholar]
- Greiner, J.W.; Morillon, Y.M.; Schlom, J. NHS-IL12, a Tumor-Targeting Immunocytokine. Immunotargets Ther. 2021, 10, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Morillon, Y.M.; Su, Z.; Schlom, J.; Greiner, J.W. Temporal Changes within the (Bladder) Tumor Microenvironment That Accompany the Therapeutic Effects of the Immunocytokine NHS-IL12. J. Immunother. Cancer 2019, 7, 150. [Google Scholar] [CrossRef] [PubMed]
- Vandeveer, A.J.; Schlom, J.; Greiner, J.W. Abstract 1480: Systemic Immunotherapeutic Efficacy of an Immunocytokine, NHS-muIL12, in a Superficial Murine Orthotopic Bladder Cancer Model. Cancer Res. 2016, 76 (Suppl. S14), 1480. [Google Scholar] [CrossRef]
- Strauss, J.; Heery, C.R.; Kim, J.W.; Jochems, C.; Donahue, R.N.; Montgomery, A.S.; McMahon, S.; Lamping, E.; Marté, J.L.; Madan, R.A.; et al. First-in-Human Phase I Trial of a Tumor-Targeted Cytokine (NHS-IL12) in Subjects with Metastatic Solid Tumors. Clin. Cancer Res. 2019, 25, 99–109. [Google Scholar] [CrossRef] [PubMed]
- Gatti-Mays, M.E.; Tschernia, N.P.; Strauss, J.; Madan, R.A.; Karzai, F.H.; Bilusic, M.; Redman, J.; Sater, H.A.; Floudas, C.S.; Toney, N.J.; et al. A Phase I Single-Arm Study of Biweekly NHS-IL12 in Patients with Metastatic Solid Tumors. Oncologist 2023, 28, 364-e217. [Google Scholar] [CrossRef] [PubMed]
- Rudman, S.; Jameson, M.; McKeage, M.; Savage, P.; Jodrell, D.; Harries, M.; Acton, G.; Erlandsson, F.; Spicer, J. A Phase 1 Study of AS1409, a Novel Antibody-Cytokine Fusion Protein, in Patients with Malignant Melanoma or Renal Cell Carcinoma. Clin. Cancer Res. 2011, 17, 1998–2005. [Google Scholar] [CrossRef] [PubMed]
- Midulla, M.; Verma, R.; Pignatelli, M.; Ritter, M.A.; Courtenay-Luck, N.S.; George, A.J.T. Source of Oncofetal ED-B-Containing Fibronectin: Implications of Production by Both Tumor and Endothelial Cells1. Cancer Res. 2000, 60, 164–169. [Google Scholar] [PubMed]
- Zardi, L.; Carnemolla, B.; Siri, A.; Petersen, T.E.; Paolella, G.; Sebastio, G.; Baralle, F.E. Transformed Human Cells Produce a New Fibronectin Isoform by Preferential Alternative Splicing of a Previously Unobserved Exon. EMBO J. 1987, 6, 2337–2342. [Google Scholar] [CrossRef] [PubMed]
- Lo, K.-M.; Lan, Y.; Siri, A.; Petersen, T.E.; Paolella, G.; Sebastio, G.; Baralle, F.E. huBC1-IL12, an Immunocytokine Which Targets EDB-Containing Oncofetal Fibronectin in Tumors and Tumor Vasculature, Shows Potent Anti-Tumor Activity in Human Tumor Models. Cancer Immunol. Immunother. 2007, 56, 447–457. [Google Scholar] [CrossRef]
- Li, J.; Hu, P.; Khawli, L.A.; Yun, A.; Epstein, A.L. chTNT-3/Hu IL-12 Fusion Protein for the Immunotherapy of Experimental Solid Tumors. Hybrid. Hybridomics 2004, 23, 1–10. [Google Scholar] [CrossRef]
- Nadal, L.; Peissert, F.; Elsayed, A.; Weiss, T.; Look, T.; Weller, M.; Piro, G.; Carbone, C.; Tortora, G.; Matasci, M.; et al. Generation and In Vivo Validation of an IL-12 Fusion Protein Based on a Novel Anti-Human FAP Monoclonal Antibody. J. Immunother. Cancer 2022, 10, e005282. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, T.; Guarino, S.R.; Scietti, L.; Palamini, M.; Wulhfard, S.; Neri, D.; Villa, A.; Forneris, F. Inference of Molecular Structure for Characterization and Improvement of Clinical Grade Immunocytokines. J. Struct. Biol. 2021, 213, 107696. [Google Scholar] [CrossRef]
