Recent Progress in Multifunctional Stimuli-Responsive Combinational Drug Delivery Systems for the Treatment of Biofilm-Forming Bacterial Infections
Abstract
:1. Introduction
2. Drug Antimicrobial Mechanisms and Encapsulation
2.1. Drug Carrier Types
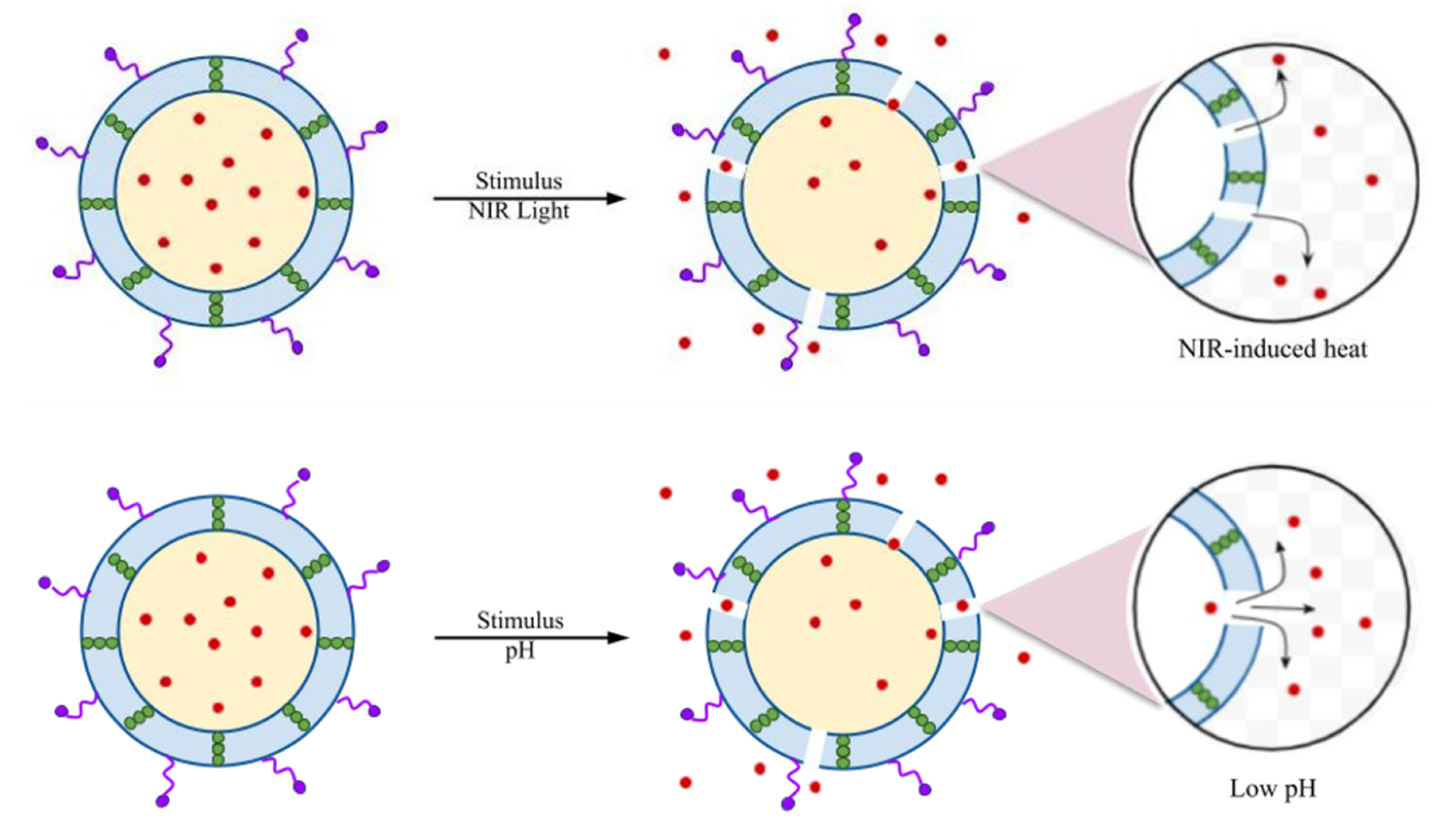
2.2. Targeted Stimuli for Bacterial Therapy
2.3. Mechanisms of Bacterial Therapy
3. Controlled Antibacterial Drug Delivery Development
3.1. Polymer-Based Exosome Modification
3.2. Inorganic Nanomaterials-Based Modifications
4. Combination Therapy
5. Conclusions and Future Prospects
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cascioferro, S.; Carbone, D.; Parrino, B.; Pecoraro, C.; Giovannetti, E.; Cirrincione, G.; Diana, P. Therapeutic Strategies to Counteract Antibiotic Resistance in MRSA Biofilm-Associated Infections. ChemMedChem 2021, 16, 65–80. [Google Scholar] [CrossRef] [PubMed]
- Hassoun, A.; Linden, P.K.; Friedman, B. Incidence, prevalence, and management of MRSA bacteremia across patient populations—A review of recent developments in MRSA management and treatment. Crit. Care 2017, 21, 211. [Google Scholar] [CrossRef] [PubMed]
- Fishovitz, J.; Hermoso, J.A.; Chang, M.; Mobashery, S. Penicillin-binding protein 2a of methicillin-resistant Staphylococcus aureus. IUBMB Life 2014, 66, 572–577. [Google Scholar] [CrossRef] [PubMed]
- Hausner, M.; Wuertz, S. High Rates of Conjugation in Bacterial Biofilms as Determined by Quantitative In Situ Analysis. Appl. Environ. Microbiol. 1999, 65, 3710–3713. [Google Scholar] [CrossRef] [PubMed]
- Bridier, A.; Briandet, R.; Thomas, V.; Dubois-Brissonnet, F. Resistance of bacterial biofilms to disinfectants: A review. Biofouling 2011, 27, 1017–1032. [Google Scholar] [CrossRef] [PubMed]
- Flemming, H.-C.; Wingender, J.; Szewzyk, U.; Steinberg, P.; Rice, S.A.; Kjelleberg, S. Biofilms: An emergent form of bacterial life. Nat. Rev. Microbiol. 2016, 14, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. An overview of the antimicrobial resistance mechanisms of bacteria. AIMS Microbiol. 2018, 4, 482–501. [Google Scholar] [CrossRef] [PubMed]
- Jia, R.; Teng, L.; Gao, L.; Su, T.; Fu, L.; Qiu, Z.; Bi, Y. Advances in Multiple Stimuli-Responsive Drug-Delivery Systems for Cancer Therapy. Int. J. Nanomed. 2021, 16, 1525–1551. [Google Scholar] [CrossRef] [PubMed]
- Jacob, P.L.; Brugnoli, B.; Del Giudice, A.; Phan, H.; Chauhan, V.M.; Beckett, L.; Gillis, R.B.; Moloney, C.; Cavanagh, R.J.; Krumins, E.; et al. Poly (diglycerol adipate) variants as enhanced nanocarrier replacements in drug delivery applications. J. Colloid Interface Sci. 2023, 641, 1043–1057. [Google Scholar] [CrossRef]
- Jacob, P.L.; Ruiz Cantu, L.A.; Pearce, A.K.; He, Y.; Lentz, J.C.; Moore, J.C.; Machado, F.; Rivers, G.; Apebende, E.; Fernandez, M.R.; et al. Poly (glycerol adipate) (PGA) backbone modifications with a library of functional diols: Chemical and physical effects. Polymer 2021, 228, 123912. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, J.; Liu, X.; Wang, G.; Qi, Y.; Wang, T.; Song, Y.; Li, Y.; Ning, G. Dual Stimuli-Responsive smart fibrous membranes for efficient Photothermal/Photodynamic/Chemo-Therapy of Drug-Resistant bacterial infection. Chem. Eng. J. 2022, 432, 134351. [Google Scholar] [CrossRef]
- Lin, X.; Wu, X.; Chen, X.; Wang, B.; Xu, W. Intellective and stimuli-responsive drug delivery systems in eyes. Int. J. Pharm. 2021, 602, 120591. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Jiang, X.; Lai, H.; Zhang, X. Smart Bacteria-Responsive Drug Delivery Systems in Medical Implants. J. Funct. Biomater. 2022, 13, 173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Liu, X.; Li, B.; Zheng, Y.; Han, Y.; Chen, D.-f.; Yeung, K.W.K.; Cui, Z.; Liang, Y.; Li, Z.; et al. Near-Infrared Light Triggered Phototherapy and Immunotherapy for Elimination of Methicillin-Resistant Staphylococcus aureus Biofilm Infection on Bone Implant. ACS Nano 2020, 14, 8157–8170. [Google Scholar] [CrossRef] [PubMed]
- Qu, J.; Zhao, X.; Ma, P.X.; Guo, B. Injectable antibacterial conductive hydrogels with dual response to an electric field and pH for localized “smart” drug release. Acta Biomater. 2018, 72, 55–69. [Google Scholar] [CrossRef] [PubMed]
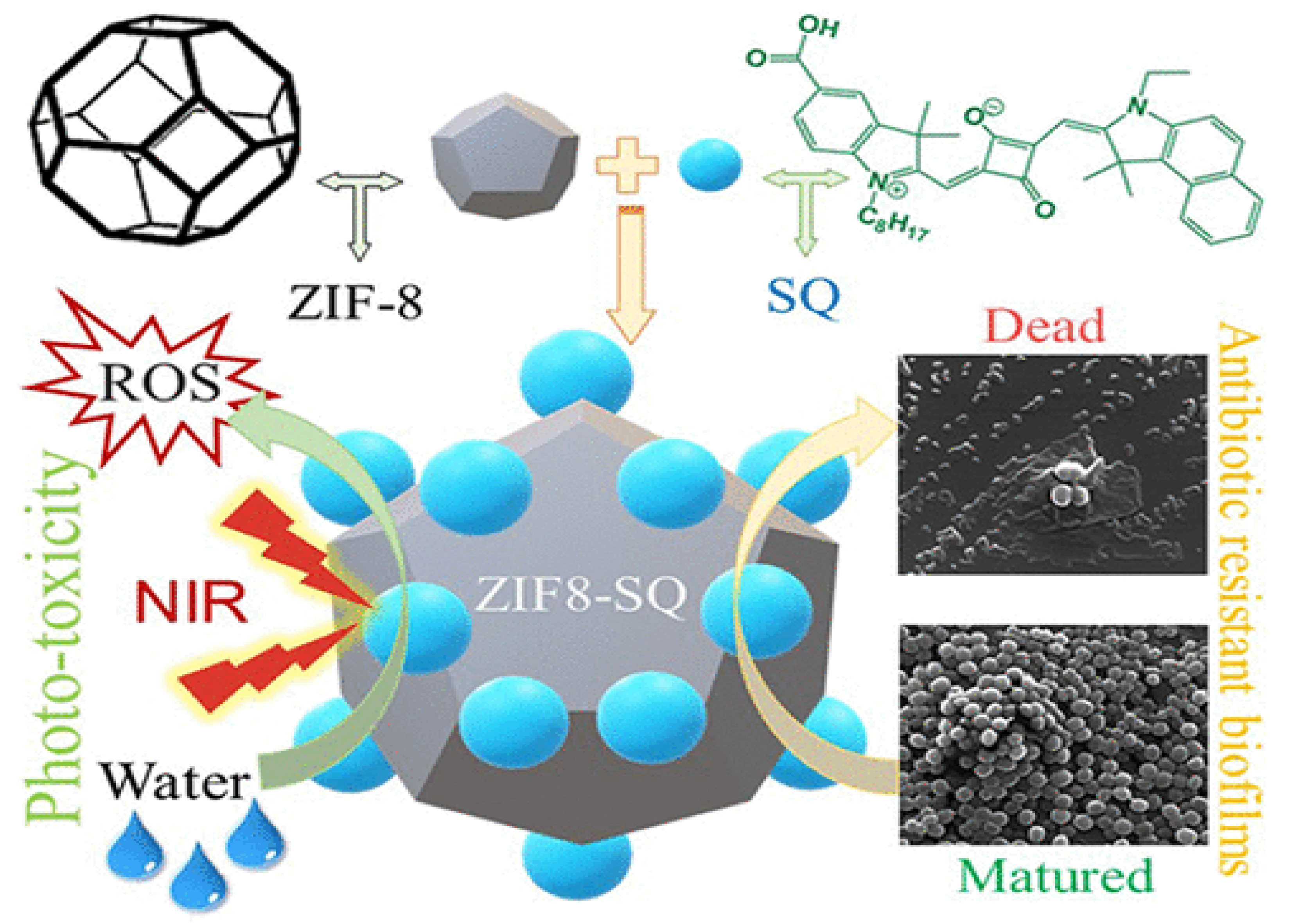