- Pasche, N.; Wulhfard, S.; Pretto, F.; Carugati, E.; Neri, D. The Antibody-Based Delivery of Interleukin-12 to the Tumor Neovasculature Eradicates Murine Models of Cancer in Combination with Paclitaxel. Clin. Cancer Res. 2012, 18, 4092–4103. [Google Scholar] [CrossRef]
- Halin, C.; Rondini, S.; Nilsson, F.; Berndt, A.; Kosmehl, H.; Zardi, L.; Neri, D. Enhancement of the Antitumor Activity of Interleukin-12 by Targeted Delivery to Neovasculature. Nat. Biotechnol. 2002, 20, 264–269. [Google Scholar] [CrossRef]
- Sommavilla, R.; Pasche, N.; Trachsel, E.; Giovannoni, L.; Roesli, C.; Villa, A.; Neri, D.; Kaspar, M. Expression, Engineering and Characterization of the Tumor-Targeting Heterodimeric Immunocytokine F8-IL12. Protein Eng. Des. Sel. 2010, 23, 653–661. [Google Scholar] [CrossRef] [PubMed]
- Ongaro, T.; Matasci, M.; Cazzamalli, S.; Gouyou, B.; De Luca, R.; Neri, D.; Villa, A. A Novel Anti-Cancer L19-Interleukin-12 Fusion Protein with an Optimized Peptide Linker Efficiently Localizes In Vivo at the Site of Tumors. J. Biotechnol. 2019, 291, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Song, D.H.; Youn, S.-J.; Kim, J.W.; Cho, G.; Kim, S.C.; Lee, H.; Jin, M.S.; Lee, J.-O. Crystal Structures of Mono- and Bi-Specific Diabodies and Reduction of Their Structural Flexibility by Introduction of Disulfide Bridges at the Fv Interface. Sci. Rep. 2016, 6, 34515. [Google Scholar] [CrossRef] [PubMed]
- Ishihara, J.; Ishihara, A.; Sasaki, K.; Lee, S.S.-Y.; Williford, J.-M.; Yasui, M.; Abe, H.; Potin, L.; Hosseinchi, P.; Fukunaga, K.; et al. Targeted Antibody and Cytokine Cancer Immunotherapies through Collagen Affinity. Sci. Transl. Med. 2019, 11, eaau3259. [Google Scholar] [CrossRef]
- Bhatia, V.; Kamat, N.V.; Pariva, T.E.; Wu, L.-T.; Tsao, A.; Sasaki, K.; Sun, H.; Javier, G.; Nutt, S.; Coleman, I.; et al. Targeting Advanced Prostate Cancer with STEAP1 Chimeric Antigen Receptor T Cell and Tumor-Localized IL-12 Immunotherapy. Nat. Commun. 2023, 14, 2041. [Google Scholar] [CrossRef]
- Spolski, R.; Leonard, W.J. Interleukin-21: A Double-Edged Sword with Therapeutic Potential. Nat. Rev. Drug Discov. 2014, 13, 379–395. [Google Scholar] [CrossRef]
- Zeng, R.; Spolski, R.; Finkelstein, S.E.; Oh, S.; Kovanen, P.E.; Hinrichs, C.S.; Pise-Masison, C.A.; Radonovich, M.F.; Brady, J.N.; Restifo, N.P.; et al. Synergy of IL-21 and IL-15 in Regulating CD8+ T Cell Expansion and Function. J. Exp. Med. 2005, 201, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Petrella, T.M.; Mihalcioiu, C.L.D.; McWhirter, E.; Belanger, K.; Savage, K.J.; Song, X.; Hamid, O.; Cheng, T.; Davis, M.L.; Lee, C.W.; et al. Final Efficacy Results of NCIC CTG IND.202: A Randomized Phase II Study of Recombinant Interleukin-21 (rIL21) in Patients with Recurrent or Metastatic Melanoma (MM). JCO 2013, 31 (Suppl. S15), 9032. [Google Scholar] [CrossRef]
- Zorzi, A.; Linciano, S.; Angelini, A. Non-Covalent Albumin-Binding Ligands for Extending the Circulating Half-Life of Small Biotherapeutics. Medchemcomm 2019, 10, 1068–1081. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Wang, R.; An, D.; Liu, H.; Ye, F.; Li, B.; Zhang, J.; Liu, P.; Zhang, X.; Yao, S.; et al. An Engineered IL-21 with Half-Life Extension Enhances Anti-Tumor Immunity as a Monotherapy or in Combination with PD-1 or TIGIT Blockade. Int. Immunopharmacol. 2021, 101, 108307. [Google Scholar] [CrossRef]
- Wu, S.; Sun, R.; Tan, B.; Chen, B.; Zhou, W.; Gao, D.S.; Zhong, J.; Huang, H.; Jiang, J.; Lu, B. The Half-Life-Extended IL21 Can Be Combined with Multiple Checkpoint Inhibitors for Tumor Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 779865. [Google Scholar] [CrossRef] [PubMed]
- Deng, S.; Sun, Z.; Qiao, J.; Liang, Y.; Liu, L.; Dong, C.; Shen, A.; Wang, Y.; Tang, H.; Fu, Y.-X.; et al. Targeting Tumors with IL-21 Reshapes the Tumor Microenvironment by Proliferating PD-1intTim-3–CD8+ T Cells. JCI Insight 2020, 5, e132000. [Google Scholar] [CrossRef]
- Di Nitto, C.; Neri, D.; Weiss, T.; Weller, M.; De Luca, R. Design and Characterization of Novel Antibody-Cytokine Fusion Proteins Based on Interleukin-21. Antibodies 2022, 11, 19. [Google Scholar] [CrossRef]
- Durm, G.; Frentzas, S.; Rasmussen, E.; Najmi, S.; Sadraei, N.H. Abstract CT205: Design and Rationale of a Phase 1 Study Evaluating AMG 256, a Novel, Targeted, PD-1 Antibody x IL-21 Mutein Bifunctional Fusion Protein, in Patients with Advanced Solid Tumors. Cancer Res. 2021, 81 (Suppl. S13), CT205. [Google Scholar] [CrossRef]
- Shen, S.; Sckisel, G.; Sahoo, A.; Lalani, A.; Otter, D.D.; Pearson, J.; DeVoss, J.; Cheng, J.; Casey, S.C.; Case, R.; et al. Engineered IL-21 Cytokine Muteins Fused to Anti-PD-1 Antibodies Can Improve CD8+ T Cell Function and Anti-Tumor Immunity. Front. Immunol. 2020, 11, 832. [Google Scholar] [CrossRef]
- Chaudhry, A.; Rudensky, A.Y. Control of Inflammation by Integration of Environmental Cues by Regulatory T Cells. J. Clin. Investig. 2013, 123, 939–944. [Google Scholar] [CrossRef]
- Glocker, E.-O.; Kotlarz, D.; Boztug, K.; Gertz, E.M.; Schäffer, A.A.; Noyan, F.; Perro, M.; Diestelhorst, J.; Allroth, A.; Murugan, D.; et al. Inflammatory Bowel Disease and Mutations Affecting the Interleukin-10 Receptor. N. Engl. J. Med. 2009, 361, 2033–2045. [Google Scholar] [CrossRef] [PubMed]
- Neven, B.; Mamessier, E.; Bruneau, J.; Kaltenbach, S.; Kotlarz, D.; Suarez, F.; Masliah-Planchon, J.; Billot, K.; Canioni, D.; Frange, P.; et al. A Mendelian Predisposition to B-Cell Lymphoma Caused by IL-10R Deficiency. Blood 2013, 122, 3713–3722. [Google Scholar] [CrossRef] [PubMed]
- Berg, D.J.; Davidson, N.; Kühn, R.; Müller, W.; Menon, S.; Holland, G.; Thompson-Snipes, L.; Leach, M.W.; Rennick, D. Enterocolitis and Colon Cancer in Interleukin-10-Deficient Mice Are Associated with Aberrant Cytokine Production and CD4(+) TH1-like Responses. J. Clin. Investig. 1996, 98, 1010–1020. [Google Scholar] [CrossRef] [PubMed]