- Xiao, Y.; Xu, M.; Lv, N.; Cheng, C.; Huang, P.; Li, J.; Hu, Y.; Sun, M. Dual stimuli-responsive metal-organic framework-based nanosystem for synergistic photothermal/pharmacological antibacterial therapy. Acta Biomater. 2021, 122, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Sharma, S.; Parveen, R.; Chatterji, B.P. Toxicology of Nanoparticles in Drug Delivery. Curr. Pathobiol. Rep. 2021, 9, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Violatto, M.B.; Casarin, E.; Talamini, L.; Russo, L.; Baldan, S.; Tondello, C.; Messmer, M.; Hintermann, E.; Rossi, A.; Passoni, A.; et al. Dexamethasone Conjugation to Biodegradable Avidin-Nucleic-Acid-Nano-Assemblies Promotes Selective Liver Targeting and Improves Therapeutic Efficacy in an Autoimmune Hepatitis Murine Model. ACS Nano 2019, 13, 4410–4423. [Google Scholar] [CrossRef]
- Rabin, N.; Zheng, Y.; Opoku-Temeng, C.; Du, Y.; Bonsu, E.; Sintim, H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015, 7, 493–512. [Google Scholar] [CrossRef]
- El-Feky, G.S.; Sharaf, S.S.; El Shafei, A.; Hegazy, A.A. Using chitosan nanoparticles as drug carriers for the development of a silver sulfadiazine wound dressing. Carbohydr. Polym. 2017, 158, 11–19. [Google Scholar] [CrossRef]
- Li, J.; Ding, Z.; Li, Y.; Miao, J.; Wang, W.; Nundlall, K.; Chen, S. Reactive oxygen species-sensitive thioketal-linked mesoporous silica nanoparticles as drug carrier for effective antibacterial activity. Mater. Des. 2020, 195, 109021. [Google Scholar] [CrossRef]
- Akolpoglu, M.B.; Alapan, Y.; Dogan, N.O.; Baltaci, S.F.; Yasa, O.; Aybar Tural, G.; Sitti, M. Magnetically steerable bacterial microrobots moving in 3D biological matrices for stimuli-responsive cargo delivery. Sci. Adv. 2022, 8, eabo6163. [Google Scholar] [CrossRef] [PubMed]
- Caddeo, C.; Miglionico, R.; Rinaldi, R.; Nigro, I.; Lamorte, D.; Chiummiento, L.; Lupattelli, P.; Funicello, M.; D’Orsi, R.; Valenti, D.; et al. PEGylated Liposomes Loaded with Carbamate Inhibitor ANP0903 Trigger Apoptosis by Enhancing ER Stress in HepG2 Cancer Cells. Int. J. Mol. Sci. 2023, 24, 4552. [Google Scholar] [CrossRef]
- Simonis, B.; Vignone, D.; Gonzalez Paz, O.; Donati, E.; Falchetti, M.L.; Bombelli, C.; Cellucci, A.; Auciello, G.; Fini, I.; Galantini, L.; et al. Transport of cationic liposomes in a human blood brain barrier model: Role of the stereochemistry of the gemini amphiphile on liposome biological features. J. Colloid Interface Sci. 2022, 627, 283–298. [Google Scholar] [CrossRef]
- Ma, M.; Cheng, Y.; Xu, Z.; Xu, P.; Qu, H.; Fang, Y.; Xu, T.; Wen, L. Evaluation of polyamidoamine (PAMAM) dendrimers as drug carriers of anti-bacterial drugs using sulfamethoxazole (SMZ) as a model drug. Eur. J. Med. Chem. 2007, 42, 93–98. [Google Scholar] [CrossRef]
- Andoy, N.M.O.; Jeon, K.; Kreis, C.T.; Sullan, R.M.A. Multifunctional and Stimuli-Responsive Polydopamine Nanoparticle-Based Platform for Targeted Antimicrobial Applications. Adv. Funct. Mater. 2020, 30, 2004503. [Google Scholar] [CrossRef]
- Ge, J.; Li, M.; Fan, J.; Celia, C.; Xie, Y.; Chang, Q.; Deng, X. Synthesis, characterization, and antibacterial activity of chitosan-chelated silver nanoparticles. J. Biomater. Sci. Polym. Ed. 2024, 35, 45–62. [Google Scholar] [CrossRef]
- Xu, J.; Lin, Q.; Sheng, M.; Ding, T.; Li, B.; Gao, Y.; Tan, Y. Antibiofilm Effect of Cinnamaldehyde-Chitosan Nanoparticles against the Biofilm of Staphylococcus aureus. Antibiotics 2022, 11, 1403. [Google Scholar] [CrossRef] [PubMed]
- Fazly Bazzaz, B.S.; Khameneh, B.; Zarei, H.; Golmohammadzadeh, S. Antibacterial efficacy of rifampin loaded solid lipid nanoparticles against Staphylococcus epidermidis biofilm. Microb. Pathog. 2016, 93, 137–144. [Google Scholar] [CrossRef]
- Zafari, M.; Adibi, M.; Chiani, M.; Bolourchi, N.; Barzi, S.M.; Shams Nosrati, M.S.; Bahari, Z.; Shirvani, P.; Noghabi, K.A.; Ebadi, M.; et al. Effects of cefazolin-containing niosome nanoparticles against methicillin-resistant Staphylococcus aureus biofilm formed on chronic wounds. Biomed. Mater. 2021, 16, 035001. [Google Scholar] [CrossRef]
- Tong, F.; Wang, P.; Chen, Z.; Liu, Y.; Wang, L.; Guo, J.; Li, Z.; Cai, H.; Wei, J. Combined Ferromagnetic Nanoparticles for Effective Periodontal Biofilm Eradication in Rat Model. Int. J. Nanomed. 2023, 18, 2371–2388. [Google Scholar] [CrossRef] [PubMed]
- Horev, B.; Klein, M.I.; Hwang, G.; Li, Y.; Kim, D.; Koo, H.; Benoit, D.S.W. pH-Activated Nanoparticles for Controlled Topical Delivery of Farnesol to Disrupt Oral Biofilm Virulence. ACS Nano 2015, 9, 2390–2404. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Zhou, T.; Yuan, Y.; Natalie Kłodzińska, S.; Zheng, T.; Sternberg, C.; Mørck Nielsen, H.; Sun, Y.; Wan, F. Synthesis of carbon quantum dot-poly lactic-co-glycolic acid hybrid nanoparticles for chemo-photothermal therapy against bacterial biofilms. J. Colloid Interface Sci. 2020, 577, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Li, K.; Xia, J.; Chen, C.; Liu, Y.; Lang, S.; Yu, L.; Liu, G. Commercial soft contact lenses engineered with zwitterionic silver nanoparticles for effectively treating microbial keratitis. J. Colloid Interface Sci. 2022, 610, 923–933. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Sathiyaseelan, A.; Zhang, X.; Wang, M.-H. Synthesis and Characterization of Bimetallic Platinum/Selenium (Pt/Se) Nanoparticles for Synergistic Antibacterial Activity. BioNanoScience 2024, 14, 630–642. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Zhang, X.; Choi, M.; Wang, M.-H. Bimetallic (Ag and MgO) nanoparticles, Aloe vera extracts loaded xanthan gum nanocomposite for enhanced antibacterial and in-vitro wound healing activity. Int. J. Biol. Macromol. 2023, 242, 124813. [Google Scholar] [CrossRef]
- Jin, Y.; Lu, Y.; Sathiyaseelan, A.; Saravanakumar, K.; Zhang, X.; Wang, M.-H. Characterization, cytotoxicity, and antibacterial activity of paeoniflorin-loaded mesoporous silica oxide nanoparticles. J. Drug Deliv. Sci. Technol. 2023, 84, 104551. [Google Scholar] [CrossRef]
- Zhang, X.; Saravanakumar, K.; Sathiyaseelan, A.; Park, S.; Wang, M.-H. Synthesis, characterization, and comparative analysis of antibiotics (ampicillin and erythromycin) loaded ZrO2 nanoparticles for enhanced antibacterial activity. J. Drug Deliv. Sci. Technol. 2023, 82, 104293. [Google Scholar] [CrossRef]
- Park, S.; Saravanakumar, K.; Zhang, X.; Seon Jeong, M.; Wang, M.-H. Tetracycline-loaded zirconium oxide nanoparticles synthesized by Lactobacillus rhamnosus effectively eradicate bacterial biofilms. Inorg. Chem. Commun. 2022, 145, 109978. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Sathiyaseelan, A.; Manivasagan, P.; Jeong, M.S.; Choi, M.; Jang, E.-S.; Priya, V.V.; Wang, M.-H. Photothermally responsive chitosan-coated iron oxide nanoparticles for enhanced eradication of bacterial biofilms. Biomater. Adv. 2022, 141, 213129. [Google Scholar] [CrossRef]
- Chen, Y.; Gao, Y.; Huang, Y.; Jin, Q.; Ji, J. Inhibiting Quorum Sensing by Active Targeted pH-Sensitive Nanoparticles for Enhanced Antibiotic Therapy of Biofilm-Associated Bacterial Infections. ACS Nano 2023, 17, 10019–10032. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.; Sun, Y.; Li, W.; Guo, X.; Liu, X.; Wu, W.; Yu, W.; Wang, J.; Shan, A. Self-Assembly of Antimicrobial Peptide-Based Micelles Breaks the Limitation of Trypsin. ACS Appl. Mater. Interfaces 2023, 15, 494–510. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiu, Q.; Liu, G.; Ren, H.; Wang, X.; Lovell, J.F.; Zhang, Y. Traceless antibiotic-crosslinked micelles for rapid clearance of intracellular bacteria. J. Control. Release 2022, 341, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Tănase, M.A.; Soare, A.C.; Diţu, L.M.; Nistor, C.L.; Mihaescu, C.I.; Gifu, I.C.; Petcu, C.; Cinteza, L.O. Influence of the Hydrophobicity of Pluronic Micelles Encapsulating Curcumin on the Membrane Permeability and Enhancement of Photoinduced Antibacterial Activity. Pharmaceutics 2022, 14, 2137. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.; Ren, J.; Li, Y.; Yuan, S.; Wang, G. Preparation of caffeic acid grafted chitosan self-assembled micelles to enhance oral bioavailability and antibacterial activity of quercetin. Front. Vet. Sci. 2023, 10, 1218025. [Google Scholar] [CrossRef] [PubMed]
- Qian, Y.; Hu, X.; Wang, J.; Li, Y.; Liu, Y.; Xie, L. Polyzwitterionic micelles with antimicrobial-conjugation for eradication of drug-resistant bacterial biofilms. Colloids Surf. B Biointerfaces 2023, 231, 113542. [Google Scholar] [CrossRef] [PubMed]
- Padaga, S.G.; Ch, S.; Paul, M.; Wable, B.D.; Ghosh, B.; Biswas, S. Chitosan oligosaccharide/pluronic F127 micelles exhibiting anti-biofilm effect to treat bacterial keratitis. Carbohydr. Polym. 2024, 330, 121818. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Yan, S.; You, J.; Wu, X. Antibacterial Micelles-Loaded Carboxymethyl Chitosan/Oxidized Konjac Glucomannan Composite Hydrogels for Enhanced Wound Repairing. ACS Appl. Mater. Interfaces 2024, 16, 13563–13572. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Wang, R.; Zhao, B.; Yin, B.; Zhang, H.; Liang, C.; Hu, X. Enzyme-Triggered Polyelectrolyte Complex for Responsive Delivery of α-Helical Polypeptides to Optimize Antibacterial Therapy. Biomacromolecules 2024, 25, 3112–3121. [Google Scholar] [CrossRef]