- Emmerich, J.; Mumm, J.B.; Chan, I.H.; LaFace, D.; Truong, H.; McClanahan, T.; Gorman, D.M.; Oft, M. IL-10 Directly Activates and Expands Tumor-Resident CD8+ T Cells without De Novo Infiltration from Secondary Lymphoid Organs. Cancer Res. 2012, 72, 3570–3581. [Google Scholar] [CrossRef] [PubMed]
- Naing, A.; Infante, J.R.; Papadopoulos, K.P.; Chan, I.H.; Shen, C.; Ratti, N.P.; Rojo, B.; Autio, K.A.; Wong, D.J.; Patel, M.R.; et al. PEGylated IL-10 (Pegilodecakin) Induces Systemic Immune Activation, CD8+ T Cell Invigoration and Polyclonal T Cell Expansion in Cancer Patients. Cancer Cell 2018, 34, 775–791.e3. [Google Scholar] [CrossRef] [PubMed]
- Hecht, J.R.; Lonardi, S.; Bendell, J.; Sim, H.-W.; Macarulla, T.; Lopez, C.D.; Van Cutsem, E.; Muñoz Martin, A.J.; Park, J.O.; Greil, R.; et al. Randomized Phase III Study of FOLFOX Alone or with Pegilodecakin as Second-Line Therapy in Patients with Metastatic Pancreatic Cancer That Progressed after Gemcitabine (SEQUOIA). J. Clin. Oncol. 2021, 39, 1108–1118. [Google Scholar] [CrossRef] [PubMed]
- Spigel, D.; Jotte, R.; Nemunaitis, J.; Shum, M.; Schneider, J.; Goldschmidt, J.; Eisenstein, J.; Berz, D.; Seneviratne, L.; Socoteanu, M.; et al. Randomized Phase 2 Studies of Checkpoint Inhibitors Alone or in Combination with Pegilodecakin in Patients with Metastatic NSCLC (CYPRESS 1 and CYPRESS 2). J. Thorac. Oncol. 2021, 16, 327–333. [Google Scholar] [CrossRef]
- Galeazzi, M.; Sebastiani, G.; Voll, R.; Viapiana, O.; Dudler, J.; Zufferey, P.; Selvi, E.; Finzel, S.; Bootz, F.S.; Neri, D.; et al. FRI0118 Dekavil (F8IL10)—Update on the Results of Clinical Trials Investigating the Immunocytokine in Patients with Rheumatoid Arthritis. Ann. Rheum. Dis. 2018, 77 (Suppl. S2), 603–604. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Z.; Dong, C.; Luan, Y.; Zhang, A.; Moore, C.; Fu, K.; Peng, J.; Wang, Y.; Ren, Z.; et al. Targeting Tumors with IL-10 Prevents Dendritic Cell-Mediated CD8+ T Cell Apoptosis. Cancer Cell 2019, 35, 901–915.e4. [Google Scholar] [CrossRef]
- Chang, Y.-W.; Hsiao, H.-W.; Chen, J.-P.; Tzeng, S.-F.; Tsai, C.-H.; Wu, C.-Y.; Hsieh, H.-H.; Carmona, S.J.; Andreatta, M.; Di Conza, G.; et al. A CSF-1R-Blocking Antibody/IL-10 Fusion Protein Increases Anti-Tumor Immunity by Effectuating Tumor-Resident CD8+ T Cells. Cell Rep. Med. 2023, 4, 101154. [Google Scholar] [CrossRef]
- Kumar, A.; Taghi Khani, A.; Sanchez Ortiz, A.; Swaminathan, S. GM-CSF: A Double-Edged Sword in Cancer Immunotherapy. Front. Immunol. 2022, 13, 901277. [Google Scholar] [CrossRef]
- Liu, B.L.; Robinson, M.; Han, Z.-Q.; Branston, R.H.; English, C.; Reay, P.; McGrath, Y.; Thomas, S.K.; Thornton, M.; Bullock, P.; et al. ICP34.5 Deleted Herpes Simplex Virus with Enhanced Oncolytic, Immune Stimulating, and Anti-Tumour Properties. Gene Ther. 2003, 10, 292–303. [Google Scholar] [CrossRef] [PubMed]