- Xiao, J.; Yin, M.; Yang, M.; Ren, J.; Liu, C.; Lian, J.; Lu, X.; Jiang, Y.; Yao, Y.; Luo, J. Lipase and pH-responsive diblock copolymers featuring fluorocarbon and carboxyl betaine for methicillin-resistant staphylococcus aureus infections. J. Control. Release 2024, 369, 39–52. [Google Scholar] [CrossRef]
- Gupta, C.; Hazra, C.; Poddar, P.; Dhara, D.; Byram, P.K.; Chakravorty, N.; Sen, R.; Ghosh, S.K. Development and performance evaluation of self-assembled pH-responsive curcumin-bacterial exopolysaccharide micellar conjugates as bioactive delivery system. Int. J. Biol. Macromol. 2024, 263, 130372. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Song, Z.; Wang, H.; Han, H. Endogenous stimulus-powered antibiotic release from nanoreactors for a combination therapy of bacterial infections. Nat. Commun. 2019, 10, 4464. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.; Liu, Y.; Chen, Y.; Xiang, Q.; Huang, Y.; Liu, Z.; Xue, W.; Guo, R. Injectable Antibacterial Hydrogel with Asiaticoside-Loaded Liposomes and Ultrafine Silver Nanosilver Particles Promotes Healing of Burn-Infected Wounds. Adv. Healthc. Mater. 2023, 12, 2203201. [Google Scholar] [CrossRef] [PubMed]
- Schito, A.M.; Schito, G.C.; Alfei, S. Synthesis and Antibacterial Activity of Cationic Amino Acid-Conjugated Dendrimers Loaded with a Mixture of Two Triterpenoid Acids. Polymers 2021, 13, 521. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wang, Z.; Feng, X.; Bai, E.; Xiong, Y.; Zhu, X.; Shen, B.; Duan, Y.; Huang, Y. Platensimycin-Encapsulated Poly(lactic-co-glycolic acid) and Poly(amidoamine) Dendrimers Nanoparticles with Enhanced Anti-Staphylococcal Activity in Vivo. Bioconjugate Chem. 2020, 31, 1425–1437. [Google Scholar] [CrossRef] [PubMed]
- Zhu, B.; Li, Y.; Huang, F.; Chen, Z.; Xie, J.; Ding, C.; Li, J. Promotion of the osteogenic activity of an antibacterial polyaniline coating by electrical stimulation. Biomater. Sci. 2019, 7, 4730–4737. [Google Scholar] [CrossRef] [PubMed]
- Foudah, A.I.; Alqarni, M.H.; Ross, S.A.; Alam, A.; Salkini, M.A.; Kumar, P. Site-Specific Evaluation of Bioactive Coumarin-Loaded Dendrimer G4 Nanoparticles against Methicillin Resistant Staphylococcus aureus. ACS Omega 2022, 7, 34990–34996. [Google Scholar] [CrossRef]
- Fallah, F.; Zargar, M.; Yousefi, M.; Alam, A.N. Synthesis of the erythromycin-conjugated nanodendrimer and its antibacterial activity. Eur. J. Pharm. Sci. 2018, 123, 321–326. [Google Scholar] [CrossRef]
- Dongargaonkar, A.A.; Bowlin, G.L.; Yang, H. Electrospun Blends of Gelatin and Gelatin–Dendrimer Conjugates as a Wound-Dressing and Drug-Delivery Platform. Biomacromolecules 2013, 14, 4038–4045. [Google Scholar] [CrossRef]
- Wang, Z.; Peng, Y.; Zhou, Y.; Zhang, S.; Tan, J.; Li, H.; He, D.; Deng, L. Pd-Cu nanoalloy for dual stimuli-responsive chemo-photothermal therapy against pathogenic biofilm bacteria. Acta Biomater. 2022, 137, 276–289. [Google Scholar] [CrossRef]
- Karimzadeh, Z.; Javanbakht, S.; Namazi, H. Carboxymethylcellulose/MOF-5/Graphene oxide bio-nanocomposite as antibacterial drug nanocarrier agent. Bioimpacts 2019, 9, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Lüchow, M.; Zhang, Y.; Lin, J.; Fortuin, L.; Mohanty, S.; Brauner, A.; Malkoch, M. Nanogel Encapsulated Hydrogels as Advanced Wound Dressings for the Controlled Delivery of Antibiotics. Adv. Funct. Mater. 2021, 31, 2006453. [Google Scholar] [CrossRef]
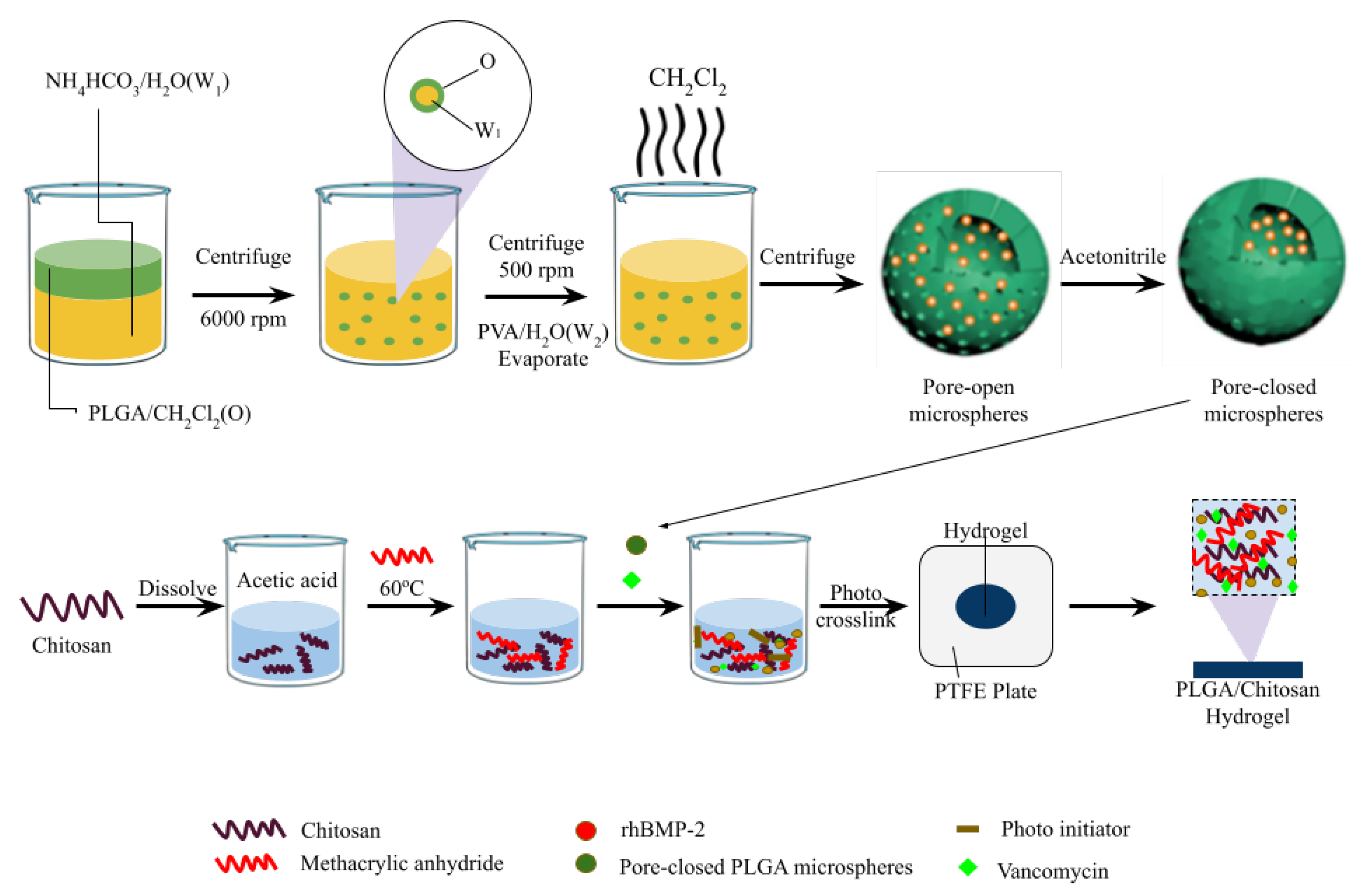
- Song, W.; Xiao, Y. Sequential drug delivery of vancomycin and rhBMP-2 via pore-closed PLGA microparticles embedded photo-crosslinked chitosan hydrogel for enhanced osteointegration. Int. J. Biol. Macromol. 2021, 182, 612–625. [Google Scholar] [CrossRef]
- Yang, X.; Shi, G.; Guo, J.; Wang, C.; He, Y. Exosome-encapsulated antibiotic against intracellular infections of methicillin-resistant Staphylococcus aureus. Int. J. Nanomed. 2018, 13, 8095–8104. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xie, B.; Peng, H.; Shi, G.; Sreenivas, B.; Guo, J.; Wang, C.; He, Y. Eradicating intracellular MRSA via targeted delivery of lysostaphin and vancomycin with mannose-modified exosomes. J. Control. Release 2021, 329, 454–467. [Google Scholar] [CrossRef] [PubMed]
- Kamboj, N.; Rodriguez, M.; Rahmani Ahranjani, R.; Prashanth, K.G.; Hussainova, I. Bioceramic scaffolds by additive manufacturing for controlled delivery of the antibiotic vancomycin. Proc. Est. Acad. Sci. 2019, 68, 185–190. [Google Scholar] [CrossRef]
- Yadav, S.K.; Khan, G.; Bonde, G.V.; Bansal, M.; Mishra, B. Design, optimization and characterizations of chitosan fortified calcium alginate microspheres for the controlled delivery of dual drugs. Artif. Cells Nanomed. Biotechnol. 2018, 46, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, X.; Tan, L.; Cui, Z.; Yang, X.; Yeung, K.W.K.; Pan, H.; Wu, S. Porous Iron-Carboxylate Metal–Organic Framework: A Novel Bioplatform with Sustained Antibacterial Efficacy and Nontoxicity. ACS Appl. Mater. Interfaces 2017, 9, 19248–19257. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Huang, L.; Zhang, W.; Wang, R.; Yue, T.; Sun, J.; Li, G.; Wang, J. The highly efficient elimination of intracellular bacteria via a metal organic framework (MOF)-based three-in-one delivery system. Nanoscale 2019, 11, 9468–9477. [Google Scholar] [CrossRef]
- Bagchi, D.; Bhattacharya, A.; Dutta, T.; Nag, S.; Wulferding, D.; Lemmens, P.; Pal, S.K. Nano MOF Entrapping Hydrophobic Photosensitizer for Dual-Stimuli-Responsive Unprecedented Therapeutic Action against Drug-Resistant Bacteria. ACS Appl. Bio Mater. 2019, 2, 1772–1780. [Google Scholar] [CrossRef]
- Song, Z.; Wu, Y.; Cao, Q.; Wang, H.; Wang, X.; Han, H. pH-Responsive, Light-Triggered on-Demand Antibiotic Release from Functional Metal–Organic Framework for Bacterial Infection Combination Therapy. Adv. Funct. Mater. 2018, 28, 1800011. [Google Scholar] [CrossRef]
- Yang, Y.; Hu, Q.; Zhang, Q.; Jiang, K.; Lin, W.; Yang, Y.; Cui, Y.; Qian, G. A Large Capacity Cationic Metal–Organic Framework Nanocarrier for Physiological pH Responsive Drug Delivery. Mol. Pharm. 2016, 13, 2782–2786. [Google Scholar] [CrossRef]
- Nasrabadi, M.; Ghasemzadeh, M.A.; Zand Monfared, M.R. The preparation and characterization of UiO-66 metal–organic frameworks for the delivery of the drug ciprofloxacin and an evaluation of their antibacterial activities. New J. Chem. 2019, 43, 16033–16040. [Google Scholar] [CrossRef]
- Al Neyadi, S.S.; Al Blooshi, A.G.; Nguyen, H.L.; Alnaqbi, M.A. UiO-66-NH2 as an effective solid support for quinazoline derivatives for antibacterial agents against Gram-negative bacteria. New J. Chem. 2021, 45, 20386–20395. [Google Scholar] [CrossRef]
- Silva, I.M.P.; Carvalho, M.A.; Oliveira, C.S.; Profirio, D.M.; Ferreira, R.B.; Corbi, P.P.; Formiga, A.L.B. Enhanced performance of a metal-organic framework analogue to MIL-101(Cr) containing amine groups for ibuprofen and nimesulide controlled release. Inorg. Chem. Commun. 2016, 70, 47–50. [Google Scholar] [CrossRef]
- Nagaraja, K.; Rao, K.M.; Reddy, G.V.; Rao, K.S.V.K. Tragacanth gum-based multifunctional hydrogels and green synthesis of their silver nanocomposites for drug delivery and inactivation of multidrug resistant bacteria. Int. J. Biol. Macromol. 2021, 174, 502–511. [Google Scholar] [CrossRef]
- Ahmed, S.A.; Nur Hasan, M.; Bagchi, D.; Altass, H.M.; Morad, M.; Althagafi, I.I.; Hameed, A.M.; Sayqal, A.; Khder, A.E.R.S.; Asghar, B.H.; et al. Nano-MOFs as targeted drug delivery agents to combat antibiotic-resistant bacterial infections. R. Soc. Open Sci. 2020, 7, 200959. [Google Scholar] [CrossRef]
- Esfahanian, M.; Ghasemzadeh, M.A.; Razavian, S.M.H. Synthesis, identification and application of the novel metal-organic framework Fe3O4@PAA@ZIF-8 for the drug delivery of ciprofloxacin and investigation of antibacterial activity. Artif. Cells Nanomed. Biotechnol. 2019, 47, 2024–2030. [Google Scholar] [CrossRef]
- Song, F.; Gong, J.; Tao, Y.; Cheng, Y.; Lu, J.; Wang, H. A robust regenerated cellulose-based dual stimuli-responsive hydrogel as an intelligent switch for controlled drug delivery. Int. J. Biol. Macromol. 2021, 176, 448–458. [Google Scholar] [CrossRef]
- Türk, S.; Altınsoy, I.; Efe, G.Ç.; Ipek, M.; Özacar, M.; Bindal, C. A novel multifunctional NCQDs-based injectable self-crosslinking and in situ forming hydrogel as an innovative stimuli responsive smart drug delivery system for cancer therapy. Mater. Sci. Eng. C 2021, 121, 111829. [Google Scholar] [CrossRef]
- Gu, C.; Wang, C.; Ma, W.; Gao, Y.; Li, J.; Jin, Q.; Wu, X. Drug-Loaded Konjac Glucomannan/Metal–Organic Framework Composite Hydrogels as Antibacterial and Anti-Inflammatory Cell Scaffolds. ACS Appl. Mater. Interfaces 2023, 15, 41287–41298. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Ji, Y.; Zhu, H.; Shi, Z.; Li, M.; Yu, Q. Gallium-based metal–organic frameworks loaded with antimicrobial peptides for synergistic killing of drug-resistant bacteria. J. Mater. Chem. B 2023, 11, 10446–10454. [Google Scholar] [CrossRef] [PubMed]
- Tian, M.; Zhou, L.; Fan, C.; Wang, L.; Lin, X.; Wen, Y.; Su, L.; Dong, H. Bimetal-organic framework/GOx-based hydrogel dressings with antibacterial and inflammatory modulation for wound healing. Acta Biomater. 2023, 158, 252–265. [Google Scholar] [CrossRef] [PubMed]