- Spitler, L.E.; Weber, R.W.; Allen, R.E.; Meyer, J.; Cruickshank, S.; Garbe, E.; Lin, H.-Y.; Soong, S. Recombinant Human Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF, Sargramostim) Administered for 3 Years as Adjuvant Therapy of Stages II(T4), III, and IV Melanoma. J. Immunother. 2009, 32, 632–637. [Google Scholar] [CrossRef]
- Spitler, L.E.; Cao, H.; Piironen, T.; Whiteside, T.L.; Weber, R.W.; Cruickshank, S. Biological Effects of Anti-Granulocyte-Macrophage Colony-Stimulating Factor (GM-CSF) Antibody Formation in Patients Treated With GM-CSF (Sargramostim) as Adjuvant Therapy of Melanoma. Am. J. Clin. Oncol. 2017, 40, 207–213. [Google Scholar] [CrossRef] [PubMed]
- Pampena, M.B.; Cartar, H.C.; Cueto, G.R.; Levy, E.M.; Blanco, P.A.; Barrio, M.M.; Mordoh, J. Dissecting the Immune Stimulation Promoted by CSF-470 Vaccine Plus Adjuvants in Cutaneous Melanoma Patients: Long Term Antitumor Immunity and Short Term Release of Acute Inflammatory Reactants. Front. Immunol. 2018, 9, 2531. [Google Scholar] [CrossRef]
- Mordoh, A.; Aris, M.; Carri, I.; Bravo, A.I.; Podaza, E.; Pardo, J.C.T.; Cueto, G.R.; Barrio, M.M.; Mordoh, J. An Update of Cutaneous Melanoma Patients Treated in Adjuvancy with the Allogeneic Melanoma Vaccine VACCIMEL and Presentation of a Selected Case Report with In-Transit Metastases. Front. Immunol. 2022, 13, 842555. [Google Scholar] [CrossRef]
- Brunsvig, P.F.; Guren, T.K.; Nyakas, M.; Steinfeldt-Reisse, C.H.; Rasch, W.; Kyte, J.A.; Juul, H.V.; Aamdal, S.; Gaudernack, G.; Inderberg, E.M. Long-Term Outcomes of a Phase I Study with UV1, a Second Generation Telomerase Based Vaccine, in Patients with Advanced Non-Small Cell Lung Cancer. Front. Immunol. 2020, 11, 572172. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, S.; Han, N.; Jiang, J.; Xu, Y.; Ma, D.; Lu, L.; Guo, X.; Qiu, M.; Huang, Q.; et al. A Neoantigen-Based Peptide Vaccine for Patients with Advanced Pancreatic Cancer Refractory to Standard Treatment. Front. Immunol. 2021, 12, 691605. [Google Scholar] [CrossRef] [PubMed]
- Hong, I.-S. Stimulatory versus Suppressive Effects of GM-CSF on Tumor Progression in Multiple Cancer Types. Exp. Mol. Med. 2016, 48, e242. [Google Scholar] [CrossRef]
- Serafini, P.; Carbley, R.; Noonan, K.A.; Tan, G.; Bronte, V.; Borrello, I. High-Dose Granulocyte-Macrophage Colony-Stimulating Factor-Producing Vaccines Impair the Immune Response through the Recruitment of Myeloid Suppressor Cells. Cancer Res. 2004, 64, 6337–6343. [Google Scholar] [CrossRef]
- Dela Cruz, J.S.; Trinh, K.R.; Morrison, S.L.; Penichet, M.L. Recombinant Anti-Human HER2/Neu IgG3-(GM-CSF) Fusion Protein Retains Antigen Specificity and Cytokine Function and Demonstrates Antitumor Activity1. J. Immunol. 2000, 165, 5112–5121. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Ge, X.; Liu, Y.; Li, H.; Zhang, Z. The Role of Toll-like Receptor Agonists and Their Nanomedicines for Tumor Immunotherapy. Pharmaceutics 2022, 14, 1228. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Chen, J.J.W.; Shen, K.-Y.; Chang, L.-S.; Yeh, Y.-C.; Chen, I.-H.; Chong, P.; Liu, S.-J.; Leng, C.-H. Recombinant Lipidated HPV E7 Induces a Th-1-Biased Immune Response and Protective Immunity against Cervical Cancer in a Mouse Model. PLoS ONE 2012, 7, e40970. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.-L.; Wu, C.-C.; Shen, K.-Y.; Liu, S.-J. Activation of GM-CSF and TLR2 Signaling Synergistically Enhances Antigen-Specific Antitumor Immunity and Modulates the Tumor Microenvironment. J. Immunother. Cancer 2021, 9, e002758. [Google Scholar] [CrossRef]