- Zhan, X.-P.; Zeng, Y.-N.; Li, B.-X.; Zheng, H.-Q.; Feng, H.-X.; Xu, Z.; Liu, J.; Lin, Z.-J. Silver Nanoparticle-Loaded Titanium-Based Metal–Organic Framework for Promoting Antibacterial Performance by Synergistic Chemical–Photodynamic Therapy. Inorg. Chem. 2024, 63, 677–688. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, Y.; Chen, Y.; Fang, Q.; Hussain, M.I.; Wang, L.-N. Flexible Curcumin-Loaded Zn-MOF Hydrogel for Long-Term Drug Release and Antibacterial Activities. Int. J. Mol. Sci. 2023, 24, 11439. [Google Scholar] [CrossRef] [PubMed]
- Vadivelmurugan, A.; Sharmila, R.; Pan, W.-L.; Tsai, S.-W. Preparation and Evaluation of Aminomalononitrile-Coated Ca–Sr Metal–Organic Frameworks as Drug Delivery Carriers for Antibacterial Applications. ACS Omega 2023, 8, 41909–41917. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Zhang, W.; Chen, Z. Magnetic Fe3O4@ZIF-8 nanoparticles as a drug release vehicle: pH-sensitive release of norfloxacin and its antibacterial activity. Colloids Surf. B Biointerfaces 2023, 223, 113170. [Google Scholar] [CrossRef]
- Qiu, L.; Ouyang, C.; Zhang, W.; Liu, J.; Yu, L.; Chen, G.; Ren, L. Zn-MOF hydrogel: Regulation of ROS-mediated inflammatory microenvironment for treatment of atopic dermatitis. J. Nanobiotechnol. 2023, 21, 163. [Google Scholar] [CrossRef]
- Kumar, P.; Behera, A.; Tiwari, P.; Karthik, S.; Biswas, M.; Sonawane, A.; Mobin, S.M. Exploring the antimicrobial potential of isoniazid loaded Cu-based metal–organic frameworks as a novel strategy for effective killing of Mycobacterium tuberculosis. J. Mater. Chem. B 2023, 11, 10929–10940. [Google Scholar] [CrossRef]
- Mo, F.; Zhong, S.; You, T.; Lu, J.; Sun, D. Aptamer and DNAzyme-Functionalized Cu-MOF Hybrid Nanozymes for the Monitoring and Management of Bacteria-Infected Wounds. ACS Appl. Mater. Interfaces 2023, 15, 52114–52127. [Google Scholar] [CrossRef]
- Costa, B.A.; Abuçafy, M.P.; Barbosa, T.W.L.; da Silva, B.L.; Fulindi, R.B.; Isquibola, G.; da Costa, P.I.; Chiavacci, L.A. ZnO@ZIF-8 Nanoparticles as Nanocarrier of Ciprofloxacin for Antimicrobial Activity. Pharmaceutics 2023, 15, 259. [Google Scholar] [CrossRef]
- Perveen, S.; Zhai, R.; Chen, X.; Kanwal, T.; Shah, M.R.; Lu, M.; Ding, B.; Jin, M. Synthesis of high-performance antibacterial agent based on incorporated vancomycin into MOF-modified lignin nanocomposites. Int. J. Biol. Macromol. 2024, 274, 133339. [Google Scholar] [CrossRef]
- Chang, Y.; Xu, K.-Q.; Yang, X.-L.; Xie, M.-H.; Mo, Z.; Li, M.-L.; Ju, H.-X. Zinc hexacyanoferrate/g-C3N4 nanocomposites with enhanced photothermal and photodynamic properties for rapid sterilization and wound healing. Colloids Surf. B Biointerfaces 2024, 240, 113998. [Google Scholar] [CrossRef] [PubMed]
- Zulfiqar, S.; Sharif, S.; Nawaz, M.S.; Shahzad, S.A.; Bashir, M.M.; Iqbal, T.; ur Rehman, I.; Yar, M. Cu-MOF loaded chitosan based freeze-dried highly porous dressings with anti-biofilm and pro-angiogenic activities accelerated Pseudomonas aeruginosa infected wounds healing in rats. Int. J. Biol. Macromol. 2024, 271, 132443. [Google Scholar] [CrossRef]
- Zheng, Y.; Cai, X.; Chen, G.; Xiang, D.; Shi, W.; Shen, J.; Xiang, B. Single Atom-Dispersed Silver Incorporated in ZIF-8-Derived Porous Carbon for Enhanced Photothermal Activity and Antibacterial Activities. Int. J. Nanomed. 2024, 19, 4253–4261. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Jin, W.; Dong, L.; Wang, J.; Li, W.; Song, P.; Tao, Y.; Gui, L.; Zhang, W.; Ge, F. Photothermal/photodynamic synergistic antibacterial study of MOF nanoplatform with SnFe2O4 as the core. Biochem. Biophys. Res. Commun. 2024, 720, 150131. [Google Scholar] [CrossRef]
- Nabipour, H.; Sadr, M.H.; Bardajee, G.R. Synthesis and characterization of nanoscale zeolitic imidazolate frameworks with ciprofloxacin and their applications as antimicrobial agents. New J. Chem. 2017, 41, 7364–7370. [Google Scholar] [CrossRef]


| Nanomaterial | Name of DDS System | Drug | Targeted Bacterial Infections | Mechanism of Action | Ref. |
|---|---|---|---|---|---|
| Nanoparticles | mPEG-TK-MSN | Vancomycin | S. aureus infected wound healing therapy | Cell membrane/cell wall partial disintegration | [21] |
| Antimicrobial peptide-Polydopamine nanoparticles (PdNPs-AMP) | Antimicrobial peptides (AMP) | E. coli | Structural deterioration | [26] | |
| CS-AgNPs | Silver nanoparticles | E. coli and S. aureus | - | [27] | |