- Ni, L.; Lu, J. Interferon Gamma in Cancer Immunotherapy. Cancer Med. 2018, 7, 4509–4516. [Google Scholar] [CrossRef]
- Pujade-Lauraine, E.; Guastalla, J.P.; Colombo, N.; Devillier, P.; François, E.; Fumoleau, P.; Monnier, A.; Nooy, M.; Mignot, L.; Bugat, R.; et al. Intraperitoneal Recombinant Interferon Gamma in Ovarian Cancer Patients with Residual Disease at Second-Look Laparotomy. JCO 1996, 14, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Windbichler, G.H.; Hausmaninger, H.; Stummvoll, W.; Graf, A.H.; Kainz, C.; Lahodny, J.; Denison, U.; Müller-Holzner, E.; Marth, C. Interferon-Gamma in the First-Line Therapy of Ovarian Cancer: A Randomized Phase III Trial. Br. J. Cancer 2000, 82, 1138–1144. [Google Scholar] [CrossRef] [PubMed]
- Alberts, D.S.; Marth, C.; Alvarez, R.D.; Johnson, G.; Bidzinski, M.; Kardatzke, D.R.; Bradford, W.Z.; Loutit, J.; Kirn, D.H.; Clouser, M.C.; et al. Randomized Phase 3 Trial of Interferon Gamma-1b plus Standard Carboplatin/Paclitaxel versus Carboplatin/Paclitaxel Alone for First-Line Treatment of Advanced Ovarian and Primary Peritoneal Carcinomas: Results from a Prospectively Designed Analysis of Progression-Free Survival. Gynecol. Oncol. 2008, 109, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Ebbinghaus, C. An Antibody-Interferon Gamma Fusion Protein for Cancer Therapy. Ph.D. Thesis, ETH Zurich, Zurich, Switzerland, 2004; p. 134S. [Google Scholar] [CrossRef]
- Hemmerle, T.; Neri, D. The Dose-Dependent Tumor Targeting of Antibody–IFNγ Fusion Proteins Reveals an Unexpected Receptor-Trapping Mechanism In Vivo. Cancer Immunol. Res. 2014, 2, 559–567. [Google Scholar] [CrossRef]
- Di Nitto, C.; Gilardoni, E.; Mock, J.; Nadal, L.; Weiss, T.; Weller, M.; Seehusen, F.; Libbra, C.; Puca, E.; Neri, D.; et al. An Engineered IFNγ-Antibody Fusion Protein with Improved Tumor-Homing Properties. Pharmaceutics 2023, 15, 377. [Google Scholar] [CrossRef]
- Mizokami, M.M.; Hu, P.; Khawli, L.A.; Li, J.; Epstein, A.L. Chimeric TNT-3 Antibody/Murine Interferon-γ Fusion Protein for the Immunotherapy of Solid Malignancies. Hybrid. Hybridomics 2003, 22, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Belardelli, F.; Ferrantini, M.; Proietti, E.; Kirkwood, J.M. Interferon-Alpha in Tumor Immunity and Immunotherapy. Cytokine Growth Factor Rev. 2002, 13, 119–134. [Google Scholar] [CrossRef]
- Baron, S.; Tyring, S.K.; Fleischmann, W.R., Jr.; Coppenhaver, D.H.; Niesel, D.W.; Klimpel, G.R.; Stanton, G.J.; Hughes, T.K. The Interferons: Mechanisms of Action and Clinical Applications. JAMA 1991, 266, 1375–1383. [Google Scholar] [CrossRef] [PubMed]
- Farkas, Á.; Kemény, L. Interferon-α in the Generation of Monocyte-derived Dendritic Cells: Recent Advances and Implications for Dermatology. Br. J. Dermatol. 2011, 165, 247–254. [Google Scholar] [CrossRef]
- Borden, E.C.; Parkinson, D. A Perspective on the Clinical Effectiveness and Tolerance of Interferon-Alpha. Semin. Oncol. 1998, 25 (Suppl. S1), 3–8. [Google Scholar]
- Xuan, C.; Steward, K.K.; Timmerman, J.M.; Morrison, S.L. Targeted Delivery of Interferon-Alpha via Fusion to Anti-CD20 Results in Potent Antitumor Activity against B-Cell Lymphoma. Blood 2010, 115, 2864–2871. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhu, Y.; Li, C.; Trinh, R.; Ren, X.; Sun, F.; Wang, Y.; Shang, P.; Wang, T.; Wang, M.; et al. Anti-VEGFR2-Interferon-A2 Regulates the Tumor Microenvironment and Exhibits Potent Antitumor Efficacy against Colorectal Cancer. OncoImmunology 2017, 6, e1290038. [Google Scholar] [CrossRef]
- Shang, P.; Gao, R.; Zhu, Y.; Zhang, X.; Wang, Y.; Guo, M.; Peng, H.; Wang, M.; Zhang, J. VEGFR2-Targeted Antibody Fused with IFNαmut Regulates the Tumor Microenvironment of Colorectal Cancer and Exhibits Potent Anti-Tumor and Anti-Metastasis Activity. Acta Pharm. Sin. B 2021, 11, 420–433. [Google Scholar] [CrossRef]
- Hutmacher, C.; Neri, D. Antibody-Cytokine Fusion Proteins: Biopharmaceuticals with Immunomodulatory Properties for Cancer Therapy. Adv. Drug Deliv. Rev. 2019, 141, 67–91. [Google Scholar] [CrossRef]
- Puskas, J.; Skrombolas, D.; Sedlacek, A.; Lord, E.; Sullivan, M.; Frelinger, J. Development of an Attenuated Interleukin-2 Fusion Protein That Can Be Activated by Tumour-Expressed Proteases. Immunology 2011, 133, 206–220. [Google Scholar] [CrossRef]
- Garcin, G.; Paul, F.; Staufenbiel, M.; Bordat, Y.; Van der Heyden, J.; Wilmes, S.; Cartron, G.; Apparailly, F.; De Koker, S.; Piehler, J.; et al. High Efficiency Cell-Specific Targeting of Cytokine Activity. Nat Commun 2014, 5, 3016. [Google Scholar] [CrossRef] [PubMed]
- Hank, J.A.; Gan, J.; Ryu, H.; Ostendorf, A.; Stauder, M.C.; Sternberg, A.; Albertini, M.; Lo, K.-M.; Gillies, S.D.; Eickhoff, J.; et al. Immunogenicity of the Hu14.18-IL2 Immunocytokine Molecule in Adults With Melanoma and Children With Neuroblastoma. Clinical Cancer Research 2009, 15, 5923–5930. [Google Scholar] [CrossRef] [PubMed]
- Prodi, E.; Corbellari, R.; Matasci, M.; Neri, D.; De Luca, R. Abstract 1877: Tripokin: Potential Best-in-Class for Tumor Targeted Interleukin-2 (IL2) Potentiated by Tumor Necrosis Factor (TNF). Cancer Res. 2023, 83 (Suppl. S7), 1877. [Google Scholar] [CrossRef]
- Schanzer, J.M.; Fichtner, I.; Baeuerle, P.A.; Kufer, P. Antitumor Activity of a Dual Cytokine/Single-Chain Antibody Fusion Protein for Simultaneous Delivery of GM-CSF and IL-2 to Ep-CAM Expressing Tumor Cells. J. Immunother. 2006, 29, 477–488. [Google Scholar] [CrossRef]
- Tian, Z.; Liu, M.; Zhang, Y.; Wang, X. Bispecific T Cell Engagers: An Emerging Therapy for Management of Hematologic Malignancies. J. Hematol. Oncol. 2021, 14, 75. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