| CSNP-CAs | Cinnamaldehyde | S. aureus biofilms eradication | Cell wall damage and permeability | [28] | |
| Rifampin-SLN-P-SA6 | Rifampin | S. epidermidis biofilm eradication | - | [29] | |
| Cefazolin-containing niosome nanoparticles | Cefazolin | MRSA | Biofilm removal | [30] | |
| Ferromagnetic Nanoparticles (Fe3O4@PDA@Mino) | Minocycline (Mino) | Periodontal biofilm eradication | Regulation of inflammatory response | [31] | |
| p(DMAEMA-co-BMA-co-PAA) | Farnesol | Treatment of rodent dental caries (S. mutans biofilms) | Bacterial biofilm penetration | [32] | |
| Quantum dots-Poly lactic-co-glycolic acid (PLGA) (CQD-PLGA NPs) | Azithromycin and tobramycin | Eradication of P. aeruginosa biofilms | Increases bacterial membrane permeability | [33] | |
| PCBDA@AgNPs-CL | AgNPs | Microbe-induced ocular infections (C. albicans) | Resist–kill–remove | [34] | |
| Pt-Se NPs | - | S. enterica, E. coli, L. monocytogenes, S. aureus, and B. cereus | Bacterial cell damage | [35] | |
| XG-AVE-Ag/MgO NCs | Ag and MgO, nanoparticles, Aloe vera extracts | E. coli biofilm removal | Cell wall damage | [36] | |
| Pae-SiO2 NPs | Paeoniflorin | S. aureus and B. cereus | - | [37] | |
| ZrO2-Amp NPs and ZrO2-Ery NPs | Ampicillin and erythromycin | E. coli and B. cereus, in vitro wound healing | Protein and DNA damage | [38] | |
| Tetracycline-loaded ZrO2 NPs | Tetracycline | S. entrica and S. aureus biofilm eradication | Penetration inside the biofilm | [39] | |
| CS-FeNPs | Fe NPs | E. coli biofilms eradication | Protein leakage, cell wall permeability | [40] | |
| Anti-CD54@Cur-DA NPs | Curcumin | Treatment of chronic lung infection (P. aeruginosa) | Inhibiting efflux pump-related genes | [41] | |
| Micelles | Nanostructured antimicrobial micelles (CT9W1000 micelles) | Antimicrobial peptides (T9W) | P. aeruginosa lung infection | ROS production and anti-inflammatory effect | [42] |
| SIR-micelles conjugated mannose targeting ligands | Inflammatory cytokines | Treatment of pneumonia infection of multidrug-resistant K. pneumoniae | Regulate the inflammatory cytokines | [43] | |
| Curcumin-loaded polymeric micelles | Curcumin | S. aureus, E. coli and C. albicans | - | [44] | |
| Caffeic acid graft chitosan copolymer loaded QR micelles (CA-g-CS/QR) | Quercetin | E. coli | In vivo antibacterial activity in broiler chickens | [45] | |
| Polyzwitterionic micelles | Triclosan | S. aureus infection | Drug penetration inside the biofilm kills bacteria | [46] | |
| Chitosan oligosaccharide lactate (COL)-pluronic F127 polymers, loaded with gatifloxacin (Gati@FCOL1/Gati@FCOL2 micelles) | Gatifloxacin | Eradication of P. aeruginosa and S. aureus and treatment of bacterial keratitis | Anti-quorum sensing (QS) effect | [47] | |
| Antibacterial Micelles- Carboxymethyl Chitosan (CC)/Oxidized Konjac Glucomannan (OKG) stevioside-stabilized honokiol (HS) (CC45/OKG40/HS hydrogel) | Honokiol | S. aureus infected Wound Healing | Eradicate the bacterial infection and regulate the inflammatory response | [48] | |
| PEG-b-PPTyr micelles | α-helical cationic polypeptide | E. coli infected wound healing | Eradication of bacterial biofilm and regulating the anti-inflammatory response | [49] | |
| CIP@FCBMs | Ciprofloxacin | Eradication of biofilms and MRSA-infected wound healing | Targeting the bacterial proteins and nucleic acid synthesis | [50] | |
| Cur-EPS conjugate-based polymeric micelles | Curcumin | Antioxidant, eradication of E. coli, S. aureus, P. aeruginosa, S. typhimurium, and S. marcescens biofilms | Antibacterial, antibiofilm, and antioxidant mechanisms | [51] | |
| Liposomes | Liposome-based nanoreactor (RFP-CaO2@PCM@Lec) | Eutectic antimicrobial mixture | Treatment of MRSA-infected wounds | Antimicrobial release through pore formation | [52] |
| Liposome-based bacterial microbats | Liposomal drug | E. coli infection | Lipid bilayer permeabilization | [22] | |
| Asiaticoside-Loaded Liposomes (rColMA/QCSG/LIP@AS/Ag@MOF (RQLAg) hydrogel | Asiaticoside | E. coli and S. aureus | Destroy the cell membrane | [53] | |
| Dendrimers | Amino acid-conjugated cationic dendrimers (CDs) | UOACDs | E. coli, K. pneumoniae, MRSA, and MRSE | - | [54] |
| PLGA/PTM; PAMAM/PTM NPs | PTM | S. aureus (mouse peritonitis model) | S. aureus cell membranes interactions | [55] | |
| Ag-loaded poly(amide-amine) dendrimer | Ag | E. coli and S. aureus | - | [56] | |
| Dendrimer G4 poloxamer nanoparticles | Coumarin | MRSA | Drug penetration and uptake, cellular damage | [57] | |
| Erythromycin-conjugated nano dendrimer | Erythromycin | S. aureus, S. epidermidis, S. saprophyticus, and P. aeruginosa | Membrane permeability and bacterial lysis | [58] | |
| Gelatin and gelatin Star-shaped polyamidoamine (PAMAM) dendrimer G3.5 (sIPN NCs) | Silver acetate | S. aureus and P. aeruginosa | Release kill mechanism | [59] |
| Composite\Carrier Composition | MOF Average Size | Drug | Bioactivity | Drug Loading Capacity | Reference |
|---|---|---|---|---|---|
| van@ZIF-8@PDA | 175.9 ± 2.74 nm | Vancomycin | Eradication of S. aureus biofilms and treatment of bacteria-infected wounds | 6.71% | [16] |
| Pd-Cu nanoalloy ZIF-8 | 155.3 nm | Amoxicillin | Eradication of P. aeruginosa and S. aureus biofilm; S. aureus infected wound healing | - | [60] |
| ZJU-101 | 300 nm | Diclofenac sodium | - | (∼0.546 g/g) | [72] |
| UiO-66 | 1.22 nm pore diameter | Ciprofloxacin | 24 mm (E. coli) 22 mm (S. aureus) inhibition zones | 84%. | [73] |
| UiO-66-NH2 | 200 nm | Quinazoline | 0.25–0.7 mg m/L MIC 0.25–4 mg m/L MBC | [74] | |
| MIL-101(Cr) | SBET—103 (m2 g−1 Vp—2.50 (cm3 g−1) | Ibuprofen and nimesulide | - | IBU, NMS (850, 443 mg g−1) | [75] |
| TGIAVE-Ag | 25 nm | 5-FU | Inhibited K. pneumonia, P. aeruginosa, E. coli, and S. aureus | 89.13 ± 1.4% | [76] |
| Rifampicin@ZIF-8 | 157.96 ± 1.07 nm | Rifampicin | Inhibited S. aureus | [77] | |
| Fe3O4@PAA@ZIF-8 | 50–200 nm | Ciprofloxacin | Inhibited E. coli and S. aureus | [78] | |
| Hydrogel (CMC/PNIPAM-co-PAM). | 39.782–38.235 g/g | Tetracycline | >85% scavenging efficiency | [79] | |
| NCQDs/Dox/HA | 4–6 nm, 4.89 nm diameter | Doxorubicin | Inhibited S. aureus | [80] | |
| KGM/MOF Hydrogels | - | Honokiol, caffeic acid, osthole, baicalein, palmatine, pterostilbene, quercetin, and luteolin | S. aureus | 0.09 mg/mg–0.157 mg/mg | [81] |
| MEL-loaded MOF (MM) | <1 µM | Antimicrobial peptides | MRSA | - | [82] |
| MOF(Fe-Cu)/GOx-polyacrylamide (PAM) gel | 280 nm | Fe-Cu | E. coli and S. aureus; infected wound healing by modulation of antibacterial and inflammatory | - | [83] |
| Ag NPs@ACM-1 | 370 to 700 nm | AgNPs | E. coli and S. aureus | - | [84] |
| Curcumin-Loaded Zn-MOF Hydrogel | - | Curcumin | E. coli and S. aureus | - | [85] |
| Ca–Sr–AMN–MOF | Ca, Sr | E. coli | - | [86] | |
| NOR-Fe3O4@ZIF-8 nanoparticles | 20 nm | Norfloxacin | E. coli | - | [87] |
| Zn-MOF(ZIF-8)-PVA-Gel | 98.72 nm | Zn-MOF(ZIF-8) | Infected wound healing and antibacterial activity against S. aureus | - | [88] |
| Isoniazid-loaded Cu-based metal-organic frameworks | - | Isoniazid | Inhibition of Mycobacterium tuberculosis biofilm | 10% | [89] |
| CoCu-ZIF and ZnCu-ZIF | - | G-quadruplex/hemin DNAzyme-aptamer functionalized | MRSA | - | [90] |
| ZnO@ZIF-8 | 1.29 ± 0.45 μm | ZnO | S. aureus, and P. aeruginosa | 30.23% | [91] |
| Lig-Van-MOF | 242.48 ± 12.20 nm | Vancomycin | E. coli and S. aureus | 84.25 ± 2.50% | [92] |
| Zn3[Fe(CN)6]/g-C3N4 | 500 nm | zinc hexacyanoferrate | E. coli and S. aureus and wound healing effect | - | [93] |
| Cu-MOF/CS | Pore size: 11.56 μm | Cu | E. coli, P. aeruginosa, S. aureus, and MRSA and P. aeruginosa infected wound healing | - | [94] |
| AgSA-ZDPC | 40–50 nm | Single atom-dispersed silver | S. aureus and E. coli | - | [95] |
| SnFe2O4-PBA/Ce6@ZIF-8 (SBC@ZIF-8) | 50–100 nm | 3-aminobenzeneboronic acid (PBA) and dihydroporphyrin e6 (Ce6) | MDR S. aureus infected wound healing | - | [96] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
MubarakAli, D.; Saravanakumar, K.; Ganeshalingam, A.; Santosh, S.S.; De Silva, S.; Park, J.U.; Lee, C.-M.; Cho, S.-H.; Kim, S.-R.; Cho, N.; et al. Recent Progress in Multifunctional Stimuli-Responsive Combinational Drug Delivery Systems for the Treatment of Biofilm-Forming Bacterial Infections. Pharmaceutics 2024, 16, 976. https://doi.org/10.3390/pharmaceutics16080976
MubarakAli D, Saravanakumar K, Ganeshalingam A, Santosh SS, De Silva S, Park JU, Lee C-M, Cho S-H, Kim S-R, Cho N, et al. Recent Progress in Multifunctional Stimuli-Responsive Combinational Drug Delivery Systems for the Treatment of Biofilm-Forming Bacterial Infections. Pharmaceutics. 2024; 16(8):976. https://doi.org/10.3390/pharmaceutics16080976
Chicago/Turabian StyleMubarakAli, Davoodbasha, Kandasamy Saravanakumar, Archchana Ganeshalingam, Sugavaneswaran Siva Santosh, Shanali De Silva, Jung Up Park, Chang-Min Lee, Su-Hyeon Cho, Song-Rae Kim, Namki Cho, and et al. 2024. "Recent Progress in Multifunctional Stimuli-Responsive Combinational Drug Delivery Systems for the Treatment of Biofilm-Forming Bacterial Infections" Pharmaceutics 16, no. 8: 976. https://doi.org/10.3390/pharmaceutics16080976






